Abstract
Selection of patients who may benefit from extracorporeal life support (ECLS) as a bridge to lung transplant (LTx) is crucial. The aim was to assess if validated prognostic scores could help in selecting patients who may benefit from ECLS-bridging predicting their outcomes. Clinical data of patients successfully ECLS-bridged to LTx from 2009 to 2021 were collected from two European centers. For each patient, we calculated Sequential Organ Failure Assessment (SOFA), Simplified Acute Physiology Score III (SAPS III), Acute Physiology and Chronic Health Evaluation II (APACHE II), before placing ECLS support, and then correlated with outcome. Median values of SOFA, SAPS III, and APACHE II were 5 (IQR 3–9), 57 (IQR 47.5–65), and 21 (IQR 15–26). In-hospital, 30 and 90 days mortality were 21%, 14%, and 22%. SOFA, SAPS III, and APACHE II were analyzed as predictors of in-hospital, 30 and 90 days mortality (SOFA C-Index: 0.67, 0.78, 0.72; SAPS III C-index: 0.48, 0.45, 0.51; APACHE II C-Index: 0.49, 0.45, 0.52). For SOFA, the score with the best performance, a value ≥9 was identified to be the optimal cut-off for the prediction of the outcomes of interest. SOFA may be considered an adequate predictor in these patients, helping clinical decision-making. More specific and simplified scores for this population are necessary.
Introduction
The utilization of extracorporeal life support (ECLS) as a bridge to lung transplantation (LTx) has allowed critically ill patients to remain eligible for transplant.
The selection of patients who may benefit from ECLS as a bridge to LTx is a crucial aspect: highly urgent patients, with a high predicted pre-transplant mortality, are often the ones who would benefit the most from ECLS but at the same time they could be too compromised to be suitable candidates for this support [1].
The patients who can derive the greatest benefit from ECLS-bridge are generally those with cardiopulmonary dysfunction severe enough to limit their ability to maintain the necessary physical condition to tolerate a transplant (such as oxygen saturation <90% with high-flow levels and with non-invasive oxygenation devices, hemodynamic instability, and use of positive pressure ventilation that could lead to further lung injury and secondary organ dysfunctions) and it is mostly recommended in patients who have already been evaluated for LTx [1–4].
The effect of ECLS as a bridge to LTx and the consequences on recipients’ clinical outcomes remain undetermined, indeed the results reported in current literature are divergent [5, 6].
Some authors [7, 8] reported negative experiences with ECLS as a bridge to LTx, showing a worse overall survival in bridged patients compared to unsupported ones. On the other hand, in more recent times, different authors have reported good outcomes for successfully bridged patients on ECLS with satisfying survival rates [2, 3, 9–11].
It is widely established that a careful patient selection, high volume transplant centers, and multidisciplinary teams are the key factors to obtain improvements in ECLS bridging strategies [1], even though a homogeneous consensus on which factors might help the clinicians in predicting outcomes of patients bridged to LTx with ECLS supports is still lacking. This is also demonstrated by the fact that currently no clinically validated tools, except the Recipient STratification Risk Analysis in Bridging Patients to Lung Transplant on ECMO (STABLE) score [12], exist to predict outcomes in this population. However, given the increasing use of different bridging devices (not only extracorporeal membrane oxygenation, ECMO) and strategies in the modern era, it is mandatory to define if validated prognostic scores might predict mortality in this population, helping to better select patients who may benefit from ECLS bridging in relation to post-operative outcomes.
Scoring systems, such as Sequential Organ Failure Assessment (SOFA), Simplified Acute Physiology Score III (SAPS III), and Acute Physiology and Chronic Health Evaluation II (APACHE II), are commonly used for risk assessment in critically ill patients, especially to predict in-hospital mortality [13–15].
The aim of this study was to assess the predictive ability of these scores in a population of patients bridged to LTx on ECLS in terms of in-hospital, 30 and 90 days mortality.
These findings might play an important role in guiding physician decision making to better select patients who might benefit from a bridge from ECLS to LTx, also facilitating evidence-based rationing of limited healthcare resources in the future.
Materials and Methods
All clinical data of 70 patients successfully ECLS-bridged to LTx from 2009 to 2021 were retrospectively collected from two European centers (Thoracic Surgery Unit of University Hospital of Padua, Italy and Department of Thoracic Surgery of University Hospital of Zurich, Switzerland) as anonymized records. The study was approved by the Ethical Committee of the University Hospital of Padua (4539/AO/18). Informed consent was waived due to the retrospective nature of this work.
