Abstract
Lifelong immunosuppression is necessary to prevent rejection in vascularized composite allotransplantation (VCA). Animal models play a pivotal role in developing innovative immunosuppressive strategies. This systematic review and case report focuses on the most impactful swine VCA models while offering insights gained from the Yale Swine Allotransplantation Vascularized Experiment (Y-SAVE). 22 studies on swine VCA models were included. Key swine breeds included SLA-matched and mismatched MGH miniature swine, Yucatan miniature swine, and outbred domestic swine. Transplantation models varied, with 10 (45%) using osteomyocutaneous flaps and only 2 (9%) involving hemifacial flaps. While 16 (73%) studies utilized heterotopic models, 5 (23%) relied on orthotopic models. Novel strategies such as preconditioning and localized drug delivery emerged, alongside immunosuppression regimens combining tacrolimus with experimental therapies. We further introduced a modified heterotopic hemiface VCA model, demonstrating its feasibility for studying immune dynamics in facial transplants while preserving oral function and enabling serial skin and mucosal biopsies. Overall, our review highlights a notable gap in models that specifically investigate facial VCAs. Given the unique immunological environment of facial allografts, models such as the heterotopic hemiface transplant may offer critical insights into immune mechanisms and may provide a platform for refining targeted immunosuppressive strategies.
Introduction
Vascularized composite allotransplantation (VCA) represents an innovative surgical approach to restore form and function of patients with devastating deformities [1–5]. Moving beyond the boundaries of conventional reconstructive approaches (such as autologous free tissue transfer and local tissue re-arrangement), VCA surgery has emerged as a valuable therapeutic option for patients with severe injuries or irreversible tissue loss [6, 7]. Over the past decades, a growing number of VCAs have been performed, yielding positive short- and long-term outcomes [8, 9]. VCAs include different tissues such as skin, mucosa, muscle, bone, lymphatics, vasculature and nerves. The inclusion of different tissue types with varying antigenicity is associated with a strong immune response by the recipient [10, 11]. In particular, epithelial surface tissues such as the skin and mucosa seem to be the primary targets of alloreactivity, mainly via a lymphocyte mediated adaptive immune response [12, 13].
Graft rejection (both acute and chronic) persists as the main barrier in VCA surgery, limiting its more widespread application. To control allograft rejection, recipients are administered lifelong immunosuppressive (IS) regimens, typically consisting of tacrolimus, mycophenolate mofetil (MMF) and prednisolone [14, 15]. Such immunosuppressants have a variety of side effects, for instance nephrotoxicity and an increased risk of malignancy and opportunistic infections. Despite high intensity IS protocols, ∼85% of VCA recipients still experience rejection episodes during the first year post-transplant and continue to reject almost annually, underlining the insufficiency of current immunomodulating strategies in VCA surgery [13, 16]. Besides acute graft rejection, patients face additional challenges such as chronic rejection which may lead to loss of functon and structure of the graft over time [17–19].
Large animal models, particularly swine, are invaluable for investigating novel immunomodulatory strategies with potential applications in human VCA recipients [20]. However, there is a notable lack of comprehensive research consolidating the current knowledge of swine models in this field. This gap represents an untapped opportunity to enhance in vivo experimentation and accelerate the translation of findings from the laboratory to clinical practice. To address this, we systematically reviewed the existing literature on experimental swine models in VCA, examining their indications, strengths, and limitations. Additionally, we detail the planning and outcomes of the Yale Swine Allotransplantation Vascularized Experiment (Y-SAVE). This research aims to advance the refinement of swine models and address persistent challenges in VCA surgery, ultimately improving their utility and translatability.
Methods
Search Strategy
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [21]. The MEDLINE database (PubMed) and Google Scholar were queried for relevant articles published until November 13th, 2024. All studies had to be written in English. Only articles presenting original data were included. Only articles discussing experimental swine models for vascularized composite allotransplantation and immunosuppression were eligible.
Data Extraction and Quality and Bias Assessment
The search strategy for PubMed/MEDLINE and Google Scholar was developed (Supplementary Table S1). Two reviewers (LK, FK) independently screened all articles by title and abstract. Articles were subsequently analyzed in greater depth through full-text assessment to determine eligibility. Any disagreements regarding the inclusion of individual studies were resolved through consultation with a third author (MK). For included articles, citation searching was carried out on Google Scholar. Data extraction was performed independently by two authors (LK, FK) to ensure accuracy and consistency. During the blinded, dual-review process, we extracted the following variables for each study included: Digital Object Identifier (DOI), first author, study title, year of publication, region of publication, number of animals, mean age, gender, follow-up (mean and range), and the specifics of performed procedures. To evaluate the quality and risk of bias of the included studies, the SYRCLE risk of bias (RoB) tool for animal studies was employed [22]. The detailed risk of bias assessments for all studies are presented in Supplementary Table S2.
Case Report
To complement the findings of this systematic review, we included a representative case report describing a novel heterotopic hemifacial VCA model in swine. This model was developed in response to gaps identified in the literature, particularly the lack of large-animal models incorporating facial tissue and permitting mucosal assessment. The case report provides detailed procedural insights and demonstrates the feasibility of serial mucosal and skin biopsies in a controlled, minimally invasive manner. Its inclusion offers practical context and supports the translational relevance of emerging strategies for immune monitoring in facial allotransplantation.
Results
After full-text analysis, a total of 22 eligible studies were included in the qualitative synthesis. A PRISMA flowchart of study identification, screening, and inclusion is presented in Figure 1.
FIGURE 1
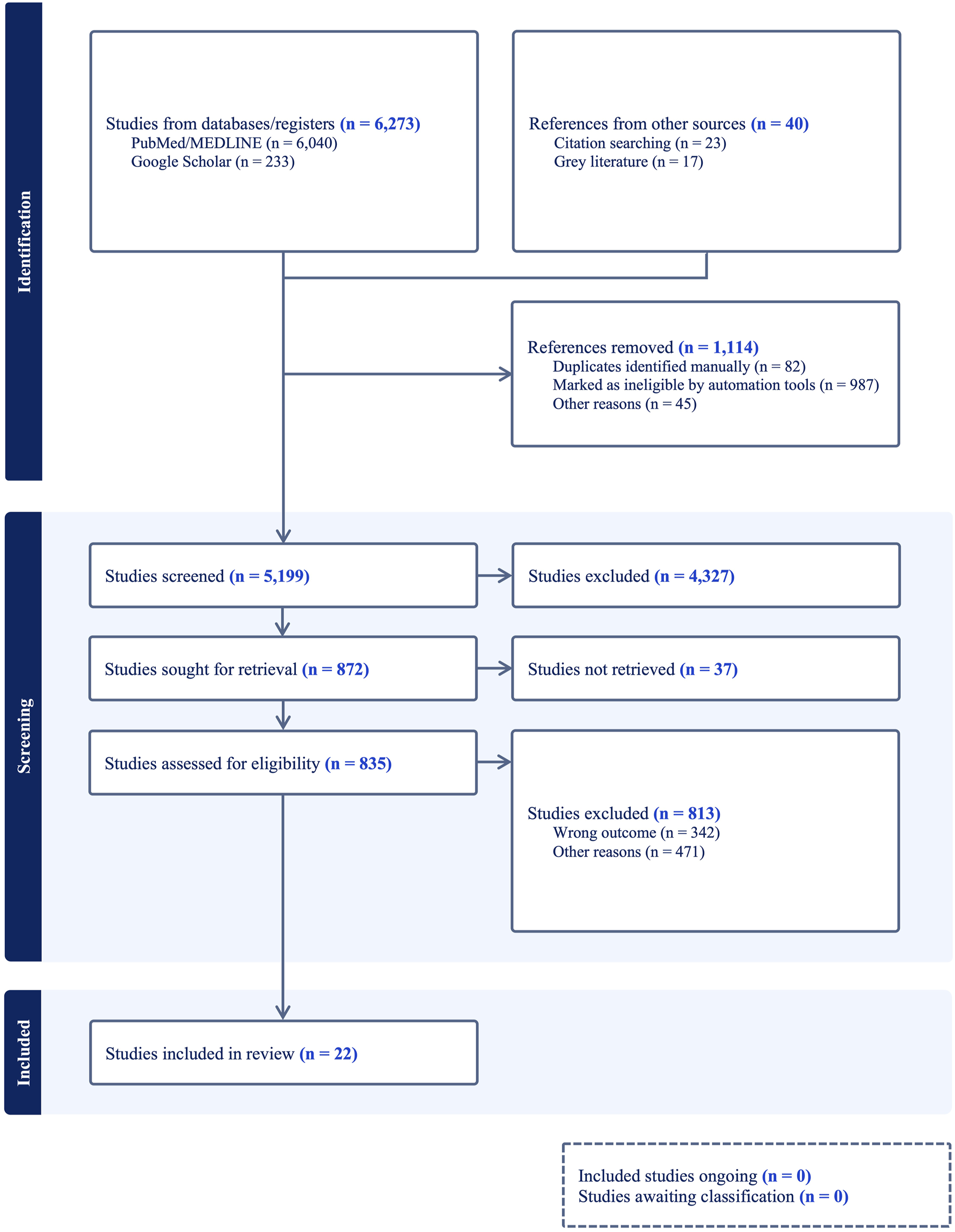
PRISMA 2020 Flowchart of the conducted methodology.
Swine Models
We identified various swine breeds that were employed to examine VCA. The primary models included MGH miniature swine [23–26], Yucatan miniature swine [27, 28], and outbred domestic swine [29–31], which were selected for their genetic similarities to human immunologic responses. In particular, MGH miniature swine and Yucatan miniature swine were frequently chosen for their manageable size and robust immunological profiles. Other studies utilized outbred Yorkshire swine and Swiss Landrace pigs [32–34], adding diversity in immune response due to genetic variability, which allowed for a comprehensive analysis of transplantation outcomes across multiple immune phenotypes. Details are reported in Table 1.
