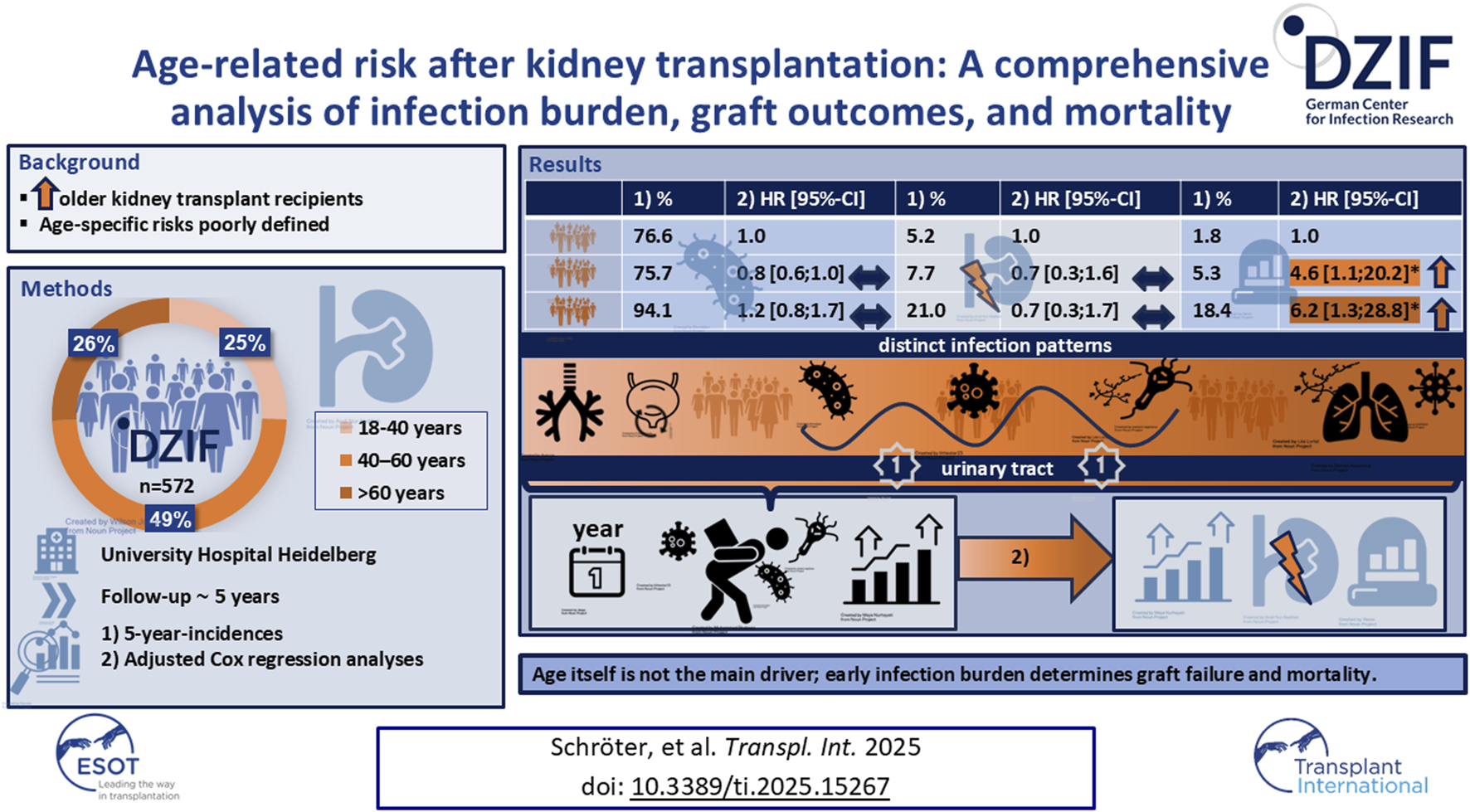
Abstract
Given the increasing number of kidney transplantation in elderly recipients, understanding age-specific risks is essential for optimized post-transplant care. We analyzed 572 kidney transplant recipients from the DZIF Transplant Cohort (2012–2023), stratified by age: <40 (n = 146), 40–60 (n = 279), >60 years (n = 147). Outcomes included infection burden, graft outcomes, and mortality over a median follow-up of 5 years. Multivariable Cox models with inverse probability weighting, adjusted for clinical confounders, was applied. In older recipients, the unadjusted 5-year rates of graft failure, mortality, and infections were significantly higher—both overall and for specific types, including pneumonia, urinary tract infections, invasive opportunistic infections, and multidrug-resistant infections. After adjustment, age remained only independently associated with mortality (HR = 6.21, p = 0.02), but not with overall infection burden or graft loss. Older patients exhibited a shift in pathogen prevalence, particularly for Pseudomonas aeruginosa and more severe herpesvirus infections, as well as higher infection-related morbidity, which contributed to graft failure. The first post-transplant year was critical, with infection burden strongly predicting graft failure (HR 1.16, p < 0.01). Age alone generally does not predict adverse transplant outcomes. Post-transplant care in elderly recipients should focus on early infection control with pathogen-targeted surveillance.
Graphical Abstract
Introduction
The prevalence of end-stage renal disease (ESRD) continues to rise in the aging population, making kidney transplantation increasingly common among older recipients [1–3]. This trend reflects demographic shifts and the increasing recognition of transplantation as a viable treatment option for selected elderly patients [1, 2, 4, 5]. However, older recipients face particular challenges due to immunosenescence, which alters immune system function and increases sensitivity to immunosuppressive therapy [6, 7]. This, together with greater frailty and a higher burden of comorbidities, complicates clinical management [7].
Guidelines emphasize emphasize individualized assessment rather than age-based exclusion [2]. Nevertheless, the protocols for immunosuppression, prophylaxis, and post-transplant monitoring are largely consistent across all age groups, due to limited data on age-specific complications [8, 9]. Older age is generally considered a universal risk factor for higher infection rates, early hospital readmissions, reduced graft function, increased graft loss, and mortality [7, 10–22]. The increasing number of older transplant recipients underscores the urgent need for a more nuanced understanding of age-related risks. However, there are a few studies that comprehensively evaluate these outcomes in parallel and adequately account for confounding variables. Furthermore, long-term data from Western European cohorts remain scarce, particularly on the type, severity, and timing of infections in older recipients. Most studies are limited by small sample sizes and focus primarily on infections occurring within the first year [11, 17, 18, 23]. This study investigates the association between recipient age and various short- and long-term post-transplant outcomes, including infection burden, graft function, acute rejection, graft loss, and mortality. The primary objective is to assess whether age independently predicts these outcomes after adjusting for relevant confounders. Secondary objectives are to characterize infection types, their timing, severity, and pathogens in different age groups. We hypothesize that although older age is initially associated with worse outcomes, this association will largely persist after extensive adjustment for selected endpoints. Furthermore, we expect to identify age-related differences in infection profiles, potentially leading to more targeted prevention and surveillance strategies.
Patients and Methods
Study Cohort, Ethics, and Follow-Up
The DZIF Transplant Cohort is a multicenter, prospective study within the framework of the German Center for Infection Research (DZIF) that focuses on transplant recipients and their infection risks [24]. This study specifically includes adult patients who received a kidney or simultaneous pancreas-kidney transplant at the University Hospital Heidelberg between January 2012 and December 2023. The Ethics Committee of the Medical Faculty of Heidelberg University approved for the study (No. S-585/2013), and all participants provided written informed consent. Clinical events were systematically recorded and evaluated, including all events up to December 2024. Follow-up examinations were performed at baseline, 3, 6, 9, and 12 months after transplantation, with additional annual visits thereafter or as clinically needed in case of complications. Clinical, laboratory, and demographic data were collected from medical records [24]. All recipients were followed for at least 1 year post-transplantation, unless death or graft loss occurred earlier.
Immunosuppressive Regimen
The standard immunosuppressive protocol consisted of a calcineurin inhibitor - either tacrolimus (Tac) or ciclosporin A (CsA) - in combination with mycophenolate sodium (1.44 g/day) or mycophenolate mofetil (2 g/day), and methylprednisolone. Target trough (C0) levels for Tac were: month 1 (6–9 ng/mL), month 3 (5–8 ng/mL), thereafter (4–7 ng/mL); and for CsA: month 1 (150–180 ng/mL), month 3 (100–150 ng/mL), thereafter (80–120 ng/mL). Induction therapy included either basiliximab or thymoglobulin. Patients with previous transplants or high levels of donor-specific anti-HLA antibodies (DSA) were classified as highly sensitized. Additional desensitization strategies such as plasmapheresis or immunoadsorption were used in these patients, as well as in AB0-incompatible recipients.
Prophylaxis and Surveillance Strategy
Prophylaxis and monitoring were performed according to the KDIGO 2009 guidelines [25]. For Pneumocystis jirovecii prophylaxis, trimethoprim-sulfamethoxazole (800 mg/160 mg) was routinely administered three times a week for the first 6 months after transplantation. Standard antiviral prophylaxis with valganciclovir was administered to all Cytomegalovirus (CMV) immunoglobulin G (IgG)–positive recipients and recipients of CMV IgG–positive donor organs for at least 3 months. In high-risk (D+/R–) cases, 6 months of antiviral prophylaxis was recommended. The dosage was adjusted according to renal function. Candida prophylaxis with oral nystatin was provided within the first one to 3 months if more than 20 mg of methylprednisolone was administered daily.
Definitions of Infectious Complications
All infections requiring hospitalization were included. Diagnoses were made by the treating physician. Data collected included clinical presentation, laboratory and microbiological/virological findings, diagnostic procedures, treatment, disease course, and infection-related outcomes. Detailed infection definitions are provided in the (Supplementary Table S1).
Acute Rejections
Acute rejection was diagnosed by histopathological examination of renal biopsies by a trained local kidney pathologists based on morphological assessment and immunohistochemical markers. Rejection episodes were classified according to the Banff criteria [26].
Statistical Methods
Baseline characteristics were summarized using means ± standard deviations or medians with interquartile ranges for continuous variables and number with percentages for categorical variables. Group comparisons were performed using Pearson’s chi-squared test or Fisher’s exact test for categorical data, and Student’s t-test or Mann–Whitney U test for continuous variables, as appropriate. Survival probabilities were estimated using the Kaplan–Meier method and compared using the log-rank test. The association between recipient age (<40, 40–60, >60 years) was first explored through univariable Cox proportional hazards regression models. To estimate independent effects, propensity scores were derived from a multinomial logistic regression model including key baseline covariates. Inverse probability weighting (IPW) based on these propensity scores was then applied to create a weighted pseudo-population minimizing residual confounding. Subsequently, IPW-weighted multivariable Cox regressions were fitted to assess independent associations between recipient age and outcomes. Variables with a p-value <0.1 in the weighted univariate analyses and clinically relevant variables were entered into multivariable models, while age group was retained in all models. Hazard ratios (HRs) with 95% confidence intervals (CIs) were reported; p < 0.05 was considered statistically significant.