Patients bridged to lung retransplantation (LReTx) or heart-lung transplantation (HLTx) with ECLS were excluded. Fifty-eight patients were finally enrolled in the study (Figure 1).
FIGURE 1
Demographic and clinical data, intra-operative characteristics, peri and post-operative outcomes were collected for each patient from both centers. Follow-up was achieved in each center by indirect contact via the treating physician. For each patient, organ dysfunction (SOFA) and illness severity (APACHE II, SAPS III) scores were collected when already available otherwise calculated retrospectively at the ICU arrival, before positioning the ECLS device and then correlated with outcomes. A comparison between the variables utilized in the abovementioned scoring systems is shown in Table 1.
TABLE 1
| SOFA (range 0–20) | APACHE II (range 0–71) | SAPS III (range 16–217) | |
|---|---|---|---|
| Variables | - PaO2/FiO2 (and if MV/CPAP) | - Temperature | - Age |
| - Platelets | - Age | - LOS before ICUA | |
| - GCS | - MAP | - In- hospital location (OR, ER, other ICU) | |
| - Bilirubin | - HR | - Cancer therapy (yes/no) | |
| - MAP (and if vasoactive agents required) | - RR | - Chronic heart failure (yes/no) | |
| - Creatinine | - pH | - Hematological cancer (yes/no) | |
| - Sodium | - Cirrhosis (yes/no) | ||
| - Potassium | - AIDS (yes/no) | ||
| - Creatinine | - Cancer (yes/no) | ||
| - Hematocrit | - Vasoactive drugs before ICUA (yes/no) | ||
| - WBC | - ICUA (planned/unplanned) | ||
| - Chronic organ failure (heart, lung, liver, kidney) | - Reason for admission (cardiovascular, hepatic, digestive, neurologic) | ||
| - GCS | - Surgical status at ICUA (scheduled, emergency, no surgery) | ||
| - FiO2 | - site of surgery (transplant, trauma, cardiac, neurosurgery) | ||
| - acute infection at ICUA (nosocomial, respiratory) | |||
| - GCS | |||
| - Bilirubine | |||
| - Temperature | |||
| - Creatinine | |||
| - HR | |||
| - WBC | |||
| - pH | |||
| - Platelets | |||
| - SBP | |||
| - pO2/FiO2 | |||
| - MV (yes/no/CPAP) |
Variables employed in SOFA, APACHE II, SAPS III scoring systems.
APACHE II, acute physiology and chronic health evaluation II; CPAP, continuous positive pressure ventilation; ER, emergency room; FiO2, fraction of inspired oxygen; GCS, Glasgow coma scale; HR, heart rate; ICU, intensive care unit; ICUA, intensive care unit admission; LOS, length of stay; MAP, mean arterial pressure; MV, mechanical ventilation; OR, operative room; paO2, partial pressure of oxygen; RR, respiratory rate; WBC, white blood cells; SBP, systolic blood pressure; SAPS III, simplified acute physiology score III; SOFA, sequential organ failure assessment.
Statistical Analyses
Descriptive statistics were reported as I quartile/median/III quartile for continuous variables and as percentages (absolute numbers) for categorical variables.
Survival distribution was evaluated using the Kaplan-Meier method. To assess the predictive ability of the scores (SOFA, APACHE II, SAPS III) on the outcomes of interest (in-hospital, 30 and 90 days mortality) logistic regression models were estimated. After models’ validation using bootstrap resampling, the Harrel’s C index, also known as “concordance index” [16] was computed.
Furthermore, the optimal cut-off for SOFA in predicting outcomes of interest was identified as the value that maximizes the sum of sensitivity and specificity.
Analyses were performed using R software version 4.1.3 [17] within the packages rms [18] and cutpointr [19].
Results
Study Population
The main clinical and demographic characteristics of 58 patients bridged to LTx on ECLS support are presented in Table 2.
