Abstract
Previous studies have reported comparable oncologic outcome between ABO-incompatible (ABOi) living donor liver transplantation (LDLT) and ABO-compatible (ABOc) LDLT in patients with hepatocellular carcinoma (HCC). We aimed to analyze the relationship between number of therapeutic plasma exchanges (TPE) before LDLT and HCC outcomes in ABOi LDLT. In this single-center retrospective study, 428 adult LDLT recipients with HCC were categorized into three groups according to ABO incompatibility and the number of pretransplant TPE: ABOc (n = 323), ABOi/TPE ≤5 (n = 75), and ABOi/TPE ≥6 (n = 30). The RFS and HCC recurrence rates were compared. Three groups showed similar characteristics in most demographics, pretransplant tumor markers and pathologies. The median initial isoagglutinin (IA) titer was 1:64 (range negative-1:512) in ABOi/TPE ≤5 group and 1:512 (range 1:128–1:4,096) in ABOi/TPE ≥6 group. Five-year RFS was significantly lower (75.7% vs. 72.7% vs. 50.0%, P = 0.005) and HCC recurrence was significantly higher in the ABOi/TPE ≥6 group than in the other groups(16.4% vs. 17.0% vs. 39.4%, P = 0.014). In multivariable Cox regression analysis, ABOi/TPE ≥6 was an independent risk factor for RFS (aHR 1.99, 95% CI:1.02–3.86, P = 0.042) and HCC recurrence (aHR 2.42, 95% CI:1.05–5.57, P = 0.037). More than six pretransplant TPE sessions may increase the risk of HCC recurrence after ABOi LDLT. Reducing TPE sessions to fewer than six should be considered while maintaining immunological stability through IA titer control.
Introduction
Liver transplantation (LT) is an effective, and sometimes the only, treatment option for unresectable hepatocellular carcinoma (HCC). However, owing to organ shortages, not all patients can receive timely LT. Consequently, the demand for living donor liver transplantation (LDLT) for HCC is increasing worldwide, and numerous studies have reported comparable oncological outcomes between LDLT and deceased donor liver transplantation (DDLT) [1–7].
When an ABO-incompatible (ABOi) living donor is the only available option, ABO-incompatible LDLT (ABOi LDLT) with proper desensitization becomes a viable choice [8–15]. Despite the need for pretransplant antibody treatment and an increased risk of posttransplant infections, ABOi LDLT has been reported as a feasible treatment for patients with end-stage liver disease, offering substantial survival benefits even for those with high Model for End-Stage Liver Disease (MELD)scores [11, 12, 16, 17]. Additionally, several Korean centers have reported that ABOi LDLT has a similar impact on HCC outcomes compared to ABO-compatible (ABOc) LDLT (ABOc LDLT) [12, 18–21].
Despite these reports, ABOi LDLT necessitates more potent immunosuppression, including B-cell depleting agents, therapeutic plasma exchange (TPE), and higher maintenance immunosuppressants, which raises concerns about potentially adverse oncologic outcomes [22, 23]. Furthermore, ABOi LDLT requires additional pretransplant TPE sessions as the titer of blood group antibodies increases. However, there are no published studies examining the differences in HCC outcomes based on the degree of desensitization required.
Therefore, this study aimed to analyze the effect of the number of pretransplant TPE sessions, a critical component of pretransplant treatment, on HCC outcomes in ABOi LDLT.
Materials and Methods
Study Material
In this retrospective cohort study, we analyzed single-center data from 466 patients who underwent LDLT for HCC between January 2011, when ABOi LDLT was initiated at our institution, and December 2022. The baseline characteristics and details of explant pathology were retrieved from a prospectively collected institutional database. The exclusion criteria were as follows: mixed cholangiocellular carcinoma on pathology (n = 29), liver cancer other than HCC (n = 2), LDLT from a dual living donor (n = 3), and missing data (n = 4) (Supplementary Figure S1, study population).
A total of 428 eligible patients were categorized according to ABO incompatibility and the number of pretransplant TPE sessions: ABO-compatible (ABOc group, n = 323, 75.5%), ABO-incompatible with fewer than 5 TPE sessions (ABOi/TPE ≤5 group, n = 75, 17.5%), and ABO-incompatible with six or more TPE sessions (ABOi/TPE ≥6 group, n = 30, 7.5%). The cutoff for the number of TPE sessions (6 times) was determined based on the spline curve for recurrence-free survival (RFS), where the hazard began to significantly increase (Supplementary Figure S2, spline curve).
Data Collection and Outcomes
All relevant information regarding recipients, donors, and LDLT surgery was retrieved from the institutional database. The underlying liver diseases associated with HCC included hepatitis B, hepatitis C, and non-B/non-C. Detailed information on explant pathology and tumor markers, such as alpha-fetoprotein (AFP) and protein induced by vitamin K absence or antagonist-II (PIVKA-II) at the time of LDLT, was obtained. Additionally, data on pretransplant locoregional and systemic treatments, as well as previous hepatectomies, were collected for patients with HCC. RFS and HCC recurrence (time to recurrence) were the primary outcomes.
Pretransplant Desensitization for ABO Incompatibility
Our institutional protocol for desensitization in ABOi LDLT mainly consisted of rituximab and TPE, as described previously [24, 25]. Every pretransplant TPE sessions and desensitization protocols were performed within 2 weeks prior to ABOi LDLT. A recently revised version of this protocol is provided in Supplementary Figures S3-1–S3-2 (Desensitization protocol for ABOi LDLT). For the initial and target isoagglutinin (IA) titers, higher IgM or IgG anti-A/B titers were employed. The number of preoperative TPE sessions was determined based on the initial IA titer, the response to TPE, and the decrease in the ABO titer. Splenectomy and postoperative TPE were performed in patients at high risk of rejection, specifically those with an IA titer greater than 1:64 at the time of LT. Additional rounds of TPE were conducted postoperatively in cases of clinical rejection or IA titer rebound, defined as a resurgence to 1:64 and a minimum two-fold increase. Following TPE, intravenous immunoglobulin (IVIG) was administered at a dose of 500–800 mg/kg on an individualized basis, depending on ABO antibody levels and infection risk.
Statistical Analysis
Depending on the type of variable, data are presented either as numbers (percentages) or as medians (interquartile range [IQR]). The Mann–Whitney U test or chi-square test was employed to compare continuous and categorical variables, respectively, when appropriate. HCC outcomes were analyzed using Kaplan–Meier curves and log-rank tests. Multivariable Cox regression was performed to evaluate HCC outcomes in the entire cohort, including covariates with significant P values <0.1 from the univariable analysis. In the risk analysis of HCC recurrence, non-HCC death was considered a competing risk, utilizing the Fine and Gray method [26] for competing risk regression. In the ABOi LDLT groups, the 5-year estimates of HCC recurrence were compared based on the number of TPE sessions (≤5 vs. ≥6) across various subgroups categorized by tumor burden, which reflects the tumor size, tumor number, and AFP and PIVKA-II levels [27–30], as well as ABO antibody strength, postoperative rebound of IA titer and TPE, and splenectomy status. Subgroup analyses were conducted in a univariate manner due to the small size of each group. All statistical analyses were performed using the R statistical package, version 4.3.0 for macOS1, with the significance threshold set at P < 0.05.
Statement of Ethics
This study was performed in accordance with the Declaration of Helsinki and the Declaration of Istanbul and was approved by the Institutional Review Board of Severance Hospital, Yonsei University Health System (IRB number 4-2024-0977). The requirement of informed consent was waived due to the retrospective nature of the study.
