Abstract
Lung transplantation has become an established life-saving treatment for selected patients with end-stage pulmonary disease. In December 2024, our center reached the milestone of 1,500 lung transplants, providing an opportunity to evaluate long-term trends, outcomes, and challenges. We analyzed donor and recipient demographics, procedural evolution, and graft survival. Contemporary guidelines and consensus recommendations were also reviewed to contextualize current practice and highlight unmet needs. Median graft survival improved markedly across eras: 3.5 years between 1991 and 2000, 9.9 years between 2001 and 2010, and 11.2 years between 2011 and 2020 (p < 0.0001). Shifts in procedure type, donor selection, and transplant indications mirrored broader developments in the field (all p < 0.0001). Donor and recipient age increased significantly over time, with older recipients experiencing poorer long-term outcomes. Despite these advances, chronic lung allograft dysfunction (CLAD) remains the most important barrier to durable success, with median CLAD-free survival of 6.7 years in the modern era (2010–2024) and a retransplantation rate of 4%. While survival now exceeds a decade in many recipients, extended longevity presents new challenges, including management of comorbidities and optimization of CLAD prevention, treatment, and retransplantation strategies. Continued translational research and evidence-based approaches remain critical to improving long-term results.
Graphical Abstract
Introduction
The first human lung transplantation (LuTx), performed by James Hardy in 1963, demonstrated technical feasibility of this procedure, but initial post-transplant outcome was poor [1]. Introduction of cyclosporine A into clinical practice in the early 1980s, combined with advances in surgical techniques, marked the beginning of the modern era of LuTx [2]. Since then, the annual number of LuTx procedures has steadily increased, now estimated to globally exceed over 5,500 transplantations per year, with in total more than 70,000 procedures performed to date [3, 4]. Notably, current registries (i.e., International Society for Heart and Lung Transplantation (ISHLT), Organ Procurement and Transplantation Network (OPTN), Collaborative Transplant Study (CTS), etc.) fail to capture all individual procedures performed world-wide, since reporting is not mandatory in every transplant center, and exact global transplant numbers are therefore unclear – and likely underestimated [3, 4]. Moreover, not all centers provide data on post-transplant outcomes, making actual graft survival—particularly long-term results—often uncertain. This underscores the need for better reporting of transplant centers’ outcomes.
Today, LuTx has become an established treatment option for carefully selected patients with end-stage pulmonary diseases. Advances in medical therapies and management strategies for respiratory conditions such as chronic obstructive pulmonary disease (COPD), interstitial lung diseases (ILD), pulmonary arterial hypertension, and cystic fibrosis (CF)—the main indications for LuTx—have significantly influenced patient selection and referral patterns over the past decades, as well as post-transplant outcomes [3]. These developments also shaped ISHLT guidelines and referral recommendations over time [5, 6]. In parallel, improvements in donor management and optimized surgical techniques, along with introduction of novel, innovative technological approaches such as extracorporeal life support bridging, controlled temperature organ preservation, and ex vivo lung perfusion, are nowadays transforming LuTx from an urgent, unplanned intervention into a more predictable, even scheduled, surgical procedure [7–9]. While ISHLT-endorsed recommendations have provided valuable guidance for pre-, peri- and post-transplant patient care ([3, 10–26]; Supplementary Table S1), immune-mediated complications remain a major challenge for improving long-term outcomes. Chronic lung allograft dysfunction (CLAD) continues to limit long-term survival, with current median graft survival reported at just 6.3 years, according to the ISHLT Registry [27].
At our center, the 1,500th LuTx was performed in December 2024, an achievement that prompted a comprehensive analysis of our cohort’s donor and recipient characteristics, surgical approaches, and long-term outcomes, to evaluate trends and progress over time, and to identify current challenges and conceptional unmet needs to further improve future long-term patient care.
Materials and Methods
Study Population
This retrospective, single-center study analyzed all 1,500 consecutive LuTx procedures performed at University Hospitals Leuven between July 1991 and December 12, 2024. Data collected included donor demographics (age and donation type) and recipient characteristics (age at LuTx, sex, transplant indication, date of transplant, procedure type, time to CLAD, CLAD phenotype, and graft survival), with follow-up censored on 31 December 2024. Patients were categorized by transplantation era (1991–2000, 2001–2010, 2011–2020, and 2021–2024) and by procedure type: unilateral LuTx (single LuTx), bilateral LuTx (sequential single LuTx), or combined LuTx (LuTx combined with heart, liver, and/or kidney transplantation). Transplant indications were grouped into four categories: obstructive, restrictive, vascular, and CF) (Supplementary Table S2) for outcome analyses. For patients transplanted since 2010, CLAD phenotyping was performed according to the 2019 ISHLT consensus [12], as earlier data were insufficient for detailed classification. Institutional Ethical Review Board approval was waived for this retrospective observational study (S51577/S63978).
Statistical Analysis
Statistical analyses and visualizations were performed using GraphPad Prism 10.4.0 (San Diego, CA, United States). Categorical variables were analyzed with Fisher’s exact test and Chi-square test, while continuous variables were assessed using the Kruskal-Wallis test. Survival outcomes were evaluated using log-rank tests and illustrated with Kaplan-Meier curves.
