Abstract
Drug abuse and related disorders are a global public health crisis affecting millions, but to date, limited treatment options are available. Abused drugs include but are not limited to opioids, cocaine, nicotine, methamphetamine, and alcohol. Drug abuse and human immunodeficiency virus-1/acquired immune deficiency syndrome (HIV-1/AIDS) are inextricably linked. Extensive research has been done to understand the effect of prolonged drug use on neuronal signaling networks and gut microbiota. Recently, there has been rising interest in exploring the interactions between the central nervous system and the gut microbiome. This review summarizes the existing research that points toward the potential role of the gut microbiome in the pathogenesis of HIV-1-linked drug abuse and subsequent neuroinflammation and neurodegenerative disorders. Preclinical data about gut dysbiosis as a consequence of drug abuse in the context of HIV-1 has been discussed in detail, along with its implications in various neurodegenerative disorders. Understanding this interplay will help elucidate the etiology and progression of drug abuse-induced neurodegenerative disorders. This will consequently be beneficial in developing possible interventions and therapeutic options for these drug abuse-related disorders.
Introduction
Drug abuse is a significant global problem prevalent in those infected with Human Immunodeficiency Virus-1 (HIV-1). The most commonly abused drugs in HIV-1 infected individuals are opioids, alcohol, cocaine, cannabis, methamphetamine (Meth), and nicotine. Among all the drugs used, opioid abuse is a growing problem since opioids are often the mainstay of pain management in infected individuals. While these drugs effectively control the pain associated with HIV-1, their long-term use is associated with addiction, tolerance, and neurocognitive impairment, adding to the burden of behavioral deficits in HIV-1-infected individuals. When HIV-1-affected individuals use morphine, it may cause a loss of functional connectivity between the amygdala and the frontal cortex of the brain, insula, and striatum leading to neurodegenerative effects (1). Alcohol consumption in the form of ethanol is both toxic and has metabolic and addictive effects on the brain, accumulating over time with age, dose, and duration of exposure. Severe debilitating diseases of the central nervous system (CNS) and the peripheral nervous system are known to manifest due to alcohol consumption. For example, it is well established that prenatal alcohol exposure paves the way for lifelong behavioral, cognitive, and psychological problems, which account for a range of cognitive dysfunctions referred to as fetal alcohol spectrum disorders (2). Prolonged heavy alcohol abuse has been shown to lead to neurodegeneration and proportionate loss of cerebral white matter. The affected regions in chronic alcohol-related metabolic injury and degeneration include the cerebellum (especially the vermis), cortical-limbic circuits, skeletal muscle, and peripheral nerves (3). Specifically, alcohol impairs neuronal and glial cell functionality (3). Also, alcohol exerts prolonged effects at the cellular and systemic levels of the neurological networks, leading to neurodegeneration. Excess alcohol exposure is associated with specific diseases such as dementias, ataxias, and Niemann-Pick disease (4). Both excess and heavy alcohol consumption contribute to the development of neurodegenerative diseases, such as amyotrophic lateral sclerosis (ALS) and Alzheimer’s disease (AD). The brain is a major organ of alcohol accumulation, and this is linked to brain damage. Long-term alcohol abuse increases glutamate excitotoxicity and oxidative stress, resulting in neuronal damage (5). Besides alcohol, psychostimulants like cocaine, amphetamines, and nicotine have also been implicated in disruption of blood-brain-barrier (BBB), neural plasticity, and neuroinflammation (6, 7). There are case reports suggesting the association of cocaine overuse with accelerated neurodegeneration exhibiting symptoms similar to that found in Parkinson’s disease (8). It has also been shown that iron metabolism regulation and storage lead to dopamine accumulation in cocaine-abusing individuals, resulting in neuroadaptive changes in the basal ganglia (9). Other than genetic events, epigenetic events also play a major role in neurodegeneration mediated by abuse of substances such as cocaine and Meth, as well as opioids. Epigenetic changes are established by classical pathways, including the class III histone deacetylase-sirtuin family modifications by the stimulatory effects of drugs in the form of psychostimulants (10). Drugs of abuse have also been extensively reported to cause dysbiosis of the gut microbiome and, there is significant amount of evidence that links the dysbiotic gut microbiome to mental health and neurodegeneration (11-14). In this review, we summarize existing research, including preclinical and clinical studies about correlation between HIV-1-linked drug abuse and the intestinal microbiota, and the potential role of the resultant dysbiotic gut microbiome in the pathogenesis of neurodegenerative disorders (Figure 1).
FIGURE 1
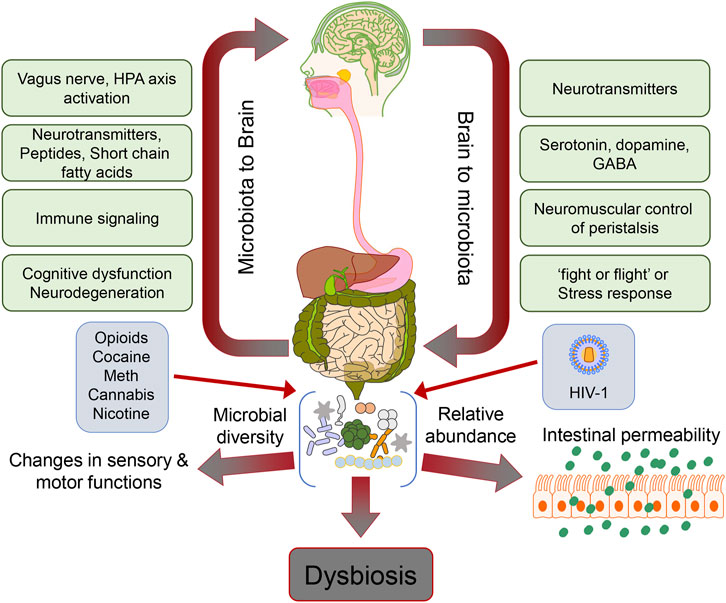
Schematic depicting the gut microbiome dysbiosis and its effects on the gut-brain axis in the context of HIV-1 and drug abuse.
Gut microbiome and the gut-brain axis
The gut microbiome comprises a highly diverse repertoire of trillions of microbes that dwell in the gut linings and has been identified as a marker associated with various disease conditions. There is a standard composition of microbes in the gut for the metabolism and energy assimilation of the body. Several environmental, nutritional and genetic factors influence the multiplication of gut microorganisms and their compositional modifications (15). The human gut microbiome consists of various microbes that are beneficial to the body for metabolism and involved in a communication pathway to the CNS via the bidirectional “microbiota-gut-brain axis,” which was initially termed the “gut-brain axis.” In the 1960s, the brain was thought to control gut function which served as the basis for coining the term “gut-brain axis.” Later, bidirectional interactions between the gut microbiota and the CNS was discovered and was defined as the “microbiota-gut-brain axis (16-19). Recent findings implicate the role of the gut-brain axis in regulating behavior and responses to drugs and, thus, underpinning its role in reward and satiety (20-22). The vagus nerve physically connects the gut and the brain through an interplay of neurotransmitters and metabolites (23-26). The existence of specific microbes in the gut is known to regulate both the immune system (27-30) and inflammation (31-34). Both preclinical and clinical studies have demonstrated a pivotal role of the gut microbiota in brain functioning (35), mood (36), and behavior (37, 38). Gut microbiota regulates the differentiation and function of immune cells of the intestine, periphery, and brain (39-41). Growing evidence also points to the critical role of the gut microbiota and the immune system in regulating the pathogenesis of neurodevelopmental and neurodegenerative diseases (12).
