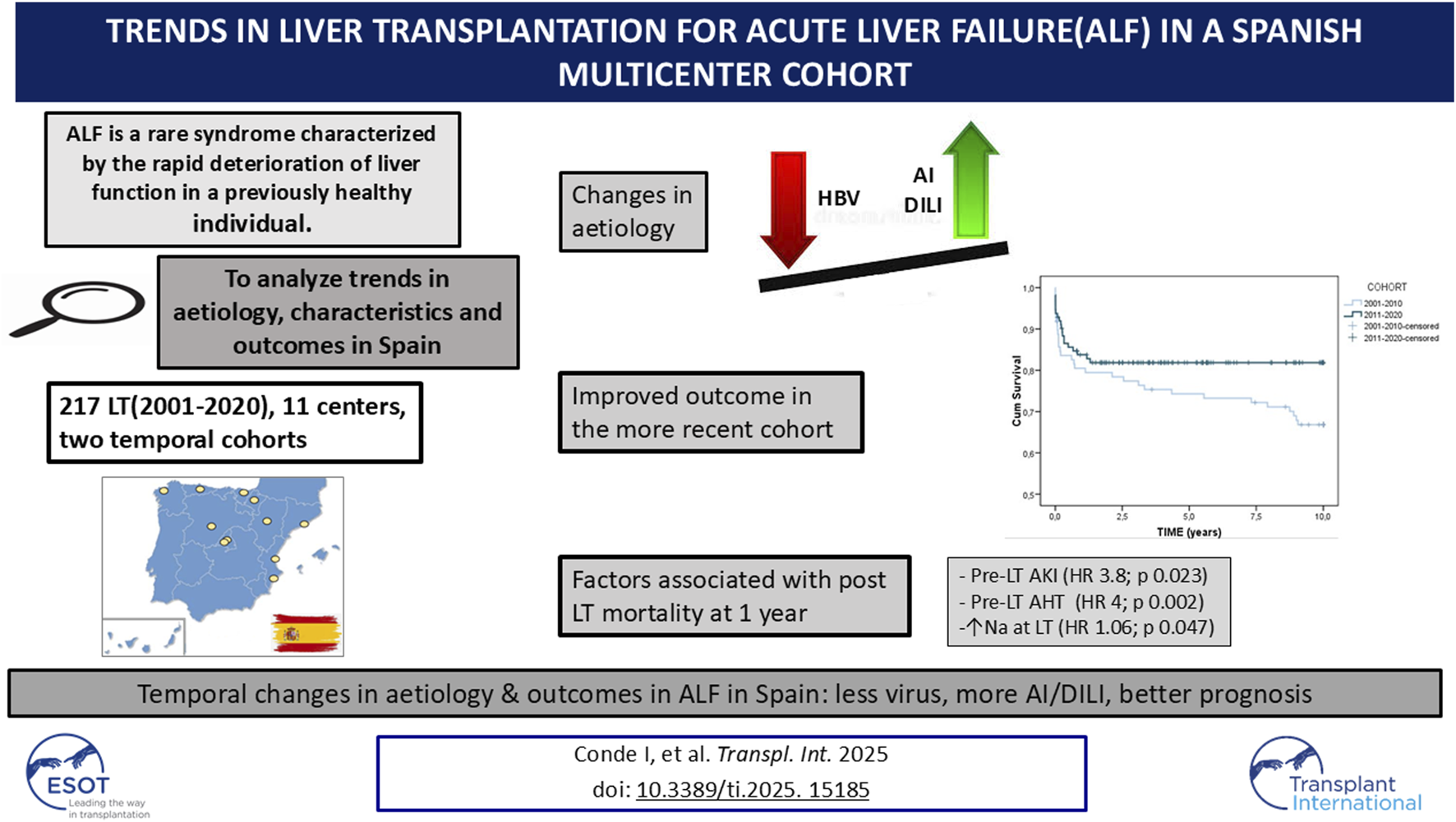
Abstract
Background and Aims:
Acute liver failure (ALF) is a rare and severe condition with high mortality. Liver transplantation (LT) has improved patient outcomes. This study analysed trends in aetiology, characteristics, and outcomes of ALF patients undergoing LT in Spain.
Methods:
We retrospectively reviewed 217 adult ALF-LT cases from 11 Spanish centers (2001 -2020), divided into two 10-year periods. Clinical, biochemical, and outcome data were collected, and predictors of mortality were identified.
Results:
217 adult ALF-LT patients were included (61.8% women, mean age: 41 years). Common aetiologies were cryptogenic (26.7%), autoimmune (26.3%), and viral (18%), with sex differences. Over time, autoimmune and drug-induced liver injury increased (22.3% vs 29.8% and 13.6% vs 21.1%), with a low prevalence of acetaminophen toxicity, and hepatitis B virus declined (23.3% vs 11.4%). Despite higher infection rates (52.5% vs 66.2%) linked to stronger immunosuppression, respiratory failure (29.1% vs 16.1%), chronic kidney disease (27.1% vs 13.6%), cardiovascular events (10.6% vs 1%), and mortality (37.6% vs 17.9%) decreased. Pre-LT hypertension, pre-LT acute kidney injury, and hypernatremia at LT were independently associated with worse survival. This large multicenter study revealed temporal changes in aetiologies, immunosuppressive treatment, and post-LT complications, with an improvement in outcome.
Graphical Abstract
Introduction
Acute liver failure (ALF) is a rare syndrome characterized by the rapid deterioration of liver function in a previously healthy individual. Although its prevalence is low, the exact incidence remains poorly defined. A retrospective Spanish study estimated an incidence of 1.4 cases per million population [1], while an American study reported 5.5 cases per million population [2], both published in 2007. Despite its infrequency, ALF is associated with significant morbidity and mortality, accounting for 6% of deaths related to liver disease [3].
ALF is highly heterogeneous in terms of aetiology, clinical presentation, and progression. These variations underscore the knowledge gaps in the field and the lack of large, high-quality studies. The natural history of ALF is also variable. In 10%–20% of patients, the condition is reversible, and liver regeneration occurs, leading to full recovery. However, in the remaining patients, complications such as cerebral oedema, renal failure, sepsis, and multiorgan failure are common, resulting in high mortality. The introduction of liver transplantation (LT) has significantly improved prognosis, with one-year survival rates approaching 90% in recent studies [4]. According to data from the Spanish National Transplant Organization (ONT), between 1984 and 2022, ALF accounted for 4.9% of LT indications, rising to 22% among individuals aged 16–39 years [5].
The aetiology of ALF varies depending on geographical location and age at presentation. Causes include viral hepatitis, drug overdose, idiosyncratic drug reactions, toxic ingestion, autoimmune diseases, and metabolic disorders [6, 7]. Descriptive studies have shown that in the United Kingdom and the United States, acetaminophen overdose is the most common cause [8, 9], while in highly endemic regions like India, acute hepatitis E is the leading cause [10]. In Germany, hepatotoxicity unrelated to acetaminophen was the most frequent aetiology [11]. In Spain, the most common cause of ALF was acute hepatitis due to hepatitis B virus (HBV), followed by drug and toxic substance ingestion. In more than 30% of cases, the cause of ALF could not be determined [1, 12]. Additionally, the incidence of drug-induced liver injury (DILI) has risen globally, likely due to the introduction of new pharmaceuticals, increased life expectancy, polypharmacy, and the widespread use of herbal products. DILI has become the leading cause of fulminant hepatic failure in both the United States and Europe [8, 13].
