Dear Editors, Cytomegalovirus (CMV) infection remains a major cause of morbidity and mortality following lung transplantation (LTx), with lung recipients facing particularly high risk due to substantial lung-associated lymphoid tissue harbouring latent CMV [1]. Beyond direct effects, CMV infection increases risks for acute rejection, chronic allograft dysfunction, and opportunistic infections. While international guidelines provide recommendations for CMV management [2–4], real-world adherence in LTx centres remains poorly characterized, particularly given that they represented only 15% of transplant centres in recent broader surveys despite bearing the highest CMV burden [5].
We conducted a cross-sectional survey of 10 French-speaking LTx centres [9 out of 11 French centres (82%) and 1 out of 4 Belgian centres (25%)] between September 2022 and February 2023, using a comprehensive questionnaire addressing CMV prevention, diagnosis, treatment, and resistance management. Fifteen physicians participated, with 13 of 15 (86%) reporting adherence to centre-specific protocols that varied between institutions. All physicians surveyed were pulmonologists and lung transplant specialists, who routinely manage LTx patients and CMV infection in this population. Details regarding our methodology, the questionnaire in itself, as well as the full responses, are available in our Supplementary Material.
Our findings revealed substantial heterogeneity in CMV management practices with significant deviations from established guidelines (Figure 1). Most strikingly, prophylaxis duration showed concerning variability: in seropositive recipients (R+), 5 of 15 respondents (33%) used only 3 months of prophylaxis despite guidelines recommending 6–12 months [3, 4], while 9 of 15 (60%) used 6 months and 1 of 15 (7%) used 12 months. For high-risk donor-positive/recipient-negative (D+/R-) patients, 11 of 15 (73%) appropriately used 12-month prophylaxis, though 4 of 15 (27%) used shorter durations. In R+ patients with short telomere syndrome, which is associated with impaired CMV immunity and increased treatment toxicity [6], 10 of 13 respondents (84%) used standard valganciclovir prophylaxis, with 2 of 13 (16%) employing alternative approaches such as anti-CMV immunoglobulins or valaciclovir.
FIGURE 1
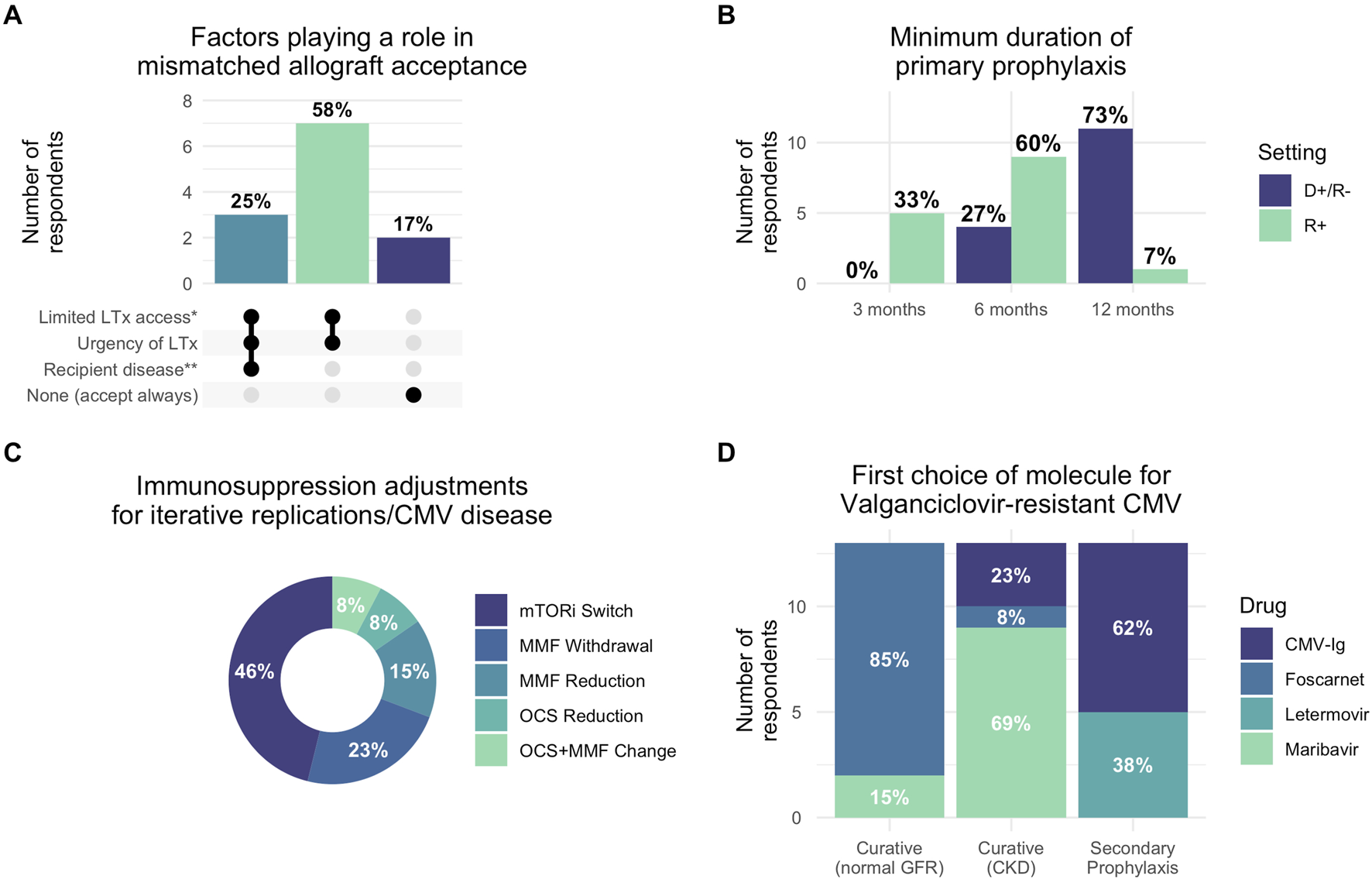