There is an altered influx and efflux of microbial metabolites and immune mediators between the gut and brain, leading to impaired neurotransmission and the advent of many neuropsychiatric and neurological disorders (42). Microbiome changes, termed dysbiosis, result in acute and chronic stages of several diseases, such as depression (43). Further, dysfunctional glutamate neurotransmission is involved in the D-glutamate signaling pathway observed in AD models in which the gut microbiome metabolized D-glutamate influences the glutamate N-methyl-D-aspartate receptors and cognitive function in dementia patients (44). The gut microbiota has also been linked to the development of schizophrenia (42). The “gut-brain axis” encompasses several key signaling pathways. The immune system, the vagus nerve, or microbiota-modulating neuroactive compounds may drive these pathways. Existing literature also points toward the fact that bacteria present in the gut microbiome are responsible for the production and consumption of several mammalian neurotransmitters, such as dopamine, serotonin, norepinephrine, or gamma-aminobutyric acid (GABA). Reports suggest that, on the one hand, any change in the levels of these neurotransmitters by bacteria could impact host physiology, while on the other hand, any form of microbiota-based interventions could also alter neurotransmitter levels (45). A prime example of this regulation is the impact of the gut microbiome on tryptophan metabolism and the serotonergic system (46). In such a scenario, the interaction between gut microbes with drugs of abuse is complex since gut microbes can directly impact the response of an individual to a specific drug by enzymatically modifying the structure of the drug and, in turn, affecting its availability, activity, or toxicity in the system. The drugs of abuse can also influence the microbiome composition (47-51).
Drug abuse and gut microbiome
Recent evidence implicates the gut-brain axis in the regulation of not only behavior but also a response to drugs in terms of reward and satiety. The vagus nerve connects the gut and brain, but several metabolites, hormones, and neurotransmitters regulate this connection. Such an influence of gut microbes on brain functions has been supported by studies in both preclinical and clinical models (52). During drug abuse, the gut-brain axis is disrupted, leading to modifications in the normal microbiota composition and dysregulated expression of neurotransmitters, bile acids, and metabolites, such as short-chain fatty acids (SCFA). Alterations in SCFA levels mediate tight junction dysfunction resulting in aberrant permeability of the gut epithelium, which can activate a wide range of proinflammatory signaling pathways (53). The hypothalamic-pituitary axis is linked to this inflammation in the gut, which subsequently sends feedback to the CNS, resulting in pain, stress, and anxiety (52). Herein, we discuss the role of the microbiota-gut-brain communication in the context of drug abuse in people living with HIV-1 (PLWH).
Opioids and HIV
Opioids comprise a large class of compounds with different mechanisms of action and include heroin, morphine, oxycodone, fentanyl, methadone, buprenorphine, and nalorphine, among several others (54). Opioid receptors are widely distributed in the central and peripheral nervous systems and the digestive tract (55, 56). Prescription opioid drugs are used to treat moderate to severe chronic pain. Recently, the use of various opioid drugs and their abuse, which can lead to tolerance and dependence, has become a severe public health issue (57). According to the Centers for Disease Control and Prevention, out of the 92000 people who died from a drug overdose in 2020, 75% were due to prescription or illicit opioid use (58). Most studies on the gut-brain axis and opioid abuse are based on the exogenous opioid compound morphine. Severe constipation is a primary physiological manifestation of chronic morphine use and has been linked to disruption of the gut epithelium and microbial dysbiosis (59). Animal models commonly used to study the pathways involved in the interaction between the host-gut microbiome and opioid drugs involve rodents, particularly mice and rats, primarily for economic reasons. However, recent studies have also focused on non-human primates (NHPs) as they are both physiologically and genetically closer to humans (60). The major outcome of these studies is a gut microbial imbalance or dysbiosis due to opioid use. Preclinical animal studies show that morphine exposure increases the abundance of pathogenic bacteria (Flavobacterium, Enterococcus, Fusobacterium, Sutterella, Clostridium, Rikenellaceae, and Ruminococcus). Once tolerance is developed, it causes a significant decrease in the quantities of beneficial bacteria (Lactobacillus and Bifidobacterium) (59, 61). It is difficult to extrapolate the data from the rodent preclinical models to the effect of morphine on human microbiota due to several factors such as genetic background, geographical setting, and lifestyle (60, 62). Human clinical studies also display variations in the presence of Bacteroidetes, Firmicutes, and Actinobacteria phylum of microbiota, consistent with rodent studies. However, there are only a limited number of studies utilizing NHPs to comment on any close association between the effect of opioids on gut microbiota in humans and that of NHPs.
It has been reported that opioid-induced gut dysbiosis, which causes structural changes in the gut epithelium, is responsible for tolerance and withdrawal behaviors. Disruption of the gut epithelium, in turn, allows bacteria and their toxic products to enter the host circulatory system, subsequently activating several inflammatory pathways and neuroinflammation. Withdrawal and tolerance linked to chronic opioid use have been related to this neuroinflammation (61, 63). The integrity of the gut epithelium depends on several factors, like the disruption of tigh-junction (TJ) organization and the restoration of the depleted epithelial layer by intestinal stem cells. The toll-like receptor (TLR) signaling is responsible for regulating intestinal TJ protein (TJP) organization. It has also been reported that morphine can disrupt the arrangement of the TJPs via modulation of myosin light chain kinase signaling (MLCK) in a TLR-dependent manner (64).
Opioid-induced microbial dysbiosis is responsible for continuous immune activation leading to HIV-1 disease progression. Several studies report that opioid addicts are at a greater risk of HIV-1 infection (65). Several factors, including the usage of contaminated needles and the nutritional status of the infected individual, could likely play a role in the heightened susceptibility of opioid abusers to HIV-1 infection. However, reports indicate that opioid use alone can also increase the risk of HIV-1 infection (66). There is ample evidence suggesting that HIV-1 infection disrupts the structure and function of the gut epithelium, leading to AIDS progression. Reports suggest that HIV-1 modulates tight junctions by disrupting CD4+ T cells, which are responsible for maintaining tight junctions (67). HIV-1 proteins such as Tat (transactivator of transcription) and gp120 have also been reported to disrupt tight junctions on epithelial cells in culture (68). Studies also report that simian immunodeficiency virus (SIV) infection results in early upregulation of proinflammatory cytokine IL-1β in the colon of the rhesus macaques (69) as well as in the intestine of HIV-1-infected patients (70), which, in turn, could activate the MLCK, resulting in mucosal damage. SIV-infected African green monkeys exhibit an accelerated depletion of CD4+ T cells in the intestine (71). An identical phenomenon is found in HIV-1-infected humans and SIV-infected rhesus macaques, suggesting that microbial translocation through the disrupted gut epithelium affects SIV disease progression.
Opioid users have been reported to display rapid HIV-1 disease progression while demonstrating severe long-term effects such as neurocognitive disorders (72). Certain opioid abusers infected with HIV-1 show elevated levels of lipopolysaccharide (LPS) in their serum compared to non-users, thus underscoring that disruption of the gut epithelium is more acute in HIV-1 patients who use opioid drugs (73). Preclinical and clinical studies done in HIV-1-infected patients indicate that morphine-mediated disruption of intestinal tight junctions involves activation of MLCK. This has also been validated in rodent models where combination of opioids and HIV-1 infection either synergistically and/or additively activate MLCK, leading to increased gut epithelium permeability, which is observed in HIV-1-infected patients misusing opioids (74). Opioids have also been reported to promote HIV-1 disease progression by disrupting the intestinal epithelial self-repair mechanism and reducing epithelial proliferation in bone marrow-liver-thymus humanized mice and in opioid-using HIV-1+ patients (75). Cumulatively these studies underscore the pivotal role of gut microbiota in the disease progression of HIV-1 infection while also demonstrating that opioid abuse by HIV-1 patients can lead to severe disruption of gut homeostasis, resulting in an accelerated progression of the disease in comparison to drug naïve, infected individuals.
Cannabis and HIV
Despite controlling the HIV-1 viral load with combined antiretroviral therapy (cART), gut epithelium defects and intestinal CD4+ cell depletion continue to persist. In HIV-1 infected patients compromised gut barrier function is aided by the increase in apoptosis, and chronic inflammatory signals on the one hand and the decrease in proliferation and repair of epithelial cells, on the other hand. Alterations in tryptophan metabolism leading to defects in microbes that produce butyrate in PLWH and likely contribute to increased gut permeability have been reported (76-78). A dysfunctional gut epithelium allows inflammatory microbial products such as LPS in the periphery to be translocated (79-82). In particular, defects in the gut epithelium make HIV-1+ individuals vulnerable to increased exposure to proinflammatory ligands produced by gut microbiota (78, 83, 84). These alterations lead to poor HIV-1 disease outcomes, including associated neurocognitive disorders (77).