Sex-based differences have been observed in the aetiology of liver disease leading to LT, with several studies documenting sex inequities in access to LT [14, 15]. Specifically, women are at higher risk of developing DILI, and they tend to experience more severe outcomes and increased susceptibility to hepatotoxicity-related ALF [16, 17]. A recent article showed sex disparities in waitlisting and LT for ALF [18].
There are few studies on ALF in Spain, and none have been conducted in the past decade. Previous studies include a multicentre retrospective analysis of cases from 1992 to 2000 and a unicentric prospective study covering 2000–2010 [1, 12]. Despite Spain having one of the highest rates of LT per capita, the outcomes of LT in ALF patients have not been specifically analyzed.
This project aims to fill this gap by evaluating the recent indications, management, and outcomes of LT in ALF patients in Spain. Data were gathered from 11 large, renowned LT centers, that performed a total of over 400 LTs annually as of 2020, according to the Spanish Registry of Liver Transplantation (RETH). [5]. Our primary objectives were to describe: (i) the evolution of indications for LT in ALF, (ii) the changes over time in ALF-LT outcome, and (iii) the predictors of early post-LT mortality at 1 year. Our secondary objective was to highlight sex-based differences in ALF-LT.
Materials and Methods
Study Design
We conducted a retrospective Spanish multicenter study involving 11 centers with extensive experience in LT. These centers accounted for 43% of the total number of LTs performed in Spain in 2020 [5].
Urgent LT performed in patients >18 years due to ALF between 2001–2020 were included. Criteria for ALF were a severe acute liver injury lasting less than 26 weeks, with jaundice, liver synthetic failure (INR ≥1.5 or prothrombin rate <40%), and hepatic encephalopathy (HE) in a patient without known chronic liver disease.
Exclusion criteria comprised patients under 18 years old and patients with pre-existing liver disease. The acute manifestation of certain chronic liver diseases (Wilson’s disease, HBV reactivation in a non-cirrhotic liver, acute Budd-Chiari, and autoimmune hepatitis) was included as an exception. Patients with prior LT and acute liver injury due to primary graft nonfunction or other causes were excluded.
Data were acquired from each LT center through a review of medical records.
Indications of LT in ALF and Legal Situation in Spain
In Spain, when a patient experiences ALF, the criteria to indicate an urgent LT are based on either fulfilling King`s College Criteria (KCC) [19], Clichy criteria [20] or presence of HE. A national urgent code is activated, enabling the allocation of the first suitable organ available within the country to the ALF patient. The median time until a liver is offered is approximately 40 h, and around 50% of patients receive a LT within 24 h [21].
Ethical Statement
This study was approved by the Ethics Committee of Clinical Research of La Fe Universitari and Politécnic Hospital (ref number: 2021-096-1) and was conducted according to the standards of Good Clinical Practice, adhering to the ethical principles outlined in the 1975 Declaration of Helsinki.
An exemption from the requirement for informed consent was granted due to the retrospective nature of the study. Some patients had been relocated or were no longer reachable during the study period.
To ensure confidentiality, patient information included in the database was anonymized and identified by a numerical code, in compliance with data protection legislation.
Collected Variables
The recorded variables included donor and recipient demographic features, epidemiological information, clinical and biochemical data before and after LT, clinical post-LT outcomes, patient and graft survival and variables associated with mortality.
Recipient variables (demographics, co-morbidities and toxics abuse)
Variables pre-LT associated with ALF: aetiology, type of presentation, clinical data, hepatic and extra-hepatic complications, KCC and Clichy criteria, management (antibiotic prophylaxis, N-Acetylcysteine (NAC) and Molecular Adsorbent Recirculating System (MARS)), days of admission until LT and on the waiting list (WL)
Biochemical tests before LT (at admission, on days 3, 7, and the day of LT).
Donor and surgical related-variables
Histology of the explanted liver (massive or sub-massive necrosis)
Early post-LT follow-up (1st–3rd month): days in the ICU and total hospitalization days, hepatic and extra-hepatic complications.
Late post-LT follow-up: long term hepatic and extra-hepatic complications.
Immunosuppression
Outcome: re-LT and/or death, and causes.
Operational Definitions
The diagnosis of cryptogenic ALF was reached after excluding any other aetiology through an exhaustive pre-LT differential diagnosis and the explant biopsy. Patients who received a LT in the context of an AI hepatitis fulfilled the criteria for ALF. No evidence of liver cirrhosis was found in the explants. The temporal classification of ALF (hyper acute, acute and sub-acute) was defined according to the interval between the onset of jaundice and the development of hepatic encephalopathy (published by O’Grady JG in 1993) [22].
Regarding pre- and post-LT complications, acute kidney injury (AKI) and chronic kidney disease (CKD) were established following KDIGO criteria [23, 24]. Renal replacement therapy (RRT) included both intermittent haemodialysis and continuous RRT. Infections were confirmed with positive culture or resolution after antibiotic treatment. Respiratory failure was defined as the necessity for mechanical ventilation, rather than in the context of HE. Early graft dysfunction was based on the definition proposed by Olthoff et al. [25], and acute liver allograft rejection was categorized following the Banff classification [26]. Finally, graft steatosis was assessed by biopsy.
Statistical Analysis
A descriptive analysis was conducted for all the studied variables. Continuous variables are described as means or medians with standard deviation (SD) or quartiles 1 (Q1) and 3 (Q3) as appropriate, and qualitative variables as absolute and relative frequencies.
The normal distribution of outcome variables was confirmed using the Kolmogorov-Smirnov test. Chi-square and Fisher’s exact test were used to assess the degree of association between categorical variables, Student’s t and ANOVA model to compare quantitative variables, and non-parametric Mann-Whitney and Kruskal-Wallis tests to analyse the distribution of at least ordinal variables in 2 or more independent groups.
Graft and patient survival analyses were performed with Kaplan-Meier survival curves.
Variables associated with mortality and re-transplantation were determined using univariate and multivariate Cox regression tests and expressed by hazard ratio (HR) and 95% confidence interval (CI). The initial multivariate model included the variables with a p value < 0.10 in the univariate analysis. Variables with a p value above this threshold could be included if considered clinically relevant by the investigators.
A p-value of <0.05 was considered significant for all analyses.
Data analysis was performed using SPSS version 22.0 (IBM, Chicago, USA).
Results
Baseline Features and Management Before LT
A total of 217 adult patients received urgent LT due to ALF between January 2001 and December 2020. Among them, 134 were women (61.8%). The overall median age was 41 years old (IQR 32–53). Baseline clinical variables and pre-LT management are shown in Table 1, and analytical data on the LT Day in Supplementary Table 1.