TABLE 2
| Variable | Total (N = 58) |
|---|---|
| Female sex | 34 (59%) |
| Age at LTx (y) | 42 (24–49) |
| Diagnosis | |
| CF | 33 (57%) |
| ILD | 16 (27%) |
| COPD | 4 (7%) |
| LAM | 1 (2%) |
| Other | 4 (7%) |
| BMI | 19.5 (17–24) |
| Waiting list time (d) | 69 (14–240) |
| Type of LTx | |
| BLTX | 57 (98%) |
| SLTX | 1 (2%) |
| Pre LTx MV | |
| No | 10 (17%) |
| Yes | 48 (83%) |
| Awake ECLS | 10 (17%) |
| Time from ECLS bridge to LTx (d) | 10 (3–18) |
| Initial ECLS bridge configuration | |
| VV-ECMO | 37 (64%) |
| VA-ECMO | 8 (14%) |
| ECCO2-R | 13 (22%) |
| ECLS bridge configuration change | 18 (30%) |
| SOFA | 5 (3–9) |
| pSOFA (age <18 y) | 5 (3–10) |
| APACHE II | 21 (15–26) |
| SAPS III | 57 (47.5–65) |
| Intraoperative ECLS configuration | |
| VV-ECMO | 18 (31%) |
| pVA-ECMO | 12 (21%) |
| cVA-ECMO | 15 (26%) |
| CEC | 4 (7%) |
| VAV | 9 (15%) |
| Prolonged post-operative ECLS | |
| NO | 28 (47%) |
| YES | 30 (51%) |
| De novo | 1 (2%) |
| Post-operative ECLS duration (d) | 3 (2–8) |
| EVLP | 4 (7%) |
| Lobar transplantation | 17 (29%) |
Patients characteristics.
APACHE II, Acute Physiology and Chronic Health Evaluation II; BLTX, bilateral lung transplant; c, central; d, days; ECCO2-R, extracorporeal carbon dioxide removal; ECLS, extracorporeal life support; ECMO, extracorporeal membrane oxygenation; EVLP, ex-vivo lung perfusion; f, female; LTx, lung transplant; MV, mechanical ventilation; p, peripheral; SOFA, Sequential Organ Failure Assessment; SAPS III, Simplified Acute Physiology Score III; y, years; VA, venoarterial; VAV, venoarterial venous; VV, venovenous.
Data are reported as median (I-III interquartile range) for continuous variables and as absolute number (relative frequencies %) for categorical variables.
Thirty-four patients (59%) were females, 24 were males (41%) with a median age at time of LTx of 42 years-old (IQR 24–49). Seven patients (12%) were in pediatric age (age <18 years). The median BMI was 19.5 (IQR 17–24). The most common indication for LTx was cystic fibrosis (CF) (57%) followed by interstitial lung disease (ILD) (27%); almost all patients (98%) underwent bilateral lung transplantation while only a 65 year-old patient affected by ILD was submitted to single LTx.
The median waiting list time was 69 days (IQR 14–240). The most common ECLS bridge configuration was veno-venous (VV) (37 patients, 64%) although in 30% of cases an upgrading to another ECLS configuration was necessary during bridging. During ECLS bridging, 48 patients (83%) were mechanically ventilated while 10 patients (17%) were awake. The median time from ECLS bridge to LTx was 10 days (IQR 3–18). Median SOFA, APACHE II, and SAPS III values at the ICU arrival were respectively 5 (IQR 3–10), 21 (IQR 15–26), 57 (IQR 47.5–65). Median pediatric (p) SOFA, a special score tailored on pediatric patients [19], was also calculated but the median value did not differ from the one obtained in adults (5, IQR 3–10). The most common intra-operative ECLS configuration was the VV (18 patients, 31%) followed by the central veno-arterial (VA) ECLS (15 patients, 26%). 30 patients (51%) needed prolonged post-operative ECLS with a median duration of 3 days (IQR 2–8).
In 4 cases (7%) ex-vivo lung perfusion (EVLP) methods were used to recondition the organs before the implantation because of extended criteria donors and in 17 patients (29%) a size reduction with lobar LTx was performed due to the donor and the recipient size mismatch.
Short-Term Outcomes
Table 3 summarizes the main post-operative outcomes. The median duration of mechanical ventilation (MV) was 96 h (IQR 48–480) and in 28 patients (48%) a tracheostomy was performed for respiratory weaning. The median duration of ICU and hospital stay were respectively 11 days (IQR 6–28) and 44 days (IQR 31–71). Twenty-four patients (41%) required post-operative continuous veno-venous hemofiltration (CVVH) or dialysis for renal failure. In-hospital, 30, and 90 days mortality were respectively 21%, 14%, and 22%.
TABLE 3
| Variable | Total (N = 58) |
|---|---|
| ICU stay (post LTx, d) | 11 (6–28) |
| MV duration (post LTx, h) | 96 (48–480) |
| Post-operative tracheostomy | 28 (48%) |
| Post-operative CVVH/dialysis | 24 (41%) |
| Hospital stay (d) | 44 (31–71) |
| CLAD | 8 (14%) |
| In hospital mortality | 12 (21%) |
| 30 d mortality | 8 (14%) |
| 90 d mortality | 13 (22%) |
Clinical course and outcomes.
