Abstract
Donor-specific HLA antibodies (DSA) are related to antibody-mediated rejection (ABMR) and graft failure. The rationale and frequency of screening for anti-HLA antibodies in stable patients are not established. The aim of our study is to evaluate the impact of early DSA appearance in the first month post-transplant on graft outcomes. All kidney transplant recipients between 1/1/2012–12/31/2022 with anti-HLA antibody screening by Luminex during the first month post-transplant were included. Patients with preformed or historical DSA and those with DSA detection after graft loss were excluded. The mean fluorescence intensity cut-off was 1,500. Three hundred fifty-three patients were included and the median time from transplant to first antibody sample was 30.0 days. During 3.8 years of follow-up, graft loss occurred in 9.1% and 19.5% had ABMR. A total of 8.5% developed early-DSA in the first month. Patients with early-DSA detection had more HLA sensitization at the time of transplant (p < 0.001). Multivariable analysis showed that the presence of early-DSA was an independent risk factor for ABMR. In conclusion, sensitized patients at the time of transplant have a higher risk of DSA formation in the first month, probably reflecting alloimmune memory, therefore early HLA antibody screening should be performed in this high-risk population.
Introduction
Donor-specific HLA antibodies (DSA) are a key factor for the diagnosis of antibody-mediated rejection (ABMR) and are associated with poor outcomes after kidney transplantation [1–6]. Immunological risk assessment before and after transplant has improved with solid-phase immunoassays in the Luminex system that provide sensitive and specific information on HLA antibodies with screening and single-antigen bead (SAB) assays, but their results must be interpreted appropriately [7–14]. In particular, the semiquantitative value of mean fluorescence intensity (MFI) and the problem of establishing a fixed and universal MFI positivity threshold hinder the correlation of DSA with clinical outcomes and the unification of results [15, 16].
Preformed and de novo DSA (dnDSA) are related to alloimmune injury [17–19]. The risk factors for dnDSA development are, among others, under-immunosuppression, graft inflammation and high HLA mismatch [17, 20–24]. Although dnDSA may appear at any time after transplantation, the STAR Working Group notes that the development of DSA between 2 weeks and 3 months post-transplant may represent a memory response [15, 25]. A history of HLA sensitizing events such as previous transplants, pregnancies or blood transfusions, and the presence of non-DSA HLA antibodies prior to transplant are risk factors for latent alloimmune memory [15].
Despite the impact of DSA on graft outcomes, there is still no consensus on the indication for DSA screening after transplantation in stable patients. Recently, the OuTSMART trial demonstrated that optimization of baseline immunosuppression after DSA detection had no impact on graft survival [26], showing that universal screening may be controversial, and the cost-effectiveness of this strategy is not determined [27, 28]. Furthermore, the frequency of routine surveillance for HLA antibodies is not established [15], and a recent ESOT Working Group proposed a monitoring scheme with screening in the first 3–6 months after transplant and annually thereafter (2C recommendation) [29]. Although an earlier assessment of HLA antibodies has been suggested in patients at potential risk of latent memory [15, 30], the exact timing of the first post-transplant HLA determination is not currently settled. Moreover, most series have described early DSA as those detected in the first year posttransplant [31–35], and there are few data on the specific evaluation of HLA monitoring in the first month.
The aim of our study is to evaluate the impact of early HLA antibody screening in the first month posttransplant on kidney graft outcomes and identify patients at risk of early DSA formation.
Materials and Methods
Study Population
For this retrospective analysis we included all kidney transplant recipients from 1/1/2012–12/31/2022 at Marqués de Valdecilla University Hospital with HLA antibody screening during the first month post-transplant (range 10–60 days). Those patients with preformed or historical DSA described in pre-transplant sera and with positive flow cytometry crossmatch (FCXM) were excluded. Patients with graft failure in the first 60 days and those who developed DSA after graft loss were also excluded. The primary outcome variable was time to antibody-mediated rejection. The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the regional Ethics Committee of our institution (2024.196).
Demographic and clinical data, including recipient and donor data (type, age), induction immunosuppression, cold ischemia time (CIT), delayed graft function (DGF), HLA mismatch, early blood transfusions - within the first 30 days after transplant, and biopsy data were collected from the prospectively maintained database of renal transplant patients at our center. All rejections were categorized according to Banff 2019 classification [36]. Allograft biopsies were performed by clinical indication and by protocol 1-year after transplantation.
Our induction protocol consisted of anti-thymocyte globulin (ATG) in highly sensitized patients and in some patients with a previous transplant lost due to rejection. Anti-IL2R was administered in patients at high risk of post-transplant acute tubular necrosis, primarily due to advanced donor age, prolonged expected CIT, or donation after circulatory death. The corticosteroid treatment protocol at our center was an intravenous pulse of 500 milligrams (mg) of methylprednisolone for induction. Oral prednisone was continued at 20 mg for the first 2 weeks after transplant, 15 mg of prednisone 2 weeks later until the first month and 10 mg at 1 month after transplant, with a subsequent reduction to 7.5 mg after 2 months and 5 mg after 3 months. Discontinuation or maintenance of baseline prednisone after 3 months was performed individually and according to clinical indication.
HLA Antibodies
Regular monitoring of HLA antibodies was performed in the first- and sixth-month post-transplant and annually thereafter as routine clinical practice in our center, and in case of signs of impaired allograft function or clinical request. Patients had pre- and post-transplant sera screened using a mixed panel beads (LABScreen Mixed Class I and II, One Lambda, Canoga Park, CA) and if a positive result was detected, further LABScreen® SAB assay class-I and class-II (One Lambda, Canoga Park, CA) was performed by Luminex® technology. Pre-transplant sera before 2012 were assessed by enzyme-linked immunosorbent assay (ELISA) as this was the available technique, but all patients had at least one pre-transplant serum screened by Luminex®. According to the policy of our center, anti-HLA antibody testing was performed every 3 months in patients on the transplant waiting list. An additional anti-HLA antibody sample was collected on day 0 if sensitizing events, such as blood transfusions, occurred between the day of transplant and the last serum sample. The last pretransplant anti-HLA antibody sample was used to calculate pretransplant cPRA, but all pretransplant sera were reviewed to exclude patients with preformed or historical DSA.
The general positivity threshold in our laboratory was set at 1,500 MFI, and the presence of DSA was defined by the Histocompatibility laboratory considering the MFI positivity cut-off and other factors such as the evolution of HLA antibodies posttransplant or epitope sharing phenomena [15, 16]. In laboratory routine, we included dilution sera in highly sensitized patients and in those with suspected prozone, as described [37].The most probable 2-field HLA typing of the donor [38] and haplotype frequencies [39, 40] for missing information on specific HLA loci were considered to assign DSA. Calculated panel-reactive antibody (cPRA) was obtained through the Virtual PRA Calculator of the Eurotransplant Reference Laboratory [41], and delta cPRA >0% was recorded (difference between cPRA in the first serum at 1-month post-transplant and cPRA in the last pre-transplant serum).
Patient Groups
Patients with early DSA detection in the first month (10–60 days) posttransplant were categorized as “early-DSA,” and those patients with first DSA detection >60 days - and without DSA in the first month - were categorized as “late-DSA.” Patients without DSA detection during the follow-up period were classified as “no-DSA.” “Transient” DSA was defined as disappearance of DSA at 3 and/or 6 months after first detection (if >1 DSA per patient, disappearance of at least one DSA).
