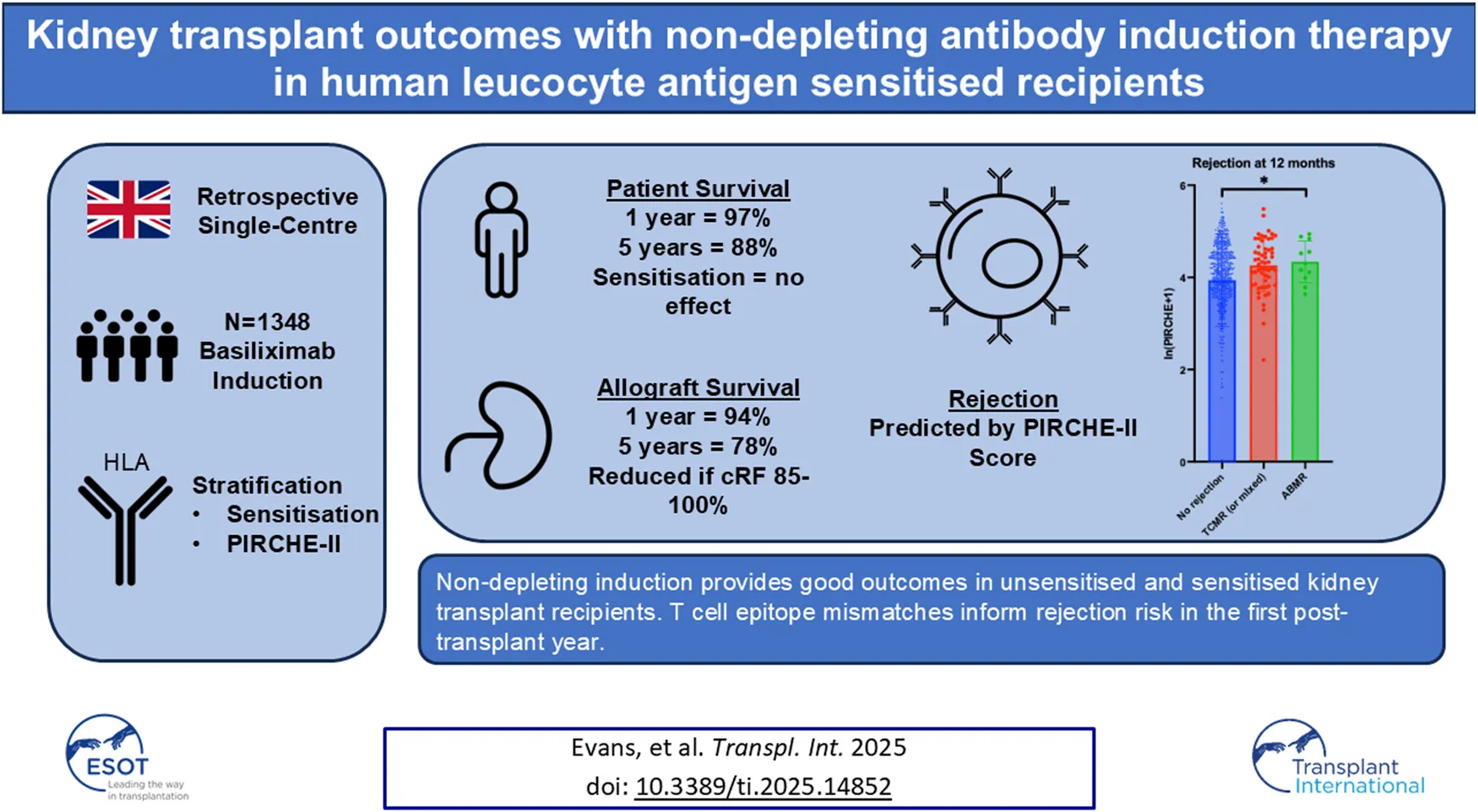
Abstract
Lymphocyte depleting induction is recommended for kidney transplant recipients (KTRs) at high immunological risk, which traditionally includes those with detectable anti-human leucocyte antigen antibodies. Data to support this approach in the modern era of histocompatibility testing are limited. We investigated outcomes in KTRs who underwent Basiliximab induction between 2012–2023 in the UK. We stratified outcomes by levels of sensitisation and T cell epitope mismatch (PIRCHE-II) scores. 1348 KTRs were included; 859 (63.7%) were unsensitised, 351 (26.0%) sensitised (calculated reaction frequency [cRF] 1%–84%), and 138 (10.3%) highly sensitised (cRF 85%–100%). Patient survival, allograft survival, and death-censored graft survival (DCGS) were 97%, 94%, and 97% at 1 year, and 88%, 78%, and 84% at 5 years respectively. There were no differences in outcomes between unsensitised and sensitised recipients; graft survival was lower in highly sensitised patients. T cell epitope mismatch scores were higher in those with rejection at 1 year (ln[PIRCHE+1] 3.94 ± 1.01 no rejection vs. 4.25 ± 0.58 rejection, p = 0.02) and epitope mismatch was associated with early rejection in multivariable analyses (Odds Ratio 1.58, 95% CI 1.01–2.62). Hence, non-depleting induction provides good outcomes in unsensitised and sensitised KTRs. T cell epitope mismatches inform rejection risk in the first post-transplant year.
Introduction
Induction immunosuppression in the form of antibody therapy is utilised in most kidney transplant procedures. These agents primarily modulate the T cell response to foreign human leucocyte antigens (HLAs). This results in reduced rates of acute rejection and allows for a reduction in the use of other immunosuppressive agents, such as calcineurin inhibitors and corticosteroids, that have unwanted side effects when used at high dose [1].
Induction antibody therapy may be classified into agents that deplete T cells (e.g., Antithymocyte globulin [ATG] and Alemtuzumab [Campath]), B cells or complement, and agents that are non-depleting, which act by inhibiting cytokine signalling important in T cell activation and proliferation, e.g., IL-2 receptor antagonists (IL2-RAs) such as Basiliximab. In general, depleting agents provide more profound immunosuppression which is counterbalanced by increased infectious and malignant complications as well as an increased cost [2].
The choice of which induction agent to use continues to be a source of debate amongst transplant professionals globally, with marked variation in practice between centres even within the same country [3–5]. International guidelines published by Kidney Disease Improving Global Outcomes (KDIGO) recommend basing the choice of induction agent on an assessment of immunological risk, with IL2-RAs recommended as first line, and depleting antibodies used in cases at increased risk [6]. This approach is supported by guidelines from the United Kingdom (UK) [7].
One of the key determinants of immunological risk is the presence of preformed antibodies to HLAs. Traditionally, the presence of such antibodies was detected and identified using a panel of lymphocytes, with the relatively non-specific and subjective complement dependent cytotoxicity test, which was then reported as percentage panel reactive antibodies (PRA) [8]. Significant advances in histocompatibility methods, including the development of single antigen bead testing using Luminex based technology, have meant the identification of HLA antibodies now occurs with exquisite sensitivity and specificity [9]. The presence of antibodies is now quantified by the calculated PRA (cPRA), or the calculated reaction frequency (cRF) in the UK, with immunological risk primarily due to antibodies that are donor specific [10, 11]. These advancements in HLA antibody identification methodology have occurred in parallel with advancements in molecular HLA typing methods which have enabled the HLA typing of transplant pairs at all loci to a high resolution. Subsequent computational algorithms have been developed to inform HLA matching according to differences in structure at the epitope level [12, 13].
The KDIGO guidelines, published 13 years ago, are primarily based on studies that pre-date these advancements in histocompatibility and immunogenetics [6]. For example, there have been 2 large trials comparing ATG with IL2-RA induction in patients at increased immunological risk, and inclusion was based on the historic assessment of HLA sensitisation with PRA in both [14, 15]. Moreover, the pivotal study by Brennan et al. compared ATG to Basiliximab in the setting of maintenance immunosuppression with cyclosporin, subsequently shown to be inferior to tacrolimus based regimens [16]. As such, the relevance of these historic guidelines to the contemporary management of kidney transplant recipients should be questioned, and cohorts reporting outcomes in patients managed in the modern era of histocompatibility testing are required.
For over a decade, our centre protocol has been to use IL2-RA induction for all kidney transplant recipients. This provides a unique opportunity for the assessment of kidney transplant outcomes when non-depleting induction therapy is used across a range of immunological risk. In this study, we determine patient and allograft outcomes of kidney transplants undertaken with Basiliximab induction. We assess outcomes stratified by current standard and novel measures of immune risk.
Patients and Methods
Study Design, Setting, and Participants
We undertook a single-centre, observational, cohort study of kidney transplant recipients who underwent transplantation at the Royal Free Hospital, London, UK. Adult patients (aged >18 years) who underwent kidney alone transplantation and had induction therapy with Basiliximab between 1st January 2012 and 31st December 2022 were included. Patients who underwent multiorgan transplant, and those who had induction with depleting antibodies, or in whom the induction agent was unclear, were excluded.
Our centre provides kidney transplant services to a multi-ethnic population from a large geographical area in north central London and Hertfordshire. Approximately 130 kidney alone transplants are undertaken each year. For the entire study period, the unit protocol was for all patients to undergo induction with Basiliximab, 20 mg administered intravenously on the day of transplant, repeated on postoperative day 4. The maintenance immunosuppression and infectious prophylaxis protocols are outlined in Supplementary File 1 [1]. Ultimately around 70% of recipients are managed steroid free long term [17]. We follow a pre-emptive strategy for the management of cytomegalovirus (CMV) and protocol biopsies are not performed. HLA antibodies are routinely measured at 1-, 3-, 6- and 12-month after transplant, and yearly thereafter. Biopsies are performed if an HLA antibody is donor specific and its development is associated with evidence of graft dysfunction (e.g., change in creatinine or development of proteinuria).
