Abstract
The development of de novo donor-specific anti-HLA antibodies (dnDSA) after lung transplantation (LuTX) has been increasingly linked to the onset of antibody-mediated rejection (AMR), chronic lung allograft dysfunction (CLAD), and impaired long-term outcomes. However, the therapeutic impact of intravenous immunoglobulin (IVIG) therapy in patients with dnDSA remains unclear. We conducted a retrospective single-center study of LuTX recipients (2015–2019) who developed dnDSA post-transplantation and received IVIG-based therapy. Patients were classified as responders or non-responders based on post-treatment antibody clearance. Clinical, immunological and functional outcomes were compared. Among 47 patients with dnDSA and IVIG-based therapy, 23 (48.9%) achieved complete antibody elimination. Preemptive treatment, defined as initiation of IVIG therapy before onset of clinical symptoms, was found to be an independent predictor of antibody clearance (odds ratio 29.5; p = 0.013). Responders showed significantly lower baseline MFI. While differences in CLAD-free survival favored responders, they did not reach statistical significance. Preemptive IVIG therapy in asymptomatic dnDSA-positive LuTX recipients may enhance antibody clearance and reduce CLAD risk. These findings support early intervention strategies and underscore the need for prospective trials to define optimal therapeutic thresholds and timing.
Introduction
Lung transplantation (LuTX) remains the only definitive therapeutic option for patients with end-stage lung diseases (ELD) who have exhausted all other treatment modalities [1–3]. While short-term outcomes have improved markedly over recent decades, largely due to advances and standardization in immunosuppressive regimens and perioperative care, long-term survival remains critically constrained [4–6]. One of the principal challenges in this field is the complex immunological interplay between donor and recipient, which manifests in various forms of allograft rejection and contributes significantly to graft failure and patient mortality [3, 7, 8]. Acute cellular rejection (ACR) is a well-recognized complication during the first year following transplantation. Although typically responsive to corticosteroid therapy and not often fatal in the acute phase, ACR has been linked to increased long-term risk of developing chronic lung allograft dysfunction (CLAD), the predominant cause of late graft failure [9–12].
Beyond cellular rejection, humoral responses have gained recognition as major contributors to graft injury. The development of donor-specific anti-HLA antibodies (DSA) post-transplant (de novo DSA) is increasingly implicated in the pathogenesis of CLAD and reduced allograft survival [13–15]. Notably, the presence of dnDSAs has been associated with increased risk for both acute and chronic rejection, including the emergence of antibody-mediated rejection (AMR), a distinct and often insidious form of immune-mediated injury [16, 17]. The immunological basis for these processes lies in the human leukocyte antigen (HLA) system, a highly polymorphic set of genes that encodes the major histocompatibility complex (MHC) proteins. These molecules play an essential role in presenting antigens to T and B cells. Mismatches between donor and recipient HLA profiles are a potent trigger of alloimmune responses [18]. Formation of anti-HLA antibodies, whether preformed or de novo, can initiate a cascade of immune events involving complement activation, endothelial injury and eventual tissue destruction [19].
The diagnosis of pulmonary AMR remains difficult, in part due to its heterogeneous clinical presentation and the lack of definitive biomarkers. The consensus guidelines propose a multifactorial diagnostic framework involving the presence of DSAs, histological evidence of capillaritis, complement deposition (specifically C4d), evidence of graft dysfunction, and the exclusion of other etiologies [20]. Notably, subclinical AMR, defined by immunologic and histologic findings in the absence of functional impairment, is increasingly recognized as an early phase in the spectrum of humoral rejection [21]. Current therapeutic approaches for AMR are largely derived from treatment protocols in kidney and heart transplantation. These include intravenous immunoglobulin (IVIG), therapeutic plasma exchange (tPE), B-cell depletion with rituximab, and more recently, proteasome inhibitors (carfilzomib) and complement-blocking agents (eculizumab) [22–24]. Despite these efforts, treatment outcomes remain inconsistent. Studies have demonstrated variable antibody clearance rates and a persistently high risk of CLAD and death in patients with clinically manifest AMR [25]. Emerging evidence suggests that preemptive intervention in patients with newly detected dnDSAs, before the onset of overt graft dysfunction, may hold promise [21, 26, 27]. These findings suggest that early immunologic intervention could interrupt the pathogenic cascade that leads to chronic dysfunction, marking a potential shift in clinical strategy from reactive to preventive care.
Despite this progress, many critical questions remain unanswered. There is no consensus on the optimal treatment regimen, and randomized controlled trials are lacking. Furthermore, predictive markers that could guide patient selection and treatment decisions remain elusive. The significant heterogeneity in clinical response underscores the need for further mechanistic studies and controlled trials to identify which patients are most likely to benefit from specific therapeutic interventions. Ultimately, improving long-term outcomes in lung transplantation will depend not only on controlling cellular rejection, but on understanding and modulating the humoral immune response with greater precision.
Materials and Methods
This retrospective single-center study was conducted at the Division of Thoracic surgery, LMU University Hospital, Munich. It was approved by the institutional review board of the faculty of medicine, Ludwig-Maximilians University Munich (UE No. 22-0123) and conducted in accordance with the ethical principles of the declaration of Helsinki. Only adult patients who underwent LuTX between 2015 and 2019 were eligible for inclusion. For the purpose of this study, only individuals who developed dnDSA postoperatively were included in further analyses. Follow-up included routine clinical controls and HLA antibody screening at standardized intervals: 1, 3, 6, 12, 18, 24, 30, 36, and 48 months postoperatively. Immunosuppression followed a standard triple-drug regimen consisting of corticosteroids, tacrolimus and mycophenolate mofetil.