TABLE 1
| Variable | N | |
|---|---|---|
| Age (years) | 217 | 41 (32–53) |
| Sex (women) | 217 | 134 (61.8) |
| Race | 217 | |
| Caucasian | 181 (83.4) | |
| Other | 36 (16.6) | |
| BMI (kg/m2) | 152 | 25 (21.3–27) |
| AHT | 216 | 25 (11.6) |
| Diabetes | 216 | 8 (3.7) |
| Dyslipidaemia | 216 | 18 (8.3) |
| Aetiology | 217 | |
| HBV | 37 (17.1) | |
| Other viruses | 9 (5.1) | |
| AI | 57 (26.3) | |
| DILI | 38 (17.1) | |
| Acetaminophen | 9 (4.1) | |
| Cryptogenic | 58 (26.7) | |
| Other | 17 (7.9) | |
| Clinical presentation | 217 | |
| Hyperacute | 68 (31.3) | |
| Acute | 88 (40.6) | |
| Subacute | 59 (27.2) | |
| Encephalopathy | 212 | |
| I-II | 62 (29.2) | |
| III-IV | 150 (70.8) | |
| Ascites | 208 | 89 (42.8) |
| Respiratory failure (MV) | 212 | 51 (24.1) |
| Infection | 215 | 30 (14) |
| GI haemorrhage | 216 | 12 (5.6) |
| AKI | 213 | 83 (39) |
| RRT | 213 | 47 (22.1) |
| Antibiotic prophylaxis | 187 | 137 (73.3) |
| NAC | 215 | 39 (18.1) |
| MARS | 216 | 11 (5.1) |
| Time on waiting list (Days) | 208 | 1 (1–2) |
| Meet KCC criteria | 205 | 188 (91.7) |
| Meet clichy criteria | 59 | 33 (55.9) |
| MELD - LT day | 142 | 25 (19–29) |
Clinical characteristics pre-LT.
Data are given as median (IQR) or number (percentage).
Abbreviations: BMI, Body mass index; AHT, Arterial hypertension; HBV, Hepatitis B virus; AI, Autoimmune; DILI, Drug Induced Liver Injury; MV, Mechanical ventilation; GI, Gastrointestinal; AKI, Acute Kidney Injury; RRT, Renal Replacement Therapy; NAC, N-Acetylcysteine; MARS, Molecular Adsorbent Recirculating System; KCC, Kings College Criteria; MELD, Model for End-Stage Liver Disease; LT, Liver Transplant.
A small number of patients had concomitant diseases or toxic habits. The prevalence of arterial hypertension (AHT), diabetes and dyslipidaemia were 11.6%, 3.7% and 8.3%, respectively. Regarding toxic substances, the smoking rate was 27.8%, 15% consumed alcohol regularly and 7.5% were drug users. A concomitant autoimmune non-liver disease was present in 14.4%, and 12.1% reported a psychiatric disease.
The predominant aetiologies of ALF were cryptogenic (26.7%) and autoimmune (26.3%). Viral aetiologies accounted for less than 25%, with hepatitis B (HBV) being the most common (17.1%). Drug-induced liver injury (DILI) represented 17% of LT indications. Only 4.1% of patients who underwent LT due to DILI-ALF did so in the context of acetaminophen intake.
In terms of temporality, most cases were acute (40.6%) and hyperacute (31.3%). The most frequent complications were ascites (42.8%) and AKI (39%), while infections and haemorrhagic complications were uncommon. RRT was used in 22.1%. The median MELD (Model for end-stage Liver Disease) score on the day of LT was 25.
Before LT, antibiotic prophylaxis was widely implemented (73.3%). The use of NAC and especially MARS had little relevance in our cohort of patients.
All the patients were transplanted with a national urgent priority, resulting in a median time on the WL of only 1 day (IQR: 1–2 days).
Compliance with the KCC and Clichy criteria was 91.7% and 55.9%, respectively. Of note, only a limited number of patients (n = 59) had Factor V determination performed, especially during the early years.
Evolution of Indications of LT in ALF
The cohort was subdivided into two 10-year periods (2001–2010 and 2011–2020). The number of ALF-LT remained stable overtime: 113 patients (3.6%) in 2001–2010 and 114 patients (3.1%) in 2011–2020.
Cryptogenic and autoimmune were the most common aetiologies of ALF-LT overall. Autoimmune (22.3% vs. 29.8%) and DILI (13.6% vs. 21.1%) aetiologies increased with time while HBV showed a decline (23.3% vs. 11.4%), although without reaching statistical significance (p 0.115). Despite the increase in DILI, Acetaminophen toxicity was not particularly prevalent and even decreased with time (8.7% and 3.5%). Cryptogenic ALF remained stable. Other viruses, such as HAV (1% and 1.8%) or HEV (1% and 0.9%) were extremely uncommon in both periods (Figure 1).
FIGURE 1
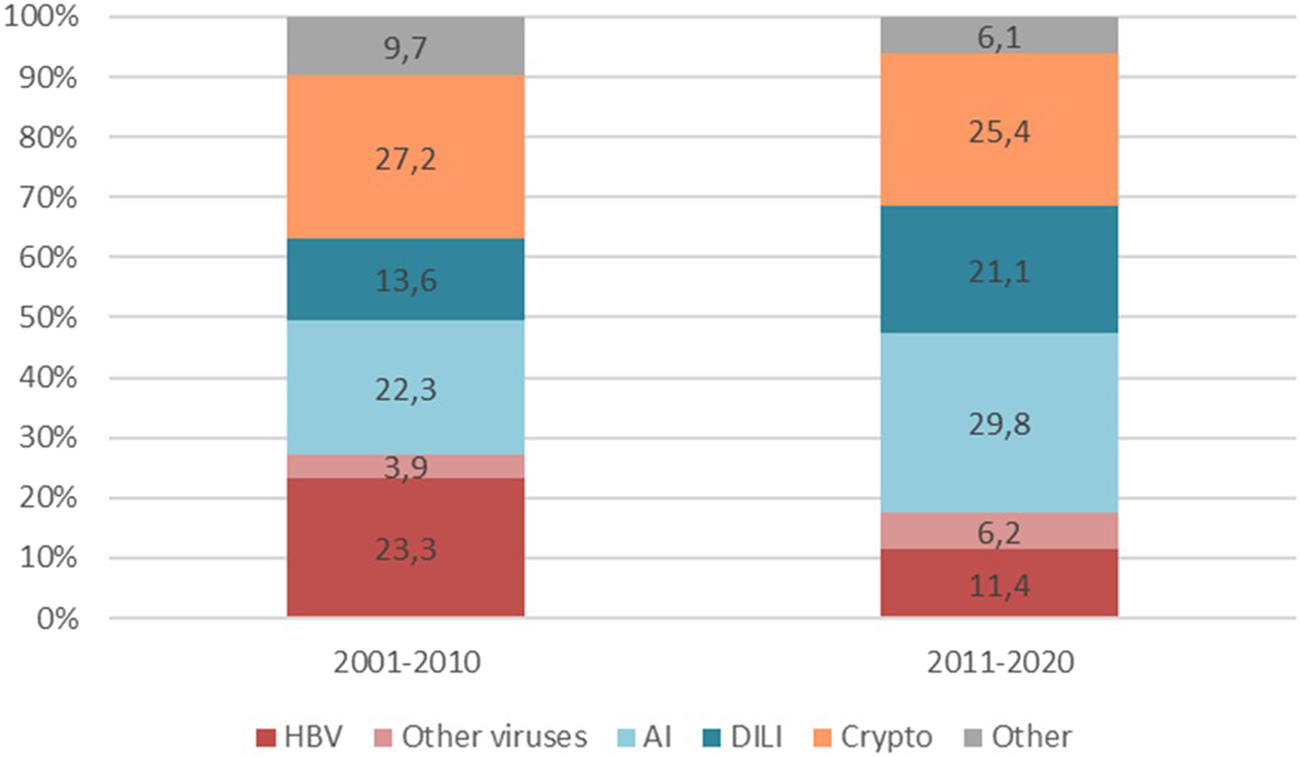
Evolution of ALF aetiologies in LT candidates Differences in ALF aetiologies between the two time periods 2001–2010 and 2011–2020 (p-value 0.115). Abbreviations: HBV, Hepatitis B virus; AI, Autoimmune; DILI, Drug Induced Liver Injury.