Variables, Data Sources and Measurement
Data were documented prospectively within electronic health records and retrospectively analysed. Clinical variables related to the donor (age, sex, and donor type), recipient (age, sex, ethnicity, and cause of end stage kidney disease), and the transplant (pre-emptive, previous transplant, and mismatch at HLA-A, -B, and -DR loci) were recorded.
Assessment of HLA Sensitisation
Patients were grouped according to levels of HLA sensitisation. Levels of sensitisation were determined using the cRF at the time of transplantation. This measure represents the percentage of the previous 10,000 blood group identical kidney donors against whom the recipient has HLA antibodies. The inclusion of blood group distinguishes the cRF from the assessment of sensitisation using the calculated panel reactive antibody, which is the predominant method outside of the UK [18]. Details of the techniques used for antibody identification and HLA typing are outlined in Supplementary File 1 [2]. Patients were categorised as unsensitised (cRF 0%), sensitised (cRF 1%–84%), and highly sensitised (cRF 85%–100%); a subgroup analysis was undertaken in patients who were very highly sensitised (cRF 98%–100%).
Assessment of T cell Epitope Mismatch
In a subset of patients with the necessary molecular HLA typing, T cell epitope mismatches were determined. T cell epitope mismatches were quantified using Predicted Indirectly ReCognizable HLA Epitope (PIRCHE-II) scores.1 This scoring system employs a computational algorithm using in silico antigen presentation pathway analysis to predict the number of mismatched HLA peptides that can be presented in the context of recipient HLA class II [13]. The PIRCHE-II score is the sum of all donor-derived candidate peptides that have a predicted binding affinity to the recipients HLA class II of less than 1000 nM [19]. Scores were transformed using the natural logarithm for analysis [20], with a score of 1 added to all recipients to allow inclusion of patients with a PIRCHE-II score of 0, as has been undertaken in previous analyses [21].
Outcome Measures
Patient and allograft outcomes were recorded at 12-, 36-, and 60-month after transplantation. Primary outcome measures were patient survival, graft survival, and death-censored graft survival. We also determined graft function (creatinine and estimated glomerular filtration rate [eGFR]), rates and type of biopsy proven rejection, rejection free allograft survival, infectious complications including CMV and BK viremia, and the development of malignancy. Outcomes were stratified by cRF and PIRCHE-II scores.
Statistical Methods
Data are reported as number and percentages for categorical variables and mean and standard deviation (SD) or median and interquartile range (IQR) for numerical variables depending on data distribution. Categorical variables were compared using the Fisher’s exact or Chi-squared test. Numerical variables were compared between 2 groups using the Mann–Whitney or an unpaired t test, and across greater than 2 groups with a one-way analysis of variance. Kaplan-Meier survival curves were plotted for patient and allograft outcomes, with differences between groups assessed using the log-rank test. Multivariable logistic regression analyses were undertaken to determine clinical variables associated with rejection at 12-month. Odds ratios (OR) and 95% confidence intervals (CIs) were determined for each variable. Multivariable cox regression analyses were undertaken to determine clinical variables associated with patient survival, graft survival, DCGS, and rejection-free allograft survival over 60-month of follow-up. Hazard ratios (HR) and 95% CIs were determined for each variable. Variables included in multivariable models were recipient demographic variables and clinical variables with a p value of <0.05 in univariable analyses. These included recipient age, sex, and ethnicity, transplant type (live/DBD/DCD; pre-emptive or not; first or subsequent graft), HLA-mismatch, cRF, and ln(PIRCHE+1). Models were developed with cRF and ln(PIRCHE+1) as both continuous and categorical variables. Analysis was performed using GraphPad Prism version 10.2 A p-value of ≤0.05 was considered statistically significant.
Ethics Statement
The study involved the retrospective analysis of routinely collected clinical data and, as such, was exempt from formal review board approval.
Results
Cohort Description
1389 kidney transplants were undertaken during the study period. Of these, 1359 (97.8%) were kidney alone transplants that underwent induction with Basiliximab and were included in the analysis (Figure 1). Recipients had a mean age of 50.0 ± 14.0 years, 494 (36.7%) were female, 615 (45.3%) were of white ethnicity, and diabetes was the cause of ESKD in 307 (22.6%) patients (Table 1). 374 (27.5%) patients underwent living donor kidney transplant, and 985 (72.5%) patients underwent deceased donor kidney transplant. Transplants were pre-emptive in 323 (23.8%) patients and represented a first kidney transplant in 1168 (86.0%) cases.
FIGURE 1
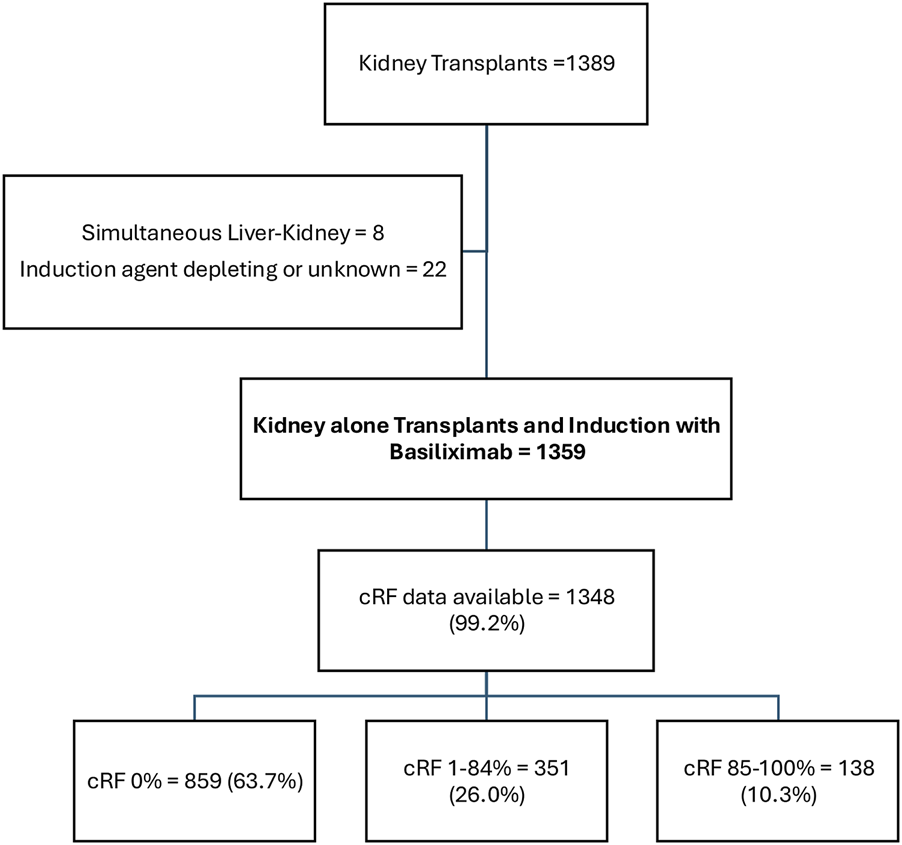
Cohort Description.
TABLE 1
| Clinical Variable | Whole population | cRF 0% | cRF 1%–84% | cRF 85%–100% | P-value |
|---|---|---|---|---|---|
| Number of patients | 1359 | 859 | 351 | 138 | |
| Donor Variables | |||||
| Age (mean; SD) | 48.30 (14.67) | 48.86 (14.60) | 47.77 (14.31) | 45.99 (15.81) | 0.07 |
| Donor type | |||||
| Live | 374 (27.5) | 254 (29.6) | 102 (29.1) | 18 (13.1) | <0.0001 |
| Donor after Brain Death (DBD) | 628 (46.2) | 367 (42.7) | 167 (47.6) | 86 (62.3) | |
| Donor after Cardiac Death (DCD) | 357 (26.3) | 238 (27.7) | 82 (23.4) | 34 (24.6) | |
| Recipient Variables | |||||
| Age (mean; SD) | 49.95 (13.98) | 50.51 (14.39) | 49.43 (13.34) | 47.28 (13.00) | 0.032 |
| Sex (n = female; %) | 494 (36.4) | 249 (29.0) | 166 (47.3) | 73 (52.9) | <0.0001 |
| Ethnicity | |||||
| White (n; %) | 615 (45.3) | 414 (48.2) | 146 (41.6) | 51 (37.0) | 0.0063 |
| Asian (n; %) | 408 (30.0) | 255 (29.7) | 113 (32.2) | 38 (27.5) | |
| Black (n; %) | 336 (24.7) | 190 (22.1) | 92 (26.2) | 49 (35.5) | |
| Cause of ESKD | |||||
| Diabetes (n; %) | 307 (22.6) | 217 (25.3) | 64 (18.2) | 23 (18.2) | 0.0094 |
| Polycystic kidney (n; %) | 105 (7.7) | 73 (8.5) | 22 (8.5) | 10 (6.3) | |
| Other/unknown (n: %) | 947 (69.7) | 569 (66.2) | 265 (66.2) | 105 (75.5) | |
| Transplant Variables | |||||
| Pre-emptive (n; %) | 323 (23.8) | 219 (25.5) | 85 (22.9) | 16 (11.5) | 0.0008 |
| First transplant (n; %) | 1168 (86.0) | 821 (95.6) | 288 (82.1) | 51 (37.0) | <0.0001 |
| Total HLA-A, -B, -DR Mismatch (mean; SD) | 2.99 (1.37) | 3.08 (1.31) | 2.96 (1.37) | 2.41 (1.49) | <0.0001 |
| Total HLA-A, -B, -DR Mismatch 0–3 (n; %) | 900 (66.2) | 561 (65.3) | 231 (65.8) | 105 (76.1) | 0.0396 |
Clinical characteristics of the cohort.
