Abstract
Achieving donor-specific immune tolerance has the potential to eliminate the need for lifelong immunosuppression in transplant recipients, but translating this goal into clinical practice remains challenging. Unlike laboratory rodents, humans are exposed to a variety of pathogens that generate memory T cells, which can interfere with tolerance induction. Establishing full donor hematopoietic chimerism, whether spontaneous or induced, can support robust immune tolerance. However, it often relies on graft-versus-host (GvH) reactivity, which carries significant risks, including graft-versus-host disease (GVHD) and infection. Although non-myeloablative conditioning protocols have shown promise, their broader use is limited by concerns about toxicity and the need to carefully balance GvH responses. Mixed and transient chimerism represents a less toxic alternative, but its effectiveness in humans is hindered by limited durability and resistance from memory T cells. Thymus transplantation offers another strategy by promoting central tolerance through donor-specific thymic education of developing T cells. Regulatory cell therapies combined with reduced immunosuppression have emerged as a safer approach. Early clinical trials have yielded encouraging results. Innovations in IL-2 pathway modulation and genetic engineering, including CAR-redirected regulatory T cells, may further enhance the precision, durability, and safety of strategies aimed at achieving transplantation tolerance.
Immune Tolerance
The concept of immune tolerance in transplantation refers to the immune system’s inability to mount an effector response selectively against donor antigens. In experimental models, the acceptance of a second graft from the same donor in the absence of immunosuppressive therapy, contrasted with the rejection of a third-party allograft, defines donor-specific tolerance. In humans, such experimental validation is not feasible. Instead, the term operational tolerance is used to describe a state in which the graft maintains normal function and histology in the absence of immunosuppressive treatment, and the recipient shows no increased susceptibility to infections, indicating overall preservation of immune competence.
Achieving a stable and robust state of donor-specific tolerance in clinical transplantation would allow for the elimination of long-term immunosuppression and its many associated complications, notably infections and malignancies. The aim of this review is not to provide an exhaustive account of all ongoing protocols, an effort already comprehensively undertaken in the report from the 6th International Sam Strober Workshop on Transplantation Tolerance [1], but rather to offer a critical and balanced perspective on current results and potential avenues for improvement.
Challenges in Translating Tolerogenic Protocols to Clinical Transplantation
Numerous immunomodulatory strategies have successfully induced tolerance to allogeneic tissues or organs in experimental models, particularly in mice [2]. However, the translation of these tolerogenic protocols to the clinical setting has often yielded disappointing or even negative results, thus limiting the translational relevance of rodent-based models [2]. It is essential to examine the reasons behind these failures in order to adapt these strategies to the specificities of the human host.
One major difference lies in the microbial exposure of humans (and other large mammals), which contrasts sharply with the controlled environments in which laboratory rodents are bred, typically under specific pathogen-free (SPF) or even more stringent conditions (SOPF) [3]. As a consequence, humans develop a substantial compartment of memory T cells [3], including donor-reactive memory T cells generated through heterologous immunity [4]. These cells contribute to a relative resistance to the induction of transplantation tolerance [5]. It is well established that laboratory mice exhibit a naïve-to-memory T cell ratio comparable to that of human neonates [3]. Remarkably, mice derived from pet stores or farms, by contrast, show a distribution of memory T cells within lymphoid organs and peripheral tissues similar to that observed in adult humans [3]. Furthermore, infection of a laboratory mouse with a single pathogenic virus can render it refractory to tolerance induction via peripheral immunomodulation, an approach otherwise highly effective in uninfected animals [6]. A dose-dependent effect has also been demonstrated: co-infection with multiple pathogens further increases resistance to tolerance induction [6].
In addition, despite numerous promising studies [7], there are still no universally validated biomarkers of tolerance in transplantation. This lack of reliable markers continues to preclude the safe and personalized tapering or withdrawal of immunosuppressive therapy [1, 8, 9].
Spontaneous Mixed Hematopoietic Chimerism Following Solid Organ Transplantation
In 2008, a landmark publication reported the spontaneous development of full hematopoietic chimerism in a 9-year-old girl following liver transplantation, in the absence of any myeloablative conditioning regimen [10]. This case demonstrated, first, that a transplanted liver can harbor a sufficient number of hematopoietic stem cells (HSCs) to support complete, multilineage, and durable hematopoiesis [10]. More importantly, it highlighted the capacity of graft-versus-host (GvH) reactivity, mediated by donor-derived T cells, to mimic the effects of bone marrow transplant conditioning.
This facilitating role of GvH reactivity includes two key mechanisms [1]: suppression of the host-versus-graft (HvG) immune response, and [2] clearance of hematopoietic niches via destruction of host HSCs, thereby enabling donor cell engraftment [10].
We recently reported a similar case following isolated kidney transplantation [11, 12]. Durable engraftment of HSCs derived from the renal graft was established in the recipient’s bone marrow [12]. In this case as well, the induction of full chimerism was associated with robust GvH reactivity [12]. In both instances, immunosuppressive therapy was successfully discontinued without subsequent graft rejection, despite restoration of immune competence, thereby meeting the criteria for operational tolerance [10, 12].
To further elucidate the mechanisms linking GvH reactivity and hematopoietic chimerism, the group led by Megan Sykes at Columbia University investigated patients undergoing intestinal and multivisceral transplantation, in whom graft survival without rejection has been shown to correlate with the volume of transplanted tissue [13, 14]. A direct relationship was identified between the number of transplanted organs and the extent of hematopoietic chimerism observed post-transplant [15]. Notably, donor-derived T lymphocytes from visceral grafts were found to mediate GvH reactivity that supported the persistence of hematopoietic chimerism not only in the graft itself [16], but also in the recipient’s peripheral blood and bone marrow [17, 18].
Collectively, these observations in solid organ transplant recipients, none of whom underwent myeloablative conditioning, underscore the critical role of GvH reactivity in the establishment and maintenance of hematopoietic chimerism.
Induction of Full Donor Hematopoietic Chimerism
The induction of stable immune tolerance associated with full donor chimerism was first achieved through sequential transplantation of hematopoietic progenitor cells and a kidney from the same HLA-incompatible donor in patients undergoing treatment for hematologic malignancies [19]. When full donor chimerism is established, donor-derived dendritic cells colonize the recipient’s thymus (Figure 1). This allows newly developing thymocytes to undergo negative selection if they are reactive to donor or recipient antigens, presented respectively by donor dendritic cells and recipient medullary thymic epithelium (Figure 1). Tolerance is thus predominantly mediated through central mechanisms and requires a stable, long-term dominance or completeness of donor hematopoiesis [20].
FIGURE 1
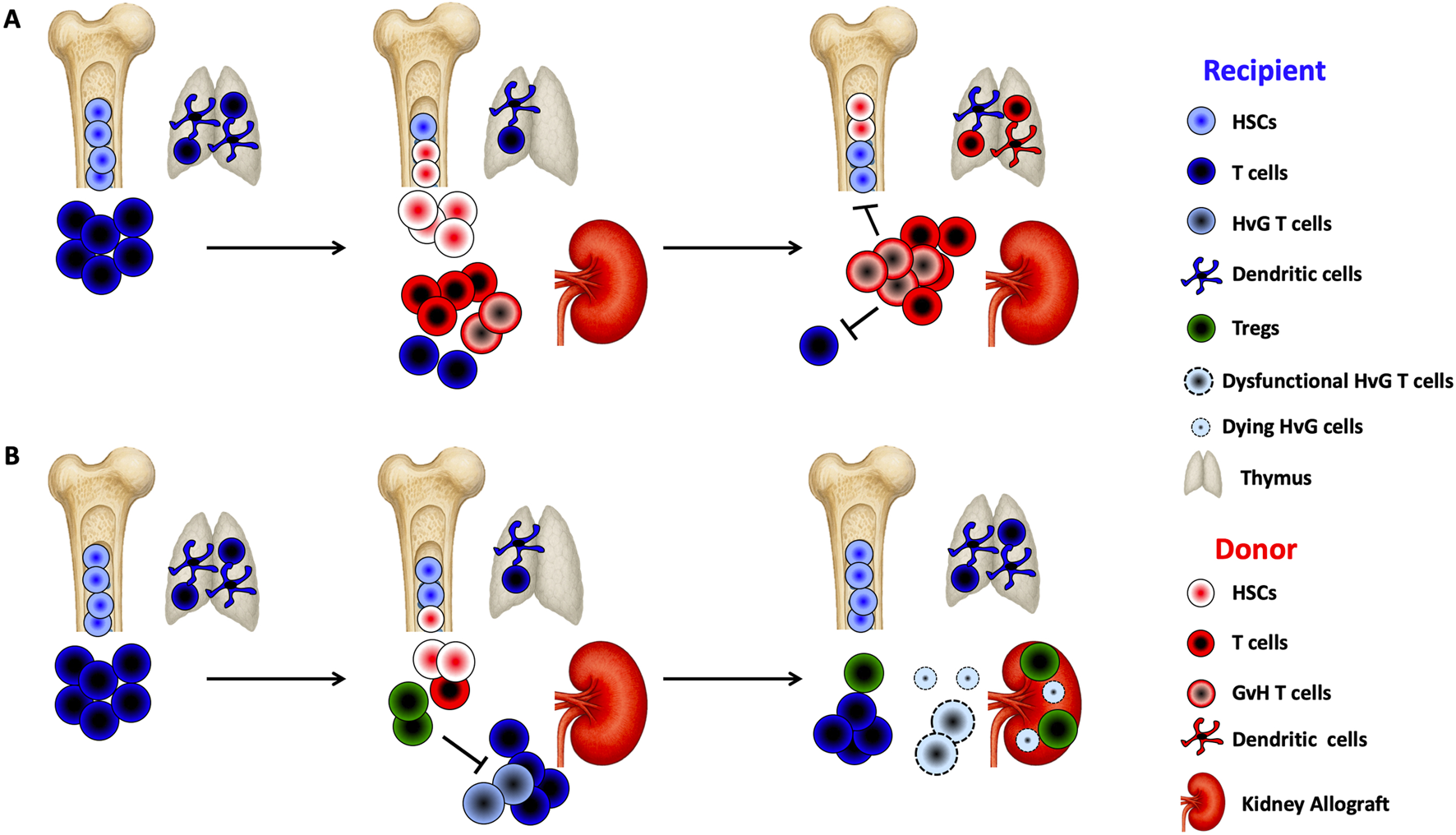
Chimerism-based transplant tolerance (A) Sustained Full Chimerism: Following non-myeloablative conditioning, recipients receive a large number of donor CD34+ hematopoietic stem cells (HSCs) and donor T cells along with the kidney allograft. Graft-versus-host (GvH) reactivity may promote the expansion of donor T cells, which eliminate recipient T cells and hematopoietic cells, thereby creating space in the bone marrow for donor HSCs to engraft. Once engrafted, donor HSCs continuously supply the thymus with T cell precursors and dendritic cell progenitors, promoting central donor-specific tolerance. These conditions support the establishment of sustained full chimerism. (B) Transient Mixed Chimerism: After non-myeloablative conditioning with T cell-depleting induction, recipients receive unfractionated donor bone marrow, including HSCs and T cells, alongside the kidney allograft. The conditioning regimen induces lymphopenia while sparing regulatory T cells (Tregs), leading to early Treg expansion. In the presence of donor antigen, this Treg-dominant environment suppresses the activation of host-versus-graft (HvG)-reactive T cells and fosters peripheral deletion of donor-reactive T cells. These mechanisms enable the development of transient mixed chimerism.