Results
Patient Cohort and Graft Survival
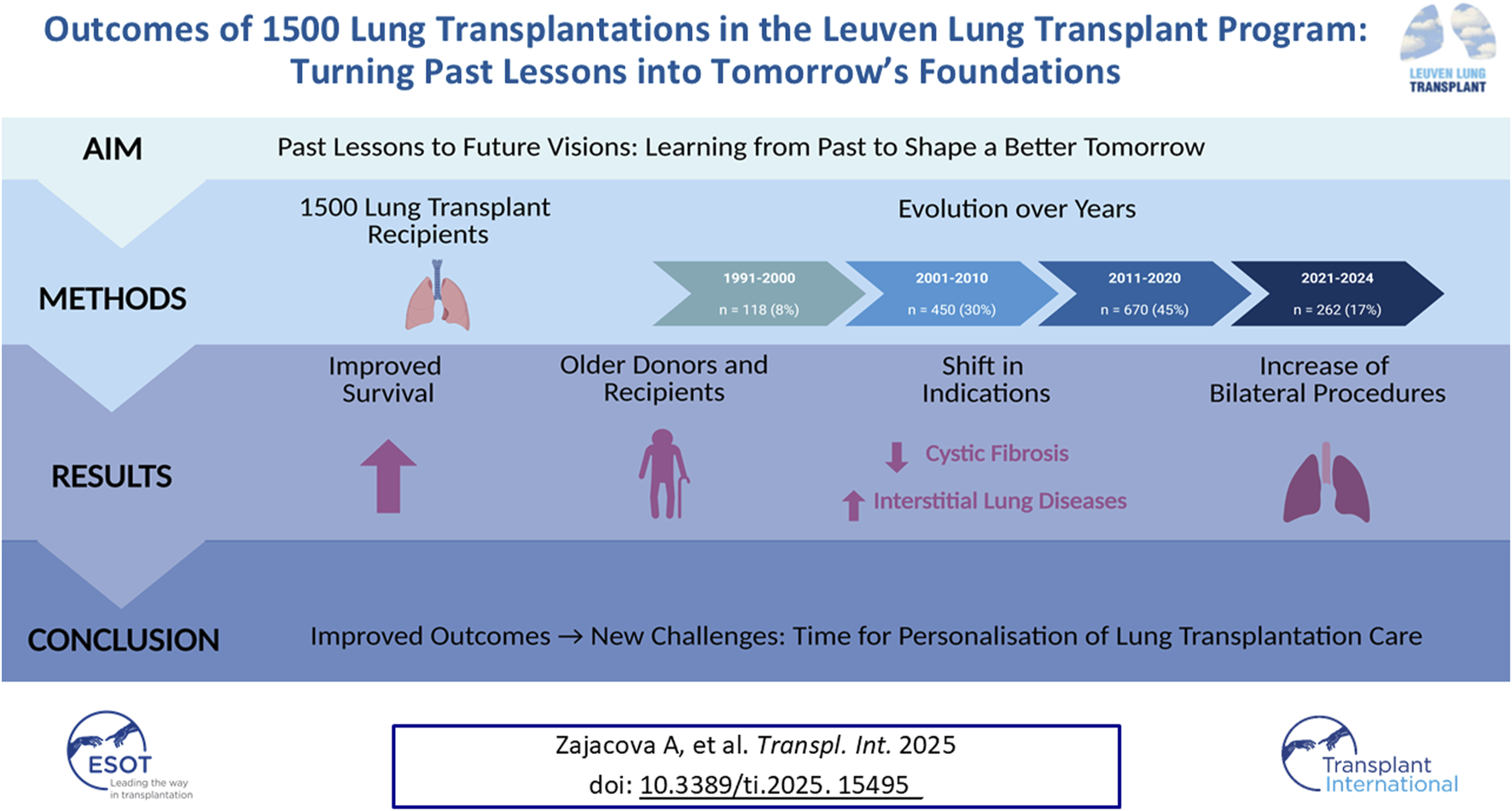
The number of transplant procedures increased steadily over time: 8% of our cohort underwent transplantation between 1991 and 2000, 30% between 2001 and 2010, 45% between 2011 and 2020, and the remaining 17% of transplantations were performed between 2021 and 2024. Recipient and donor demographics are summarized in Supplementary Tables S3, S4. At the censoring date, 746 recipients (50%) were alive and followed up in our center.
Overall graft survival among all 1,500 patients was 88% at 1 year, 78% at 3 years, 70% at 5 years, 50% at 10 years, 33% at 15 years, and 22% at 20 years. Graft survival improved significantly across eras, with median survival increasing from 3.5 years (1991–2000; 95% CI 1.9–6) to 9.9 years (2001–2010; 95% CI 8.9–11.4), and 11.2 years (2011–2020; 95% CI 9.8–NA) (p < 0.0001; Figure 1A). Conditional 1-year graft survival also improved, from 7.8 years (1991–2000; 95% CI 5.5–10.6) to 11.5 years (2001–2010; 95% CI 10.4–12.6), and 12.6 years (2011–2020; 95% CI 11.44–NA) (p = 0.003).
FIGURE 1
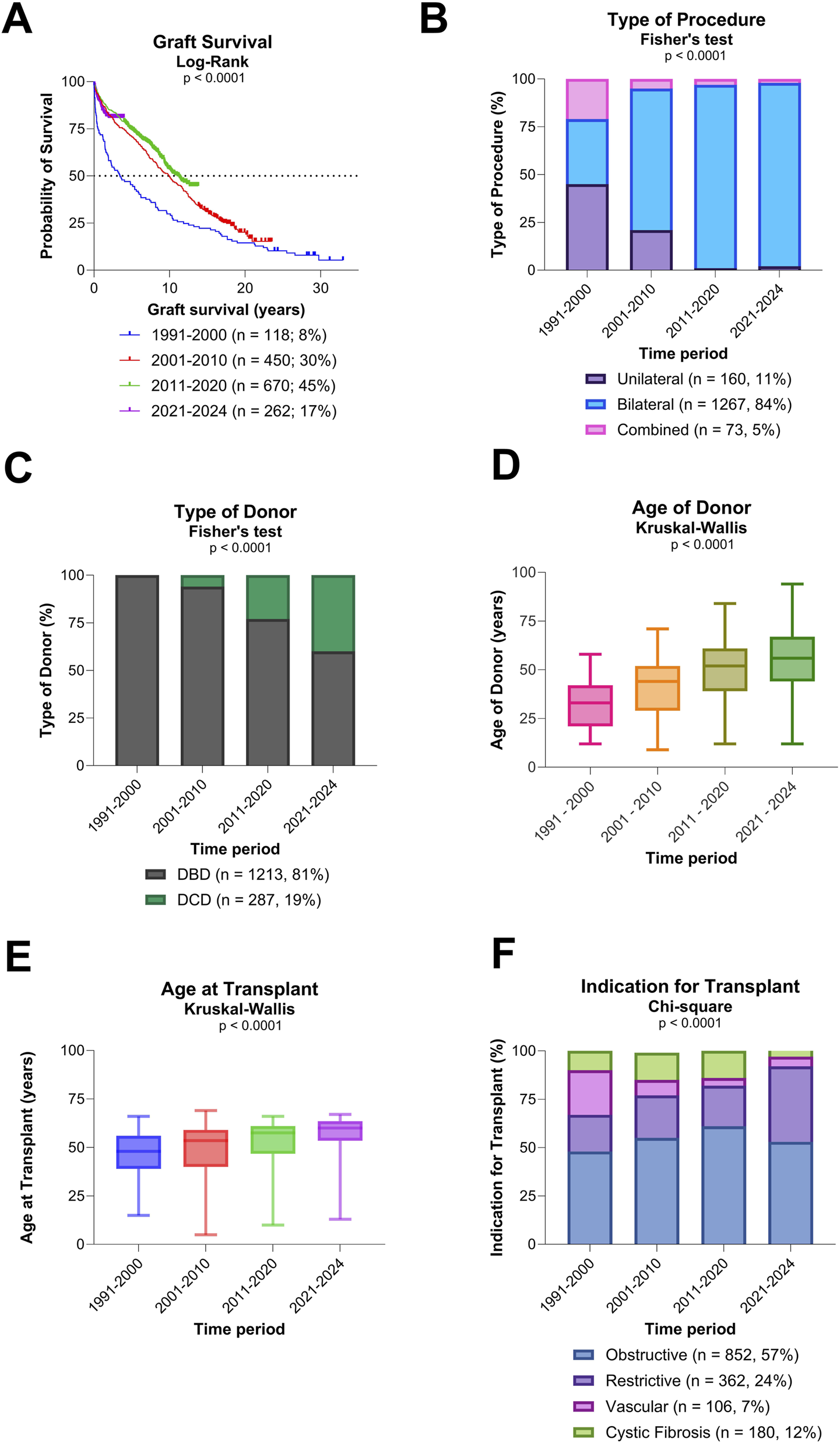
Overall graft survival, type of procedure, and donor/recipient characteristics (A) Evolution of overall graft survival over time. (B) Evolution of type of procedure over time. (C) Evolution of type of donors over time. (D) Evolution of age of donors over time. (E) Evolution of recipient age at transplantation over time. (F) Evolution of indication for transplantation over time.