Significant results are highlighted in bold.
cRF data were available in 1348 (99.2%) patients. 859 (63.7%), 351 (26.0%), and 138 (10.3%) patients had cRFs of 0% (unsensitised), 1%–84% (sensitised), and 85%–100% (highly sensitised) respectively. 59 (4.4%) patients had a donor specific antibody (DSA) detectable at the time of transplant (antibodies against all HLA loci were represented, median fluorescence intensity ranged 971–8000), and 36 (2.8%) patients had a DSA detectable in historical sera. Highly sensitised patients were younger, more commonly female, less commonly white, less frequently underwent living or pre-emptive kidney transplantation, and had a better total match at HLA-A, -B, and -DR loci.
Patient and Allograft Outcomes
1080 (79.5%) patients were followed up to 12 months, 844 (62.1%) patients to 36 months, and 659 (48.5%) patients to 60 months. In the entire cohort, patient survival was 96.9%, 93.6%, and 87.6%, allograft survival was 93.9%, 88.4%, and 78.3%, and DCGS was 96.9%, 92.1%, and 84.5% at 12-, 36-, and 60-month respectively (Table 2). Patient survival was not different according to cRF categories, whereas allograft survival and DCGS were lower in highly sensitised patients compared to other cRF groups (Figure 2). Subgroup analyses of outcomes in very highly sensitised patients (cRF 98%–100%) and in deceased donor kidney transplants are outlined in Supplementary File 1 [3], [4]; patient and allograft outcomes followed similar trends to the wider cohort, albeit did not reach statistical significance for all outcomes. There was no difference in patient or allograft survival between sensitised patients with and without a preformed DSA (detected either at the time of transplant or historically).
TABLE 2
| Outcome | Whole population | cRF 0% | cRF 1%–84% | cRF 85%–100% | P-value (comparing all cRF categories) | P-value (cRF 0% vs. cRF 1%–84%) | P-value (cRF 0% vs. cRF 85%–100%) | P-value (cRF 1%–84% vs. cRF 85%–100%) |
|---|---|---|---|---|---|---|---|---|
| Patient survival | ||||||||
| 12 months | 1047/1080 (96.94) | 653/669 (97.61) | 290/298 (97.32) | 104/113 (92.04) | 0.16 | 0.82 | 0.0054 | 0.02 |
| 36 months | 790/844 (93.60) | 482/514 (93.77) | 240/252 (95.24) | 68/78 (87.18) | 0.39 | 0.51 | 0.054 | 0.019 |
| 60 months | 577/659 (87.56) | 349/400 (87.25) | 183/202 (90.59) | 45/57 (78.95) | 0.34 | 0.28 | 0.10 | 0.02 |
| Graft Survival | ||||||||
| 12 months | 1014/1080 (93.89) | 635/669 (94.92) | 284/298 (95.30) | 95/113 (84.07) | 0.0018 | 0.87 | 0.001 | 0.0004 |
| 36 months | 746/844 (88.39) | 455/514 (88.52) | 229/252 (90.87) | 62/78 (79.49) | 0.16 | 0.38 | 0.04 | 0.0092 |
| 60 months | 516/659 (78.30) | 306/400 (76.50) | 170/202 (84.15) | 40/57 (70.18) | 0.027 | 0.034 | 0.32 | 0.02 |
| Death Censored Allograft Survival | ||||||||
| 12 months | 1014/1047 (96.85) | 635/653 (97.24) | 284/290 (97.93) | 95/104 (91.35) | 0.09 | 0.66 | 0.007 | 0.0053 |
| 36 months | 746/810 (92.10) | 455/490 (92.86) | 229/244 (93.86) | 62/76 (81.58) | 0.0324 | 0.76 | 0.0033 | 0.0024 |
| 60 months | 516/611 (84.45) | 306/364 (84.07) | 170/191 (89.01) | 40/56 (71.43) | 0.0079 | 0.13 | 0.04 | 0.0024 |
Patient and Allograft outcomes at 12-, 36-, and 60-month in the whole population, and in unsensitised (cRF 0%), sensitised (cRF 1%–84%), and highly sensitised (cRF 85%–100%) patients.
Significant results are highlighted in bold.
FIGURE 2
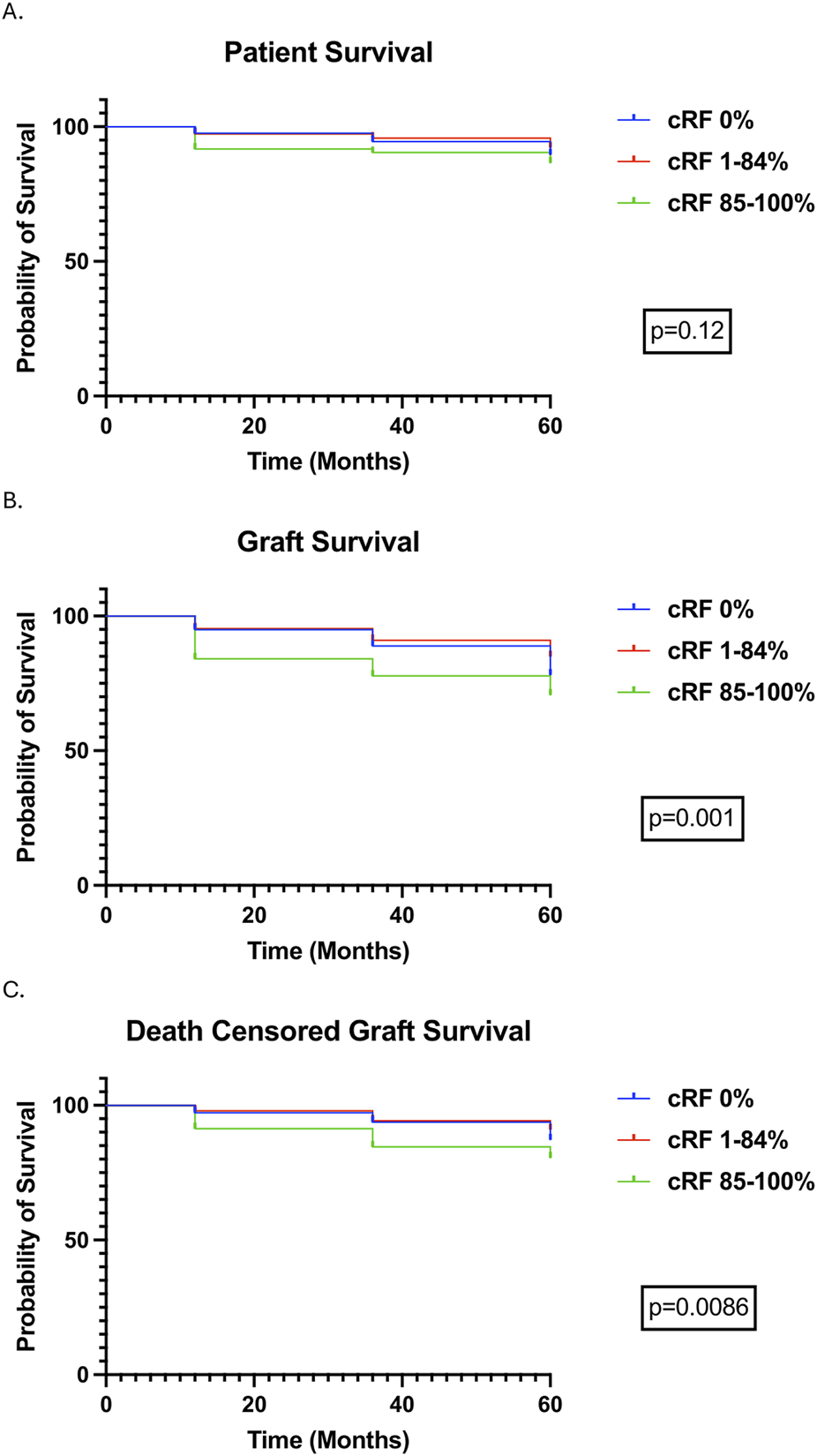
Transplant Outcomes over a follow-up period of 5 years stratified by cRF. Survival curves were compared using the logrank test. (A) Patient Survival, (B) Allograft Survival, (C) Death Censored Allograft Survival.
Graft Function, Rejection, Infection and Malignancy
Median (IQR) creatinine of all patients was 125 (102–158) μmol/L, 129 (102–175) μmol/L, and 130 (102–187) μmol/L at 12-, 36-, and 60-month post-transplant; eGFR was 50 (38–64) mL/min, 47 (33–64) mL/min, and 48 (32–64) mL/min at the same timepoints respectively (Table 3; Supplementary File 1 [5]). There were no differences in GFR between cRF categories at any of the follow-up time points (Table 3, Supplementary File 1 [6]).