Sensitivity analyses included unweighted and trimmed (2.5th–97.5th percentile) IPW models, as well as models with and without adjustment for first-year infection burden (Supplementary Table S3). To account for potential COVID-19 bias, follow-up was additionally censored at 1 March 2020, or modeled with a pandemic indicator (March 2020–December 2022).
Death before graft failure was treated as a censoring event. Variables were handled by complete-case analysis within each model. Given the overall low proportion of missing data (<5% across key variables), multiple imputation was not deemed necessary.
Risk factors for infection burden within the first post-transplant year were analyzed using negative binomial regression.
All statistical analyses were conducted using R software version 2024.12.0 + 467.
Results
A total of 572 adult kidney transplant recipients (62.6% male, mean age 49 ± 14 years) were included and divided into three age groups: <40 years (25.5%), 40–60 years (48.8%), and >60 years (25.7%). Clinical and demographic characteristics are summarized in Table 1. The median follow-up was 61 months (IQR 30–87), with no significant differences between groups.
TABLE 1
| Total | <40 Years | 40–60 Years | >60 Years | |
|---|---|---|---|---|
| No. of patients | 572 | 147 | 279 | 146 |
| Demographics | ||||
| Age (mean ± SD) | 49.4 ± 13.5 | 30.8 ± 5.5 | 52.2 ± 5.5 | 65.6 ± 3.4 |
| Male gender (%) | 62.6 | 59.9 | 62.4 | 65.8 |
| Clinical data | ||||
| Body mass index (mean ± SD) in kg/m2 | 26.5 ± 4.7 | 25.0 ± 4.6 | 27.0 ± 4.9 | 27.0 ± 4.1 |
| Diabetes mellitus (%) | 20.6 | 9.6 | 15.8 | 25.3 |
| Cause of ESRD | ||||
| Glomerulonephritis (%) | 26.9 | 17.7 | 30.5 | 29.5 |
| ADPKD (%) | 17.5 | 10.2 | 18.3 | 23.3 |
| Diabetes mellitus (%) | 8.2 | 5.4 | 9.3 | 8.9 |
| Nephrosclerosis (%) | 10.5 | 6.8 | 10.0 | 14.4 |
| Intestinal nephritis (%) | 3.0 | 2.7 | 3.2 | 2.7 |
| Vasculitis and collagenoses (%) | 9.3 | 12.9 | 9.4 | 5.5 |
| Urological diseases (%) | 4.7 | 10.2 | 3.2 | 2.1 |
| Other hereditary diseases (%) | 4.9 | 15.0 | 2.2 | 1.4 |
| Other (%) | 15.0 | 19.0 | 14.0 | 12.3 |
| Donor characteristics | ||||
| Donor age | 55.4 ± 14.9 | 52.2 ± 11.1 | 52.2 ± 14.6 | 64.7 ± 15.0 |
| Male donor | 43.3 | 44.5 | 44.1 | 40.4 |
| CMV IgG serology | ||||
| D+/R- (%) | 20.2 | 19.0 | 20.6 | 20.5 |
| D+/R+ (%) | 33.2 | 34.0 | 30.0 | 38.4 |
| D-/R+ (%) | 24.6 | 23.1 | 23.8 | 27.4 |
| D-/R- (%) | 22.1 | 23.8 | 25.6 | 13.7 |
| Type of transplantation and immunology | ||||
| Living donation (%) | 33.6 | 53.7 | 33.7 | 13.0 |
| AB0i (%) | 4.6 | 6.8 | 4.3 | 2.7 |
| High sensitization (%) | 9.3 | 10.2 | 11.8 | 3.4 |
| ESP (%) | 12.2 | 0.0 | 0.0 | |
| Pancreas-kidney (%) | 3.3 | 4.1 | 4.7 | 0.0 |
| Cold ischemia time (min), mean ± SD | 565.5 ± 378.5 | 448.5 ± 392.1 | 587.2 ± 397.8 | 641.0 ± 292.7 |
| Mean number of HLA matches (±SD) | 2.9 ± 1.6 | 3.1 ± 1.4 | 3.0 ± 1.6 | 2.4 ± 1.6 |
| Mean number of HLA mismatches (±SD) | 2.8 ± 1.6 | 2.7 ± 1.3 | 2.7 ± 1.7 | 3.3 ± 1.8 |
| Immunosuppression | ||||
| Induction therapy | ||||
| Basiliximab (%) | 75.0 | 73.5 | 72.0 | 82.1 |
| Thymoglobuline (%) | 25.0 | 26.5 | 28.0 | 17.9 |
| Rituximab (%) | 9.4 | 14.3 | 10.0 | 3.4 |
| Plasmapheresis (%) | 20.1 | 26.5 | 21.9 | 10.3 |
| Immunadsorption (%) | 3.8 | 5.4 | 3.9 | 2.1 |
| Maintenance therapy at discharge | ||||
| Tacrolimus + MPA/MMF + Steroids (%) | 75.2 | 80.3 | 76.3 | 67.8 |
| Ciclosporine A + MPA/MMF + Steroids (%) | 24.0 | 19.0 | 23.7 | 29.5 |
| Antimicrobial prophylaxis | ||||
| Valganciclovir (%) | 62.9 | 65.3 | 59.9 | 65.8 |
| Cotrimoxazol (%) | 84.1 | 88.4 | 83.5 | 80.8 |
| Dapson (%) | 15.9 | 11.6 | 16.5 | 19.2 |
| Post-operative course | ||||
| Delayed graft function [1] | 27.0 | 22.4 | 24.5 | 36.3 |
Clinical and demographic characteristics of the total cohort and stratified by age group.
Missing values were excluded. Variables were handled by complete case analysis. Abbreviations: AB0i = AB0 incompatibility, ADPKD = autosomal dominant polycystic kidney disease, C0 = trough level, CMV = cytomegalovirus, D+/− = CMV IgG donor positive/negative, ESRD = end-stage renal disease, ESP = Eurotransplant Senior Program, HLA = human leukocyte antigen, IgG = immunoglobulin G, IQR = interquartile range, Md = median, MMF = mycophenolate mofetil, MPA = mycophenolic acid, R+/− = CMV IgG recipient positive/negative, SD = standard deviation [1]. Defined as the requirement for dialysis within the first 7 days after kidney transplantation, excluding dialysis performed solely for hyperkalemia.
Figures 1a–d displays Kaplan–Meier curves for long-term infection-free (a), graft-failure-free (b), rejection-free (c), and overall survival (d), stratified by age.
FIGURE 1
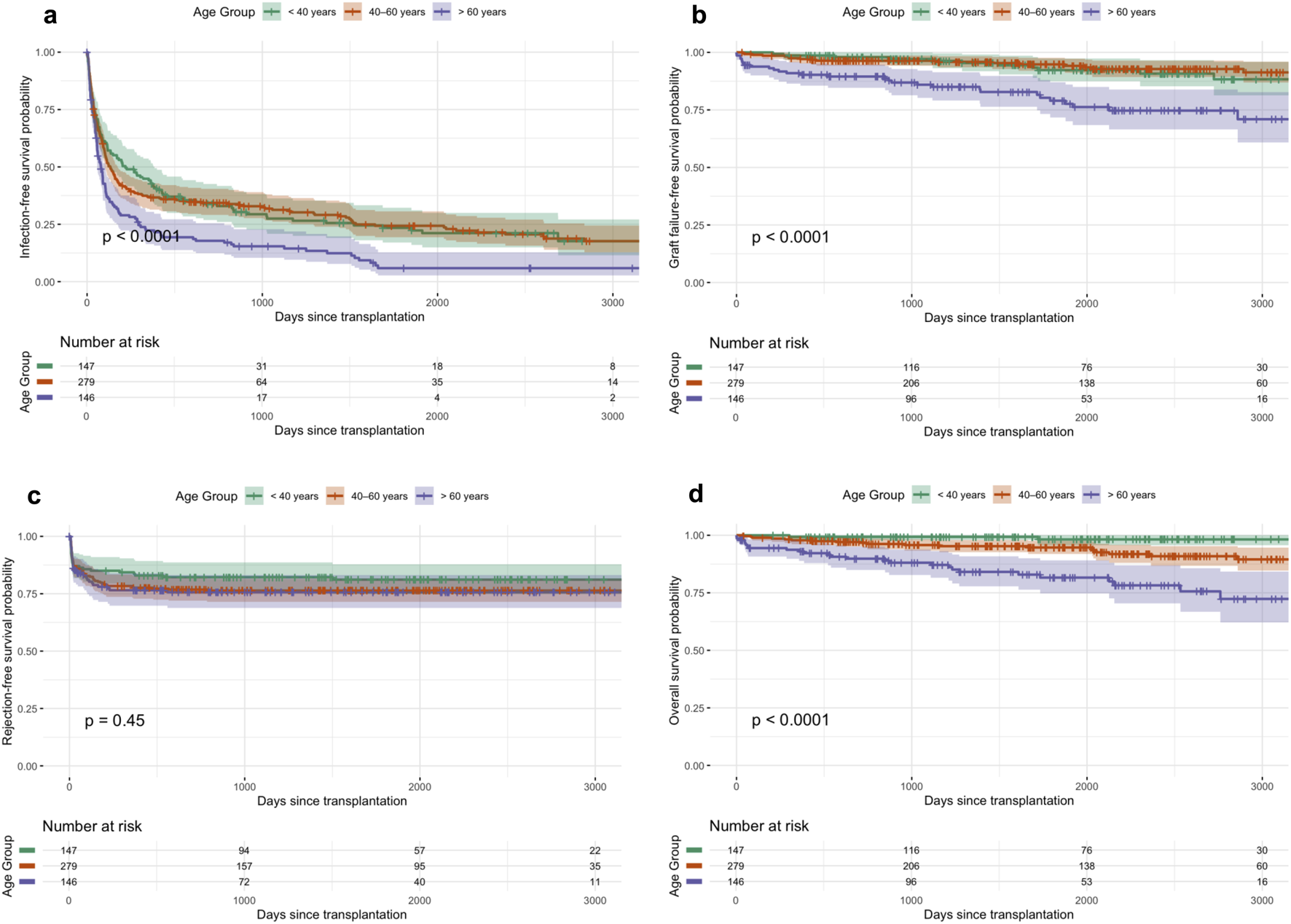
(a–d) Kaplan-Meier curves for long-term infection-free (a), graft-failure-free (b), rejection-free survival (c), and overall survival (d) across age groups.