CLAD, chronic lung allograft dysfunction; CVVH, continous venovenous hemofiltration; d, days; h, hours; ICU, intensive care unit; LTx, lung transplant; MV, mechanical ventilation.
Data are reported as median (I-III interquartile range) for continuous variables and as absolute number (relative frequencies %) for categorical variables.
Long-Term Outcomes
One, 3, and 5 years survival rates were 72 % (95% CI 0.61–0.84), 55% (95% CI 0.43–0.70), and 51% (95% CI 0.38–0.66), respectively (Figure 2).
FIGURE 2
Predictive Ability of SOFA, APACHE II, and SAPS III
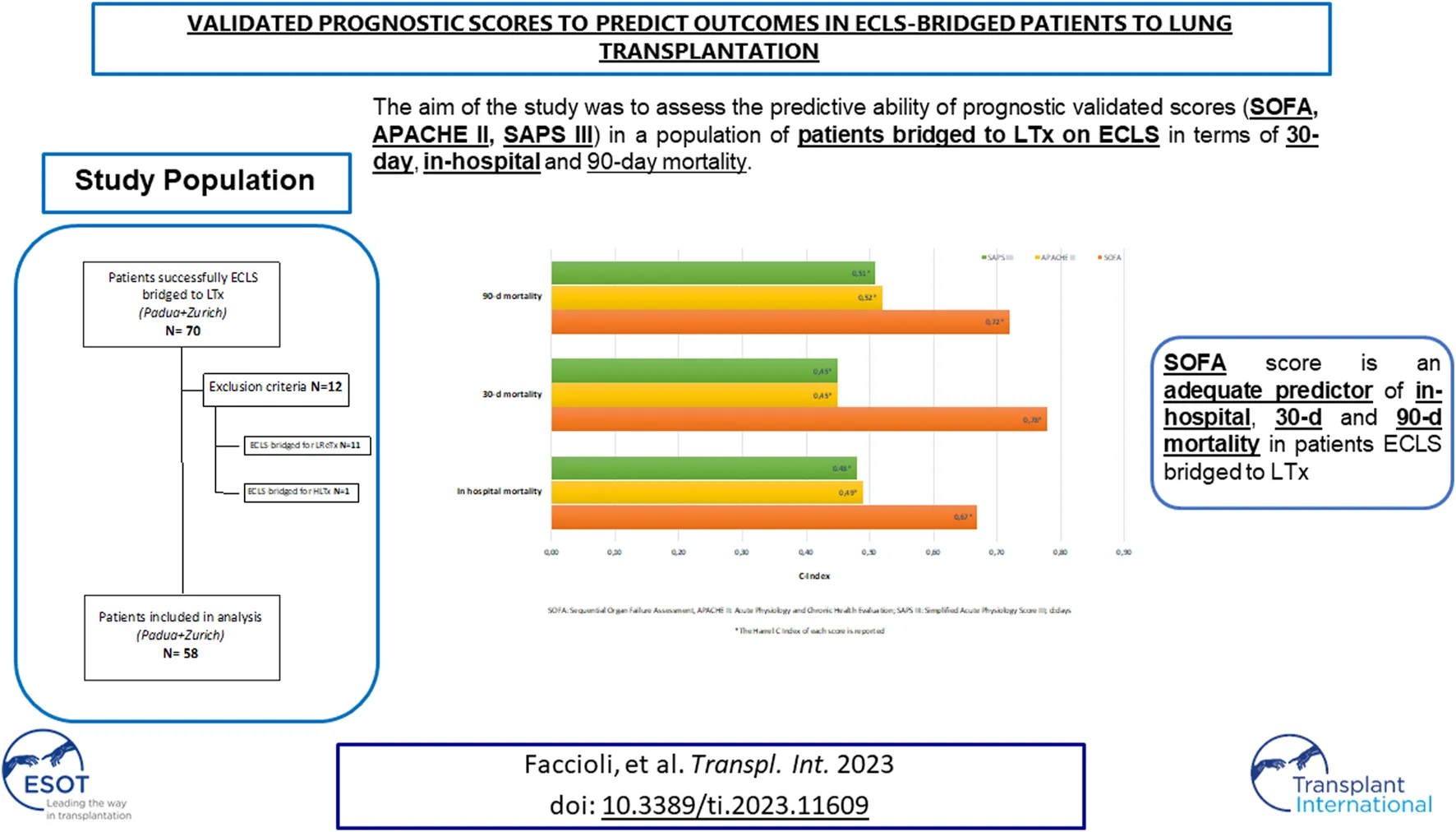
The ability of SOFA, APACHE II, and SAPS III in predicting post-LTx outcomes in ECLS-bridged patients is presented as C Index in Table 4.