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation (SD) or median and interquartile range (IQR) according to their distribution. Categorical variables were described as relative frequencies. Continuous variables with non-normal distribution were compared using non-parametric tests (Mann-Whitney U test to compare 2 groups and Kruskal-Wallis test to compare 3 groups). A chi-square test was used to compare the average values of categorical variables. Univariable and multivariable Cox regression were performed to determine which variables were associated with ABMR, and hazard ratios (HR) were reported with 95% confidence intervals. To evaluate the predictive capacity of DSA by Cox regression, patients with ABMR before DSA appearance were eliminated from this analysis. Time-to-event outcome data were assessed by Kaplan–Meier plots and log-rank tests. A p-value <0.05 was defined as statistical significance. Statistical analysis was conducted using the SPSS statistical software package (Version 25.0. Armonk, NY: IBM Corp.).
Results
Baseline Characteristics
In total, we included 353 patients with early HLA antibody screening in the first month post-transplant (Figure 1). The time between transplant and last pre-transplant antibody sample was 42.0 days (IQR 16.0–73.0), and the median time from transplant to first anti-HLA antibody test was 30.0 days (IQR 26.0–37.0). Most patients (297, 84.1%) had systematic early HLA screening by protocol, and 56/353 (15.9%) underwent early HLA screening in the first month also for clinical indication (rise in creatinine and/or proteinuria). No significant differences were observed between DSA groups regarding early HLA antibody testing solely by protocol or by clinical indication (p = 0.178). At the time of early HLA antibody screening, the median creatinine (Cr) value was 1.49 mg/dL (IQR 1.13–1.97), the estimated glomerular filtration rate (eGFR) by CKD-EPI was 49.3 mL/min/1.73 m2 (IQR 34.4–67.1), and the median urine albumin-to-creatinine ratio was 69.6 mg/g (IQR 27.5–176.9). The time of initial hospital admission for transplant was 17.0 days (IQR 11.0–25.0), and most early HLA determinations in the first month (279/353, 79.0%) were performed on an outpatient basis, after first hospital admission.
FIGURE 1
The study cohort comprised mainly patients with a first single-kidney transplant from a deceased donor (Table 1) with a median follow-up of 3.8 years (IQR 2.1–6.4). By design of the study, none of the patients had pretransplant DSA and most patients were not sensitized (median cPRA 0.0%). Specifically analyzing patients with a first kidney transplant (n = 257, 72.8%), 20.6% had a pregnancy before transplantation. In this group of first transplants, pregnancy before transplant was significantly higher in sensitized patients with cPRA ≥5% (32.1% vs. 4.9%, p < 0.001) and cPRA ≥85% (7.5% vs. 0.5%, p = 0.001).
TABLE 1
| Baseline characteristics | All patients (n = 353) | No-DSA (n = 304) | Early-DSA (n = 30) | Late-DSA (n = 19) | p |
|---|---|---|---|---|---|
| Recipient age (years)a | 57.0 (45.0–65.0) | 58.0 (46.0–65.0) | 49.5 (41.0–64.2) | 45.0 (32.0–63.0) | 0.235 |
| Recipient sex (male) (n, %)a | 245 (69.4%) | 217 (71.4%) | 17 (56.7%) | 11 (57.9%) | 0.133 |
| Recipient sex (female) (n, %) • Pregnancy before transplant | 108 (30.6%) 65 (60.2%) | 87 (28.6%) 53 (60.9%) | 13 (43.3%) 8 (61.5%) | 8 (42.1%) 4 (50.0%) | 0.133 0.829 |
| Death-censored graft failure (n, %) | 32 (9.1%) | 19 (6.3%) | 6 (20.0%) | 7 (36.8%) | <0.001 |
| Death (n, %) | 37 (10.5%) | 31 (10.2%) | 5 (16.7%) | 1 (5.3%) | 0.406 |
| Donor age (years)a | 55.0 (45.0–63.0) | 55.0 (45.0–63.0) | 55.0 (44.7–63.2) | 52.0 (41.0–69.0) | 0.982 |
| Donor sex (male) (n, %) | 210 (59.5%) | 180 (59.2%) | 18 (60.0%) | 12 (63.2%) | 0.942 |
| Donor type (deceased) (n, %) • DBD (n, %) • DCD (n, %) | 337 (95.5%) 213 (60.3%) 124 (35.1%) | 293 (96.4%) 183 (60.2%) 110 (36.2%) | 27 (90.0%) 18 (60.0%) 9 (30.0%) | 17 (89.5%) 12 (63.2%) 5 (26.4%) | 0.120 0.542 |
| Induction immunosuppression (n, %) • ATG (n, %) • Anti-IL2R (n, %) | 253 (71.7%) 120 (47.4%) 133 (52.6%) | 216 (71.1%) 92 (42.6%) 124 (57.4%) | 23 (76.7%) 18 (78.3%) 5 (21.7%) | 14 (73.7%) 10 (71.4%) 4 (28.6%) | 0.793 0.001 |
| First kidney transplant (n, %) | 257 (72.8%) | 228 (75.0%) | 18 (60.0%) | 11 (57.9%) | 0.069 |
| Retransplant (n, %) • Repeated HLA mismatch with previous donors (n, %) | 96 (27.2%) 30 (31.3%) | 76 (25.0%) 23 (30.3%) | 12 (40.0%) 5 (41.7%) | 8 (42.1%) 2 (25.0%) | 0.069 0.675 |
| Combined transplant (n, %) • Pancreas-kidney (n, %) • Liver-kidney (n, %) | 23 (6.5%) 20 (87.0%) 3 (13.0%) | 20 (6.6%) 17 (85.0%) 3 (15.0%) | 2 (6.7%) 2 (100.0%) 0 (0.0%) | 1 (5.3%) 1 (100.0%) 0 (0.0%) | 0.974 0.772 |
| CIT (hours)a | 19.0 (10.0–23.0) | 19.0 (10.0–23.0) | 20.0 (11.2–22.2) | 12.0 (5.0–22.0) | 0.506 |
| DGF (n, %) | 91 (25.8%) | 82 (27.0%) | 6 (20.0%) | 3 (15.8%) | 0.746 |
| Early blood transfusion (n, %)b | 199 (56.4%) | 169 (55.6%) | 19 (63.3%) | 11 (57.9%) | 0.710 |
| cPRA at the time of transplant (%)a | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 40.4 (0.0–87.8) | 0.0 (0.0–36.3) | <0.001 |
| cPRA ≥5% at the time of transplant (n, %) | 79 (22.4%) | 56 (18.4%) | 17 (56.7%) | 6 (31.6%) | <0.001 |
| cPRA ≥85% at the time of transplant (n, %) | 33 (9.3%) | 24 (7.9%) | 8 (26.7%) | 1 (5.3%) | 0.003 |
| HLA-A mismatch >0 (n, %) | 312 (88.4%) | 266 (87.5%) | 29 (96.7%) | 17 (89.5%) | 0.323 |
| HLA-B mismatch >0 (n, %) | 322 (91.2%) | 277 (91.1%) | 27 (90.0%) | 18 (94.7%) | 0.838 |
| HLA-DRB1 mismatch >0 (n, %) | 297 (84.1%) | 257 (84.5%) | 24 (80.0%) | 16 (84.2%) | 0.810 |
| IS regimen at the time of early HLA screening (n, %) • Tacrolimus, MMF/MPA, prednisone • Tacrolimus, mTORi, prednisone • Others | 344 (97.5%) 7 (2.0%) 2 (0.6%) | 296 (97.4%) 7 (2.3%) 1 (0.3%) | 29 (96.7%) 0 (0.0%) 1 (3.3%) | 19 (100.0%) 0 (0.0%) 0 (0.0%) | 0.748 0.562 0.106 |
| Early HLA screening only by protocol (n, %) | 297 (84.1%) | 260 (85.5%) | 22 (73.3%) | 15 (78.9%) | 0.178 |
| Plasmapheresis before early HLA screening (n, %)c | 35 (9.9%) | 26 (8.6%) | 7 (23.3%) | 2 (10.5%) | 0.035 |
| IVIG before early HLA screening (n, %)c | 30 (8.5%) | 21 (6.9%) | 7 (23.3%) | 2 (10.5%) | 0.008 |
| ABMR (Banff 2019 Classification) (n, %)d • Time from transplant to ABMR (months)a | 69 (19.5%) 3.3 (0.6–12.1) | 38 (12.5%) 2.7 (0.5–12.0) | 16 (53.3%) 1.7 (0.5–3.6) | 15 (78.9%) 11.4 (1.5–35.7) | <0.001 0.027 |
| Biopsy with ABMR diagnosis by clincal indication or 1-year protocol • Clinical indication (graft dysfunction and/or DSA appearance) (n, %) - Only for DSA (n, %) - Graft dysfunction (n, %) • 1-year-Protocol (n, %) | 54 (78.3%) 15 (21.7%) | 26 (68.4%) 12 (31.6%) | 16 (100.0%) 5 (31.3%) 11 (68.8%) 0 (0.0%) | 12 (80.0%) 2 (16.7%) 10 (83.3%) 3 (20.0%) | 0.036 |
| TCMR (Banff 2019 Classification) (n, %)d | 96 (27.2%) | 75 (24.7%) | 11 (36.7%) | 10 (52.6%) | 0.014 |
Baseline characteristics of patients included in our study.