TABLE 1
| Author and year | Study design | Animals used | Transplant model | Heterotopic/orthotopic | Facial VCA (yes/no) | Tissue type | Donor | Recipient |
|---|---|---|---|---|---|---|---|---|
| Barone et al. [23] | In vitro | Complete MHC mismatched MGH miniature swine | Gracilis VCA transplanted to the cervical region | Heterotopic | No | Myocutaneous | MGH miniature swine | MGH miniature swine |
| Berkane et al. [33] | Ex vivo | Female Yorkshire pigs | Bilateral partial hindlimb VCA | N/A | No | Osteomyocutaneous | Female Yorkshire pigs | N/A |
| Blades et al. [43] | In vivo | Sinclair and Yucatan pigs | VRAM flap transplanted to the neck region | Heterotopic | No | Myocutaneous | Sinclair pigs | Yucatan pigs |
| Elgendy et al. [26] | In vitro | SLA- mismatched MGH miniature swines | VRAM flap transplanted to dorso-lateral neck region | Heterotopic | No | Myocutaneous | MGH miniature swine | MGH miniature swine |
| Fries et al. [35] | In vivo | SH- mismatched miniature swine | Radio-ulnar forelimb VCA | Orthotopic | No | Osteomyocutaneous | SH- mismatched Yucatan miniature pigs | SH- mismatched Yucatan miniature pigs with four SLA-HS |
| Ibrahim et al. [36] | In vivo | MHC-defined inbred MGH miniature swine | Hind limb VCA transplanted to subcutaneous abdominal wall pockets | Heterotopic | No | Osteomyocutaneous | Male MGH miniature swine | Female miniature swine |
| Kim et al. [25] | In vivo | Fully MHC mismatched MGH miniature swine | Hind-limb VCA model | Heterotopic | No | N/A | MGH mini-swine | MGH mini-swine |
| Kotsougiani et al. [28] | In vivo | Yucatan miniature pig | Tibial defect VCA model | Orthotopic | No | Osteomyocutaneous | Yucatan mini pig tibia (SLA- and blood type compatibility) | Yucatan mini pig tibia, age and size matched (SLA- and blood type compatibility) |
| Kuo et al. [29] | In vitro | Outbred miniature swine (genotypes: GPI-BB and PGD-AA) | Hind limb VCA transplanted to subcutaneous abdominal wall pockets | Heterotopic | No | Osteomyocutaneous | Outbred miniature swine (lan-yu strain; age 3 months; weight 12–20 kg) | Outbred miniature swine (lan-yu strain; age 3 months; weight 12–20 kg) |
| Kuo et al. [29] | In vivo | Outbred miniature swine (Lan-Yu and Hwa-Ban strains) | Hemi-facial flap (skin, muscle, ear cartilage, nerve, parotid gland, surrounding tissue) | Orthotopic | Yes | Chondromyocutaneous | Lan-Yu and Hwa-Ban strain | Lan-Yu strain |
| Kuo et al. [30] | In vitro | Outbred miniature swine | Hind limb VCA transplanted to subcutaneous abdominal wall pockets | Heterotopic | No | Osteomyocutaneous | Outbred miniature swine lan-yu strain; age 3 months; weight 12–20 kg | Outbred miniature swine lan-yu strain; age 3 months; weight 12–20 kg |
| Kuo et al. [31] | Ex vivo | Outbred miniature swine | Hind limb VCA transplanted to subcutaneous abdominal wall pockets | Heterotopic | No | Osteomyocutaneous | Female outbred miniature swine | Male outbred miniature swine |
| Leonard et al. [24] | In vitro | MGH miniature swine | Hind limb VCA transplanted to neck or abdominal wall region | Heterotopic | No | Fasciocutaneous | MGH miniature swine with PAA-positive SLA | MGH miniature Swine with PAA-negative SLA |
| Mathes et al. [32] | In utero and in vitro | MGH miniature swine and outbred Yorkshire sows and boars | Hind limb VCA transplanted to subcutaneous abdominal wall pockets | Heterotopic | No | Osteomyocutaneous | SLA homozygous MGH miniature swine | Outbred Yorkshire sow and boar fetuses (negative for SLA class Ic) |
| Park et al. [44] | In vivo | Domestic swine | Hemi-facial flap (skin, mucosa, subcutaneous fat tissue, ear, maxilla and mandibular bone) | Orthotopic | Yes | Osteochondrocutaneous | Domestic swine | Domestic swine |
| Shanmugarajah et al. [38] | In vitro | Miniature MGH swine model | Hind limb VCA transplanted to the neck region | Heterotopic | No | Fasciocutaneous | HC miniature swine model (SLAgg class Ic/iid) | HC miniature swine model (SLAcc class Ic/iic) |
| Tratnig-Frankl et al. [39] | In vivo/ex vivo | MHC-defined miniature swine | Gracilis VCA model | Orthotopic | No | Myocutaneous | MHC-defined miniature swine | MHC-defined miniature swine (group 1: class I and class II match; group 2: class I and class II missmatch) |
| Wachtman et al. [27] | In vitro | Yucatan miniature swine | Hind limb VCA transplanted to subcutaneous abdominal wall pockets | Heterotopic | No | Osteomyocutaneous | Yucatan miniature swine | Yucatan miniature swine |
| Waldner et al. [40] | In vivo | Partially inbred SLA–mismatched miniature swine (homozygous HC alleles) | VRAM flap transplanted to the neck region | Heterotopic | No | Myocutaneous | Miniature swine (hetero- and homozygous for HC; 2-3 months old; weight between 10 and 20 kg; full SLA mismatch | Miniature swine, (hetero- and homozygous for HC; 3-5 months old; weight between 20 and 30 kg; full SLA mismatch |
| Wang et al. [45] | Ex vivo | Yorkshire swines (SLA-mismatch in one) | Gracilis VCA transplanted to the neck region | Heterotopic | No | Myocutaneous | Yorkshire swine | Yorkshire swine |
| Wu et al. [42] | In vivo | SLA- mismatch swine | Gracilis VCA model | Heterotopic | No | Myocutaneous | Swine with single SLA mismatch | Swine with single SLA mismatch |
| Zhang et al. [34] | In vivo | MHC-mismatched Swiss landrace pigs | Knee VCA transplanted to subcutaneous abdominal wall pockets | Heterotopic | No | Osteomyocutaneous | Swiss landrace pigs (MHC-mismatched; aged 11–14 weeks) | Swiss landrace pigs (MHC-mismatched) |
Overview of studies on Experimental Swine VCA models.
MGH, Massachusetts General Hospital; SLA, Swine leukocyte antigen; HC, histocompatibility complex; MHC, major histocompatibility complex; VCA, vascularized composite allotransplant; SH, single haplotype; PAA, pig allelic antigen; CS, Cold Storage; TAC, tacrolimus; MMF, Mycophenolate Mofetil; MPDN, Methylprednisolone; CXA, cyclosporine A; CD3-IT, CD3-Immunotoxin; (CTLA4-Ig), cytotoxic T-lymphocyte antigen 4 immunoglobulin; POD, postoperative day; AR, acute rejection; DSAs, donor specific antibodies; N/A, not applicable; TGMS, triglycerol monostearate; VRAM, vertical rectus abdominus myocutaneous flap, ASC, adipose-derived stem cell; AV, arteriovenous.
Transplant Models and Interventions
Various transplantation models were utilized, including heterotopic hind-limb transplantation, gracilis myocutaneous flaps, vertical rectus abdominis myocutaneous (VRAM) flaps, osteomyocutaneous flaps, partial hindlimb models, forelimb models, and tibial VCA. Of the n = 22 studies included, n = 16 (73%) applied heterotopic VCA models, while n = 5 (23%) used orthotopic models [23, 24, 26–32, 34–44]. Notably, n = 1 study (5%) did not categorize the approach as either heterotopic or orthotopic and n = 10 (45%) studies included osteomyocutaneous VCAs [27, 28, 30–37]. Furthermore, n = 2 (9%) studies performed hemi-facial VCAs, with n = 1 involving transplantation of the maxillo-mandibular complex [29, 44]. Lastly, assessment of mucosal tissue was reported in n = 1 (5%) of studies included.
Orthotopic models were employed by fewer authors. Fries et al. utilized an orthotopic mismatched porcine forelimb VCA model in SH-mismatched Yucatan miniature pigs [35]. Kotsougiani et al. implemented an orthotopic tibial defect VCA model in SLA- and blood type-compatible Yucatan miniature pigs [28]. Tratnig-Frankl et al. used an orthotopic gracilis myocutaneous free flap model in MHC-defined miniature swine to assess the impact of antioxidant therapies on graft survival [39]. Interestingly, Kuo et al. employed an orthotopic hemi-facial chondromyocutaneous flap, including skin, muscle, ear cartilage, and parotid gland in Lan-Yu miniature swine to study rejection dynamics and Park et al. utilized an orthotopic hemi-facial osteochondrocutaneous flap, incorporating skin, mucosa, subcutaneous tissue, ear cartilage and the maxillo-mandibular complex in domestic swine to investigate vascular and skeletal fixation techniques [29, 44]. More information is provided in Table 1.
Immunosuppressive Strategies
Multiple immunosuppressive strategies were employed across different VCA models. These approaches included total body irradiation (TBI), thymic irradiation, T-cell depletion, bone marrow transplantation (BMT), and targeted drug therapies such as tacrolimus (TAC), cyclosporine A (CXA), mycophenolate mofetil (MMF), and mTOR inhibitors (e.g., rapamycin). Outcomes varied based on the immunosuppressive regimen and dosages used.