Reported clinical practices for CMV management in lung transplantation. (A) Factors influencing acceptance of CMV-mismatched allografts based on responses. Dark dots indicate factors considered by each group. (B) Minimum duration of primary CMV prophylaxis by donor/recipient serostatus (D+/R- vs. R+). (C) Immunosuppression adjustment strategies preferred for recurrent CMV replication or disease. (D) First-choice antiviral therapies for valganciclovir-resistant CMV across different treatment contexts (curative treatment for patients with normal glomerular filtration rate or patients with chronic kidney disease, and secondary prophylaxis). Percentages indicate proportion of responses selecting each factor. *Limited LTx access: recipient factors anticipated to limit access to compatible allografts, such as hyperimmunization, rare ABO group or extreme height, favored mismatched allograft acceptance; **Recipient disease: respondents cited mainly short-telomere syndrome-associated pulmonary fibrosis or systemic sclerosis as situations precluding mismatched allograft acceptance. Abbreviations: D+/R-: donor-positive recipient-negative serostatus; R+: recipient-positive serostatus; CMV: cytomegalovirus; LTx: lung transplantation; CKD: chronic kidney disease; GFR: glomerular filtration rate mTORi: mTOR inhibitor; MMF: mycophenolate mofetil; OCS: oral corticosteroids; CMV-Ig: CMV-specific hyperimmune globulin.
Secondary prophylaxis practices diverged markedly from 2018 guidelines that recommended against routine use [3]. After CMV reactivation, 5 of 14 respondents (36%) systematically initiated secondary prophylaxis with an additional 2 of 14 (14%) using it conditionally. Following CMV disease, these proportions increased to 8 of 14 (57%) and 3 of 14 (21%), respectively. All respondents maintained secondary prophylaxis for 3 months. For patients with iterative replications, 11 of 14 (79%) used long-term prophylaxis with durations varying from 3 to 12 months. This widespread adoption likely reflects the clinical reality that LTx recipients experience higher CMV recurrence rates compared to other solid organ transplant recipients.
Post-prophylaxis monitoring also showed substantial variation, with 6 of 15 respondents (40%) performing monthly monitoring in R+ patients, while in D+/R- patients, 5 of 15 (33%) performed monthly monitoring and 4 of 15 (27%) performed weekly monitoring. This heterogeneity emerged despite 2018 guidelines not supporting surveillance after prophylaxis, though updated 2025 guidelines now suggest monitoring in high-risk patients [4]. CMV-specific cellular immune response testing was used by only 4 of 13 respondents (31%), reflecting limited adoption of these newer diagnostic tools despite their potential for personalized management.