Donor and Recipient Characteristics
A significant shift towards bilateral LuTx was observed, along with a significant increased use of donors after circulatory death (DCD) (both p < 0.0001; Figures 1B,C). Additionally, both donor and recipient ages rose significantly over time (both p < 0.0001; Figures 1D,E). There was a notable change in the indications for LuTx: the proportion of patients transplanted for CF declined, while those with ILD increased (p < 0.0001; Figure 1F).
Post-transplant survival varied significantly by type of LuTx, recipient age, and indication for transplantation (all p < 0.0001; Figures 2A–C). Trends toward different survival outcomes were observed by donor type (p = 0.06; Figure 2D) and donor age (p = 0.09; Figure 2E), though these did not reach statistical significance. Outcomes for each indication group (obstructive, restrictive, vascular, and CF) and single LuTx alone are shown in Supplementary Figures S1–S5.
FIGURE 2
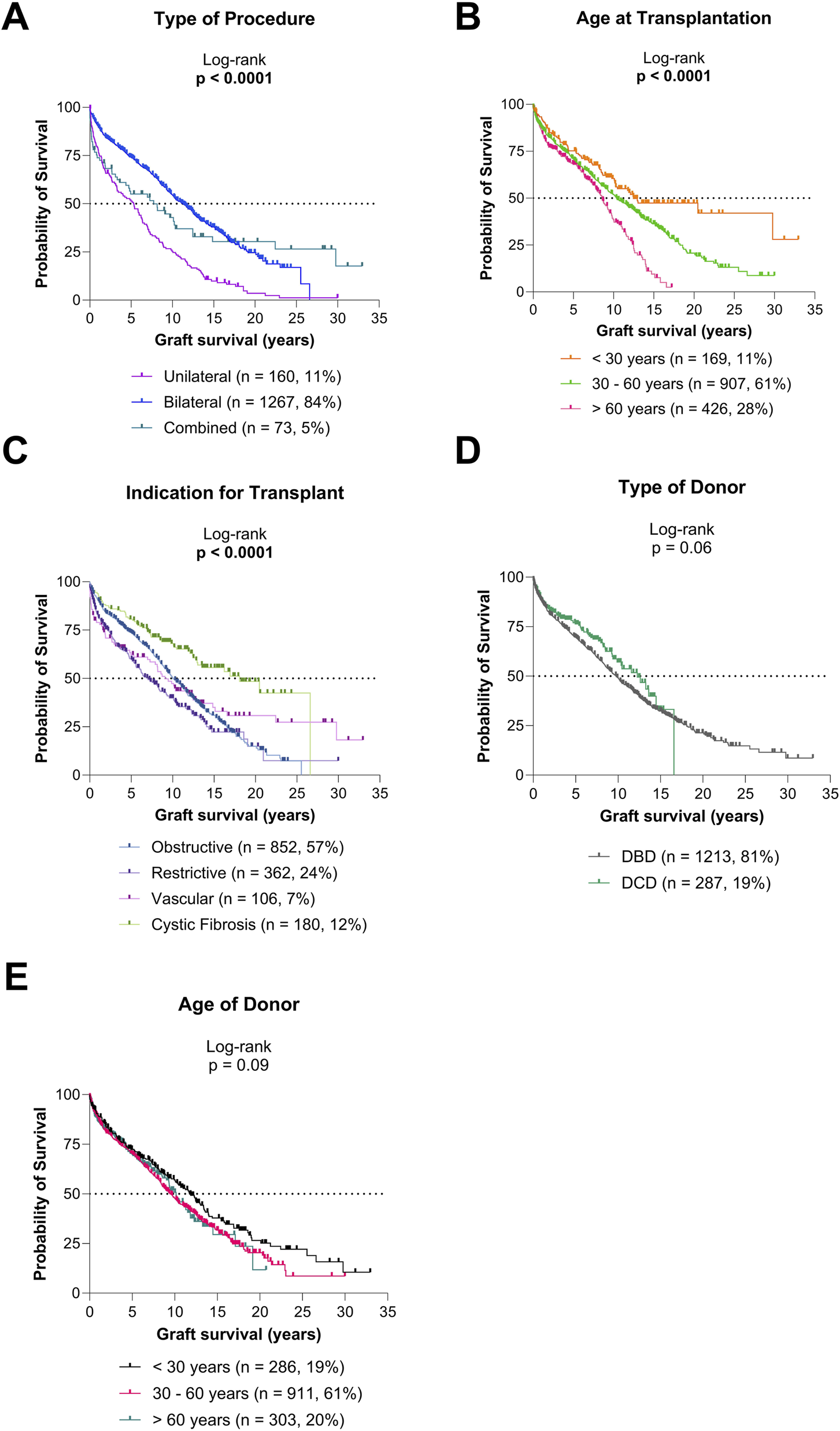
Graft survival according to type of procedure and donor/recipient characteristics (A) Graft survival for diverse types of procedures. (B) Graft survival for diverse recipient age groups. (C) Graft survival for diverse indications for transplantation. (D) Graft survival based on type of donor. (E) Graft survival based on age of donor.