TABLE 4
| Score | Outcomes | |||
|---|---|---|---|---|
| In hospital mortality | 30 d mortality | 60 d mortality | 90 d mortality | |
| SOFA | 0.67 | 0.78 | 0.60 | 0.72 |
| APACHE II | 0.49 | 0.45 | 0.42 | 0.52 |
| SAPS III | 0.48 | 0.45 | 0.42 | 0.51 |
Predictive ability of SOFA, APACHE II, SAPS III scores in predicting outcomes.
APACHE II, Acute Physiology and Chronic Health Evaluation II; d, days; SAPS III, Simplified Acute Physiology Score III; SOFA, Sequential Organ Failure Assessment.
The Harrel’s C Index of each score for in-hospital, 30, 60, and 90 days mortality is reported.
SOFA, SAPS III, and APACHE II were analyzed as predictors of in-hospital, 30, and 90 days mortality respectively (SOFA C Index: 0.67, 0.78, 0.72; SAPS III C Index: 0.48, 0.45, 0.51; APACHE II C Index: 0.49, 0.45, 0.52). For SOFA, the score with the best performance in this population, a value ≥9, was identified to be the optimal cut-off for the prediction of all the outcomes of interest (Table 5).
TABLE 5
| Outcome | Sensitivity | Specificity | AUC |
|---|---|---|---|
| In-hospital mortality | 0.714 | 0.780 | 0.775 |
| 30 d mortality | 0.6 | 0.789 | 0.680 |
| 90 d mortality | 0.636 | 0.810 | 0.717 |
Performance of SOFA value ≥9.
AUC, area under curve; d, days.
Discussion
Patients bridged to LTx with ECLS are often critically ill with a severe deterioration of clinical conditions. The investigation of predictors of outcomes in this population is mandatory, especially in a context of donors’ paucity as well as to facilitate evidence-based rationing of limited healthcare resources in the future.
We decided to analyze the predictive ability of three scores (SOFA, APACHE II, SAPS III), which are widespread known and easily accessible for every patient at the ICU arrival, to predict post-operative outcomes in ECLS bridged patients. These scores have already been extensively validated as predictors of mortality in several clinical settings [20–22] including transplantation field [23] and in patients on ECMO for cardiac or acute respiratory failure [24, 25]. However, they were not validated in ECLS-bridged patients to LTx. In addition, a number of specific scores in VA ECMO settings like prEdictioN of Cardiogenic shock OUtcome foR AMI patients salvaGed by VA ECMO (ENCOURAGE), Survival After Veno-arterial ECMO (SAVE), and pRedicting mortality in patients undergoing veno-arterial Extracorporeal MEMBrane oxygenation after coronary artEry bypass gRafting (REMEMBER) have been proposed to predict mortality in selective cardiogenic shock subsets [26–28], limiting their application in our study population.
On the other hand, The Respiratory Extracorporeal Membrane Oxygenation Survival Prediction (RESP) Score predicts survival for patients receiving ECMO for severe acute respiratory failure [29] but again it is not tailored to chronic end-stage lung disease and it does not take into account those patients with an associated hemodynamic instability (like in idiopathic pulmonary hypertension).
To the best of our knowledge, the only available predictive tool for risk stratification in ECMO bridge patients to LTx is the STABLE score [12] but some of its limitations made it not applicable to our entire population: firstly, it is validated only in adults but our population was composed for 12% of pediatric patients. In our study, in accordance with what has been reported by some of the most consistent studies on ECLS bridge [2, 8], more than a half (57%) of our population had cystic fibrosis which is the most common indication in pediatric population, therefore a score also applicable in a pediatric population (<18 years old) is mandatory. In our pediatric patients, we have also calculated for each of them the pSOFA, an adapted and validated pediatric version of the SOFA score [20], finding the same median value of the adult population. Concerning the other two scores, APACHE II has already been utilized for pediatrics in other clinical setting [30] and in the SAPS III calculator, ages of <18 years old can be inserted, so we felt authorized to use these scores also in pediatrics.
Secondly, among extracorporeal supports, the STABLE score only considers ECMO and not other devices such as extracorporeal carbon dioxide removal (ECCO2-R), which was utilized in 22% of our population as a bridge to LTx. Finally, this score was created on a big number of patients extracted from United Network for Organ Sharing (UNOS) database but it was externally validated only on 31 American patients and so it could not be representative of the European reality.