Cannabis effectively alleviates symptoms associated with HIV-1 disease and other conditions such as cancer and neuropathic pain (85). Cannabinoids act on inflammatory pathways through mechanisms distinct from agents such as non-steroidal anti-inflammatory drugs (NSAIDs) (86). Naturally occurring endocannabinoids, including cannabis, have antioxidative and anti-inflammatory characteristics that help in healing and restoration and thus can be used as adjunctive therapy. As a class, cannabinoids are generally free from the adverse effects of NSAIDs. A concise survey of the anti-inflammatory actions of the phytocannabinoids Δ9-tetrahydrocannabinol (THC), cannabidiol, cannabichromene, and cannabinol has been reported (85-90). Meta-analyses of several clinical trials have established the efficacy of cannabis in HIV-1-related neuropathic pain and nausea (85-92), although dosing and administration routes varied widely. Some studies suggest that titrating dosing to effectiveness and side effects is a valuable strategy for dose selection. While acute cannabis exposure disturbs cognition, how its long-term use affects brain function in the context of HIV-1 is yet to be elucidated clearly (93, 94). Medicinal use of cannabis is becoming rapidly accepted, and a state-level authorized disease management strategy (95, 96). Healthcare providers identify the potential benefits of cannabis by understanding the potential benefits of symptom management. However, a few clinical studies on patients using cannabis as therapy showed potential dependence or possible adverse effects (93, 97). A better understanding of the strategic use of cannabis could aid clinicians in better treatment and therapeutic options with their patients. Since not much research has been done to assess the effects of cannabis in PLWH, there is a dearth of reliable data for cannabis use recommendations in the clinical field.
The endocannabinoid system is a complex network of receptors and enzymes involved in synthesizing and detecting endogenous lipid ligands (98-100). Most human tissues express cannabinoid (type-1 and -2) receptors (98, 99). Cannabinoid receptors type-2 are densely expressed in diverse immune cell types, including macrophages, microglia, splenocytes, monocytes, and T-cells resident in the thymus, spleen, and bone marrow tonsils (98-100). Endocannabinoid system signaling pathways are essential in HIV-1 infection for several reasons and has been pursued as a target for future pharmacotherapy to reduce inflammation (98-100). In HIV-1 infection, cannabis use has been shown to reduce systemic inflammation and activate the immune system (101). Furthermore, HIV-1 DNA is reported to decline more rapidly in individuals taking antiretroviral therapy and using cannabis than those not using cannabis (102). Cannabis use in PLWH leads to aggravated dysbiosis and epithelial barrier dysfunction of the gut, along with chronic inflammation and consequential ill effect on overall health (79, 81, 82, 103). Chronic cannabis use is reported to lower the abundance of Prevotella and increase the abundance of Bacteriodes bacteria in the gut microbiome. Lower abundance of Prevotella leads to systemic mitochondrial dysfunction and reduction of gut SCFA production in cannabis users which is linked to impairment in cognitive function (104). It is also reported that administration of cannabidiol-rich cannabis extract resulted in increased abundance of A. muciniphila and significant decrease in Alistipes finegoldii, Lachnoclostridium sp. YL32, and Ruminiclostridium sp. KB18 alongwith remarkable downregulation of mucin-2 which is associated with maintenance of gut integrity. The study also found upregulation of inflammatory markers IL-1β, CXCL1, and CXCL2 which points towards the disruptive effect of long-term cannabis use (105).
Cocaine and HIV
Cocaine is one of the most commonly abused drugs among PLWH, and it has been suggested that it accelerates AIDS progression. Based on the evidence that the limbic system of the brain, comprising a set of interconnected regions regulating pleasure and motivation, is the primary site of action for cocaine helps explain its high potential for addiction and relapse. Cocaine, a commonly used psychostimulant among PLWH, is a cofactor for HIV-1 infection and progression to AIDS. Globally almost 22.5 million people worldwide are affected by cocaine use disorder, thus making it a significant public health crisis with a high socioeconomic burden (106). Although cocaine is known to have immunomodulatory functions (107-109), the underlying mechanism(s) by which cocaine accentuates HIV-1 replication remains unclear. There are reports that cocaine increases HIV-1 infection/replication by inhibiting HIV-1 protective chemokines and/or upregulating the HIV-1 entry co-receptor (110, 111). Cocaine is a potent vasoconstrictor and brain stimulant. Its abuse leads to severe neurological (fainting attacks, hemorrhagic brain strokes, CNS vasculitis, and encephalopathies), cardiovascular (cardiac arrhythmia and heart attacks), and gastrointestinal complications (112-117).
Cocaine abuse has been reported to alter the gut microbiota composition which in turn affects the uptake and clearence of neurotransmitters. One particular study reports higher accumulation of norepinephrine in intestines of cocaine-administred mice helped the resident Citrobacter rodentium to flourish which resulted in depletion of the intestinal neurotransmitter glycine. This also resulted in glycine depletion in circulation and cerebrospinal fluid of cocaine-administered mice, which was in correlation with increased hyperlocomotion and escalation of drug-seeking behavior (118). The authors also reported alteration of synaptic plasticity pathways at the transcriptome-level in the nucleus accumbens of the cocaine-administered mice, and also that the behavioral changes were reversed with dietary supplementation of glycine or sarcosine (118). Another study reports that cocaine administration in mice reduces the abundance of Mucispirillum, Butrycicoccus, Ruminococcaceae, Pseudoflavonifractor, and Lachnospiracea species of bacteria in the gut microbiota which are the involved in the synthesis of SCFAs involved in maintaining mucosal epithelium integrity. Cocaine administration resulted in alteration of TJPs of the gut membrane, upregulated expression of proinflammatory markers NF-ĸB and IL-1β, and also disruption of the mucosal permeability via MAPK/ERK1/2 signaling pathway (106). Also, in case of mice with reduced gut-bacteria, cocaine admistration resulted in increased sensitivity towards drug reward as well as increased locomotor-sensitivity (119). These studies reveal the critical role of gut microbiome in the behavioral effects of cocaine addiction. The research on the gut microbiome and its relationship with drug abuse is currently in its infancy with a bright future, and still a long way to go.
Methamphetamine and HIV
Similar to other drugs of abuse, several preclinical and clinical studies have demonstrated that Meth induces alterations in the gut microbiome (49,120-123). However, there is a lack of evidence directly linking the gut microbiota with Meth-induced brain dysfunction (124). Meth has been reported to promote the release of norepinephrine and dopamine, leading to a markedly decreased intestinal contractility and motor capacity (125). This decrease in intestinal muscle tone is associated with oxidative and nitrosative stress, which, in turn, can cause neuronal injury and death in the intestine and disrupt intestinal barrier functioning (126). Disruption of the intestinal mucosal barrier increases the permeability of the gut epithelium and plays an essential role in contributing to anxiogenic behavior (127), stress (128), depression (129), cognitive decline (130), and eating and sleep disorders (131). Disruption of the intestinal barrier also leads to the leakage of several inflammatory factors (like TNF-α, interferon-γ, IL-6), microbes, and metabolites from the gut epithelium to the circulatory and lymphatic systems (132). It has been reported that Meth use can increase the permeability of the blood-brain barrier (133), thereby facilitating the entry of microbial communities and metabolites to enter the brain (134). In mouse models, Meth-exposure has been reported to increase the abundance of pathogenic bacteria in the fecal microbiota (120), with increased inflammation, reduced TJP expression in the intestine, and decreased relative quantity of probiotics and fecal metabolites. Further, Meth exposure was also shown to enhance the intestinal autophagy-associated flora, concomitantly leading to the induction of autophagy in the CNS (123). Intestinal inflammatory biomarkers, including the proinflammatory cytokines, are upregulated in Meth abusers and have been reported to infiltrate the brain regions related to depression (135), causing alterations in neurotransmitter metabolism, neuroendocrine function, and neuroplasticity. A recent study has also shown that gene sequencing of the 16S rRNA of the rectal swab samples collected from individuals using Meth, showed increased presence of bacterial species such as Finegoldia, Peptoniphilus, Parvimonas, and Porphyromonas and depletion of species like Faecalibacterium and Butyricicoccus (122). In line with this study, other studies have also shown that there were alterations in the composition of microbes present in the gut of Meth users with decrease in quantity of Bacteroidaceae and Deltaproteobacteria, and increased abundance of Sphingomonadales, Xanthomonadales, Romboutsia and Lachnospiraceae (49). Interestingly, these alterations have been reported in those bacterial species which had previously been demonstrated to be altered in individuals with psychotic syndromes, thus pointing towards a potential link between Meth abuse and psychotic disorders (49). Forouzan et al. showed that Meth exposure and withdrawal in rats resulted in gut dysbiosis, which was linked to depression-like behavior as evidenced by the forced swim test. However, the authors reported no alterations in anxiety-like behaviors which was assessed by either the elevated plus maze or the open field test (136).