TABLE 3
| Outcomes | Whole population | cRF 0% | cRF 1%–84% | cRF 85%–100% | P-value (comparing all cRF categories) | P-value (cRF 0% vs. cRF 1%–84%) | P-value (cRF 0% vs. cRF 85%–100%) | P-value (cRF 1%–84% vs. cRF 85%–100%) |
|---|---|---|---|---|---|---|---|---|
| 12-month outcomes | ||||||||
| Creatinine (μmol/l; median, IQR) | 125 (102–158) | 129 (104–162) | 118 (98–145) | 126 (96–166) | 0.0009 | 0.0005 | >0.99 | 0.26 |
| eGFR (ml/min; median, IQR) | 50 (38–65) | 50 (38–64) | 52 (40–66) | 47 (36–69) | 0.19 | 0.29 | >0.99 | 0.51 |
| Rejection (n; %) | 101/1080 (9.35) | 63/669 (9.42) | 20/298 (6.71) | 18/113 (15.93) | 0.021 | 0.17 | 0.044 | 0.0068 |
| TCMR (n; % of rejection) | 81/101 (80.20) | 56/63 (88.89) | 13/20 (65.00) | 12/18 (66.67) | 0.014 | 0.03 | 0.03 | >0.99 |
| ABMR (n; % of rejection) | 9/101 (8.91) | 2/63 (3.17) | 4/20 (20.00) | 3/18 (16.67) | 0.020 | 0.03 | 0.07 | >0.99 |
| Mixed/Both TCMR and ABMR (n; % of rejection) | 11/101 (10.89) | 5/63 (7.94) | 3/20 (15.00) | 3/18 (16.67) | 0.42 | 0.39 | 0.37 | >0.99 |
| CMV viremia (n; %) | 259/1080 (23.98) | 154/669 (23.02) | 77/298 (25.84) | 27/113 (23.89) | 0.63 | 0.37 | 0.81 | 0.80 |
| BK viremia (any level) (n; %) | 137/1080 (12.69) | 90/669 (13.45) | 30/298 (10.07) | 17/113 (15.04) | 0.24 | 0.17 | 0.66 | 0.17 |
| BK viremia (>10,000 copies/mL) (n; %) | 65/1080 (6.02) | 44/669 (6.58) | 13/298 (4.36) | 8/113 (7.08) | 0.35 | 0.24 | 0.84 | 0.31 |
| 36-month outcomes | ||||||||
| Creatinine (μmol/l; median, IQR) | 129 (102–175) | 132 (107–179) | 121 (96–165) | 145 (102–193) | 0.0039 | 0.006 | >0.99 | 0.07 |
| eGFR (ml/min; median, IQR) | 47 (33–64) | 47 (32–62) | 50 (35–68) | 41 (28–69) | 0.089 | 0.21 | >0.99 | 0.19 |
| Rejection (n; %) | 87/844 (10.31) | 50/514 (9.73) | 17/252 (6.75) | 20/78 (25.64) | <0.0001 | 0.22 | 0.0002 | <0.0001 |
| TCMR (n; % of rejection) | 66/87 (75.86) | 43/50 (86.00) | 9/17 (52.94) | 14/20 (70.00) | 0.020 | 0.015 | 0.17 | 0.32 |
| ABMR (n; % of rejection) | 7/87 (8.05) | 1/50 (2.00) | 3/17 (17.65) | 3/20 (15.00) | 0.026 | 0.048 | 0.07 | >0.99 |
| Mixed/Both TCMR and ABMR (n; % of rejection) | 14/87 (16.09) | 6/50 (12.00) | 5/17 (29.41) | 3/20 (15.00) | 0.22 | 0.13 | 0.71 | 0.43 |
| Malignancy (n; %) | 52/844 (6.16) | 37/514 (7.20) | 10/252 (3.97) | 5/78 (6.41) | 0.21 | 0.11 | >0.99 | 0.36 |
| Cardiovascular event (n; %) | 34/844 (4.03) | 19/514 (3.70) | 12/252 (4.76) | 3/78 (3.85) | 0.74 | 0.56 | >0.99 | >0.99 |
| 60-month outcomes | ||||||||
| Creatinine (μmol/l; median, IQR) | 130 (102–187) | 132 (105–192) | 128 (98–171) | 122 (96–201) | 0.15 | 0.19 | >0.99 | >0.99 |
| eGFR (ml/min; median, IQR) | 48 (32–64) | 47 (31–63) | 50 (32–66) | 46 (28–72) | 0.64 | >0.99 | >0.99 | >0.99 |
| Rejection (n; %) | 76/659 (11.53) | 45/400 (11.25) | 16/202 (7.92) | 15/57 (26.32) | 0.0016 | 0.25 | 0.005 | 0.0007 |
| TCMR (n; % of rejection) | 55/76 (72.37) | 33/45 (73.33) | 10/16 (62.50) | 12/15 (80.00) | 0.56 | 0.53 | 0.74 | 0.43 |
| ABMR (n; % of rejection) | 8/76 (10.53) | 5/45 (11.11) | 1/16 (6.25) | 2/15 (13.33) | 0.88 | >0.99 | >0.99 | 0.60 |
| Mixed/Both TCMR and ABMR (n; % of rejection) | 13/76 (17.11) | 7/45 (15.56) | 5/16 (31.25) | 1/15 (6.67) | 0.22 | 0.27 | 0.67 | 0.17 |
| Malignancy (n; %) | 53/659 (8.04) | 34/400 (8.50) | 13/202 (6.44) | 6/57 (10.53) | 0.51 | 0.42 | 0.62 | 0.39 |
| Cardiovascular event (n; %) | 39/659 (5.91) | 23/400 (5.75) | 14/202 (6.93) | 2/57 (3.51) | 0.67 | 0.59 | 0.76 | 0.54 |
Outcomes 12-, 36-, and 60-month in unsensitised (cRF 0%), sensitised (cRF 1%–84%), and highly sensitised (cRF 85%–100%) recipients.
Significant results are highlighted in bold.
Rejection rates (cumulative) were 9.35%, 10.31%, and 11.53% in patients followed-up to 12-, 36-, and 60-month. Rejection was more common in highly sensitised patients at all time points and there was more antibody mediated rejection (ABMR) within the first 12 months as levels of sensitisation increased (Table 3). 11 (61%) of 18 highly sensitised patients that experienced rejection at 12-month were very highly sensitised (cRF 98%–100%); outcomes in very highly sensitised patients are summarised in Supplementary File 1 [7]. As with highly sensitised patients, rejection was higher in very highly sensitised compared to other cRF categories at all time points, and ABMR occurred more frequently, albeit T cell mediated rejection (TCMR) remained the commonest type of rejection overall. Rejection free allograft survival was worse in highly sensitised and very highly sensitised patients compared to other cRF groups, primarily driven by increased rejection in the first post-transplant year (Figures 3A,B). Rejection free allograft survival was also worse in sensitised patients with a preformed DSA detected either at the time of transplant or in historical sera, compared to sensitised patients without a preformed DSA (Figure 3C).
FIGURE 3
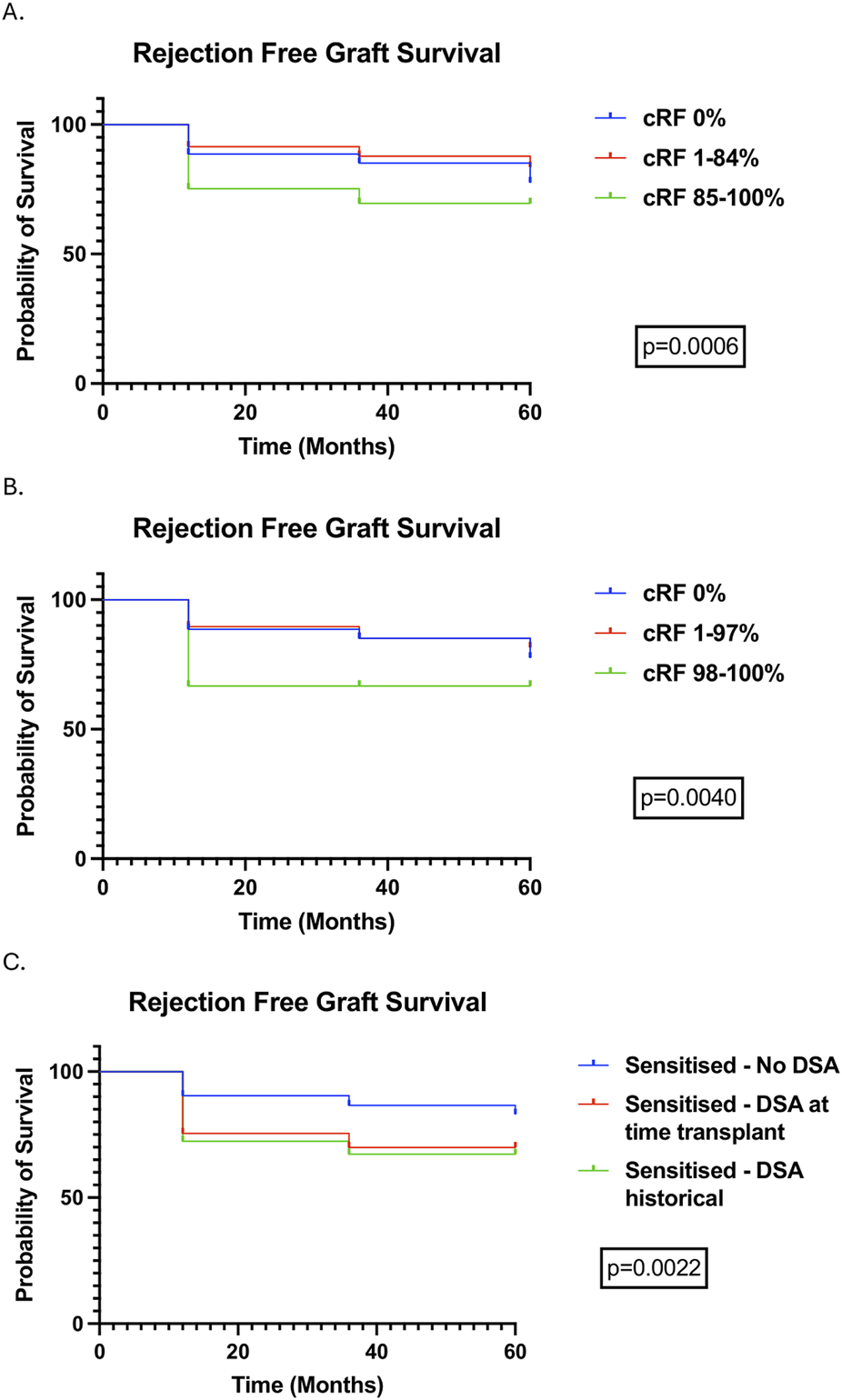
Rejection free allograft survival (censored for death) stratified by cRF categories, demonstrating outcomes in highly sensitised (A) and very highly sensitised (B) patients, and stratified by the presence of DSAs, detectable at the time of transplant and historically (C).
CMV viremia occurred in 259 (23.98%) patients within the first post-transplant year. BK viremia of any level occurred in 137 (12.69%) patients and BK viremia >104 copies/mL occurred in 65 (6.02%) patients. There was no difference in the prevalence of either infection between cRF categories (Table 3). At 60-month post-transplant, 53 (8.04%) patients had developed a malignancy, and 39 (5.91%) patients had experienced a cardiovascular event. There were also no differences in these events between cRF categories (Table 3).