Among the three scores utilized in our analysis, the predictor of in-hospital, 30- and 90-days mortality with the best performance was the SOFA with a cut-off value of 9. SOFA score is the easiest to calculate and based on easy repeatable variables available in all institutions. Originally, it was designed to describe morbidity expressing different degrees of organ failure, but then it has been extensively externally validated as a good predictor of hospital mortality [14, 25].
In contrast to our finding [24], in a previous study, compared the prognostic ability of different scores in ECMO patients, showing that APACHE had a superior ability to SOFA in predicting hospital mortality. Their study did not focus on patients bridged to LTx and furthermore the scores were calculated only on the first day of ECMO support and not at the ICU admission as these models were originally developed and this may have affected the results. The low accuracy of APACHE II and SAPS III in predicting in-hospital mortality in transplant patients has already been established [23]. We also reported the same finding in ECLS bridged patients to LTx; this may be due to the multitude of physiologic aspects (such as for examples sodium, potassium, hematocrit, white blood cells, and platelets count) accounted by these two scores compared to the SOFA. These parameters are usually out of normality range in this population and tend to have a large and rapid variability during the pre- and post-transplant periods, making these scores unreliable in our patients.
Although in our study, a SOFA score of higher than or equal to 9 was associated with a poor short-term prognosis, this value should not be intended to arbitrarily exclude patients from life-sustaining therapies or from the possibility of a lung transplantation but just as a useful tool to better select the most appropriate LTx candidate or to help clinicians to identify which patients would need a stricter follow-up in the early post-operative period.
In conclusion, the results of this study serve as a first external validation of these scores in ECLS-bridged patients to LTX but it has some limitations. Even though it reflects the reality of two European lung transplant centers, the main limitation is the small number of patients and the absence of a control group. Then, in some situations, difficulties exist in performing this analysis as clinical/laboratory data to calculate the scores are not always collected at the same moment for all the subjects with a tendency towards high variability during pre-operative course during ECLS bridging. Again, it would be necessary to evaluate the evolution of these scores in different moments by sequential measurements (daily, weekly) and not only at the ICU admission, providing a more robust prediction of mortality.
Further studies are necessary to validate our results and to find a promising and accurate score in this peculiar subgroup of patients.
Statements
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Comitato Etico Azienda Ospedale-Università di Padova. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin due to the retrospective nature of the study.
Author contributions
EF: conceptualization, investigation, data curation, writing-original draft. GL: data curation software, methodology. DS: writing-review and editing. AD’A: writing-review and editing. SH: writing-review and editing. MS: writing-review and editing. CC: writing-review and editing. DG: data curation software, methodology. FR: writing-review and editing. IO: writing-review and editing. II: conceptualization, data curation, investigation, supervision, writing-original draft.
Acknowledgments
This study was presented at the ESTS Annual Meeting 2022 and at the SITO Annual Meeting 2022.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
APACHE II, Acute Physiology and Chronic Health Evaluation II; CIFs, cumulative incidence functions; CLAD, chronic lung allograft dysfunction; ECCO2-R, extracorporeal carbon dioxide removal; ECLS, extracorporeal life support; ECMO, extracorporeal membrane oxygenation; ENCOURAGE, prEdictioN of Cardiogenic shock OUtcome foR AMI patients salvaGed by VA ECMO; EVLP, ex-vivo lung perfusion; HLTx, heart-lung transplantation; ICU, intensive care unit; IQR, interquartile range; MV, mechanical ventilation; LTx, lung transplantation; LreTx, lung retransplantation; pSOFA, pediatric Sequential Organ Failure Assessment; REMEMBER, pRedicting mortality in patients undergoing veno-arterial Extracorporeal MEMBrane oxygenation after coronary artEry bypass gRafting; RESP, Respiratory Extracorporeal Membrane Oxygenation Survival Prediction; SAPS III, Simplified Acute Physiology Score III; SAVE, Survival After Veno-arterial ECMO; SOFA, Sequential Organ Failure Assessment; STABLE, Recipient STratification Risk Analysis in Bridging Patients to Lung Transplant on ECMO; UNOS, United Network for Organ Sharing; VV, veno-venous; VA, veno-arterial.
References
1.
LeardLEHolmAMValapourMGlanvilleARAttawarSAversaMet alConsensus Document for the Selection of Lung Transplant Candidates: An Update From the International Society for Heart and Lung Transplantation. J Heart Lung Transpl (2021) 40:1349–79. 10.1016/j.healun.2021.07.005
2.