All included patients had undergone routine pre- and post-transplant immunological monitoring. Genotyping was performed using sequence-specific oligonucleotide probes (SSO; LabType, One Lambda, Canoga Park, CA, United States), targeting HLA-A, -B, -C, DRB1, -DRB3/4/5, and DQB1 loci. Antibody screening and identification were performed using Luminex-based bead assays (LABScreen™, One Lambda), enabling high-sensitivity detection of HLA class I and II IgG antibodies. Mean fluorescence intensity (MFI) values greater than 1,000 were considered positive.
Within the dnDSA-positive cohort, patients were retrospectively categorized based on their immunological response to IVIG therapy. The standard therapeutic approach at our center for newly detected dnDSA is to initiate IVIG at 1 g/kg body weight, followed by three subsequent doses of 0.5 g/kg at 4-week intervals. The subset of patients who received IVIG-therapy, was divided into two groups: (1) those who achieved complete elimination of dnDSA following treatment (responders), and (2) those with persistent antibodies after therapy (non-responders). Complete antibody elimination was defined as the absence of previously detected donor-specific HLA antibodies in follow-up screenings, without subsequent recurrence.
Bronchoscopic surveillance was performed at regular intervals during the first 2 years post-transplant and subsequently based on clinical indication. Transbronchial biopsies (TBB) and histological diagnosis of ACR followed the International Society for Heart and Lung Transplantation (ISHLT) criteria, classifying rejection grades A0–A4 and lymphocytic bronchiolitis grades B0–B2R [28]. The 2016 ISHLT consensus report provided the basis for defining antibody-mediated rejection (AMR), distinguishing between clinical AMR, marked by detectable declines in lung function, which may occur without symptoms, and subclinical AMR, in which lung function remains preserved despite immunologic evidence of rejection [19, 20]. Lung function testing (forced expiratory volume in the first second, FEV1) was conducted in parallel with antibody assessments. To evaluate treatment efficacy, FEV1 measurements taken before therapy (baseline) were compared to post-treatment averages within 1 year after treatment initiation. CLAD was defined according to international consensus guidelines [29]. In this study, only bronchiolitis obliterans syndrome (BOS) was used as a clinical endpoint and diagnosed based on sustained FEV1 decline in the absence of alternative explanations [30]. Clinical, immunological, and procedural data were extracted from institutional records and the ET-database, including demographic information, transplant type, donor characteristics and intraoperative parameters (e.g., allograft ischemia time).
Statistical Analysis
Statistical analyses were conducted using R (version 4.0.5) and RStudio (version 1.4.1106). Comparisons between groups were performed using the chi-square test or Fisher’s exact test for categorical variables and the Student’s t-test or Wilcoxon–Mann–Whitney test for continuous variables, as appropriate. Time-dependent outcomes were analyzed using Kaplan–Meier curves with log-rank tests. Logistic regression models were applied to identify factors independently associated with antibody elimination. Statistical significance was defined as a two-tailed p-value ≤0.05.
Results
A total of 47 LuTX-recipients met the inclusion criteria and were enrolled in the analysis, among which 22 were female (46.8%) and 25 male (53.2%). The most common underlying disease was interstitial lung disease (ILD) (n = 21; 44.7%), followed by cystic fibrosis (CF) (n = 12; 25.5%) and chronic obstructive pulmonary disease (COPD) (n = 9; 19.1%). The median follow-up time post-transplantation was 18 months (range: 3–48 months). Antibody elimination was achieved in 48.9% of all patients (n = 23; responder), while 51% (n = 24; non-responder) exhibited persistent dnDSA throughout follow-up.
Responders vs. Non-Responders
As depicted in Table 1, comparison of demographic characteristics between responders and non-responders revealed no statistically significant differences in recipient or donor age, sex distribution, transplant indication or surgical approach. Both groups had similar proportions of bilateral versus single lung transplants and comparable ischemia times.
TABLE 1
| Non-responders and responders | Non-responders (n = 24) | Responders (n = 23) | (p) | ||
|---|---|---|---|---|---|
| (mean) | (sd) | (mean) | (sd) | ||
| Age (years) | |||||
| Recipient | 51.2 | 12.1 | 46.8 | 15.2 | 0.28 |
| Donor | 44.7 | 16.9 | 48.8 | 15.6 | 0.4 |
| Ischemia time (minutes) | 522 | 125 | 510 | 128 | 0,75 |
| (n) | (%) | (n) | (%) | (p) | |
| Sex | |||||
| Female | 11 | 45.8% | 11 | 47.8% | 1,00 |
| Male | 13 | 44.2% | 12 | 52.2% | |
| Underlying Diagnosis for LuTX | |||||
| ILD | 11 | 45.8% | 10 | 43.5% | 1,00 |
| COPD | 4 | 16.7% | 5 | 21.7% | |
| CF | 6 | 25.0% | 6 | 26.1% | |
| other | 3 | 12.5% | 2 | 8.7% | |
| One-year Survival | 20 | 83.3% | 21 | 91.3% | 0.66 |
| Lung Allograft Rejection | |||||
| ACR | 8 | 33.3% | 6 | 26.1% | 0.75 |
| Clinical AMR | 16 | 66.7% | 1 | 4.3% | <0.001 |
| CLAD | 7 | 29.2% | 0 | 0.0% | 0.009 |
Non-Responders vs. Responders.
ACR, acute cellular rejection; AMR, antibody-mediated rejection; CF, cystic fibrosis; CLAD, chronic lung allograft dysfunction; COPD, chronic obstructive pulmonary disease; LuTX, lung transplantation.
When analyzing HLA class distribution of dnDSA, it became apparent that non-responders were more likely to develop HLA class II antibodies (95.8%; n = 23 vs. 78.3%; n = 18 in the responder group); however, this difference did not reach statistical significance (p = 0.097). As shown in Figure 1, MFI prior to therapy was significantly higher in the non-responder group compared to responders (mean 11,683 ± 7,055 vs. 7,152 ± 5,912; p = 0.019). Notably, the time to development of dnDSA was also significantly longer in non-responders than in responders (median: 94 days (IQR 35-343) vs. 39 days (IQR 22-65); p = 0.0025).