Starting with Barone et al., the authors combined low-dose total body irradiation (100cGy 2 days prior to surgery or 200cGy divided in 2 × 100 cGy doses on preoperative day 2 and 3), T-cell depletion with CD3 immunotoxin (0.05 mg/kg i.v., twice daily from preoperative day 4 to day 0), CXA (target level 400–800 ng/mL), and donor bone marrow cell infusion (7.8 × 108 to 4 × 109 cells/kg of recipient body weight) alongside VCA to achieve mixed chimerism, though this was insufficient for complete tolerance induction [23]. Ibrahim et al. employed short-term TAC monotherapy (target levels of 10–15 ng/mL) in a VCA model with intact vascularized bone marrow, demonstrating long-term graft survival with viable vascularized bone marrow and successful immune monitoring [36]. Kim et al. utilized a 30-day TAC course combined with adipose-derived stem cell (ASC) therapy (1.0 × 106 cells/kg administered intravenously on postoperative day (POD) 7), achieving rejection-free survival for over 200 days while significantly upregulating T-regulatory cells and donor-specific unresponsiveness. Elgendy et al. compared the efficacy of mTOR inhibitors, finding that TAC (0.1–0.125 mg/kg) significantly delayed acute rejection (grade I AR on POD 30 and grade IV AR on POD 74) compared to rapamycin (0.02–0.2 mg/kg), which led to rapid rejection (grade IV AR by POD 17-20) [26]. Conversely, Fries et al. employed low-dose TAC (49 mg) administered via an enzyme-responsive hydrogel platform, which prolonged graft survival, whereas high doses (91 mg) caused poor tolerance and complications such as weight loss and pancreatitis [35]. Kotsougiani et al. used a combination of TAC (target levels of 5–30 ng/mL), MMF (target levels of 1–3.5 ng/mL), and methylprednisolone (tapered to 0.1 mL for maintenance), achieving graft survival and enhancing vascular remodeling without rejection during the 4-month follow-up [28]. Meanwhile, Kuo et al. combined irradiation, BMT, and CXA with mesenchymal stem cells (MSCs) in variying dosages, resulting in significantly prolonged graft survival and reduced acute rejection. Here, increased regulatory T-cell populations (CD4+/CD25+ and CD4+/FoxP3+) were found [30, 31, 37]. Leonard et al. applied 100 cGy total body irradiation, T-cell depletion with CD3 immunotoxin (50 μg/kg), and hematopoietic cell transplantation (15 × 109 cells/kg), achieving stable mixed chimerism and long-term graft acceptance without signs of rejection up to POD 504 [24]. Mathes et al. pioneered an in utero bone marrow transplantation approach, achieving multilineage macrochimerism and donor-specific tolerance without prolonged post-transplant immunosuppression. The authors relied on CXA (target levels of 400–800 ng/mL) post-bone marrow infusion (2 × 109 cells/kg) to maintain donor-specific tolerance, demonstrating effective rejection prevention in chimeric animals [32]. Furthermore, Shanmugarajah et al. utilized T-cell depletion with CD3 immunotoxin (50 μg/kg), 100 cGy TBI, and a 45-day CXA regimen (target levels of 400–800 ng/mL) to achieve immune tolerance in MHC class II mismatched chimeras, although MHC class I mismatched animals experienced rejection [38]. Meanwhile, Kuo et al. demonstrated that CXA delayed rejection from POD 7 to 28 in untreated controls to POD 38 to 49 in their hemi-facial VCA model [29]. Additionally, strategies explored by Wu et al. focused on enzyme-responsive and TAC-eluting hydrogels. The authors demonstrated prolonged survival using hydrogel-administered TAC (28 mg/4 cc and 49 mg/4 cc), effectively delaying grade IV AR to POD 20 and 28 [42].
Overall, five studies did not administer immunosuppressive therapies. For instance, Blades et al. observed flap rejection between POD 5 and 9 without immunosuppressive treatment and Park et al. by POD 14 to 18 [43, 44]. Tratnig-Frankl et al. and Wang et al. did not administer immunosuppression to avoid skewing of rejection periods in novel treatment approaches. Tratnig-Frankl et al. investigated H2S and NaI treatments but observed no significant differences in graft survival or immunological outcomes compared to saline controls [39]. In Wang et al. the experimental group received hyperoxygenated University of Wisconsin solution and showed significantly later onset of grade 1 AR, compared to the control group [45]. Lastly, in Berkane et al. the study protocol did not foresee immunosuppression [33]. Further information can be found in Table 2.
TABLE 2
| Author and year | Interventions | Immunosuppresion | Outcomes | Complications |
|---|---|---|---|---|
| Barone et al. [23] | Bone marrow transplantation | Low-dose total body irradiation (100cGy 2 days prior to surgery or 200cGy divided in 2 × 100 cGy doses on preoperative day 2 and 3), T-cell depletion wirh CD3-IT (0.05 mg/kg), CXA (target level 400–800 ng/mL), donor bone marrow cells (7.8 × 108 to 4 × 109 cells/kg of recipient body weight) | Bone marrow infusion led to better clinical outcomes; chimerism detected but insufficient for tolerance | Mixed chimerism after bone marrow transplantat; VCA appeared insufficient for tolerance induction |
| Berkane et al. [33] | Two study groups: supercooling intervention group and cold storage control group undergoing subsequent normothermic machine perfusion | No immunosuppressive therapy used | Supercooled VCAs restored vascular flow and had lower resistance during machine perfusion | N/A |
| Blades et al., 2024 [43] | Investigation of possible surgical complications | No immunosuppressive therapy used | All flaps survived initially, with adequate perfusion for 4 days. Flap rejection occurred between POD 5 and POD 9 in all animals | Minimal erythema observed post-transplant, no surgery-related deaths or infections |
| Elgendy et al. [26] | Treatment with Co-stimulation blockade and mTOR inhibitor, with or without preceding short-term calcineurin inhibitor therapy | mTOR inhibitor (rapamycin [0.02–0.2 mg/kg] or tacrolimus [0.1–0.125 mg/kg]) | TAC delayed AR (grade-I AR on POD 30, grade-IV on POD 74); rapid rejection with rapamycin (grade-I AR by POD 2 and 7, grade-IV AR by POD 17–20) | Rejection of allograft, erythema, severe necrotizing T cell mediated rejection with deep dermal arterial thrombosis |
| Fries et al. [35] | Tacrolimus eluting hydrogel implants with various concentrations (91 mg, high dose/49 mg, low dose) | Graft-implanted enzyme-responsive, TAC eluting hydrogel platform | Low-dose TAC prolonged survival; high-dose TAC caused poor tolerance (grade IV AR from POD 56–93) | High dose TAC group: one sample excluded due to flap failure on POD 1; four animals showed poor feeding and weight loss, requiring early euthanasia; four animals from high dose TAC group developed pancreatitis |
| Ibrahim et al. [36] | Development of novel tranlational VCA research model | Short-term tacrolimus monotherapy (target levels of 10–15 ng/mL) with or without bone marrow infusion | Long-term graft survival (>150 days) with viable vascularized bone marrow; successful immune monitoring | Venous thrombus in one case resolved by reanastomosis, no graft-versus-host disease |
| Kim et al. [25] | Treatment with tacrolimus for 30 days and ASC therapy (donor-derived ASCs [1.0 × 10^6 cells/kg]) | TAC, ASC-therapy | Adipose-derived stem cells demonstrated grade IV AR on POD 119 and rejection-free survival over POD 200 as well as upregulated T-regulatory cells | The control group reached Banff grade 4 acute rejection by an average of 7.5 days after transplantation. Allografts treated with ASCs demonstrated grade 4 rejection on day 119 |
| Kotsougiani et al. [28] | AV-bundle implantation in tibial allotransplant | TAC (target levels of 5–30 ng/mL), MMF (target levels of 1–3.5 ng/mL), MPDN (tapered to 0.1 mL) | Micro-CT showed bone formation and remodeling at the distal allograft junction; allograft survived without any healing problems or limited hindlimb perfusion during the 4-month follow-up | N/A |
| Kuo et al. [29] | Treatment with various dosages of mesenchymal stem cells, CXA, bone marrow transplantation and irradiation | Irradiation, bone marrow trnsplantation and CXA | Mesenchymal cells extended graft survival, combined CXA and stem cells showed significantly better survival, allografts with CXA exhibited delayed AR, examination of bromodeoxyuridine-labeled mesenchymal stem cells revealed donor mesenchymal stem cells engraftment into the recipient and donor skin | Graft-versus-host disease evident in CXA group |
| Kuo et al. [29] | Comparison of rejection in untreated, control and CXA-treatment groups | CXA in treatment group, untreated and control: N/A | 100% survival rate, CXA treatment delayed flap rejection significantly (POD 38-49), no significant difference in rejection signs in allo-cartilage | Swelling for 2 weeks (postoperative saliva gland hypersecretion), control group: progressive rejection by POD 7-28, lymphoid gland tissue and skin were susceptible to early rejection |
| Kuo et al. [30] | Various combinations of mesenchymal stem cells cyclosporine or irradiation | Mesenchymal stem cells, CXA, irradiation | Mesenchymal stem cells with irradiation and CXA: significantly increased allograft survival compared with other groups (>120 days; p < 0.01); histology showed lowest degree of AR in grafted skin and interstitial muscle layers in mesenchymal stem cell/irradiation/CXA group; significant increase in percentage of CD4+/CD25+ and CD4+/FoxP3+ T in the mesenchymal stem cell/irradiation/CXA group | Rejection episodes |
| Kuo et al. [31] | Various dosages of ASCs, tacrolimus or irradiation | TAC, irradiation | Multiple injections of adipose-derived stem cells, irradiation and TAC increased allograft survival significantly | Lymphocyte infiltration in the alloskin and interstitial muscle layers of treatment group |