Results
Baseline Characteristics
No significant difference was noted in most baseline patient characteristics (Table 1). The distribution of LT years was also not statistically significant (P = 0.069); however, a higher proportion of transplants in the ABOi groups occurred between 2016 and 2019. Most patients had hepatitis B as the underlying cause of HCC across all groups, with no statistical significance (76.8% in ABOc, 69.3% in ABOi/TPE ≤5, and 86.7% in ABOi/TPE ≥6, P = 0.401). Notably, the ABOi/TPE ≥6 group required a significantly higher number of red blood cell transfusions (median 4.5 packs) than the ABOc and ABOi/TPE ≤5 groups (median two packs, P = 0.014). No significant differences were noted in the pretransplant AFP and PIVKA-II levels. Additionally, history of hepatectomy, locoregional therapy (LRT), and systemic treatment were similar across the groups.
TABLE 1
| Variables | ABOc (n = 323) | ABOi/TPE ≤5 (n = 75) | ABOi/TPE ≥6 (n = 30) | P |
|---|---|---|---|---|
| Age, years | 56.8 ± 7.0 | 57.1 ± 6.9 | 55.6 ± 7.3 | 0.608 |
| Sex, female | 58 (18.0) | 17 (22.7) | 7 (23.3) | 0.539 |
| BMI | 23.8 (22.3–26.1) | 24.9 (23.4–26.3) | 24.0 (21.9–25.9) | 0.065 |
| LT year | 0.069 | |||
| 2011–2015 | 112 (34.7) | 15 (20.0) | 7 (23.3) | |
| 2016–2019 | 104 (32.2) | 33 (44.0) | 14 (46.7) | |
| 2020–2022 | 107 (33.1) | 27 (36.0) | 9 (30.0) | |
| Underlying liver disease for HCC | 0.401 | |||
| Hepatitis B | 248 (76.8) | 52 (69.3) | 26 (86.7) | |
| Hepatitis C | 23 (7.1) | 8 (10.7) | 1 (3.3) | |
| Non-B, Non-C | 52 (16.1) | 15 (20.0) | 3 (10.0) | |
| Hypertension | 74 (22.9) | 21 (28.0) | 9 (30.0) | 0.490 |
| Diabetes mellitus | 97 (30.0) | 27 (36.0) | 12 (40.0) | 0.367 |
| Pretransplant MELD | 10 (8–14) | 10 (8–13) | 11.5 (8–14) | 0.674 |
| Donor age, years | 31 (24–40) | 34 (26–40.5) | 35 (25–41) | 0.203 |
| Donor sex, female | 130 (40.2) | 26 (34.7) | 11 (36.7) | 0.647 |
| GRWRa <0.8 | 27 (8.4) | 5 (6.7) | 2 (6.7) | 0.856 |
| Macrovesicular steatosis ≥10% | 46 (15.2) | 9 (12.3) | 2 (6.9) | 0.422 |
| Cold ischemic time, min | 126 (106–150) | 126 (102–152.5) | 128.5 (96–180) | 0.884 |
| Transfusion RBC, packs | 2 (0–6) | 2 (0–7.5) | 4.5 (2–9) | 0.014 |
| AFP at LT, ng/mL | 6.6 (3.3–23.1) | 6.4 (3.3–14.0) | 4.3 (2.2–27.2) | 0.545 |
| PIVKA at LT, mAU/mL | 38 (22–112) | 38 (23.5–141) | 47 (20–232) | 0.666 |
| Hepatectomy history | 62 (19.2) | 13 (17.3) | 7 (23.3) | 0.779 |
| Pretransplant LRT | 246 (76.2) | 59 (78.7) | 23 (76.7) | 0.899 |
| Systemic treatment | 45 (13.9) | 9 (12.0) | 5 (16.7) | 0.812 |
| Explant pathology | ||||
| Total necrosis | 57 (17.6) | 13 (17.3) | 4 (13.3) | 0.836 |
| Viable tumor number | 1 (1–3) | 2 (1–3) | 2 (1–3) | 0.485 |
| Maximum tumor size, cm | 1.7 (1.0–3.0) | 1.8 (0.8–3.0) | 2.4 (1.3–3.7) | 0.139 |
| Microvascular invasion | 76 (23.5) | 20 (26.7) | 9 (30.0) | 0.656 |
| Poor differentiation | 107 (33.1) | 22 (29.3) | 13 (43.3) | 0.388 |
| Satellite nodule | 35 (10.8) | 8 (10.7) | 8 (26.7) | 0.035 |
| PVTT | 5 (1.5) | 2 (2.7) | 1 (3.3) | 0.673 |
Baseline characteristics of patients, according to ABO incompatibility and the number of pretransplant therapeutic plasma exchange.
Results presented as number (percentage) or median (interquartile range) values.
Graft weight was directly measured during operation.
ABOc, ABO, compatible; ABOi, ABO incompatible; AFP, alpha-feto protein; BMI, body mass index; GRWR, graft recipient weight ratio; HCC, hepatocellular carcinoma; LRT, locoregional treatment; LT, liver transplantation; MELD, model for end-stage liver disease; PIVKA, protein induced by vitamin K antagonist-II; PVTT, portal vein tumor thrombosis; TPE, therapeutic plasma exchange.
Most characteristics from explant pathology were similar across the groups, including the incidence of portal vein tumor thrombosis (PVTT), total necrosis, number of viable tumors, maximum tumor size, microvascular invasion, and poor differentiation. However, the presence of satellite nodules was significantly higher in the ABOi/TPE ≥6 group (26.7%) than in the other groups (10.8% in ABOc and 10.7% in the ABOi/TPE ≤5, P = 0.035).
Detailed Information on Recipient of ABOi LDLT
Almost all patients who underwent ABOi LDLT received rituximab and at least one cycle of TPE for desensitization. Table 2 presents details regarding ABO incompatibility and desensitization protocols for patients in the ABOi group, categorized by the number of pretransplant TPE sessions. A significantly higher proportion of A to O transplants was observed in the ABOi/TPE ≥6 group (56.7%) than in the ABOi/TPE ≤5 group (17.3%, P < 0.001). The median IA titer was significantly higher in the ABOi/TPE ≥6 group than in the ABOi/TPE ≤5 group at initial assessment (1:64 vs. 1:512, P < 0.001), at the time of LT (1:8 vs. 1:32, P < 0.001), and after LT (1:16 vs. 1:32, P < 0.001).