HIV-1 has been reported to alter the human intestinal microbiome. An exciting study showed significant changes in the microbiome in the context of drug abuse and sexual behavior during HIV-1 infection. Rectal swab samples, urine drug test results, along with responses to substance use and sex behavior questionnaires were collected from 37 HIV-1-positive individuals at two-time points, in a 6-month gap period, in a group that was being evaluated for the effects of drug abuse in men who have sex with men. The samples were subjected to 16S ribosomal RNA gene sequencing, and the association of the data with behavioral factors was examined using 0-inflated negative binomial regression. Further analyses demonstrated that abuse of Meth and marijuana exhibit unique associations. Meth use was linked with increased Granulicatella and Porphyromonas organisms in HIV-1 patients and a decrease in abundance of Collinsella, Ruminococcus, and Parabacteroides organisms. In contrast, marijuana use was associated with an increased abundance of Clostridium cluster IV, Ruminococcus, Fusobacterium, and Solobacterium organisms and decreased Acidaminococcus, Dialister, Prevotella, Anaerostipes, and Dorea organisms. From this study, it can be concluded that drug use and sexual behavior are important factors associated with intestinal dysbiosis during chronic HIV-1 infection among young men who have sex with men (137). Further, studies are warranted in the field, specifically in association with HIV-1 infection and drug abuse-related disorders.
Nicotine and HIV
Several reports have, on the other hand, demonstrated an association between nicotine and microbiome dysregulation (138-142). In one study aimed at assessing the link between the smoking status of an individual and their intensity of smoking with the relative abundance of gut microbial species in 249 Bangladesh participants, it was reported that there was an increase in the relative abundance of Erysipelotrichi and Catenibacterium in current smokers in comparison to those who had never smoked (139). Another interesting study showed that long-term nicotine administration in rats resulted in alterations of gut microbiota, which was more prominent in rodents fed a high-fat diet than a regular chow diet, thus indicating diet-dependent changes (142). In line with this study, another study showed that cigarette smoke altered gut microbiota composition, which was linked to modifications in the distribution of primary bile acids and cholesterol homeostasis (138). Another study also showed that oral administration of nicotine in mice differentially reorganized the gut microbiome in a gender-specific manner and, furthermore, modified the levels of metabolites such as GABA and glutamate, which are involved in gut-brain communication (142). A recent study has also demonstrated that nicotine altered the gut microbiome and metabolites involved in the gut-brain axis in a sex-specific manner. This study employed high-throughput sequencing and gas chromatography-mass spectrometry to evaluate the effect of nicotine exposure on the gut microbiome and its metabolism in C57BL/6J mice in a sex-dependent manner, with special emphasis on the signaling pathways involved in the gut-brain axis. The 16S sequencing results from this study indicated that the composition of the gut microbiome was differentially altered by nicotine in both females and males. Also, the differential changes in the bacterial carbohydrate metabolic pathways were consistent with lower body weight gain in nicotine-administered males. Genes related to oxidative stress response and DNA repair were also explicitly upregulated in the gut microbiome of the nicotine-treated male mice. Analysis of the fecal metabolome demonstrated that several neurotransmitters, such as glutamate, GABA, and glycine, and neuroactive metabolites-leucine and uric acid, were also differentially altered in female versus male mice. This study showed a sex-dependent effect of nicotine on gut microbiome composition, functional bacterial genes, and the fecal metabolome (141). However, studies are lacking on gut-brain axis in the context of nicotine and HIV.
Conclusion and future perspectives
Understanding the impact of the gut microbiome on gut-brain axis communication has been the topic of momentous research over the past few years. There is a mounting effort to delineate the mechanism(s) of this communication at all axis nodes. It has been now well-established that gut microbiota is crucial for the proper development and maintenance of brain functions. Additionally, as discussed above, there is accumulating evidence from preclinical and clinical studies that implicate the role of microbial dysbiosis in various psychiatric, neurological, and neurodegenerative diseases in the context of HIV-1 and drug abuse. However, it is still a very nascent field of research, and caution must be exerted in over-interpreting these studies. Many unanswered questions remain regarding the beneficial effects of probiotics, with extensive work required to test optimal dosing, strain, and timing in therapeutic applications. The emphasis in the field must shift from correlative analyses to prospective longitudinal study design, causative and mechanistic investigations, and larger-scale trials of potential therapeutic approaches, especially in the case of HIV-1 and drug abuse comorbidity. One big conundrum in microbiota-based research is the ideal definition of healthy microbiota. Inter-individual differences in the gut microbiota composition can be very critical, making it challenging to apply a “one size fits all” approach to target the microbiota. However, this also provides future opportunities for practical personalized medicine approaches. We have also moved from focusing on single bacterial strains as pathogens to an emphasis on nurturing an entire community of microbes, lest they become pathological entities. There are many challenges to conventional wisdom at play, with the possibility that the alterations in the gut microbiota noted in many CNS disorders may have a causal role in symptomatology and that many of the drugs used to treat those disorders could be toxic to or support the diversity of our gut microbes.
Statements
Author Contributions
Study conception and design: SR, PP, and SB; Draft manuscript preparation: SR, SS, MK, and PP. All authors reviewed the contents and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1
Nass SR Ohene-Nyako M Hahn YK Knapp PE Hauser KF . Neurodegeneration within the amygdala is differentially induced by opioid and HIV-1 Tat exposure. Front Neurosci (2022) 16:804774. 10.3389/fnins.2022.804774
2
Arzua T Yan Y Jiang C Logan S Allison RL Wells C et al Modeling alcohol-induced neurotoxicity using human induced pluripotent stem cell-derived three-dimensional cerebral organoids. Transl Psychiatry (2020) 10(1):347. 10.1038/s41398-020-01029-4
3
de la Monte SM Kril JJ . Human alcohol-related neuropathology. Acta Neuropathol (2014) 127(1):71–90. 10.1007/s00401-013-1233-3
4
Araujo I Henriksen A Gamsby J Gulick D . Impact of alcohol abuse on susceptibility to rare neurodegenerative diseases. Front Mol Biosci (2021) 8:643273. 10.3389/fmolb.2021.643273
5
Peng B Yang Q B Joshi R Liu Y Akbar M Song BJ et al Role of alcohol drinking in Alzheimer's disease, Parkinson's disease, and amyotrophic lateral sclerosis. Int J Mol Sci, 2020. 21, 2316. 10.3390/ijms21072316
6
Clark KH Wiley CA Bradberry CW . Psychostimulant abuse and neuroinflammation: Emerging evidence of their interconnection. Neurotox Res (2013) 23(2):174–88. 10.1007/s12640-012-9334-7
7
Sajja RK Rahman S Cucullo L . Drugs of abuse and blood-brain barrier endothelial dysfunction: A focus on the role of oxidative stress. J Cereb Blood Flow Metab (2016) 36(3):539–54. 10.1177/0271678X15616978
8
Illes A Balicza P Molnar V Bencsik R Szilvasi I Molnar MJ . Dynamic interaction of genetic risk factors and cocaine abuse in the background of Parkinsonism - a case report. BMC Neurol (2019) 19(1):260. 10.1186/s12883-019-1496-y
9
Ersche KD Acosta-Cabronero J Jones PS Ziauddeen H van Swelm RPL Laarakkers CMM et al Disrupted iron regulation in the brain and periphery in cocaine addiction. Transl Psychiatry (2017) 7(2):e1040. 10.1038/tp.2016.271
10
Sivalingam K Doke M Khan MA Samikkannu T . Influence of psychostimulants and opioids on epigenetic modification of class III histone deacetylase (HDAC)-sirtuins in glial cells. Sci Rep (2021) 11(1):21335. 10.1038/s41598-021-00836-z
11
Shreiner AB Kao JY Young VB . The gut microbiome in health and in disease. Curr Opin Gastroenterol (2015) 31(1):69–75. 10.1097/MOG.0000000000000139
12
Fung TC Olson CA Hsiao EY . Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci (2017) 20(2):145–55. 10.1038/nn.4476
13
Capuco A Urits I Hasoon J Chun R Gerald B Wang JK et al Gut microbiome dysbiosis and depression: A comprehensive review. Curr Pain Headache Rep (2020) 24(7):36. 10.1007/s11916-020-00871-x
14
Mossad O Erny D . The microbiota-microglia axis in central nervous system disorders. Brain Pathol (2020) 30(6):1159–77. 10.1111/bpa.12908
15
Gomaa EZ . Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek (2020) 113(12):2019–40. 10.1007/s10482-020-01474-7
16
Rhee SH Pothoulakis C Mayer EA . Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol (2009) 6(5):306–14. 10.1038/nrgastro.2009.35
17
Holzer P Farzi A . Neuropeptides and the microbiota-gut-brain axis. Adv Exp Med Biol (2014) 817:195–219. 10.1007/978-1-4939-0897-4_9
18
Carabotti M Scirocco A Maselli MA Severi C . The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol (2015) 28(2):203–9.