CLAD and Retransplantation
For the cohort included in CLAD analysis (2011–2024, n = 989; 66%), median CLAD-free survival was 7.3 years (95% CI 6.6–8.2; Figure 3A). CLAD-free survival was comparable in patients transplanted in 2011–2020 vs. 2021–2024 (p = 0.17). Among the 238 patients (25.5%) who developed CLAD, 66% had bronchiolitis obliterans syndrome (BOS), 27% restrictive allograft syndrome (RAS), 1% mixed phenotype, and 6% undefined phenotype. Post-CLAD survival differed significantly by phenotype: 5.5 years for BOS (95% CI 3.8–8.5), 1.6 years for RAS (95% CI 1–2.2), 1.2 years for mixed (95% CI 0.37-NA), and 3.4 years for undefined phenotypes (95% CI 3.13-NA; p < 0.0001; Figure 3B).
FIGURE 3
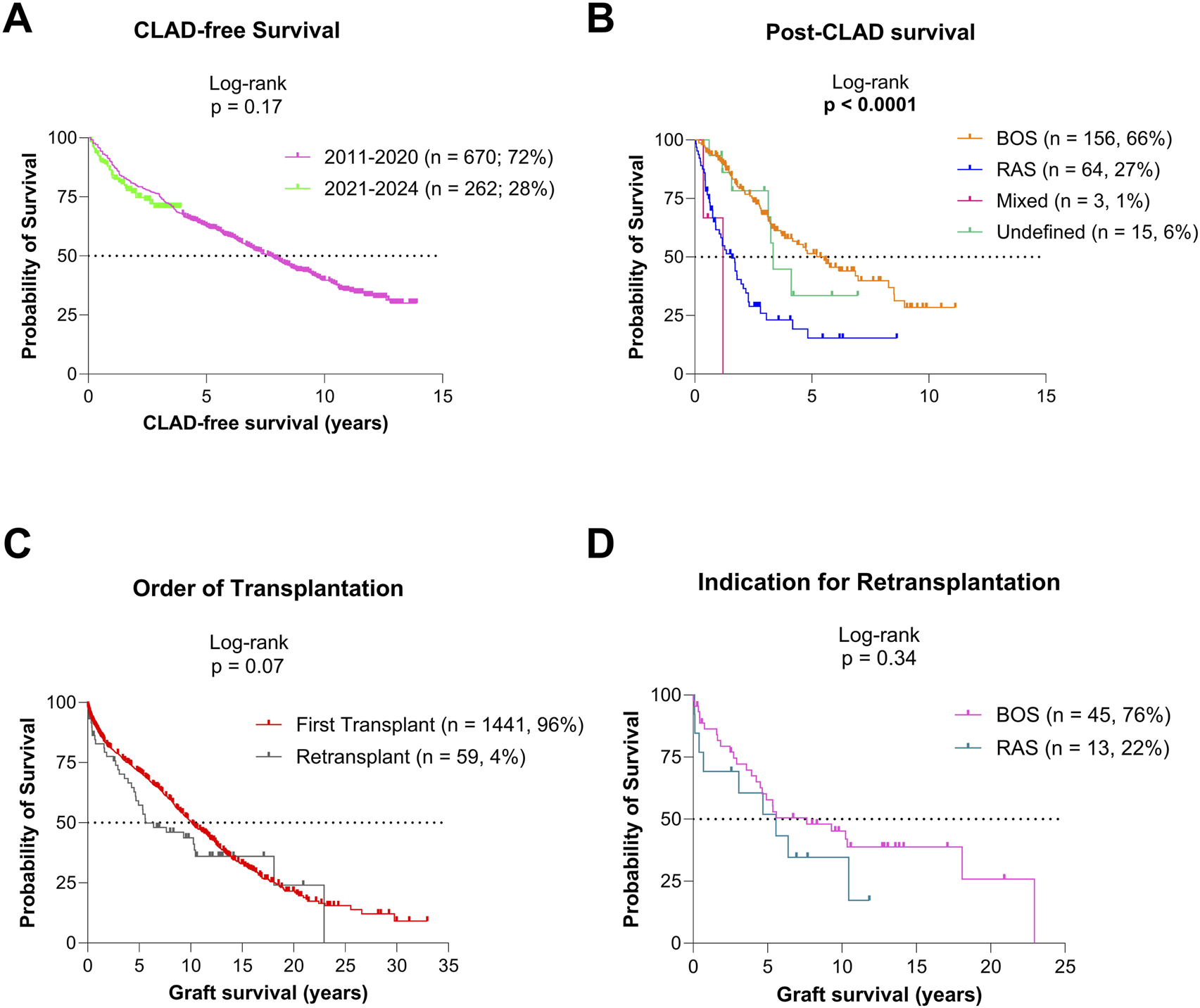
Graft survival based on CLAD status (A) CLAD-free survival for the patients transplanted since 2011. (B) Post-CLAD survival for patients transplanted since 2011. (C) Graft survival for patients after primary transplantation and retransplantation. (D) Graft survival after retransplantation for BOS and RAS CLAD phenotypes.
In total, 59 lung retransplantations (reLuTx; 4%) were performed: 45 (76%) for BOS, 13 (22%) for RAS, and 1 (2%) for early postoperative pulmonary venous occlusion. Graft survival after reLuTx tended to be lower than after primary LuTx (p = 0.07), but survival between BOS and RAS indications was similar (p = 0.34; Figures 3C,D).
Discussion
Over the course of 1,500 lung transplants in Leuven, graft survival has significantly increased from 3.5 to 11.2 years, notwithstanding major changes in procedure types, and donor and recipient profiles -trends that align with ISHLT Registry data and findings from other large cohorts [3, 28]. These improvements cannot be attributed to a single factor, but rather reflect the cumulative effect of multiple, coordinated advances in surgical techniques, perioperative management, immunosuppressive therapies, infection prophylaxis, and long-term follow-up care of recipients. These changes, combined with general improvements in medical management, complicate direct comparisons of patient cohorts across different time periods and centers. This emphasizes the importance of contemporary, near real-time outcome assessments over reliance on pooled historical registry data.