However, this approach typically necessitates myeloablative conditioning, which carries unacceptable toxicity in patients without malignancy. In transplantation, several strategies have been developed to induce full chimerism, and thereby stable tolerance, without resorting to myeloablation [20].
The first such protocol, developed at Stanford University, combines total lymphoid irradiation, anti-thymocyte globulin (ATG), and infusion of a limited number of donor T cells (1 × 106/kg). This approach achieved durable chimerism and successful immunosuppression withdrawal in more than 80% (24/29) of recipients receiving combined kidney and hematopoietic stem cell transplants from HLA-identical donors [21, 22]. However, in HLA-incompatible settings, this protocol generally results in low-level and transient chimerism, even when higher doses of donor T cells are administered (up to 100 × 106/kg) [22]. Critically, loss of chimerism in this context is often rapidly followed by renal graft rejection [22].
A second strategy, developed at Northwestern University, involves a more intensive conditioning regimen [23], comprising total body irradiation, fludarabine, and cyclophosphamide administered both pre- and post-transplant, along with donor T cells (3.8 × 106/kg). Cyclophosphamide post-transplant, in combination with co-infusion of CD8+ TCR− immunomodulatory “facilitator” cells (FCR001), is designed to mitigate the risk of graft-versus-host disease (GVHD) associated with the transfer of mature donor T cells [23]. Over 80% (26/32) of patients in this protocol achieved high and stable levels of chimerism, with successful discontinuation of immunosuppressive therapy in the vast majority (25/26) [24]. In this context, the establishment of full donor chimerism represents the most reliable biomarker of successful tolerance induction [25].
Nevertheless, two major adverse events have emerged as limitations to widespread implementation. Three patients experienced severe infections resulting in graft loss (n = 2) or death (n = 1) [26]. In addition, two cases of GVHD occurred, one of which was fatal, and the other developed into chronic GVHD [26]. These findings emphasize both the necessity and the inherent risk of robust GvH reactivity for maintaining high-level chimerism in the absence of myeloablation [20].
Regarding GVHD risk mitigation, Alice Bertaina and colleagues at Stanford recently reported a novel approach in three patients with Schimke immuno-osseous dysplasia, a syndrome characterized by severe combined immunodeficiency, skeletal dysplasia, and early-onset glomerular kidney failure [27]. This condition is caused by mutations in SMARCAL1, a gene involved in DNA repair, rendering patients highly vulnerable to cytoreductive treatments and increasing mortality following hematopoietic stem cell transplantation [28]. The Stanford protocol reduces this risk through the use of reduced-intensity conditioning and grafts depleted of TCRαβ+ T cells and CD19+ B cells. All three patients achieved full donor hematopoietic chimerism and maintained excellent renal graft function from the same donor, in the complete and sustained absence of immunosuppressive therapy [27]. The investigators propose expanding this protocol to broader patient populations beyond those with inborn errors of immunity and hematopoiesis [27]. However, favorable outcomes with TCRαβ/CD19-depleted grafts have so far been primarily observed in patients with inborn errors of immunity [29].
Additionally, the case of a child with Schimke syndrome who spontaneously developed acute GVHD and full hematopoietic chimerism following isolated kidney transplantation illustrates the unique pathophysiological context of this condition [12]. This case highlights how host cells in this syndrome, due to their limited proliferative capacity and functional impairment, are at a competitive disadvantage, particularly under immunosuppressive therapy, which favors engraftment of donor-derived hematopoietic stem cells [29, 30].
Collectively, these pilot studies indicate that in patients without preexisting immune deficiency, achieving and maintaining high-level, durable hematopoietic chimerism in the context of HLA incompatibility and without myeloablation requires a degree of GvH reactivity that may carry life-threatening complications.
Induction of Mixed and Transient Donor Hematopoietic Chimerism
Mixed chimerism refers to the coexistence of donor- and recipient-derived hematopoietic cells in the peripheral blood and indicates the preservation of the recipient’s hematopoietic system [20]. In laboratory mice, stable mixed chimerism can be readily achieved through the administration of donor hematopoietic stem cells in combination with various tolerance-inducing regimens. Historically, the foundation for a clinically translatable strategy was laid using non-myeloablative conditioning that combined cytoreductive agents with thymic irradiation [31]. To mitigate treatment-related toxicity, cytoreduction was successfully replaced in murine models by either co-stimulatory blockade [32] or regulatory T cell-based therapy [33].
However, in humans and non-human primates, prior exposure to pathogens leads to the development of alloreactive memory T cells via heterologous immunity, which impairs the induction of mixed chimerism through immunomodulation alone [6, 34]. The first clinical protocol for tolerance induction via mixed chimerism therefore incorporated siplizumab, an anti-CD2 monoclonal antibody that effectively targets memory T cells [35]. Notably, both siplizumab and alefacept are able to inhibit the expansion of CD2high CD28− pro-inflammatory T cells, which are resistant to CTLA-4-Ig [36, 37]. The development of new agents targeting memory T cells, such as OX40-specific antibodies, may ultimately restore the tolerogenic potential of co-stimulatory blockade in humans, a mechanism currently best demonstrated in murine and non-human primate models [38]. In this context, it is important to highlight the recent communication at the ESOT 2025 congress regarding the first use in humans (NCT07020156) of a monoclonal anti-OX40 antibody (OX118).
Under the leadership of the Massachusetts General Hospital (MGH) team, the mixed chimerism protocol was successfully translated to non-human primates [39] and humans [40, 41]. However, in contrast to murine models, the level and duration of donor chimerism achieved in these species were substantially lower and more transient (lasting only a few weeks). This short-lived chimerism was associated with reduced tolerance efficacy: three of the first ten patients developed de novo donor-specific antibodies (DSAs) or acute rejection episodes, precluding immunosuppression withdrawal. Among the remaining seven patients, three had to resume immunosuppression due to chronic rejection or recurrence of native kidney disease [26].
Several modifications have since been introduced to enhance protocol efficacy and compensate for the unavailability of specific therapeutic agents. The inclusion of four doses of rituximab helped prevent de novo DSA development, which had been observed in early patients [41]. More recently, the protocol was adapted to address “chimerism transition syndrome,” characterized by acute kidney injury and chimerism loss during rapid immune reconstitution. The revised MGH protocol now includes fludarabine, a reduced dose of cyclophosphamide, and omits post-transplant rituximab [1]. In parallel, the PANORAMA trial (NCT04803006), led by Joshua Weiner at Columbia University, is investigating modified siplizumab dosing to enhance memory T cell depletion, with encouraging preliminary results [1].
At the Samsung Medical Center, where siplizumab is unavailable, the protocol was adapted using anti-thymocyte globulin (ATG) instead. Infectious complications, including BK virus nephritis, prompted dose reductions of both fludarabine and ATG, and a switch from tacrolimus to sirolimus at 1-month post-transplant [42]. In this Korean cohort, immunosuppression was discontinued for over 1 year in five of eight patients. However, one patient experienced T cell-mediated rejection following a respiratory infection, highlighting the fragility of the tolerogenic state [42].