| Leonard et al. [24] | Stem cell transfusion | 100 cGy irradiation, T cell depletion with CD3-IT (50 μg/kg), hematopoietic cell transplantation (15 × 109 cells/kg) | Following withdrawal of immunosuppression both VCAs transplanted into stable chimeras Recipients of hematopoietic cell transplantation displayed no clinical signs of AR up to POD 504 |
Two animals developed skin graft versus host disease |
| Mathes et al. [32] | Treatment with CXA and bone marrow transplantation (2 × 109 cells/kg) | CXA (target levels of 400–800 ng/mL) | Donor cell engraftment and multilineage macro chimerism after in utero transplantation of adult bone marrow cells, and chimeric animals were unresponsive to donor antigens in vitro; both control VCAs rejected by POD 21; chimeric animals accepted VCAs (no DSAs or alloreactivity) | All grafts demonstrated some mild lymphocytic infiltration at the day 7 biopsy. All of the animals developed a severe dermal perivascular lymphocytic infiltration with scattered eosinophils and went on to reject their donor skin grafts |
| Park et al. [44] | Vascular anastomosis of the carotid artery and jugular vein, fixation of the maxillo-mandibular complex with titanium plates | No immunosuppressive therapy used | Successful transplant without early arterial or venous insufficiency, acute rejection from POD 7-8 onwards | Acute rejection POD 7-8, pink discoloration, edema, erythematous papule with flap necrosis on POD 14–18 |
| Shanmugarajah et al. [38] | Hematopoietic stem cell transplantant, irradiation | T cell depletion with CD3-IT (50 μg/kg), 100 cGy TBI and 45 days of CXA (target levels of 400–800 ng/mL) | HC class II–mismatched chimeras were tolerant of VCAs; HC class I–mismatched animals rejected VCA skin, (infiltration of CD8+ lymphocytes) |
One HC class II mismatched model displayed clinical features of chronic graft versus host disease (euthanized on POD 190) |
| Tratnig-Frankl et al. [39] | Treatment with either saline (control), sodium iodide (NaI), or hydrogen sulfide (H2S) injections | No postoperative immunosuppression | No effect of H2S or NaI treatment in comparison to NaCl in delaying AR, flap survival and histology revealed no significant differences between the groups | One technical failure occurred in the saline MISMATCH subgroup |
| Wachtman et al. [27] | Bone marrow infusion and irradiation | Total body (100 cGy) and thymic (700 cGy) irradiation, bone marrow infusion, tacrolimus (0.1 mg/kg/day), CTLA4-Ig (20 mg/kg) | Experimental groups rejected allografts (skin and muscle) on POD 5 to 30; skin and muscle histology in all long-term survivors were normal | Rejection episodes |
| Waldner et al. [40] | Investigation of VRAM flap applicability in VCA research | TAC, rapamycin, CTLA4-Ig | POD 5: all grafts demonstrated pale-pink skin color without edema; follow-up showed improved correlation between clinical appearance and progression of graft rejection in histology |
Intraoperative cardiac arrest in one sample (death due to anesthesia); one recipient experienced flap loss due to venous compromise; Banff grade I AR with erythemous and edematous grafts |
| Wang et al. [45] | Treatment with sub-normothermic ex-vivo perfusion using hyper-oxygenated University of Wisconsin (UW) solution | No immunosuppressive therapy used | Experimental group showed significantly later onset of grade 1 AR at 13.7 days (SD = 0.52, p < 0.05); by POD 15 75% of the flaps showed no evidence of grade 4 AR | Rejection episodes |
| Wu et al. [42] | Treatment with various dosages (28 mg/4cc and 49 mg/4cc) of tacrolimus-eluting hydrogel injected into the donor flap | TAC-eluting hydrogel (28 mg/4cc and 49 mg/4cc) | TAC-eluting hydrogel prolonged graft survival in both groups (grade 4 AR on average by POD 20 and 28) | Rejection episodes |
| Zhang et al. [34] | Treatment with various combinations of TGMS and TAC | Locally administered TAC-loaded on-demand drug delivery system | Repeated intra-graft TGMS-TAC administrations prolong graft survival | Grade III-IV rejection |
Overview of Interventions, Immunosuppressive Strategies and Outcomes of studies included.
MGH, Massachusetts General Hospital; SLA, Swine leukocyte antigen; HC, histocompatibility complex; MHC, major histocompatibility complex; VCA, vascularized composite allotransplant; SH, single haplotype; PAA, pig allelic antigen; CS, Cold Storage; TAC, tacrolimus; MMF, Mycophenolate Mofetil; MPDN, Methylprednisolone; CXA, cyclosporine A; CD3-IT, CD3-Immunotoxin; (CTLA4-Ig), cytotoxic T-lymphocyte antigen 4 immunoglobulin; POD, postoperative day; AR, acute rejection; DSAs, donor specific antibodies; N/A, not applicable; TGMS, triglycerol monostearate; VRAM, vertical rectus abdominus myocutaneous flap; ASC, adipose-derived stem cell; AV, arteriovenous.
Major Findings
In several models, immunosuppressive therapies and interventions significantly improved graft survival, with some protocols achieving long-term graft acceptance and reduced acute rejection (AR). For instance, Ibrahim et al. reported long-term graft survival exceeding 150 days with short-term TAC therapy and bone marrow infusion, highlighting the effectiveness of combining localized and systemic immunosuppression [36]. Similarly, Kim et al. observed prolonged rejection-free graft survival beyond 200 days using adipose-derived stem cell therapy combined with TAC, correlating the upregulation of regulatory T-cells (Tregs) with sustained graft tolerance [25]. Leonard et al. demonstrated stable chimerism and long-term graft survival up to 504 days following hematopoietic cell transplantation with irradiation and T-cell depletion, suggesting the importance of chimerism in inducing robust immune tolerance [24]. Additionally, Mathes et al. demonstrated that cyclosporine treatment combined with in utero bone marrow transplantation resulted in long-term chimeric stability and VCA acceptance, highlighting the effectiveness of early hematopoietic intervention [32].
Most studies reported significant success in prolonging graft survival with tailored immunosuppressive regimens. For example, Wang et al. observed a delay in acute rejection in flaps treated with systemic immunosuppression, with 75% of experimental flaps showing no rejection by day 15 [45]. Treatments combining stem cells with immunosuppressantsoften resulted in prolonged graft survival with reduced rejection rates, as seen in the work of Kuo et al. [30, 31, 37] In contrast, Elgendy et al. found that TAC significantly delayed AR compared to rapamycin, where rapid AR was observed [26]. Additionally, Fries et al. revealed that while low-dose TAC via enzyme-responsive hydrogels prolonged graft survival, high doses led to poor outcomes, including weight loss, pancreatitis, and early euthanasia [35]. Localized immunosuppressive delivery methods demonstrated promising results. Wu et al. utilized tacrolimus-eluting hydrogels, which effectively prolonged graft survival and delayed grade IV AR [42]. Similarly, Zhang et al. employed a localized tacrolimus-loaded drug delivery system, resulting in repeated intra-graft administration that significantly extended graft survival [34]. Kuo et al. found CXA to significantly delay rejection of hemi-facial flaps. Lastly, some treatments failed to demonstrate significant efficacy: Tratnig-Frankl et al. found no significant difference in graft survival or histological outcomes after antixodative therapy compared to controls [39]. Details are provided in Table 2.
Complications
Complications varied with the immunosuppressive approach and transplant model. While many studies reported successful outcomes in terms of prolonged graft survival and delayed rejection, complications often arose from either the intervention protocols themselves or the adverse effects of immunosuppressive regimens. For example, Fries et al. reported weight loss, poor feeding, and pancreatitis in animals subjected to high-dose TAC therapy, with some requiring early euthanasia [35]. Similarly, Elgendy et al. noted rapid rejection with rapamycin treatment compared to TAC [26]. Furthermore, vascular complications such as venous thrombosis were reported. These complications were resolved through re-anastomosis without long-term graft loss [36]. Blades et al. observed flap rejection between POD 5 and 9, despite initial adequate perfusion, and noted minimal erythema as an early rejection marker [43]. Additionally, Tratnig-Frankl et al. reported a technical failure in one saline subgroup, emphasizing the role of surgical precision in preventing graft loss [39]. Systemic complications related to immunosuppression were also observed. Shanmugarajah et al. documented chronic graft-versus-host disease (GvHD) in one animal, requiring euthanasia by POD 190 [38]. Similarly, Kuo et al. noted GvHD in animals treated with cyclophosphamide and irradiation, indicating the risks associated with preconditioning regimens [30, 31, 37]. Additionally, Wachtman et al. reported histological evidence of graft rejection in skin and muscle components, despite long-term survival in other grafted tissues [27]. Surgical mortality due to anesthesia-related complications was also recorded. Waldner et al. described an intraoperative cardiac arrest in one recipient, as well as venous compromise leading to flap loss in another case [40]. Furthermore, complications such as poor perfusion, erythema, and edema were commonly cited as markers of early graft rejection, requiring close monitoring for timely intervention [40, 43]. More information is presented in Table 2.
In summary, these studies underline the potential of swine models to explore VCA and immunosuppressive strategies, revealing that combinations of traditional drugs like TAC and cyclosporine with novel agents or delivery systems can extend graft survival and reduce immune responses.