GFR Dynamics and Immunosuppression
The incidence of delayed graft function (DGF) increased significantly with recipient age, rising from 22.4% in recipients <40 years to 36.3% in those >60 years (p = 0.012, Table 1). Across all age groups, there was a significant improvement in estimated glomerular filtration rate (eGFR) from month 1 to month 3, with the greatest relative increase observed in recipients >60 years (median +50.2% from the baseline median of 24.5 mL/min/1.73 m2). Younger recipients maintained higher absolute eGFR levels throughout the first year (Figure 3a). In recipients >60 years, lower tacrolimus doses were required to achieve comparable and adequate drug levels (Figure 3b).
Overall Infection Incidences and Age-Related Patterns of Bacterial, Viral, and Fungal Infections
Short and long-term infection incidences are provided in Table 2. Older recipients had the highest overall infection rates, with a 5-year incidence of 94.1% (p < 0.001; Table 2). Bacterial infections were more frequent in older patients (>60: 77.0%, p < 0.0001), while viral infections occurred at similar rates across all age groups (p = 0.761). Fungal infections were significantly more common in older recipients (>60: 9.8% vs. <40: 0.8%, 40–60: 3.3%; p = 0.002).
TABLE 2
| Outcome and time interval | Total | <40 Years | 40–60 Years | >60 Years | p |
|---|---|---|---|---|---|
| Mortality | |||||
| Overall day 0–30 day 0–180 day 0–365 year 2 year 3 year 5 |
17.1 [11.5; 25.5] 0.5 [0.3; 1.6] 1.9 [1.1; 3.5] 3.0 [1.9; 4.8] 4.2 [2.8; 6.2] 5.2 [3.6; 7.5] 7.6 [5.5; 10.4] |
1.8 [0.4; 7.5] 0.0 [0.0; 0.0] 0.0 [0.0; 0.0] 0.7 [0.1; 0.5] 0.7 [0.1; 0.5] 0.7 [0.1; 0.5] 1.8 [0.4; 7.5] |
12.3 [7.5; 20.1] 0.0 [0.0; 0.0] 1.1 [0.4; 3.3] 2.2 [1.0; 3.8] 2.9 [1.5; 5.8] 4.3 [2.4; 7.6] 5.3 [3.1; 9.1] |
51.8 [28.8; 93.1] 2.1 [0.7; 6.3] 5.6 [2.8; 10.9] 7.0 [3.9; 12.8] 10.2 [6.2; 16.7] 11.9 [7.5; 18.9] 18.4 [12.4; 27.2] |
<0.0001 |
| Graft failure | |||||
| Overall day 0–30 day 0–180 day 0–365 year 2 year 3 year 5 |
14.4 [10.9; 19.0] 0.9 [0.4; 2.1] 2.3 [1.3; 3.9] 4.2 [2.9; 6.3] 5.0 [3.5; 7.1] 6.1 [4.3; 8.4] 9.8 [7.4; 12.9] |
8.7 [5.2; 14.6] 0.0 [0.0; 0.0] 1.4 [0.5; 3.8] 2.9 [1.5; 5.7] 3.6 [2.0; 6.7] 3.6 [2.0; 6.7] 5.2 [3.0; 8.9] |
11.7 [6.3; 21.8] 0.0 [0.0; 0.0] 0.0 [0.0; 0.0] 1.4 [0.3; 5.4] 2.1 [0.7; 6.4] 2.9 [1.1; 7.6] 7.7 [4.1; 14.6] |
29.0 [20.0; 42.2] 3.4 [1.4; 8.1] 6.2 [3.3; 11.6] 9.7 [5.0; 16.0] 10.5 [6.5; 16.9] 14.0 [9.2; 21.4] 21.0 [14.6; 30.2] |
<0.0001 |
| Acute rejection | |||||
| Overall day 0–30 day 0–180 day 0–365 year 2 year 3 year 5 |
22.6 [19.3; 26.3] 13.9 [11.3; 17.0] 19.2 [16.3; 22.8] 20.9 [17.8; 24.5] 22.0 [18.9; 25.7] 22.3 [19.1; 26.0] 22.6 [19.3; 26.3] |
18.9 [13.4; 26.6] 12.9 [8.5; 19.7] 15.0 [10.2; 22.0] 15.7 [10.8; 22.8] 17.8 [12.5; 25.2] 17.8 [12.5; 25.2] 18.9 [13.4; 26.6] |
23.6 [19.1; 29.2] 14.0 [10.4; 18.7] 20.2 [16.0; 25.5] 22.4 [18.0; 27.9] 23.2 [18.7; 28.7] 23.6 [19.1; 29.2] 23.6 [19.1; 29.2] |
24.3 [18.1; 32.7] 14.6 [9.8; 21.7] 22.0 [16.1; 30.0] 23.5 [17.4; 31.7] 24.3 [18.1; 32.7] 24.3 [18.1; 32.7] 24.3 [18.1; 32.7] |
0.4480 |
| Infection (any) | |||||
| Overall day 0–30 day 0–180 day 0–365 year 2 year 3 year 5 |
85.4 [81.8; 89.1] 21.7 [18.6; 25.4] 57.4 [53.4; 61.6] 65.3 [61.5; 69.4] 69.9 [66.2; 73.9] 73.7 [70.0; 77.5] 80.6 [77.0; 84.3] |
82.4 [75.1; 90.3] 21.8 [16.0; 29,6] 46.3 [38.9; 55.1] 57.2 [49.8; 65.8] 66.2 [58.8; 74.5] 72.5 [65.3; 80.5] 76.6 [69.5; 84.4] |
82.3 [76.8; 88.3] 19.7 [15.6; 25.0] 57.0 [51.5; 63.2] 63.3 [57.9; 69.3] 65.7 [60.3; 71.6] 68.7 [63.3; 74.6] 75.7 [70.3; 81.5] |
94.1 [89.7; 98.8] 25.6 [19.4; 33.8] 69.6 [62.4; 77.7] 77.7 [71.1; 84.9] 82.2 [76.1; 88.9] 84.6 [787; 90.9] 94.1 [89.7; 98.8] |
<0.0001 |
| Bacterial infection | |||||
| Overall day 0–30 day 0–180 day 0–365 year 2 year 3 year 5 |
64.5 [59.6; 69.9] 17.5 [14.7; 20.9] 35.3 [31.6; 39.5] 40.8 [36.9; 45.0] 44.9 [41.0; 49.3] 49.7 [45.6; 54.1] 56.7 [52.5; 61.4] |
62.8 [53.7; 73.5] 17.0 [11.9; 24.3] 25.2 [19.0; 33.3] 32.0 [25.3; 40.6] 37.1 [30.0; 45.9] 42.9 [35.4; 52.0] 54.0 [45.8; 63.6] |
55.6 [48.6; 63.7] 15.4 [11.7; 20.3] 32.2 [27.1; 38.2] 35.5 [30.3; 41.6] 39.0 [33.6; 45.2] 42.4 [36.8; 48.8] 47.9 [41.9; 54.6] |
85.2 [75.1; 96.6] 22.1 [16.3; 30.0] 52.0 [44.4; 61.0] 59.5 [51.8; 68.2] 64.8 [57.3; 73.3] 71.1 [63.7; 79.4] 77.0 [69.6; 85.1] |
<0.0001 |
| Viral infection | |||||
| Overall day 0–30 day 0–180 day 0–365 year 2 year 3 year 5 |
64.8 [52.7; 79.8] 2.3 [1.3; 3.9] 28.4 [24.9; 32.9] 36.2 [32.4; 40.4] 41.4 [37.4; 45.7] 45.1 [41.0; 49.5] 49.5 [45.3; 54.1] |
76.0 [48.9;-] 2.7 [1.0; 7.2] 25.9 [19.7; 34.0] 35.5 [28.5; 44.1] 42.1 [34.7; 51.0] 46.4 [38.8; 55.6] 47.5 [39.8; 56.7] |
57.4 [49.9; 65.9] 2.2 [1.0; 4.7] 29.0 [24.1; 34.9] 37.1 [31.8; 43.2] 40.5 [35.1; 46.8] 43.8 [38.2; 50.2] 48.7 [42.8; 55.4] |
67.3 [53.9; 84.1] 2.1 [0.7; 6.4] 30.6 [23.8; 39.4] 35.2 [28.0; 44.2] 42.6 [34.9; 51.9] 46.3 [38.4; 55.9] 53.6 [45.1; 63.7] |
0.761 |
| Fungal infection | |||||
| Overall day 0–30 day 0–180 day 0–365 year 2 year 3 year 5 |
4.6 [3.0; 6.9] 0.7 [0.3; 1.9] 1.9 [1.1:3.5] 2.5 [1.5; 4.2] 3.3 [2.1; 5.1] 3.9 [2.6; 6.0] 4.2 [2.8; 6.3] |
0.8 [0.1; 5.4] - - - 0.8 [0.1; 5.4] 0.8 [0.1; 5.4] 0.8 [0.1; 5.4] |
4.0 [2.1; 7.6] 0.4 [0.1; 2.5] 2.2 [1.0; 4.8] 2.5 [1.2; 5.3] 3.3 [1.7; 6.2] 3.3 [1.7; 6.2] 3.3 [1.7; 6.2] |
9.8 [5.7; 16.9] 2.1 [0.7; 6.4] 3.5 [1.5; 8.3] 5.0 [2.4; 10.4] 5.8 [3.0; 11.4] 8.7 [4.9; 15.5] 9.8 [5.7; 16.9] |
0.002 |
| Urinary tract infection | |||||
| Overall | 29.8 [26.2; 33.8] | 22.5 [16.7; 30.4] | 28.4 [23.5; 34.2] | 40.4 [33.0; 49.5] | 0.003 |
| Pneumonia | |||||
| Overall | 23.9 [19.4; 29.6] | 20.0 [12.3; 32.6] | 18.1 [13.3; 24.7] | 45.1 [30.6; 66.5] | <0.0001 |
| Upper respiratory tract infection | |||||
| Overall | 23.6 [19,0.1; 29.3] | 25.5 [18.1; 35.8] | 24.1 [18.0; 32.4] | 22.2 [12.2; 40.4] | 0.167 |
| Gastrointestinal infection | |||||
| Overall | 27.9 [17.4; 44.5] | 25.1 [16.8; 37.5] | 24.2 [10.4; 56.2] | 35.1 [23.1; 53.4] | <0.0001 |
| Sepsis | |||||
| Overall | 21.8 [17.3; 27.5] | 22.7 [14.1; 36.6] | 20.1 [14.3; 28.1] | 23.5 [15.9; 34.7] | 0.236 |
| Invasive opportunistic infection | |||||
| Overall | 9.1 [6.3; 13.1] | 3.4 [1.3; 9.0] | 10.0 [5.9; 16.9] | 12.7 [7.8; 20.6] | 0.011 |
Age-stratified short- and long-term incidences of clinical outcomes.