19
Baj A Moro E Bistoletti M Orlandi V Crema F Giaroni C . Glutamatergic signaling along the microbiota-gut-brain Axis. Int J Mol Sci (2019) 20(6):1482. 10.3390/ijms20061482
20
Bliss ES Whiteside E . The gut-brain Axis, the human gut microbiota and their integration in the development of obesity. Front Physiol (2018) 9:900. 10.3389/fphys.2018.00900
21
Han W Tellez LA Perkins MH Perez IO Qu T Ferreira J et al A neural circuit for gut-induced reward. Cell (2018) 175(3):887–8. 10.1016/j.cell.2018.10.018
22
Van Oudenhove L . Does the gut-brain axis control anticipatory food reward? Novel insights from bariatric surgery. Gut (2014) 63(6):868–9. 10.1136/gutjnl-2013-305488
23
Brookes SJ Spencer NJ Costa M Zagorodnyuk VP . Extrinsic primary afferent signalling in the gut. Nat Rev Gastroenterol Hepatol (2013) 10(5):286–96. 10.1038/nrgastro.2013.29
24
Cryan JF Dinan TG . Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat Rev Neurosci (2012) 13(10):701–12. 10.1038/nrn3346
25
Forsythe P Kunze W Bienenstock J . Moody microbes or fecal phrenology: What do we know about the microbiota-gut-brain axis?BMC Med (2016) 14:58. 10.1186/s12916-016-0604-8
26
Sarkar A Lehto SM Harty S Dinan TG Cryan JF Burnet PWJ . Psychobiotics and the manipulation of bacteria-gut-brain signals. Trends Neurosci (2016) 39(11):763–81. 10.1016/j.tins.2016.09.002
27
Belkaid Y Hand TW . Role of the microbiota in immunity and inflammation. Cell (2014) 157(1):121–41. 10.1016/j.cell.2014.03.011
28
Gensollen T Iyer SS Kasper DL Blumberg RS . How colonization by microbiota in early life shapes the immune system. Science (2016) 352(6285):539–44. 10.1126/science.aad9378
29
Lazar V Ditu LM Pircalabioru GG Gheorghe I Curutiu C Holban AM et al Aspects of gut microbiota and immune system interactions in infectious diseases, immunopathology, and cancer. Front Immunol (2018) 9:1830. 10.3389/fimmu.2018.01830
30
Zhao Q Elson CO . Adaptive immune education by gut microbiota antigens. Immunology (2018) 154(1):28–37. 10.1111/imm.12896
31
Blander JM Longman RS Iliev ID Sonnenberg GF Artis D . Regulation of inflammation by microbiota interactions with the host. Nat Immunol (2017) 18(8):851–60. 10.1038/ni.3780
32
Clemente JC Manasson J Scher JU . The role of the gut microbiome in systemic inflammatory disease. BMJ (2018) 360:j5145. 10.1136/bmj.j5145
33
Lobionda S Sittipo P Kwon HY Lee YK . The role of gut microbiota in intestinal inflammation with respect to diet and extrinsic stressors. Microorganisms (2019) 7(8):271. 10.3390/microorganisms7080271
34
Tilg H Zmora N Adolph TE Elinav E . The intestinal microbiota fuelling metabolic inflammation. Nat Rev Immunol (2020) 20(1):40–54. 10.1038/s41577-019-0198-4
35
Mohajeri MH La Fata G Steinert RE Weber P . Relationship between the gut microbiome and brain function. Nutr Rev (2018) 76(7):481–96. 10.1093/nutrit/nuy009
36
Huang TT Lai JB Du YL Xu Y Ruan LM Hu SH . Current understanding of gut microbiota in mood disorders: An update of human studies. Front Genet (2019) 10:98. 10.3389/fgene.2019.00098
37
Li M Wang B Zhang M Rantalainen M Wang S Zhou H et al Symbiotic gut microbes modulate human metabolic phenotypes. Proc Natl Acad Sci U S A (2008) 105(6):2117–22. 10.1073/pnas.0712038105
38
Marchesi JR Adams DH Fava F Hermes GDA Hirschfield GM Hold G et al The gut microbiota and host health: A new clinical frontier. Gut (2016) 65(2):330–9. 10.1136/gutjnl-2015-309990
39
Microbiology: Gut bacteria linked to Parkinson's. Nature (2016) 540(7632):172–3. 10.1038/540172d
40
Erny D Prinz M . Microbiology: Gut microbes augment neurodegeneration. Nature (2017) 544(7650):304–5. 10.1038/nature21910
41
Sampson TR Debelius JW Thron T Janssen S Shastri GG Ilhan ZE et al Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson's disease. Cell (2016) 167(6):1469–80. 10.1016/j.cell.2016.11.018
42
From the American Association of Neurological Surgeons (AANS) American Society of Neuroradiology (ASNR) Cardiovascular and Interventional Radiology Society of Europe (CIRSE) Canadian Interventional Radiology Association (CIRA) Congress of Neurological Surgeons (CNS) European Society of Minimally Invasive Neurological Therapy (ESMINT) et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke (2018) 13(6):612–32. 10.1177/1747493018778713
43
Capuco A Urits I Hasoon J Chun R Gerald B Wang JK et al Current perspectives on gut microbiome dysbiosis and depression. Adv Ther (2020) 37(4):1328–46. 10.1007/s12325-020-01272-7
44
Chang CH Lin CH Lane HY . D-Glutamate and gut microbiota in Alzheimer's disease. Int J Mol Sci (2020) 21(8):2676. 10.3390/ijms21082676
45
Strandwitz P . Neurotransmitter modulation by the gut microbiota. Brain Res (2018) 1693:128–33. 10.1016/j.brainres.2018.03.015
46
O'Mahony SM Clarke G Borre YE Dinan TG Cryan JF . Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res (2015) 277:32–48. 10.1016/j.bbr.2014.07.027
47
Wang F Roy S . Gut homeostasis, microbial dysbiosis, and opioids. Toxicol Pathol (2017) 45(1):150–6. 10.1177/0192623316679898
48
Mutlu EA Gillevet PM Rangwala H Sikaroodi M Naqvi A Engen PA et al Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol (2012) 302(9):G966–78. 10.1152/ajpgi.00380.2011
49
Yang Y Yu X Liu X Liu G Zeng K Wang G . Altered fecal microbiota composition in individuals who abuse methamphetamine. Sci Rep (2021) 11(1):18178. 10.1038/s41598-021-97548-1
50
Salavrakos M Leclercq S De Timary P Dom G . Microbiome and substances of abuse. Prog Neuropsychopharmacol Biol Psychiatry (2021) 105:110113. 10.1016/j.pnpbp.2020.110113
51
Weersma RK Zhernakova A Fu J . Interaction between drugs and the gut microbiome. Gut (2020) 69(8):1510–9. 10.1136/gutjnl-2019-320204
52
Simpson S Mclellan R Wellmeyer E Matalon F George O . Drugs and bugs: The gut-brain Axis and substance use disorders. J Neuroimmune Pharmacol (2021) 17:33–61. 10.1007/s11481-021-10022-7
53
Meng J Sindberg GM Roy S . Disruption of gut homeostasis by opioids accelerates HIV disease progression. Front Microbiol (2015) 6:643. 10.3389/fmicb.2015.00643
54
Ren M Lotfipour S . The role of the gut microbiome in opioid use. Behav Pharmacol (2020) 31(2&3):113–21. 10.1097/FBP.0000000000000538
55
Cussotto S Clarke G Dinan TG Cryan JF . Psychotropics and the microbiome: A chamber of secrets. Psychopharmacology (Berl) (2019) 236(5):1411–32. 10.1007/s00213-019-5185-8
56
Trang T Al-Hasani R Salvemini D Salter MW Gutstein H Cahill CM . Pain and poppies: The good, the bad, and the ugly of opioid analgesics. J Neurosci (2015) 35(41):13879–88. 10.1523/JNEUROSCI.2711-15.2015
57
Kalkman GA Kramers C van Dongen RT van den Brink W Schellekens A . Trends in use and misuse of opioids in The Netherlands: A retrospective, multi-source database study. Lancet Public Health (2019) 4(10):e498–e505. 10.1016/S2468-2667(19)30128-8
58
Hedegaard H Minino AM Spencer MR Warner M . Drug overdose deaths in the United States, 1999-2020. NCHS Data Brief (2021) 2021(426):1–8.
