Dear Editors,
Human cytomegalovirus (CMV) is the most frequent opportunistic infection in the early months after heart transplantation with reported DNAemia in up to 40%–50% of high risk recipients and CMV disease in 10%–15% within the first year post-transplantation. CMV continues to exert a major negative impact, contributing to acute rejection, opportunistic infections, coronary allograft vasculopathy, graft dysfunction and increased healthcare costs [1].
Post-transplant risk stratification is based on donor–recipient serostatus (D+/R–as high risk, R+ as intermediate, and D–/R–as low); paradoxically CMV transmission and infection may still occur even in settings where immunity or prophylaxis should confer protection, reflecting organ-specific variability in transmission risk and indicating that serology alone does not reliably reflect protective immunity, thereby highlighting the need for additional immune biomarkers [2].
Memory/effector T cells help control CMV through responses to IE-1 and pp65 antigens and the IFN-γ ELISpot assay enables the detection of CMV-reactive T cells and prediction of infection risk [3]. CMV-specific cellular immunity (CMI) can identify higher-risk kidney transplant recipients who are more likely to develop CMV infection, while preserved CMV-CMI responses confer protection against post-transplant replication and disease [4, 5]. Recent multicenter trials have further supported the role of immunoguided prophylaxis in solid-organ transplantation. In kidney and liver transplant recipients, immune monitoring based on CMV-specific T-cell responses safely reduced antiviral exposure without increasing infection rates [6], whereas in lung transplantation, CMV-CMI–guided prophylaxis proved non-inferior to standard strategies while minimizing antiviral toxicity and adverse events [7].
However, most studies assessing CMV-specific cellular immunity have been conducted in kidney transplantation, and their findings may not be fully extrapolable to heart transplant recipients, who face higher perioperative morbidity and mortality [5].
Current Spanish and international guidelines for heart transplantation provide only low-level evidence, and CMV-CMI monitoring is still recommended based solely on expert opinion, particularly in seropositive (R+) recipients [8, 9].
Preventive strategies currently rely on universal prophylaxis with valgancicloviror PCR-guided preemptive therapy. While effective, both have limitations such as toxicity, late-onset infections, and viral resistance [8, 10].