Cumulative-incidences with 95%-confidence-intervals.
Urinary Tract Infections
UTIs were the most common infection type across all age groups, accounting for approximately 34%–35% of all infections. Although older age was initially associated with a higher risk of UTI, this association was no longer significant after multivariable adjustment (Figure 2; Supplementary Table S2).
FIGURE 2
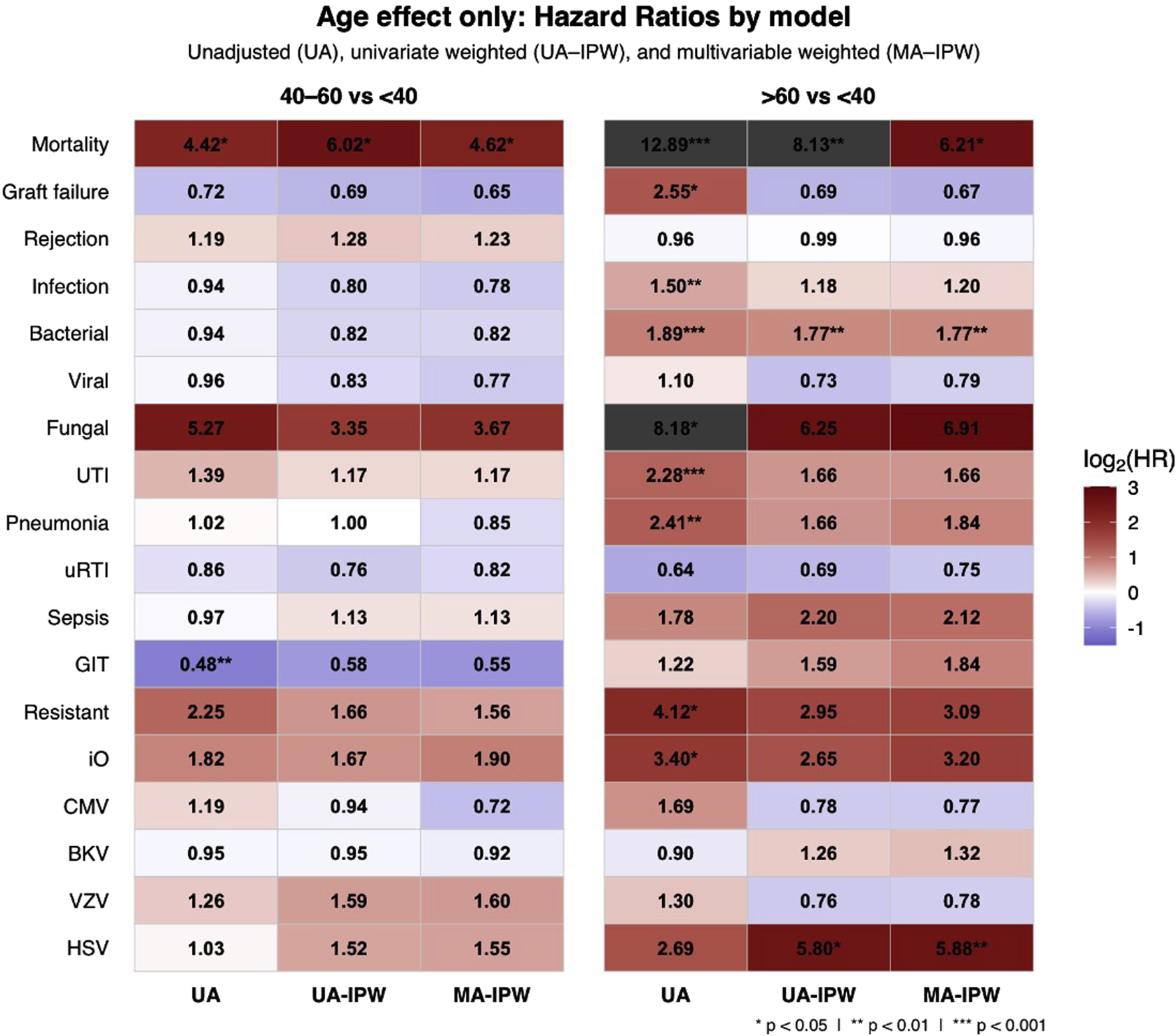
Heatmap of log2-transformed hazard ratios (HRs) showing the independent effect of age on clinical outcomes by age group using a three-step IPW-adjusted modeling approach. The heatmap displays HRs with 95% confidence intervals (CIs) comparing age groups 40–60 and >60 years to the reference group <40 years. Colors indicate effect size. The three-step approach includes unadjusted Cox (UA), inverse probability weighted univariate (UA-IPW), and IPW-adjusted multivariable (MA-IPW) models, where age group and variables with p < 0.1 in UA-IPW and clinically relevant variables were included in the MA-IPW model. All covariates analyzed and included are detailed in Table 2 and Supplementary Table S2. Abbreviations: UTI; urinary tract infection; uRTI; upper respiratory tract infection; GIT – gastrointestinal infection; iO – invasive opportunistic; CMV – cytomegalovirus; BKV – BK polyomavirus; VZV – varicella zoster virus; HSV – herpes simplex virus.
The number of UTIs per infected patient was highest in recipients <40 years (3.6 episodes), followed by >60 years (3.2) and 40–60 years (2.7). Across groups, E. coli (42%, 38%, 35%) and Enterococcus spp. (30%, 22%, 30%) were the leading uropathogens. Pseudomonas aeruginosa prevalence increased with age (6% in <40 vs. 18% in >60), as did Enterobacter spp. (2%, 4%, 5%), while Klebsiella spp. decreased (21%, 18%, 12%) (Supplementary Figure S2). Resistant uropathogens were more frequent in recipients >60 years (17.6% vs. 6.7% in <40), but age was not an independent risk factor for resistant infections after full adjustment (Figure 2; Supplementary Table S2).
Older recipients more often had multidrug-resistant Gram-negative infections causing urosepsis or transplant pyelonephritis (45.4%). In recipients <40 years, resistant infections were limited to uncomplicated lower UTIs. Patients with resistant infections averaged 7.7 ± 5.8 episodes during follow-up and 3.9 ± 3.2 in the first post-transplant year. Their 5-year mortality was 28.4% (95%-CI, 19.3–38.4). Graft failure occurred in 33.3%. Cumulative incidence rose with age (from 4.8% to 11.6%), but age was not an independent predictor after full adjustment (HR = 1.66, p = 022.39; Table 2; Figure 2).
Distribution of Other Infection Types
Other types of infection showed age-related patterns. Pneumonia accounted for a higher proportion of infections with increasing age: 6.9% (<40 years) vs. 11.8% (>60 years) with a higher incidence in the elderly (Table 2), but this difference lost significance after adjustment (Supplementary Table S2). In contrast, upper respiratory tract infections decreased with age (10.3% vs. 3.2%). Gastrointestinal infections, pyelonephritis, and infections of unknown origin remained stable across all age groups (Table 3; Figure 3). Viral GI infections were more common in younger recipients, while Clostridium difficile was more prevalent in older groups. Sepsis rates remained stable across all age groups (8%–9%). The majority of sepsis cases (89.2%) originated from UTIs, mainly caused by Gram-negative bacteria (89.2%), particularly E. coli (52%) and Klebsiella spp. (20.4%). Sepsis due to pneumonia or soft tissue infections was rare, both accounted for 3.1% of all sepsis cases.