FIGURE 1
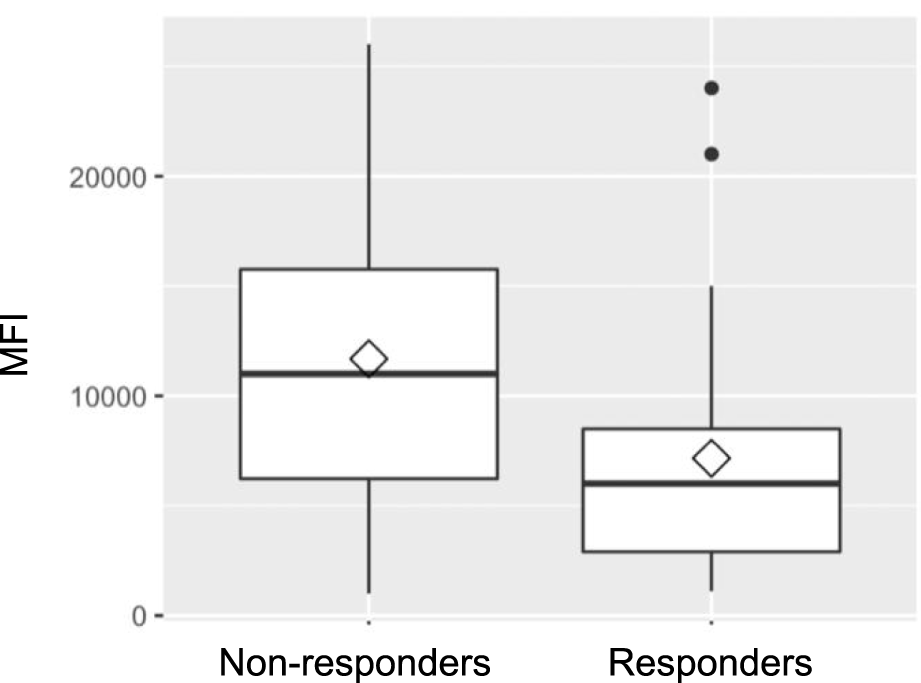
Mean fluorescence intensity (MFI).
Lung Function
Baseline lung function, assessed by FEV1 prior to treatment, was retrospectively found to be significantly better in future responders (75.0% of predicted) than in non-responders (54.8%; p = 0.019). Lung function testing at 1 year following IVIG-therapy initiation, showed consistently higher FEV1 values (78.8% vs. 63.3%) in responders, although the difference between the groups was no longer statistically significant (p = 0.07). Figures 2, 3 illustrate FEV1 prior to and 1 year after IVIG-therapy.
FIGURE 2
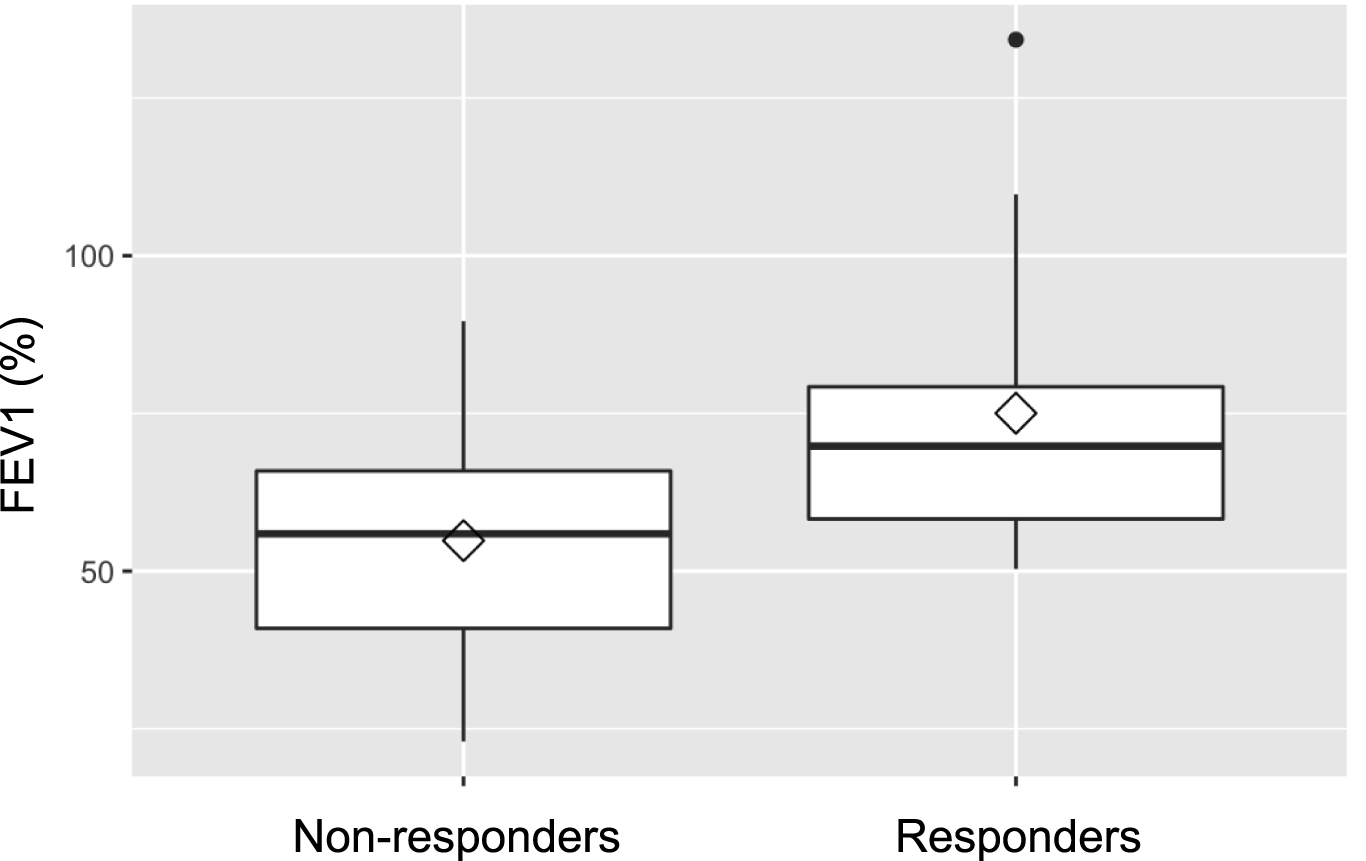
FEV1 pre IVIG-therapy.