Case Report
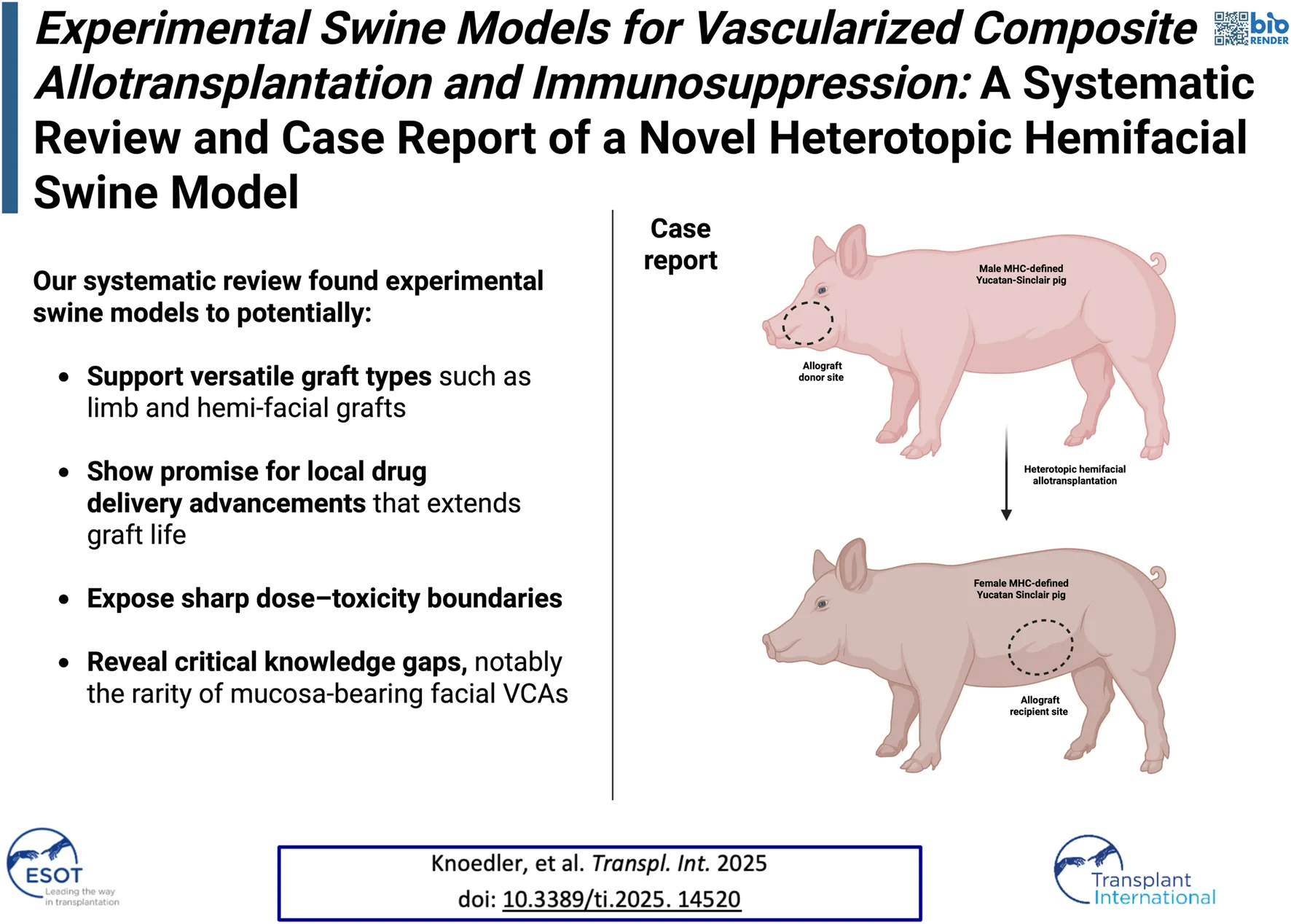
Based on these findings, we decided to perform a heterotopic hemiface transplantation procedure using an MHC-defined Yucatan Sinclair strain to establish a novel swine model consisting of heterotopic hemiface vascularized composite allotransplantation (VCA) to the groin area. This model specifically enables frequent biopsies due to the accessibility of the flap but more importantly including donor mucosa while allowing the receipient to ingeste, feeding, or persue related activities. In contrast, orthotopic transplantation risks confounding mucosal assessment, as the animal may chew on or manipulate the graft. This setup enables detailed analysis of immunological interactions at the skin and mucosa interfaces, thus providing valuable insights into tissue rejection dynamics and tolerance in a way that conventional graft sites may not accommodate as effectively. To conclude, the Yucatan Sinclair strain is furthermore well-suited for this purpose, given its immunologic compatibility in modeling human responses.
Animals
A heterotopic hemiface vascularized composite allotransplantation to the groin area was performed from a male donor pig to a female recipient of MHC-defined Yucatan Sinclair strain. We performed the study following the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health [46]. Experiments were conducted according to a protocol approved by Yale University’s Institutional Animal Care and Use Committee (protocol number 2022-20476). More details are depicted in Figure 2.
FIGURE 2
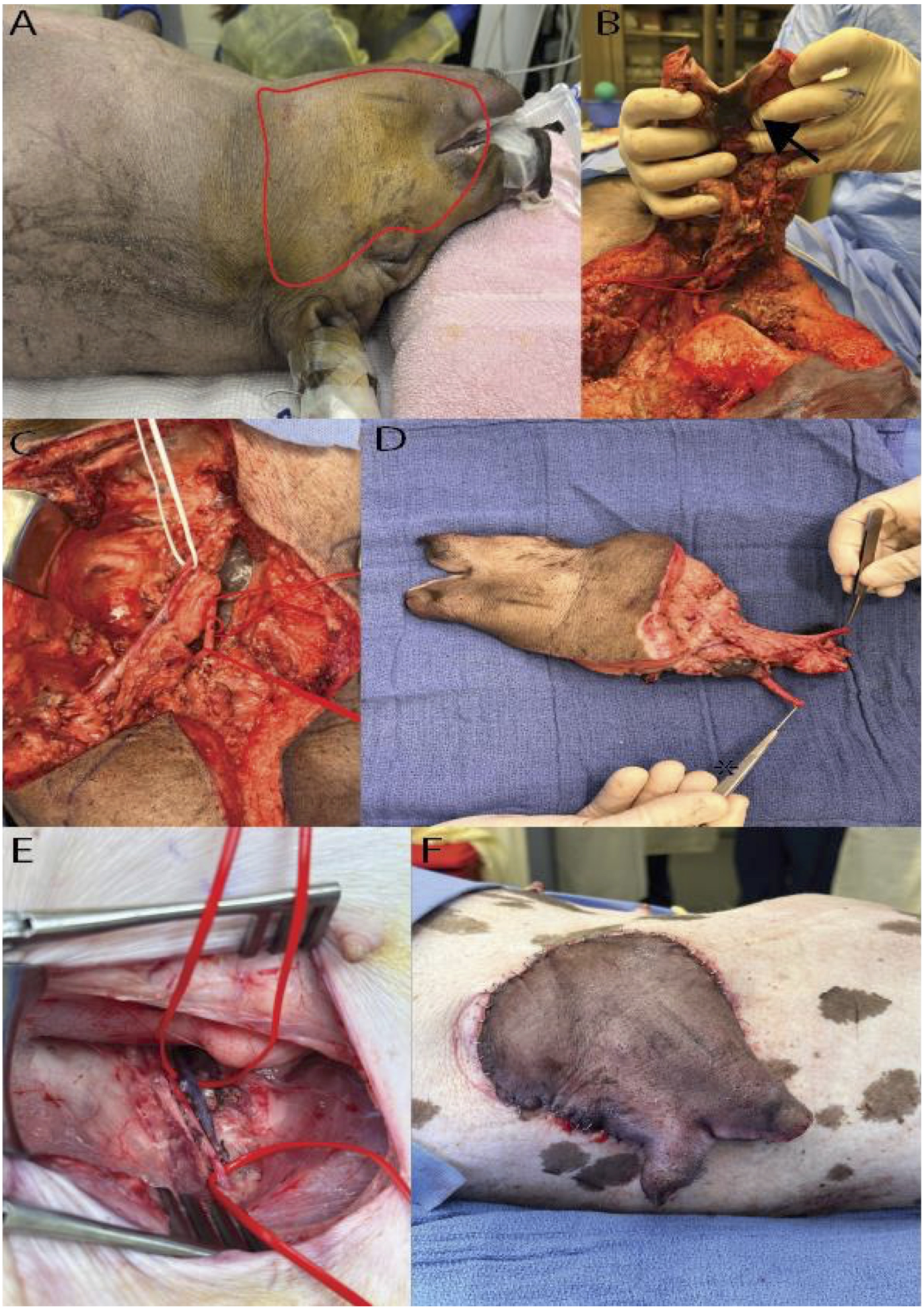
Hemifacial Heterotopic Transplant Model. (A) Outline of the hemifacial transplant. (B) Underside of the hemifacial graft after dissection, with an arrowhead marking the intraoral mucosa. (C) Demonstration of the vascular pedicle of the graft, with white vessel loop identifying the external jugular vein and red loops marking the common carotid artery. (D) Explanted hemifacial graft showing the vascular pedicle. (E) Dissected femoral vessels used for vascular anastomosis. (F) Hemifacial graft inset in the lateral abdominal wall post-transplantation.
Donor Preparation and Allograft Harvest
The donor pig was positioned supine on heat support, under isoflurane anesthesia (0.8%–2%). Following connection to monitoring equipment and IV fluid administration, the donor received prophylactic antibiotics (cefazolin) and analgesics (meloxicam, buprenorphine) alongside local anesthesia with bupivacaine at key surgical sites. Antiseptic preparation with povidone-iodine was applied to the head and neck.
A hemifacial flap was carefully marked on the donor pig’s face. Skin incisions were made along the brachiocephalic muscle and the neck, sparing the ear and eye, while advancing dissection above the periosteal plane in the nasal and fronto-parietal areas. The dissection proceeded superiorly to the mandible, preserving the external jugular vein. In the facial region, meticulous incisions were made around the auricular, eyelid, and oral areas, incorporating buccal mucosa and securing the salivary glands. Further, the submandibular gland was removed after ligating its vascular branches, and the facial artery and nerve were identified. The facial nerve was transected near the stylomastoid foramen, and the external carotid artery and external jugular vein served as the flap’s vascular pedicle. Following tissue elevations along the masseter muscle and excisions in the neck area, the sternomastoideus muscle was detached, exposing central vessels including the common carotid and its branches. Key arteries, such as the internal carotid, were ligated and transected.
The graft was perfused in situ until the recipient’s vasculature was ready. The donor’s central vessels were ligated, and the graft was flushed with heparin solution, followed by euthanasia with sodium pentobarbital as per established veterinary protocols.
Recipient Preparation and Hemiface Graft Inset
The recipient pig was anesthetized and positioned supine with a 30° rotation to expose the dorsolateral side, allowing simultaneous preparation with the donor. A groin incision exposed the femoral vessels, isolated to allow anastomosis. A subcutaneous pocket was created from the groin to the dorsolateral abdominal wall, where the graft would be placed. The hemiface flap was inset dorsolaterally to facilitate immune monitoring.
Following ligation of the donor’s femoral vessels, the graft was prepared for anastomosis. Venous anastomoses were conducted with a vascular coupling device (2.5 mm size), while arterial anastomoses were sutured with 9-0 nylon. Once vascular patency was confirmed, the graft was secured in place with sutures to the abdominal wall muscles while the skin and mucosa paddle were exteriorized for monitoring. The groin incision was closed in layers and covered with a Tegaderm® patch to prevent infection. Analgesia was administered via a fentanyl patch, and postoperative antibiotics were given. The recipient pig was monitored until full recovery.