TABLE 3
| Outcome and covariates | 1) Univariate HR (95% CI) | p-value | 2) Univariate IPW HR (95% CI) | p-value | 3) Multivariate IPW HR (95% CI) | p-value |
|---|---|---|---|---|---|---|
| Mortality | ||||||
| Agegroup 2 | 4.14 [1.02;19.11] | 0.0047 | 5.99 [1.06;8.25] | 0.0191 | 4.62 [1.05;20.23] | 0.0424 |
| Agegroup 3 | 13.20 [3.09;56.36] | 0.0005 | 8.07 [1.70;38.33] | 0.0086 | 6.21 [1.34;28.81] | 0.0198 |
| Male gender | 1.37 [0.69; 2.69] | 0.3663 | 1.80 [0.78; 4.14] | 0.1671 | ||
| BMI (per 5 kg/m2) | 1.09 [1.03;1.15] | 0.0032 | 1.95 [1.41;2.69] | <0.001 | 1.61 [1.11;2.33] | 0.0112 |
| Diabetes mellitus | 1.64 [0.83:3.21] | 0.1536 | 2.68 [1.07;6.72] | 0.0361 | 1.77 [0.68; 4.65] | 0.2439 |
| Donor age (per 10 years) | 1.07 [1.04;1.09] | <0.0001 | 1.79 [1.18;2.72 | 0.0061 | 1.13 [0.99;1.29] | 0.0073 |
| Male donor | 0.68 [0.37; 1.23] | 0.2088 | 0.59 [0.26; 1.24] | 0.1538 | ||
| Deceased donation | 4.59 [1.78;11.85] | 0.0017 | 2.96 [1.06;8.25] | 0.0380 | 1.39 [0.32; 6.00] | 0.6601 |
| High sensitization | 0.80 [0.25; 2.61] | 0.8191 | 0.80 [0.24; 2.72] | 0.7236 | ||
| Thymoglobuline | 0.80 [0.35; 1.83] | 0.5214 | 0.51 [0.20;1.27] | 0.1480 | ||
| HLA mismatches (no.) | 1.03 [0.84; 1.26] | 0.7703 | 1.07 [0.84; 1.36] | 0.5936 | ||
| AB0i | 0.98 [0.58; 4.49] | 0.3602 | 1.63 [0.38; 7.06] | 0.5128 | ||
| Cold ischemia time (min) | 1.00 [1.00;1.00] | 0.0038 | 1.00 [1.00;1.00] | 0.0070 | 1.00 [1.00; 1.00] | 0.4204 |
| Delayed graft function | 1.79 [0.94;3.40] | 0.0146 | 1.63 [0.76; 3.49] | 0.2082 | ||
| CKD-EPI W2 | 0.97 [0.95;0.98] | 0.0002 | 0.73 [0.60;0.88] | 0.0008 | 0.88 [0.70; 1.10] | 0.2629 |
| CMV IgG D+ | 1.57 [0.80;3.07] | 0.1877 | 1.02 [0.46; 2.27] | 0.9582 | ||
| CMV IgG R+ | 0.95 [0.50;1.78] | 0.8619 | 0.75 [0.35; 1.61] | 0.4561 | ||
| CMV IgG D+/R- | 1.19 [0.56;2.50] | 0.6493 | 0.91 [0.36; 2.29] | 0.8438 | ||
| No. of infections (y1) | 1.28 [1.15;1.44] | <0.0001 | 1.13 [1.05;1.29] | 0.0307 | ||
| Graft failure | ||||||
| Agegroup 2 | 0.71 [0.33; 1.55] | 0.3892 | 0.70 [0.29; 1.67] | 0.4188 | 0.65 [0.26; 1.62] | 0.3537 |
| Agegroup 3 | 2.50 [1.22;5.14] | 0.0124 | 0.70 [0.27; 1.76] | 0.4444 | 0.67 [0.26; 1.69] | 0.3924 |
| Male gender | 0.93 [0.52; 1.65] | 0.8051 | 0.88 [0.42; 1.84] | 0.7332 | ||
| BMI (per 5 kg/m2) | 1.01 [0.95; 1.07] | 0.7424 | 1.12 [0.84; 1.49] | 0.4498 | ||
| Diabetes mellitus | 1.94 [1.03;3.65] | 0.0411 | 1.67 [0.78; 3.57] | 0.1876 | ||
| Donor age (per 10 years) | 1.06 [1.03;1.08] | <0.0001 | 1.69 [1.29;2.23] | 0.0002 | 1.60 [1.21;2.12] | 0.0009 |
| Male donor | 1.53 [0.87; 2.68] | 0.1381 | 2.15 [1.04;4.42] | 0.0381 | 2.21 [1.01;4.86] | 0.0474 |
| Deceased donation | 4.12 [1.75;9.69] | 0.0012 | 3.62 [1.32;9.90] | 0.0121 | ||
| High sensitization | 1.81 [0.85; 3.85] | 0.1260 | 2.11 [0.90; 4.95] | 0.0848 | 2.63 [0.98; 7.07] | 0.0560 |
| Thymoglobuline | 0.85 [0.41; 1.77] | 0.6692 | 0.62 [0.26; 1.48] | 0.2802 | ||
| HLA mismatches (no.) | 1.10 [0.91; 1.32] | 0.0509 | 1.18 [0.97; 1.44] | 0.1008 | 1.12 [0.85; 1.46] | 0.4244 |
| AB0i | 0.42 [0.06; 3.03] | 0.3887 | 0.75 [0.11; 5.17] | 0.7682 | ||
| Cold ischemia time (min) | 1.00 [1.00;1.00] | 0.0096 | 1.00 [1.00;1.00] | 0.0083 | 1.00 [1.00; 1.00] | 0.9494 |
| Delayed graft function | 2.32 [1.32;4.08] | 0.0034 | 2.67 [1.31;5.43] | 0.0068 | 1.15 [0.42; 3.18] | 0.7822 |
| CKD-EPI W2 | 0.97 [0.96;0.99] | 0.0001 | 0.76 [0.64;0.89] | 0.0009 | 0.91 [0.74; 1.12] | 0.3946 |
| CMV IgG D+ | 1.62 [0.87;3.01] | 0.1276 | 1.34 [0.63; 2.85] | 0.4528 | ||
| CMV IgG R+ | 1.29 [0.70;2.38] | 0.4098 | 1.19 [0.57; 2.49] | 0.6442 | ||
| CMV IgG D+/R- | 0.86 [0.40;1.84] | 0.6831 | ||||
| No. of infections (y1) | 1.29 [1.17;1.42] | <0.0001 | 1.29 [1.17;1.42] | <0.0001 | 1.16 [1.03;1.31] | 0.0114 |
| Acute rejection | ||||||
| Agegroup 2 | 1.24 [0.79; 1.96] | 0.3577 | 1.28 [0.75; 2.20] | 0.3621 | 1.23 [0.71; 2.15] | 0.4613 |
| Agegroup 3 | 1.17 [0.69; 2.00] | 0.5582 | 0.99 [0.37; 2.66] | 0.9810 | 0.96 [0.39; 2.35] | 0.9250 |
| Male gender | 1.20 [0.82; 1.76] | 0.5582 | 2.l11 [1.20;3.70] | 0.0092 | 1.83 [1.05; 3.20] | 0.0340 |
| BMI (per 5 kg/m2) | 1.01 [0.97; 1.05] | 0.6167 | 1.04 [0.83; 1.30] | 0.7228 | ||
| Diabetes mellitus | 0.77 [0.45; 1.30] | 0.3276 | 0.64 [0.33; 1.21] | 0.1690 | ||
| Donor age (per 10 years) | 1.01 [1.00; 1.02] | 0.0724 | 1.10 [0.86; 1.39] | 0.4427 | 0.52 [0.31;0.88] | 0.0146 |
| Male donor | 0.64 [0.44;0.95] | 0.0247 | 0.48 [0.27;0.83] | 0.0092 | ||
| Deceased donation | 0.97 [0.67; 1.42] | 0.8879 | 0.74 [0.40; 1.35] | 0.3276 | ||
| High sensitization | 1.36 [0.79; 2.34] | 0.2615 | 1.01 [0.50; 2.01] | 0.9829 | ||
| Thymoglobuline | 1.21 [0.79; 1.86] | 0.3873 | 0.94 [0.47; 1.89] | 0.8639 | ||
| HLA mismatches (no.) | 1.12 [0.99; 1.26] | 0.0723 | 1.10 [0.93; 1.30] | 0.2681 | ||
| AB0i | 1.01 [0.41; 2.48] | 0.9797 | 1.02 [0.31; 3.32] | 0.9801 | ||
| Cold ischemia time (min) | 1.00 [1.00; 1.00] | 0.9620 | 1.00 [1.00; 1.00] | 0.5842 | ||
| Delayed graft function | 1.05 [0.70; 1.58] | 0.8108 | 0.98 [0.57; 1.69] | 0.9372 | ||
| CKD-EPI W2 | 0.99 [0.98; 1.00] | 0.0014 | 0.94 [0.80; 1.10] | 0.4634 | ||
| CMV IgG D+ | 1.06 [0.72; 1.55] | 0.7217 | 0.73 [0.42; 1.29] | 0.2811 | ||
| CMV IgG R+ | 1.12 [0.76; 1.65] | 0.5608 | 1.06 [0.58; 1.97] | 0.8443 | ||
| CMV IgG D+/R- | 1.00 [0.63; 1.60] | 0.9951 | 0.72 [0.37; 1.42] | 0.3480 | ||
| Infection (any) | ||||||
| Agegroup 2 | 0.95 [0.75; 1.20] | 0.6526 | 0.79 [0.61; 1.02] | 0.0742 | 0.78 [0.59; 1.01] | 0.0635 |
| Agegroup 3 | 1.46 [1.12;1.91] | 0.0053 | 1.17 [0.82; 1.67] | 0.3950 | 1.20 [0.82; 1.74] | 0.3563 |
| Male gender | 1.12 [0.92; 1.37] | 0.2732 | 1.27 [1.02;1.60] | 0.0347 | 1.33 [1.00; 1.76] | 0.0466 |
| BMI (per 5 kg/m2) | 1.01 [0.99; 1.03] | 0.5705 | 1.08 [0.95; 1.22] | 0.2431 | ||
| Diabetes mellitus | 1.26 [0.98;1.63] | 0.0704 | 1.21 [0.86; 1.69] | 0.2744 | ||
| Donor age (per 10 years) | 1.01 [1.00;1.02] | 0.0198 | 1.03 [0.96; 1.11] | 0.4540 | ||
| Male donor | 1.03 [0.85; 1.26] | 0.7404 | 1.00 [0.79; 1.27] | 0.9980 | ||
| Deceased donation | 1.43 [1.16;1.76] | 0.0009 | 1.40 [1.04; 1.88] | 0.2830 | 1.25 [0.80; 1.98] | 0.3301 |
| High sensitization | 1.03 [0.75;1.41] | 0.0704 | 1.12 [0.75; 1.67] | 0.5871 | ||
| Thymoglobuline | 1.11 [0.88; 1.40] | 0.3857 | 1.10 [0.84; 1.45] | 0.4900 | ||
| HLA mismatches (no.) | 1.04 [0.98; 1.11] | 0.2191 | 1.00 [0.93; 1.07] | 0.9038 | ||
| AB0i | 1.12 [0.70; 1.79] | 0.6439 | 1.05 [0.61; 1.82] | 0.8504 | ||
| Cold ischemia time (min) | 1.00 [1.00;1.00] | 0.0329 | 1.00 [1.00; 1.00] | 0.0554 | 1.00 [1.00; 1.00] | 0.6809 |
| Delayed graft function | 1.28 [1.03;1.58] | 0.0250 | 1.20 [0.93; 1.54] | 0.1720 | ||
| CKD-EPI W2 | 0.99 [0.99;1.00] | 0.0046 | 0.95 [0.89; 1.01] | 0.1080 | ||
| CMV IgG D+ | 0.97 [0.80; 1.19] | 0.7832 | 0.82 [0.64; 1.06] | 0.1287 | ||
| CMV IgG R+ | 1.18 [0.96; 1.44] | 0.1139 | 1.15 [0.89; 1.49] | 0.2763 | ||
| CMV IgG D+/R- | 1.11 [0.87; 1.41] | 0.4197 | 0.99 [0.78; 1.28] | 0.9663 | ||
Risk analyses for clinical outcomes using a three-step IPW-adjusted model.