FIGURE 3
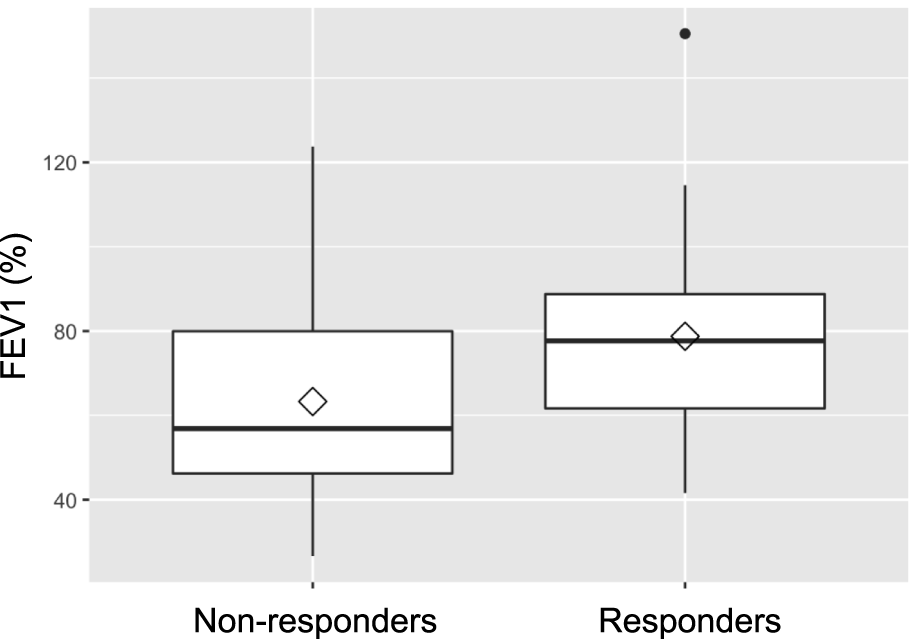
FEV1 1 year after IVIG-therapy initiation.
Acute Cellular Rejection (ACR)
ACR was identified in a total of 14 patients, corresponding to 29.8% of the entire study cohort. Among these cases, the majority were categorized as grade A1 rejection, which is characterized histologically by minimal perivascular mononuclear infiltrates without evidence of tissue injury. These milder rejection episodes were evenly distributed across both study groups (6/23 in the responder group vs. 6/24 in the non-responder group). Notably, a single episode of grade A2 rejection and one episode of grade A3 rejection were each observed and both occurred within the non-responder group. When comparing the incidence of ACR between treatment groups, no statistically significant difference was observed: 6 out of 23 responders (26.1%) experienced ACR, compared to 8 out of 24 non-responders (33.3%) (p = 0.75). These findings suggest that the rate of ACR was comparable between groups, regardless of treatment response.
Antibody-Mediated Rejection (AMR)
The prevalence of AMR at the time of IVIG initiation differed between responders and non-responders (Figure 4). For clinical AMR, only 1 of 23 responders (4.3%) met the criteria for possible clinical AMR, compared to 16 of 24 non-responders (66.7%). In contrast, subclinical AMR was more frequent among responders: 22 responders were classified with subclinical AMR at baseline, whereas only 8 non-responders fell into this category. Clinical AMR was therefore predominantly observed in non-responders, while subclinical AMR was more commonly seen in responders. Multivariable logistic regression analysis identified preemptive treatment, defined as initiation of therapy during subclinical AMR prior to the onset of clinical symptoms, as a significant independent predictor of dnDSA clearance (odds ratio 29.5 (OR); p = 0.013). Other variables assessed, including age, sex, MFI levels, antibody class, and timing of dnDSA detection were not significantly associated with antibody elimination.