59
Banerjee S SindberG G Wang F Meng J Sharma U Zhang L et al Opioid-induced gut microbial disruption and bile dysregulation leads to gut barrier compromise and sustained systemic inflammation. Mucosal Immunol (2016) 9(6):1418–28. 10.1038/mi.2016.9
60
Nagpal R Wang S Solberg Woods LC Seshie O Chung ST Shively CA et al Comparative microbiome signatures and short-chain fatty acids in mouse, rat, non-human primate, and human feces. Front Microbiol (2018) 9:2897. 10.3389/fmicb.2018.02897
61
Zhang L Meng J Ban Y Jalodia R Chupikova I Fernandez I et al Morphine tolerance is attenuated in germfree mice and reversed by probiotics, implicating the role of gut microbiome. Proc Natl Acad Sci U S A (2019) 116(27):13523–32. 10.1073/pnas.1901182116
62
Park JC Im SH . Of men in mice: The development and application of a humanized gnotobiotic mouse model for microbiome therapeutics. Exp Mol Med (2020) 52(9):1383–96. 10.1038/s12276-020-0473-2
63
Lee K Vuong HE Nusbaum DJ Hsiao EY Evans CJ Taylor AMW . The gut microbiota mediates reward and sensory responses associated with regimen-selective morphine dependence. Neuropsychopharmacology (2018) 43(13):2606–14. 10.1038/s41386-018-0211-9
64
Meng J Yu H Ma J Wang J Banerjee S Charboneau R et al Morphine induces bacterial translocation in mice by compromising intestinal barrier function in a TLR-dependent manner. PLoS One (2013) 8(1):e54040. 10.1371/journal.pone.0054040
65
Nath A Hauser KF Wojna V Booze RM Maragos W Prendergast M et al Molecular basis for interactions of HIV and drugs of abuse. J Acquir Immune Defic Syndr (2002) 31(2):S62–9. 10.1097/00126334-200210012-00006
66
Roy S Ninkovic J Banerjee S Charboneau RG Das S Dutta R et al Opioid drug abuse and modulation of immune function: Consequences in the susceptibility to opportunistic infections. J Neuroimmune Pharmacol (2011) 6(4):442–65. 10.1007/s11481-011-9292-5
67
Shanahan F . Intestinal lymphoepithelial communication. Adv Exp Med Biol (1999) 473:1–9. 10.1007/978-1-4615-4143-1_1
68
Nazli A Chan O Dobson-Belaire WN Ouellet M Tremblay MJ Gray-Owen SD et al Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. Plos Pathog (2010) 6(4):e1000852. 10.1371/journal.ppat.1000852
69
Hirao LA Grishina I Bourry O Hu WK Somrit M Sankaran-Walters S et al Early mucosal sensing of SIV infection by paneth cells induces IL-1β production and initiates gut epithelial disruption. Plos Pathog (2014) 10(8):e1004311. 10.1371/journal.ppat.1004311
70
McGowan I Elliott J Fuerst M Taing P Boscardin J Poles M et al Increased HIV-1 mucosal replication is associated with generalized mucosal cytokine activation. J Acquir Immune Defic Syndr (2004) 37(2):1228–36. 10.1097/01.qai.0000131846.12453.29
71
Pandrea IV Gautam R Ribeiro RM Brenchley JM Butler IF Pattison M et al Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence. J Immunol (2007) 179(5):3035–46. 10.4049/jimmunol.179.5.3035
72
Byrd DA Fellows RP Morgello S Franklin D Heaton RK Deutsch R et al Neurocognitive impact of substance use in HIV infection. J Acquir Immune Defic Syndr (2011) 58(2):154–62. 10.1097/QAI.0b013e318229ba41
73
Ancuta P Kamat A Kunstman KJ Kim EY Autissier P Wurcel A et al Microbial translocation is associated with increased monocyte activation and dementia in AIDS patients. PLoS One (2008) 3(6):e2516. 10.1371/journal.pone.0002516
74
Sindberg GM Sharma U Banerjee S Anand V Dutta R Gu CJ et al An infectious murine model for studying the systemic effects of opioids on early HIV pathogenesis in the gut. J Neuroimmune Pharmacol (2015) 10(1):74–87. 10.1007/s11481-014-9574-9
75
Meng J Banerjee S Zhang L Sindberg G Moidunny S Li B et al Opioids impair intestinal epithelial repair in HIV-infected humanized mice. Front Immunol (2019) 10:2999. 10.3389/fimmu.2019.02999
76
Dillon SM Kibbie J Lee EJ Guo K Santiago ML Austin GL et al Low abundance of colonic butyrate-producing bacteria in HIV infection is associated with microbial translocation and immune activation. AIDS (2017) 31(4):511–21. 10.1097/QAD.0000000000001366
77
Dillon SM Lee EJ Kotter CV Austin GL Dong Z Hecht DK et al An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol (2014) 7(4):983–94. 10.1038/mi.2013.116
78
Jenabian MA El-Far M Vyboh K Kema I Costiniuk CT Thomas R et al Immunosuppressive tryptophan catabolism and gut mucosal dysfunction following early HIV infection. J Infect Dis (2015) 212(3):355–66. 10.1093/infdis/jiv037
79
Brenchley JM Douek DC . The mucosal barrier and immune activation in HIV pathogenesis. Curr Opin HIV AIDS (2008) 3(3):356–61. 10.1097/COH.0b013e3282f9ae9c
80
Brenchley JM Price DA Schacker TW Asher TE Silvestri G Rao S et al Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med (2006) 12(12):1365–71. 10.1038/nm1511
81
Somsouk M Estes JD Deleage C Dunham RM Albright R Inadomi JM et al Gut epithelial barrier and systemic inflammation during chronic HIV infection. AIDS (2015) 29(1):43–51. 10.1097/QAD.0000000000000511
82
Vujkovic-Cvijin I Dunham RM Iwai S Maher MC Albright RG Broadhurst MJ et al Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci Transl Med (2013) 5(193):193ra91. 10.1126/scitranslmed.3006438
83
McHardy IH Li X Tong M Ruegger P Jacobs J Borneman J et al HIV Infection is associated with compositional and functional shifts in the rectal mucosal microbiota. Microbiome (2013) 1(1):26. 10.1186/2049-2618-1-26
84
Sandler NG Douek DC . Microbial translocation in HIV infection: Causes, consequences and treatment opportunities. Nat Rev Microbiol (2012) 10(9):655–66. 10.1038/nrmicro2848
85
Abrams DI Jay CA Shade SB Vizoso H Reda H PreSS S et al Cannabis in painful HIV-associated sensory neuropathy: A randomized placebo-controlled trial. Neurology (2007) 68(7):515–21. 10.1212/01.wnl.0000253187.66183.9c
86