T cell Epitope Mismatch and Rejection
T cell epitope mismatch data were available in 825 patients; the baseline clinical characteristics of these patients are outlined in Supplementary File 1 [8]. 740 (89.7%) and 335 (40.6%) patients completed follow-up to 12 and 60 months respectively. Mean ln(PIRCHE+1) scores were 4.249 ± 0.583 and 3.973 ± 1.005 in patients with and without rejection at 12-month (p = 0.022), and 4.102 ± 0.669 and 3.909 ± 0.987 in patients with and without rejection at 60-month (p = 0.27) (Figures 4a, b).
FIGURE 4
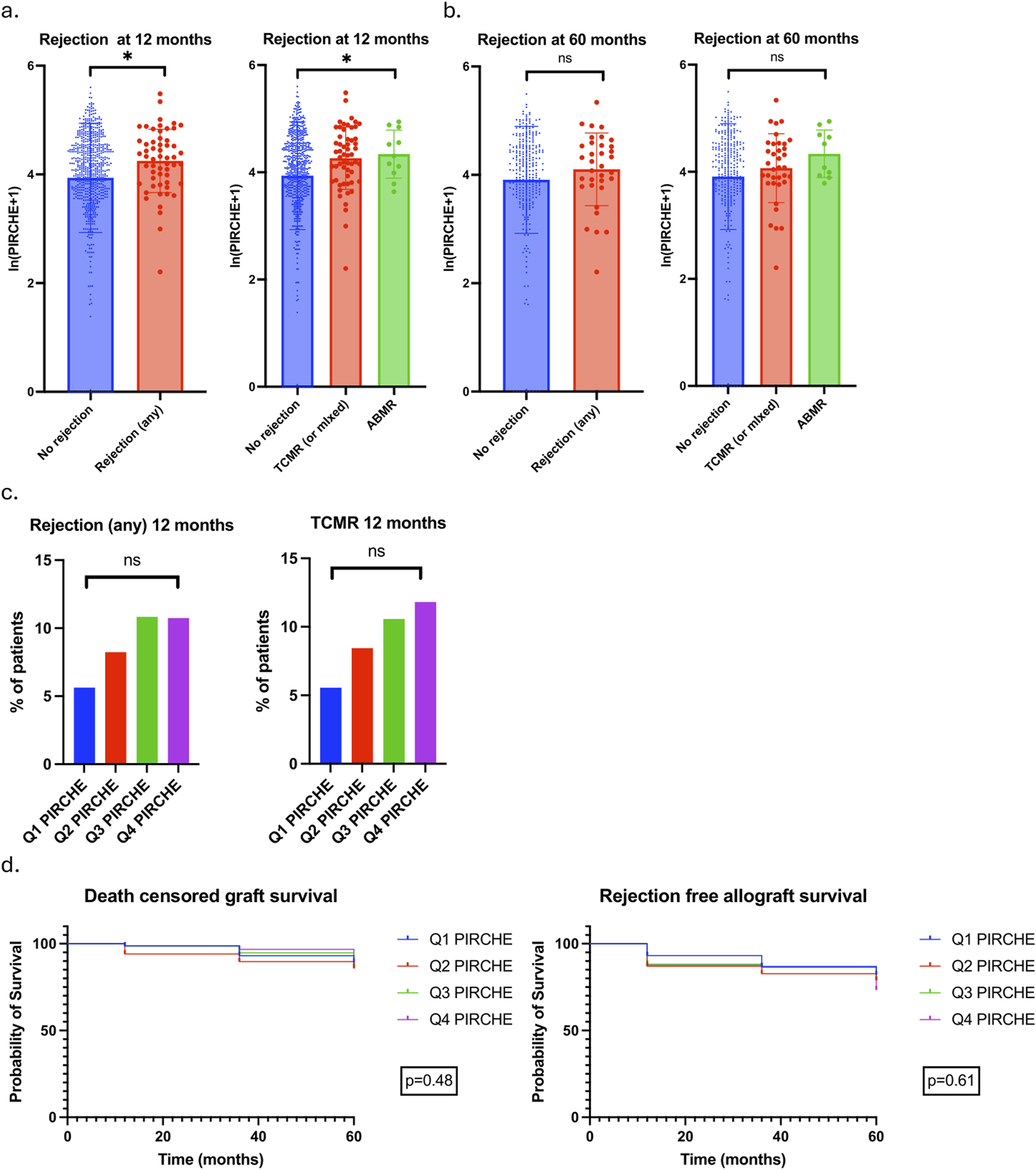
Association between PIRCHE scores and rejection. (a) ln(PIRCHE+1) scores in patients with no rejection, any rejection, TCMR/mixed rejection and ABMR at 12 months. Mean and SD values plotted. No rejection 3.937 ± 1.005, Any rejection 4.249 ± 0.583, TCMR/mixed 4.262 ± 0.581, ABMR 4.330 ± 0.447. P value for unpaired T-test comparing No rejection to any rejection 0.022; P value for one-way ANOVA comparing no rejection and TCMR/mixed and ABMR 0.024. (b) ln(PIRCHE+1) scores in patients with no rejection, any rejection, TCMR/mixed rejection and ABMR at 60 months. Mean and SD values plotted. No rejection 3.909 ± 0.987, Any rejection 4.102 ± 0.669, TCMR/mixed 4.067 ± 0.644, ABMR 4.336 ± 0.444. P value for unpaired T-test comparing No rejection to any rejection 0.27; P value for one-way ANOVA comparing no rejection and TCMR/mixed and ABMR 0.27. (c) 12-month rejection rates in patients divided into PIRCHE score quartiles. Rates of any rejection and TCMR/mixed rejection plotted. (d) DCGS and rejection free allograft survival in patients stratified by PIRCHE quartile.
Patients were divided into PIRCHE score quartiles with quartile 1 (Q1 PIRCHE) having the lowest and quartile 4 (Q4 PIRCHE) having the highest PIRCHE scores. Rejection of any type at 12 months occurred in 9 (5.63%) patients in Q1 PIRCHE and 16 (10.74%) patients in Q4 PIRCHE (p = 0.29); TCMR occurred in 8 (5.56%) and 15 (11.81%) patients in Q1 and Q4 PIRCHE respectively (p = 0.27). There was a stepwise increase in rejection (both any rejection and TCMR) with each increase in PIRCHE quartile but this did not reach statistical significance (Figure 4c). There were no differences in DCGS or rejection free allograft survival when patients were stratified by PIRCHE scores (Figure 4d).
Multivariable logistic regression analyses were undertaken to determine clinical variables associated with rejection at 12-month (Table 4). Ln(PIRCHE+1) was associated with rejection at 12-month (Odds Ratio 1.576, 95% CI 1.006–2.618) whereas cRF was not.
TABLE 4
| Clinical Variable | Odds Ratio for Rejection at 12-month | 95% confidence interval |
|---|---|---|
| Age at Transplant | 0.9844 | 0.9629 to 1.006 |
| Male Sex [reference = female] | 0.7775 | 0.4319 to 1.423 |
| Ethnicity [black; reference = white] | 0.9112 | 0.4449 to 1.825 |
| Ethnicity [Asian; reference = white] | 0.7587 | 0.3572 to 1.548 |
| DBD transplant [reference = live transplant] | 0.5766 | 0.2690 to 1.246 |
| DCD transplant [reference = live transplant] | 0.9076 | 0.4140 to 2.005 |
| Total HLA Mismatch at HLA-A, -B, -DR loci | 1.060 | 0.8121 to 1.382 |
| cRF 1%–84% (sensitised) [reference = unsensitised) | 0.6298 | 0.2819 to 1.296 |
| cRF 85%–100% (highly sensitised) [reference = unsensitised) | 1.856 | 0.7282 to 4.475 |
| Pre-emptive transplant [reference = not pre-emptive] | 1.336 | 0.6619 to 2.586 |
| Multiple grafts [reference = first graft] | 1.271 | 0.5613 to 2.708 |
| ln(PIRCHE+1) | 1.576 | 1.006 to 2.618 |
Multivariable logistic regression analyses of clinical variables associated with rejection (any type) at 12-month. Odds ratios and 95% confidence intervals are provided for each variable included within the model. cRF is included as a categorical variable and ln(PIRCHE+1) as a continuous variable.
Significant results are highlighted in bold.
Multivariable Analyses of Patient and Graft Outcomes
Cox regression analyses were undertaken to determine clinical variables associated with patient survival, graft survival, DCGS, and rejection-free allograft survival (Table 5; Supplementary File 1 [9], [10]). A higher PIRCHE score (HR 1.350, 95% CI 1.028–1.817) was associated with worse rejection-free allograft survival, whereas cRF was not associated with any outcome.