The recipient pig recovered from the operation without any complications, exhibiting normal eating and drinking behavior and full mobility. Frequent monitoring revealed a viable flap, with the recipient site in the groin well-tolerated by the pig. After 24 h, the pig was euthanized according to protocol. These findings demonstrate the feasibility of our model for heterotopic hemiface vascularized composite allotransplantation in evaluating graft viability and immune response in a controlled and accessible site.
Discussion
Our review highlights that specific experimental variables play a critical role in shaping long-term outcomes in swine VCA models. Graft composition emerged as a key determinant of immunogenicity and tolerance induction. Grafts that incorporate vascularized bone marrow (VBM) or osteomyocutaneous tissues consistently demonstrate enhanced tolerance induction, prolonged survival, and the establishment of mixed chimerism, compared to purely fasciocutaneous grafts. Multiple studies in large animal and rodent models show that inclusion of vascularized bone or bone marrow within the graft provides a continuous source of donor-derived hematopoietic stem cells, facilitating stable mixed chimerism and promoting donor-specific tolerance [47–49]. For example, in swine, protocols combining non-myeloablative conditioning, bone marrow infusion, and osteomyocutaneous VCA have achieved stable mixed chimerism and long-term graft survival across MHC barriers, with evidence of donor-specific hyporesponsiveness and regulatory T cell expansion. Similarly, in rodent models, VBM-containing grafts result in higher chimerism and longer allograft survival than non-osseous grafts, and removal of the VBM component abrogates tolerance [50, 51]. In contrast, purely fasciocutaneous or skin-only VCAs are more immunogenic and typically undergo earlier rejection, even under similar immunomodulatory protocols, and rarely achieve durable chimerism or tolerance [27, 49, 52]. The skin component remains the most challenging tissue for tolerance induction, and its rejection is accelerated in the absence of VBM [27, 38].
In addition, immunosuppressive regimens were found to vary widely, with tacrolimus serving as a cornerstone agent. Localized delivery of tacrolimus, such as via a hydrogel platform, has been shown to extend graft survival and reduce systemic toxicity compared to high-dose systemic regimens, as demonstrated by Fries et al. Low-dose tacrolimus hydrogel delayed acute rejection and was better tolerated, while high-dose regimens led to toxicity and poorer tolerability [35, 53–56]. Combination therapies, including tacrolimus with mycophenolate mofetil and methylprednisolone, are commonly used and have been associated with improved graft viability, bone remodeling, and minimal complications in large animal models, as described in systematic reviews and preclinical studies [57, 58]. Cellular therapies, such as mesenchymal stem cells (MSCs) and adipose-derived stem cells, have also been shown to modulate immune responses, promote regulatory T cell expansion, and extend rejection-free intervals, particularly when combined with short-course tacrolimus [59, 60].
Furthermore, monitoring strategies most commonly rely on clinical observation and histological grading of acute rejection, with relatively few studies employing serial biopsies or advanced immunophenotyping. The literature highlights that clinical assessment and histopathology—often using adaptations of the Banff criteria—are the mainstays for diagnosing and grading rejection, but there is a lack of standardized, reproducible protocols across studies, and serial or multimodal monitoring is not routine. Leonard et al. and Waldner et al. are exceptions to this general trend [58, 61, 62]. Leonard et al. correlated the presence of mixed chimerism with the histologic absence of acute rejection in swine VCA recipients, with tolerance and rejection-free survival documented up to postoperative day 504, integrating both chimerism analysis and histopathology for longitudinal monitoring. Waldner et al. specifically emphasized the correlation between clinical graft appearance and histological findings, using serial punch biopsies to confirm the progression of rejection in a swine myocutaneous VCA model [40, 63]. These variations in monitoring approaches highlight the need for standardized, reproducible protocols that integrate graft design, immunosuppressive regimens, and multimodal monitoring—including clinical, histological, and immunological parameters—to improve the translational value and comparability of swine VCA research.
Future research may also investigate swine VCA xenotransplants. Recent advancements in the field have introduced genetic engineering strategies to reduce the expression of swine xenogeneic antigens identifiable by human immunoglobulins, ultimately lessening the immunological rejection against xenotransplantation. For instance, Yoon et al. used CRISPR-CAS9 to target xeno-reactive genes GGTA1, CMAH, and B4GALNT2 from Jeju Native Pigs and develop triple-knockout pigs [64]. Genetically engineered pigs showed reduced expression of galactose-alpha-1,3-galactose and N-glycolylneuraminic acid, which have been previously identified as key drivers of xenorejection [65–67]. Overall, the removal of the three genes significantly reduced xenograft rejection and binding by human IgM and IgG antibodies [64]. Interestingly, another study used galactose-alpha-1,3-galactose/N-glycolylneuraminic acid double-knockout pig lungs that were perfused for up to 6 h with fresh heparinized human blood. The authors reported reduced antibody-mediated inflammation and activation of the coagulation cascade, as well as a delayed rise in pulmonary vascular resistance when compared to galactose-alpha-1,3-galactose single-knockout pig lungs [68]. Here, the authors highlighted that the additional N-glycolylneuraminic acid helps mediate the innate immune antigenicity in xenogenically perfused porcine lungs. Additional research has also underpinned the key role of the GGTA1, CMAH, β4GalNT2 and CIITA genes in activating human CD4+ T cells in 4-gene knockout pigs [69]. With these recent advancements on the clinical horizon, wild-type pigs may become increasingly obsolete, both scientifically and due to evolving regulatory standards. Vice versa, knockout pigs may serve as a valuable donor pool to catalyze the widespread clinical adoption of VCA and pave the way toward the first vascularized composite xenotransplantation (VCX) case [2].
The insights gained from this systematic review and our heterotopic hemiface model underscore the importance of swine models in particular in translating immunosuppressive strategies for human VCA. Our model’s ability to support serial biopsies of skin and mucosa provides a unique tool for examining the dynamics of immune tolerance and rejection, potentially improving clinical outcomes in patients undergoing complex tissue transplants. By optimizing immunosuppressive strategies to balance efficacy and safety, our model offers valuable guidance for refining VCA protocols, supporting the development of safer, more effective treatment paradigms in clinical transplantation.
Limitations
This study is limited by the inherent heterogeneity of the swine models and experimental protocols reviewed, which complicates direct comparisons and generalizations across studies. The small sample sizes across the included studies reduce the generalizability of our findings and limit our ability to perform a robust quantitative meta-analysis. The rarity of VCA studies in swine also introduces potential publication bias, as studies with negative or inconclusive outcomes may be underreported.
Additionally, our heterotopic hemiface model, while valuable for serial biopsies, may not fully represent the complexity of vascular integration seen in orthotopic models, potentially limiting its direct applicability to specific clinical scenarios in VCA. Moreover, while the described heterotopic hemifacial VCA model was primarily designed to ensure surgical feasibility and facilitate serial mucosal and skin biopsies, we acknowledge the limitation that the transplanted mucosa is no longer located within its native anatomical environment. As such, it is exposed to non-physiological conditions, including external microbial flora and mechanical influences at the abdominal implantation site. These factors may affect local immune responses and limit the interpretability of biopsy-derived data with respect to natural mucosal immunity. Nevertheless, the model remains valuable for studying epithelial immune activation and early alloimmune events in a controlled and accessible setting, and it offers an important proof-of-concept for future refinements toward orthotopic models.
Conclusion
Swine models have significantly advanced our understanding of VCA immunology through diverse composite grafts and immunomodulatory approaches. However, our review highlights a notable gap in models that specifically investigate facial VCAs, particularly those including the oral mucosa. Given the unique immunological environment of facial allografts, models such as the heterotopic hemiface transplant offer critical insights into immune mechanisms and provide a platform for refining targeted immunosuppressive strategies. By enabling serial biopsies and localized immune monitoring, this model addresses key challenges such as graft rejection and the systemic effects of immunosuppression. These advancements are essential for developing safer, more effective transplantation protocols, ultimately improving patient outcomes in facial VCA.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Yale University’s Institutional Animal Care and Use Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
LK and FK conceived the study and developed the overall research strategy, including the design of the systematic review and case report. LH and MK-N were responsible for planning and executing the experimental procedures, from donor preparation to the performance of the heterotopic hemiface transplant model. SK, TS, and TN played key roles in the collection and management of experimental data. SB and SBr optimized the immunosuppressive regimens and assisted in monitoring graft function and animal recovery throughout the study. BP provided critical clinical insight, advising on surgical technique refinements and ensuring the translational relevance of our approach. MK-N supervised the entire project, coordinated ethical approvals, and led the manuscript drafting and revision process. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the German Research Foundation (DFG), grant KL 3846/2-1 (recipient Felix J Klimitz).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2025.14520/full#supplementary-material
References
1.
Kauke-Navarro M Knoedler S Panayi AC Knoedler L Haller B Parikh N et al Correlation between Facial Vascularized Composite Allotransplantation Rejection and Laboratory Markers: Insights from a Retrospective Study of Eight Patients. J Plast Reconstr and Aesthet Surg (2023) 83:155–64. 10.1016/j.bjps.2023.04.050
2.
Kauke-Navarro M Knoedler S Panayi AC Knoedler L Noel OF Pomahac B . Regulatory T Cells: Liquid and Living Precision Medicine for the Future of VCA. Transplantation (2023) 107(1):86–97. 10.1097/TP.0000000000004342
3.
Kauke M Panayi AC Safi AF Haug V Perry B Kollar B et al Full Facial Retransplantation in a Female Patient-Technical, Immunologic, and Clinical Considerations. Am J Transpl (2021) 21(10):3472–80. 10.1111/ajt.16696
4.
Kauke M Panayi AC Tchiloemba B Diehm YF Haug V Kollar B et al Face Transplantation in a Black Patient - Racial Considerations and Early Outcomes. N Engl J Med (2021) 384(11):1075–6. 10.1056/NEJMc2033961
5.
Huelsboemer L Kauke-Navarro M Boroumand S Parikh N Hosseini H Yu CT et al Ten-Year Follow-Up after Face Transplantation-A Single-Center Retrospective Cohort Study. Am J Transpl (2024) 25:611–22. 10.1016/j.ajt.2024.10.007
6.