The ELISPOT-TC trial will address this gap by evaluating whether prophylaxis guided by CMV-specific CMI, assessed by IFN-γ ELISpot (T-SPOT.CMV), is non-inferior to universal valganciclovir prophylaxis in seropositive heart transplant recipients.
It will be a phase IV, multicenter, open-label, randomized (2:1), non-inferiority clinical trial conducted across 11 Spanish transplant centers. A total of 188 adult CMV-seropositive recipients will be enrolled (125 experimental, 63 control) as shown in Figure 1.
FIGURE 1
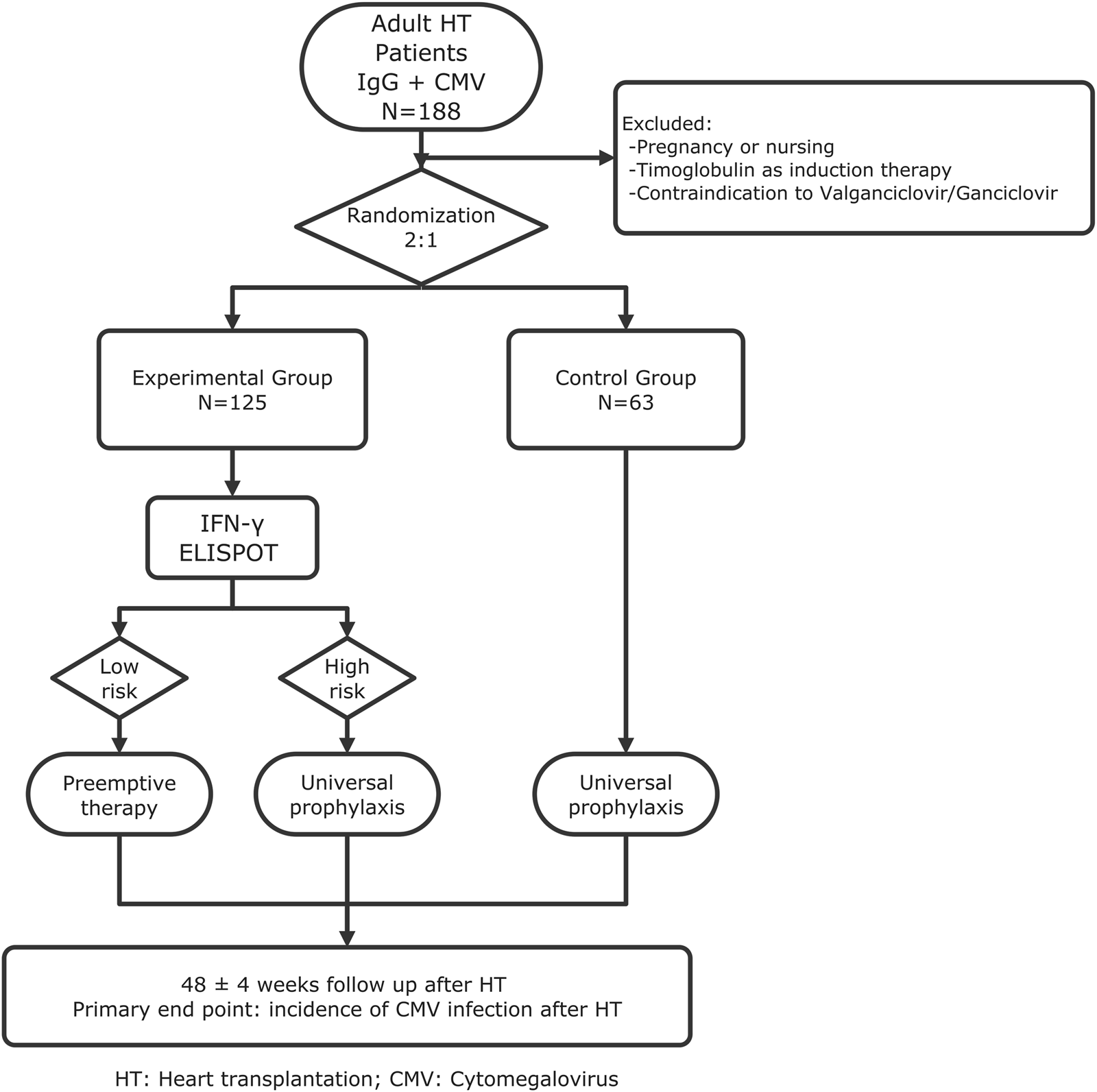
Flowchart of the trial.
Patients will be randomly assigned by a centralized computer system to either:
- -
Group 1: CMI-guided prevention based on T-SPOT. CMV results, or
- -
Group 2: Standard universal valganciclovir prophylaxis for 3 months.
Randomized will be centralized and stratified by center. The assigned preventive strategy will not be masked to investigators or participants.
Eligible participants will be adult (≥18 years) CMV-seropositive recipients able to provide informed consent. All patients will undergo T-SPOT. CMV testing at day 10 and at month 3 post-transplant,Viral load monitoring by quantitative nucleic acid testing (QNAT) will be performed throughout follow-up according to a standardized schedule.
In the experimental arm, T-SPOT. CMV testing at day 10 post-transplant will classify patients as high or low risk, guiding either initiation of antiviral prophylaxis in those without CMV-specific cellular immunity (high-risk) or a pre-emptive strategy in those with preserved immunity (low-risk).
Patients will be followed for 48 weeks with scheduled visits to monitor outcomes.
The primary endpoint will be the cumulative incidence of CMV infection at 1 year. Secondary endpoints will include the classification of CMV infection into clinically significant and no-clinically significant categories (according to standardized, consensus-based viral load thresholds uniformly applied across centers and the presence of CMV-related symptoms or disease) as well as CMV disease, graft rejection, opportunistic infections, hematological adverse events, mortality, and cost-effectiveness.
Analysis is planned both per-protocol and by intention-to-treat. Comparisons between groups will use standard tests for continuous and categorical variables, Cox proportional hazards models for time-to-event analyses, and Kaplan-Meier survival curves. Non-inferiority will be assessed with a 10% margin. The trial will comply with the Declaration of Helsinki and Spanish legislation and has received approval from the national regulatory authority and local ethics committees. It has been registered at ClinicalTrials.gov (NCT04278547).