FIGURE 4
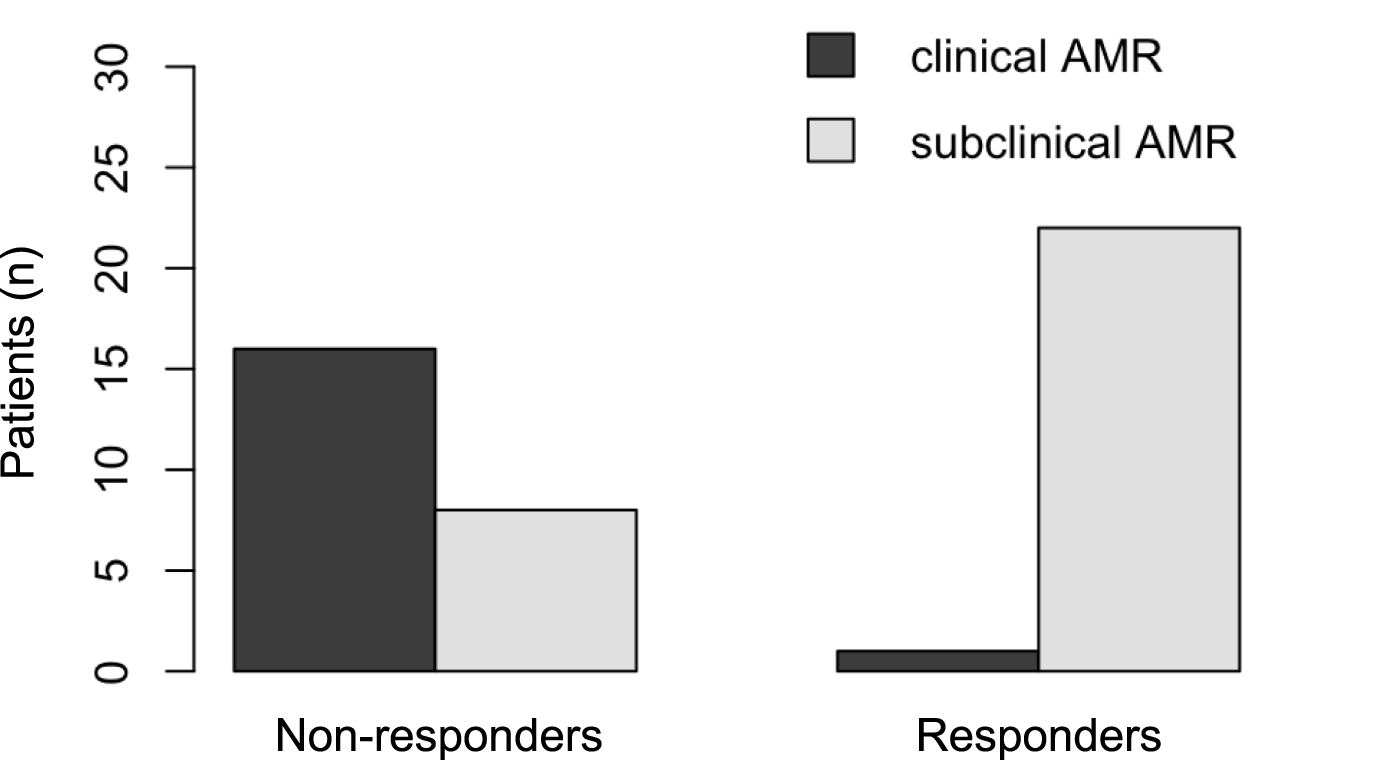
Antibody-mediated rejection (AMR) pre IVIG-therapy.
Chronic Lung Allograft Dysfunction (CLAD)
CLAD, defined according to standard criteria for BOS, was observed exclusively among patients in the non-responder group. A total of 7 non-responders (29.2%) were diagnosed with BOS during the observation period, whereas no responder developed this form of chronic allograft dysfunction. This difference was statistically significant (p = 0.009). Importantly, all cases of BOS were diagnosed prior to the initiation of IVIG therapy.
Survival
One-year post-transplant survival in the overall cohort was 87.2%. Of the ten patients who died, eight were from the non-responder group and two from the responder group. Both deaths among responders were attributed to causes not directly related to the transplant (intracranial hemorrhage and cardiac failure). Although 1-year survival was numerically higher in responders compared to non-responders (91.3% vs. 83.3%), the difference was not statistically significant (p = 0.66). A similar trend was observed in 1-year survival following dnDSA detection: 91.3% in responders versus 71.4% in non-responders (p = 0.13). Survival 1 year after initiation of IVIG therapy was also higher in responders (90.9%) compared to non-responders (66.7%), although this difference did not reach statistical significance (p = 0.11). Kaplan–Meier analysis demonstrated non-significant differences in survival between groups following dnDSA detection (log-rank p = 0.11) and IVIG initiation (log-rank p = 0.06). Overall survival probabilty showed no differences between the two groups (log-rank p = 0.2).
Discussion
The development of dnDSA following LuTX and their association with the onset of CLAD, impaired graft function and reduced survival have been well documented in numerous studies over recent years [13–16, 19, 31]. To date, the management of AMR remains heterogeneous, with no standardized protocols or universally accepted treatment strategies in place [21, 26, 32].
Our findings are consistent with growing evidence in literature that early immunological intervention can mitigate the development of chronic allograft dysfunction and extend graft survival [20, 33, 34]. While IVIG is well established in the treatment of AMR, its role in asymptomatic dnDSA or subclinical AMR is less clearly defined. We demonstrated that early intervention, particularly in asymptomatic patients with subclinical AMR, may significantly improve dnDSA clearance rates and associated clinical outcomes. Preemptive therapy, defined as the initiation of treatment before the onset of clinical symptoms, proved to be an independent predictor of successful dnDSA elimination in multivariable analysis (OR 29.5; p = 0.013). This observation aligns with data from Ius et al. (2018), who reported a 92% clearance rate following preemptive IVIG therapy in asymptomatic recipients, with graft survival comparable to DSA-negative controls [21]. Hachem et al. (2010) similarly found that preemptive therapy with IVIG solely or IVIG in combination with Rituximab resulted in DSA clearance in 65% of patients. Furthermore, patients witnessing successful antibody-depletion were less likely to develop BOS throughout the follow-up period [32]. Recent evidence presented by McDermott et al. provides important insight [27]. In a cohort of asymptomatic LuTX-recipients with dnDSA, preemptive IVIG monotherapy resulted in substantial reduction in antibody strength, with complete clearance in more than half of the patients. Moreover, they observe a trend toward lower rates of subsequent ACR among patients achieving DSA clearance.
The timing of dnDSA detection also appeared to influence treatment success. In our cohort, responders exhibited significantly earlier detection and received treatment sooner (median 15.0 vs. 36.5 days), suggesting that patients with early-onset dnDSA may benefit more from antibody-directed intervention. These observations are supported by Ensor et al. (2017) and Vacha et al. (2017), who reported that earlier therapy initiation was associated with improved antibody clearance and better clinical outcomes. Delayed treatment may permit maturation of antibodies and acquisition of complement-binding properties, which are known to reduce responsiveness to desensitization [24, 25].