TABLE 2
| Variables | ABOi/TPE ≤5 (n = 75) | ABOi/TPE ≥6 (n = 30) | P |
|---|---|---|---|
| ABO type | <0.001 | ||
| A | 36 (48.0) | 2 (6.7) | |
| B | 16 (21.3) | 1 (3.3) | |
| O | 23 (30.7) | 27 (90.0) | |
| Donor ABO type | 0.008 | ||
| A | 23 (30.7) | 17 (56.7) | |
| AB | 25 (33.3) | 2 (6.7) | |
| B | 27 (36.0) | 11 (36.7) | |
| A to O | 13 (17.3) | 17 (56.7) | <0.001 |
| IA titer at initial | 1:64 (1:24–1:128) | 1:512 (1:256–1:1024) | <0.001 |
| IA titer at LT | 1:8 (1:4–1:16) | 1:32 (1:16–1:64) | <0.001 |
| Pretransplant TPE number | 3 (2–4) | 6.5 (6–7) | <0.001 |
| Pretransplant IVIGa | 8 (10.7) | 16 (53.3) | <0.001 |
| Rituximab | 73 (97.3) | 30 (100.0) | 0.910 |
| Rituximab conventional doseb | 54 (72.0) | 26 (86.7) | 0.078 |
| Pretransplant duration of MMF | 7 (4–8) | 7 (0–8) | 0.645 |
| Pretransplant MMF total dose, mg | 3,500 (3,000–4,000) | 3,500 (2000–4,000) | 0.284 |
| Splenectomy | 4 (5.3) | 7 (23.3) | 0.018 |
| Posttransplant IA titer reboundc | 19 (25.3) | 8 (26.7) | 0.986 |
| Posttransplant maximum IA titer | 1:16 (1:4–1:48) | 1:32 (1:16–1:128) | 0.001 |
| Posttransplant TPEd | 14 (18.7) | 11 (36.7) | 0.049 |
| Posttransplant IVIGa | 6 (8.0) | 9 (30.0) | 0.009 |
Details for ABO incompatibility and desensitization of ABO incompatible group patients, according to therapeutic plasma exchange number.
Results presented as number (percentage) or median (interquartile range) values.
Pretransplant IVIG total dose range was 7.5–50 g in ABOi/TPE ≤5 group, and 5.5–136 g in ABOi/TPE ≥6 group. Posttransplant IVIG total dose range was 15–127.5 g in ABOi/TPE ≤5 group, and 1–458 g in ABOi/TPE ≥6 group.
375 ± 25 mg per body surface area (m2).
Defined as IA titer increased to more than 1:64 after transplantation.
Posttransplant TPE number ranges 0–10 in ABOi/TPE ≤5 group, and 0–15 in ABOi/TPE ≥6 group.
ABOi, ABO incompatible; IA, isoagglutinin; IVIG, intravenous immunoglobulin; LT, liver transplantation; MMF, mycophenolate mofetil; TPE, therapeutic plasma exchange.
Additionally, a significantly higher proportion of patients in the ABOi/TPE ≥6 group underwent splenectomy (5.3% vs. 23.3%, P = 0.018), pretransplant IVIG (10.7% vs. 53.3%, P < 0.001), posttransplant IVIG (8.0% vs. 30.0%, P = 0.009), and posttransplant TPE (18.7% vs. 36.7%, P = 0.049). The univariate analysis showed no significant association between recipient or donor ABO blood type and 5-year HCC recurrence, regardless of TPE sessions. Similarly, A to O donor-recipient mismatches did not show a significant impact on recurrence risk (Supplementary Table S1).
HCC Outcomes
As shown in the Kaplan-Meier curves in Figure 1, a significant difference was observed in RFS between the ABOc group and the ABOi/TPE ≥6 group (5-year survival: 75.7% in the ABOc group vs. 50.0% in the ABOi/TPE ≥6 group, P = 0.005). Additionally, the HCC recurrence rates also differed significantly (5-year survival: 16.4% vs. 39.4%, P = 0.014).
FIGURE 1
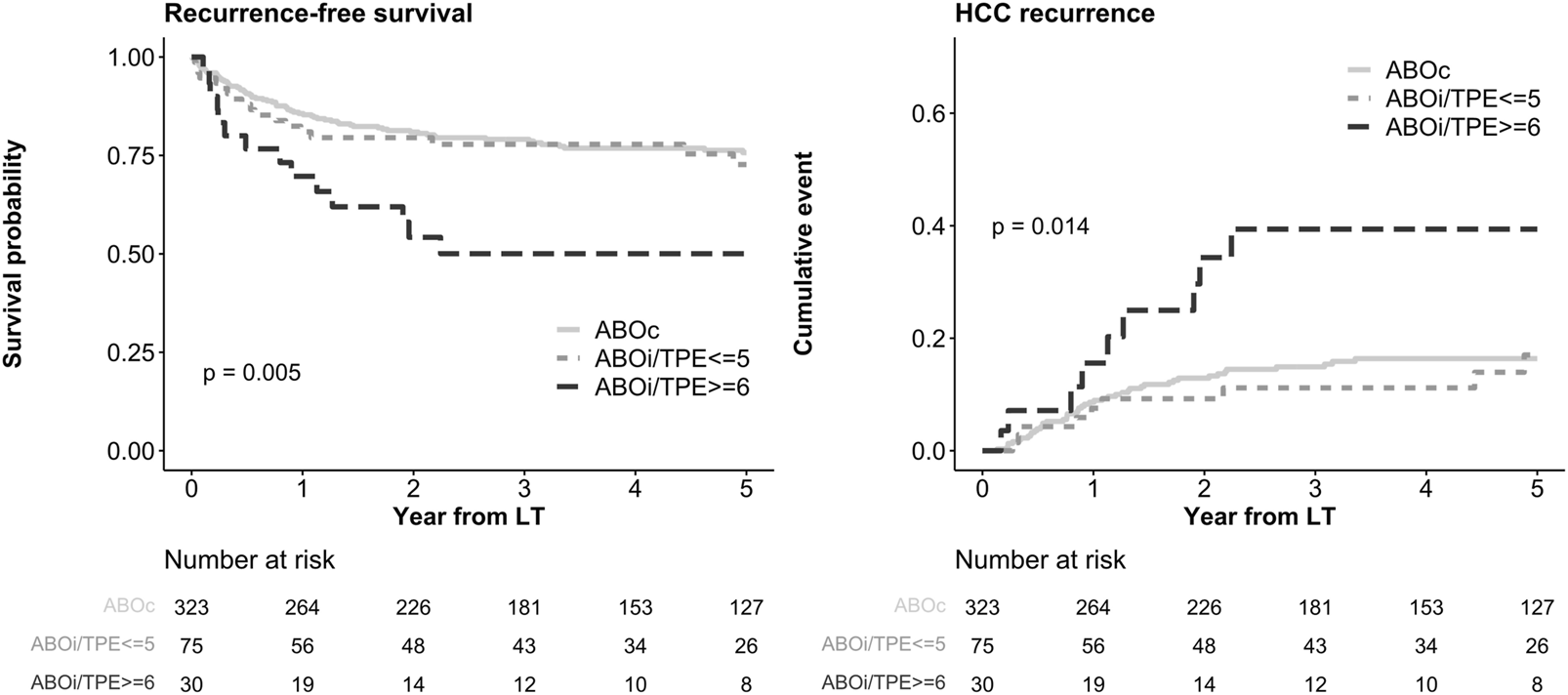
Kaplan-Meier curve of RFS and HCC recurrence according to ABO incompatibility and plasma exchange numbers. RFS, recurrence free survival; HCC, hepatocellular carcinoma; ABOc, ABO compatible; ABOi, ABO incompatible; TPE, therapeutic plasma exchange; LT, liver transplantation.
To further evaluate the impact of TPE on oncologic outcomes, we categorized the ABO incompatibility group into subgroups based on the number of TPE sessions: ≤3 sessions (5-year RFS: 76.2%, 5-year HCC recurrence: 17.4%), 4-5 sessions (5-year RFS: 68.5%, 5-year HCC recurrence: 16.5%), and ≥6 sessions (5-year RFS: 50.0%, 5-year HCC recurrence: 39.4%). Although these results were not statistically significant, a trend related to the number of TPE sessions was observed (P = 0.056 for RFS and P = 0.051 for HCC recurrence, Supplementary Figure S4).