Cox regression was performed with and without inverse probability weighting (IPW) based on propensity scores for the exposure age group (reference: age group 1 = recipient age <40 years). The three-step approach includes unadjusted cox (UA), inverse probability weighted univariate (UA-IPW), and IPW-adjusted multivariable (MA-IPW) models, where age group and variables with p < 0.1 in UA-IPW were included in the MA-IPW model. IPW was derived from a multinomial logistic model using clinical and demographic covariates. Only complete cases were analyzed. In the multivariable model, variables with p < 0.1 in IPW-univariable models and clinically relevant variables were included, along with age group regardless of significance.
Abbreviations: Age group 1 = recipients <40 years, age group 2 = recipients 40–60 years, age group 3 = recipients >60 years, AB0i = AB0-incompatible transplantation, BMI = body Mass index (kg/m2), CKD-EPI W2 = estimated glomerular filtration rate at week 2 post-transplant (ml/min/1.73 m2, CKD-EPI formula), CMV = cytomegalovirus, D+ = donor positive, R+ = recipient positive, R− = recipient negative, D+/R− = donor positive/recipient negative serostatus, DGF = delayed graft function, HR = hazard ratio, CI = confidence interval, HLA = human leukocyte antigen, IPW = inverse probability weighting, MA-IPW = multivariable IPW-weighted model, UA = unadjusted model, UA-IPW = univariate IPW-weighted model, no. of infections (y1) = number of infections during the first post-transplant year. Thymoglobulin = used for induction therapy. Delayed graft function = Defined as the requirement for dialysis within the first 7 days after kidney transplantation, excluding dialysis performed solely for hyperkalemia. Bold values indicate statistically significant results (p < 0.05).
FIGURE 3
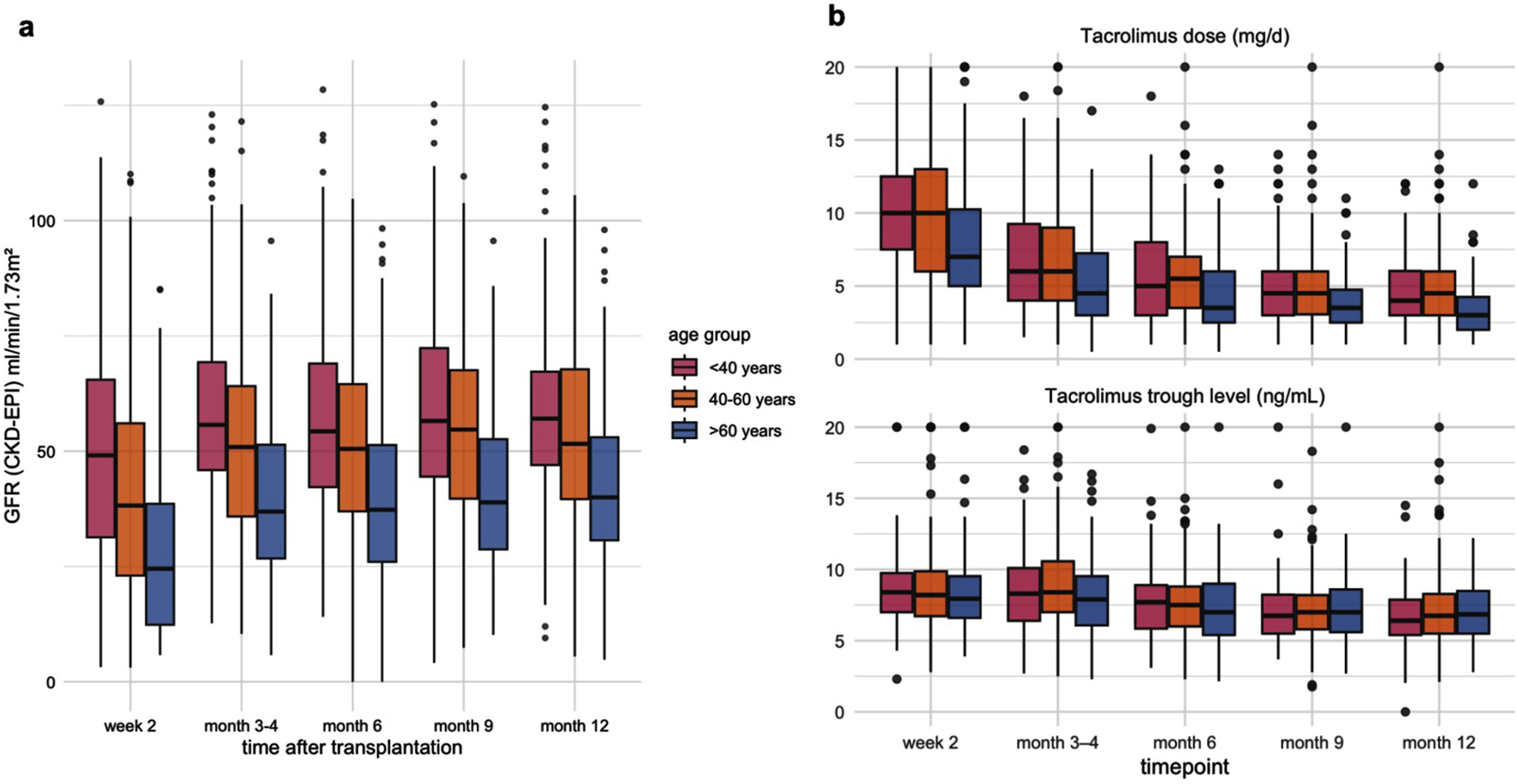
(a) Dynamics of estimated glomerular filtration rate (eGFR, CKD-EPI) during the first post-transplant year, stratified by recipient age group. Boxplots show median, interquartile range, and range (whiskers) of eGFR (mL/min/1.73 m2) at week 2, months 3–4, 6, 9, and 12 after kidney transplantation. (b) Tacrolimus dose and trough levels during the first post-transplant year by recipient age group. Boxplots display median values, interquartile ranges, and outliers at each time point (week 2, months 3–4, 6, 9, and 12).
BKV
BKV viremia was most common in the 40–60 years age group. In the first year post-transplant, the cumulative incidence ranged from 11.6% in recipients <40 years to 15.1% in those >60 years (Supplementary Table S2). New BKV viremia cases appeared in the >60 years group after the first year. Recipients aged 40–60 and >60 years had higher initial plasma viral loads than younger recipients, while those <40 years had the highest peak viral loads. Older recipients (40–60 and >60 years) also had shorter intervals between successive BKV viremia peaks (Supplementary Figure S1). The highest BKV nephropathy (BKVN) rate was found in the 40–60 years group (19.3%), compared to 5.3% in <40 years and 12.5% in >60 years.
Herpesviruses
CMV viremia was similar across age groups in the first post-transplant year: 13.6% in recipients <40 years, 14.6% in 40–60 years, and 16.4% in those >60 years. Beyond the first year, the CMV incidence continued to rise in older recipients, reaching 21.5% at 5 years (Supplementary Table S2). Peak viral load was highest in recipients >60 years (103,500 IU/mL), followed by <40 years (66,988 IU/mL) and 40–60 years (53,325 IU/mL). CMV-related organ complications occurred more frequent in recipients >60 years (19.2%) than in recipients between 40 and 60 years (17.4%) and <40 years (4.8%). Reinfection rates were significantly higher in recipients >60 years (46.2%) and 40–60 years (45.7%) than in those <40 years (28.6%, p = 0.03). De novo CMV infections occurred more frequently in >60 years (46.7%) than in 40–60 years (39.7%) and <40 years (39.3%). Reactivation rates followed a similar trend: 20.0% in >60 years, 14.8% in 40–60 years, and 11.9% in <40 years.
Non-CMV herpesvirus infections had a cumulative incidence of 6.1% at year one, increasing to 11.8% at 5 years. The overall incidence was 13.8%, with most cases occurring after the first year. VZV infections (all herpes zoster) increased from 2.5% at year one to 7.2% at 5 years. Notably, 90% of VZV infections in recipients >60 years occurred after the first year, with a median onset of 27 months (IQR 16–40), compared to 8 months (IQR 2–15) in those <40 years.
HSV infections had a cumulative incidence of 3.1% at year one, 4.5% at 5 years, and 6.0% overall. Nearly 46% of cases were diagnosed after the first year, particularly in older recipients. 61.5% of these cases presented with pneumonia, while all HSV cases in younger recipients were limited to herpes labialis.
Four cases of post-transplant lymphoproliferative disorder (PTLD) were reported, with a median onset of 266 days (IQR 241–335). Seventy-five percent of these cases occurred in recipients >60 years, including one who required transplant nephrectomy.
Opportunistic Infections With Organ Involvement
The 5-year cumulative incidence of opportunistic infections with organ involvement increased with age, from 3.4% in recipients <40 years to 12.7% in those >60 years (Table 2). Bacterial infections predominated in the youngest group (57.1%), while fungal infections were most common in the 40–60 years group (43.5%), followed by viral (34.8%) and bacterial (21.7%) infections. In recipients >60 years, fungal and viral infections occurred equally (40% each).
The median onset of infections was late (Md = 420 days, IQR 104–948), particularly in older recipients. Pneumonia was the most frequent manifestation, caused by pathogens including HSV (n = 11), Pneumocystis jirovecii (n = 11), Aspergillus spp. (n = 9), CMV (n = 4), Mycobacterium tuberculosis (n = 2), Legionella (n = 1), and Rhodococcus (n = 1). CMV colitis occurred in 10 cases. Although one case of disseminated Bartonella infection was identified in a 24-year-old, disseminated infections occurred predominantly in recipients >60 years and included severe disease such as mucormycosis (resulting in death within 26 days post-transplant), nocardiosis with brain abscess, invasive aspergillosis, multifocal CMV disease, and cryptococcal pyelonephritis requiring nephrectomy.
Age >60 years was initially associated with a higher risk of opportunistic infections but lost significance after adjustment (HR = 3.20, p = 0.1275; Figure 2), while high sensitization and high BMI remained significant predictors (Supplementary Table S2).
Aspergillus infections occurred exclusively in recipients >40 years of age, with an incidence of 1.8% in the 40–60 years group and 4.6% in those >60 years. Pneumocystis jirovecii pneumonia (PjP) occurred in all age groups but was least common in recipients <40 years (0.6% vs. 2.6% in older groups). Aspergillus infections appeared significantly earlier in the 40–60 years group (median 62 days, IQR 45–78) than in those >60 years (291 days, IQR 63–866; p = 0.037). The majority of PjP cases (90.9%) occurred after prophylaxis was stopped, with no age-related differences in time course. Intensive induction therapy was administered more frequently in PjP cases (33.3%) than in Aspergillus cases (8.3%). Recipients with Aspergillus infections had a higher infection burden in the first post-transplant year (mean 3.6 episodes) than in recipients with PjP (mean 2.2 episodes). Aspergillus-related mortality was 28.3% in recipients >60 years, while no deaths were observed in the 40–60 years group. Although 33.3% of PjP patients died, none of the deaths were directly attributable to PjP.