While MFI values were not independently predictive of treatment success, we observed significantly higher pre-treatment MFI levels in non-responders, with the median nearly double that of responders. This observation is consistent with findings by [21, 24], who reported that lower baseline MFI values were associated with a higher likelihood of antibody clearance [21, 24]. In contrast, Timofeeva et al. (2021) did not find a significant correlation between pre-treatment MFI levels and overall survival; however, they observed that lower post-treatment MFI values following therapeutic plasma exchange (tPE) were significantly associated with improved survival [35].
Clinically, lung function and survival outcomes tended to be more favorable in the responder group. However, these differences were not statistically significant, which may be attributable to small sample size and ceiling effects, as many responders already had preserved baseline FEV1 values. Both groups showed only modest relative improvements in lung function and therapy in responders was more likely aimed at preventing decline rather than reversing damage. Similar associations between DSA elimination and improved BOS-free survival have been reported in other studies [24, 32, 35].
This study has several limitations that should be considered when interpreting the findings. First, the retrospective and single-center design inherently limits the generalizability of the results and may introduce selection or information bias. Although the study was conducted at a high-volume transplant center over a 5-year period, the overall sample size remained relatively small, thereby limiting statistical power and increasing the risk of type II error, particularly in subgroup analyses and survival comparisons. Furthermore, the classification of treatment response was based solely on dnDSA elimination, without histopathologic correlates such as C1q-binding capacity or tissue deposition. Due to institutional standards, uniform C4d staining was not performed, which may limit the ability to fully characterize the histopathologic phenotype of AMR and subclinical graft injury. However, given the limited sensitivity and specificity of C4d staining in lung allografts and the variability in its expression across AMR phenotypes, as highlighted in the ISHLT consensus report and subsequent studies, we consider its absence to have limited clinical impact in the context of this study [19]. While our study focused on IVIG monotherapy, the heterogeneity of dnDSA profiles and immune status across patients suggests that individualized or combination therapies may be required, an area not addressed in this analysis.
All cases of BOS in our cohort occurred prior to IVIG initiation and exclusively among non-responders, which may reflect inherent biological or immunologic differences between patients who did and did not achieve dnDSA clearance. The question of whether this observation strengthens or weakens the association between IVIG therapy and DSA clearance cannot be answered in the retrospective analysis and requires prospective data. This temporal pattern limits the ability to directly attribute the absence of BOS in responders to the effect of IVIG therapy.
Given the retrospective nature of the study, it did not include a control group of dnDSA-positive patients who did not receive IVIG therapy. This design inherently limits the ability to fully distinguish treatment effects from the natural course of dnDSA evolution. Future prospective studies with appropriate control cohorts will be important to confirm the therapeutic impact of IVIG. Lastly, the observational nature of the study precludes causal inference, and although multivariable analysis was performed, unmeasured confounders may still influence the associations observed.
Taken together, our findings highlight the importance of early, preemptive intervention in lung transplant recipients with dnDSA, particularly those who are asymptomatic and have moderate antibody burden. While high MFI and class II specificity may suggest reduced clearance probability, they should not preclude therapeutic attempts when clinical stability allows. DSA elimination remains a critical therapeutic goal, not only to limit immunologic injury but also to preserve long-term graft function and survival. Our findings underscore the need for early detection and individualized intervention in dnDSA-positive patients. Prospective multicenter trials are essential to validate risk-adapted treatment algorithms and define standardized thresholds for therapeutic initiation.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of the Faculty of Medicine, Ludwig-Maximilians University Munich. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because observational and non-interventional retrospective study.
Author contributions
Concept and design: MV, PD, TK, AD, and JW; Data collection: PD and TK; Data interpretation: MV, PD, GY, OG, JK, JW, AD, SM, CS, MZ, JB, and TK (all authors); Statistics: PD, JW; Writing and editing: MV, PC, GY, OG, JK, JW, AD, SM, CS, MZ, JB, and TK (all authors); Critical revision: MV, PC, GY, OG, JK, JW, AD, SM, CS, MZ, JB, and TK (all authors). All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The content of this manuscript is based on research data that was previously presented in the context of a doctoral thesis submitted to the Ludwig-Maximilians-Universität München. The thesis, entitled “Intravenöse Immunglobulin-Therapie bei Antikörper-vermittelter Abstoßungsreaktion nach Lungentransplantation Analyse prädiktiver Faktoren für die Elimination de novo donorspezifischer Antikörper” [36], includes the full dataset analyzed in this study. The thesis is publicly accessible through the university’s library repository and has not been published in any peer-reviewed journal. A full reference to the thesis is included in the reference list of this manuscript. The use of generative AI in this manuscript was limited to language refinement and editing. No AI tools were used for data analysis, interpretation, or content generation. All use adheres to applicable ethical guidelines.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2025.15350/full#supplementary-material
Abbreviations
ACR, Acute Cellular Rejection; AMR, Antibody-mediated Rejection; BOS, Bronchiolitis Obliterans Syndrome; CLAD, Chronic Lung Allograft Dysfunction; COPD, Chronic Obstructive Pulmonary Disease; dnDSA, De Novo Donor-Specific Antibody; DSA, Donor-Specific Antibody; ELD, End-Stage Lung Disease; FEV1, Forced Expiratory Volume in One Second; HLA, Human Leukocyte Antigen; ILD, Interstitial Lung Disease; IQR, Interquartile Range; ISHLT, International Society for Heart and Lung Transplantation; IVIG, Intravenous Immunoglobulin; LuTX, Lung Transplantation; MFI, Mean Fluorescence Intensity; MHC, Major Histocompatibility Complex; OR, Odds Ratio; SD, Standard Deviation; SSO, Sequence-Specific Oligonucleotide; TBB, Transbronchial Biopsy; tPE, Therapeutic Plasma Exchange.