Unilateral LuTx, once predominant between 1991 and 2000, has declined steadily since 2011. This change likely reflects advances in donor management, expanding donor criteria with increase in DCD donations and broader use of older donors [29], improved graft preservation techniques, and refined surgical practices, which altogether have expanded the donor pool while reducing surgical risks and complications of bilateral transplantation, which is now the preferred procedure in most centers. Given that unilateral LuTx confers inferior long-term graft survival compared to bilateral LuTx, this may raise ethical concerns when prioritizing unilateral over bilateral LuTx, despite donor shortages and patient factors (e.g., older age) which could favor its use. Unfortunately, evidence-based guidelines for the selection of appropriate candidates for ‘split’ (dividing two suitable donor lungs between recipients) or ‘isolated’ single LuTx (where one donor lung is transplanted, and the other is declined) are still lacking [30]. In general, most centers nowadays mainly reserve unilateral LuTx for selected cases with either ILD or emphysema - in both of which conditions significant challenges may arise during long-term follow-up (i.e., infections or malignancy in the native lung, progressive fibrosis in ILD, hyperinflation in emphysema), which may compromise patient outcome.
Due to the aging LuTx population, other challenges also arise. The proportion of COPD recipients over 60 years increased from ∼25% (1992–2000) to over 50% (2010–2018) per ISHLT data [31]. This is in line with evolving ISHLT candidate selection recommendations: in 2014, age >65 years was a relative contraindication, while in 2021, age 65–70 years became a risk factor only [6, 7]. However, older recipients face worse long-term outcomes [3], as also seen in our cohort, which again may raise ethical concerns when listing elderly patients. Our center’s LuTx listing age limit is 65 years (67 for ILD), based on ISHLT registry data consistently demonstrating an increased risk for post-transplant mortality in patients aged ≥60 years [27]. Especially for elderly candidates, thorough comorbidity and frailty assessments, prehabilitation, and enhanced recovery protocols are essential [32–34]. Yet, optimal frailty evaluation and management remain undefined, highlighting the need for guidelines on pretransplant frailty assessment, prehabilitation, and post-transplant physiotherapy protocols along with comorbidity management.
CLAD remains one of the main factors limiting long-term survival after lung transplantation. Despite advances in graft monitoring strategies and improved recognition of CLAD and its clinical phenotypes over the past decade, little progress has been made in the treatment of this devastating complication. Consequently, the development of evidence-based guidelines for CLAD prevention and management, together with sustained translational research to elucidate its underlying mechanisms and clinical trials testing novel therapies, represents a critical and unmet need. Meanwhile, reLuTx—still the only curative option for CLAD—is becoming increasingly common worldwide, yet clear referral and listing criteria remain absent [3, 35]. Importantly, long-term use of maintenance immunosuppressive therapies–the cornerstone of transplant medicine–frequently leads to non-respiratory comorbidities (i.e., cardiovascular and renal disease, diabetes, or malignancy), which may contribute to poorer outcomes in older patients following primary LuTx, and especially after reLuTx, where the cumulative burden of immunosuppressive therapy increases substantially over time [36, 37]. Notably, reLuTx may pose significant technical challenges, especially in restrictive CLAD/RAS (i.e., pleural adhesions), which may contribute to the worse outcomes in reLuTx compared to primary LuTx [36, 37]. While previous studies reported significantly shorter post-transplant survival in reLuTx for restrictive CLAD/RAS compared to obstructive CLAD/BOS, our cohort did not fully mirror these findings [38, 39]. More strict candidate selection following recent ISHLT recommendations [6] and increased surgical experience with performing reLuTx over time [35] may have contributed to this result [6, 35]. Interestingly, the incidence of RAS as indication for reLuTx rose sharply over time in our cohort, from 6% (2001–2010) to 29% (2011–2020), and 40% (2021–2024) (Supplementary Table S3), likely reflecting improved recognition of this phenotype since its description in 2011 [40], as well as the worse prognosis associated with this phenotype compared to BOS, which may skew referrals towards reLuTx listing. Importantly, given the overall poor prognosis associated with CLAD, timely reLuTx evaluation in patients with CLAD—particularly at CLAD stage 4 (FEV1 ≤35% of post-transplant baseline)—is critical. Thorough multidisciplinary pre-reLuTx assessment is essential, and despite formal reLuTx criteria are currently missing, listing otherwise eligible patients could be considered when FEV1 and/or DLCO are ≤30% predicted, especially in the presence of pulmonary arterial hypertension or exertional hypoxemia.
While extending long-term survival and enhancing quality of life remain the primary goals of LuTx, appropriate end-of-life care is an essential component of management for all recipients. Early and proactive advance care planning is particularly important for patients who develop respiratory complications such as CLAD or who experience severe non-respiratory comorbidities. Future recommendations should address terminal-stage management, including symptom management, reduction of polypharmacy, and palliative care. Finally, with an ever-growing LuTx population requiring extended and often complex healthcare, also adequate healthcare organization—including logistical coordination, budgetary management, workload control, and physicians’ well-being—requires attention. Adequate staffing to ensure state-of-the-art, life-long patients’ follow-up and to avoid burnout in healthcare practitioners is challenging, yet essential for sustainable high-quality transplant care [41, 42]. Table 1 highlights some of the essential areas for future clinical guidelines, which may be pivotal in further optimizing long-term outcomes. Supplementary Table S5 provides a list of ISHLT-endorsed guidelines currently in development.
TABLE 1
| Pretransplant care |
| Frailty assessment criteria |
| Prehabilitation strategies |
| Lung retransplant criteria |
| Peritransplant care |
| Donor optimization strategies |
| Organ preservation strategies |
| Immunosuppression strategies and management of immunologically sensitized recipients |
| Management of surgical complications, including bronchial anastomosis problems |
| Early recovery after surgery protocols and post-transplant revalidation strategies |
| Posttransplant care |
| Maintenance immunosuppression regimen strategies |
| Prevention and treatment of chronic lung allograft dysfunction |
| Screening and management of comorbidities |
| Patient reported outcomes |
| Advanced care planning and end-of-life management |
| Healthcare practitioners’ involvement |
| Healthcare organization, including logistics, workload and budgetary management |
Key areas of focus for future clinical guidelines to improve long-term outcomes.
In conclusion, long-term outcomes post-LuTx are nowadays favorable, with median graft survival exceeding a decade in our center. However, changing donor and recipient profiles, and longer post-transplant survival conveys new challenges, of which the most important remains CLAD. Our findings may also help inform future clinical guidelines by illustrating how evolving donor and recipient characteristics, shifting indications, and changes in transplant types impact outcomes. Ongoing translational research efforts, systematic outcome assessment, and evidence-based strategies are essential to address these challenges and further improve long-term results in LuTx. Recommendations on evidence-based strategies regarding frailty assessment and management, post-transplant comorbidities, CLAD prevention and treatment, reLuTx criteria, and end-of-life care are crucial to advancing current lung transplant care.