BenazzoASchwarzSFrommletFScweigherTJakschPSchellongowskiPet alTwenty-Year Experience With Extracorporeal Life Support as Bridge to Lung Transplantation. J Thorac Cardiovasc Surg (2019) 157:2515–25.e10. 10.1016/j.jtcvs.2019.02.048
3.
HoetzeneckerKDonahoeLYeungJCAzadSFanEFergusonNDet alExtracorporeal Life Support as a Bridge to Lung Transplantation-Experience of a High-Volume Transplant Center. J Thorac Cardiovasc Surg (2018) 155:1316–28. 10.1016/j.jtcvs.2017.09.161
4.
AbramsDBrodieDArcasoySM. Extracorporeal Life Support in Lung Transplantation. Clin Chest Med (2017) 38:655–66. 10.1016/j.ccm.2017.07.006
5.
OhDKHongSBShimTSKimDKChoiSLeeGDet alEffects of the Duration of Bridge to Lung Transplantation With Extracorporeal Membrane Oxygenation. PloS ONE (2021) 16(7):e0253520. 10.1371/journal.pone.0253520
6.
FaccioliETerziSPangoniALomanginoIRossiSLloretAet alExtracorporeal Membrane Oxygenation in Lung Transplantation: Indications, Techniques and Results. World J Transpl (2021) 11:290–302. 10.5500/wjt.v11.i7.290
7.
MasonDPThuitaLNowickiERMurthySCPetterssonGBBlackstoneEH. Should Lung Transplantation Be Performed for Patients on Mechanical Respiratory Support? The US Experience. J Thorac Cardiovasc Surg (2010) 139:765–73. 10.1016/j.jtcvs.2009.09.031
8.
InciIKlinzingSSchneiterDSchuepbachRAKestenholzPHillingerSet alOutcome of Extracorporeal Membrane Oxygenation as a Bridge to Lung Transplantation: An Institutional Experience and Literature Review. Transplantation (2015) 99:1667–71. 10.1097/TP.0000000000000653
9.
ToddEMBiswasRSHashimiASSerroneRPanchanathanRKangPet alExtracorporeal Membrane Oxygenation as a Bridge to Lung Transplantation: A Single-Center Experience in the Present Era. J Thorac Cardiovasc Surg (2017) 154:1798–809. 10.1016/j.jtcvs.2017.06.063
10.
HakimAHAhmadUMcCurryKRJohnstonDRPetterssonGBBudevMet alContemporary Outcomes of Extracorporeal Membrane Oxygenation Used as Bridge to Lung Transplantation. Ann Thorac Surg (2018) 106:192–8. 10.1016/j.athoracsur.2018.02.036
11.
IusFNatanovRSalmanJKuehnCSommerWAvsarMet alExtracorporeal Membrane Oxygenation as a Bridge to Lung Transplantation May Not Impact Overall Mortality Risk After Transplantation: Results From a 7-Year Single-Centre Experience. Eur J Cardiothorac Surg (2018) 54:334–40. 10.1093/ejcts/ezy036
12.
HabertheuerARichardsTSerticFMolinaMVallabhajosyulaPSuzukiYet alStratification Risk Analysis in Bridging Patients to Lung Transplant on ECMO: The STABLE Risk Score. Ann Thorac Surg (2020) 110:1175–84. 10.1016/j.athoracsur.2020.03.078
13.
MarkgrafRDeutschinoffGPientkaLScholtenTLorenzC. Performance of the Score Systems Acute Physiology and Chronic Health Evaluation II and III at an Interdisciplinary Intensive Care Unit, After Customization. Crit Care (2001) 5:31–6. 10.1186/cc975
14.
FerreiraFLPeres BotaDBrossAMélotCVincentJ-L. Serial Evaluation of the SOFA Score to Predict Outcome in Critically Ill Patients. JAMA (2001) 286:1754–8. 10.1001/jama.286.14.1754
15.
SakrYKraussCAmaralACKBRea-NetoASpechtMReinhartKet alComparison of the Performance of SAPS II, SAPS 3, Apache II, and Their Customized Prognostic Models in a Surgical Intensive Care Unit. Br J Anaesth (2008) 101:798–803. 10.1093/bja/aen291
16.
HarrellFERobertMCaliffRMLeeKLRosatiRA. Evaluating the Yield of Medical Tests. JAMA (1982) 247:2543–6. 10.1001/jama.1982.03320430047030
17.
R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing (2022). Available from: https://www.R-project.org/ (Accessed January 30, 2022).
18.