TABLE 5
| Clinical Variable | Patient survival | Graft survival | Death-censored graft survival | Rejection-free allograft survival | ||||
|---|---|---|---|---|---|---|---|---|
| Hazard ratio | 95% confidence interval | Hazard ratio | 95% confidence interval | Hazard ratio | 95% confidence interval | Hazard ratio | 95% confidence interval | |
| Age at Transplant | 1.069 | 1.040 to 1.102 | 1.025 | 1.008 to 1.042 | 1.002 | 0.9805 to 1.025 | 0.9884 | 0.9735 to 1.003 |
| Male Sex [reference = female] | 1.168 | 0.6102 to 2.357 | 0.7787 | 0.5059 to 1.211 | 0.5220 | 0.2934 to 0.9274 | 0.6102 | 0.4110 to 0.9089 |
| Ethnicity [black; reference = white] | 0.2849 | 0.09454 to 0.7038 | 0.7934 | 0.4692 to 1.318 | 1.392 | 0.7266 to 2.690 | 1.051 | 0.6621 to 1.658 |
| Ethnicity [Asian; reference = white] | 0.8578 | 0.4248 to 1.673 | 0.7817 | 0.4656 to 1.287 | 0.7682 | 0.3573 to 1.590 | 0.7088 | 0.4213 to 1.164 |
| DBD transplant [reference = live transplant] | 1.456 | 0.5695 to 4.503 | 1.639 | 0.8542 to 3.423 | 1.837 | 0.7645 to 5.155 | 0.9129 | 0.5388 to 1.579 |
| DCD transplant [reference = live transplant] | 0.9462 | 0.3476 to 3.023 | 1.772 | 0.8911 to 3.802 | 2.906 | 1.179 to 8.311 | 1.340 | 0.7796 to 2.346 |
| Total HLA Mismatch at HLA A-, B-, and DR-loci | 0.9653 | 0.7345 to 1.266 | 0.8341 | 0.6869 to 1.011 | 0.7680 | 0.5869 to 1.002 | 0.9243 | 0.7672 to 1.111 |
| cRF 1%–84% [reference = cRF 0%) | 0.9219 | 0.4399 to 1.828 | 0.8069 | 0.4804 to 1.315 | 0.7633 | 0.3803 to 1.461 | 0.6852 | 0.4154 to 1.097 |
| cRF 85%–100% [reference = cRF 0%) | 0.6697 | 0.1315 to 2.542 | 1.138 | 0.4991 to 2.407 | 1.348 | 0.5105 to 3.231 | 1.600 | 0.8361 to 2.918 |
| Pre-emptive transplant [reference = not pre-emptive] | 0.07196 | 0.004035 to 0.3371 | 0.3434 | 0.1504 to 0.6822 | 0.5652 | 0.2242 to 1.228 | 1.123 | 0.6878 to 1.779 |
| Multiple grafts [reference = first graft] | 1.747 | 0.5514 to 4.799 | 1.093 | 0.5253 to 2.128 | 0.9386 | 0.3539 to 2.181 | 1.277 | 0.7167 to 2.181 |
| ln(PIRCHE+1) as continuous variable | 1.197 | 0.7712 to 1.942 | 1.140 | 0.8571 to 1.551 | 1.124 | 0.7819 to 1.679 | 1.350 | 1.028 to 1.817 |
Cox regression analyses of clinical variables associated with patient survival, graft survival, death-censored graft survival and rejection-free allograft survival. Hazard ratios and 95% confidence intervals are provided for each variable included within the model. cRF is included as a categorical variable and ln(PIRCHE+1) as a continuous variable.
Significant results are highlighted in bold.
Discussion
Key Results
Induction immunosuppression is widely used in kidney transplantation but there is marked variation in which induction regimen is used. The relevance of guidelines that recommend depleting antibody induction based on traditional assessments of immune risk to the contemporary management of kidney transplant recipients is unknown. We therefore assessed outcomes in kidney transplant recipients who underwent induction with non-depleting antibody therapy in the modern era of histocompatibility testing, with outcomes stratified by HLA sensitisation determined by single antigen bead testing, and T cell epitope mismatches determined by PIRCHE-II scores.
We included an ethnically diverse cohort of 1359 kidney transplant recipients who underwent induction with Basiliximab. Just over one third of the cohort were sensitised, and 1 in 10 recipients were highly sensitised to HLA antigens. Patient survival, graft survival and DCGS were 88%, 78%, and 84% at 5 years respectively, representing favourable outcomes compared to registry data [22, 23]. There were no differences in these primary outcomes in sensitised compared to unsensitised recipients; a reduction in graft survival and DCGS were restricted to highly sensitised recipients but remained 70% and 71% at 5 years. The 12-month rejection rate was 9% overall and the major rejection type was TCMR. As was seen with the outcome data, there was no difference in rejection rates in sensitised compared to unsensitised recipients; there was an increase in rejection restricted to the highly sensitised group at all follow-up timepoints, with a cumulative rejection rate of 26% in highly sensitised recipients who were followed up to 5 years. Rejection free allograft survival was worse in sensitised patients with a preformed DSA. The association of T cell epitope mismatches with outcomes was assessed in 825 recipients and there was an increase in PIRCHE-II scores in those with rejection in the first year. T cell epitope mismatch, but not cRF, was associated with early rejection and rejection free allograft survival in multivariable analyses.
Interpretation
The 2012 KDIGO guidelines on the management of kidney transplant recipients recommends basing the choice of induction therapy on an assessment of immunological risk. Non-depleting antibody therapy is recommended first line, and depleting antibody induction is recommended for patients at high immunological risk, defined by several factors including any level of HLA sensitisation [6]. This recommendation is underpinned by a meta-analysis published shortly before guideline development that highlighted a 25% reduction in graft loss at 1 year with IL2-RA compared with no antibody induction, and a 30% increase in risk of biopsy-proven acute rejection at 1 year when IL2-RA was compared to ATG; this came at the cost of a 75% increase in malignancy and 32% increase in CMV disease [2]. Two multicentre randomised studies have compared IL2-RA induction with ATG specifically in patients at increased immunological risk, with one of these studies making this comparison in the setting of cyclosporin based immunosuppression [14, 15]. Both studies demonstrated a reduction in rejection with ATG compared to IL2-RA at 1- and 5-years, but importantly no difference in patient or allograft outcomes were demonstrated, with follow up now reported out to 10 years [24–26]. A comparison of IL2-RA with ATG induction coupled with early steroid withdrawal in a predominantly white low immunological risk population was made in the Harmony study, which demonstrated no difference in rejection rates, patient or allograft outcomes at 1- and 5-years between the arms [27, 28]. A more recent pilot study demonstrated no difference between depleting and non-depleting induction in sensitised recipients without preformed DSAs [29]. The lack of proven benefit of depleting antibody induction on hard outcomes (i.e., patient and allograft survival), coupled with a more adverse side effect profile and cost, underlies our unit policy for Basiliximab induction in all. In this study we provide unique real-world data on outcomes from the use of this uniform approach in a large contemporary cohort of patients undergoing kidney transplantation across a range of immunological risk.
The outcomes of this strategy are summarised in Supplementary File 2 and outlined alongside those seen in previous large randomised controlled trials of induction therapy that include high [14, 15, 24–26], low [16, 27, 28, 30, 31], and mixed [32, 33] immunological risk populations, in addition to recent registry data from the US [22] and the UK [23], and other large registry analyses [34, 35]. Patient survival in our cohort was 96.9% at 1-year and 87.6% at 5-years (96.1% and 85.4% in deceased donors), similar to patient outcomes previously reported. For example, patient survival was 95%–97% at 1-year and 85%–90% at 5-years in the low immunological risk Harmony population [27, 28]; UK-wide patient survival after deceased donor kidney transplant is currently 96% and 85% at 1- and 5-years respectively [23]. Graft survival (censored for death) was 96.9% and 84.5% at 1- and 5-years (96.0% and 82.1% in deceased donors), providing favourable outcomes compared to recently reported 5-year US graft survival of 66.1%–82.2% after deceased donor kidney transplantation [22]. Graft survival in our cohort did not differ in sensitised compared to unsensitised recipients, and in highly sensitised recipients was 91.4% and 71.4% at 1- and 5-years. These graft outcomes are similar to the outcomes in the high immunological risk population that underwent induction with ATG in the TAXI study, where graft survival was 85% and 76% at 1- and 5-years [15, 25]. Hence, the use of non-depleting antibody induction in our cohort in recipients across a range of immunological risk provided comparable patient and graft outcomes to previous cohorts where depleting antibody induction has been used. This occurred in an ethnically diverse population, which was predominantly managed steroid free.
Acute rejection rates have steadily declined over the last 2 decades. In 2000, rejection within the first year occurred in 17%–24% of kidney transplant recipients in the US [35], and early rejection occurred in 15%–16% of high immunological risk patients managed with depleting antibodies and 26%–27% of patients managed with non-depleting antibodies in the older clinical trials [14, 15]. 1-year rejection rates reduced to 8%–10% in the US in 2012, and the most recent data demonstrate rates of 5.7% and 6.0% in patients undergoing induction with non-depleting and depleting induction respectively [22]. The current low rates of acute rejection encountered in routine clinical practice may lead us to question whether this outcome remains as relevant in contemporary analyses and clinical trials. Rejection within the first year in our cohort occurred in 9.4% of patients, consistent with US registry data from the last decade, and the 11% acute rejection rate seen in Harmony when Basiliximab induction was combined with a steroid free regimen [27]. Clinically relevant BK viremia (>104 copies/mL) was relatively infrequent (6%) in our cohort, and the non-depleting induction facilitated a pre-emptive approach to CMV management with acceptable rates of viremia. Cumulative 5-year rejection rates were 15% in Harmony [28], and 24.2% when IL2-RA induction was combined with steroid withdrawal in the Astellas corticoid study group trial [30, 31], with both these studies investigating populations at low immunological risk. Biopsy proven rejection within the first 5 years occurred in 11.5% of our cohort, despite the inclusion of patients across a range of HLA sensitisation. Two thirds of our cohort had a total HLA A-, B-, DR-mismatch of 3 or less, and our outcomes would support the current UK practice of including HLA matching within allocation schemes, especially in younger recipients. Our data would suggest that acceptable rejection rates and good graft outcomes are achievable with non-depleting induction in all when HLA antibody detection occurs using sensitive methods and efforts are made to HLA match transplant pairs.
Stratification of immunological risk in transplantation has traditionally involved an assessment of the total level of sensitisation to HLA antibodies [36]. Guidelines suggest that any level of sensitisation leads to increased risk and the need for depleting antibody induction, but our data would not support this given no difference in rejection rates or graft outcomes between unsensitised and sensitised recipients. Moreover, cRF was not associated with rejection or graft outcomes in multivariable models. An increase in rejection and a reduction in graft survival was seen, however, in highly sensitised recipients.
