Dear Editors,
Normothermic machine perfusion (NMP) has become a routine technique in liver transplantation, allowing preservation and assessment of grafts prior to implantation [1]. Current commercially approved systems are limited to 24 h, prompting interest in prolonged perfusion to further improve graft conditioning. During extended NMP, microbial contamination is a potential risk, as warm, humid conditions promote bacterial growth [2]. To mitigate this, antimicrobials are commonly added to the perfusate, although the pharmacokinetics under NMP conditions - characterized by a small volume of distribution and absence of renal clearance - remain insufficiently understood.
Piperacillin, a broad-spectrum β-lactam, has been used experimentally for extended NMP [3]. The present study characterizes its pharmacokinetics during liver NMP, including perfusate levels, tissue concentrations via microdialysis, and bile excretion.
In this study, the livers of eight domestic pigs were used and perfused with 1,500 mL leukocyte-depleted whole blood from the donor animal. A microdialysis double lumen catheter with a semi-permeable membrane at the tip was inserted into the liver tissue. Prior to piperacillin administration, the relative recovery of each microdialysis probe was determined by retrodialysis using Ringer’s solution (Fresenius Kabi Austria GmbH, Graz, Austria) containing 80 μg mL−1 piperacillin. The piperacillin concentrations in the retroperfusate (CRP) and the corresponding retrodialysate (CRD) were used to calculate the relative recovery using the formula: relative recovery = [1 - (CRD/CRP)] × 100%.
After calibration, 400 mg piperacillin was added into the reservoir of the NMP system.
The catheter was perfused with Ringer’s solution at a flow rate of 1 μL min−1 to facilitate the exchange of piperacillin between the liver interstitial space fluid (ISF) and the microdialysis perfusate across the membrane. The piperacillin concentration measured in the resulting microdialysate (CMD), corrected for the relative recovery of the probe, was used to estimate the piperacillin concentration in the ISF (CISF = CMD × 100%/relative recovery). Microdialysate samples were collected at 20-minute intervals for 2 h and at 60-minute intervals for up to 8 h after piperacillin administration. NMP perfusate samples were collected 5 min, 15, 30, 60 min and 3, 4, 6 and 24 h after piperacillin administration. Bile samples were analyzed 4 and 8 h after piperacillin administration (in four grafts due to technical limitations). The concentrations of piperacillin were determined by HPLC-UV.
Stable perfusion and organ function was achieved over the entire study period. The piperacillin concentration in perfusate samples and ISF of liver tissue during NMP are shown in Figure 1. After piperacillin application (t = 0 min), the first samples of NMP perfusate, taken at 5 min (n = 5) or 15 min (n = 3) showed the highest measured concentrations of total piperacillin: 109.9 ± 25.1 mg L−1 at 5 min (n = 5) or 78.7 ± 29.9 mg L−1 at 15 min (n = 3; corresponding to 108.5 ± 36.7 mg L−1 at 5 min).
FIGURE 1
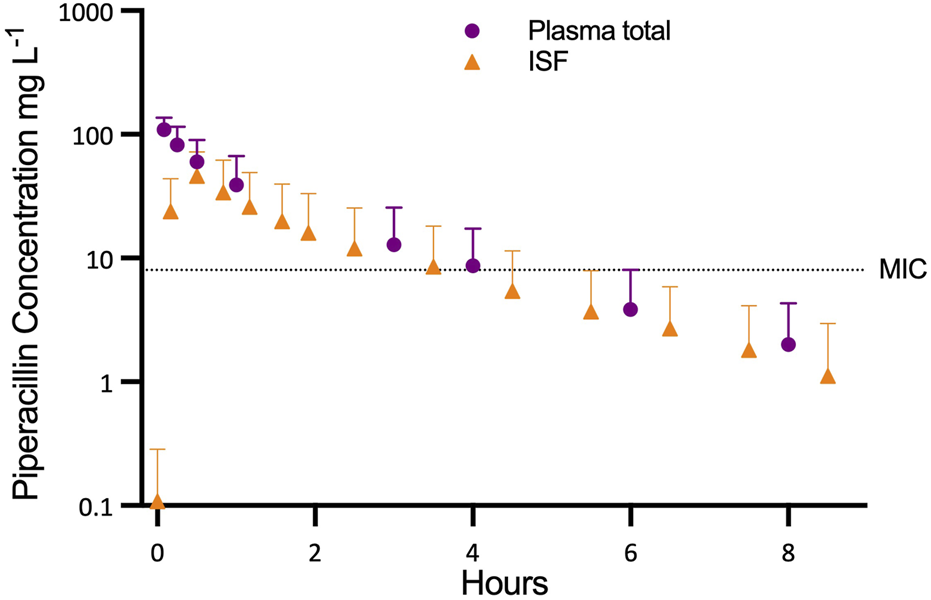
Progression of piperacillin concentration in plasma and interstitial fluid (ISF) in relation to the minimum inhibitory concentration (MIC).