HarrellFEJr. RMS: Regression Modelling Strategies. R Package Version 6.2-0 (2021). Available from: https://CRAN.R-project.org/packege=rms (Accessed January 30, 2022).
19.
ThieleCHirschfeldG. Cutpointr: Improved Estimation and Validation of Optimal Cutpoints in R. J Stat Softw (2021) 11:127. 10.18637/jss.v098.i11
20.
MaticsTJSanchez-PintoN. Adaptation and Validation of a Pediatric Sequential Organ Failure Assessment Score and Evaluation of the Sepsis-3 Definitions in Critically Ill Children. JAMA Pediatr (2017) 171:e172352. 10.1001/jamapediatrics.2017.2352
21.
KrasseltMBaerwaldCPetrosSSeifertO. Sepsis Mortality Is High in Patients With Connective Tissue Diseases Admitted to the Intensive Care Unit (ICU). J Intensive Care Med (2022) 37:401–7. 10.1177/0885066621996257
22.
RaithEPUdyAABaileyMMcGloughlinSMacIsaacCBellomoRet alPrognostic Accuracy of the SOFA Score, SIRS Criteria, and qSOFA Score for In-Hospital Mortality Among Adults With Suspected Infection Admitted to the Intensive Care Unit. JAMA (2017) 317:290–300. 10.1001/jama.2016.20328
23.
De OliveiraVMBraunerJSFilhoERSusinRGADraghettiVBolzanSTet alIs SAPS 3 Better Than Apache II at Predicting Mortality in Critically Ill Transplant Patients?Clinics (2013) 68:153–8. 10.6061/clinics/2013(02)oa06
24.
LinCYTsaiFCTianYJenqCCChenYCFangJTet alEvaluation of Outcome Scoring Systems for Patients on Extracorporeal Membrane Oxygenation. Ann Thorac Surg (2007) 84:1256–62. 10.1016/j.athoracsur.2007.05.045
25.
LaimoudMAlanziM. The Validity of SOFA Score to Predict Mortality in Adult Patients With Cardiogenic Shock on Venoarterial Extracorporeal Membrane Oxygenation. Crit Care Res Pract (2020) 2020:3129864. 10.1155/2020/3129864
26.
MullerGFlecherELebretonGLuytCETrouilletJLBréchotNet alThe ENCOURAGE Mortality Risk Score and Analysis of Long-Term Outcomes After VA ECMO for Acute Myocardial Infarction With Cardiogenic Shock. Intensive Care Med (2016) 42:370–8. 10.1007/s00134-016-4223-9
27.
SchmidMBurrellARobertsLBaileyMSheldrakeJRycusPTet alPredicting Survival After ECMO for Refractory Cardiogenic Shock: The Survival After Veno-Arterial ECMO (SAVE)-Score. Eur Heart J (2015) 36:2246–56. 10.1093/eurheartj/ehv194
28.
WangLYangFWangXXieHFanEOginoMet alPredicting Mortality in Patients Undergoing VA-ECMO After Coronary Artery Bypass Grafting: The REMEMBER Score. Crit Care (2019) 23:11. 10.1186/s13054-019-2307-y
29.
SchmidMBaileyMSheldrakeJHodgsonCAubronCRycusPTet alPredicting Survival After Extracorporeal Membrane Oxygenation or Severe Acute Respiratory Failure. Am J Respir Crit Care (2014) 189:1374–89. 10.1164/rccm.201311-2023OC
30.
QiXDongYLinXXinW. Value of Neutrophil to Lymphocyte Ratio, Platelet to Lymphocyte Ratio, and Red Blood Cell Distribution Width in Evaluating the Prognosis of Children With Severe Pneumonia. Evid Based Complement Alternat Med (2021) 24:2021–8. 10.1155/2021/1818469
Summary
Keywords
outcomes, lung transplantation, bridge to transplant, SOFA, extracorporeal life support
Citation
Faccioli E, Lorenzoni G, Schneiter D, Dell’Amore A, Hillinger S, Schiavon M, Caviezel C, Gregori D, Rea F, Opitz I and Inci I (2023) Validated Prognostic Scores to Predict Outcomes in ECLS-Bridged Patients to Lung Transplantation. Transpl Int 36:11609. doi: 10.3389/ti.2023.11609
Received
24 May 2023
Accepted
16 October 2023
Published
30 October 2023
Volume
36 - 2023
Updates
Copyright
© 2023 Faccioli, Lorenzoni, Schneiter, Dell’Amore, Hillinger, Schiavon, Caviezel, Gregori, Rea, Opitz and Inci.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eleonora Faccioli, eleonora.faccioli@unipd.it
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.