Median and interquartile range. For continuous variables with non-normal distribution, the Kruskal-Wallis test was used to compare the 3 groups.
Early blood transfusion: at least one blood transfusion within the first 30 days after transplant.
Treatment with plasmapheresis and/or IVIG, before the first early HLA, determination was performed as rejection prophylaxis in high-risk patients, suspected rejection and inability to perform a biopsy, or biopsy-proven ABMR.
Rejection episodes were categorized according to Banff 2019 Classification. Borderline rejection was included in the category of T-cell mediated rejection (TCMR).
DBD: donor brain death. DCD: donor circulatory death. ATG, antithymocyte globulin; Anti-IL2R, anti-interleukin-2, receptor. CIT: cold ischemia time. DGF: delayed graft-function. cPRA: calculated panel-reactive antibody (Eurotransplant). IS: immunosuppression. MMF/MPA: Mycophenolate mofetil/Mycophenolic acid. mTORi: mTOR, inhibitor. IVIG: Intravenous immunoglobulin. ABMR: antibody-mediated rejection. TCMR: T-cell mediated rejection.
Graft failure occurred in 9.1% of patients and 19.5% had ABMR. Delta cPRA >0% developed in 54 patients (15.3%) whereas 30 out of 353 (8.5%) had early-DSA detection in the first month, and the total number of patients with DSA appearance during the complete follow-up period was 49/353 (13.9%). In our cohort, the median time from transplant to first DSA detection was 1.5 months (IQR 1.0–11.4) (Supplementary Figure S1).
When evaluating the characteristics of patient groups (Table 1), they were comparable in terms of recipient age, recipient sex, donor age and donor type. 71.7% of patients received induction immunosuppression, with more induction with anti-thymocyte globulin (ATG) in the early-DSA group (p = 0.001). Patients in the early-DSA group had more HLA sensitization at the time of transplant compared to patients with late-DSA and no-DSA (cPRA 40.4% vs. 0.0% vs. 0.0%, p < 0.001). There was a higher proportion of patients receiving plasmapheresis and intravenous immunoglobulin (IVIG) treatment before early screening in the early-DSA group (p = 0.035 and p = 0.008, respectively). There were differences between groups in T-cell mediated rejection (TCMR) episodes, with a higher incidence in patients with late-DSA (p = 0.014). Patients with early-DSA and late-DSA had more proportion of ABMR compared to patients without DSA (53.3% vs. 78.9% vs. 12.5%, p < 0.001), and the median time from transplant to first ABMR episode was shorter in the early-DSA group (p = 0.027). A higher proportion of biopsies with ABMR diagnosis were performed for clinical indication in the early and late-DSA groups (p = 0.036). Histological data are shown in Supplementary Tables S1–S3.
Patients With Early-DSA
Most patients with early-DSA had class-I (56.7%), 33.3% had class-II and 10.0% had class-I and class-II. Regardless of the antibody class, 33.3% had >1 DSA in the same first serum after transplant. The median MFI level at first early-DSA detection was 4,912.0 (IQR 2,505.7–7,235.2). “Transient” early-DSA were detected in 20/30 patients (66.7%), and 60.0% (12/20) had early-DSA negativity after rejection and specific active treatment whereas 40.0% (8/20) had “spontaneous” disappearance of early-DSA without treatment for rejection.
The median values of estimated glomerular filtration rate and urine albumin-creatinine ratio in the early-DSA group at the time of early-DSA appearance were 37.5 mL/min/1.73 m2 (IQR 28.7–60.0) and 91.0 mg/g (IQR 43.8–332.7), respectively. At least one allograft biopsy was performed in 83.3% of patients with early-DSA. Technical difficulty, high-risk due to anticoagulation, patient refusal or “transient” early-DSA without evidence of graft dysfunction were the reasons for not performing a biopsy in 5/30 patients. ABMR was present in 53.3% of patients with early-DSA, and 46.7% had “subclinical” early-DSA without evidence of ABMR. In patients with ABMR, 31.3% (5/16) had rejection before early-DSA appearance in the first month and 68.8% (11/16) presented ABMR at the time or after early-DSA detection. Patients with early-DSA and ABMR were associated with lower allograft survival compared to those patients with “subclinical” early-DSA (log rank p = 0.012) (Supplementary Figure S2). 36.7% of patients with early-DSA (11/30) presented TCMR, and 36.4% of these patients (4/11) had TCMR before first early-DSA appearance. The characteristics of DSA in patients with early-DSA and late-DSA are illustrated in Table 2. The evolution of eGFR in patients with DSA is shown in Supplementary Table S4 and Supplementary Figures S3, S4.
TABLE 2
| DSA characteristics | Early-DSA (n = 30) | Late-DSA (n = 19) | p |
|---|---|---|---|
| Class of DSA (n, %) • Class-I • Class-IIa • Both class-I and class-IIa | 17 (56.7%) 10 (33.3%) 3 (10.0%) | 6 (31.6%) 10 (52.6%) 3 (15.8%) | 0.230 |
| Number of DSA per patient: >1 DSA in the first sample (n, %) | 10 (33.3%) | 7 (36.8%) | 0.801 |
| “Transient” DSA (n, %) | 20 (66.7%) | 7 (36.8%) | 0.041 |
| MFI at DSA first occurrenceb | 4912.0 (2505.7–7235.2) | 2428.5 (1432.5–11,109.0) | 0.365 |
| IS at the time of first DSA detection • Tacrolimus (n, %) • MMF/MPA (n, %) • Prednisone (n, %) • mTORi (n, %) | 29 (96.7%) 29 (96.7%) 29 (96.7%) 0 (0.0%) | 18 (94.7%) 18 (94.7%) 17 (89.5%) 2 (10.5%) | 0.739 0.739 0.306 0.070 |
| ABMR (Banff 2019 Classification) (n, %) • ABMR before DSA detection • ABMR at the time/after DSA detection Time from DSA to ABMR diagnosis (months)b | 16 (53.3%) 5 (31.3%) 11 (68.8%) 1.4 (0.3–3.1) | 15 (78.9%) 7 (46.7%) 8 (53.3%) 3.2 (2.0–5.2) | 0.070 0.370 |
DSA characteristics in patients with early-DSA and late-DSA.