Knoedler L Dean J Diatta F Thompson N Knoedler S Rhys R et al Immune Modulation in Transplant Medicine: A Comprehensive Review of Cell Therapy Applications and Future Directions. Front Immunol (2024) 15:1372862. 10.3389/fimmu.2024.1372862
7.
Homsy P Huelsboemer L Barret JP Blondeel P Borsuk DE Bula D et al An Update on the Survival of the First 50 Face Transplants Worldwide. JAMA Surg (2024) 159(12):1339–45. 10.1001/jamasurg.2024.3748
8.
Kaufman CL Ouseph R Marvin MR Manon-Matos Y Blair B Kutz JE . Monitoring and Long-Term Outcomes in Vascularized Composite Allotransplantation. Curr Opin Organ Transpl (2013) 18(6):652–8. 10.1097/MOT.0000000000000025
9.
Lantieri L Grimbert P Ortonne N Suberbielle C Bories D Cebrián S et al Face Transplant: Long-Term Follow-Up and Results of a Prospective Open Study. Lancet (London, England) (2016) 388:1398–407. 10.1016/S0140-6736(16)31138-2
10.
Iske J Nian Y Maenosono R Maurer M Sauer IM Tullius SG . Composite Tissue Allotransplantation: Opportunities and Challenges. Cell Mol Immunol (2019) 16(4):343–9. 10.1038/s41423-019-0215-3
11.
Sun J Adil A Biniazan F Haykal S . Immunogenicity and Tolerance Induction in Vascularized Composite Allotransplantation. Front Transplant (2024) 3:1350546. 10.3389/frtra.2024.1350546
12.
Kauke-Navarro M Tchiloemba B Haug V Kollar B Diehm Y Safi AF et al Pathologies of Oral and Sinonasal Mucosa Following Facial Vascularized Composite Allotransplantation. J Plast Reconstr Aesthet Surg (2021) 74(7):1562–71. 10.1016/j.bjps.2020.11.028
13.
Leonard DA Amin KR Giele H Fildes JE Wong JKF . Skin Immunology and Rejection in VCA and Organ Transplantation. Curr Transplant Rep (2020) 7(4):251–9. 10.1007/s40472-020-00310-1
14.
Kauke M Safi AF Zhegibe A Haug V Kollar B Nelms L et al Mucosa and Rejection in Facial Vascularized Composite Allotransplantation: A Systematic Review. Transplantation (2020) 104(12):2616–24. 10.1097/TP.0000000000003171
15.
Knoedler L Knoedler S Allam O Remy K Miragall M Safi AF et al Application Possibilities of Artificial Intelligence in Facial Vascularized Composite Allotransplantation-A Narrative Review. Front Surg (2023) 10:1266399. 10.3389/fsurg.2023.1266399
16.
Huelsboemer L Boroumand S Kochen A Dony A Moscarelli J Hauc SC et al Immunosuppressive Strategies in Face and Hand Transplantation: A Comprehensive Systematic Review of Current Therapy Regimens and Outcomes. Front Transpl (2024) 3:1366243. 10.3389/frtra.2024.1366243
17.
Messner F Grahammer J Hautz T Brandacher G Schneeberger S . Ischemia/reperfusion Injury in Vascularized Tissue Allotransplantation: Tissue Damage and Clinical Relevance. Curr Opin Organ Transpl (2016) 21(5):503–9. 10.1097/MOT.0000000000000343
18.
Kueckelhaus M Turk M Kumamaru KK Wo L Bueno EM Lian CG et al Transformation of Face Transplants: Volumetric and Morphologic Graft Changes Resemble Aging after Facial Allotransplantation. Am J Transplant (2016) 16(3):968–78. 10.1111/ajt.13544
19.
Huelsboemer L Boroumand S Boroumand T Vafa AZ Parikh N Chiarella LS et al Long-Term Posttransplant-Related Bone Volumetric Changes in Eight Face Transplant Recipients - A Single-Center Retrospective Case Series. J Plast Reconstr Aesthet Surg (2025) 101:220–30. 10.1016/j.bjps.2024.11.025
20.
Huelsboemer L Kauke-Navarro M Reuter S Stoegner VA Feldmann J Hirsch T et al Tolerance Induction in Vascularized Composite Allotransplantation-A Brief Review of Preclinical Models. Transpl Int (2023) 36:10955. 10.3389/ti.2023.10955
21.
Liberati A Altman DG Tetzlaff J Mulrow C Gøtzsche PC Ioannidis JP et al The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies that Evaluate Healthcare Interventions: Explanation and Elaboration. Bmj (2009) 339:b2700. 10.1136/bmj.b2700
22.
Hooijmans CR Rovers MM de Vries RB Leenaars M Ritskes-Hoitinga M Langendam MW . SYRCLE's Risk of Bias Tool for Animal Studies. BMC Med Res Methodol (2014) 14:43. 10.1186/1471-2288-14-43
23.
Leto Barone AA Kurtz JM Albritton A Mallard CA Shanmugarajah K Torabi R et al Effects of Transient Donor Chimerism on Rejection of MHC-Mismatched Vascularized Composite Allografts in Swine. Vascularized Compos Allotransplantation (2015) 2(1):1–8. 10.1080/23723505.2015.1039692
24.
Leonard DA Kurtz JM Mallard C Albritton A Duran-Struuck R Farkash EA et al Vascularized Composite Allograft Tolerance across MHC Barriers in a Large Animal Model. Am J Transpl (2014) 14(2):343–55. 10.1111/ajt.12560
25.
Kim D Waldner M Zhang W Solari M Washington K Marra K et al Abstract 41: Adipose-Derived Stem Cell-Based Immunomodulatory Therapy in a Translational Porcine Limb Transplant Model. Plast Reconstr Surg – Glob Open (2018) 6(4S):32–3. 10.1097/01.gox.0000533906.10398.74
26.
Elgendy TY Waldner M Zhang W Kim DY Minervini MI Komatsu C et al Tacrolimus before CTLA4Ig and Rapamycin Promotes Vascularized Composite Allograft Survival in MGH Miniature Swine. Transpl Immunol (2022) 75:101696. 10.1016/j.trim.2022.101696
27.
Wachtman GS Wimmers EG Gorantla VS Lin CH Schneeberger S Unadkat JV et al Biologics and Donor Bone Marrow Cells for Targeted Immunomodulation in Vascularized Composite Allotransplantation: A Translational Trial in Swine. Transpl Proc (2011) 43(9):3541–4. 10.1016/j.transproceed.2011.10.010
28.
Kotsougiani D Hundepool CA Bulstra LF Friedrich PF Shin AY Bishop AT . Recipient-Derived Angiogenesis with Short Term Immunosuppression Increases Bone Remodeling in Bone Vascularized Composite Allotransplantation: A Pilot Study in a Swine Tibial Defect Model. J Orthop Res (2017) 35(6):1242–9. 10.1002/jor.23378
29.
Kuo YR Shih HS Lin CC Huang CC Yang JC Wu WS et al Swine Hemi-Facial Composite Tissue Allotransplantation: A Model to Study Immune Rejection. J Surg Res (2009) 153(2):268–73. 10.1016/j.jss.2008.03.050
30.
Kuo YR Chen CC Shih HS Goto S Huang CW Wang CT et al Prolongation of Composite Tissue Allotransplant Survival by Treatment with Bone Marrow Mesenchymal Stem Cells Is Correlated with T-Cell Regulation in a Swine Hind-Limb Model. Plast Reconstr Surg (2011) 127(2):569–79. 10.1097/PRS.0b013e318200a92c
31.
Kuo YR Chen CC Chen YC Chien CM . Recipient Adipose-Derived Stem Cells Enhance Recipient Cell Engraftment and Prolong Allotransplant Survival in a Miniature Swine Hind-Limb Model. Cell Transpl (2017) 26(8):1418–27. 10.1177/0963689717724534
32.
Mathes DW Solari MG Gazelle GS Butler PE Wu A Nazzal A et al Stable Mixed Hematopoietic Chimerism Permits Tolerance of Vascularized Composite Allografts across a Full Major Histocompatibility Mismatch in Swine. Transpl Int (2014) 27(10):1086–96. 10.1111/tri.12380
33.
Berkane Y Filz von Reiterdank I Tawa P Charlès L Goutard M Dinicu AT et al VCA Supercooling in a Swine Partial Hindlimb Model. Sci Rep (2024) 14(1):12618. 10.1038/s41598-024-63041-8
34.
Zhang L Arenas Hoyos I Helmer A Banz Y Haenni B Lese I et al Immune Rejection of Cartilage in a Swine Vascularized Composite Allotransplantation Model. Transpl Proc (2024) 56(8):1896–903. 10.1016/j.transproceed.2024.08.042
35.
Fries CA Lawson SD Wang LC Slaughter KV Vemula PK Dhayani A et al Graft-Implanted, Enzyme Responsive, Tacrolimus-Eluting Hydrogel Enables Long-Term Survival of Orthotopic Porcine Limb Vascularized Composite Allografts: A Proof of Concept Study. PLoS One (2019) 14(1):e0210914. 10.1371/journal.pone.0210914
36.
Ibrahim Z Cooney DS Shores JT Sacks JM Wimmers EG Bonawitz SC et al A Modified Heterotopic Swine Hind Limb Transplant Model for Translational Vascularized Composite Allotransplantation (VCA) Research. J Vis Exp (2013)(80):50475. 10.3791/50475
37.
Kuo YR Goto S Shih HS Wang FS Lin CC Wang CT et al Mesenchymal Stem Cells Prolong Composite Tissue Allotransplant Survival in a Swine Model. Transplantation (2009) 87(12):1769–77. 10.1097/TP.0b013e3181a664f1
38.
Shanmugarajah K Powell H Leonard DA Mallard C Albritton A Harrington E et al The Effect of MHC Antigen Matching between Donors and Recipients on Skin Tolerance of Vascularized Composite Allografts. Am J Transpl (2017) 17(7):1729–41. 10.1111/ajt.14189
39.
Tratnig-Frankl P Andrews AR Berkane Y Guinier C Goutard M Lupon E et al Exploring Iodide and Hydrogen Sulfide as ROS Scavengers to Delay Acute Rejection in MHC-Defined Vascularized Composite Allografts. Antioxidants (Basel) (2024) 13(5):531. 10.3390/antiox13050531
40.