Mechanistic studies have demonstrated a biphasic process in tolerance induction: initial enrichment of regulatory T cells during post-induction lymphopenia [43], followed by progressive deletion of alloreactive T cell clones over time (Figure 1) [44]. The renal allograft likely contributes to this functional inactivation of donor-specific responses (Figure 1). Indeed, patients who received hematopoietic stem cells under the same induction regimen but without a kidney transplant retained anti-donor T cell reactivity upon chimerism loss [45], unlike those with combined kidney-bone marrow transplantation [44]. This hypothesis is supported by observations in non-human primates: combined (simultaneous or sequential) heart and bone marrow transplantation from the same donor failed to induce tolerance [46]. In contrast, triple transplantation of heart, kidney, and bone marrow from the same donor, using identical conditioning, resulted in higher levels of donor chimerism, prevented the formation of DSAs and anti-donor cytotoxic responses, and most importantly, enabled successful withdrawal of immunosuppression [46]. This kidney-specific protective effect was associated with an accumulation of regulatory T cells within the renal graft, suggesting their role in local suppression and potentially in the deletion of alloreactive clones [43, 46, 47].
In line with these findings, during the Sixth International Workshop on Clinical Transplantation Tolerance, several investigators reported the presence of organized infiltrates enriched in FOXP3+ regulatory T cells within the grafts of operationally tolerant patients [1]. These structures are reminiscent of regulatory tertiary lymphoid organs observed in renal allografts of tolerant mice [48]. Advances in spatial transcriptomics may soon clarify the prognostic and mechanistic significance of these structures [1].
In conclusion, protocols based on mixed chimerism have yielded mixed results, with variable and often temporary efficacy in inducing tolerance. Further optimization of immunomodulatory regimens accompanying hematopoietic stem cell transplantation will be required to enhance both their clinical effectiveness and safety profile.
Thymus Transplantation
Thymus transplantation enables the induction of central, donor-specific immune tolerance, particularly when the donor is juvenile, provided two key conditions are met (Figure 2). First, the recipient must undergo thymectomy to ensure that all developing thymocytes are educated within the donor thymic microenvironment [49]. Second, profound peripheral lymphodepletion is required to eliminate pre-existing alloreactive T cells generated prior to thymus transplantation [49, 50].
FIGURE 2
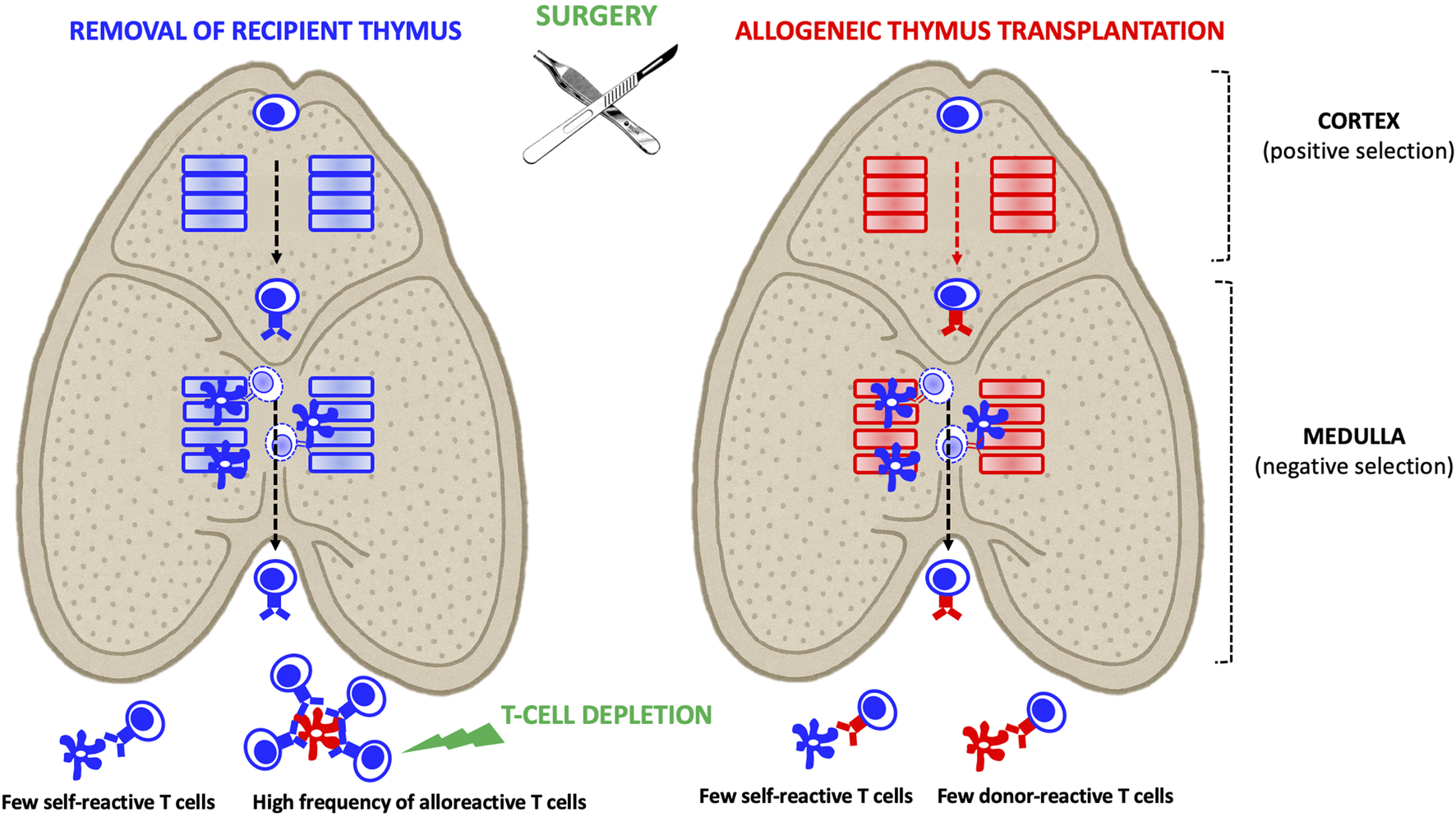
Allogeneic Thymus Transplantation: In the recipient thymus, thymocytes derived from T cell progenitors undergo positive selection by cortical epithelial cells. This positive selection process determines the T cells’ restriction to self-HLA antigens (depicted by a blue TCR) that were recognized in the cortex. Subsequently, developing thymocytes undergo negative selection in the medulla, where those that strongly react to self-antigens presented by medullary epithelial cells and dendritic cells are eliminated. As a result, mature thymocytes exiting the thymus are devoid of self-reactive T cells but may still include alloreactive T cells. In the context of allogeneic thymus transplantation, recipient-derived thymocytes are positively selected on donor HLA molecules (depicted by a red TCR). As they migrate through the medulla, they undergo negative selection, eliminating those that strongly react to recipient HLA (presented by medullary dendritic cells) or donor HLA (presented by medullary epithelial cells). Consequently, the mature thymocytes that exit the donor thymus are tolerant to both recipient and donor antigens. Combining thymus and organ transplantation from the same donor represents a potent strategy to induce immune tolerance. However, two key conditions must be met to achieve this: 1- The recipient’s native thymus must be removed to eliminate a source of donor-reactive T cells. 2- Pre-existing peripheral donor-reactive T cells generated before thymus transplantation must be eliminated.
In murine models, transplantation of a neonatal donor thymus under the kidney capsule of a thymectomized and lymphodepleted recipient enables long-term acceptance of a heart graft from the same donor strain without the need for ongoing immunosuppression [50]. However, the critical role of thymic vascularization in maintaining the tolerogenic function of the thymic epithelium became apparent when this approach was translated to large animal models. To address this, various surgical techniques have been developed to optimize thymic graft perfusion.
One such strategy, known as the “thymokidney” approach, involves transplanting the donor’s thymus under the capsule of one of their own kidneys several weeks prior to allogeneic transplantation [51]. This interval allows the thymus to revascularize and regain functional capacity in an autologous environment before subsequent transplantation of the composite “thymokidney” graft. This approach was notably employed by Robert Montgomery’s team during the first porcine thymokidney xenotransplant into brain-dead human recipients [52]. Thymic perfusion may also be preserved through microsurgical anastomosis of donor thymic vessels [49], or via en bloc transplantation of combined thymic and cardiac grafts.
Nevertheless, transplantation of an intact thymus that retains mature donor-derived thymocytes carries a significant risk of GVHD, especially in immunodeficient recipients. To mitigate this risk in athymic infants, researchers at Duke University developed a strategy involving the transplantation of thymic epithelial tissue devoid of donor thymocytes. These thymocytes are eliminated by culturing thymic slices for approximately 10 days prior to implantation. This approach, now FDA-approved under the commercial name RETHYMIC® (allogeneic processed thymus tissue), has dramatically improved outcomes for children with congenital athymia [53]. Its potential to induce alloimmune tolerance has been demonstrated in a rat heart transplantation model [54] and is currently under investigation in clinical transplantation settings [55–57].
Regulatory Cell Therapy
The iatrogenic risks associated with hematopoietic stem cell transplantation have sparked interest in peripheral immunomodulation strategies using regulatory cell therapies, including T lymphocytes and myeloid cells such as dendritic cells and macrophages [1]. The ONE Study consortium, comprising eight European and American centers, jointly analyzed the clinical and immunological impact of distinct regulatory cell products administered to kidney transplant recipients, using a shared protocol for follow-up [58]. Combined analysis of these trials showed that, when regulatory cell therapy was paired with reduced immunosuppression, infection rates were lower and rejection rates comparable to standard immunosuppressive regimens [58].