The mean area under the curve (AUC(0.8)) for total piperacillin in NMP perfusate was 135 ± 92.91 mg L−1 h, AUCINFINITY was 144 ± 103 mg L−1 h, the elimination half-life time was 1.43 ± 0.42 h. The mean volume of distribution was 8.22 ± 4.83 L and the mean clearance of piperacillin was 4.54 ± 3.25 L h−1.
The unbound fraction (fu) of piperacillin in NMP perfusate was 93.8% ± 1.31%. Mean fAUC(0.8) and fAUCINFINITY was 128 ± 86.6 mg L−1 h and 136 ± 95.9 mg L−1 h, respectively.
Relative recovery of the microdialysis probes was high (95.4% ± 4.67%) and ranged from 83.0% to 100%. Peak concentrations (Cmax) in ISF were reached at 10 min (n = 1), 30 min (n = 5) or after 50 min (n = 2). Mean Cmax in ISF amounted to 48.6 ± 26.4 mg L−1. Mean elimination half-life (1.50 ± 0.36 h) was similar to that of the NMP perfusate.
Mean AUC(0.8) and AUCINFINITY for piperacillin in ISF (87.18 ± 76.18 and 90.8 ± 81.3 mg L−1 h) were lower than in NMP perfusate (p = 0.016). The penetration ratio, defined as the ratio of the AUCINFINITY for piperacillin in ISF to AUCINFINFINITY for free piperacillin in NMP perfusate (AUCINFINITY_ISF/fAUCINFINITY_perfusate), was 0.653 ± 0.300.
The grafts produced 118.25 (±44.74) mL of bile during the first 8 h of perfusion with a mean piperacillin concentration of 1.48 ± 1.11 g L−1, corresponding to 168.15 ± 68.4 mg of piperacillin excreted in bile per graft.
Piperacillin rapidly achieved high concentrations in graft tissue during NMP, followed by swift elimination. Perfusate levels consistently exceeded tissue concentrations, with elimination predominantly occurring via bile in the absence of renal excretion. The reduced volume of distribution inherent to isolated liver perfusion, combined with rapid recirculation of a small perfusate volume, explains both the fast tissue penetration and rapid clearance.
Although piperacillin elimination in vivo is largely renal, biliary excretion represents a known alternative route, particularly in renal insufficiency [4]. This pathway likely accounts for the significant biliary concentrations observed in this study despite absent renal clearance.
Protein binding in NMP perfusate was markedly lower than in human plasma (20%–30%), attributed to the leukocyte-depleted whole blood used here [5]. In clinical NMP with red cell concentrates and colloids [6], protein binding would be negligible.
Using the EUCAST minimum inhibitory concentration breakpoint for piperacillin/tazobactam-sensitive strains (8 mg L−1), graft tissue levels were below this threshold within 4 hours of receiving a 400 mg bolus dose [7]. This suggests that a single-dose strategy provides only transient antimicrobial protection during NMP. Continuous infusion could maintain therapeutic levels, but dosing must account for the high inter-graft variability observed (coefficient of variation 72%).
These findings underscore that drug pharmacokinetics during NMP differ markedly from in vivo conditions, necessitating dedicated dosing studies for medications administered in this setting. Limitations include the use of an animal model [8], perfusate composition differing from clinical practice (although protein binding was low even with whole blood), and the lack of metabolite measurements.