Waldner M Elgendy TY Kim DY Öksüz S Schweizer R Zhang W et al Heterotopic Transplantation of Allogeneic Vertical Rectus Abdominis Myocutaneous Flaps in Miniature Swine. J Surg Res (2020) 254:175–82. 10.1016/j.jss.2020.04.026
41.
Wang S Liu Y Fang D Shi S . The Miniature Pig: A Useful Large Animal Model for Dental and Orofacial Research. Oral Dis (2007) 13(6):530–7. 10.1111/j.1601-0825.2006.01337.x
42.
Wu KDMR Gorantla VS Lawson SD Cindass R Karp J Vemula P et al GRAFT-Implanted Tacrolimus-Eluting Hydrogels Prolong Survival after Vascularized Composite Allotransplantation. Vascularized Compos Allotransplantation (2016) 3(1-2):60. 10.1080/23723505.2016.1234226
43.
Blades CM Greyson MA Dumanian ZP Yu JW Wang Y Li B et al Development of a Porcine VCA Model Using an External Iliac Vessel-Based Vertical Rectus Abdominus Myocutaneous Flap. J Reconstr Microsurg (2024) 41:339–46. 10.1055/s-0044-1788812
44.
Park J Yim S Eun SC . Experimental Design for Composite Face Transplantation. J Craniofac Surg (2016) 27(4):843–5. 10.1097/SCS.0000000000002511
45.
Wang LC Lawson SD Fries CA Davis MR . Hyperbaric Sub-Normothermic Ex-Vivo Perfusion Delays the Onset of Acute Rejection in a Porcine VCA Model. Plast Reconstr Surg (2015) 136(4S):34. 10.1097/01.prs.0000472317.36007.b1
46.
Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. 8th ed. ed. Washington, DC: National Academies Press (2011).
47.
Huang WC Lin JY Wallace CG Chuang WY Wei FC Liao SK . Vascularized Bone Grafts within Composite Tissue Allotransplants Can Autocreate Tolerance through Mixed Chimerism with Partial Myeloablative Conditioning: An Experimental Study in Rats. Plast Reconstr Surg (2010) 125(4):1095–103. 10.1097/PRS.0b013e3181d0ab80
48.
Lellouch AG Andrews AR Saviane G Ng ZY Schol IM Goutard M et al Tolerance of a Vascularized Composite Allograft Achieved in MHC Class-I-Mismatch Swine via Mixed Chimerism. Front Immunol (2022) 13:829406. 10.3389/fimmu.2022.829406
49.
Lin CH Zhang W Ng TW Zhang D Jiang J Pulikkottil B et al Vascularized Osteomyocutaneous Allografts Are Permissive to Tolerance by Induction-Based Immunomodulatory Therapy. Am J Transpl (2013) 13(8):2161–8. 10.1111/ajt.12275
50.
Lin CH Anggelia MR Cheng HY Hsieh YH Chuang WY Yang HY et al The Intragraft Vascularized Bone Marrow Induces Secondary Donor-Specific Mystacial Pad Allograft Tolerance. Front Immunol (2022) 13:1059271. 10.3389/fimmu.2022.1059271
51.
Lin CH Anggelia MR Cheng HY Wang AYL Chuang WY Lin CH et al The Intragraft Vascularized Bone Marrow Component Plays a Critical Role in Tolerance Induction after Reconstructive Transplantation. Cell Mol Immunol (2021) 18(2):363–73. 10.1038/s41423-019-0325-y
52.
Chen J Zhang D Zhang T Chen C Song Y Liu S et al Effect of the Vascularized Bone Components on the Survival of Vascularized Composite Allografts. J Surg Res (2018) 224:132–8. 10.1016/j.jss.2017.03.050
53.
Ben Brahim B Arenas Hoyos I Zhang L Vogelin E Olariu R Rieben R . Tacrolimus-Loaded Drug Delivery Systems in Vascularized Composite Allotransplantation: Lessons and Opportunities for Local Immunosuppression. Transplantation (2025) 109(1):142–52. 10.1097/TP.0000000000005049
54.
Feturi FG Unadkat JV Zhang W Hag ME Wang Y Komatsu C et al Tacrolimus-Eluting Disk within the Allograft Enables Vascularized Composite Allograft Survival with Site-Specific Immunosuppression without Systemic Toxicity. Pharm Res (2022) 39(9):2179–90. 10.1007/s11095-022-03345-4
55.
Safi AF Kauke M Nelms L Palmer WJ Tchiloemba B Kollar B et al Local Immunosuppression in Vascularized Composite Allotransplantation (VCA): A Systematic Review. J Plast Reconstr Aesthet Surg (2021) 74(2):327–35. 10.1016/j.bjps.2020.10.003
56.
Olariu R Denoyelle J Leclere FM Dzhonova DV Gajanayake T Banz Y et al Intra-Graft Injection of Tacrolimus Promotes Survival of Vascularized Composite Allotransplantation. J Surg Res (2017) 218:49–57. 10.1016/j.jss.2017.05.046
57.
Feturi FG Zhang W Erbas VE Dong L Sahin H Zhang Z et al Topical Tacrolimus and Mycophenolic Acid Therapy Synergizes with Low Dose Systemic Immunosuppression to Sustain Vascularized Composite Allograft Survival. J Pharm Sci (2024) 113(6):1607–15. 10.1016/j.xphs.2024.01.015
58.
Kauke M Safi AF Panayi AC Palmer WJ Haug V Kollar B et al A Systematic Review of Immunomodulatory Strategies Used in Skin-Containing Preclinical Vascularized Composite Allotransplant Models. J Plast Reconstr Aesthet Surg (2022) 75(2):586–604. 10.1016/j.bjps.2021.11.003
59.
Plock JA Schnider JT Zhang W Schweizer R Tsuji W Kostereva N et al Adipose- and Bone Marrow-Derived Mesenchymal Stem Cells Prolong Graft Survival in Vascularized Composite Allotransplantation. Transplantation (2015) 99(9):1765–73. 10.1097/TP.0000000000000731
60.
Schweizer R Taddeo A Waldner M Klein HJ Fuchs N Kamat P et al Adipose-Derived Stromal Cell Therapy Combined with a Short Course Nonmyeloablative Conditioning Promotes Long-Term Graft Tolerance in Vascularized Composite Allotransplantation. Am J Transpl (2020) 20(5):1272–84. 10.1111/ajt.15726
61.
Etra JW Grzelak MJ Fidder SAJ Kolegraff K Bonawitz S Shores J et al A Skin Rejection Grading System for Vascularized Composite Allotransplantation in a Preclinical Large Animal Model. Transplantation (2019) 103(7):1385–91. 10.1097/TP.0000000000002695
62.
Sarhane KA Khalifian S Ibrahim Z Cooney DS Hautz T Lee WP et al Diagnosing Skin Rejection in Vascularized Composite Allotransplantation: Advances and Challenges. Clin Transpl (2014) 28(3):277–85. 10.1111/ctr.12316
63.
Leonard DA Powell HR Defazio MW Shanmugarajah K Mastroianni M Rosales IA et al Cutaneous Leukocyte Lineages in Tolerant Large Animal and Immunosuppressed Clinical Vascularized Composite Allograft Recipients. Am J Transpl (2021) 21(2):582–92. 10.1111/ajt.16230
64.
Yoon S Lee S Park C Choi H Yoo M Lee SC et al An Efficacious Transgenic Strategy for Triple Knockout of Xeno-Reactive Antigen Genes GGTA1, CMAH, and B4GALNT2 from Jeju Native Pigs. Vaccines (Basel) (2022) 10(9):1503. 10.3390/vaccines10091503
65.
Joziasse DH Oriol R . Xenotransplantation: The Importance of the Galalpha1,3Gal Epitope in Hyperacute Vascular Rejection. Biochim Biophys Acta (1999) 1455(2-3):403–18. 10.1016/s0925-4439(99)00056-3
66.
Sandrin MS McKenzie IF . Gal Alpha (1,3)Gal, the Major Xenoantigen(s) Recognised in Pigs by Human Natural Antibodies. Immunol Rev (1994) 141:169–90. 10.1111/j.1600-065x.1994.tb00877.x
67.
Tector AJ Mosser M Tector M Bach JM . The Possible Role of Anti-Neu5Gc as an Obstacle in Xenotransplantation. Front Immunol (2020) 11:622. 10.3389/fimmu.2020.00622
68.
Chaban R Habibabady Z Hassanein W Connolly MR Burdorf L Redding E et al Knock-Out of N-Glycolylneuraminic Acid Attenuates Antibody-Mediated Rejection in Xenogenically Perfused Porcine Lungs. Xenotransplantation (2022) 29(6):e12784. 10.1111/xen.12784
69.
Xu J Ren J Xu K Fang M Ka M Xu F et al Elimination of GGTA1, CMAH, β4GalNT2 and CIITA Genes in Pigs Compromises Human versus Pig Xenogeneic Immune Reactions. Anim Model Exp Med (2024) 7(4):584–90. 10.1002/ame2.12461
Summary
Keywords
swine models, vascularized composite allotransplantation, immunosuppression, graft rejection, preclinical research
Citation
Knoedler L, Klimitz FJ, Huelsboemer L, Niederegger T, Schaschinger T, Knoedler S, Boroumand S, Brown S, Pomahac B and Kauke-Navarro M (2025) Experimental Swine Models for Vascularized Composite Allotransplantation and Immunosuppression: A Systematic Review and Case Report of a Novel Heterotopic Hemifacial Swine Model. Transpl. Int. 38:14520. doi: 10.3389/ti.2025.14520
Received
21 February 2025
Accepted
11 July 2025
Published
29 July 2025
Volume
38 - 2025
Updates
Copyright
© 2025 Knoedler, Klimitz, Huelsboemer, Niederegger, Schaschinger, Knoedler, Boroumand, Brown, Pomahac and Kauke-Navarro.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Leonard Knoedler, leonard.knoedler@charite.de; Martin Kauke-Navarro, kauke-navarro.martin@yale.edu
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.