In the multivariable Cox analyses (Table 3), the ABOi/TPE ≥6 group was significantly associated with RFS [hazard ratio (HR) = 1.99, 95% confidence interval (CI): 1.02–3.86, P = 0.042] and HCC recurrence (HR = 2.42, 95% CI: 1.05–5.57, P = 0.037).
TABLE 3
| Variables | Recurrence free survival HR (95% CI) | P | HCC recurrencea HR (95% CI) | P |
|---|---|---|---|---|
| ABOi group | ||||
| ABOc | Reference | Reference | ||
| ABOi/TPE ≤5 | 1.08 (0.63–1.85) | 0.777 | 0.97 (0.46–2.01) | 0.928 |
| ABOi/TPE ≥6 | 1.99 (1.02–3.86) | 0.042 | 2.42 (1.05–5.57) | 0.037 |
| Age, years | - | - | 0.96 (0.92–1.00) | 0.048 |
| BMI | 0.95 (0.89–1.01) | 0.102 | - | - |
| Pretransplant MELD | 1.06 (1.03–1.09) | <0.001 | - | - |
| Cold ischemic time, min | 1.00 (1.00–1.01) | 0.622 | - | - |
| Transfusion RBC, pack | 1.02 (1.01–1.04) | 0.002 | - | - |
| Log_AFP at LT | 1.11 (0.98–1.25) | 0.093 | 1.09 (0.94–1.25) | 0.260 |
| Log_PIVKA at LT | 1.03 (0.90–1.18) | 0.659 | 1.14 (0.98–1.34) | 0.091 |
| Pretransplant LRT, yes | 2.91 (1.45–5.84) | 0.003 | 7.00 (2.02–24.26) | 0.002 |
| Systemic treatment, yes | 2.20 (1.37–3.53) | 0.001 | 2.10 (1.16–3.82) | 0.015 |
| Viable tumor number | 1.02 (1.01–1.04) | 0.007 | 1.04 (1.01–1.07) | 0.004 |
| Maximum tumor size, cm | 0.90 (0.82–0.98) | 0.019 | 0.92 (0.82–1.03) | 0.150 |
| Microvascular invasion, yes | 1.77 (0.97–3.22) | 0.062 | 2.07 (1.01–4.24) | 0.046 |
| Poor differentiation, yes | 1.30 (0.82–2.05) | 0.268 | 1.69 (0.95–3.02) | 0.076 |
| Satellite nodule, yes | 1.67 (0.90–3.10) | 0.101 | 1.83 (0.92–3.63) | 0.085 |
| PVTT, yes | 2.83 (0.98–8.16) | 0.054 | 2.49 (0.57–10.86) | 0.226 |
Multivariable Cox analysis for recurrence free survival and hepatocellular carcinoma recurrence.
Variables which result p < 0.1 in univarible Cox analysis were included and represented at multivariable Cox analysis. Full univariate and multivariate results are represented at Supplementary Tables S2, S3.
Multivariable analysis for HCC recurrence was performed treating non-HCC death as competing risk.
ABOc, ABO compatible; ABOi, ABO incompatible; AFP, alpha-feto protein; BMI, body mass index; CI, confidence interval; HCC, hepatocellular carcinoma; HR, hazard ratio; LRT, locoregional treatment; MELD, model for end-stage liver disease; PIVKA, protein induced by vitamin K antagonist-II; PVTT, portal vein tumor thrombosis; TPE, therapeutic plasma exchange.
Subgroup Analysis for HCC Recurrence
In the subgroup analysis (Table 4), the 5-year HCC recurrence rates were higher across all subgroups in the ABOi/TPE ≥6 group. Although this trend is numerically apparent, the small sample size limits the ability to confirm statistical significance. Interestingly, among patients with a tumor marker-based MoRAL score ≥100, the recurrence rate was significantly higher in the ABOi/TPE ≥6 group (56.7%) than in the ABOi/TPE ≤5 group with a MoRAL score ≥100 (16.1%, P = 0.017). However, patients with a MoRAL score <100 exhibited similar 5-year HCC recurrence rates between the two groups (17.6% vs. 20.5%, P = 0.75). Supplementary Figure S5 illustrates HCC recurrence based on the number of TPE sessions and the MoRAL score. As observed, a marked difference was evident between the ABOi/TPE ≥6 group with a high MoRAL score and the other groups (P = 0.0042).
TABLE 4
| Subgroups | Patient number | 5 years HCC recurrence | P | ||
|---|---|---|---|---|---|
| ABOi/TPE ≤5 (n = 75) | ABOi/TPE ≥6 (n = 30) | ABOi/TPE ≤5 (n = 75) | ABOi/TPE ≥6 (n = 30) | ||
| Milan criteria | |||||
| Within | 44 | 15 | 9.9% | 35.2% | 0.025 |
| Above | 31 | 15 | 27.2% | 43.8% | 0.267 |
| Up-to-7 | |||||
| Within | 65 | 22 | 16.1% | 35.5% | 0.033 |
| Above | 10 | 8 | 27.1% | 47.5% | 0.569 |
| French risk score | |||||
| ≤2 | 56 | 20 | 14.8% | 31.8% | 0.082 |
| >2 | 19 | 10 | 21.3% | 55.0% | 0.117 |
| MoRAL score | |||||
| <100 | 49 | 16 | 17.6% | 20.5% | 0.750 |
| ≥100 | 26 | 14 | 16.1% | 56.7% | 0.017 |
| IA titer at initial | |||||
| ≤1:128 | 65 | 6 | 17.9% | 50.0% | 0.035 |
| ≥1:256 | 10 | 24 | 0.0% | 64.1% | 0.177 |
| IA titer at LT | |||||
| ≤1:16 | 61 | 11 | 19.4% | 31.8% | 0.287 |
| ≥1:32 | 14 | 19 | 0.0% | 43.5% | 0.026 |
| IA titer rebound | |||||
| No | 56 | 22 | 12.9% | 28.6% | 0.073 |
| Yes | 19 | 8 | 28.9% | 66.7% | 0.103 |
| Post LT TPE | |||||
| No | 61 | 19 | 12.1% | 24.0% | 0.203 |
| Yes | 14 | 11 | 37.3% | 59.1% | 0.246 |
| Splenectomy | |||||
| No | 71 | 23 | 17.9% | 36.9% | 0.075 |
| Yes | 4 | 7 | 0.0% | 46.4% | 0.137 |
Subgroup analysis of 5-year hepatocellular carcinoma recurrence according to therapeutic plasma exchange numbers in ABO incompatible group.
ABOi, ABO incompatible; HCC, hepatocellular carcinoma; IA, isoagglutinin; LT, liver transplantation; MoRAL, model of recurrence after liver transplant; TPE, therapeutic plasma exchange.
Regarding the tumor burden criteria, the ABOi/TPE ≤5 group of patients within the Milan criteria exhibited a significantly lower 5-year HCC recurrence rate (9.9%) than the ABOi/TPE ≥6 group (35.2%, P = 0.025). Additionally, the 5-year HCC recurrence rate was significantly lower in the ABOi/TPE ≤5 group of patients within the Up-to-7 criteria (16.1%) than in the ABOi/TPE ≥6 group (35.5%, P = 0.033).
In the subgroup analysis based on immunological classification, patients with an initial IA titer ≤1:128 demonstrated a significantly higher recurrence rate in the ABOi/TPE ≥6 group (50.0%) than in the ABOi/TPE ≤5 group (17.9%, P = 0.035). However, patients with an IA titer ≥1:32 at LT had a significantly higher recurrence rate in the ABOi/TPE ≥6 group (43.5%) than in the ABOi/TPE ≤5 group (0.0%, P = 0.026).