Statements
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: The datasets analyzed during this study are not publicly available because of patient privacy concerns and institutional data protection policies. Requests to access these datasets should be directed to robin.vos@uzleuven.be.
Ethics statement
The requirement of ethical approval was waived by Institutional Ethical Review Board (S51577/S63978) for the studies involving humans because it is a retrospective observational study. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
Conceptualization, manuscript drafting, editing and finalization: AZ, LD, PD, LC, and RV. Statistical analyses: AZ and RV. Reviewing: AZ, LD, PD, LC, and RV. All authors contributed to the article and approved the submitted version.
Group Members of Leuven Lung Transplant Group
Saskia Bos: University Hospitals Leuven, Department of Respiratory Diseases, KU Leuven, Department CHROMETA, BREATHE, Leuven, Belgium; Laurens De Sadeleer: University Hospitals Leuven, Department of Respiratory Diseases, KU Leuven, Department CHROMETA, BREATHE, Leuven, Belgium; Laurent Godinas: University Hospitals Leuven, Department of Respiratory Diseases, KU Leuven, Department CHROMETA, BREATHE, Leuven, Belgium; Dirk E. Van Raemdonck, KU Leuven, Department CHROMETA, BREATHE, Leuven, Belgium, University Hospitals Leuven, Department of Thoracic Surgery; Lieven Depypere, KU Leuven, Department CHROMETA, BREATHE, Leuven, Belgium, University Hospitals Leuven, Department of Thoracic Surgery; Hans Van Veer, KU Leuven, Department CHROMETA, BREATHE, Leuven, Belgium, University Hospitals Leuven, Department of Thoracic Surgery; Yanina Jansen, KU Leuven, Department CHROMETA, BREATHE, Leuven, Belgium, University Hospitals Leuven, Department of Thoracic Surgery; Catherine Ingels, University Hospitals Leuven, Department of Intensive Care; Karen Denaux, University Hospitals Leuven, Organ Donation and Transplant Coordination; Arne P. Neyrinck, University Hospitals Leuven, Department of Anesthesiology; Filip Rega, KU Leuven, Department CHROMETA, BREATHE, Leuven, Belgium, University Hospitals Leuven, Department of Thoracic Surgery; Bart M. Vanaudenaerde, KU Leuven, Department CHROMETA, BREATHE, Leuven, Belgium. All listed group members contributed to the reviewing of this article.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. AZ is supported by a Transplant Fellowship of the European Society for Organ Transplantation (ESOT). LC is supported by research foundation Flanders (FWO) as a Senior Clinical Researcher (18E2B24N) and by a University chair supported by Medtronic. RV is supported by research foundation Flanders (FWO) as a Senior Clinical Researcher (1803521N) and by a research grant (G060322N).
Acknowledgments
The authors sincerely thank Maurits Demedts, Marc Decramer, Geert M. Verleden, Hans Schreinemakers, Eugène Vandermeersch, Toni Lerut, Willy Coosemans, Herbert Decaluwé, Philippe Nafteux, Lori van Rozendaal, Wim Daenen, Johan Van Haecke, Peter Lauwers, Greet Van den Berghe, Alexander Wilmer, Steffen Rex, Sofian Bouneb, Leo Roels, Bruno Desschans, Stijn Dirix, Dirk Claes, Nele Grossen, Delphine Kumps, Eddy Van De Zande, Karlien Degezelle, Dirk Delva, Marie-Paule Emonds, Steffi De Pelsmaeker, Jacques Pirenne, Stijn Vanstraelen, An-Lies Provoost, Jan Van Slambrouck, Annalisa Barbarossa, Cedric Vanluyten, and all other current and former procurement surgeons, anesthesiologists, intensive care unit physicians, transplant coordinators, lung transplant research fellows, nursing staff, physiotherapists, and allied healthcare workers for their invaluable clinical contributions to this work. Their dedication and expertise as part of the Leuven Lung Transplant Group have been instrumental in advancing research and patient care in the field of lung transplantation. We deeply appreciate their support, collaboration, and commitment to excellence.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2025.15495/full#supplementary-material
Abbreviations
CF, Cystic fibrosis; CLAD, Chronic lung allograft dysfunction; CTS, Collaborative Transplant Study; COPD, Chronic obstructive pulmonary disease; BOS, Bronchiolitis obliterans syndrome; DBD, Donation after brain death; DCD, Donation after circulatory death; DLCO, Diffusing capacity of the lungs for carbon monoxide; FEV1, Forced expiratory volume in one second; ILD, Interstitial lung diseases; ISHLT, International Society for Heart and Lung Transplantation; LuTx, Lung transplantation; OPTN, Organ Procurement and Transplantation Network; RAS, Restrictive allograft syndrome; reLuTx, Lung retransplantation.
References
1.
Panchabhai TS Chaddha U McCurry KR Bremner RM Mehta AC . Historical Perspectives of Lung Transplantation: Connecting the Dots. J Thorac Dis (2018) 10(7):4516–31. 10.21037/jtd.2018.07.06
2.
Heusler K Pletscher A . The Controversial Early History of Cyclosporin. Swiss Med Wkly (2001) 131(21-22):299–302. 10.4414/smw.2001.09702
3.
Chambers DC Cherikh WS Harhay MO Hayes D Jr Hsich E Khush KK et al The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-Sixth Adult Lung and Heart-Lung Transplantation Report-2019; Focus Theme: Donor and Recipient Size Match. J Heart Lung Transpl (2019) 38(10):1042–55. 10.1016/j.healun.2019.08.001
4.
Singh TP Hsich E Cherikh WS Perch M Hayes D Jr Lewis A et al The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: 2025 Annual Report of Heart and Lung Transplantation. J Heart Lung Transpl (2025). 10.1016/j.healun.2025.04.014
5.
Weill D Benden C Corris PA Dark JH Davis RD Keshavjee S et al A Consensus Document for the Selection of Lung Transplant Candidates: 2014--An Update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transpl (2015) 34(1):1–15. 10.1016/j.healun.2014.06.014
6.
Leard LE Holm AM Valapour M Glanville AR Attawar S Aversa M et al Consensus Document for the Selection of Lung Transplant Candidates: An Update From the International Society for Heart and Lung Transplantation. J Heart Lung Transpl (2021) 40(11):1349–79. 10.1016/j.healun.2021.07.005
7.