In summary, piperacillin during NMP demonstrates rapid hepatic penetration and biliary elimination, with therapeutic levels declining within 4 hours. For prolonged or long-term NMP, continuous dosing strategies may be required to ensure sustained antimicrobial protection.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Institutional Animal Care and Use Committee of the Medical University of Innsbruck and the Austrian Ministry of Science, Research and Economy (Nr.: 2022-0.386.456). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
SM participated in research design, performance of research, writing of the paper and data analysis. GP participated in research design, writing of the paper and data analysis. JM participated in research design, writing of the paper and data analysis. TR participated in performance of research and writing of the paper. CB participated in performance of research. MD participated in performance of research. JD participated in performance of research. FN participated in performance of research. NS participated in performance of research. MB participated in performance of research. TH participated in writing of the paper. JH participated in writing of the paper. SS participated in writing of the paper. CD participated in research design, performance of research, writing of the paper and data analysis. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This work was funded by the “In Memoriam Dr. Gabriel Salzner Foundation.”
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2025.15348/full#supplementary-material
References
1.
Krendl FJ Cardini B Fodor M Singh J Ponholzer F Messner F et al Normothermic Liver Machine Perfusion at a Large European Center: Real-World Outcomes Following 238 Applications. Ann Surg (2025) 281:872–83. 10.1097/SLA.0000000000006634
2.
Lau NS Ly M Dennis C Toomath S Huang JL Huang J et al Microbial Contamination During Long-Term Ex Vivo Normothermic Machine Perfusion of Human Livers. Transplantation (2024) 108:198–203. 10.1097/TP.0000000000004653
3.
Eshmuminov D Becker D Bautista Borrego L Hefti M Schuler MJ Hagedorn C et al An Integrated Perfusion Machine Preserves Injured Human Livers for 1 Week. Nat Biotechnol (2020) 38:189–98. 10.1038/s41587-019-0374-x
4.
Brogard JM Jehl F Blickle JF Dorner M Arnaud JP Monteil H . Biliary Pharmacokinetic Profile of Piperacillin: Experimental Data and Evaluation in Man. Int J Clin Pharmacol Ther Toxicol (1990) 28:462–70.
5.
Sörgel F Kinzig M . The Chemistry, Pharmacokinetics and Tissue Distribution of Piperacillin/Tazobactam. J Antimicrob Chemother (1993) 31(Suppl. A):39–60. 10.1093/jac/31.suppl_a.39
6.
Ravikumar R Jassem W Mergental H Heaton N Mirza D Perera MT et al Liver Transplantation After Ex Vivo Normothermic Machine Preservation: A Phase 1 (First-in-Man) Clinical Trial. Am J Transpl (2016) 16:1779–87. 10.1111/ajt.13708
7.
The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 15.0. Available online at: https://www.eucast.org (Accessed June 8, 2025).
8.
Pontoppidan LL Bue M Houlind KC Knudsen AR Pedersen JB Hvistendahl MA et al Piperacillin Reaches High Concentrations in Bile and Target Tissues of the Biliary System: An Experimental Study in Pigs. Antimicrob Agents Chemother (2025) 69:e0079225. 10.1128/aac.00792-25
Summary
Keywords
normothermic machine perfusion, piperacillin, microdialysis, liver transplant, pharmacokinetic analysis
Citation
Mathis S, Putzer G, Martini J, Resch T, Bogensperger C, Dullnig M, Dunz J, Nawabi F, Staier N, Bordt M, Hautz T, Hofmann J, Schneeberger S and Dorn C (2026) Pharmacokinetics of Piperacillin in an Experimental Porcine Liver Model During Normothermic Machine Perfusion. Transpl. Int. 38:15348. doi: 10.3389/ti.2025.15348
Received
30 July 2025
Revised
06 October 2025
Accepted
16 December 2025
Published
06 January 2026
Volume
38 - 2025
Updates
Copyright
© 2026 Mathis, Putzer, Martini, Resch, Bogensperger, Dullnig, Dunz, Nawabi, Staier, Bordt, Hautz, Hofmann, Schneeberger and Dorn.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gabriel Putzer, gabriel.putzer@i-med.ac.at
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.