This study will represent the first randomized multicenter trial in heart transplantation to use a validated and commercially available IFN-γ ELISpot assay (T-SPOT.CMV) for guiding CMV prevention. As a randomized clinical trial, the study design will ensure strong internal validity and methodological robustness, while stratification by center will enhance the generalizability of the results across diverse clinical settings. By prospectively integrating immunological monitoring into a clinical decision algorithm, this trial takes an essential step toward individualized prevention.
The trial will not only examines efficacy and safety but will also address the economic impact, including both direct antiviral costs and indirect healthcare-related costs.
Universal valganciclovir prophylaxis is effective but costly and associated with frequent adverse events such as leukopenia and neutropenia. A CMI-guided approach has the potential to reduce unnecessary drug exposure, minimize toxicity, and lower costs while maintaining protection. This aligns with the broader movement toward precision medicine in transplantation [8, 9].
Certain limitations should be acknowledged: variability among participating laboratories may influence CMV DNAemia quantification despite standardized procedures; the exact proportion of patients who will ultimately receive valganciclovir prophylaxis versus preemptive therapy cannot be determined in advance; and the 48-week follow-up period will be insufficient to fully assess indirect CMV effects such as cardiac allograft vasculopathy, which has traditionally been one of the main arguments supporting universal prophylaxis.
Despite these challenges, the ELISPOT-TC trial could support a paradigm shift in CMV prevention. Instead of exposing all patients to antiviral prophylaxis, immune monitoring could allow for targeted use of therapy, reducing adverse events and improving cost-effectiveness. The ELISPOT-TC trial thus represents a landmark step toward personalized prevention strategies in transplantation [9].
Detailed methods, inclusion criteria, statistical analyses, and full CONSORT checklist will be provided in the Supplementary Material.
Sincerely,
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Bellvitge University Hospital Ethics Committee, Barcelona, Spain. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
Conceptualization/Design: EG-R, CD-L, DK, VD, MG-C, CO-B, FH-P, DC-M, FG-V, LF-G, LL-L, AG-T, MG-M, PC, LH, LR, SI, NS, JC-C, OB, and JG-C. Methodology/Investigation: All authors. Writing – Original Draft and Review: EG-R, CD-L, DK, VD, MG-C, CO-B, FH-P, DC-M, FG-V, LF-G, LL-L, AG-T, MG-M, PC, LH, NS, JC-C, OB, and JG-C. Resources/Analytic Tools: DK, LD, and OB. All authors contributed to the article and approved the submitted version.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This work was supported by the Instituto de Salud Carlos III (Grant number PI19/01610), co-founded by the Spanish Cardiology Society (Grant number 19PSJ017) and by the Spanish Platform for Clinical Research and Clinical Trials, (Spanish Clinical Research Network), financed by the ISCIII-General Subdirectorate of Evaluation and Promotion of Research, through project PT17/0017/0010 and PT20/00008 integrated into the I+D+I 2013–2016 State Plan and co-financed by the European Regional Development Fund (FEDER). We thank CERCA Programme/Generalitat de Catalunya for institutional support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2025.15565/full#supplementary-material
References
1.
Kotton CN Kamar N . New Insights on CMV Management in Solid Organ Transplant Patients: Prevention, Treatment, and Management of Resistant/Refractory Disease. Infect Dis Ther (2023) 12(2):333–42. 10.1007/s40121-022-00746-1
2.
Hernandez C Mabilangan C Burton C Doucette K Preiksaitis J . Cytomegalovirus Transmission in Mismatched Solid Organ Transplant Recipients: Are Factors Other than Antiviral Prophylaxis at Play?Am J Transpl (2021) 21(12):3958–70. 10.1111/ajt.16734
3.
Lúcia MCE Melilli E Cruzado JM Luque S Llaudó I Preformed Frequencies of Cytomegalovirus (CMV)-Specific Memory T and B Cells Identify Protected CMV-Sensitized Individuals Among Seronegative Kidney Transplant Recipients. Clin Infect Dis (2014) 59(11):1622–30. 10.1093/cid/ciu678
4.