Discussion
The study evaluated the impact of pretransplant TPE sessions on HCC recurrence in patients undergoing ABOi LDLT and determined if limiting TPE sessions to fewer than six can enhance oncologic outcomes. We found that in the ABOi LDLT group, patients who underwent more than six pretransplant TPE sessions exhibited significantly worse HCC RFS and recurrence outcomes, with a similar trend observed in the subgroup analysis. Interestingly, the MoRAL score, which includes biomarkers, revealed that poorer oncologic outcomes were particularly pronounced in the high MoRAL score group. This suggests that in patients requiring a greater number of TPE sessions, biomarkers, in addition to tumor size, may play a crucial role in influencing HCC outcomes.
Moreover, the immunologic status at the time of transplantation is a critical factor influencing HCC recurrence [31, 32]. The need for multiple pretransplant TPE sessions may reflect an underlying immune dysregulation that could contribute to an increased risk of HCC recurrence. In particular, alterations in immune surveillance due to intensified desensitization protocols may affect the tumor microenvironment, potentially facilitating HCC recurrence [33, 34]. Similarly, ischemia-reperfusion injury (IRI) plays a crucial role in shaping the post-transplant microenvironment, influencing oncologic outcomes. Recent studies suggest that machine perfusion may help reduce HCC recurrence by mitigating IRI-induced inflammation and creating a more favorable post-transplant microenvironment [35–37]. Thus, assessing and managing the pretransplant immunologic status is essential for optimizing long-term oncologic outcomes in ABOi LDLT. A tailored approach that considers both desensitization requirements and immune profiling may help refine patient selection and improve posttransplant HCC prognosis. The strengths of our study include a well-organized dataset and a standardized desensitization protocol within the context of ABOi LDLT.
Globally, there has been a growing demand for LT as a definitive treatment for HCC, particularly for LDLT and ABOi LDLT due to organ shortages [15, 38]. In many countries outside East Asia, there is greater availability of deceased donors (DD), resulting in a predominant reliance on DDLT [38–40]. Consequently, these regions have limited cases and data regarding ABOi LDLT and the frequent use of TPE. In contrast, due to extreme shortages of deceased donors in Korea, LDLT is commonly performed for HCC [40, 41]. Paradoxically, this societal impact of deceased donor shortages has contributed to the accumulation of extensive data on ABOi LDLT, particularly in cases with high ABO antibody titers and a greater number of TPE sessions.
TPE is an intervention that involves the extracorporeal removal, return, or exchange of blood plasma or its components [42, 43]. The fundamental mechanism of this procedure is achieved through centrifugation or filtration using semipermeable membranes [44, 45]. In ABOi LDLT, the primary purpose of TPE is to remove IA. However, because this procedure is not selective, other immune-related factors in the blood are also removed, which presents a theoretical concern. Consequently, TPE is typically used as a primary or adjunctive treatment for conditions such as neurological diseases—including multiple sclerosis, amyotrophic lateral sclerosis, and myasthenia gravis—as well as autoimmune diseases like systemic lupus erythematosus and Kawasaki disease. Recent studies in this field have indicated that TPE promotes the differentiation and function of regulatory T cells [46–53].
Upon reviewing prior studies, it was noted that desensitization through pretransplant TPE or induction therapy in immunologically high-risk groups is associated with an increased cancer risk in certain malignancies (Table 5) [22, 23, 54, 55]. Although specific studies on ABOi LDLT are lacking, and the existing literature did not establish consistent protocols for TPE in kidney transplantation, direct comparisons with our study are challenging. Nevertheless, these findings underscore the relevance of desensitization and induction therapy concerning cancer risk, which was considered in our research.
TABLE 5
| Study | Yang, C.Y., et al.a | Motter, J.D., et al.b |
|---|---|---|
| Country | Taiwan | USA |
| Study period | 2007–2013 | 1997–2016 |
| Transplantation | Kidney | Kidney |
| Compared groups | DSA+ (n = 22) vs DSA – (n = 152) | ABOi LDKT (n = 858) vs ABOc LDKT (n = 12,239) |
| Plasmapheresis number | At least 4 cycles in DSA+ group | Not provided |
| Cancer type | Urothelial, endometrial, colon, and thyroid cancer | Colorectal cancer |
| Cancer incidence | DSA+ 19.6% vs DSA- 8.5% for 5 years (HR = 7.81, p = 0.028) | ABOi 0.6% vs ABOc 0.3% (HR = 3.27, p = 0.002) |
| Hypothesis for higher cancer incidence | Desensitization therapy for DSA+ including TPE might increase cancer | Desensitization therapy might increase cancer |
Previous studies regarding pretransplant desensitization and cancer risk.
Yang, C.Y., et al., Renal transplantation across the donor-specific antibody barrier: Graft outcome and cancer risk after desensitization therapy. J Formos Med Assoc, 2016. 115 (6): p. 426–33.
Motter, J.D., et al., Cancer Risk Following HLA-Incompatible Living Donor Kidney Transplantation. transplant direct, 2023. 9 (8): p. e1505.
ABOc, ABO compatible; ABOi, ABO incompatible; DSA, donor specific antibody; HR, hazard ratio; LDKT, living donor kidney transplantation; TPE, therapeutic plasma exchange.
Recent trends suggest that the outcomes of ABOi LDLT, including HCC outcomes and oncologic survival benefits, are comparable to those of ABOc LT [18, 19, 21]. However, these studies did not account for the cumulative and long-term effects of TPE, which prompted the initiation of our research.
In our study, the data indicated that ABOi patients requiring six or more pretransplant TPE sessions exhibited significantly poorer RFS and higher rates of HCC recurrence than ABOc patients. Additionally, our subgroup analysis shows that a higher number of pretransplant TPE sessions (≥6) was associated with a statistically significant increase in the 5-year HCC recurrence rate across several subgroups, including those within the Milan and Up-to-7 criteria, those with a high MoRAL score, and those with lower initial IA titers and higher IA titers at the time of LT.
Notably, within the size-based criteria, the ABOi/TPE ≥6 group exhibited a significantly higher recurrence rate. In contrast, regarding the tumor marker-based MoRAL score, a higher recurrence rate was observed in the ABOi/TPE ≥6 group only among patients with a score above 100. This suggests that among patients with a lower size-based tumor burden and a higher biologic-based tumor burden, those requiring more TPE sessions tended to experience poorer oncologic outcomes. Furthermore, this implies that the number of TPE sessions may be a more critical factor than the IA titer in influencing these outcomes.
Unlike previous studies, we focused on the immunomodulatory effects of T-regulatory (T-reg) cells induced by TPE and their association with HCC recurrence. As discussed earlier, while it is well established that T-reg cells are effective in treating autoimmune and neurological disorders, there are theoretical concerns that this process may reduce patient resistance to cancer [56, 57]. The literature indicates that the activation of T-reg cells can increase the risk of cancers such as HCC, with CD4+CD25+FoxP3+ T cells playing a significant role in this risk [58–60]. Although the exact cytokines and mechanisms through which these cells interact with others remain unclear, their differentiation within the tumor microenvironment (TME) has been observed [33, 34, 61], suggesting a potential increase in poor long-term cancer outcomes in various malignancies, including HCC. This information is illustrated in Supplementary Figure S6.