Of patients with class-II DSA, in both groups (alone or together with class-I DSA), 18/26 (69.2%) had anti-DQB, 5/26 (19.2%) had anti-DQA, and 1/16 (3.8%) had anti-DP DSA.
Median and interquartile range. For continuous variables with non-normal distribution, the Mann-Whitney U test was used to compare the 2 groups.
DSA: donor-specific HLA, antibody. MFI: mean fluorescence intensity. IS: immunosuppression. MMF/MPA: Mycophenolate mofetil/Mycophenolic acid. mTORi: mTOR, inhibitor.
DSA Status, Rejection and Graft Survival
The presence of early-DSA and late-DSA was associated with lower death-censored allograft survival (log rank p = 0.001), as shown in Figure 2. Independently of DSA status, patients with ABMR were associated with lower allograft survival compared to patients without ABMR (log rank p = 0.001). Similarly, the presence of TCMR was associated with lower graft survival (log rank p = 0.006). These results are shown in Supplementary Figures S5, S6.
FIGURE 2
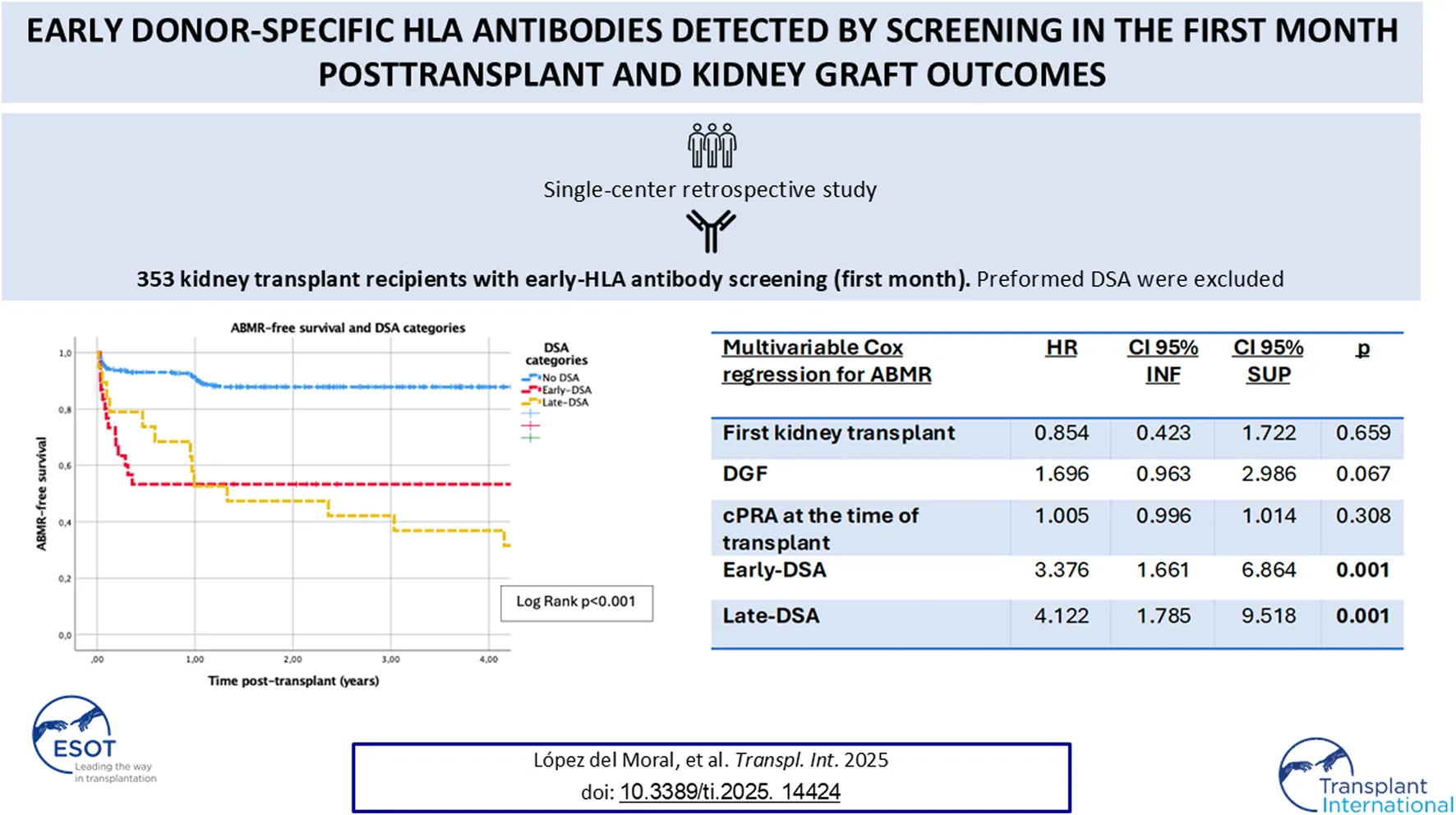
Analyzing the relationship between DSA and ABMR, the presence of DSA was associated with lower ABMR-free survival (log rank p < 0.001) (Figure 3). Different patient characteristics were associated with ABMR in univariable Cox regression analyses (Table 3). Specifically analyzing factors that were associated with ABMR posttransplant by multivariable Cox regression (Table 4), early-DSA and late-DSA were independent risk factors for ABMR (HR 3.3, CI 95% 1.6–6.8, p = 0.001 and HR 4.1, CI 95% 1.7–9.5, p = 0.001, respectively). Conversely, first kidney transplant, DGF or HLA sensitization at the time of transplant were not contributors in the multivariable model.
FIGURE 3
TABLE 3
| Univariable cox regression for ABMRa | HR | CI 95% INF | CI 95% SUP | p |
|---|---|---|---|---|
| Recipient age | 0.985 | 0.966 | 1.006 | 0.158 |
| Donor age | 0.982 | 0.964 | 1.001 | 0.061 |
| Deceased donor | 2.000 | 0.798 | 5.015 | 0.139 |
| DCD | 0.910 | 0.519 | 1.519 | 0.743 |
| Induction immunosuppression | 1.478 | 0.795 | 2.747 | 0.216 |
| First kidney transplant | 0.523 | 0.308 | 0.888 | 0.016 |
| Combined transplant | 0.645 | 0.201 | 2.068 | 0.461 |
| CIT | 1.022 | 0.986 | 1.059 | 0.235 |
| DGF | 1.779 | 1.026 | 3.083 | 0.040 |
| cPRA at the time of transplant | 1.009 | 1.003 | 1.016 | 0.007 |
| HLA-A mismatch >0 | 1.506 | 0.601 | 3.773 | 0.382 |
| HLA-B mismatch >0 | 1.237 | 0.448 | 3.418 | 0.682 |
| HLA-DRB1 mismatch >0 | 2.059 | 0.822 | 5.157 | 0.123 |
| HLA mismatch – sum of A, B and DRB1 mismatch | 1.069 | 0.884 | 1.293 | 0.490 |
| Early-DSA | 3.279 | 1.696 | 6.339 | <0.001 |
| Late-DSA | 3.981 | 1.882 | 8.421 | <0.001 |
Univariable Cox regression analysis for antibody-mediated rejection (ABMR).
ABMR: antibody-mediated rejection. DCD: donor circulatory death. CIT: cold ischemia time. DGF: delayed graft-function. cPRA: calculated panel-reactive antibody (Eurotransplant). DSA: donor-specific HLA, antibody.
To evaluate the predictive capacity of DSA, by Cox regression, patients with ABMR before DSA appearance were eliminated from this analysis.