Changes Over Time in ALF-LT Characteristics and Outcomes
We first conducted an analysis of LT characteristics and post-LT evolution of the whole cohort (Supplementary Table 2). Most grafts were total (98.6%), with AB0 compatibility (isogroup 62.5% and compatible 37%), and showed minimal steatosis (<10% in 91.3% of grafts) and most donors were brain dead. In the early post-LT period, the main complications were infections (60.7%) and AKI (61%), while in the late post-LT period, AHT (30.3%), biliary complications (27.4%) and CKD (19.7%) predominated. The mortality rate was 27.2%, with infections (41.5%) and liver-related complications (20.8%) being the leading causes of death. The survival rates at 1, 5, and 10 years were 82%, 78% and 72%, respectively (Supplementary Figure 1).
Regarding differences over time in pre-LT characteristics and management (Table 2), there was a trend towards an increase of women (55.3% vs. 67.3%) approaching statistical significance (p 0.065) and a decline in the rate of Caucasian race (90.3% vs. 77.2%; p 0.001). Alcohol consumption was reported less frequently in recent years (21.6% vs. 8.9%; p 0.01). Antibiotic prophylaxis and, notably, the use of NAC significantly increased (65.4% vs. 78.9%; p 0.04% and 4% vs. 30.1%; p < 0.001).
TABLE 2
| Variable | 2001–2010 | 2011–2020 | p-value | |||
|---|---|---|---|---|---|---|
| n = 103 | n = 114 | |||||
| n | n | |||||
| Clinical characteristics and management pre-LT | ||||||
| Sex (women) | 103 | 57 (55.3) | 114 | 77 (67.5) | 0.065 | |
| Race | 103 | 114 | 0.001 | |||
| Caucasian | 93 (90.3) | 68 (77.2) | ||||
| Other | 10 (9.7) | 26 (22.8) | ||||
| Alcohol | 102 | 22 (21.6) | 112 | 10 (8.9) | 0.010 | |
| Aetiology | 103 | 114 | 0.115 | |||
| HBV | 24 (23.3) | 13 (11.4) | ||||
| Other viruses | 4 (3.9) | 8 (6.2) | ||||
| AI | 23 (22.3) | 34 (29.8) | ||||
| DILI | 14 (13.6) | 24 (21.1) | ||||
| Acetaminophen | 5 (8.7) | 4 (3.5) | ||||
| Cryptogenic | 28 (27.2) | 29 (25.4) | ||||
| Other | 10 (9.7) | 7 (6.1) | ||||
| Antibiotic prophylaxis | 78 | 51 (65.4) | 109 | 86 (78.9) | 0.040 | |
| NAC | 102 | 5 (4.0) | 113 | 34 (30.1) | <0.001 | |
| MARS | 102 | 8 (7.8) | 114 | 3 (2.6) | 0.082 | |
| Donor | ||||||
| Steatosis | 82 | 80 | 0.037 | |||
| <10% | 78 (95.1) | 70 (87.5) | ||||
| 10%–30% | 2 (2.4) | 9 (11.3) | ||||
| >30% | 2 (2.4) | 1 (1.3) | ||||
| Immunosuppression | ||||||
| Induction IS | 97 | 110 | <0.001 | |||
| Triple IS | 60 (61.9) | 87 (79.1) | ||||
| Double IS | 33 (34) | 12 (10.9) | ||||
| Other | 4 (4.1) | 11 (10) | ||||
| Basiliximab | 82 | 28 (34.1) | 107 | 61 (67) | 0.002 | |
| Early post-LT complications | ||||||
| Resuscitation unit (days) | 99 | 6 (4–11) | 113 | 5 (3–9) | 0.077 | |
| Infection | 101 | 53 (52.5) | 110 | 75 (68.2) | 0.020 | |
| Respiratory insufficiency | 103 | 30 (29.1) | 112 | 18 (16.1) | 0.022 | |
| Late post-LT complications | ||||||
| CKD | 85 | 23 (27.1) | 103 | 14 (13.6) | 0.021 | |
| CV event | 85 | 9 (10.6) | 103 | 1 (1) | 0.003 | |
| Death | 101 | 38 (37.6) | 112 | 20 (17.9) | 0.001 | |
| Death 1yr | 19 (19) | 18 (16.1) | 0.575 | |||
Differences over time in ALF-LT.
Data are given as median (IQR) or number (percentage). The bold values indicate variables that are statistically significant (p < 0.05).
Abbreviations: HBV, Hepatitis B virus; AI, Autoimmune; DILI, Drug Induced Liver Injury; NAC, N-Acetylcysteine; MARS, Molecular Adsorbent Recirculating System; IS, Immunosuppression; CKD, Chronic Kidney Disease; CV, Cardiovascular.
We also examined the changes in post-transplant management and outcome between the first and second decade (Table 2). There was a higher use of triple immunosuppression (61.9%–79.1%; p < 0.001) and basiliximab (34.1%–67%; p 0.002) in the recent cohort. Some differences were found in post-LT complications. Infection rates increased overtime (52.5% vs. 66.2%; p 0.02) while respiratory insufficiency decreased (29.1% vs. 16.1%; p 0.022). In the long-term, there was a reduction in CKD (27.1% vs. 13.6%; p 0.022), cardiovascular events (10.6% vs. 1%; p 0.003) and mortality (37.6% vs. 17.9%; p 0.001) in recent years. One year mortality improved with time, not reaching statistical significance (19% in the first cohort vs. 16.1% in the latter, p 0.575). Evolution of patient survival rates between the two time periods is shown in Figure 2. (80.5%, 74% and 67% vs 84%, 82% and 82% at 1, 5, and 10 years, respectively).