Donor specificity of antibodies is a key determinant of immunological risk and previous studies provide conflicting evidence regarding outcomes on the use of Basiliximab in the setting of pre-existing DSAs. Some reports demonstrate that in the absence of a preformed DSA, sensitisation does not impact rejection and outcomes after Basiliximab induction [29, 37], whereas others have found that it does [38]. Our data demonstrate worse rejection free allograft survival in the presence of a preformed DSA, but no difference in other patient or allograft outcomes. Whether depleting induction therapy would have improved outcomes in the highly sensitised subgroup or in patients with a preformed DSA is not answered by this study.
Given most rejection episodes were T cell mediated across all cRF categories, we investigated if T cell epitope mismatches, determined using PIRCHE scores, were associated with rejection. These mismatches predict the risk of de novo T cell alloimmune responses, and higher mismatch scores have previously been associated with the development of DSAs, rejection, and graft survival in kidney transplantation [20, 39, 40]. Moreover, scores have been shown to provide additive information to traditional HLA matching [41], especially in sensitised recipients, and they may inform outcomes in patients with TCMR [42, 43]. Our data support a role for T cell epitope mismatch scores in the immunological assessment of transplant pairs undergoing non-depleting antibody induction. Scores were higher in patients with rejection at 1 year and were associated with early rejection and rejection free allograft survival in multivariable models, whereas levels of sensitisation were not.
Limitations
In this study, we provide unique data on the use of non-depleting antibody induction in a large, ethnically diverse, cohort of kidney transplant recipients with outcomes stratified by HLA sensitisation at the time of transplant and T cell epitope mismatch scores. We report these outcomes from a centre that manages most patients steroid free, albeit we lack data on the maintenance immunosuppression regimens used at the individual level. We provide medium term outcomes to 5 years but lack outcome data thereafter. We anticipate our outcomes are generalizable to many healthcare systems, albeit the ethnic diversity of the cohort, the inclusion of HLA matching within organ allocation, and the ability to perform HLA typing at medium to high resolution may mean it is not applicable to all settings. Moreover, our results should be interpreted in the knowledge that there were relatively few patients in the highly sensitised group, and a larger number of patients would be required to confirm the findings of multivariable analyses. We assess the impact of T cell epitope mismatches in a large proportion of the cohort, but not all. We report rejection that was clinically apparent, but our lack of protocol biopsies may underestimate true prevalence [44]. Moreover, we lack data on the development of DSAs, which has been shown to be impacted by choice of induction therapy, especially in sensitised recipients [45]. We provide data on some complications of immunosuppression, including infection, malignancy, and cardiovascular disease, but we lack more granular data on the development of diabetes, hyperlipidaemia and blood pressure control, which are increasingly relevant to the transplant population we manage. Moreover, we lack patient reported outcomes on the uniform use of this non-depleting induction strategy.
Conclusion
In summary, non-depleting antibody induction provides good outcomes for kidney transplant recipients managed using contemporary histocompatibility techniques across a range of immunological risk. Depleting antibody induction may not be necessary in all patients who are sensitised to HLA antigens, but may be considered in highly sensitised recipients and those with a preformed DSA. T cell epitope mismatch scores provide useful information during the immunological assessment of transplants being undertaken with non-depleting antibody induction. We propose that guidelines for induction therapy in kidney transplantation should be reviewed and updated.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the studies involving humans because The study involved the retrospective analysis of routinely collected clinical data and, as such, was exempt from formal review board approval. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements because The study involved the retrospective analysis of routinely collected clinical data and, as such, was exempt from formal review board approval.
Author contributions
Participated in research conception and design: RN, RE, RF, AS, MH, and GJ. Undertook data acquisition, analysis and interpretation: RN, KB, NT, AH, AG, MJ, FK, AK, AN, GS, HS, SV, FT, NM, SF, RF, and RE. Drafted the initial manuscript: RN and RE. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
RE has received honorarium from Chiesi and Therakos.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2025.14852/full#supplementary-material
SUPPLEMENTARY FILE S2Summary of outcomes in the cohort in this study, and in cohorts from previous randomised trials and registry analyses.
Abbreviations
ABMR, Antibody mediated rejection; ATG, Anti-thymocyte globulin; CI, Confidence interval; CMV, Cytomegalovirus; cPRA, calculated panel reactive antibodies; cRF, calculated reaction frequency; DBD, Donor after brain death; DCD, Donor after cardiac death; DCGS, Death censored graft survival; DSA, Donor-specific antibody; eGFR, estimated glomerular filtration rate; HLA, Human leucocyte antigen; HR, Hazard ratio; IL2-RA, Interleukin-2 receptor antagonist; IQR, Interquartile range; KDIGO, Kidney Disease Improving Global Outcomes; KTR, Kidney transplant recipient; OR, Odds ratio; PIRCHE, Predicted Indirectly ReCognizable HLA Epitope; SD, Standard deviation; TCMR, T cell mediated rejection; UK, United Kingdom; US, United States.
References
1.
HalloranPF. Immunosuppressive Drugs for Kidney Transplantation. N Engl J Med (2004) 351(26):2715–29. 10.1056/NEJMra033540
2.
WebsterACRusterLPMcGeeRMathesonSLHigginsGYWillisNSet alInterleukin 2 Receptor Antagonists for Kidney Transplant Recipients. Cochrane Database Syst Rev (2010) 2010(1):CD003897. 10.1002/14651858.CD003897.pub3
3.
DharnidharkaVRNaikASAxelrodDASchnitzlerMAZhangZBaeSet alCenter Practice Drives Variation in Choice of US Kidney Transplant Induction Therapy: A Retrospective Analysis of Contemporary Practice. Transpl Int Off J Eur Soc Organ Transpl (2018) 31(2):198–211. 10.1111/tri.13079
4.
HellemansRBosmansJLAbramowiczD. Induction Therapy for Kidney Transplant Recipients: Do We Still Need Anti-IL2 Receptor Monoclonal Antibodies?Am J Transpl Off J Am Soc Transpl Am Soc Transpl Surg (2017) 17(1):22–7. 10.1111/ajt.13884
5.
FlemingJNTaberDJPilchNASrinivasTRChavinKD. Yes, We Still Need IL-2 Receptor Antagonists. Am J Transpl Off J Am Soc Transpl Am Soc Transpl Surg (2016) 16(11):3308–9. 10.1111/ajt.13930
6.
Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO Clinical Practice Guideline for the Care of Kidney Transplant Recipients. Am J Transpl Off J Am Soc Transpl Am Soc Transpl Surg (2009) 9(Suppl. 3):S1–155. 10.1111/j.1600-6143.2009.02834.x
7.
BakerRJardineAAndrewsP. Renal Association Clinical Practice Guideline on Post-operative Care of the Kidney Transplant Recipient. Nephron Clin Pract (2011) 118(Suppl. 1):c311–347. 10.1159/000328074
8.
GebelHMBrayRANickersonP. Pre-Transplant Assessment of Donor-Reactive, HLA-Specific Antibodies in Renal Transplantation: Contraindication vs. Risk. Am J Transpl Off J Am Soc Transpl Am Soc Transpl Surg (2003) 3(12):1488–500. 10.1046/j.1600-6135.2003.00273.x
9.
PeiRLeeJHShihNJChenMTerasakiPI. Single Human Leukocyte Antigen Flow Cytometry Beads for Accurate Identification of Human Leukocyte Antigen Antibody Specificities. Transplantation (2003) 75(1):43–9. 10.1097/00007890-200301150-00008
10.
CeckaJM. Calculated PRA (CPRA): The New Measure of Sensitization for Transplant Candidates. Am J Transpl Off J Am Soc Transpl Am Soc Transpl Surg (2010) 10(1):26–9. 10.1111/j.1600-6143.2009.02927.x
11.
MamodeNBestardOClaasFFurianLGriffinSLegendreCet alEuropean Guideline for the Management of Kidney Transplant Patients with HLA Antibodies: By the European Society for Organ Transplantation Working Group. Transpl Int Off J Eur Soc Organ Transpl (2022) 35:10511. 10.3389/ti.2022.10511
12.
DuquesnoyRJAskarM. HLAMatchmaker: A Molecularly Based Algorithm for Histocompatibility Determination. V. Eplet Matching for HLA-DR, HLA-DQ, and HLA-DP. Hum Immunol (2007) 68(1):12–25. 10.1016/j.humimm.2006.10.003
13.
GeneugelijkKSpieringsE. PIRCHE-II: An Algorithm to Predict Indirectly Recognizable HLA Epitopes in Solid Organ Transplantation. Immunogenetics (2020) 72(1-2):119–29. 10.1007/s00251-019-01140-x
14.
BrennanDCDallerJALakeKDCibrikDDel CastilloD, Thymoglobulin Induction Study Group. Rabbit Antithymocyte Globulin versus Basiliximab in Renal Transplantation. N Engl J Med (2006) 355(19):1967–77. 10.1056/NEJMoa060068
15.
NoëlCAbramowiczDDurandDMouradGLangPKesslerMet alDaclizumab versus Antithymocyte Globulin in High-Immunological-Risk Renal Transplant Recipients. J Am Soc Nephrol JASN (2009) 20(6):1385–92. 10.1681/ASN.2008101037
16.
EkbergHTedesco-SilvaHDemirbasAVítkoSNashanBGürkanAet alReduced Exposure to Calcineurin Inhibitors in Renal Transplantation. N Engl J Med (2007) 357(25):2562–75. 10.1056/NEJMoa067411
17.
BTS-NHSBT. BTS-NHSBT-2025-Abstract-Book-Final-3.pdf. Available online at: https://bts.org.uk/wp-content/uploads/2025/03/BTS-NHSBT-2025-Abstract-Book-final-3.pdf (Accessed April 9, 2025).
18.
GragertLKadatzMAlcornJStewartDChangDGillJet alABO-Adjusted Calculated Panel Reactive Antibody (cPRA): A Unified Metric for Immunologic Compatibility in Kidney Transplantation. Am J Transpl Off J Am Soc Transpl Am Soc Transpl Surg (2022) 22(12):3093–100. 10.1111/ajt.17175
19.