In summary, our hypothesis suggests that plasmapheresis induces the activation of T-reg cells, particularly CD4+ with CD25high, FoxP3+ effector T-reg cells, leading to an immunosuppressive effect within the tumor microenvironment that may facilitate tumor progression in various malignancies, including HCC. While some aspects of this pathway remain unexplained in the current foundational research, further studies are warranted to elucidate these mechanisms. Notably, the cumulative effect of TPE in the context of ABOi LT has not been extensively studied, underscoring the significance of our research.
This study has some limitations, including its retrospective and non-randomized design, the low number of ABOi/TPE ≥6 patients from a single center (n = 30), and the lack of fully established theoretical hypotheses or evidence to support our claims. Also, patients requiring more pretransplant TPE sessions may have additional unknown risk factors for HCC recurrence, highlighting the need for prospective studies to assess their impact on posttransplant outcomes [62]. However, despite these limitations, our study is significant, as it is the first to investigate the relationship between HCC outcomes and the number of preoperative TPE sessions and emphasizes the importance of comprehensive pretransplant evaluations in refining risk assessment for ABOi LDLT. In the future, we aim to address these limitations by increasing the sample size and establishing a more robust theoretical framework.
Conclusion
This study demonstrated that the administration of more than six pretransplant TPE sessions in patients with HCC undergoing ABOi LDLT was associated with poorer oncologic outcomes. Based on our clinical findings and the theoretical association between TPE and HCC oncologic outcomes, we propose that limiting the number of TPE sessions to fewer than six may improve cancer outcomes in patients with HCC receiving ABOi LDLT. A strategy to reduce the number of TPE sessions to fewer than five should be implemented if possible when planning ABOi LDLT for HCC patients, ensuring adequate immunological stability through isoagglutinin titer control and maintaining comparable levels of immunological risk.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Severance HospitalYonsei University Health System. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because This study is a retrospective research model conducted using clinical data, posing minimal risk to the participants. The exemption itself does not negatively impact the welfare or rights of the participants, and the study cannot be practically conducted without the exemption. Additionally, participants will be provided with any relevant information after participation, if necessary.
Author contributions
YY and DJ had full access to all aspects of the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. YY, D-GK, and DJ participated in the research design. YY, D-GK, E-KM, SY, MC, H-HK, MK, JL, MK, and DJ participated in the performance of the research. YY, D-GK, and DJ participated in the data acquisition. YY and D-GK participated in the statistical analysis. YY and D-GK participated in the writing of the paper. DJ supervised the study process. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to thank Editage (www.editage.co.kr) for English language editing and MID (Medical Illustration and Design), as a member of the Medical Research Support Services of Yonsei University College of Medicine, providing excellent support with medical illustration.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2025.14304/full#supplementary-material
Abbreviations
ABOc, ABO-compatible; ABOi, ABO-incompatible; AFP, Alpha-fetoprotein; aHR, Adjusted Hazards ratio; CD, Cluster of differentiation; CI, Confidence interval; DDLT, Deceased donor liver transplantation; MELD, Model for end-stage liver disease; HCC, Hepatocellular carcinoma; HR, Hazards ratio; IA, Isoagglutinin; LDLT, Living donor liver transplantation; LT, Liver transplantation; MoRAL, Model of Recurrence After Liver transplant; PIVKA-II, protein induced by vitamin K absence or antagonist II; PVTT, portal vein tumor thrombosis; RFS, Recurrence-free survival; TPE, Therapeutic plasma exchange.
Footnotes
References
1.
Lee SG . A Complete Treatment of Adult Living Donor Liver Transplantation: A Review of Surgical Technique and Current Challenges to Expand Indication of Patients. Am J Transplant (2015) 15(1):17–38. 10.1111/ajt.12907
2.
Goldaracena N Barbas AS . Living Donor Liver Transplantation. Curr Opin Organ Transplant (2019) 24(2):131–7. 10.1097/MOT.0000000000000610
3.
Akamatsu N Sugawara Y Kokudo N . Living-Donor Vs deceased-donor Liver Transplantation for Patients with Hepatocellular Carcinoma. World J Hepatol (2014) 6(9):626–31. 10.4254/wjh.v6.i9.626
4.
Ninomiya M Shirabe K Facciuto ME Schwartz ME Florman SS Yoshizumi T et al Comparative Study of Living and Deceased Donor Liver Transplantation as a Treatment for Hepatocellular Carcinoma. J Am Coll Surgeons (2015) 220(3):297–304.e3. 10.1016/j.jamcollsurg.2014.12.009
5.
Ogawa K Takada Y . Living Vs. deceased-donor Liver Transplantation for Patients with Hepatocellular Carcinoma. Transl Gastroenterol Hepatol (2016) 1:35. 10.21037/tgh.2016.04.03
6.
Azoulay D Audureau E Bhangui P Belghiti J Boillot O Andreani P et al Living or brain-dead Donor Liver Transplantation for Hepatocellular Carcinoma: A Multicenter, Western, intent-to-treat Cohort Study. Ann Surg (2017) 266(6):1035–44. 10.1097/SLA.0000000000001986
7.
Lai Q Sapisochin G Gorgen A Vitale A Halazun KJ Iesari S et al Evaluation of the intention-to-treat Benefit of Living Donation in Patients with Hepatocellular Carcinoma Awaiting a Liver Transplant. JAMA Surg (2021) 156(9):e213112-e. 10.1001/jamasurg.2021.3112
8.
Gugenheim J Samuel D Bismuth H Reynes M . Liver Transplantation Across ABO Blood Group Barriers. The Lancet (1990) 336(8714):519–23. 10.1016/0140-6736(90)92082-s
9.
Gordon RD Iwatsuki S Esquivel CO Tzakis A Todo S Starzl TE . Liver Transplantation Across ABO Blood Groups. Surgery (1986) 100(2):342–8.
10.
Tanabe M Shimazu M Wakabayashi G Hoshino K Kawachi S Kadomura T et al Intraportal Infusion Therapy as a Novel Approach to Adult ABO-Incompatible Liver Transplantation. Transplantation (2002) 73(12):1959–61. 10.1097/00007890-200206270-00021
11.
Egawa H Teramukai S Haga H Tanabe M Mori A Ikegami T et al Impact of Rituximab Desensitization on blood-type-incompatible Adult Living Donor Liver Transplantation: A Japanese Multicenter Study. Am J Transplant (2014) 14(1):102–14. 10.1111/ajt.12520
12.
Kim JM Kwon CHD Joh J-W Kang E-S Park JB Lee JH et al ABO-Incompatible Living Donor Liver Transplantation Is Suitable in Patients Without ABO-Matched Donor. J Hepatol (2013) 59(6):1215–22. 10.1016/j.jhep.2013.07.035
13.
Lee C-F Cheng C-H Wang Y-C Soong R-S Wu T-H Chou H-S et al Adult Living Donor Liver Transplantation Across ABO-incompatibility. Medicine (2015) 94(42):e1796. 10.1097/MD.0000000000001796
14.
Lee SD Kim SH Kong S-Y Kim Y-K Lee S-A Park S-J . ABO-Incompatible Living Donor Liver Transplantation Without Graft Local Infusion and Splenectomy. HPB (2014) 16(9):807–13. 10.1111/hpb.12215
15.
Matsuno N Iwamoto H Nakamura Y Hama K Kihara Y Konno O et al ABO-incompatible Adult Living Donor Liver Transplantation for Hepatocellular Carcinoma. Transplant Proc (2008) 40(8):2497–500. 10.1016/j.transproceed.2008.07.054
16.