Acute Rejections and Transplant Biopsy Findings
Acute rejection occurred in 22.6% of recipients (95% CI: 19.3–26.3), with no significant differences between age groups (Table 2; Figure 1c). However, the timing of rejection varied by age: the earliest rejections were observed in recipients >60 years (median 37 days, IQR 15–118), followed by 40–60 years (65 days, IQR 16–162), and the latest in recipients <40 years (78 days, IQR 13–414).
Recurrent rejection occurred most frequently in the youngest group (8.2%) compared to 5.4% in those >60 years. Biopsy findings revealed only minor age-related differences. Borderline rejection was the most common finding, occurring in 37.8%–47.3% of cases. Acute tubular necrosis increased with age, peaking at 13.3% in recipients >60 years. T-cell–mediated rejection (12.4%) and BKVN (11.8%) occurred most frequently in the 40–60 years group.
Graft Failure
The 5-year graft failure rate increased significantly with recipient age, from 5.2% (95% CI, 3.0–8.9) in recipients <40 years to 21.0% (95%-CI, 14.6–33.2) in those >60 years (p 0.0001, Table 2; Figure 1b). The unadjusted analysis showed a higher risk of graft loss in recipients >60 years (Figure 2; Table 3). Graft failure also occurred earlier in older recipients, with a median time to failure of 34 months (IQR 18–56) in the >60 group, compared to 52 months (IQR 31–74) in <40 years.
Kidney transplant removal was more often necessary in older recipients (50.2% in >60 years vs. 18.2% in <40 years, p < 0.0001). Infections and acute or chronic rejection were the main causes of graft failure in all age groups. In recipients <40 years, infections accounted for more than half of graft losses, while infections contributed to 40% of graft failures in those >60 years. These infections included BKVN, parainfectious complications, and other graft-related infections.
After adjustment, recipient age >60 years was no longer a significant predictor of graft failure (HR = 0.67, p = 0.3924; Figure 2; Table 3). Independent predictors included donor age, male donor and the number of infections during the first post-transplant year (Table 3).
Mortality
Five-year mortality rate rised significantly with increasing age, from 1.8% (<40 years) to 18.4% (>60 years) (p < 0.0001) (Table 2; Figure 1d). The unadjusted analysis showed a strong association between age >60 years and mortality risk (Figure 2; Table 3). Even after multivariable adjustment, it remained a significant predictor of mortality (HR = 1.15, p = 0.07), along with higher BMI, donor age and an increased infection burden in the first year after transplantation (Table 3). Sensitivity analyses confirmed the robustness of the association between older recipient age and mortality, whereas no consistent association was observed for graft failure (Supplementary Table S3). Analyses accounting for the COVID-19 pandemic period did not materially change the results (Supplementary Table S4).
Infections were the most common cause of death at 40% of cases. Pulmonary infections were the most frequent, affecting 55.6% of patients. Fatal cases included COVID-19 pneumonia, pulmonary aspergillosis, and Legionella pneumonia. Bloodstream infections and sepsis were the second most common causes of death. Pathogens were detected in approximately one-third of deaths, with Aspergillus spp., HSV, and E. coli being most common.
In recipients >60 years, the median time to death was shortest (24 months), compared with 28 months in the 40–60 years group and 33 months in <40 years (p = 0.034).
Risk Factors for First-Year Infection Burden
The first post-transplant year emerged as a critical period linking early infection burden to long-term outcomes. Higher infection burden during the first post-transplant year was independently associated with recipient age >60 years, CMV D+/R− serostatus, higher donor age, and greater HLA mismatch count (Supplementary Table S5).
Discussion
Our findings demonstrate that recipient age significantly influences post-transplant outcomes, affecting both infection patterns and long-term patient and graft survival. Consistent with previous reports, unadjusted analyses showed higher rates of bacterial and fungal infections, graft failure, and mortality in older recipients [15, 21, 23, 27–32]. They experienced higher rates of pneumonia, UTIs, invasive opportunistic infections, and infections caused by multidrug-resistant organisms. While unadjusted analyses showed broad age-related differences, only a restricted set of associations persisted after adjustment, indicating that many apparent age effects are mediated through modifiable clinical and immunologic factors.
Consistent with Esnault et al., age independently predicted mortality but not overall opportunistic infections [31]. Older recipients were more prone to invasive fungal infections and more severe viral disease, particularly CMV and HSV indicating a pathogen-specific rather than generalized susceptibility. While the overall incidence of CMV infection did not differ by age, older recipients exhibited higher viral loads, more frequent organ involvement, and a greater proportion of late reactivations. Bacterial infection profiles also differed across age groups: P. aeruginosa was markedly more prevalent in older recipients and frequently associated with complicated UTIs and urosepsis. Younger recipients, by contrast, tended to experience recurrent but clinically milder lower UTIs. These findings emphasize the importance of age-adjusted infection profiling to tailor prophylaxis and surveillance strategies individually. In older recipients, extended CMV monitoring beyond standard prophylaxis and early assessment of bacterial resistance patterns may be particularly beneficial. Notably, our recent findings within the DZIF transplant cohort revealed that center-specific prophylaxis and monitoring strategies substantially influence herpes- and polyomavirus infection rates, highlighting additional opportunities for optimizing local protocols.
Infections remain one of the leading challenges in post-transplant care, with 54.6% of recipients experiencing at least one infectious complication within the first year [33]. Our analysis revealed that the first year after transplantation is a crucial period for long-term outcomes. A higher infection burden in the early phase was independently associated with recipient age over 60 years, CMV serostatus D+/R–, older donor age, and greater HLA incompatibility. These factors likely interact by increasing infectious exposure and immune stress in the early post-transplant phase, reducing graft resilience and recovery capacity. Although the infection burden contributed to the risk of mortality, it did not fully explain the excess mortality observed in older patients, suggesting reduced physiological and immunological resilience that limits the ability to compensate for infectious and inflammatory stress.
Consistent with previous findings [33], pneumonia was a leading cause of death in recipients over 60, highlighting the need for targeted respiratory surveillance, age-adapted vaccinations, and regular assessment of the net sate of immunosuppression [34]. Fungal infections should be a central component of the differential diagnosis of respiratory symptoms in older recipients. Closer respiratory monitoring, including vigilant clinical examination, early oxygen saturation checks, and early mycological testing can facilitate earlier detection.
Older recipients experienced fewer but delayed acute rejection episodes, whereas younger recipients had more frequent recurrent and early episodes. Consistent with previous reports [27, 35], infection-related morbidity and graft explantation rates were higher in older recipients despite similar rejection rates, indicating that excessive, rather than insufficient immunosuppression in this group. This likely reflects age-related immune alterations that weaken immune defenses and increase infection susceptibility. [36]. Although older recipients received lower tacrolimus doses, trough levels were comparable to younger recipients. Tailoring immunosuppression through pharmacodynamic monitoring may help to balance infection risk [37].
The strengths of this study include the large cohort, the long follow-up period, and the detailed characterization of infectious complications in relation to graft and patient outcomes.
Our multivariable, IPW-based approach minimized confounding and allowed robust assessment of independent age effects. Limitations arise from the non-centralized infection diagnosis, which may have led to some observer variability but reflects clinical practice. The exclusion of incomplete cases may have introduced bias, and some subgroup analyses were limited in their statistical power due to small sample sizes.
In conclusion, age should not be considered a fixed risk factor, but rather a composite marker for immunologic, infectious, and physiological vulnerability. Importantly, the infection burden within the first post-transplant year emerged as a strong independent predictor of both graft failure and mortality, defining a critical, modifiable window for preventive interventions. Post-transplant care in the elderly should therefore primarily include strict infection control, enhanced CMV monitoring, and the early detection of Pseudomonas and fungal infections in the first year. Optimizing immunosuppression and minimizing the use of nephrotoxic medications can further protect graft function. Future multicenter studies integrating immunomonitoring, pharmacodynamic profiling, and long-term infection surveillance are needed to further refine these findings.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Medical Faculty of Heidelberg University (No. S-585/2013). The studies were conducted in accordance with the local legislation and institutional requirements. The Ethics Committee of the Medical Faculty of Heidelberg University approved for the study (No. S-585/2013), and all participants provided written informed consent.
Author contributions
IS collected and analyzed data and wrote the manuscript; DS conducted the study and collected data; MZ discussed the manuscript; TG supervised the study conception and performance of the study, CS designed the study, recruited patients, analyzed data and wrote the manuscript. All authors contributed to the article and approved the submitted version.
Group Members of the Transplant Cohort of THE German Center for Infection Research (DZIF Transplant Cohort)
Christine S. Falk, Nele Kanzelmeyer, Anette Melk, Thomas F. Schulz, Susanne Delecluse, Philipp Ehlermann, Uta Merle, Burkhard Tönshoff, Joachim Andrassy, Martin Hildebrandt, Michael Neuenhahn, Tina Ganzemüller, Thomas Iftner, Peter Lang, Berit Lange, Carolina Klett-Tammen, Bärbel Fösel, Lutz Renders, and Thomas Illig.
Funding
The author(s) declared that financial support was not received for this work and/or its publication.
Acknowledgments
This study was conducted with resources provided by the DZIF transplant cohort e.V. (https://www.dzif.de/en/working-group/transplant-cohort), support code TTU 07.701. This study was supported by the scientific steering committee and the executive board of the DZIF transplant cohort. We thank the study coordinators at all participating facilities of the Transplant Cohort of the German Center for Infection Research (DZIF Transplant Cohort) Consortium for their excellent support.
Conflict of interest
The authors(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2025.15267/full#supplementary-material
References
1.
Hariharan S Rogers N Naesens M Pestana JM Ferreira GF Requião-Moura LR et al Long-Term Kidney Transplant Survival Across the Globe. Transplantation (2024) 108:e254–e263. 10.1097/tp.0000000000004977
2.