TABLE 4
| Multivariable cox regression for ABMRa | HR | CI 95% INF | CI 95% SUP | p |
|---|---|---|---|---|
| First kidney transplant | 0.854 | 0.423 | 1.722 | 0.659 |
| DGF | 1.696 | 0.963 | 2.986 | 0.067 |
| cPRA at the time of transplant | 1.005 | 0.996 | 1.014 | 0.308 |
| Early-DSA | 3.376 | 1.661 | 6.864 | 0.001 |
| Late-DSA | 4.122 | 1.785 | 9.518 | 0.001 |
Multivariable Cox regression analysis for antibody-mediated rejection (ABMR).
ABMR: antibody-mediated rejection. DGF: delayed graft-function. cPRA: calculated panel-reactive antibody (Eurotransplant). DSA: donor-specific HLA, antibody.
To evaluate the predictive capacity of DSA, by Cox regression, patients with ABMR before DSA appearance were eliminated from this analysis.
Discussion
Despite the widely described impact of DSA on graft outcomes in kidney transplantation [1–6], the indication of universal post-transplant HLA antibody screening remains unclear [26, 29]. Data on the cost-effectiveness of DSA monitoring are scarce, and different strategies have been proposed to select high-risk patients [27, 28]. The frequency of HLA screening is also not determined and current recommendations have a low level of evidence [15, 29, 30]. In our center we established regular DSA screening in the first month posttransplant more than 10 years ago and in this study, we describe a large cohort of mostly non-sensitized patients without preformed or historical DSA and with early HLA antibody screening by Luminex in the first month. After 3.8 years of follow-up, DSA develop in 13.9% of patients with a median time to first DSA appearance of 1.5 months post-transplantation. Early-DSA are predominant (8.5%) and are an independent risk factor for ABMR. Patients with early-DSA had more HLA sensitization at the time of transplant, presumably reflecting alloimmune memory even in the absence of preformed DSA, therefore we suggest that early-DSA screening should be performed in this high-risk population. Consequently, with our data we highlight that assessing the HLA sensitization status and immunological risk of patients may be the best tool to generate a post-transplant DSA monitoring scheme.
The timing of DSA appearance after transplantation is variable and most series have described a higher incidence in the first year, ranging from 3% to 20%, with a lower annual rate thereafter [17, 29, 33]. A recent study showed that 77% of DSA detected by screening appeared in the first 100 days post-transplant [32]. However, some series have shown that dnDSA can appear up to 10 years after transplant [16, 29], demonstrating that the risk of developing DSA should be considered at any time during functional graft life. In our cohort, the incidence of DSA is almost 14% (49/353) over about 4 years of follow-up, and although the number of patients with DSA is not very high, our incidence is in line with previous observations [5, 34, 42]. Surprisingly, most of them (30/49) are detected early in the first month. These results are probably explained by the more intensive monitoring in our center with a first HLA determination in the first month, allowing rapid detection of DSA. A relevant proportion of patients with early-DSA (8/30) present “spontaneous” negativity at 3 or 6 months after first appearance, and these “transient” early-DSA cannot be detected if first early HLA determination is omitted, which could explain our high incidence. Also, our study describes two exclusive categories of patients based on first detection of DSA, and patients of the early-DSA group may develop new DSA later after transplantation. Despite these considerations, our study shows a relevant proportion of patients with DSA appearance in the first month, emphasizing that early HLA screening allows prompt detection of patients at potential risk of poor outcomes.
The STAR Working Group noted that DSA up to the third month after transplant are likely preformed and reflect alloimmune memory [15, 25]. Previous transplants are a risk factor for memory responses [15] and in patients undergoing retransplantation it has been described that re-exposure to mismatched HLA antigens may be associated with increased immunological risk [43]. In our cohort there are no differences between groups in the proportion of first transplants. 31.3% of our retransplanted patients present a repeated HLA mismatch with previous donors, without differences between patient groups, being in line with previous observations showing that a repeated HLA mismatch within negative FCXM and without described preformed DSA is not associated with DSA detection after transplant [44]. The presence of non-DSA HLA antibodies at the time or before transplantation may also be a risk factor for latent alloimmune memory responses [15]. In our study, patients with HLA sensitization present a higher risk of early-DSA detection in the first month, probably reflecting a memory response even with a negative FCXM and in the absence of preformed DSA. ATG induction is higher in the early-DSA group, possibly explained by the greater number of highly sensitized patients. Because of this high risk, patients with early-DSA also more frequently received plasmapheresis and/or IVIG prior to early HLA screening in the first month and early-DSA detection, showing that current treatments are not fully effective in suppressing DSA. Despite this, there is an important proportion of non-sensitized patients (cPRA = 0% in 12/30) who develop DSA rapidly in the first month. These data likely show that the percentage of cPRA in an isolated serum is not completely informative about the HLA sensitization status or the risk of memory responses, and a cPRA of 0% does not necessarily represent an immunologically naïve patient [15]. Patients with prior exposure to alloantigens through previous transplants, pregnancies or transfusions may not have detectable anti-HLA antibodies, and preexisting DSA below the positivity threshold could be present but not detected by initial screening [13] and triggered by transplantation. In fact, to study alloimmune memory beyond the presence or absence of anti-HLA antibodies, other immune assays that detect donor-specific B and T cell memory, such as the interferon-γ enzyme-linked immunospot (ELISpot), have been described as a monitoring tool to assess cellular immune risk [45–48]. Therefore, with our data we highlight the complexity of the clinical measurement of immune memory and the importance of stratifying the immunological risk of patients to predict the risk of developing DSA.
The development of ABMR regardless of DSA status is significantly associated with lower graft survival in our cohort (log rank p = 0.001), emphasizing ABMR as a fundamental cause of allograft failure, as widely described [1–6]. In our study, more than half of the patients with ABMR (55.0%) did not have detectable DSA, highlighting that the presence of C4d staining in the biopsy is a sufficient factor that allows ABMR diagnosis without serological evidence of DSA [36, 49]. The presence of DSA is associated with ABMR and graft failure, however the clinical evolution of patients with DSA is variable [50]. The MFI level may have predictive capacity, but the relationship of a single MFI value with clinical outcomes is not established, and some characteristics of DSA such as class, specificity, IgG subclass or complement binding capacity may be prognostic factors [16, 17, 51–53]. In our cohort, 19.5% of patients develop ABMR, with a higher incidence in patients with DSA. Despite this, almost half of patients with early-DSA have “subclinical” DSA without evidence of rejection and 26.6% present “transient” early-DSA with “spontaneous” disappearance, underscoring the different clinical course of patients with DSA and the urgent need to improve our knowledge of DSA characteristics and prognostic factors to determine patients at highest risk of rejection after DSA detection [50, 53].
Our study shows early-DSA as a determining factor of poor outcomes, since the presence of early-DSA is associated with a 3.3-fold higher risk of ABMR in multivariable analysis independently of other variables such as DGF, first kidney transplant or HLA sensitization. Furthermore, the presence of late-DSA is significantly associated with lower allograft survival (log rank p = 0.001) and ABMR-free survival (log rank p < 0.001), and remains a strong, independent risk factor for ABMR in multivariable analysis (HR 4.1). Altogether, our data support that the presence of DSA at any time after transplant is a consistent risk factor for ABMR, with worse outcomes in the group of patients with late-DSA. It has been described that class-II DSA usually appear later after transplant and are the most common dnDSA, being more harmful and resistant to treatment [16, 54]. Our group of patients with late-DSA presents a higher percentage of class-II, the proportion of “transient” DSA is lower as well as the number of “subclinical” DSA, and they have more chronic ABMR changes, showing a different profile of patients with DSA that could explain our findings and determine the worse outcomes compared to the early-DSA group. It has been shown that patients with dnDSA present lower allograft survival compared to preexisting DSA [19]. Moreover, it has been demonstrated that patients with preformed DSA that persist after transplant have a higher incidence of ABMR than patients without DSA, but with a significantly lower risk of ABMR compared to those with dnDSA [55]. Accordingly, these results support our hypothesis that early-DSA could represent a memory response and have a better outcome than late-DSA.