Zurier RB Burstein SH . Cannabinoids, inflammation, and fibrosis. FASEB J (2016) 30(11):3682–9. 10.1096/fj.201600646R
87
Whiting PF Wolff RF Deshpande S Di Nisio M Duffy S Hernandez AV et al Cannabinoids for medical use: A systematic review and meta-analysis. JAMA (2015) 313(24):2456–73. 10.1001/jama.2015.6358
88
Abrams DI Hilton JF Leiser RJ Shade SB Elbeik TA Aweeka FT et al Short-term effects of cannabinoids in patients with HIV-1 infection: A randomized, placebo-controlled clinical trial. Ann Intern Med (2003) 139(4):258–66. 10.7326/0003-4819-139-4-200308190-00008
89
Ellis RJ Toperoff W Vaida F van den Brande G Gonzales J Gouaux B et al Smoked medicinal cannabis for neuropathic pain in HIV: A randomized, crossover clinical trial. Neuropsychopharmacology (2009) 34(3):672–80. 10.1038/npp.2008.120
90
Lutge EE Gray A Siegfried N . The medical use of cannabis for reducing morbidity and mortality in patients with HIV/AIDS. Cochrane Database Syst Rev (2013)(4) CD005175. 10.1002/14651858.CD005175.pub3
91
Andreae MH Carter GM Shaparin N Suslov K Ellis RJ Ware MA et al Inhaled cannabis for chronic neuropathic pain: A meta-analysis of individual patient data. J Pain (2015) 16(12):1221–32. 10.1016/j.jpain.2015.07.009
92
Prospero-Garcia O Amancio-Belmont O Becerril Melendez AL Ruiz-Contreras AE Mendez-Diaz M . Endocannabinoids and sleep. Neurosci Biobehav Rev (2016) 71:671–9. 10.1016/j.neubiorev.2016.10.005
93
Brooks E Gundersen DC Flynn E Brooks-Russell A Bull S . The clinical implications of legalizing marijuana: Are physician and non-physician providers prepared?Addict Behav (2017) 72:1–7. 10.1016/j.addbeh.2017.03.007
94
Carlini BH Garrett SB Carter GT . Medicinal cannabis: A survey among health care providers in Washington state. Am J Hosp Palliat Care (2017) 34(1):85–91. 10.1177/1049909115604669
95
Azofeifa A Mattson ME Schauer G McAfee T Grant A Lyerla R . National estimates of marijuana use and related indicators - national survey on drug use and health, United States, 2002-2014. MMWR Surveill Summ (2016) 65(11):1–28. 10.15585/mmwr.ss6511a1
96
Bonn-Miller MO Oser ML Bucossi MM Trafton JA . Cannabis use and HIV antiretroviral therapy adherence and HIV-related symptoms. J Behav Med (2014) 37(1):1–10. 10.1007/s10865-012-9458-5
97
Wilson NL Azuero A Vance DE Richman JS Moneyham LD Raper JL et al Identifying symptom patterns in people living with HIV disease. J Assoc Nurses AIDS Care (2016) 27(2):121–32. 10.1016/j.jana.2015.11.009
98
Acharya N Penukonda S Shcheglova T Hagymasi AT Basu S Srivastava PK . Endocannabinoid system acts as a regulator of immune homeostasis in the gut. Proc Natl Acad Sci U S A (2017) 114(19):5005–10. 10.1073/pnas.1612177114
99
Li C Jones PM Persaud SJ . Role of the endocannabinoid system in food intake, energy homeostasis and regulation of the endocrine pancreas. Pharmacol Ther (2011) 129(3):307–20. 10.1016/j.pharmthera.2010.10.006
100
Pacher P Batkai S Kunos G . The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev (2006) 58(3):389–462. 10.1124/pr.58.3.2
101
Manuzak JA Gott TM Kirkwood JS Coronado E Hensley-McBain T Miller C et al Heavy cannabis use associated with reduction in activated and inflammatory immune cell frequencies in antiretroviral therapy-treated human immunodeficiency virus-infected individuals. Clin Infect Dis (2018) 66(12):1872–82. 10.1093/cid/cix1116
102
Chaillon A Nakazawa M Anderson C Christensen-Quick A Ellis RJ Franklin D et al Effect of cannabis use on human immunodeficiency virus DNA during suppressive antiretroviral therapy. Clin Infect Dis (2020) 70(1):140–3. 10.1093/cid/ciz387
103
Klatt NR Funderburg NT Brenchley JM . Microbial translocation, immune activation, and HIV disease. Trends Microbiol (2013) 21(1):6–13. 10.1016/j.tim.2012.09.001
104
Panee J Gerschenson M Chang L . Associations between microbiota, mitochondrial function, and cognition in chronic marijuana users. J Neuroimmune Pharmacol (2018) 13(1):113–22. 10.1007/s11481-017-9767-0
105
Skinner CM Nookaew I Ewing LE Wongsurawat T Jenjaroenpun P Quick CM et al Potential probiotic or trigger of gut inflammation - the janus-faced nature of cannabidiol-rich cannabis extract. J Diet Suppl (2020) 17(5):543–60. 10.1080/19390211.2020.1761506
106
Chivero ET Ahmad R Thangaraj A Periyasamy P Kumar B Kroeger E et al Cocaine induces inflammatory gut milieu by compromising the mucosal barrier integrity and altering the gut microbiota colonization. Sci Rep (2019) 9(1):12187. 10.1038/s41598-019-48428-2
107
Rofael HZ Turkall RM Abdel-Rahman MS . Immunomodulation by cocaine and ketamine in postnatal rats. Toxicology (2003) 188(1):101–14. 10.1016/s0300-483x(03)00081-7
108
Watzl B Watson RR . Immunomodulation by cocaine--a neuroendocrine mediated response. Life Sci (1990) 46(19):1319–29. 10.1016/0024-3205(90)90331-k
109
Halpern JH Sholar MB Glowacki J Mello NK Mendelson JH Siegel AJ . Diminished interleukin-6 response to proinflammatory challenge in men and women after intravenous cocaine administration. J Clin Endocrinol Metab (2003) 88(3):1188–93. 10.1210/jc.2002-020804
110
Nair MP Mahajan S Chadha KC Nair NM Hewitt RG Pillai SK et al Effect of cocaine on chemokine and CCR-5 gene expression by mononuclear cells from normal donors and HIV-1 infected patients. Adv Exp Med Biol (2001) 493:235–40. 10.1007/0-306-47611-8_28
111
Nair MP Chadha KC Hewitt RG Mahajan S Sweet A Schwartz SA . Cocaine differentially modulates chemokine production by mononuclear cells from normal donors and human immunodeficiency virus type 1-infected patients. Clin Diagn Lab Immunol (2000) 7(1):96–100. 10.1128/cdli.7.1.96-100.2000
112
Dash S Balasubramaniam M Villalta F Dash C Pandhare J . Impact of cocaine abuse on HIV pathogenesis. Front Microbiol (2015) 6:1111. 10.3389/fmicb.2015.01111
113
Daras M . Neurologic complications of cocaine. NIDA Res Monogr (1996) 163:43–65.