Yim SH Kim D-G Kang M Koh H-h Choi MC Min E-K et al Intention-To-Treat Analysis for Survival Benefit of ABO-Incompatible living-donor Liver Transplantation in Patients with a High Model for End-stage Liver Disease Score. Korean J Transplant (2023) 37(1):77. 10.4285/atw2023.f-6562
17.
Lee W-C Cheng C-H Lee C-F Hung H-C Lee J-C Wu T-H et al Quick Preparation of ABO-Incompatible Living Donor Liver Transplantation for Acute Liver Failure. Clin Transplant (2022) 36(3):e14555. 10.1111/ctr.14555
18.
Yoon Y-I Song G-W Lee S-G Hwang S Kim K-H Kim S-H et al Outcome of ABO-Incompatible Adult living-donor Liver Transplantation for Patients with Hepatocellular Carcinoma. J Hepatol (2018) 68(6):1153–62. 10.1016/j.jhep.2018.02.002
19.
Kim SH Lee EC Na BG Park SJ . Impact of ABO-Incompatibility on Hepatocellular Carcinoma Recurrence After Living Donor Liver Transplantation. Eur J Surg Oncol (2019) 45(2):180–6. 10.1016/j.ejso.2018.07.066
20.
Kang SH Song G-W Yoon Y-I . Outcome of ABO-Incompatible Adult living-donor Liver Transplantation for Patients with Hepatocellular Carcinoma. HPB (2019) 21:S344–5. 10.1016/j.hpb.2019.10.1937
21.
Kim JM Kwon CHD Joh J-W Han S Yoo J Kim K et al ABO-Incompatible Living Donor Liver Transplantation with Rituximab and Total Plasma Exchange does Not Increase Hepatocellular Carcinoma Recurrence. Transplantation (2018) 102(10):1695–701. 10.1097/TP.0000000000002154
22.
Lee SD Kim SH Kong S-Y Kim Y-K Park S-J . Kinetics of B, T, NK Lymphocytes and Isoagglutinin Titers in ABO Incompatible Living Donor Liver Transplantation Using Rituximab and Basiliximab. Transpl Immunol (2015) 32(1):29–34. 10.1016/j.trim.2014.11.216
23.
Miyagi S Kawagishi N Sekiguchi S Akamatsu Y Sato K Takeda I et al The Relationship Between Recurrences and Immunosuppression on Living Donor Liver Transplantation for Hepatocellular Carcinoma. Transplant Proc (2012) 44(3):797–801. 10.1016/j.transproceed.2012.01.012
24.
Choi MC Min E-K Yim SH Kim D-G Lee JG Joo DJ et al High Number of Plasma Exchanges Increases the Risk of Bacterial Infection in ABO-Incompatible Living Donor Liver Transplantation. Transplantation (2024) 108(8):1760–8. 10.1097/TP.0000000000004883
25.
Lee J Lee JG Lee JJ Kim MS Ju MK Choi GH et al Results of ABO-incompatible Liver Transplantation Using a Simplified Protocol at a Single Institution. Transplant Proc (2015) 47(3):723–6. 10.1016/j.transproceed.2015.02.004
26.
Fine JP Gray RJ . A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc (1999) 94(446):496–509. 10.1080/01621459.1999.10474144
27.
Mazzaferro V Regalia E Doci R Andreola S Pulvirenti A Bozzetti F et al Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients with Cirrhosis. New Engl J Med (2025) 334(11):693–700. 10.1056/nejm199603143341104
28.
Mazzaferro V Llovet JM Miceli R Bhoori S Schiavo M Mariani L et al Predicting Survival After Liver Transplantation in Patients with Hepatocellular Carcinoma Beyond the Milan Criteria: A Retrospective, Exploratory Analysis. The Lancet Oncol (2009) 10(1):35–43. 10.1016/S1470-2045(08)70284-5
29.
Duvoux C Roudot–Thoraval F Decaens T Pessione F Badran H Piardi T et al Liver Transplantation for Hepatocellular Carcinoma: A Model Including α-Fetoprotein Improves the Performance of Milan Criteria. Gastroenterology (2012) 143(4):986–e15. 10.1053/j.gastro.2012.05.052
30.
Lee J-H Cho Y Kim HY Cho EJ Lee DH Yu SJ et al Serum Tumor Markers Provide Refined Prognostication in Selecting Liver Transplantation Candidate for Hepatocellular Carcinoma Patients Beyond the Milan Criteria. Ann Surg (2016) 263(5):842–50. 10.1097/SLA.0000000000001578
31.
Imaoka Y Ohira M Sato S Chogahara I Bekki T Imaoka K et al Impact of a Liver Immune Status Index Among Living Liver Transplant Recipients with Hepatocellular Carcinoma. Jma j (2024) 7(2):232–9. 10.31662/jmaj.2023-0195
32.
Abdelhamed W El-Kassas M . Hepatocellular Carcinoma Recurrence: Predictors and Management. Liver Res (2023) 7(4):321–32. 10.1016/j.livres.2023.11.004
33.
Ohue Y Nishikawa H . Regulatory T (Treg) Cells in Cancer: Can Treg Cells Be a New Therapeutic Target?Cancer Sci (2019) 110(7):2080–9. 10.1111/cas.14069
34.
Fridman WH Pages F Sautes-Fridman C Galon J . The Immune Contexture in Human Tumours: Impact on Clinical Outcome. Nat Rev Cancer (2012) 12(4):298–306. 10.1038/nrc3245
35.
Parente A Flores Carvalho M Eden J Dutkowski P Schlegel A . Mitochondria and Cancer Recurrence After Liver Transplantation-What Is the Benefit of Machine Perfusion?Int J Mol Sci (2022) 23(17):9747. 10.3390/ijms23179747
36.
Garcia KB Hussein A Satish S Wehrle CJ Karakaya O Panconesi R et al Machine Perfusion as a Strategy to Decrease Ischemia-Reperfusion Injury and Lower Cancer Recurrence Following Liver Transplantation. Cancers (2024) 16(23):3959. 10.3390/cancers16233959
37.
Boteon Y Flores Carvalho MA Panconesi R Muiesan P Schlegel A . Preventing Tumour Recurrence After Liver Transplantation: The Role of Machine Perfusion. Int J Mol Sci (2020) 21(16):5791. 10.3390/ijms21165791
38.
Rela M Rammohan A . Why Are There so Many Liver Transplants from Living Donors in Asia and so Few in Europe and the US?J Hepatol (2021) 75(4):975–80. 10.1016/j.jhep.2021.05.036
39.
Lee SG Moon DB Shin H Kim KH Ahn CS Ha TY et al Living Donor Liver Transplantation for Hepatocellular Carcinoma: Current Status in Korea. Transplant Proc (2012) 44(2):520–2. 10.1016/j.transproceed.2012.02.003
40.
Hibi T Chieh AKW Chan AC-Y Bhangui P . Current Status of Liver Transplantation in Asia. Int J Surg (2020) 82:4–8. 10.1016/j.ijsu.2020.05.071
41.
Choi HJ . Current Status and Outcome of Liver Transplantation in South Korea. Clin Mol Hepatol (2022) 28(1):117–9. 10.3350/cmh.2021.0381
42.
Kaplan AA . Therapeutic Plasma Exchange: Core Curriculum 2008. Am J Kidney Dis (2008) 52(6):1180–96. 10.1053/j.ajkd.2008.02.360
43.
Schwartz J Padmanabhan A Aqui N Balogun RA Connelly-Smith L Delaney M et al Guidelines on the Use of Therapeutic Apheresis in Clinical Practice-Evidence-Based Approach From the Writing Committee of the American Society for Apheresis: The Seventh Special Issue. J Clin Apher (2016) 31(3):149–62. 10.1002/jca.21470
44.