The indication and frequency of universal HLA screening are not established, and it has been proposed a monitoring scheme with a first determination at 3–6 months post-transplant [29]. Although it seems that monitoring of preformed DSA may be useful [29, 56], high-quality data are lacking. It has been described that an early determination in the first month may be necessary in intermediate-risk patients with historical DSA or HLA sensitization [30], but there are currently no clear guidelines and the STAR 2017 Working Group suggests an early post-transplant HLA assessment in patients at risk for latent memory responses, without making specific recommendations on frequency and duration due to the absence of robust evidence [15]. In our cohort, early-DSA are consistently associated with ABMR and appear more frequently in sensitized patients. Most patients with early-DSA (68.8%) present ABMR at the time or after DSA detection. The time to ABMR diagnosis is shorter in patients with early-DSA compared to those with late-DSA (1.7 vs. 11.4 months), demonstrating that early screening potentially identifies high-risk patients and reduces the time to ABMR diagnosis, allowing prompt therapy initiation. Hence, until the clinical measurement of immune memory is better known and implemented in clinical routine to assess immunological risk, we suggest that early-DSA monitoring in the first month should be performed at least in patients with HLA sensitization, without waiting for the third or sixth month.
It should be noted that there is a time between the initial follow-up in our cohort and the determination of anti-HLA antibodies in which the appearance of DSA is not possible, mainly for analytical reasons, since there is a period without measurement of anti-HLA antibodies. Of note, all patients included in our study had a recent anti-HLA antibody sample prior to transplantation (median 42 days), and all patients underwent anti-HLA antibody testing at a specific time point or “landmark,” the first month (median 30 days). Therefore, we closely monitor pre- and post-transplant HLA antibodies, and we perform a very early first HLA determination after transplant, which allows us to correctly assess the clinical impact of DSA on graft outcomes.
Nevertheless, our study has several limitations. This is a single-center, retrospective analysis that includes patients with HLA monitoring by Luminex, with a limited sample size and a low number of patients with DSA during the follow-up period. Our study presents a cohort of patients without preformed DSA and with negative FCXM, but with a variable risk of memory responses due to the inclusion of women with previous pregnancies, retransplants, and different degrees of HLA sensitization; despite this, the fact that our cohort is heterogeneous emphasizes our fundamental finding that early-DSA appearance represents memory and allows us to identify patients at risk. Although it is the best technique available, Luminex has multiple limitations and in our center SAB assay is only performed if initial screening is positive. The MFI positivity threshold is set at 1,500 as widely accepted in the literature, however the MFI cut-off value is controversial and can be affected by several parameters. The MFI level at the time of DSA detection is recorded but not the evolution of MFI in subsequent samples. Some characteristics of DSA that may be related to poor outcomes, such as complement binding capacity, are not analyzed as they are not performed routinely. Adherence to treatment and levels of immunosuppressive drugs are also not evaluated at the time of DSA detection. Pretransplant history of transfusions to assess immune memory is not registered. Classical antigen HLA mismatch is considered, without analyzing epitope mismatch. The lack of complete donor typing, especially in DP and DQ, does not allow us to rule out previous sensitization and limits the definition of post-transplant DSA at these HLA loci. To calculate repeated HLA mismatch in retransplants, it must be noted that most previous donor typings are low resolution, and repeated mismatches are not assessed at a molecular level. Finally, the presence of non-HLA antibodies is not analyzed. The fundamental strength of our study is having a large and well-described cohort of patients with early HLA determination in the first month posttransplant. Furthermore, we have clinical and histological data available that allow us to evaluate the impact of early-DSA on graft outcomes and the clinical course of patients after routine early HLA monitoring.
While more accurate knowledge of immunological risk and clinical measurement of immune memory is needed, our study describes a relevant proportion of patients with DSA detection in the first month (8.5%), probably showing alloimmune memory even in the absence of preformed DSA described in pretransplant sera and in the context of negative FCXM. Although almost half of these early-DSA are ‘subclinical’ without evidence of humoral injury, the presence of early-DSA remains a consistent risk factor for ABMR. The risk of developing early-DSA is significantly higher in patients with HLA sensitization, therefore routine early HLA screening in the first month may be reasonably performed in these high-risk patients. In conclusion, we provide granular evidence on the impact of early-DSA detected by screening on clinical events, being strongly related to ABMR and inferior outcomes. High-quality data on the clinical course of patients with DSA detected by universal screening and cost-effectiveness studies are essential to improve results and provide an appropriate post-transplant DSA monitoring strategy.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Regional Ethics Committee of our institution: Comité de Ética de la Investigación con medicamentos de Cantabria (code: 2024.196). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because The clinical variables and sera of kidney transplant recipients included in the study have already been analyzed and were routinely collected as clinical practice after transplant, without requiring written informed consent from the patients.
Author contributions
CL and JR conceived and designed the study. CL wrote the article. MO and MM-B provided technical support and acquired data. CL analyzed and interpreted the data. DS and ML-H performed HLA antibody testing. RV, LB, MV, and ER advised on the preparation of the article and provided conceptual advice. JR supervised the research. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Instituto de Salud Carlos III RD21/0005/0010 and RD24/0004/0019 (ISCIII RICORS2040).
Acknowledgments
We acknowledge the valuable contribution of all authors.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2025.14424/full#supplementary-material
References
1.
MayrdorferMLiefeldtLWuKRudolphBZhangQFriedersdorffFet alExploring the Complexity of death-censored Kidney Allograft Failure. J Am Soc Nephrol (2021) 32(6):1513–26. 10.1681/ASN.2020081215
2.
SellarésJde FreitasDGMengelMReeveJEineckeGSisBet alUnderstanding the Causes of Kidney Transplant Failure: The Dominant Role of Antibody-Mediated Rejection and Nonadherence. Am J Transpl (2012) 12(2):388–99. 10.1111/j.1600-6143.2011.03840.x
3.
LachmannNTerasakiPISchönemannC. Donor-Specific HLA Antibodies in Chronic Renal Allograft Rejection: A Prospective Trial With a Four-Year Follow-Up. Clin Transpl (2006) 171–99.
4.
MaoQTerasakiPICaiJBrileyKCatrouPHaischCet alExtremely High Association Between Appearance of HLA Antibodies and Failure of Kidney Grafts in a five-year Longitudinal Study. Am J Transpl (2007) 7(4):864–71. 10.1111/j.1600-6143.2006.01711.x
5.
WiebeCGibsonIWBlydt-HansenTDKarpinskiMHoJStorsleyLJet alEvolution and Clinical Pathologic Correlations of De Novo Donor Specific HLA Antibody Post Kidney Transplant. Am J Transpl (2012) 12(5):1157–67. 10.1111/j.1600-6143.2012.04013.x
6.
CooperJEGrallaJCagleLGoldbergRChanLWisemanAC. Inferior Kidney Allograft Outcomes in Patients with De Novo Donor-specific Antibodies Are due to Acute Rejection Episodes. Transplantation (2011) 91(10):1103–9. 10.1097/TP.0b013e3182139da1
7.
SüsalCWettsteinDDöhlerBMorathCRuhenstrothASchererSet alAssociation of Kidney Graft Loss with De Novo Produced Donor-specific and Nondonor-specific HLA Antibodies Detected by Single Antigen Testing. Transplantation (2015) 99(9):1976–80. 10.1097/TP.0000000000000672
8.