Immunosuppression modification was considered by 5 of 13 respondents (38%) for CMV disease and 12 of 13 (92%) for recurrent infections, most commonly involving mTOR inhibitor introduction or antimetabolite reduction. For hematologic toxicity, 10 of 14 (71%) appropriately used hematologic support, though 2 of 14 (14%) modified immunosuppression and 1 of 14 (7%) reduced valganciclovir doses as first-line interventions, potentially increasing resistance risk [7].
Resistant CMV management revealed evolving practices influenced by new therapeutic options, highlighting both opportunities and challenges in this complex clinical scenario. For patients with normal renal function, 11 of 13 (85%) preferred foscarnet over maribavir (2 of 13, 15%), while in renal impairment, maribavir was preferred by 9 of 13 (69%). Anti-CMV immunoglobulins were used by 8 of 12 respondents (67%) for secondary prophylaxis in resistant cases, with letermovir usage varying widely (8 of 13 (61%) never used it, while others employed it in specific scenarios).
The availability of maribavir through compassionate use programs during our survey period and its subsequent broader approval likely influenced these preferences [8]. Nearly all respondents would test for ganciclovir resistance in case of reactivation despite preventive treatment (11 of 13, 85%) or failure of curative treatment (12 of 13, 93%). These findings underscore the challenges clinicians face when managing resistant CMV, particularly the need to balance efficacy against drug-specific toxicity profiles in an already immunocompromised population with limited access to resistance testing.
The widespread practice variation we observed is particularly significant given that participating centres employ similar immunosuppression protocols and serve comparable populations. Our sample comprised nearly all French LTx centres, suggesting these findings reflect national practice patterns. Similar variability has been reported in Italian programmes [9] and broader European surveys [10], indicating these challenges transcend national boundaries.
The clinical implications are concerning. Santos et al. demonstrated that delayed-onset CMV disease following prophylaxis discontinuation occurs in up to 14% of LTx recipients with associated mortality risk [2]. Our finding that one-third of respondents use only 3-month prophylaxis in R+ patients may have significant clinical consequences, particularly when considering that breakthrough infections may increase resistance risk, impacting long-term allograft survival. Encouragingly, many practice variations we documented have been partially addressed in updated 2025 guidelines [4], which incorporate more aggressive secondary prevention strategies and suggest post-prophylaxis monitoring in high-risk patients, reflecting growing recognition of LTx-specific challenges.
While our study has limitations, including modest sample size and focus on French-speaking centres, our comprehensive coverage of French centres provides valuable insights into an underrepresented but high-risk population. The documented practice heterogeneity, particularly deviations from evidence-based recommendations, highlights critical gaps in CMV management standardization. The fact that 86% of respondents follow centre-specific protocols suggests local guidelines themselves diverge from international recommendations. The higher CMV burden in LTx recipients compared to other solid organ transplant populations necessitates specialized management approaches addressing unique challenges including optimal prophylaxis duration and management of patients with conditions like short telomere syndrome. These findings underscore the need for enhanced education, practice standardization initiatives, and generation of LTx-specific evidence to support future guideline development.
In conclusion, this survey reveals significant heterogeneity in CMV management among French-speaking LTx centres, with notable deviations from international guidelines. Given CMV’s substantial impact on LTx outcomes, addressing these variations through enhanced education, standardized protocols, and LTx-specific evidence generation should be a priority for the transplant community.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
Conceptualization: TG and VB; Data curation: TG; Formal analysis: TG and KH; Investigation: TG, KH, AR, MB, GD, BR-P, CM, LF, BC, TV, XD, AT, DM, FC, SA, JM, VB; Methodology: TG, KH, and VB; Project administration: TG and VB; Resources: AR, MB, GD, BR-P, CM, LF, BC, TV, XD, AT, DM, FC, SA, and JM; Supervision: VB and JM; Validation: All authors; Visualization: KH; Writing – original draft: TG and KH; Writing – review and editing. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2025.15224/full#supplementary-material
References
1.