Gerhardt RE Ntoso KA Koethe JD Lodge S Wolf CJ . Acute Plasma Separation with Hemodialysis Equipment. J Am Soc Nephrol (1992) 2(9):1455–8. 10.1681/ASN.V291455
45.
Siami GA Siami FS . Membrane Plasmapheresis in the United States: A Review over the Last 20 Years. Ther Apher (2001) 5(4):315–20. 10.1046/j.1526-0968.2001.00316.x
46.
Jamshidian A Gharagozloo M . Can Plasma Exchange Therapy Induce Regulatory T Lymphocytes in Multiple Sclerosis Patients?Clin Exp Immunol (2012) 168(1):75–7. 10.1111/j.1365-2249.2011.04547.x
47.
Thonhoff J Beers D Zhao W Wen S Wang J Lay L et al Plasmapheresis Improves the Suppressive Function of Regulatory T Cells in Patients with Fast-Progressing Amyotrophic Lateral Sclerosis (P3.183). Neurology (2016) 86(16_Suppl. ment):P3.183–. 10.1212/wnl.86.16_supplement.p3.183
48.
Zhang L Liu J Wang H Zhao C Lu J Xue J et al Double Filtration Plasmapheresis Benefits Myasthenia Gravis Patients Through an Immunomodulatory Action. J Clin Neurosci (2014) 21(9):1570–4. 10.1016/j.jocn.2013.11.046
49.
Fiorini G Paracchini ML Fornasieri A . Modifications in Peripheral Blood Lymphocyte Subpopulations Induced by Plasmapheresis and Immunosuppressive Drugs. Plasma Ther Transfus Technology (1982) 3(4):389–93.
50.
Barath S Soltesz P Kiss E Aleksza M Zeher M Szegedi G et al The Severity of Systemic Lupus Erythematosus Negatively Correlates with the Increasing Number of CD4+CD25(high)FoxP3+ Regulatory T Cells During Repeated Plasmapheresis Treatments of Patients. Autoimmunity (2007) 40(7):521–8. 10.1080/08916930701610028
51.
Mehdipour M Etienne J Liu C Mehdipour T Kato C Conboy M et al Attenuation of age-elevated Blood Factors by Repositioning Plasmapheresis: A Novel Perspective and Approach. Transfus Apher Sci (2021) 60(3):103162. 10.1016/j.transci.2021.103162
52.
Koizumi K Hoshiai M Moriguchi T Katsumata N Toda T Kise H et al Plasma Exchange Downregulates Activated Monocytes and Restores Regulatory T Cells in Kawasaki disease. Ther Apher Dial (2019) 23(1):92–8. 10.1111/1744-9987.12754
53.
Lim YJ Jung JW . Clinical Outcomes of Initial Dexamethasone Treatment Combined with a Single High Dose of Intravenous Immunoglobulin for Primary Treatment of Kawasaki disease. Yonsei Med J (2014) 55(5):1260–6. 10.3349/ymj.2014.55.5.1260
54.
Yang CY Lee CY Yeh CC Tsai MK . Renal Transplantation Across the Donor-specific Antibody Barrier: Graft Outcome and Cancer Risk After Desensitization Therapy. J Formos Med Assoc (2016) 115(6):426–33. 10.1016/j.jfma.2015.11.006
55.
Motter JD Massie AB Garonzik-Wang JM Pfeiffer RM Yu KJ Segev DL et al Cancer Risk Following HLA-incompatible Living Donor Kidney Transplantation. Transpl Direct (2023) 9(8):e1505. 10.1097/TXD.0000000000001505
56.
Miyara M Sakaguchi S . Natural Regulatory T Cells: Mechanisms of Suppression. Trends Mol Med (2007) 13(3):108–16. 10.1016/j.molmed.2007.01.003
57.
Jung MK Lee JS Kwak J-E Shin E-C . Tumor Necrosis Factor and Regulatory T Cells. Yonsei Med J (2019) 60(2):126–31. 10.3349/ymj.2019.60.2.126
58.
Kalathil S Lugade AA Miller A Iyer R Thanavala Y . Higher Frequencies of GARP+CTLA-4+Foxp3+ T Regulatory Cells and Myeloid-Derived Suppressor Cells in Hepatocellular Carcinoma Patients are Associated with Impaired T-Cell Functionality. Cancer Res (2013) 73(8):2435–44. 10.1158/0008-5472.CAN-12-3381
59.
Sakaguchi S Yamaguchi T Nomura T Ono M . Regulatory T Cells and Immune Tolerance. Cell. (2008) 133(5):775–87. 10.1016/j.cell.2008.05.009
60.
Sun W Li W-J Wu C-Y Zhong H Wen W-P . CD45RA-Foxp3high but Not CD45RA+Foxp3low Suppressive T Regulatory Cells Increased in the Peripheral Circulation of Patients with Head and Neck Squamous Cell Carcinoma and Correlated with Tumor Progression. J Exp and Clin Cancer Res (2014) 33(1):35. 10.1186/1756-9966-33-35
61.
Adeegbe DO Nishikawa H . Natural and Induced T Regulatory Cells in Cancer. Front Immunol (2013) 4:190. 10.3389/fimmu.2013.00190
62.
Straś WA Wasiak D Łągiewska B Tronina O Hreńczuk M Gotlib J et al Recurrence of Hepatocellular Carcinoma After Liver Transplantation: Risk Factors and Predictive Models. Ann Transpl (2022) 27:e934924. 10.12659/AOT.934924
Summary
Keywords
ABO-incompatible living donor liver transplantation, hepatocellular carcinoma, plasma exchange, surgical oncology, oncologic outcome
Citation
Yoo YJ, Kim D-G, Min E-K, Yim SH, Choi MC, Koh H-H, Kang M, Lee JG, Kim MS and Joo DJ (2025) Number of Pretransplant Therapeutic Plasma Exchange Sessions Increase the Recurrence Risk of Hepatocellular Carcinoma in ABO-Incompatible Living Donor Liver Transplantation. Transpl. Int. 38:14304. doi: 10.3389/ti.2025.14304
Received
07 January 2025
Accepted
31 July 2025
Published
13 August 2025
Volume
38 - 2025
Updates
Copyright
© 2025 Yoo, Kim, Min, Yim, Choi, Koh, Kang, Lee, Kim and Joo.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dong Jin Joo, djjoo@yuhs.ac; Deok-Gie Kim, mppl01@yuhs.ac
‡ Present address: Mun Chae Choi, Department of Surgery, Armed Forces Capital Hospital, Seongnam, Republic of Korea Hwa-Hee Koh, Department of Surgery, Catholic Kwandong University International St. Mary's hospital, Incheon, Republic of Korea
ORCID: Young Jin Yoo, orcid.org/0000-0002-1391-048X; Deok-Gie Kim, orcid.org/0000-0001-9653-926X; Eun-Ki Min, orcid.org/0000-0003-3255-1942; Seung Hyuk Yim, orcid.org/0000-0003-2146-3592; Mun Chae Choi, orcid.org/0000-0002-2708-0755; Hwa-Hee Koh, orcid.org/0000-0001-5944-6136; Minyu Kang, orcid.org/0000-0002-6623-2774; Jae Geun Lee, orcid.org/0000-0002-6722-0257; Myoung Soo Kim, orcid.org/0000-0002-8975-8381; Dong Jin Joo, orcid.org/0000-0001-8405-1531
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.