Rando HJ Fanning JP Cho SM Kim BS Whitman G Bush EL et al Extracorporeal Membrane Oxygenation as a Bridge to Lung Transplantation: Practice Patterns and Patient Outcomes. J Heart Lung Transpl (2024) 43(1):77–84. 10.1016/j.healun.2023.06.016
8.
Novysedlak R Provoost AL Langer NB Van Slambrouck J Barbarossa A Cenik I et al Extended Ischemic Time (>15 Hours) Using Controlled Hypothermic Storage in Lung Transplantation: A Multicenter Experience. J Heart Lung Transpl (2024) 43(6):999–1004. 10.1016/j.healun.2024.02.006
9.
Ali A Hoetzenecker K Schwarz S Barturen MG Tomlinson G et al Luis Campo-Cañaveral de la Cruz J Extension of Cold Static Donor Lung Preservation at 10 °C. NEJM Evid (2023) 2(6):EVIDoa2300008. 10.1056/EVIDoa2300008
10.
Stewart S Fishbein MC Snell GI Berry GJ Boehler A Burke MM et al Revision of the 1996 Working Formulation for the Standardization of Nomenclature in the Diagnosis of Lung Rejection. J Heart Lung Transpl (2007) 26(12):1229–42. 10.1016/j.healun.2007.10.017
11.
Levine DJ Glanville AR Aboyoun C Belperio J Benden C Berry GJ et al Antibody-Mediated Rejection of the Lung: A Consensus Report of the International Society for Heart and Lung Transplantation. J Heart Lung Transpl (2016) 35(4):397–406. 10.1016/j.healun.2016.01.1223
12.
Verleden GM Glanville AR Lease ED Fisher AJ Calabrese F Corris PA et al Chronic Lung Allograft Dysfunction: Definition, Diagnostic Criteria, and Approaches to treatment-A Consensus Report from the Pulmonary Council of the ISHLT. J Heart Lung Transpl (2019) 38(5):493–503. 10.1016/j.healun.2019.03.009
13.
Crespo MM Lease ED Sole A Sandorfi N Snyder LD Berry GJ et al ISHLT Consensus Document on Lung Transplantation in Patients With Connective Tissue Disease: Part I: Epidemiology, Assessment of Extrapulmonary Conditions, Candidate Evaluation, Selection Criteria, and Pathology Statements. J Heart Lung Transpl (2021) 40(11):1251–66. 10.1016/j.healun.2021.07.014
14.
Martin AK Mercier O Fritz AV Gelzinis TA Hoetzenecker K Lindstedt S et al ISHLT Consensus Statement on the Perioperative Use of ECLS in Lung Transplantation: Part II: Intraoperative Considerations. J Heart Lung Transpl (2025). 10.1016/j.healun.2024.08.027
15.
Martin AK Mercier O Bottiger B Cypel M Fessler J Gomez-De-Antonio D et al ISHLT Consensus Statement on the Perioperative Use of ECLS in Lung Transplantation: Part III: Postoperative Considerations. J Heart Lung Transpl (2025). 10.1016/j.healun.2025.03.004
16.
Holm AM Courtwright A Olland A Zuckermann A Van Raemdonck D . ISHLT Position Paper on Thoracic Organ Transplantation in Controlled Donation After Circulatory Determination of Death (Cdcd). J Heart Lung Transpl (2022) 41(6):671–7. 10.1016/j.healun.2022.03.005
17.
Marczin N de Waal EEC Hopkins PMA Mulligan MS Simon A Shaw AD et al International Consensus Recommendations for Anesthetic and Intensive Care Management of Lung Transplantation. an EACTAIC, SCA, ISHLT, ESOT, ESTS, and AST Approved Document. J Heart Lung Transpl (2021) 40(11):1327–48. 10.1016/j.healun.2021.07.012
18.
Bermudez CA Crespo MM Shlobin OA Cantu E Mazurek JA Levine D et al ISHLT Consensus Document on Lung Transplantation in Patients With Connective Tissue Disease: Part II: Cardiac, Surgical, Perioperative, Operative, and Post-operative Challenges and Management Statements. J Heart Lung Transpl (2021) 40(11):1267–78. 10.1016/j.healun.2021.07.016
19.
Snell GI Yusen RD Weill D Strueber M Garrity E Reed A et al Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, Part I: Definition and grading-A 2016 Consensus Group Statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transpl (2017) 36(10):1097–103. 10.1016/j.healun.2017.07.021
20.
Diamond JM Arcasoy S Kennedy CC Eberlein M Singer JP Patterson GM et al Report of the International Society for Heart and Lung Transplantation Working Group on Primary Lung Graft Dysfunction, Part II: Epidemiology, Risk Factors, and outcomes-A 2016 Consensus Group Statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transpl (2017) 36(10):1104–13. 10.1016/j.healun.2017.07.020
21.
Gelman AE Fisher AJ Huang HJ Baz MA Shaver CM Egan TM et al Report of the ISHLT Working Group on Primary Lung Graft Dysfunction Part III: Mechanisms: A 2016 Consensus Group Statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transpl (2017) 36(10):1114–20. 10.1016/j.healun.2017.07.014
22.
Van Raemdonck D Hartwig MG Hertz MI Davis RD Cypel M Hayes D et al Report of the ISHLT Working Group on Primary Lung Graft Dysfunction Part IV: Prevention and Treatment: A 2016 Consensus Group Statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transpl (2017) 36(10):1121–36. 10.1016/j.healun.2017.07.013
23.
Kittleson MM DeFilippis EM Bhagra CJ Casale JP Cauldwell M Coscia LA et al Reproductive Health After Thoracic Transplantation: An ISHLT Expert Consensus Statement. J Heart Lung Transpl (2023) 42(3):e1–e42. 10.1016/j.healun.2022.10.009
24.
Crespo MM Claridge T Domsic RT Hartwig M Kukreja J Stratton K et al ISHLT Consensus Document on Lung Transplantation in Patients With Connective Tissue Disease: Part III: Pharmacology, Medical and Surgical Management of Post-Transplant Extrapulmonary Conditions Statements. J Heart Lung Transpl (2021) 40(11):1279–300. 10.1016/j.healun.2021.07.013
25.
Glanville AR Verleden GM Todd JL Benden C Calabrese F Gottlieb J et al Chronic Lung Allograft Dysfunction: Definition and Update of Restrictive Allograft Syndrome-A Consensus Report From the Pulmonary Council of the ISHLT. J Heart Lung Transpl (2019) 38(5):483–92. 10.1016/j.healun.2019.03.008
26.