Zamora MR . Cytomegalovirus and Lung Transplantation. Am J Transplant (2004) 4(8):1219–26. 10.1111/j.1600-6143.2004.00505.x
2.
Santos CAQ Brennan DC Yusen RD Olsen MA . Incidence, Risk Factors and Outcomes of Delayed-Onset Cytomegalovirus Disease in a Large Retrospective Cohort of Lung Transplant Recipients. Transplantation (2015) 99(8):1658–66. 10.1097/TP.0000000000000549
3.
Kotton CN Kumar D Caliendo AM Huprikar S Chou S Danziger-Isakov L et al The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation. Transplantation (2018) 102(6):900–31. 10.1097/TP.0000000000002191
4.
Kotton CN Kumar D Manuel O Chou S Hayden RT Danziger-Isakov L et al The Fourth International Consensus Guidelines on the Management of Cytomegalovirus in Solid Organ Transplantation. Transplantation (2025) 109:1066–110. 10.1097/TP.0000000000005374
5.
Grossi PA Kamar N Saliba F Baldanti F Aguado JM Gottlieb J et al Cytomegalovirus Management in Solid Organ Transplant Recipients: A Pre-COVID-19 Survey From the Working Group of the European Society for Organ Transplantation. Transpl Int (2022) 35:10332. 10.3389/ti.2022.10332
6.
Popescu I Mannem H Winters SA Hoji A Silveira F McNally E et al Impaired Cytomegalovirus Immunity in Idiopathic Pulmonary Fibrosis Lung Transplant Recipients with Short Telomeres. Am J Respir Crit Care Med (2019) 199(3):362–76. 10.1164/rccm.201805-0825OC
7.
Stevens DR Sawinski D Blumberg E Galanakis N Bloom RD Trofe-Clark J . Increased Risk of Breakthrough Infection Among Cytomegalovirus Donor-Positive/Recipient-Negative Kidney Transplant Recipients Receiving Lower-Dose Valganciclovir Prophylaxis. Transpl Infect Dis (2015) 17(2):163–73. 10.1111/tid.12349
8.
Avery RK Alain S Alexander BD Blumberg EA Chemaly RF Cordonnier C et al Maribavir for Refractory Cytomegalovirus Infections with or Without Resistance Post-Transplant: Results from a Phase 3 Randomized Clinical Trial. Clin Infect Dis (2022) 75(4):690–701. 10.1093/cid/ciab988
9.
Lombardi A Grossi P Mikulska M Giannella M Pascale R Marinello S et al Infections Management in the Lung Transplant Setting in Italy: A Web‐Survey. Transpl Infect Dis. Published Online (2025) 27:e14413. 10.1111/tid.14413
10.
Navarro D San-Juan R Manuel O Giménez E Fernández-Ruiz M Hirsch HH et al Cytomegalovirus Infection Management in Solid Organ Transplant Recipients Across European Centers in the Time of Molecular Diagnostics: An ESGICH Survey. Transpl Infect Dis (2017) 19(6):e12773. 10.1111/tid.12773
Summary
Keywords
cytomegalovirus, lung transplant, questionnaire, practices, guidelines
Citation
Goletto T, El Husseini K, Roux A, Briard M, Dauriat G, Renaud-Picard B, Merveilleux du Vignaux C, Falque L, Coiffard B, Villeneuve T, Demant X, Tissot A, Mouren D, Carlier FM, Alain S, Messika J and Bunel V (2025) Managing Cytomegalovirus Infection in Lung Transplant Recipients in Real Life: Results of a French Multicenter Survey. Transpl. Int. 38:15224. doi: 10.3389/ti.2025.15224
Received
04 July 2025
Accepted
20 October 2025
Published
29 October 2025
Volume
38 - 2025
Updates
Copyright
© 2025 Goletto, El Husseini, Roux, Briard, Dauriat, Renaud-Picard, Merveilleux du Vignaux, Falque, Coiffard, Villeneuve, Demant, Tissot, Mouren, Carlier, Alain, Messika and Bunel.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kinan El Husseini, kinan.elhusseini@aphp.fr
†These authors share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.