TamburARHerreraNDHaarbergKMCusickMFGordonRALeventhalJRet alAssessing Antibody Strength: Comparison of MFI, C1q, and Titer Information. Am J Transpl (2015) 15(09):2421–30. 10.1111/ajt.13295
9.
TamburARWiebeC. HLA Diagnostics: Evaluating DSA Strength by Titration. Transplantation (2018) 102(1S Suppl. 1):S23-S30–30. 10.1097/TP.0000000000001817
10.
ReedEFRaoPZhangZGebelHBrayRAGuleriaIet alComprehensive Assessment and Standardization of Solid Phase multiplex-bead Arrays for the Detection of Antibodies to HLA. Am J Transpl (2013) 13(7):1859–70. 10.1111/ajt.12287
11.
ZacharyAALucasDPDetrickBLeffellMS. Naturally Occurring Interference in Luminex Assays for HLA-specific Antibodies: Characteristics and Resolution. Hum Immunol (2009) 70(7):496–501. 10.1016/j.humimm.2009.04.001
12.
KarahanGEClaasFHJHeidtS. Technical Challenges and Clinical Relevance of Single Antigen Bead C1q/C3d Testing and Igg Subclass Analysis of HLA Antibodies. Transpl Int (2018) 31(11):1189–97. 10.1111/tri.13327
13.
BurballaCPérez-SaézMJRedondo-PachónDGarcíaCMirMArias-CabralesCet alLuminex Screening First Vs. Direct Single Antigen Bead Assays: Different Strategies for HLA Antibody Monitoring After Kidney Transplantation. Hum Immunol (2020) 81(6):293–9. 10.1016/j.humimm.2020.03.003
14.
SüsalCOvensJMahmoudKDöhlerBSchererSRuhenstrothAet alNo Association of Kidney Graft Loss With Human Leukocyte Antigen Antibodies Detected Exclusively by Sensitive Luminex single-antigen Testing: A Collaborative Transplant Study Report. Transplantation (2011) 91(8):883–7. 10.1097/TP.0b013e3182100f77
15.
TamburARCampbellPClaasFHFengSGebelHMJacksonAMet alSensitization in Transplantation: Assessment of Risk (STAR) 2017 Working Group Meeting Report. Am J Transpl (2018) 18(7):1604–14. 10.1111/ajt.14752
16.
López Del MoralCWuKNaikMOsmanodjaBAkifovaALachmannNet alThe Natural History of De Novo Donor-specific HLA Antibodies After Kidney Transplantation. Front Med (Lausanne) (2022) 9:943502. 10.3389/fmed.2022.943502
17.
ZhangR. Donor-Specific Antibodies in Kidney Transplant Recipients. Clin J Am Soc Nephrol (2018) 13(1):182–92. 10.2215/CJN.00700117
18.
ZiemannMAltermannWAngertKArnsWBachmannABakchoulTet alPreformed Donor-Specific HLA Antibodies in Living and Deceased Donor Transplantation: A Multicenter Study. Clin J Am Soc Nephrol (2019) 14(7):1056–66. 10.2215/CJN.13401118
19.
AubertOLoupyAHidalgoLDuong van HuyenJPHigginsSVigliettiDet alAntibody-Mediated Rejection due to Preexisting Versus De Novo Donor-specific Antibodies in Kidney Allograft Recipients. J Am Soc Nephrol (2017) 28(6):1912–23. 10.1681/ASN.2016070797
20.
SakamotoSIwasakiKTomosugiTNiemannMSpieringsEMiwaYet alAnalysis of T and B Cell Epitopes to Predict the Risk of De Novo Donor-specific Antibody (DSA) Production After Kidney Transplantation: A Two-Center Retrospective Cohort Study. Front Immunol (2020) 11:2000. 10.3389/fimmu.2020.02000
21.
SenevACoemansMLerutEVan SandtVKerkhofsJDaniëlsLet alEplet Mismatch Load and De Novo Occurrence of Donor-specific Anti-hla Antibodies, Rejection, and Graft Failure After Kidney Transplantation: An Observational Cohort Study. J Am Soc Nephrol (2020) 31(9):2193–204. 10.1681/ASN.2020010019
22.
GeneugelijkKNiemannMDrylewiczJvan ZuilenADJoostenIAllebesWAet alPIRCHE-II Is Related to Graft Failure After Kidney Transplantation. Front Immunol (2018) 9:321. 10.3389/fimmu.2018.00321
23.
LachmannNNiemannMReinkePBuddeKSchmidtDHalleckFet alDonor-Recipient Matching Based on Predicted Indirectly Recognizable HLA Epitopes Independently Predicts the Incidence of De Novo Donor-specific HLA Antibodies Following Renal Transplantation. Am J Transpl (2017) 17(12):3076–86. 10.1111/ajt.14393
24.
UnterrainerCDöhlerBNiemannMLachmannNSüsalC. Can PIRCHE-II Matching Outmatch Traditional HLA Matching?Front Immunol (2021) 12:631246. 10.3389/fimmu.2021.631246
25.
TamburARCampbellPChongASFengSFordMLGebelHet alSensitization in Transplantation: Assessment of Risk (STAR) 2019 Working Group Meeting Report. Am J Transpl (2020) 20(10):2652–68. 10.1111/ajt.15937
26.
StringerDGardnerLShawOClarkeBBriggsDWorthingtonJet alOptimized Immunosuppression to Prevent Graft Failure in Renal Transplant Recipients with HLA Antibodies (Outsmart): A Randomised Controlled Trial. EClinicalMedicine (2023) 56:101819. 10.1016/j.eclinm.2022.101819
27.
WiebeCBalshawRGibsonIWHoJShawJKarpinskiMet alA Rational Approach to Guide cost-effective De Novo Donor-specific Antibody Surveillance with Tacrolimus Immunosuppression. Am J Transpl (2023) 23(12):1882–92. 10.1016/j.ajt.2023.07.025
28.
KiberdBAMillerAMartinSTennankoreKK. De Novo Donor-Specific Human Leukocyte Antigen Antibody Screening in Kidney Transplant Recipients After the First Year Posttransplantation: A Medical Decision Analysis. Am J Transpl (2016) 16(11):3212–9. 10.1111/ajt.13838
29.
van den BroekDAJMeziyerhSBuddeKLefaucheurCCozziEBertrandDet alThe Clinical Utility of Post-transplant Monitoring of Donor-Specific Antibodies in Stable Renal Transplant Recipients: A Consensus Report with Guideline Statements for Clinical Practice. Transpl Int (2023) 36:11321. 10.3389/ti.2023.11321
30.
TaitBDSüsalCGebelHMNickersonPWZacharyAAClaasFHet alConsensus Guidelines on the Testing and Clinical Management Issues Associated with HLA and non-HLA Antibodies in Transplantation. Transplantation (2013) 95(1):19–47. 10.1097/TP.0b013e31827a19cc
31.
HeilmanRLNijimADesmarteauYMKhamashHPandoMJSmithMLet alDe Novo Donor-specific Human Leukocyte Antigen Antibodies Early After Kidney Transplantation. Transplantation (2014) 98(12):1310–5. 10.1097/TP.0000000000000216
32.
SharmaAJorgensenDRMehtaRBSoodPPuttarajappaCMWuCMet alThe Clinical Impact of Anti-HLA Donor Specific Antibody Detection Through First Year Screening on Stable Kidney Transplant Recipients. Transpl Int (2022) 35:10094. 10.3389/ti.2022.10094
33.
EverlyMJRebellatoLMHaischCEOzawaMParkerKBrileyKPet alIncidence and Impact of De Novo Donor-specific Alloantibody in Primary Renal Allografts. Transplantation (2013) 95(3):410–7. 10.1097/TP.0b013e31827d62e3
34.