Shah P Lowery E Chaparro C Visner G Hempstead SE Abraham J et al Cystic Fibrosis Foundation Consensus Statements for the Care of Cystic Fibrosis Lung Transplant Recipients. J Heart Lung Transpl (2021) 40(7):539–56. 10.1016/j.healun.2021.04.011
27.
Singh TP Cherikh WS Hsich E Lewis A Perch M Kian S et al Graft Survival in Primary Thoracic Organ Transplant Recipients: A Special Report from the International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation. J Heart Lung Transpl (2023) 42(10):1321–33. 10.1016/j.healun.2023.07.017
28.
Balsara KR Krupnick AS Bell JM Khiabani A Scavuzzo M Hachem R et al A Single-Center Experience of 1500 Lung Transplant Patients. J Thorac Cardiovasc Surg (2018) 156(2):894–905. 10.1016/j.jtcvs.2018.03.112
29.
Vanluyten C Vandervelde CM Vos R Van Slambrouck J Fieuws S De Leyn P et al Lung Transplant Outcome From Selected Older Donors (≥70 Years) Equals Younger Donors (<70 Years): A propensity-matched Analysis. Ann Surg (2023) 278(3):e641–e649. 10.1097/SLA.0000000000005813
30.
Asija R Fuller J Costa J Abramov A Grewal H Benvenuto L et al Single Lung Transplantation Is Safe when the Other Lung Is Declined. Eur J Cardiothorac Surg (2025) 67(2):ezaf028. 10.1093/ejcts/ezaf028
31.
Perch M Hayes D Jr Cherikh WS Zuckermann A Harhay MO Hsich E et al The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-Ninth Adult Lung Transplantation report-2022; Focus on Lung Transplant Recipients with Chronic Obstructive Pulmonary Disease. J Heart Lung Transpl (2022) 41(10):1335–47. 10.1016/j.healun.2022.08.007
32.
Singer JP Christie JD Diamond JM Anderson MA Benvenuto LA Gao Y et al Development of the Lung Transplant Frailty Scale (LT-FS). J Heart Lung Transpl (2023) 42(7):892–904. 10.1016/j.healun.2023.02.006
33.
Bourgeois N Lands LC Prévost K Poirier C Janaudis-Ferreira T . Virtual Physical Prehabilitation in Lung Transplant Candidates: A Proof-Of-Concept Study. Transpl Int (2024) 37:12355. 10.3389/ti.2024.12355
34.
Annema C De Smet S Castle EM Overloop Y Klaase JM Janaudis-Ferreira T et al European Society of Organ Transplantation (ESOT) Consensus Statement on Prehabilitation for Solid Organ Transplantation Candidates. Transpl Int (2023) 36:11564. 10.3389/ti.2023.11564
35.
Jin X Vanluyten C Orlitová M Van Slambrouck J Vos R Verleden GM et al Off-Pump Lung Re-Transplantation Avoiding Clamshell Thoracotomy Is Feasible and Safe: A Single-Center Experience. J Thorac Dis (2023) 15(10):5811–22. 10.21037/jtd-23-64
36.
Harhay MO Cherikh WS Toll AE Christie JD Stehlik J Chambers D et al Epidemiology, Risk Factors, and Outcomes of Lung Retransplantation: An Analysis of the International Society for Heart and Lung Transplantation Thoracic Transplant Registry. J Heart Lung Transpl (2022) 41(10):1478–86. 10.1016/j.healun.2022.06.022
37.
Randhawa SK Yang Z Morkan DB Yan Y Chang SH Hachem RR et al One-Year Survival Worse for Lung Retransplants Relative to Primary Lung Transplants. Ann Thorac Surg (2022) 113(4):1265–73. 10.1016/j.athoracsur.2021.03.112
38.
Kovacs Z Gottlieb J Simon S Benazzo A Jaksch P . Survival of Patients with Advanced Chronic Lung Allograft Dysfunction and the Role of Redo Transplantation. JHLT Open (2025) 8:100257. 10.1016/j.jhlto.2025.100257
39.
Verleden SE Todd JL Sato M Palmer SM Martinu T Pavlisko EN et al Impact of CLAD Phenotype on Survival After Lung Retransplantation: A Multicenter Study. Am J Transpl (2015) 15(8):2223–30. 10.1111/ajt.13281
40.
Sato M Waddell TK Wagnetz U Roberts HC Hwang DM Haroon A et al Restrictive Allograft Syndrome (RAS): A Novel Form of Chronic Lung Allograft Dysfunction. J Heart Lung Transpl (2011) 30(7):735–42. 10.1016/j.healun.2011.01.712
41.
Trindade AJ Chapin KC Gannon WD Erasmus DB Shaver CM . A Multicenter Survey Study of Lung Transplant Program Staffing. Transplantation (2023) 107(5):1013–6. 10.1097/TP.0000000000004478
42.
Sanchez-Antolín G Blanco-Fernández G Campos-Varela I Ruiz P Álamo JM Otero A et al Burnout Among Physicians of Specialties Dedicated to Liver Transplantation. Transpl Int (2024) 37:13738. 10.3389/ti.2024.13738
Summary
Keywords
lung transplantation, outcome, graft survival, evolution over time, future perspectives
Citation
Zajacova A, Dupont LJ, De Leyn P, Ceulemans LJ, Vos R and Leuven Lung Transplant Group (2025) Characteristics and Outcomes of 1500 Lung Transplantations in the Leuven Lung Transplant Program: Turning Past Lessons Into Tomorrow’s Foundations. Transpl. Int. 38:15495. doi: 10.3389/ti.2025.15495
Received
27 August 2025
Revised
11 October 2025
Accepted
20 October 2025
Published
12 November 2025
Volume
38 - 2025
Updates
Copyright
© 2025 Zajacova, Dupont, De Leyn, Ceulemans, Vos and Leuven Lung Transplant Group.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robin Vos, robin.vos@uzleuven.be
‡These authors have contributed equally to this work and share senior authorship
ORCID: Andrea Zajacova, orcid.org/0000-0002-9691-2500; Lieven J. Dupont, orcid.org/0000-0003-3961-1522; Paul De Leyn, orcid.org/0000-0002-4200-227X; Laurens J. Ceulemans, orcid.org/0000-0002-4261-7100; Robin Vos, orcid.org/0000-0002-3468-9251
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.