Bestard O Lucia M Crespo E Cruzado JM Pérez D Melilli E et al Pretransplant Immediately Early-1-Specific T Cell Responses Provide Protection for CMV Infection After Kidney Transplantation. Am J Transpl (2013) 13(7):1793–805. 10.1111/ajt.12256
5.
Jarque M Crespo E Melilli E Gutiérrez A Moreso F Guirado L et al Cellular Immunity to Predict the Risk of Cytomegalovirus Infection in Kidney Transplantation: A Prospective, Interventional, Multicenter Clinical Trial. Clin Infect Dis (2020) 71(5):2375–85. 10.1093/cid/ciz1209
6.
Manuel O Laager M Hirzel C Neofytos D Walti LN Hoenger G et al Immune Monitoring–Guided Versus Fixed Duration of Antiviral Prophylaxis Against Cytomegalovirus in Solid-Organ Transplant Recipients: A Multicenter, Randomized Clinical Trial. Clin Infect Dis (2024) 78(2):312–23. 10.1093/cid/ciad575
7.
Páez-Vega A Vaquero-Barrios JM Ruiz-Arabi E Iturbe-Fernández D Alonso R Ussetti-Gil P et al Safety and Efficacy of Immunoguided Prophylaxis for Cytomegalovirus Disease in Low-Risk Lung Transplant Recipients in Spain: A Multicentre, Open-Label, Randomised, Phase 3, Noninferiority Trial. Lancet Reg Health Eur (2025) 52:101268. 10.1016/j.lanepe.2025.101268
8.
Ruiz-Arabi E Torre-Cisneros J Aguilera V Alonso R Berenguer M Bestard O et al Management of Cytomegalovirus in Adult Solid Organ Transplant Patients: GESITRA-IC-SEIMC, CIBERINFEC, and SET Recommendations Update. Transpl Rev (2024) 38(4):100875. 10.1016/j.trre.2024.100875
9.
Kotton CN Kumar D Manuel O Chou S Hayden RT Danziger-Isakov L et al The Fourth International Consensus Guidelines on the Management of Cytomegalovirus in Solid Organ Transplantation. Transplantation (2025) 109(7):1066–110. 10.1097/TP.0000000000005374
10.
Costanzo MR Dipchand A Starling R Anderson A Chan M Desai S et al The International Society of Heart and Lung Transplantation Guidelines for the Care of Heart Transplant Recipients. Task Force 3: Long-Term Care of Heart Transplant Recipients. J Heart Lung Transpl (2010) 29(8):914–56. 10.1016/j.healun.2010.05.034
Summary
Keywords
cytomegalovirus (CMV), cellular immune response, heart transplantation, ELISpot, enzyme-linked immunosorbent spot
Citation
García-Romero E, Díez-López C, Kervella D, Donoso V, García-Cosío MD, Ortiz-Bautista C, Hernández-Pérez FJ, Couto-Mallón D, González-Vílchez F, De la Fuente-Galán L, López-López L, Grande-Trillo A, Gómez-Molina M, Catalá P, Herrador L, Rosenfeld L, Ibáñez S, Donadeu L, Sabé N, Comín-Colet J, Bestard O and González-Costello J (2025) Protocol for the ELISPOT-TC Trial: A Randomized Controlled Study Evaluating CMV-Specific Cellular Immune Monitoring in Heart Transplant Recipients. Transpl. Int. 38:15565. doi: 10.3389/ti.2025.15565
Received
10 September 2025
Revised
31 October 2025
Accepted
13 November 2025
Published
27 November 2025
Volume
38 - 2025
Updates
Copyright
© 2025 García-Romero, Díez-López, Kervella, Donoso, García-Cosío, Ortiz-Bautista, Hernández-Pérez, Couto-Mallón, González-Vílchez, De la Fuente-Galán, López-López, Grande-Trillo, Gómez-Molina, Catalá, Herrador, Rosenfeld, Ibáñez, Donadeu, Sabé, Comín-Colet, Bestard and González-Costello.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elena García-Romero, e.garcia.r@bellvitgehospital.cat
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.