A more specific analysis from the German cohort at Charité University Hospital demonstrated the feasibility of generating autologous CD4+ regulatory T cell (Treg) products from peripheral blood collected 2 weeks prior to transplantation. Three-year kidney allograft survival reached 100% in both arms of the trial, while 73% of patients who received polyclonal Tregs were maintained on tacrolimus monotherapy [59]. A recent report indicates no graft loss among the 12 patients in the United Kingdom cohort who received polyclonal Tregs, even 7 years after transplantation [1]. Surveillance biopsies from this cohort revealed focal infiltrates enriched in B cell and regulatory gene signatures [60].
These encouraging outcomes have led to the launch of the randomized controlled TWO Study, aiming to enroll 60 patients in two arms. Initially, regulatory cell therapy was scheduled to be administered 6 months after induction with alemtuzumab [61]. Seven patients were treated under this protocol before it was suspended due to concerns about COVID-19-related risks associated with prolonged lymphodepletion [61]. The trial has since resumed with a revised protocol: one arm receives standard immunosuppression after basiliximab induction, while the other includes regulatory cell therapy on day 5 post-transplantation, followed by progressive immunosuppression minimization [62].
In liver transplantation, two clinical trials evaluating donor-specific regulatory cell therapy have yielded contrasting results [63, 64]. A Japanese study achieved complete withdrawal of immunosuppression by 18 months post-transplantation in 70% of patients, with follow-up ranging from 5.4 to 10.4 years [1, 63]. The subsequent multicenter trial (NCT04950842) showed preliminary evidence of FOXP3+-enriched lymphoid infiltrates in protocol biopsies, similar to those observed in renal transplantation trials [1]. By contrast, in an American study, 4 out of 5 patients experienced acute rejection during immunosuppression tapering [64]. Notably, timing differed between the two studies: regulatory T cell therapy was administered on day 13 post-transplantation in the Japanese study, whereas in the American trial, it occurred between 2 and 7 years post-transplantation. More importantly, the Japanese protocol included a bolus of cyclophosphamide 1 week prior to Treg infusion, aimed at depleting alloreactive effector T cells that were activated and proliferating immediately following transplantation. This “debulking” effect, combined with regulatory cell therapy, shifts the immune balance in favor of tolerance.
In the American trial, deuterium-labeled cell tracking revealed rapid contraction and disappearance of infused Tregs, likely due to abrupt interleukin-2 (IL-2) withdrawal following an IL-2-rich ex vivo expansion phase [64]. Indeed, studies in type 1 diabetes have demonstrated enhanced Treg persistence when low-dose IL-2 is co-administered with cell therapy [65]. However, this strategy is more challenging in transplantation, where IL-2 may simultaneously stimulate effector responses. One liver transplant trial showed significant Treg expansion but also activation of CD8+ T cells and NK cells, resulting in unexpectedly high rejection rates and premature trial termination [66]. To enhance IL-2 selectivity for Tregs, multiple pharmaceutical efforts are underway to develop IL-2 muteins with increased affinity for the high-affinity IL-2 receptor (CD25), while minimizing interaction with lower-affinity receptors [67, 68]. Combining regulatory cell therapy with such IL-2 muteins may further amplify therapeutic efficacy.
Finally, the development of genetically engineered regulatory cells offers highly promising new avenues (Figure 3). Several groups have demonstrated that chimeric antigen receptor (CAR)-redirected Tregs targeting HLA-A2 can suppress alloreactive responses in preclinical transplant models [69, 70]. CAR-Tregs display enhanced suppressive capacity compared to donor-specific Tregs generated via co-culture with donor cells [71]. The ongoing STEADFAST (NCT04817774) and LIBERATE (NCT05234190) trials are evaluating anti-HLA-A2 CAR-Tregs in renal and liver transplantation, respectively [1]. Preliminary findings suggest the presence of FOXP3+ regulatory lymphoid structures within renal grafts from CAR-Treg-treated patients [1]. Additional genetic modifications have been proposed to further improve Treg efficacy and resilience. These include conferring resistance to immunosuppressants (e.g., tacrolimus, everolimus, sirolimus) by inactivating FKBP12 [1, 72], and autonomous IL-2 signaling [73]. We are also developing a strategy that harnesses the tolerogenic properties of anti-CD3 monoclonal antibodies [74], in combination with shielded CAR-Tregs that are protected from anti-CD3-mediated clearance via CRISPR-Cas9-based editing (Blein et al. personal communication). Additionally, the use of monoclonal antibodies currently under development - such as those targeting CD28 (FR104; NCT04837092) [75] or CD45RC [76] - in combination with targeted regulatory cell therapy, may act synergistically to shift the balance toward immune tolerance. Finally, the transgenic expression of transcription factors involved in the Treg program may help stabilize the Treg epigenetic landscape and reinforce lineage stability in inflammatory environments [77].
FIGURE 3
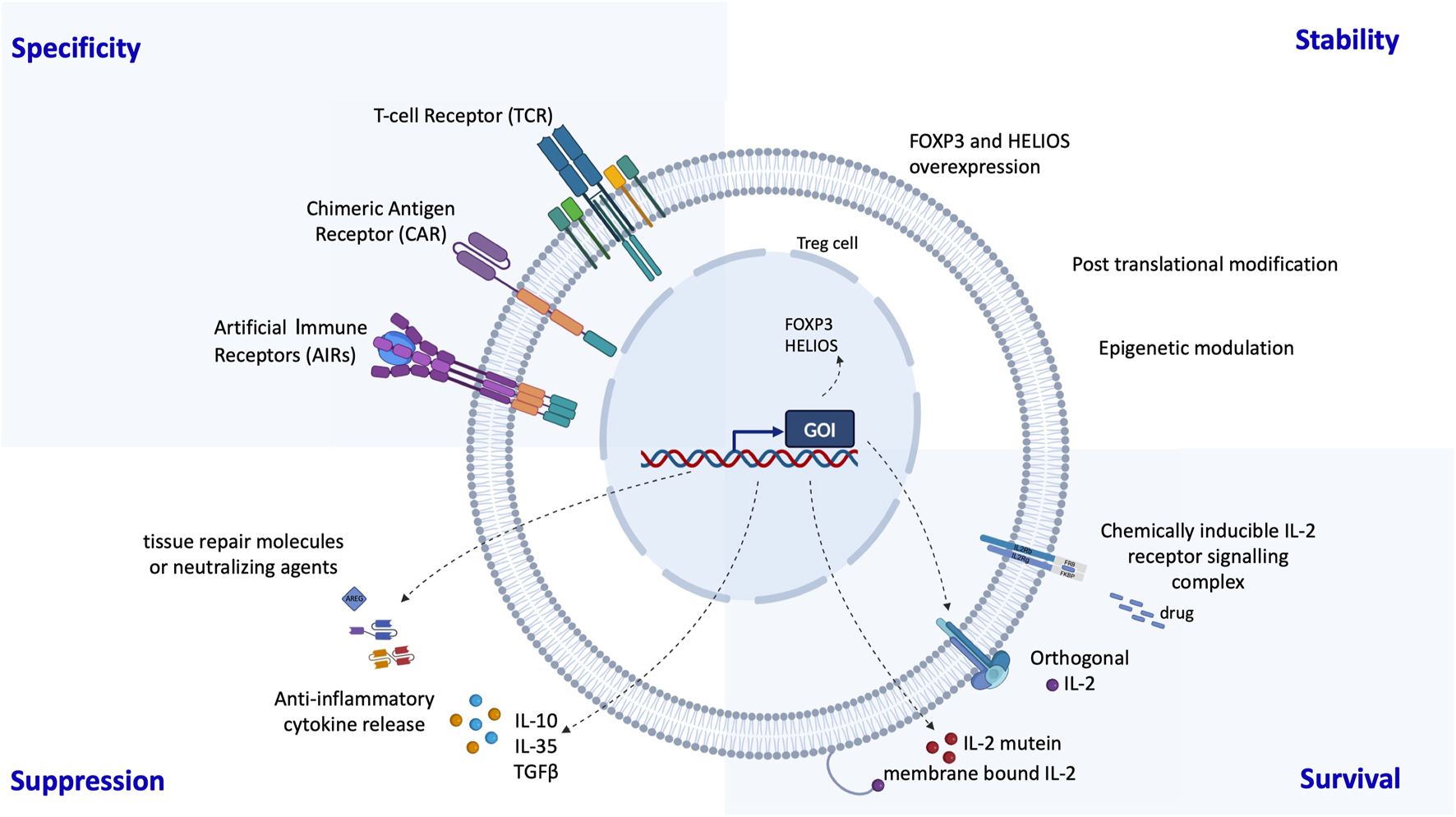
Possibilities to enhance Treg therapies through genetic engineering: Several groups have developed genetic engineering strategies to improve the specificity, stability, survival and suppression of regulatory T cells. Specificity has been greatly enhanced by the addition of chimeric antigen receptors (CARs) or synthetic T cell receptors (TCRs). Stability can be achieved by overexpression of the transcription factors FOXP3 and HELIOS and suppression can be supported by expression of anti-inflammatory cytokines and molecules. The in vivo survival of regulatory T cells can be prolonged by making them self-sufficient in a Treg specific IL-2.
Organ Engineering to Evade the Immune System
An alternative to inducing immune tolerance through immunological reprogramming is the engineering of the graft itself to evade immune detection. Pregnancy provides a compelling demonstration that multiple immunomodulatory mechanisms at the placental interface can create an immunologically privileged zone that remains invisible to alloimmune responses [78]. Failure of these mechanisms can result in placental inflammation resembling transplant rejection [79, 80].