FIGURE 2
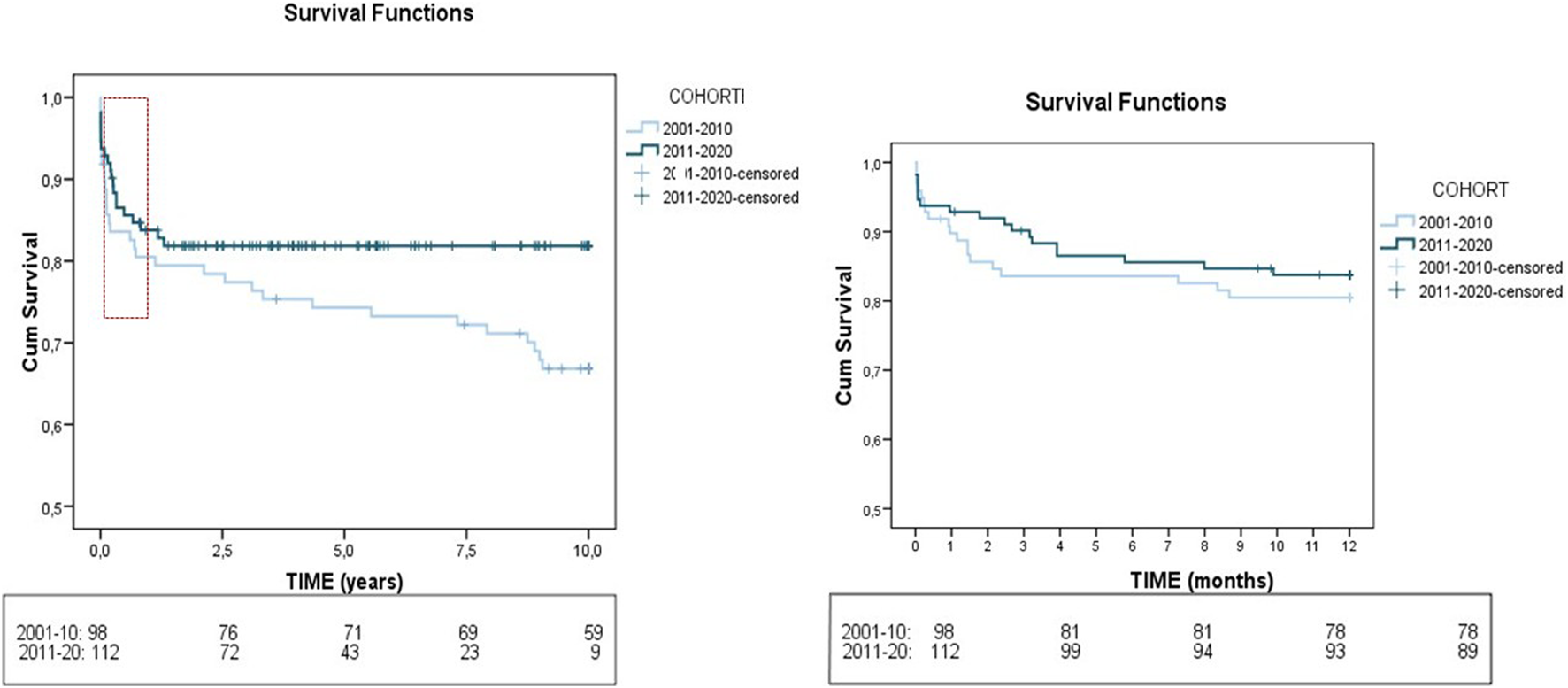
Evolution of patient survival rates between the two time periods 2001–2010 and 2011–2020. The Kaplan-Meier plot illustrates the differences in post-LT survival rates between the 103 ALF patients who underwent LT in 2001–2010 versus 114 patients who underwent LT in 2011–2020.
Given the observed increase in AI/DILI aetiologies, we implemented an analysis to determine whether there were differences in management and outcome when comparing AI/DILI ALF group to the rest of aetiologies (Supplementary Table 4). A total of 95 patients were transplanted in the context of AI or DILI ALF, and 122 patients had other ALF aetiologies. Subacute presentations were more prevalent in AI/DILI aetiologies (34.7% vs. 21.7%; p 0.044), with a different trend for hyperacute presentations. AKI was significantly less common AI/DILI subgroup (30.1% vs. 45.8%; p 0.024). In terms of post-LT outcome, differences were observed in the IS management (higher use of triple IS, p 0.034) and in early complications (lower requirement for RRT, p 0.015; higher incidence of infections, with a lower rate of bacterial infections, p 0.017; and a decrease in bleeding and CV complications, p 0.020 and p 0.021). A significant finding in late post-LT outcome was the lower rate of de novo tumours in AI/DILI aetiologies (3.6% vs. 10.6%, p 0.034), as well as lower mortality at 1-year post-LT (12% vs. 21.7%, approaching statistical significance: p 0.065).
Factors Associated With Post-LT Mortality
Given that most deaths occurred early post-LT, we determined variables independently associated with mortality at 1-year post-LT (Table 3).
TABLE 3
| Variable | N | Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | ||||
| Aetiology AI-DILI | 217 | 0.52 | 0.26 | 1.06 | 0.072 | ||||
| Obesity | 152 | 3.33 | 1.07 | 10.3 | 0.037 | 2.693 | 0.972 | 7.456 | 0.057 |
| AHT | 216 | 2.75 | 1.30 | 5.84 | 0.008 | 4.002 | 1.222 | 13.106 | 0.022 |
| Dyslipidaemia | 216 | 2.75 | 1.21 | 6.26 | 0.016 | ||||
| Acute presentation | 217 | 0.37 | 0.17 | 0.84 | 0.017 | ||||
| AKI pre-LT | 213 | 2.83 | 1.46 | 5.5 | 0.002 | 3.819 | 1.199 | 12.159 | 0.023 |
| Respiratory insuff. | 212 | 1.97 | 1.01 | 3.82 | 0.046 | ||||
| Infections | 215 | 2.41 | 1.16 | 4.97 | 0.018 | 3.120 | 0.872 | 11.162 | 0.080 |
| Vasopressors | 150 | 2.14 | 1.02 | 4.5 | 0.044 | ||||
| Na - LT day | 189 | 1.087 | 1.027 | 1.149 | 0.004 | 1.065 | 1.001 | 1.133 | 0.047 |
| Cr - LT day | 193 | 1.253 | 1.013 | 1.550 | 0.038 | ||||
| P - LT day | 76 | 1.307 | 1.044 | 1.636 | 0.019 | ||||
| Ammonium - LT day | 70 | 1.007 | 1.001 | 1.013 | 0.021 | ||||
| Lactate - LT day | 69 | 1.119 | 1.008 | 1.242 | 0.036 | ||||
| Factor V - LT day | 37 | 0.893 | 0.803 | 0.993 | 0.037 | ||||
Factors associated with post-LT mortality.
Univariate and multivariate analysis. Results of Cox multiple regression models, adjusted hazard ratio (HR), 95%CI and p-value. The bold values indicate variables that are statistically significant (p < 0.05).
Abbreviations: AI, Autoimmune; DILI, Drug Induced Liver Injury; AHT, Arterial Hypertension; LT, Liver Transplant; AKI, Acute Kidney Injury; Na, Sodium; Cr, Creatinine; P, Phosphorus.