Segall L Nistor I Pascual J Mucsi I Guirado L Higgins R et al Criteria for and Appropriateness of Renal Transplantation in Elderly Patients with End-Stage Renal Disease: A Literature Review and Position Statement on Behalf of the European Renal Association-European Dialysis and Transplant Association Descartes Working Group and European Renal Best Practice. Transplantation (2016) 100:e55–65. 10.1097/tp.0000000000001367
3.
(USRDS), USRDS: 2023 USRDS Annual Data Report. Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (2023).
4.
Heldal K Midtvedt K Lønning K Iversen T Hernæs KH Tsarpali V et al Kidney Transplantation: An Attractive and Cost-Effective Alternative for Older Patients? A Cost-Utility Study. Clin Kidney J (2019) 12:888–94. 10.1093/ckj/sfz018
5.
Huang Q Luo T Yang J Lu Y Zhou S Hei Z et al Association Between the Age-Adjusted Charlson Comorbidity Index and Complications After Kidney Transplantation: A Retrospective Observational Cohort Study. BMC Nephrol (2024) 25:457. 10.1186/s12882-024-03888-1
6.
Krenzien F ElKhal A Quante M Rodriguez Cetina Biefer H Hirofumi U Gabardi S et al A Rationale for Age-Adapted Immunosuppression in Organ Transplantation. Transplantation (2015) 99:2258–68. 10.1097/tp.0000000000000842
7.
Jallah BP Kuypers DRJ . Impact of Immunosenescence in Older Kidney Transplant Recipients: Associated Clinical Outcomes and Possible Risk Stratification for Immunosuppression Reduction. Drugs Aging (2024) 41:219–38. 10.1007/s40266-024-01100-5
8.
Chadban SJ Ahn C Axelrod DA Foster BJ Kasiske BL Kher V et al KDIGO Clinical Practice Guideline on the Evaluation and Management of Candidates for Kidney Transplantation. Transplantation (2020) 104:S11–s103. 10.1097/tp.0000000000003136
9.
Green M Blumberg EA Danziger-Isakov L Huprikar S Kotton CN Kumar D . Foreword: 4Th Edition of the American Society of Transplantation Infectious Diseases Guidelines. Clin Transpl (2019) 33:e13642. 10.1111/ctr.13642
10.
Wu DA Robb ML Forsythe JLR Bradley C Cairns J Draper H et al Recipient Comorbidity and Survival Outcomes After Kidney Transplantation: A UK-Wide Prospective Cohort Study. Transplantation (2020) 104:1246–55. 10.1097/tp.0000000000002931
11.
Veroux M Grosso G Corona D Mistretta A Giaquinta A Giuffrida G et al Age Is an Important Predictor of Kidney Transplantation Outcome. Nephrol Dial Transpl (2012) 27:1663–71. 10.1093/ndt/gfr524
12.
Salas MAP Rodriguez-Abreu RD Amaechi P Rao V Soliman K Taber D . Clinical Outcomes of Older Kidney Transplant Recipients. Am J Med Sci (2021) 362:130–4. 10.1016/j.amjms.2021.02.017
13.
McAdams-DeMarco MA Law A King E Orandi B Salter M Gupta N et al Frailty and Mortality in Kidney Transplant Recipients. Am J Transpl (2015) 15:149–54. 10.1111/ajt.12992
14.
Haugen CE King EA Bae S Bowring MG Holscher CM Garonzik-Wang J et al Early Hospital Readmission in Older and Younger Kidney Transplant Recipients. Am J Nephrol (2018) 48:235–41. 10.1159/000492338
15.
Neri F Furian L Cavallin F Ravaioli M Silvestre C Donato P et al How Does Age Affect the Outcome of Kidney Transplantation in Elderly Recipients? Clin Transpl (2017) 31:e13036. 10.1111/ctr.13036
16.
Meier-Kriesche HU Ojo AO Hanson JA Kaplan B . Exponentially Increased Risk of Infectious Death in Older Renal Transplant Recipients. Kidney Int (2001) 59:1539–43. 10.1046/j.1523-1755.2001.0590041539.x
17.
Kim JS Jeong KH Lee DW Lee SY Lee SH Yang J et al Epidemiology, Risk Factors, and Clinical Impact of Early Post-Transplant Infection in Older Kidney Transplant Recipients: The Korean Organ Transplantation Registry Study. BMC Geriatr (2020) 20:519. 10.1186/s12877-020-01859-3
18.
Ayaz CM Ceylan S Yılmaz VT Adanır H Turhan Ö . Timeline and Incidence of Infectious Complications in Older Transplant Recipients During the First Year Post-transplantation. Pathogens (2024) 13:1061. 10.3390/pathogens13121061
19.
Beerli N Denhaerynck K Binet I Dahdal S Dickenmann M Golshayan D et al Age at Time of Kidney Transplantation as a Predictor for Mortality, Graft Loss and Self-Rated Health Status: Results from the Swiss Transplant Cohort Study. Transpl Int (2021) 35:10076. 10.3389/ti.2021.10076
20.
Karim A Farrugia D Cheshire J Mahboob S Begaj I Ray D et al Recipient Age and Risk for Mortality After Kidney Transplantation in England. Transplantation (2014) 97:832–8. 10.1097/01.TP.0000438026.03958.7b
21.
Winkler S Kim MJ Fisler A Farese S Burkhalter F von Moos S et al The Impact of Patient Age on Causes of Graft Loss After Renal Transplantation. Transpl Int (2025) 38:14544. 10.3389/ti.2025.14544
22.
Ziaja J Skrabaka D Owczarek AJ Widera M Król R Kolonko A et al Long-Term Results of Kidney Transplantation in Patients Aged 60 Years and Older. J Clin Med (2024) 14:78. 10.3390/jcm14010078
23.
Hemmersbach-Miller M Alexander BD Sudan DL Pieper C Schmader KE . Infections After Kidney Transplantation. does Age Matter?Clin Transpl (2019) 33:e13516. 10.1111/ctr.13516
24.
Karch A Schindler D Kühn-Steven A Blaser R Kuhn KA Sandmann L et al The Transplant Cohort of the German Center for Infection Research (DZIF Tx-Cohort): Study Design and Baseline Characteristics. Eur J Epidemiol (2021) 36:233–41. 10.1007/s10654-020-00715-3
25.
Kidney Disease: Improving Global Outcomes KDIGO Transplant Work Group. Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group: KDIGO Clinical Practice Guideline for the Care of Kidney Transplant Recipients. Am J Transpl (2009) 9(Suppl. 3):S1–155. 10.1111/j.1600-6143.2009.02834.x
26.
Solez K Colvin RB Racusen LC Sis B Halloran PF Birk PE et al Banff '05 Meeting Report: Differential Diagnosis of Chronic Allograft Injury and Elimination of Chronic Allograft Nephropathy ('CAN'). Am J Transpl (2007) 7:518–26. 10.1111/j.1600-6143.2006.01688.x
27.
Lim JH Lee GY Jeon Y Jung HY Choi JY Cho JH et al Elderly Kidney Transplant Recipients Have Favorable Outcomes but Increased infection-related Mortality. Kidney Res Clin Pract (2022) 41:372–83. 10.23876/j.krcp.21.207
28.
Trouillhet I Benito N Cervera C Rivas P Cofán F Almela M et al Influence of Age in Renal Transplant Infections: Cases and Controls Study. Transplantation (2005) 80:989–92. 10.1097/01.tp.0000173822.05877.d7
29.
Kauffman HM McBride MA Cors CS Roza AM Wynn JJ . Early Mortality Rates in Older Kidney Recipients with Comorbid Risk Factors. Transplantation (2007) 83:404–10. 10.1097/01.tp.0000251780.01031.81
30.
Hemmersbach-Miller M Alexander BD Pieper CF Schmader KE . Age Matters: Older Age as a Risk Factor for CMV Reactivation in the CMV Serostatus-Positive Kidney Transplant Recipient. Eur J Clin Microbiol Infect Dis (2020) 39:455–63. 10.1007/s10096-019-03744-3
31.
Esnault V Hoisnard L Peiffer B Fihman V Fourati S Angebault C et al Beyond the First Year: Epidemiology and Management of Late-Onset Opportunistic Infections After Kidney Transplantation. Transpl Int (2024) 37:12065. 10.3389/ti.2024.12065
32.
Huang E Poommipanit N Sampaio MS Kuo HT Reddy P Gritsch HA et al Intermediate-Term Outcomes Associated with Kidney Transplantation in Recipients 80 Years and Older: An Analysis of the OPTN/UNOS Database. Transplantation (2010) 90:974–9. 10.1097/TP.0b013e3181f5c3bf
33.
Sommerer C Schröter I Gruneberg K Schindler D Behnisch R Morath C et al Incidences of Infectious Events in a Renal Transplant Cohort of the German Center of Infectious Diseases (DZIF). Open Forum Infect Dis (2022) 9:ofac243. 10.1093/ofid/ofac243
34.
Fishman J . Infection in Organ Transplantation. Am J Transplant (2017) 17:856–79. 10.1111/ajt.14208
35.
Kinnunen S Karhapää P Juutilainen A Finne P Helanterä I . Secular Trends in Infection-Related Mortality After Kidney Transplantation. Clin J Am Soc Nephrol (2018) 13:755–62. 10.2215/cjn.11511017
36.
Schaier M Leick A Uhlmann L Kälble F Eckstein V Ho A et al The Role of Age-Related T-Cell Differentiation in Patients with Renal Replacement Therapy. Immunol Cell Biol (2017) 95:895–905. 10.1038/icb.2017.57
37.
Sommerer C Schnitzler P Meuer S Zeier M Giese T . Pharmacodynamic Monitoring of Cyclosporin A Reveals Risk of Opportunistic Infections and Malignancies in Renal Transplant Recipients 65 Years and Older. Ther Drug Monit (2011) 33:694–8. 10.1097/FTD.0b013e318237e33c
Summary
Keywords
kidney transplantation, elderly recipients, age, infections, fungal
Citation
Schröter I, Schindler D, Zeier M, Giese T and Sommerer C (2026) Age-Related Risk After Kidney Transplantation: A Comprehensive Analysis of Infection Burden, Graft Outcomes, and Mortality. Transpl. Int. 38:15267. doi: 10.3389/ti.2025.15267
Received
14 July 2025
Revised
29 October 2025
Accepted
08 December 2025
Published
07 January 2026
Volume
38 - 2025
Updates
Copyright
© 2026 Schröter, Schindler, Zeier, Giese and Sommerer.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Iris Schröter, iris.schroeter@med.uni-heidelberg.de
ORCID: Thomas Giese, orcid.org/0000-0002-9649-0424
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.