The placenta offers multiple avenues for modulating the immunogenicity of allogeneic transplants, including the epigenetic silencing of polymorphic HLA genes [81] and Th1-skewed chemokine genes CXCL9, CXCL10, and CXCL11 [82]. Additionally, the expression of FasL [83], the enzyme indoleamine 2,3-dioxygenase [84], and immune checkpoint molecules [85] can suppress T cell responses, while specific sialylation motifs on trophoblast proteins inhibit B cell activation [86].
This conceptual framework has already inspired a successful strategy in islet transplantation models in humanized mice [87], non-human primates [88], and more recently, in a first-in-human case [89]. In these studies, three genetic modifications were introduced into allogeneic pancreatic islet cells using CRISPR-Cas12-based gene editing and lentiviral transduction. These modifications involved silencing HLA class I and II molecule expression and overexpressing CD47 [89]. The absence of polymorphic HLA prevents activation of the adaptive immune response (T and B cells), while CD47 expression neutralizes the innate immune response (NK cells and myeloid cells).
The development of normothermic perfusion machines [90, 91], along with rapid advances in cell-specific targeting of viral (lentivirus, AAV) and non-viral vectors [92, 93], opens new avenues for applying these strategies to more complex, vascularized organs beyond pancreatic islets. Even before attempting to render complex organs such as the kidney immunologically privileged, it may be feasible to first engineer the graft endothelium to resist preformed donor-specific antibodies (DSA), thereby mimicking the phenomenon of accommodation [94, 95].
Current Clinical Implications
To date, no tolerance induction strategy in clinical transplantation has been approved by the FDA or the EMA, nor validated in a completed phase III trial in comparison with standard-of-care immunosuppressive therapies. In other words, protocols designed to induce transplant tolerance have not yet entered routine clinical practice and remain within the realm of research. This observation raises important questions regarding the current limitations and obstacles faced by the strategies developed thus far (Table 1). It also underscores the need for continued research to improve both efficacy and safety (Table 1).
TABLE 1
| Tolerance induction strategy | Main Mechanism |
Advantages | Limitations | Currently explored new avenues | Challenges before regulatory approval |
|---|---|---|---|---|---|
| Full chimerism | Robust central tolerance via complete donor-derived hematopoiesis | − Enables successful withdrawal of IS drugs without rejection or development of DSA − Prevents post-transplant recurrence of immune-mediated nephropathies |
− Conditioning-related toxicity − Risk of life-threatening GVHD − Delayed immune reconstitution − Increased risk of infections |
– Approaches to reduce conditioning intensity and GVHR dependence • Use of Tregs (NCT03943238) • T- and B-cell-depleted HSC graft (NCT05508009) Delayed tolerance approaches, for patients who have already a kidney transplant • NCT03591302 • NCT01649388: terminated by sponsor |
– Phase 3 CT required to demonstrate a superior benefit/risk ratio compared to standard IS therapy – • NCT03363945 (HLA-matched LD) • NCT03995901 terminated due to high GVHD rates in initial participants |
| Mixed chimerism | Peripheral tolerogenic mechanisms, via transient, incomplete donor-derived hematopoiesis | − Lower conditioning toxicity − No risk of GVHD − IS drugs withdrawal achieved in some patients |
− Less robust tolerogenic effect (rejection or de novo DSA may occur upon IS tapering) − Potential recurrence of immune-mediated nephropathies − Risk of chimerism transition syndrome # |
Strategies to improve efficacy and safety profile • ECP-DL infusion (NCT07083830) • Combined Treg therapy (NCT03867617) Approaches to mitigate chimerism transition syndrome • Use of fludarabine and avoidance of post-Tx rituximab (NCT04540380) • Enhanced T cell depletion (NCT04803006) |
No ongoing Phase 3 CT |
| Thymus Tx | Central tolerance through intrathymic deletion of donor-reactive T cells | − No need of cytoreductive conditioning − Donor thymus can support immune reconstitution after recipient thymectomy |
− Requires thymectomy (open-chest surgery) − Profound T cell depletion needed to eliminate preexisting donor-reactive T cells − GVHD may occur following the transplantation of an intact thymus into an immunocompromised recipient − May not promote tolerance to peripheral tissue-specific antigens (as seen in xenotransplantation models) |
Combined thymus-kidney transplantation from neonatal donors in KTx recipients (NCT06715865) ## | While CTTI is approved for congenital athymia, there is currently no clinical evidence supporting its tolerogenic efficacy in solid organ transplantation |
| Regulatory T cell therapy | Peripheral tolerogenic effects by shifting the immune balance toward regulation | − No need of cytoreductive conditioning − No risk of GVHD − Potential to enhance Treg function through genetic engineering − May at least allow reduction of IS drug burden |
− Risk of lineage instability in inflammatory environments (possible drift toward pro-inflammatory phenotypes) − Short-term persistence after administration − Unknown homing capacity to the transplanted graft − Challenge of generating sufficient cells from patients with end-stage organ failure |
Approaches to improve the efficacy and robustness • Combination with donor-derived bone-marrow cells (NCT03867617) • Profound T cell depletion prior to therapy (TWO Study ISRCTN 11038572) • Use of cyclophosphamide before Treg infusion (NCT03577431; NCT03654040) Alternative regulatory T cell types • CD8+ Treg cells (NCT06777719) CAR-engineered Tregs • In kidney transplant recipients (NCT04817774) • In liver transplant recipients (NCT05234190) |
No ongoing Phase 3 CT currently The RETIRE study is a Phase 2 RCT, comparing Treg therapy combined with reduced IS versus SOC (NCT06552169) Overcoming high manufacturing costs and standardization issues |
Pros and cons, and advancements of the different tolerogenic strategies.
#: Chimerism transition syndrome: characterized by acute kidney injury, fever, loss of chimerism during reconstitution of the recipient’s immune system.
##: Available information does not clarify whether kidney transplant recipients in the study undergo thymectomy as part of the tolerogenic protocol.
Abbreviations: CTTI, cultured thymus tissue implantation; CT, clinical trial; DSA, donor-specific antibodies; ECP-DL, extracorporeal photopheresis donor lymphocytes; GVHR, graft-vs-host reactivity; GVHD, graft-vs-host disease; IS, immunosuppressive; LD, living donor; SOC, standard of care; Treg, regulatory T cell; Tx, transplantation.
Mixed and transient chimerism protocols have shown variable success, often requiring resumed immunosuppression due to rejection or donor-specific antibodies [96]. Targeting memory T cells combined with immunomodulation may improve outcomes. Full durable chimerism offers more robust tolerance [24] but carries serious risks like GVHD and infections, limiting development [26]. Reducing conditioning intensity and GVH reactivity dependence is a key challenge. Thymus transplantation shows promise in heart and lung transplants, where thymectomy is feasible, but evidence remains limited and its use in abdominal transplants raises safety concerns due to invasive surgery. Moreover, the scientific publication of the first proof-of-concept case remains pending [57]. Polyclonal or donor-specific Treg therapies alone have not safely enabled immunosuppression withdrawal. This emphasizes the need to enhance Treg function (e.g., genetic engineering) while controlling effector immune responses to improve therapeutic success.
Conclusion
Hematopoietic chimerism induction protocols have demonstrated the possibility of achieving tolerance in clinical transplantation, although sometimes at the cost of excessive iatrogenic risk. The implantation of a juvenile donor thymic epithelial template in a heart transplant recipient, thymectomized during the transplantation procedure, could represent an alternative strategy for central tolerance that should be rigorously evaluated. Finally, “augmented” regulatory cell therapies, through genetic modifications, combined with a targeted strategy of effector cell depletion and immunotherapy favoring the regulatory arm of the immune response, represent very promising strategies.
Statements
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. TB et SC are supported by the Emmanuel Boussard Foundation. This research is funded by grants from the French National Research Agency (ANR-24-CE18-6414) and the Foundation for Medical Research (FRM-PME202406019177).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
References
1.
Stark H Ho QY Cross A Alessandrini A Bertaina A Brennan D et al Meeting Report: The Sixth International Sam Strober Workshop on Clinical Immune Tolerance. Transplantation (2025) 109(4):569–79. 10.1097/TP.0000000000005311
2.
Cravedi P Riella LV Ford ML Valujskikh A Menon MC Kirk AD et al Advancing Mouse Models for Transplantation Research. Am J Transplant. (2024) 24(8):1362–8. 10.1016/j.ajt.2024.01.006
3.
Beura LK Hamilton SE Bi K Schenkel JM Odumade OA Casey KA et al Normalizing the Environment Recapitulates Adult Human Immune Traits in Laboratory Mice. Nature (2016) 532(7600):512–6. 10.1038/nature17655
4.
Amir AL D’Orsogna LJA Roelen DL van Loenen MM Hagedoorn RS de Boer R et al Allo-HLA Reactivity of Virus-specific Memory T Cells Is Common. Blood (2010) 115(15):3146–57. 10.1182/blood-2009-07-234906
5.
Benichou G Gonzalez B Marino J Ayasoufi K Valujskikh A . Role of Memory T Cells in Allograft Rejection and Tolerance. Front Immunol (2017) 8:170. 10.3389/fimmu.2017.00170
6.
Adams AB Williams MA Jones TR Shirasugi N Durham MM Kaech SM et al Heterologous Immunity Provides a Potent Barrier to Transplantation Tolerance. J Clin Invest (2003) 111(12):1887–95. 10.1172/JCI17477
7.
Danger R Chesneau M Paul C Guérif P Durand M Newell KA et al A Composite Score Associated with Spontaneous Operational Tolerance in Kidney Transplant Recipients. Kidney Int Juin (2017) 91(6):1473–81. 10.1016/j.kint.2016.12.020
8.