114
Riezzo I Fiore C De Carlo D Pascale N Neri M Turillazzi E et al Side effects of cocaine abuse: Multiorgan toxicity and pathological consequences. Curr Med Chem (2012) 19(33):5624–46. 10.2174/092986712803988893
115
Marasco CC Goodwin CR Winder DG Schramm-Sapyta NL McLean JA Wikswo JP . Systems-level view of cocaine addiction: The interconnection of the immune and nervous systems. Exp Biol Med (Maywood) (2014) 239(11):1433–42. 10.1177/1535370214537747
116
Gawin FH . Cocaine addiction: Psychology and neurophysiology. Science (1991) 251(5001):1580–6. 10.1126/science.2011738
117
Soder HE Berumen AM Gomez KE Green CE Suchting R Wardle MC et al Elevated neutrophil to lymphocyte ratio in older adults with cocaine use disorder as a marker of chronic inflammation. Clin Psychopharmacol Neurosci (2020) 18(1):32–40. 10.9758/cpn.2020.18.1.32
118
Cuesta S Burdisso P Segev A Kourrich S Sperandio V . Gut colonization by Proteobacteria alters host metabolism and modulates cocaine neurobehavioral responses. Cell Host Microbe (2022) 30(11):1615–29 e5. 10.1016/j.chom.2022.09.014
119
Kiraly DD Walker DM Calipari ES Labonte B Issler O Pena CJ et al Alterations of the host microbiome affect behavioral responses to cocaine. Sci Rep (2016) 6:35455. 10.1038/srep35455
120
Ning T Gong X Xie L Ma B . Gut microbiota analysis in rats with methamphetamine-induced conditioned place preference. Front Microbiol (2017) 8:1620. 10.3389/fmicb.2017.01620
121
Xu Y Xie Z Wang H Shen Z Guo Y Gao Y et al Bacterial diversity of intestinal microbiota in patients with substance use disorders revealed by 16S rRNA gene deep sequencing. Sci Rep (2017) 7(1):3628. 10.1038/s41598-017-03706-9
122
Cook RR Fulcher JA Tobin NH Li F Lee DJ Woodward C et al Alterations to the gastrointestinal microbiome associated with methamphetamine use among young men who have sex with men. Sci Rep (2019) 9(1):14840. 10.1038/s41598-019-51142-8
123
Chen LJ Zhi X Zhang KK Wang LB Li JH Liu JL et al Escalating dose-multiple binge methamphetamine treatment elicits neurotoxicity, altering gut microbiota and fecal metabolites in mice. Food Chem Toxicol (2021) 148:111946. 10.1016/j.fct.2020.111946
124
Deng D Su H Song Y Chen T Sun Q Jiang H et al Altered fecal microbiota correlated with systemic inflammation in male subjects with methamphetamine use disorder. Front Cel Infect Microbiol (2021) 11:783917. 10.3389/fcimb.2021.783917
125
Li Y Kong D Bi K Luo H . Related effects of methamphetamine on the intestinal barrier via cytokines, and potential mechanisms by which methamphetamine may occur on the brain-gut Axis. Front Med (Lausanne) (2022) 9:783121. 10.3389/fmed.2022.783121
126
Boschetti E Accarino A Malagelada C Malagelada JR Cogliandro RF Gori A et al Gut epithelial and vascular barrier abnormalities in patients with chronic intestinal pseudo-obstruction. Neurogastroenterol Motil (2019) 31(8):e13652. 10.1111/nmo.13652
127
Stevens BR Goel R Seungbum K Richards EM Holbert RC Pepine CJ et al Increased human intestinal barrier permeability plasma biomarkers zonulin and FABP2 correlated with plasma LPS and altered gut microbiome in anxiety or depression. Gut (2018) 67(8):1555–7. 10.1136/gutjnl-2017-314759
128
Camilleri M . Leaky gut: Mechanisms, measurement and clinical implications in humans. Gut (2019) 68(8):1516–26. 10.1136/gutjnl-2019-318427
129
Trzeciak P Herbet M . Role of the intestinal microbiome, intestinal barrier and psychobiotics in depression. Nutrients (2021) 13(3):927. 10.3390/nu13030927
130
Wang Y An Y Ma W Yu H Lu Y Zhang X et al 27-Hydroxycholesterol contributes to cognitive deficits in APP/PS1 transgenic mice through microbiota dysbiosis and intestinal barrier dysfunction. J Neuroinflammation (2020) 17(1):199. 10.1186/s12974-020-01873-7
131
Gao T Wang Z Dong Y Cao J Lin R Wang X et al Role of melatonin in sleep deprivation-induced intestinal barrier dysfunction in mice. J Pineal Res (2019) 67(1):e12574. 10.1111/jpi.12574
132
Suzuki T . Regulation of intestinal epithelial permeability by tight junctions. Cell Mol Life Sci (2013) 70(4):631–59. 10.1007/s00018-012-1070-x
133
Coelho-Santos V Leitao RA Cardoso FL Palmela I Rito M Barbosa M et al The TNF-α/NF-κB signaling pathway has a key role in methamphetamine-induced blood-brain barrier dysfunction. J Cereb Blood Flow Metab (2015) 35(8):1260–71. 10.1038/jcbfm.2015.59
134
Northrop NA Yamamoto BK . Methamphetamine effects on blood-brain barrier structure and function. Front Neurosci (2015) 9:69. 10.3389/fnins.2015.00069
135
Miller AH Maletic V Raison CL . Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol Psychiatry (2009) 65(9):732–41. 10.1016/j.biopsych.2008.11.029
136
Forouzan S Hoffman KL Kosten TA . Methamphetamine exposure and its cessation alter gut microbiota and induce depressive-like behavioral effects on rats. Psychopharmacology (Berl) (2021) 238(1):281–92. 10.1007/s00213-020-05681-y
137
Fulcher JA Hussain SK Cook R Li F Tobin NH Ragsdale A et al Effects of substance use and sex practices on the intestinal microbiome during HIV-1 infection. J Infect Dis (2018) 218(10):1560–70. 10.1093/infdis/jiy349
138
Yang Y Yang C Lei Z Rong H Yu S Wu H et al Cigarette smoking exposure breaks the homeostasis of cholesterol and bile acid metabolism and induces gut microbiota dysbiosis in mice with different diets. Toxicology (2021) 450:152678. 10.1016/j.tox.2021.152678
139
Nolan-Kenney R Wu F Hu J Yang L Kelly D Li H et al The association between smoking and gut microbiome in Bangladesh. Nicotine Tob Res (2020) 22(8):1339–46. 10.1093/ntr/ntz220
140
Kobayashi T Fujiwara K . Identification of heavy smokers through their intestinal microbiota by data mining analysis. Biosci Microbiota Food Health (2013) 32(2):77–80. 10.12938/bmfh.32.77
141
Chi L Mahbub R Gao B Bian X Tu P Ru H et al Nicotine alters the gut microbiome and metabolites of gut-brain interactions in a sex-specific manner. Chem Res Toxicol (2017) 30(12):2110–9. 10.1021/acs.chemrestox.7b00162
142
Wang R Li S Jin L Zhang W Liu N Wang H et al Four-week administration of nicotine moderately impacts blood metabolic profile and gut microbiota in a diet-dependent manner. Biomed Pharmacother (2019) 115:108945. 10.1016/j.biopha.2019.108945
Summary
Keywords
microbiome, drug abuse, neuroinflammation, HIV-1, gut-brain axis
Citation
Ray S, Sil S, Kannan M, Periyasamy P and Buch S (2023) Role of the gut-brain axis in HIV and drug abuse-mediated neuroinflammation. Adv. Drug. Alco. Res. 3:11092. doi: 10.3389/adar.2023.11092
Received
01 December 2022
Accepted
23 February 2023
Published
03 March 2023
Volume
3 - 2023
Edited by
Emmanuel Onaivi, William Paterson University, United States
Reviewed by
Yuri Persidsky, Temple University, United States
Laura Orio, Complutense University of Madrid, Spain
Updates
Copyright
© 2023 Ray, Sil, Kannan, Periyasamy and Buch.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Palsamy Periyasamy, palsamy.periyasamy@unmc.edu; Shilpa Buch, sbuch@unmc.edu
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.