WiebeCNickersonPWKosmoliaptsisV. Molecular Mismatch and the Risk for T Cell-Mediated Rejection. Am J Kidney Dis Off J Natl Kidney Found (2022) 80(6):704–6. 10.1053/j.ajkd.2022.06.005
20.
GeneugelijkKNiemannMDrylewiczJvan ZuilenADJoostenIAllebesWAet alPIRCHE-II Is Related to Graft Failure after Kidney Transplantation. Front Immunol (2018) 9:321. 10.3389/fimmu.2018.00321
21.
KokGVerstegenMMAHouwenRHJNieuwenhuisEESMetselaarHJPolakWGet alAssessment of Human Leukocyte Antigen Matching Algorithm PIRCHE‐II on Liver Transplantation Outcomes. Liver Transpl (2022) 28(8):1356–66. 10.1002/lt.26431
22.
LentineKLSmithJMLydenGRMillerJMBookerSEDolanTGet alOPTN/SRTR 2023 Annual Data Report: Kidney. Am J Transpl Off J Am Soc Transpl Am Soc Transpl Surg (2025) 25(2S1):S22–S137. 10.1016/j.ajt.2025.01.020
23.
Organ specific reports. ODT Clinical - NHS Blood and Transplant. Available online at: https://www.odt.nhs.uk/statistics-and-reports/organ-specific-reports/ (Accessed February 3, 2025).
24.
BrennanDCSchnitzlerMA. Long-Term Results of Rabbit Antithymocyte Globulin and Basiliximab Induction. N Engl J Med (2008) 359(16):1736–8. 10.1056/NEJMc0805714
25.
HellemansRHazzanMDurandDMouradGLangPKesslerMet alDaclizumab versus Rabbit Antithymocyte Globulin in High-Risk Renal Transplants: Five-Year Follow-Up of a Randomized Study. Am J Transpl Off J Am Soc Transpl Am Soc Transpl Surg (2015) 15(7):1923–32. 10.1111/ajt.13191
26.
KlLMaSHXDcB. Long-Term Safety and Efficacy of Antithymocyte Globulin Induction: Use of Integrated National Registry Data to Achieve Ten-Year Follow-Up of 10-10 Study Participants. Trials (2015) 16:365. 10.1186/s13063-015-0891-y
27.
ThomuschOWiesenerMOpgenoorthMPascherAWoitasRPWitzkeOet alRabbit-ATG or Basiliximab Induction for Rapid Steroid Withdrawal after Renal Transplantation (Harmony): An Open-Label, Multicentre, Randomised Controlled Trial. Lancet Lond Engl (2016) 388(10063):3006–16. 10.1016/S0140-6736(16)32187-0
28.
StumpfJThomuschOOpgenoorthMWiesenerMPascherAWoitasRPet alExcellent Efficacy and Beneficial Safety during Observational 5-Year Follow-Up of Rapid Steroid Withdrawal after Renal Transplantation (Harmony FU Study). Nephrol Dial Transpl Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. (2023) 39(1):141–50. 10.1093/ndt/gfad130
29.
KamarNLepageBCouziLAlbanoLDurrbachAPerninVet alA Randomized Prospective Study Comparing Anti-T-Lymphocyte Igs to Basiliximab in Highly Sensitized Kidney Transplant Patients. Kidney Int Rep (2020) 5(8):1207–17. 10.1016/j.ekir.2020.05.020
30.
WoodleESFirstMRPirschJShihabFGaberAOVan VeldhuisenPet alA Prospective, Randomized, Double-Blind, Placebo-Controlled Multicenter Trial Comparing Early (7 Day) Corticosteroid Cessation versus Long-Term, Low-Dose Corticosteroid Therapy. Ann Surg (2008) 248(4):564–77. 10.1097/SLA.0b013e318187d1da
31.
WoodleESGillJSClarkSStewartDAllowayRFirstR. Early Corticosteroid Cessation vs Long-Term Corticosteroid Therapy in Kidney Transplant Recipients: Long-Term Outcomes of a Randomized Clinical Trial. JAMA Surg (2021) 156(4):307–14. 10.1001/jamasurg.2020.6929
32.
PilchNATaberDJMoussaOThomasBDenmarkSMeadowsHBet alProspective Randomized Controlled Trial of Rabbit Antithymocyte Globulin Compared with IL-2 Receptor Antagonist Induction Therapy in Kidney Transplantation. Ann Surg (2014) 259(5):888–93. 10.1097/SLA.0000000000000496
33.
HanawayMJWoodleESMulgaonkarSPeddiVRKaufmanDBFirstMRet alAlemtuzumab Induction in Renal Transplantation. N Engl J Med (2011) 364(20):1909–19. 10.1056/NEJMoa1009546
34.
TanrioverBZhangSMacConmaraMGaoASandikciBAyvaciMUSet alInduction Therapies in Live Donor Kidney Transplantation on Tacrolimus and Mycophenolate with or without Steroid Maintenance. Clin J Am Soc Nephrol CJASN (2015) 10(6):1041–9. 10.2215/CJN.08710814
35.
TanrioverBJaikaransinghVMacConmaraMPParekhJRLeveaSLAriyamuthuVKet alAcute Rejection Rates and Graft Outcomes According to Induction Regimen Among Recipients of Kidneys from Deceased Donors Treated with Tacrolimus and Mycophenolate. Clin J Am Soc Nephrol CJASN (2016) 11(9):1650–61. 10.2215/CJN.13171215
36.
BestardOThaunatOBelliniMIBöhmigGABuddeKClaasFet alAlloimmune Risk Stratification for Kidney Transplant Rejection. Transpl Int (2022) 35:10138. 10.3389/ti.2022.10138
37.
WehmeierCHöngerGCunHAmicoPHirt-MinkowskiPGeorgalisAet alDonor Specificity but Not Broadness of Sensitization Is Associated with Antibody-Mediated Rejection and Graft Loss in Renal Allograft Recipients. Am J Transpl Off J Am Soc Transpl Am Soc Transpl Surg (2017) 17(8):2092–102. 10.1111/ajt.14247
38.
GoumardASautenetBBaillyEMiquelestorena-StandleyEProustBLonguetHet alIncreased Risk of Rejection after Basiliximab Induction in Sensitized Kidney Transplant Recipients without Pre-Existing Donor-specific Antibodies - A Retrospective Study. Transpl Int Off J Eur Soc Organ Transpl (2019) 32(8):820–30. 10.1111/tri.13428
39.
LachmannNNiemannMReinkePBuddeKSchmidtDHalleckFet alDonor-Recipient Matching Based on Predicted Indirectly Recognizable HLA Epitopes Independently Predicts the Incidence of De Novo Donor-Specific HLA Antibodies Following Renal Transplantation. Am J Transpl Off J Am Soc Transpl Am Soc Transpl Surg (2017) 17(12):3076–86. 10.1111/ajt.14393
40.
DaniëlsLNaesensMBosmansJLAbramowiczDNaglerEVan LaeckeSet alThe Clinical Significance of Epitope Mismatch Load in Kidney Transplantation: A Multicentre Study. Transpl Immunol (2018) 50:55–9. 10.1016/j.trim.2018.06.006
41.
UnterrainerCDöhlerBNiemannMLachmannNSüsalC. Can PIRCHE-II Matching Outmatch Traditional HLA Matching?Front Immunol (2021) 12:631246. 10.3389/fimmu.2021.631246
42.
SpitznagelTMatterLSKaufmannYLNilssonJvon MoosSSchachtnerT. PIRCHE-II Scores Prove Useful as a Predictive Biomarker Among Kidney Transplant Recipients with Rejection: An Analysis of Indication and Follow-Up Biopsies. Front Immunol (2022) 13:949933. 10.3389/fimmu.2022.949933
43.
LezoevaENilssonJWüthrichRMuellerTFSchachtnerT. High PIRCHE Scores May Allow Risk Stratification of Borderline Rejection in Kidney Transplant Recipients. Front Immunol (2022) 13:788818. 10.3389/fimmu.2022.788818
44.
MengelMChapmanJRCosioFGCavaillé-CollMWHallerHHalloranPFet alProtocol Biopsies in Renal Transplantation: Insights into Patient Management and Pathogenesis. Am J Transpl Off J Am Soc Transpl Am Soc Transpl Surg (2007) 7(3):512–7. 10.1111/j.1600-6143.2006.01677.x
45.
BrokhofMMSollingerHWHagerDRMuthBLPirschJDFernandezLAet alAntithymocyte Globulin Is Associated with a Lower Incidence of De Novo Donor-Specific Antibodies in Moderately Sensitized Renal Transplant Recipients. Transplantation (2014) 97(6):612–7. 10.1097/TP.0000000000000031
Summary
Keywords
rejection, survival, Epitopes, HLA allosensitization, Basiliximab
Citation
Nagpal R, Butler K, Thal N, Hobill A, Gage A, Javed M, Karst F, Khan AA, Needleman A, Shirling G, Stephens H, Vivers S, Tavarozzi F, Mayor N, Frater S, Salama A, Harber M, Jones G, Fernando R and Evans RDR (2025) Kidney Transplant Outcomes With Non-Depleting Antibody Induction Therapy in Human Leucocyte Antigen Sensitised Recipients. Transpl. Int. 38:14852. doi: 10.3389/ti.2025.14852
Received
03 May 2025
Accepted
17 September 2025
Published
30 September 2025
Volume
38 - 2025
Updates
Copyright
© 2025 Nagpal, Butler, Thal, Hobill, Gage, Javed, Karst, Khan, Needleman, Shirling, Stephens, Vivers, Tavarozzi, Mayor, Frater, Salama, Harber, Jones, Fernando and Evans.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rhys D. R. Evans, rhys.evans5@nhs.net
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.