Vionnet J Sánchez-Fueyo A . Biomarkers of Operational Tolerance After Liver Transplantation: Are we There Yet?Liver Transpl Off Publ Am Assoc Study Liver Dis Int Liver Transpl Soc. (2022) 28(1):15–6. 10.1002/lt.26270
9.
Valke LLFG van Cranenbroek B Hilbrands LB Joosten I . Soluble CD30 Does Not Predict Late Acute Rejection or Safe Tapering of Immunosuppression in Renal Transplantation. Transpl Immunol (2015) 32(1):18–22. 10.1016/j.trim.2014.10.006
10.
Alexander SI Smith N Hu M Verran D Shun A Dorney S et al Chimerism and Tolerance in a Recipient of a Deceased-Donor Liver Transplant. N Engl J Med (2008) 358(4):369–74. 10.1056/NEJMoa0707255
11.
Zuber J Boyer O Neven B Jollet I Renac V Berthaud R et al Donor-Targeted Serotherapy as a Rescue Therapy for Steroid-Resistant Acute GVHD After HLA-Mismatched Kidney Transplantation. Am J Transplant off J Am Soc Transplant Am Soc Transpl Surg. (2020) 20(8):2243–53. 10.1111/ajt.15827
12.
Sobrino S Abdo C Neven B Denis A Gouge-Biebuyck N Clave E et al Human Kidney-Derived Hematopoietic Stem Cells Can Support Long-Term Multilineage Hematopoiesis. Kidney Int (2023) 103(1):70–6. 10.1016/j.kint.2022.08.024
13.
Kato T Tzakis AG Selvaggi G Gaynor JJ Takahashi H Mathew J et al Transplantation of the Spleen: Effect of Splenic Allograft in Human Multivisceral Transplantation. Ann Surg (2007) 246(3):436–44. 10.1097/SLA.0b013e3181485124
14.
Abu-Elmagd KM Kosmach-Park B Costa G Zenati M Martin L Koritsky DA et al Long-Term Survival, Nutritional Autonomy, and Quality of Life After Intestinal and Multivisceral Transplantation. Ann Surg (2012) 256(3):494–508. 10.1097/SLA.0b013e318265f310
15.
Zuber J Rosen S Shonts B Sprangers B Savage TM Richman S et al Macrochimerism in Intestinal Transplantation: Association With Lower Rejection Rates and Multivisceral Transplants Without GVHD. Am J Transpl Off J Am Soc Transpl Am Soc Transpl Surg (2015) 15(10):2691–703. 10.1111/ajt.13325
16.
Zuber J Shonts B Lau SP Obradovic A Fu J Yang S et al Bidirectional Intragraft Alloreactivity Drives the Repopulation of Human Intestinal Allografts and Correlates with Clinical Outcome. Sci Immunol (2016) 1(4):eaah3732. 10.1126/sciimmunol.aah3732
17.
Fu J Zuber J Shonts B Obradovic A Wang Z Frangaj K et al Lymphohematopoietic Graft-Versus-Host Responses Promote Mixed Chimerism in Patients Receiving Intestinal Transplantation. J Clin Invest (2021) 131(8):e141698. 10.1172/JCI141698
18.
Fu J Zuber J Martinez M Shonts B Obradovic A Wang H et al Human Intestinal Allografts Contain Functional Hematopoietic Stem and Progenitor Cells that are Maintained by a Circulating Pool. Cell Stem Cell (2019) 24(2):227–39. 10.1016/j.stem.2018.11.007
19.
Spitzer TR Tolkoff-Rubin N Cosimi AB McAfee S Dey BR Chen YB et al Twenty-Year Follow-Up of Histocompatibility Leukocyte Antigen-Matched Kidney and Bone Marrow Cotransplantation for Multiple Myeloma With End-Stage Renal Disease: Lessons Learned. Transplantation (2019) 103(11):2366–72. 10.1097/TP.0000000000002669
20.
Zuber J Sykes M . Mechanisms of Mixed Chimerism-Based Transplant Tolerance. Trends Immunol (2017) 38(11):829–43. 10.1016/j.it.2017.07.008
21.
Scandling JD Busque S Dejbakhsh-Jones S Benike C Millan MT Shizuru JA et al Tolerance and Chimerism After Renal and Hematopoietic-Cell Transplantation. N Engl J Med (2008) 358(4):362–8. 10.1056/NEJMoa074191
22.
Busque S Scandling JD Lowsky R Shizuru J Jensen K Waters J et al Mixed Chimerism and Acceptance of Kidney Transplants After Immunosuppressive Drug Withdrawal. Sci Transl Med (2020) 12(528):eaax8863. 10.1126/scitranslmed.aax8863
23.
Leventhal J Abecassis M Miller J Gallon L Ravindra K Tollerud DJ et al Chimerism and Tolerance Without GVHD or Engraftment Syndrome in HLA-Mismatched Combined Kidney and Hematopoietic Stem Cell Transplantation. Sci Transl Med (2012) 4(124):124ra28. 10.1126/scitranslmed.3003509
24.
Senev A Tambur AR Kosmoliaptsis V Copley HC García-Sánchez C Usenko C et al HLA Molecular Mismatches and Induced Donor-Specific Tolerance in Combined Living Donor Kidney and Hematopoietic Stem Cell Transplantation. Front Immunol (2024) 15:1377535. 10.3389/fimmu.2024.1377535
25.
Leventhal J Abecassis M Miller J Gallon L Tollerud D Elliott MJ et al Tolerance Induction in HLA Disparate Living Donor Kidney Transplantation by Donor Stem Cell Infusion: Durable Chimerism Predicts Outcome. Transplantation (2013) 95(1):169–76. 10.1097/TP.0b013e3182782fc1
26.
Podestà MA Sykes M . Chimerism-Based Tolerance to Kidney Allografts in Humans: Novel Insights and Future Perspectives. Front Immunol (2021) 12:791725. 10.3389/fimmu.2021.791725
27.
Bertaina A Grimm PC Weinberg K Parkman R Kristovich KM Barbarito G et al Sequential Stem Cell-Kidney Transplantation in Schimke immuno-Osseous Dysplasia. N Engl J Med (2022) 386(24):2295–302. 10.1056/NEJMoa2117028
28.
Baradaran-Heravi A Lange J Asakura Y Cochat P Massella L Boerkoel CF . Bone Marrow Transplantation in Schimke Immuno-Osseous Dysplasia. Am J Med Genet A (2013) 161A(10):2609–13. 10.1002/ajmg.a.36111
29.
Lum SH Albert MH Gilbert P Sirait T Algeri M Muratori R et al Outcomes of HLA-Mismatched HSCT with TCRαβ/CD19 Depletion or post-HSCT Cyclophosphamide for Inborn Errors of Immunity. Blood (2024) 144(5):565–80. 10.1182/blood.2024024038
30.
Pilat N . Inadvertent Stem Cell Transfer Demonstrating a Way for Tolerance Induction. Kidney Int Janv (2023) 103(1):21–2. 10.1016/j.kint.2022.11.007
31.
Sharabi Y Sachs DH . Mixed Chimerism and Permanent Specific Transplantation Tolerance Induced by a Nonlethal Preparative Regimen. J Exp Med (1989) 169(2):493–502. 10.1084/jem.169.2.493
32.
Wekerle T Kurtz J Ito H Ronquillo JV Dong V Zhao G et al Allogeneic Bone Marrow Transplantation With Co-Stimulatory Blockade Induces Macrochimerism and Tolerance Without Cytoreductive Host Treatment. Nat Med (2000) 6(4):464–9. 10.1038/74731
33.
Pilat N Baranyi U Klaus C Jaeckel E Mpofu N Wrba F et al Treg-Therapy Allows Mixed Chimerism and Transplantation Tolerance Without Cytoreductive Conditioning. Am J Transpl Off J Am Soc Transpl Am Soc Transpl Surg. (2010) 10(4):751–62. 10.1111/j.1600-6143.2010.03018.x
34.
Adams AB Pearson TC Larsen CP . Heterologous Immunity: An Overlooked Barrier to Tolerance. Immunol Rev (2003) 196:147–60. 10.1046/j.1600-065x.2003.00082.x
35.
Podestà MA Binder C Sellberg F DeWolf S Shonts B Ho SH et al Siplizumab Selectively Depletes Effector Memory T Cells and Promotes a Relative Expansion of Alloreactive Regulatory T Cells in vitro. Am J Transpl Off J Am Soc Transpl Am Soc Transpl Surg. (2020) 20(1):88–100. 10.1111/ajt.15533
36.
Lo DJ Anderson DJ Weaver TA Leopardi F Song M Farris AB et al Belatacept and Sirolimus Prolong Nonhuman Primate Renal Allograft Survival Without a Requirement for Memory T Cell Depletion. Am J Transplant off J Am Soc Transplant Am Soc Transpl Surg (2013) 13(2):320–8. 10.1111/j.1600-6143.2012.04342.x
37.
Cvetkovski F Razavi R Sellberg F Berglund E Berglund D . Siplizumab Combination Therapy with Belatacept or Abatacept Broadly Inhibits Human T Cell Alloreactivity In Vitro. Am J Transpl Off J Am Soc Transpl Am Soc Transpl Surg (2023) 23(10):1603–11. 10.1016/j.ajt.2023.05.032
38.