Significant variables related to patient’s baseline characteristics, clinical presentation, pre-LT complications and laboratory data at LT predicted poor outcomes in the univariate logistic analysis. Obese patients were at a significantly higher risk of death than those with normal BMI (HR = 3.33; p = 0.037). AHT and dyslipidaemia significantly influenced survival time (HR = 2.75; p = 0.008 and HR = 2.75; p = 0.016). Acute presentation was related to lower mortality (HR = 0.37; p = 0.017). Among the complications detected prior to transplantation, AKI, respiratory insufficiency, infections and vasopressor use significantly worsened the prognosis (p < 0.05). Finally, an increase in creatinine, sodium, phosphorus, ammonium, and lactate levels and a decrease in Factor V were independently associated with death (p < 0.05). The remaining pre-LT variables were not statistically significant.
Multivariable logistic regression analysis was performed on selected baseline variables from the univariate analyses, including independent predictors with clinical relevance, that were previously identified as significant (p < 0.05) and that were available in a relevant number of patients. Obesity, AHT, AKI, infections and sodium level on LT-day were entered into the multivariable model, and AHT, AKI and sodium remained as independent risk factors (HR 4.002 p 0.022, HR 3.819 p 0.023 and HR 1.065 p 0.047, respectively).
Differences According to Sex in ALF-LT
There was a trend towards an increase of women over time (55.3% vs. 67.5%), although this difference was not statistically significant (p 0.065) (Table 2).
Autoimmune and cryptogenic aetiologies were more frequent in women (31% vs. 19% and 31% vs. 20%) while HBV was more common in men (29% vs. 10%) (p 0.007).
Before LT, men had a higher history of alcohol, tobacco and drug consumption (p < 0.05). AKI was more frequently observed in men (52% vs. 29%). Renal function, ALT levels, platelets count and MELD score pre-LT were worse in men (p < 0.05). No significant differences were found in other pre-LT characteristics.
Early post-LT complications such as AKI (73% vs. 53%) and haemorrhage (26% vs. 14%) were more frequent in men, while rejection was more common in women (11% vs. 22.5%) (p < 0.05). Later complications including AHT (36% vs. 27%), dyslipidaemia (25% vs. 11%), CKD (24% vs. 17%) and biliary complications (32% vs. 21%) were all more frequent in men but without reaching statistical significance. Causes of death, survival and re-LT were similar in both groups (Supplementary Table 3).
Discussion
This study includes a large multicenter cohort of patients, allowing for an accurate overview of the evolution and outcomes of LT for ALF in Spain from 2001 to 2020. The only prior Spanish multicenter study, published in 2007, evaluated ALF patients between 1992 and 2000 [1]. Our analysis covers a more recent period (2001–2020), and provides an update on the evolution of this condition in patients who eventually required LT. Additionally, there are two older European studies: a German multicentre study that included ALF patients diagnosed in 2008–2009 [11], and a second study that assessed patients included in the European Liver Transplant Registry (ELTR) database between 1988 and 2009 [27]. Although there are discrepancies in ALF epidemiology and management across Europe, our study may serve as a current benchmark for the region.
Our study highlights differences in the aetiology of ALF compared to other regions. The most frequent aetiologies in our cohort were cryptogenic, autoimmune, and viral, with a notable shift towards autoimmune and DILI aetiologies and a decreased relevance of HBV in recent years. While DILI is the most common cause in Anglo-Saxon countries, viral hepatitis remains significant in developing countries. Notably, DILI was less common in our study compared to Western countries, similar to previous Spanish data published by Escorsell in 2007 [1], but its frequency has increased over time. Another significant distinction is that acetaminophen toxicity was uncommon in our cohort and has even decreased in recent years, probably due to the implementation of NAC protocols and the fact that it is less accessible to the general population than in other countries. International cohort studies, such as one from the US, have reported similar trends, with an increase in autoimmune cases and a decrease in HBV and DILI over time [24, 28].
Some changes in the outcome of ALF-LT in recent years have been documented in our cohort. We have observed a decrease in certain short- and long-term post-LT complications: respiratory insufficiency, CKD, cardiovascular events and even mortality. The higher use of monoclonal antibodies in the induction IS facilitates the reduction of the CNI dose from the moment of transplantation, and possibly justifies the downward trend in CKD. Survival rates were consistent with data from other series reaching 82%, 78% and 72% at 1, 5 and 10 years after LT, respectively. Recent studies have reported lower mortality in recent years [1, 4, 25], with improved peri-transplant management in intensive care units being a key factor. In our study, we also detected a trend toward a decrease in 1-year post-LT mortality. However, mortality remains high in the early post-transplant period, especially during the first 3 months (13%). One notable finding is the increase in infections in the early post-LT period (although without impact on survival), which may be explained by the use of more potent immunosuppression regimes in recent years.
When compared to other aetiologies, distinct clinical characteristics were observed in the AI-DILI group. Notably, the subacute presentation was more frequent, likely associated with the early use of corticosteroids. Post-transplant, patients with AI-DILI were more frequently maintained on a triple IS regimen, and fungal and viral infections were more commonly observed. These findings may be related to pre-transplant immunosuppressive therapy, including corticosteroids, administered in an attempt to avoid LT. A significant finding was the lower rate of de novo tumours, despite the higher IS, and mortality in AI-DILI aetiologies. This may be related to the higher prevalence of women in this subgroup, the lower rate of toxic habits among them, and possibly the shorter follow up of this group of patients.
Several pre-transplant parameters were associated with 1-year mortality. The significant predictors of post-transplant survival in the univariate were baseline features such as obesity, AHT and dyslipidaemia, pre-transplant clinical complications (AKI, respiratory insufficiency, infections and vasopressors need), and laboratory variables (sodium, creatinine, phosphorus, ammonium, lactate and factor V). These variables are consistent with previously published prognostic factors linked to poor survival in ALF [29–31]. Serum sodium levels showed an inverse relationship with post-LT survival. Classically studies linked pretransplant hyponatremia with increased post-LT mortality, recent large-scale analyses have suggested that hypernatremia is associated with worse outcome in ALF [32]. Other variables reported in previous studies, such as recipient and donor age, ABO incompatibility and intracranial pressure (ICP) monitoring pre-LT [4, 27] did not reach statistical significance in our analysis. The use of high-quality donors (young, compatible, with minimal steatosis) may explain some of these results. We have no data on ICP; however, HE, and more specifically grade IV HE, was not statistically significant in the univariate analysis.
Regarding potential sex differences, we observed an increasing rate of women undergoing LT for ALF across years and a higher number of ALF due to autoimmune hepatitis and cryptogenic liver disease. The increasing prevalence of autoimmune diseases among women may explain this [33]. Men presented in worse clinical condition at the time of LT, leading to a higher rate of post-LT complications, except for rejection, which was more common in women. Long-term outcomes, however, were similar for both sexes, with no differences in mortality. This data is particularly noteworthy in contrast to previous series which showed sex differences in pre-LT disease course in favour of men [18, 34].