References
1.
Chambers DC Cherikh WS Goldfarb SB Hayes D Jr Kucheryavaya AY Toll AE et al The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-Fifth Adult Lung and Heart-Lung Transplant Report-2018; Focus Theme: Multiorgan Transplantation. J Heart Lung Transpl (2018) 37(10):1169–83. 10.1016/j.healun.2018.07.020
2.
Weill D Benden C Corris PA Dark JH Davis RD Keshavjee S et al A Consensus Document for the Selection of Lung Transplant Candidates: 2014--An Update From the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transpl (2015) 34(1):1–15. 10.1016/j.healun.2014.06.014
3.
Leard LE Holm AM Valapour M Glanville AR Attawar S Aversa M et al Consensus Document for the Selection of Lung Transplant Candidates: An Update From the International Society for Heart and Lung Transplantation. J Heart Lung Transpl (2021) 40(11):1349–79. 10.1016/j.healun.2021.07.005
4.
Valapour M Lehr CJ Skeans MA Smith JM Miller E Goff R et al OPTN/SRTR 2019 Annual Data Report: Lung. Am J Transpl (2021) 21(Suppl. 2):441–520. 10.1111/ajt.16495
5.
Dipchand AI Kirk R Edwards LB Kucheryavaya AY Benden C Christie JD et al The Registry of the International Society for Heart and Lung Transplantation: Sixteenth Official Pediatric Heart Transplantation Report--2013; Focus Theme: Age. J Heart Lung Transpl (2013) 32(10):979–88. 10.1016/j.healun.2013.08.005
6.
Nelson J Kincaide E Schulte J Hall R Levine DJ . Immunosuppression in Lung Transplantation. Handb Exp Pharmacol (2022) 272:139–64. 10.1007/164_2021_548
7.
Niehaus H Haverich A Ius F . Current Status of Transplantation Medicine in the Field of Heart and Lung Transplantation: Legal Regulations and Clinical Implementation. Anaesthesiologie (2022) 71(9):727–38. 10.1007/s00101-022-01179-8
8.
Sato M . Chronic Lung Allograft Dysfunction After Lung Transplantation: The Moving Target. Gen Thorac Cardiovasc Surg (2013) 61(2):67–78. 10.1007/s11748-012-0167-3
9.
Royer PJ Olivera-Botello G Koutsokera A Aubert JD Bernasconi E Tissot A et al Chronic Lung Allograft Dysfunction: A Systematic Review of Mechanisms. Transplantation (2016) 100(9):1803–14. 10.1097/TP.0000000000001215
10.
Bos S Vos R Van Raemdonck DE Verleden GM . Survival in Adult Lung Transplantation: Where Are We in 2020?Curr Opin Organ Transpl (2020) 25(3):268–73. 10.1097/MOT.0000000000000753
11.
Burton CM Iversen M Carlsen J Mortensen J Andersen CB Steinbrüchel D et al Acute Cellular Rejection Is a Risk Factor for Bronchiolitis Obliterans Syndrome Independent of Post-Transplant Baseline FEV1. J Heart Lung Transpl (2009) 28(9):888–93. 10.1016/j.healun.2009.04.022
12.
Khalifah AP Hachem RR Chakinala MM Yusen RD Aloush A Patterson GA et al Minimal Acute Rejection After Lung Transplantation: A Risk for Bronchiolitis Obliterans Syndrome. Am J Transpl (2005) 5(8):2022–30. 10.1111/j.1600-6143.2005.00953.x
13.
Kauke T Kneidinger N Martin B Dick A Schneider C Schramm R et al Bronchiolitis Obliterans Syndrome Due to Donor-Specific HLA-Antibodies. Tissue Antigens (2015) 86(3):178–85. 10.1111/tan.12626
14.
Safavi S Robinson DR Soresi S Carby M Smith JD . De Novo Donor HLA-Specific Antibodies Predict Development of Bronchiolitis Obliterans Syndrome After Lung Transplantation. J Heart Lung Transpl (2014) 33(12):1273–81. 10.1016/j.healun.2014.07.012
15.
Girnita AL Duquesnoy R Yousem SA Iacono AT Corcoran TE Buzoianu M et al HLA-Specific Antibodies Are Risk Factors for Lymphocytic Bronchiolitis and Chronic Lung Allograft Dysfunction. Am J Transpl (2005) 5(1):131–8. 10.1111/j.1600-6143.2004.00650.x
16.
Lobo LJ Aris RM Schmitz J Neuringer IP . Donor-Specific Antibodies Are Associated With Antibody-Mediated Rejection, Acute Cellular Rejection, Bronchiolitis Obliterans Syndrome, and Cystic Fibrosis After Lung Transplantation. J Heart Lung Transpl (2013) 32(1):70–7. 10.1016/j.healun.2012.10.007
17.
Snyder LD Wang Z Chen DF Reinsmoen NL Finlen-Copeland CA Davis WA et al Implications for Human Leukocyte Antigen Antibodies After Lung Transplantation: A 10-Year Experience in 441 Patients. Chest (2013) 144(1):226–33. 10.1378/chest.12-0587
18.
Choo SY . The HLA System: Genetics, Immunology, Clinical Testing, and Clinical Implications. Yonsei Med J (2007) 48(1):11–23. 10.3349/ymj.2007.48.1.11
19.
Levine DJ Glanville AR Aboyoun C Belperio J Benden C Berry GJ et al Antibody-Mediated Rejection of the Lung: A Consensus Report of the International Society for Heart and Lung Transplantation. J Heart Lung Transpl (2016) 35(4):397–406. 10.1016/j.healun.2016.01.1223
20.
Roux A Levine DJ Zeevi A Hachem R Halloran K Halloran PF et al Banff Lung Report: Current Knowledge and Future Research Perspectives for Diagnosis and Treatment of Pulmonary Antibody-Mediated Rejection (AMR). Am J Transpl (2019) 19(1):21–31. 10.1111/ajt.14990
21.