Kitchens WH Dong Y Mathews DV Breeden CP Strobert E Fuentes ME et al Interruption of OX40L Signaling Prevents Costimulation Blockade-Resistant Allograft Rejection. JCI Insight (2017) 2(5):e90317. 10.1172/jci.insight.90317
39.
Sasaki H Oura T Spitzer TR Chen YB Madsen JC Allan J et al Preclinical and Clinical Studies for Transplant Tolerance via the Mixed Chimerism Approach. Hum Immunol Mai (2018) 79(5):258–65. 10.1016/j.humimm.2017.11.008
40.
Kawai T Cosimi AB Spitzer TR Tolkoff-Rubin N Suthanthiran M Saidman SL et al HLA-Mismatched Renal Transplantation Without Maintenance Immunosuppression. N Engl J Med (2008) 358(4):353–61. 10.1056/NEJMoa071074
41.
Kawai T Sachs DH Sprangers B Spitzer TR Saidman SL Zorn E et al Long-Term Results in Recipients of Combined HLA-Mismatched Kidney and Bone Marrow Transplantation Without Maintenance Immunosuppression. Am J Transpl Off J Am Soc Transpl Am Soc Transpl Surg. (2014) 14(7):1599–611. 10.1111/ajt.12731
42.
Lee KW Park JB Park H Kwon Y Lee JS Kim KS et al Inducing Transient Mixed Chimerism for Allograft Survival Without Maintenance Immunosuppression with Combined Kidney and Bone Marrow Transplantation: Protocol Optimization. Transplantation (2020) 104(7):1472–82. 10.1097/TP.0000000000003006
43.
Sprangers B DeWolf S Savage TM Morokata T Obradovic A LoCascio SA et al Origin of Enriched Regulatory T Cells in Patients Receiving Combined Kidney-Bone Marrow Transplantation to Induce Transplantation Tolerance. Am J Transplant off J Am Soc Transplant Am Soc Transpl Surg (2017) 17(8):2020–32. 10.1111/ajt.14251
44.
Morris H DeWolf S Robins H Sprangers B LoCascio SA Shonts BA et al Tracking Donor-Reactive T Cells: Evidence for Clonal Deletion in Tolerant Kidney Transplant Patients. Sci Transl Med (2015) 7(272):272ra10. 10.1126/scitranslmed.3010760
45.
Shaffer J Villard J Means TK Alexander S Dombkowski D Dey BR et al Regulatory T-Cell Recovery in Recipients of Haploidentical Nonmyeloablative Hematopoietic Cell Transplantation with a Humanized anti-CD2 Mab, MEDI-507, With or Without Fludarabine. Exp Hematol (2007) 35(7):1140–52. 10.1016/j.exphem.2007.03.018
46.
Tonsho M O JM Ahrens K Robinson K Sommer W Boskovic S et al Cardiac Allograft Tolerance Can Be Achieved in Nonhuman Primates by Donor Bone Marrow and Kidney Cotransplantation. Sci Transl Med (2025) 17(782):eads0255. 10.1126/scitranslmed.ads0255
47.
Yang C Ge J Rosales I Yuan Q Szuter E Acheampong E et al Kidney-Induced Systemic Tolerance of Heart Allografts in Mice. JCI Insight. (2020) 5(18):e139331. 10.1172/jci.insight.139331
48.
Rosales IA Yang C Farkash EA Ashry T Ge J Aljabban I et al Novel Intragraft Regulatory Lymphoid Structures in Kidney Allograft Tolerance. Am J Transpl Off J Am Soc Transpl Am Soc Transpl Surg. (2022) 22(3):705–16. 10.1111/ajt.16880
49.
Kamano C Vagefi PA Kumagai N Yamamoto S Barth RN LaMattina JC et al Vascularized Thymic Lobe Transplantation in Miniature Swine: Thymopoiesis and Tolerance Induction Across Fully MHC-Mismatched Barriers. Proc Natl Acad Sci U S A. (2004) 101(11):3827–32. 10.1073/pnas.0306666101
50.
Waer M Palathumpat V Sobis H Vandeputte M . Induction of Transplantation Tolerance in Mice Across Major Histocompatibility Barrier by Using Allogeneic Thymus Transplantation and Total Lymphoid Irradiation. J Immunol Baltim Md (1990) 145(2):499–504. 10.4049/jimmunol.145.2.499
51.
Yamada K Shimizu A Utsugi R Ierino FL Gargollo P Haller GW et al Thymic Transplantation in Miniature Swine. II. Induction of Tolerance by Transplantation of Composite Thymokidneys to Thymectomized Recipients. J Immunol Baltim Md (2000) 164(6):3079–86. 10.4049/jimmunol.164.6.3079
52.
Montgomery RA Stern JM Lonze BE Tatapudi VS Mangiola M Wu M et al Results of Two Cases of Pig-to-Human Kidney Xenotransplantation. N Engl J Med (2022) 386(20):1889–98. 10.1056/NEJMoa2120238
53.
Markert ML Gupton SE McCarthy EA . Experience with Cultured Thymus Tissue in 105 Children. J Allergy Clin Immunol. (2022) 149(2):747–57. 10.1016/j.jaci.2021.06.028
54.
Kwun J Li J Rouse C Park JB Farris AB Kuchibhatla M et al Cultured Thymus Tissue Implantation Promotes Donor-specific Tolerance to Allogeneic Heart Transplants. JCI Insight. (2020) 5(11):e129983. 10.1172/jci.insight.129983
55.
Fitch ZW Kang L Li J Knechtle SJ Turek JW Kirk AD et al Introducing Thymus for Promoting Transplantation Tolerance. J Allergy Clin Immunol. (2022) 150(3):549–56. 10.1016/j.jaci.2022.05.006
56.
Kang L Markert ML Turek JW . Induction of Donor-Specific Tolerance to Heart Transplantation: From Concept to Clinical Translation. J Thorac Cardiovasc Surg (2023) 165(5):1661–6. 10.1016/j.jtcvs.2021.12.048
57.
Pullen LC . T Cells Prepare for a Test: Can They Tolerate a Donor Heart? Am J Transplant off J Am Soc Transplant Am Soc Transpl Surg. Am J Transplant (2022) 22(7):1731–2. 10.1111/ajt.16663
58.
Sawitzki B Harden PN Reinke P Moreau A Hutchinson JA Game DS et al Regulatory Cell Therapy in Kidney Transplantation (The ONE Study): A Harmonised Design and Analysis of Seven Non-randomised, single-arm, Phase 1/2A Trials. Lancet Lond Engl. (2020) 395(10237):1627–39. 10.1016/S0140-6736(20)30167-7
59.
Roemhild A Otto NM Moll G Abou-El-Enein M Kaiser D Bold G et al Regulatory T Cells for Minimising Immune Suppression in Kidney Transplantation: Phase I/IIa Clinical Trial. BMJ (2020) 371:m3734. 10.1136/bmj.m3734
60.
McCallion O Cross AR Brook MO Hennessy C Ferreira R Trzupek D et al Regulatory T Cell Therapy Is Associated With Distinct Immune Regulatory Lymphocytic Infiltrates in Kidney Transplants. Med N Y N. (2024) 6:100561. 10.1016/j.medj.2024.11.014
61.
Brook MA Hennessy C Hester J Hammad S Alzhrani A Rombach I et al Late Treatment with Autologous Expanded Regulatory T-cell Therapy After Alemtuzumab Induction Is Safe and Facilitates Immunosuppression Minimization in Living Donor Renal Transplantation. Transplantation (2024) 108(11):2278–86. 10.1097/TP.0000000000005065
62.
Brook MA Hester J Petchey W Rombach I Dutton S Bottomley MJ et al Transplantation Without Overimmunosuppression (TWO) Study Protocol: A Phase 2b Randomised Controlled single-centre Trial of Regulatory T Cell Therapy to Facilitate Immunosuppression Reduction in Living Donor Kidney Transplant Recipients. BMJ Open (2022) 12(4):e061864. 10.1136/bmjopen-2022-061864
63.
Todo S Yamashita K Goto R Zaitsu M Nagatsu A Oura T et al A Pilot Study of Operational Tolerance with a Regulatory T-cell-based Cell Therapy in Living Donor Liver Transplantation. Hepatol Baltim Md (2016) 64(2):632–43. 10.1002/hep.28459
64.
Tang Q Leung J Peng Y Sanchez-Fueyo A Lozano JJ Lam A et al Selective Decrease of donor-reactive Tregs After Liver Transplantation Limits Treg Therapy for Promoting Allograft Tolerance in Humans. Sci Transl Med (2022) 14(669):eabo2628. 10.1126/scitranslmed.abo2628
65.
Dong S Hiam-Galvez KJ Mowery CT Herold KC Gitelman SE Esensten JH et al The Effect of low-dose IL-2 and Treg Adoptive Cell Therapy in Patients with Type 1 Diabetes. JCI Insight. (2021) 6(18):e147474. 10.1172/jci.insight.147474
66.
Lim TY Perpiñán E Londoño MC Miquel R Ruiz P Kurt AS et al Low Dose interleukin-2 Selectively Expands Circulating Regulatory T Cells but Fails to Promote Liver Allograft Tolerance in Humans. J Hepatol. (2023) 78(1):153–64. 10.1016/j.jhep.2022.08.035
67.
Khoryati L Pham MN Sherve M Kumari S Cook K Pearson J et al An IL-2 Mutein Engineered to Promote Expansion of Regulatory T Cells Arrests Ongoing Autoimmunity in Mice. Sci Immunol. (2020) 5(50):eaba5264. 10.1126/sciimmunol.aba5264
68.