SchinstockCACosioFCheungpasitpornWDadhaniaDMEverlyMJSamaniego-PicotaMDet alThe Value of Protocol Biopsies to Identify Patients with De Novo Donor-specific Antibody at High Risk for Allograft Loss. Am J Transpl (2017) 17(6):1574–84. 10.1111/ajt.14161
35.
LeePCZhuLTerasakiPIEverlyMJ. HLA-specific Antibodies Developed in the First Year Posttransplant Are Predictive of Chronic Rejection and Renal Graft Loss. Transplantation (2009) 88(04):568–74. 10.1097/TP.0b013e3181b11b72
36.
LoupyAHaasMRoufosseCNaesensMAdamBAfrouzianMet alThe Banff 2019 Kidney Meeting Report (I): Updates on and Clarification of Criteria for T cell- and antibody-mediated Rejection. Am J Transpl (2020) 20(9):2318–31. 10.1111/ajt.15898
37.
Castro HernándezCde la SierraDRenuncio-GarcíaMMikhalkovichDMota-PérezNComins-BooAet alStrategies for moderate-risk Delisting in Highly Sensitized Patients. Transpl Proc (2025) 57(1):13–5. 10.1016/j.transproceed.2024.12.008
38.
GragertLMadboulyAFreemanJMaiersM. Six-Locus High Resolution HLA Haplotype Frequencies Derived from mixed-resolutionDNA Typing for the Entire US Donor Registry. Hum Immunol (2013) 74(10):1313–20. 10.1016/j.humimm.2013.06.025
39.
CrearyLEGangavarapuSMallempatiKCMontero-MartínGCaillierSJSantanielloAet alNext-Generation Sequencing Reveals New Information About HLA Allele and Haplotype Diversity in a Large European American Population. Hum Immunol (2019) 80(10):807–22. 10.1016/j.humimm.2019.07.275
40.
EberhardHPSchmidtAHMytilineosJFleischhauerKMüllerCR. Common and well-documented HLA Alleles of German Stem Cell Donors by Haplotype Frequency Estimation. HLA (2018) 92(04):206–14. 10.1111/tan.13378
41.
Eurotransplant Reference Laboratory Virtual PRA Calculator (2024). Available online at: https://www.etrl.org/vPRA.aspx (Accessed May 15, 2024).
42.
WiebeCGibsonIWBlydt-HansenTDPochincoDBirkPEHoJet alRates and Determinants of Progression to Graft Failure in Kidney Allograft Recipients with De Novo Donor-specific Antibody. Am J Transpl (2015) 15(11):2921–30. 10.1111/ajt.13347
43.
TinckamKJRoseCHariharanSGillJ. Re‐Examining Risk of Repeated HLA Mismatch in Kidney Transplantation. J Am Soc Nephrol (2016) 27(9):2833–41. 10.1681/ASN.2015060626
44.
LucisanoGThiruvengadamSHassanSGueret-WardleABrookesPSantos-NunezEet alDonor-Specific Antibodies Detected by Single Antigen Beads Alone Can Help Risk Stratify Patients Undergoing Retransplantation Across a Repeat HLA Mismatch. Am J Transpl (2020) 20(2):441–50. 10.1111/ajt.15595
45.
BestardOThaunatOBelliniMIBöhmigGABuddeKClaasFet alAlloimmune Risk Stratification for Kidney Transplant Rejection. Transpl Int (2022) 35:10138. 10.3389/ti.2022.10138
46.
MonteroNFaroukSGandolfiniICrespoEJarqueMMeneghiniMet alPretransplant Donor-specific Ifnγ ELISPOT as a Predictor of Graft Rejection: A Diagnostic Test Accuracy meta-analysis. Transpl Direct (2019) 5(5):e451. 10.1097/TXD.0000000000000886
47.
GandolfiniICrespoEBawejaMJarqueMDonadeiCLuqueSet alImpact of Preformed T-cell Alloreactivity by Means of Donor-specific and Panel of Reactive T Cells (PRT) ELISPOT in Kidney Transplantation. PLoS One (2018) 13(7):e0200696. 10.1371/journal.pone.0200696
48.
LuqueSLúciaMMelilliELefaucheurCCrespoMLoupyAet alValue of Monitoring Circulating donor-reactive Memory B Cells to Characterize antibody-mediated Rejection After Kidney Transplantation. Am J Transpl (2019) 19(2):368–80. 10.1111/ajt.15055
49.
HaasMLoupyALefaucheurCRoufosseCGlotzDSeronDet alThe Banff 2017 Kidney Meeting Report: Revised Diagnostic Criteria for Chronic Active T cell-mediated Rejection, antibody-mediated Rejection, and Prospects for Integrative Endpoints for next-generation Clinical Trials. Am J Transpl (2018) 18(2):293–307. 10.1111/ajt.14625
50.
López Del MoralCWuKNaikMOsmanodjaBAkifovaALachmannNet alPredictors of Graft Failure After First Detection of De Novo Donor-specific HLA Antibodies in Kidney Transplant Recipients. Nephrol Dial Transpl (2023) 39(1):84–94. 10.1093/ndt/gfad149
51.
FilipponeEJFarberJL. Humoral Immune Response and Allograft Function in Kidney Transplantation. Am J Kidney Dis (2015) 66(2):337–47. 10.1053/j.ajkd.2015.03.033
52.
KumbalaDZhangR. Essential Concept of Transplant Immunology for Clinical Practice. World J Transpl (2013) 3(4):113–8. 10.5500/wjt.v3.i4.113
53.
AkifovaABuddeKAmannKBuettner-HeroldMChoiMOellerichMet alDonor-Derived Cell-free DNA Monitoring for Early Diagnosis of Antibody-Mediated Rejection After Kidney Transplantation: A Randomized Trial. Nephrol Dial Transpl (2024) 40:1384–95. 10.1093/ndt/gfae282
54.
NtokouI-SAIniotakiAGKontouENDaremaMNApostolakiMDKostakisAGet alLong-Term Follow up for anti-HLA Donor Specific Antibodies Postrenal Transplantation: High Immunogenicity of HLA Class II Graft Molecules. Transpl Int (2011) 24(11):1084–93. 10.1111/j.1432-2277.2011.01312.x
55.
Redondo-PachónDPérez-SáezMJMirMGimenoJLlinásLGarcíaCet alImpact of Persistent and Cleared Preformed HLA DSA on Kidney Transplant Outcomes. Hum Immunol (2018) 79(6):424–31. 10.1016/j.humimm.2018.02.014
56.
PhillpottMDagaSHigginsRLoweDKrishnanNZehnderDet alDynamic Behaviour of Donor Specific Antibodies in the Early Period Following HLA Incompatible Kidney Transplantation. Transpl Int (2022) 35:10128. 10.3389/ti.2022.10128
Summary
Keywords
kidney transplant, antibody-mediated rejection, donor-specific antibodies, graft outcomes, HLA screening
Citation
López del Moral C, San Segundo D, Ortega MJ, Martínez-Belotto M, Valero R, Belmar L, Valentín MO, Rodrigo E, López-Hoyos M and Ruiz JC (2025) Early Donor-Specific HLA Antibodies Detected by Screening in the First Month Posttransplant and Kidney Graft Outcomes. Transpl. Int. 38:14424. doi: 10.3389/ti.2025.14424
Received
30 January 2025
Accepted
25 July 2025
Published
06 August 2025
Volume
38 - 2025
Updates
Copyright
© 2025 López del Moral, San Segundo, Ortega, Martínez-Belotto, Valero, Belmar, Valentín, Rodrigo, López-Hoyos and Ruiz.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Covadonga López del Moral, covadonga.lopezdelmoral@scsalud.es
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.