Some limitations of the study should be mentioned. The retrospective design of the study may have led to partial loss of information, especially in the early years. Inclusion of only 11 out of 26 national LT centers may potentially bias some of the results; yet we incorporated the larger centers with more expertise. Potential heterogeneity in ALF management and transplantation protocols across centers may result in biases in patient selection and therapeutic decisions. For example, antibiotics, NAC or MARS are not addressed in national protocols. In our centers, NAC was administered in all instances of acetaminophen-induced ALF. In recent years, it was also used in select cases of non-acetaminophen ALF during the early stages of HE, in accordance with published potential benefit in this clinical scenario [35]. Antibiotic prophylaxis was not universally implemented, but was consistently prescribed at the slightest suspicion of infection following clinical practice guidelines [36]. The use of MARS was minimal, probably due to the low availability of this technique in our country, the short waiting time, and the lack of evidence supporting its efficacy [37]. Finally, data from ALF patients who have not undergone LT are not available in the majority of centers. The lack of these patients may introduce a selection bias. We plan to perform a prospective study to assess this very relevant piece of information to understand the process of patient referral and LT selection.
In conclusion, with data based on 11 large reference LT centers, this study is a picture of LT for ALF in Spain and reflects the trends over time in the last 20 years. The study revealed temporal changes in aetiologies (with an increase in autoimmune and DILI aetiologies, with a marginal relevance of acetaminophen overdose, and a decreased relevance of HBV in recent years), pre-LT management, immunosuppressive treatment, and post-LT complications. Overall, outcomes in this critically ill patient group have improved with increased survival over time. Early post-LT mortality was associated with pre-transplant AHT, AKI and hypernatremia.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Comité de Ética de la Investigación con medicamentos del Hospital Universitario y Politécnico La Fe. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because it is a retrospective study.
Author contributions
IC: Conceptualization, Data acquisition, Formal analysis, Writing – original draft, Writing – review and editing. SM, AB, MS, RM, CA, MG, SL, AO, MR-S, JH, LA, and AF: Data acquisition, Writing – review and editing. MB and VA: Conceptualization, Formal analysis, Writing – review and editing, Supervision. All authors contributed to the article and approved the submitted version.
Funding
The authors declare that financial support was received for the research and/or publication of this article. MB was supported by competitive grants from the Instituto de Salud Carlos III (grants number PI23/00088, and INT24/00021) and co-funded by the European Union, by Generalitat Valenciana (grants number AICO/2021/035 and CIPROM/2023/16) and the Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd) (https://www.ciberehd.org/) co-financed by the European Regional Development Fund. VA received a grant from AEEH (Asociación Española para el Estudio del Hígado, Spain) “Beca de intensificación para investigadores” in 2021 and was supported by competitive grants from the Instituto de Salud Carlos III (grant numbers: PI13/01770 and PI18/01759), and cofinanced by European Development Regional Fund “A way to achieve Europe.”
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2025.15185/full#supplementary-material
Glossary
- AHT
Arterial Hypertension
- AI
autoimmune
- AKI
Acute Kidney Injury
- RRT
Renal Replacement Therapy
- ALF
Acute Liver Failure
- ALT
Alanine Aminotransferase
- AP
Alkaline Phosphatase
- AST
Aspartate Aminotransferase
- AZA
Azathioprine
- CKD
Chronic Kidney Disease
- CNI
Calcineurin Inhibitor
- CV
Cardiovascular
- DILI
Drug Induced Liver Injury
- GFR
Glomerular Filtration Rate
- HAV
Hepatitis A Virus
- HBV
Hepatitis B Virus
- HE
Hepatic Encephalopathy
- HEV
Hepatitis E Virus
- HR
Hazard Ratio
- ICU
Intensive Care Unit
- IQR
Interquartile Range
- INR
International Normalized Ratio
- IS
Immunosuppression
- KCC
King’s College Criteria
- LT
Liver Transplantation
- MARS
Molecular Adsorbent Recirculating System
- WL
Waiting list
- MELD
Model for End-Stage Liver Disease
- MMF
Mycophenolate Mofetil
- NAC
N-Acetylcysteine
- PDN
Prednisone
- SD
Standard Deviation
Footnotes
References
1.
Escorsell Á Mas A de la Mata M . Acute Liver Failure in Spain: Analysis of 267 Cases. Liver Transpl (2007) 13:1389–95. 10.1002/lt.21119
2.
Bower WA Johns M Margolis HS Williams IT Bell BP . Population-Based Surveillance for Acute Liver Failure. Am J Gastroenterol (2007) 102:2459–63. 10.1111/j.1572-0241.2007.01388.x
3.
Lee WM Squires RH Nyberg SL Doo E Hoofnagle JH . Acute Liver Failure: Summary of a Workshop. Hepatology (2008) 47:1401–15. 10.1002/hep.22177
4.
Karvellas CJ Leventhal TM Rakela JL Zhang J Durkalski V Reddy KR et al Outcomes of Patients with Acute Liver Failure Listed for Liver Transplantation: A Multicenter Prospective Cohort Analysis. Liver Transpl (2023) 29:318–30. 10.1002/lt.26563
5.
ONT. Organización Nacional de Trasplantes (2024). Available online at: https://www.ont.es/https-www-ont-es-informacion-a-los-profesionales-4-actividad-de-donacion-y-trasplante-4-5/.
6.
Bernal W Lee WM Wendon J Larsen FS Williams R . Acute Liver Failure: A Curable Disease by 2024?J Hepatol (2015) 62:S112–S120. 10.1016/j.jhep.2014.12.016
7.
Donnelly MC Hayes PC Simpson KJ . The Changing Face of Liver Transplantation for Acute Liver Failure: Assessment of Current Status and Implications for Future Practice. Liver Transplant (2016) 22:527–35. 10.1002/lt.24403
8.
Ostapowicz G Fontana RJ Schiødt FV Larson A Davern TJ Han SHB et al Results of a Prospective Study of Acute Liver Failure at 17 Tertiary Care Centers in the United States. Ann Intern Med (2002) 137:947–54. 10.7326/0003-4819-137-12-200212170-00007
9.
Goldberg DS Forde KA Carbonari DM Lewis JD Leidl KBF Reddy KR et al Population-Representative Incidence of Drug-Induced Acute Liver Failure Based on an Analysis of an Integrated Health Care System. Gastroenterology (2015) 148:1353–61.e3. 10.1053/j.gastro.2015.02.050
10.
Khuroo MS Kamili S . Aetiology and Prognostic Factors in Acute Liver Failure in India. J Viral Hepat (2003) 10:224–31. 10.1046/j.1365-2893.2003.00415.x
11.
Hadem J Tacke F Bruns T Langgartner J Strnad P Denk GU et al Etiologies and Outcomes of Acute Liver Failure in Germany. Clin Gastroenterol Hepatol (2012) 10:664–9.e2. 10.1016/j.cgh.2012.02.016
12.
Fábrega E Mieses MÁ Terán A Moraleja I Casafont F Crespo J et al Etiologies and Outcomes of Acute Liver Failure in a Spanish Community. Int J Hepatol (2013) 2013:1–5. 10.1155/2013/928960
13.
Meier Y Cavallaro M Roos M Pauli-Magnus C Folkers G Meier PJ et al Incidence of Drug-i’nduced Liver Injury in Medical Inpatients. Eur J Clin Pharmacol (2005) 61:135–43. 10.1007/s00228-004-0888-z
14.