Ius F Verboom M Sommer W Poyanmehr R Knoefel AK Salman J et al Preemptive Treatment of Early Donor-Specific Antibodies With IgA- and IgM-Enriched Intravenous Human Immunoglobulins in Lung Transplantation. Am J Transpl (2018) 18(9):2295–304. 10.1111/ajt.14912
22.
Bery AI Hachem RR . Antibody-Mediated Rejection After Lung Transplantation. Ann Transl Med (2020) 8(6):411. 10.21037/atm.2019.11.86
23.
Messika J Belousova N Parquin F Roux A . Antibody-Mediated Rejection in Lung Transplantation: Diagnosis and Therapeutic Armamentarium in a 21st Century Perspective. Transpl Int (2024) 37:12973. 10.3389/ti.2024.12973
24.
Ensor CR Yousem SA Marrari M Morrell MR Mangiola M Pilewski JM et al Proteasome Inhibitor Carfilzomib-Based Therapy for Antibody-Mediated Rejection of the Pulmonary Allograft: Use and Short-Term Findings. Am J Transpl (2017) 17(5):1380–8. 10.1111/ajt.14222
25.
Vacha M Chery G Hulbert A Byrns J Benedetti C Finlen Copeland CA et al Antibody Depletion Strategy for the Treatment of Suspected Antibody-Mediated Rejection in Lung Transplant Recipients: Does It Work? Clin Transpl (2017) 31(3):e12886. 10.1111/ctr.12886
26.
Halverson LP Hachem RR . Antibody-Mediated Rejection: Diagnosis and Treatment. Clin Chest Med (2023) 44(1):95–103. 10.1016/j.ccm.2022.10.008
27.
McDermott JK Castaneda SJ Mietz SM Lawson CK Gerlach JA Hadley RJ et al Preemptive Treatment of De Novo Donor Specific Anti-hla Antibodies with IVIG Monotherapy After Lung Transplantation. Transpl Int (2024) 37. 10.3389/ti.2024.13431
28.
Stewart S Fishbein MC Snell GI Berry GJ Boehler A Burke MM et al Revision of the 1996 Working Formulation for the Standardization of Nomenclature in the Diagnosis of Lung Rejection. J Heart Lung Transpl (2007) 26(12):1229–42. 10.1016/j.healun.2007.10.017
29.
Verleden GM Vos R Vanaudenaerde B Dupont L Yserbyt J Van Raemdonck D et al Current Views on Chronic Rejection After Lung Transplantation. Transpl Int (2015) 28(10):1131–9. 10.1111/tri.12579
30.
Meyer KC Raghu G Verleden GM Corris PA Aurora P Wilson KC et al An International ISHLT/ATS/ERS Clinical Practice Guideline: Diagnosis and Management of Bronchiolitis Obliterans Syndrome. Eur Respir J (2014) 44(6):1479–503. 10.1183/09031936.00107514
31.
Schmitzer M Winter H Kneidinger N Meimarakis G Dick A Schramm R et al Persistence of De Novo Donor Specific HLA-Antibodies After Lung Transplantation: A Potential Marker of Decreased Patient Survival. Hla (2018) 92:24–32. 10.1111/tan.13306
32.
Hachem RR Yusen RD Meyers BF Aloush AA Mohanakumar T Patterson GA et al Anti-Human Leukocyte Antigen Antibodies and Preemptive Antibody-Directed Therapy After Lung Transplantation. J Heart Lung Transpl (2010) 29(9):973–80. 10.1016/j.healun.2010.05.006
33.
Lefaucheur C Nochy D Andrade J Verine J Gautreau C Charron D et al Comparison of Combination Plasmapheresis/IVIg/anti-CD20 Versus High-Dose Ivig in the Treatment of Antibody-Mediated Rejection. Am J Transpl (2009) 9(5):1099–107. 10.1111/j.1600-6143.2009.02591.x
34.
Roux A Bendib Le Lan I Holifanjaniaina S Thomas KA Hamid AM Picard C et al Antibody-Mediated Rejection in Lung Transplantation: Clinical Outcomes and Donor-Specific Antibody Characteristics. Am J Transpl (2016) 16(4):1216–28. 10.1111/ajt.13589
35.
Timofeeva OA Choe J Alsammak M Yoon EJ Geier SS Mathew L et al Guiding Therapeutic Plasma Exchange for Antibody-Mediated Rejection Treatment in Lung Transplant Recipients - A Retrospective Study. Transpl Int (2021) 34(4):700–8. 10.1111/tri.13825
36.
Degenfelder P , Intravenöse Immunglobulin-Therapie Bei Antikörper-vermittelter Abstoßungsreaktion Nach Lungentransplantation Analyse Prädiktiver Faktoren Für Die Elimination De Novo Donorspezifischer Antikörper
Summary
Keywords
lung transplantation, dnDSA, AMR, ACR, CLAD
Citation
Vorstandlechner M, Degenfelder P, Yavuz G, Glueck OM, Kovács JR, Walter J, Dick A, Michel S, Schneider CP, Zoller M, Barton J and Kauke T (2025) Efficacy of Intravenous Immunoglobulin in Eliminating De Novo Donor-Specific Antibodies After Lung Transplantation: Importance of Early Intervention. Transpl. Int. 38:15350. doi: 10.3389/ti.2025.15350
Received
30 July 2025
Accepted
20 October 2025
Published
03 November 2025
Volume
38 - 2025
Updates
Copyright
© 2025 Vorstandlechner, Degenfelder, Yavuz, Glueck, Kovács, Walter, Dick, Michel, Schneider, Zoller, Barton and Kauke.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maximilian Vorstandlechner, max.vorstandlechner@med.uni-muenchen.de
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.