Tomasovic LM Liu K VanDyke D Fabilane CS Spangler JB . Molecular Engineering of Interleukin-2 for Enhanced Therapeutic Activity in Autoimmune Diseases. BioDrugs Clin Immunother Biopharm Gene Ther. (2024) 38(2):227–48. 10.1007/s40259-023-00635-0
69.
Lamarthée B Marchal A Charbonnier S Blein T Leon J Martin E et al Transient Mtor Inhibition Rescues 4-1BB CAR-Tregs From Tonic Signal-Induced Dysfunction. Nat Commun (2021) 12(1):6446. 10.1038/s41467-021-26844-1
70.
Dawson NAJ Rosado-Sánchez I Novakovsky GE Fung VCW Huang Q McIver E et al Functional Effects of Chimeric Antigen Receptor Co-Receptor Signaling Domains in Human Regulatory T Cells. Sci Transl Med (2020) 12(557):eaaz3866. 10.1126/scitranslmed.aaz3866
71.
Kurt AS Ruiz P Landmann E Elgosbi M Kan Fung T Kodela E et al Conferring Alloantigen Specificity to Regulatory T Cells: A Comparative Analysis of Cell Preparations Undergoing Clinical Development in Transplantation. Am J Transpl Off J Am Soc Transpl Am Soc Transpl Surg. (2025) 25(1):38–47. 10.1016/j.ajt.2024.09.009
72.
Amini L Wagner DL Rössler U Zarrinrad G Wagner LF Vollmer T et al CRISPR-Cas9-Edited Tacrolimus-Resistant Antiviral T Cells for Advanced Adoptive Immunotherapy in Transplant Recipients. Mol Ther J Am Soc Gene Ther. (2021) 29(1):32–46. 10.1016/j.ymthe.2020.09.011
73.
Kremer J Henschel P Simon D Riet T Falk C Hardtke-Wolenski M et al Membrane-Bound IL-2 Improves the Expansion, Survival, and Phenotype of CAR Tregs and Confers Resistance to Calcineurin Inhibitors. Front Immunol (2022) 13:1005582. 10.3389/fimmu.2022.1005582
74.
Chatenoud L Bluestone JA . CD3-Specific Antibodies: A Portal to the Treatment of Autoimmunity. Nat Rev Immunol. (2007) 7(8):622–32. 10.1038/nri2134
75.
Poirier N Azimzadeh AM Zhang T Dilek N Mary C Nguyen B et al Inducing CTLA-4-Dependent Immune Regulation by Selective CD28 Blockade Promotes Regulatory T Cells in Organ Transplantation. Sci Transl Med. (2010) 2(17):17ra10. 10.1126/scitranslmed.3000116
76.
Besnard M Sérazin C Ossart J Moreau A Vimond N Flippe L et al Anti-CD45RC Antibody Immunotherapy Prevents and Treats Experimental Autoimmune Polyendocrinopathy-Candidiasis-Ectodermal Dystrophy Syndrome. J Clin Invest (2022) 132(7):e156507. 10.1172/JCI156507
77.
Seng A Krausz KL Pei D Koestler DC Fischer RT Yankee TM et al Coexpression of FOXP3 and a Helios Isoform Enhances the Effectiveness of Human Engineered Regulatory T Cells. Blood Adv. (2020) 4(7):1325–39. 10.1182/bloodadvances.2019000965
78.
Ander S Diamond M Coyne CB . Immune Responses at the Maternal-Fetal Interface. Sci Immunol. (2019) 4(31):eaat6114. 10.1126/sciimmunol.aat6114
79.
Warren BD Ahn SH Brittain KS Nanjappa MK Wang H Wang J et al Multiple Lesions Contribute to Infertility in Males Lacking Autoimmune Regulator. Am J Pathol. (2021) 191(9):1592–609. 10.1016/j.ajpath.2021.05.021
80.
Albersammer L Leon J Martinovic J Dagobert J Lebraud E Bessières B et al Histologic and Molecular Features Shared Between Antibody-Mediated Rejection of Kidney Allografts and Chronic Histiocytic Intervillositis Support Common Pathogenesis. J Pathol. (2025) 266(2):177–91. 10.1002/path.6413
81.
Tersigni C Meli F Neri C Iacoangeli A Franco R Lanzone A et al Role of Human Leukocyte Antigens at the Feto-Maternal Interface in Normal and Pathological Pregnancy: An Update. Int J Mol Sci. (2020) 21(13):4756. 10.3390/ijms21134756
82.
Nancy P Tagliani E Tay CS Asp P Levy DE Erlebacher A . Chemokine Gene Silencing in Decidual Stromal Cells Limits T Cell Access to the Maternal-Fetal Interface. Science (2012) 336(6086):1317–21. 10.1126/science.1220030
83.
Stenqvist AC Nagaeva O Baranov V Mincheva-Nilsson L . Exosomes Secreted by Human Placenta Carry Functional Fas Ligand and TRAIL Molecules and Convey Apoptosis in Activated Immune Cells, Suggesting Exosome-Mediated Immune Privilege of the Fetus. J Immunol. (2013) 191(11):5515–23. 10.4049/jimmunol.1301885
84.
Munn DH Zhou M Attwood JT Bondarev I Conway SJ Marshall B et al Prevention of Allogeneic Fetal Rejection by Tryptophan Catabolism. Science (1998) 281(5380):1191–3. 10.1126/science.281.5380.1191
85.
Guleria I Khosroshahi A Ansari MJ Habicht A Azuma M Yagita H et al A Critical Role for the Programmed Death Ligand 1 in Fetomaternal Tolerance. J Exp Med (2005) 202(2):231–7. 10.1084/jem.20050019
86.
Rizzuto G Brooks JF Tuomivaara ST McIntyre TI Ma S Rideaux D et al Establishment of Fetomaternal Tolerance Through Glycan-Mediated B Cell Suppression. Nature (2022) 603(7901):497–502. 10.1038/s41586-022-04471-0
87.
Hu X Gattis C Olroyd AG Friera AM White K Young C et al Human Hypoimmune Primary Pancreatic Islets Avoid Rejection and Autoimmunity and Alleviate Diabetes in Allogeneic Humanized Mice. Sci Transl Med (2023) 15(691):eadg5794. 10.1126/scitranslmed.adg5794
88.
Hu X White K Young C Olroyd AG Kievit P Connolly AJ et al Hypoimmune Islets Achieve Insulin Independence After Allogeneic Transplantation in a Fully Immunocompetent Non-human Primate. Cell Stem Cell (2024) 31(3):334–40.e5. 10.1016/j.stem.2024.02.001
89.
Carlsson PO Hu X Scholz H Ingvast S Lundgren T Scholz T et al Survival of Transplanted Allogeneic Beta Cells with No Immunosuppression. N Engl J Med (2025) 393(9):887–94. 10.1056/NEJMoa2503822
90.
Stimmeder S Leber B Sucher R Stiegler P . Genetic Modulation: Future Trends Toward Graft Optimization During Machine Perfusion. Transplantation (2024) 108(3):614–24. 10.1097/TP.0000000000004738
91.
Filz von Reiterdank I Mojoudi M Bento R Taggart MS Dinicu AT Wojtkiewicz G et al Ex vivo Machine Perfusion as a Platform for Lentiviral Gene Delivery in Rat Livers. Gene Ther. (2025) 32(4):421–9. 10.1038/s41434-025-00536-7
92.
Rosales A Blondel LO Hull J Gao Q Aykun N Peek JL et al Evolving Adeno-Associated Viruses for Gene Transfer to the Kidney Via Cross-Species Cycling of Capsid Libraries. Nat Biomed Eng. (2025) 9(7):1086–100. 10.1038/s41551-024-01341-0
93.
Tietjen GT Hosgood SA DiRito J Cui J Deep D Song E et al Nanoparticle Targeting to the Endothelium During Normothermic Machine Perfusion of Human Kidneys. Sci Transl Med (2017) 9(418):eaam6764. 10.1126/scitranslmed.aam6764
94.
Kenta I Takaaki K . Molecular Mechanisms of Antibody-Mediated Rejection and Accommodation in Organ Transplantation. Nephron (2020) 144(Suppl. 1):2–6. 10.1159/000510747
95.
Liburd ST Shi AA Pober JS Tietjen GT . Wanted: An Endothelial Cell Targeting Atlas for Nanotherapeutic Delivery in Allograft Organs. Am J Transplant Off J Am Soc Transplant Am Soc Transpl Surg. (2022) 22(7):1754–9. 10.1111/ajt.17050
96.
Kawai T Sachs DH Sykes M Cosimi AB , Immune Tolerance Network. HLA-Mismatched Renal Transplantation Without Maintenance Immunosuppression. N Engl J Med. (2013) 368(19):1850–2. 10.1056/NEJMc1213779
Summary
Keywords
transplantation, thymus, hematopoietic chimerism, immune tolerance induction, regulatory cell therapy, genetic engineering
Citation
Blein T, Ayas N, Charbonnier S, Gil A, Leon J and Zuber J (2025) Tolerance Induction Strategies in Organ Transplantation: Current Status and Future Perspectives. Transpl. Int. 38:14958. doi: 10.3389/ti.2025.14958
Received
26 May 2025
Accepted
25 September 2025
Published
07 October 2025
Volume
38 - 2025
Updates
Copyright
© 2025 Blein, Ayas, Charbonnier, Gil, Leon and Zuber.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julien Zuber, julien.zuber@aphp.fr
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.