Lai JC Pomfret EA Verna EC . Implicit Bias and the Gender Inequity in Liver Transplantation. Am J Transplant (2022) 22:1515–8. 10.1111/ajt.16986
15.
Sheikh SS Locke JE . Gender Disparities in Transplantation. Curr Opin Organ Transplant (2021) 26:513–20. 10.1097/MOT.0000000000000909
16.
Floreani A Bizzaro D Shalaby S Taliani G Burra P . Sex Disparity and Drug-Induced Liver Injury. Dig Liver Dis (2023) 55:21–8. 10.1016/j.dld.2022.06.025
17.
Amacher DE . Female Gender as a Susceptibility Factor for Drug-Induced Liver Injury. Hum Exp Toxicol (2014) 33:928–39. 10.1177/0960327113512860
18.
Nephew LD Zia Z Ghabril M Orman E Lammert C Kubal C et al Sex Disparities in Waitlisting and Liver Transplant for Acute Liver Failure. JHEP Rep (2021) 3:100200. 10.1016/j.jhepr.2020.100200
19.
O’Grady JG Alexander GJM Hayllar KM Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology (1989) 97:439–445.
20.
Bernuau J Goudeau A Poynard T Dubois F. Lesage G. Yvonnet B. Degott C. et al Multivariate analysis of prognostic factors in fulminant hepatitis B. Hepatology (1986) 6:648–651.
21.
Arregui AC Bernal Monterde V Serrano Aulló MT Blesa L Zaragoza E . Fallo hepático fulminante: indicaciones de trasplante y resultados. Gastroenterol Hepatol (2008) 31 (Supl 1):46–50. Available online at: www.eltr.org.
22.
O’Grady JG Schalm SW Williams R Acute liver failure: redefining the syndromes. Lancet (1993) 342:273–275.
23.
Kellum JA . Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Supplements (2012) 2:1–138. 10.1038/kisup.2012.1
24.
Stevens PE Ahmed SB Carrero JJ Foster B Francis A Hall RK et al KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int (2024) 105:S117–S314. 10.1016/j.kint.2023.10.018
25.
Olthoff KM Kulik L Samstein B Kaminski M Abecassis M Emond J et al Validation of a Current Definition of Early Allograft Dysfunction in Liver Transplant Recipients and Analysis of Risk Factors. Liver Transpl (2010) 16:943–9. 10.1002/lt.22091
26.
Demetris AJ Batts KP Dhillon AP Ferrell L Fung J Geller SA et al Banff Schema for Grading Liver Allograft Rejection: An International Consensus Document. Hepatology (1997) 25:658–63. 10.1002/hep.510250328
27.
Germani G Theocharidou E Adam R Karam V Wendon J O'Grady J et al Liver Transplantation for Acute Liver Failure in Europe: Outcomes over 20 Years from the ELTR Database. J Hepatol (2012) 57:288–96. 10.1016/j.jhep.2012.03.017
28.
Reuben A Tillman H Fontana RJ Davern T McGuire B Stravitz RT et al Outcomes in Adults with Acute Liver Failure Between 1998 and 2013: An Observational Cohort Study. Ann Intern Med (2016) 164:724–32. 10.7326/M15-2211
29.
Karvellas CJ Artru F Coilly A Amiel IC Dhawan A Gurakar A et al Management of the Acute Liver Failure Patient and the Role of Liver Transplantation. Transplant (2025) 109:1680–91. 10.1097/TP.0000000000005451
30.
Rovegno M Vera M Ruiz A Benítez C . Current Concepts in Acute Liver Failure. Ann Hepatol (2019) 18:543–52. 10.1016/j.aohep.2019.04.008
31.
Reddy KR Ellerbe C Schilsky M Stravitz RT Fontana RJ Durkalski V et al Determinants of Outcome Among Patients with Acute Liver Failure Listed for Liver Transplantation in the United States. Liver Transpl (2016) 22:505–15. 10.1002/lt.24347
32.
Turan İ Akarca US Aladağ M Harputluoğlu M Yılmaz S Gençdal G et al Pre-Transplant Predictors of 3-Month Survival Following Liver Transplantation for Acute Liver Failure in Adult and Pediatric Patients in Türkiye. Sci Rep (2025) 15:26221. 10.1038/s41598-025-11298-y
33.
Conrad N Misra S Verbakel JY Verbeke G Molenberghs G Taylor PN et al Incidence, Prevalence, and Co-Occurrence of Autoimmune Disorders over Time and by Age, Sex, and Socioeconomic Status: A Population-Based Cohort Study of 22 Million Individuals in the UK. Lancet (2023) 401:1878–90. 10.1016/S0140-6736(23)00457-9
34.
Rubin JB Hameed B Gottfried M Lee WM Sarkar M . Acetaminophen-Induced Acute Liver Failure Is More Common and More Severe in Women. Clin Gastroenterol Hepatol (2018) 16:936–46. 10.1016/j.cgh.2017.11.042
35.
Lee WM Hynan LS Rossaro L Fontana RJ Stravitz RT Larson AM et al Intravenous N-Acetylcysteine Improves Transplant-Free Survival in Early Stage Non-Acetaminophen Acute Liver Failure. Gastroenterology (2009) 137:856–64.e1. 10.1053/j.gastro.2009.06.006
36.
European Association for the Study of the Liver. EASL Clinical Practical Guidelines on the Management of Acute (Fulminant) Liver Failure. J Hepatol (2017) 66:1047–81. 10.1016/j.jhep.2016.12.003
37.
Saliba F Camus C Durand F Mathurin P Letierce A Delafosse B et al Albumin Dialysis with a Noncell Artificial Liver Support Device in Patients with Acute Liver Failure: A Randomized, Controlled Trial. Ann Intern Med (2013) 159:522–31. 10.7326/0003-4819-159-8-201310150-00005
Summary
Keywords
liver transplantation, outcomes, acute liver failure, aetiology, sex differences
Citation
Conde Amiel I, Martínez Delgado S, Bosca Robledo A, Senosiáin Labiano M, Martín Mateos RM, Almohalla Álvarez C, González Diéguez ML, Lorente Pérez S, Otero Ferreiro A, Rodríguez-Soler M, Herrero JI, Aceituno L, Fernández Yunquera A, Berenguer M and Aguilera Sancho-Tello V (2025) Trends in Liver Transplantation for Acute Liver Failure in a Spanish Multicenter Cohort. Transpl. Int. 38:15185. doi: 10.3389/ti.2025.15185
Received
27 June 2025
Revised
22 October 2025
Accepted
24 November 2025
Published
16 December 2025
Volume
38 - 2025
Updates
Copyright
© 2025 Conde Amiel, Martínez Delgado, Bosca Robledo, Senosiáin Labiano, Martín Mateos, Almohalla Álvarez, González Diéguez, Lorente Pérez, Otero Ferreiro, Rodríguez-Soler, Herrero, Aceituno, Fernández Yunquera, Berenguer and Aguilera Sancho-Tello.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Isabel Conde Amiel, icondemiel@gmail.com
†These authors have contributed equally to this work and share senior authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.