Abstract
Health-related quality of life (HRQOL) improves after kidney transplantation (KT) but declines over time. Studies on the effect of early postoperative basal insulin therapy on HRQOL after KT, especially KTRs at high risk of developing post-transplant diabetes mellitus (PTDM) are missing. Data from a randomized controlled trial on 148 non-diabetic KTRs were analyzed. HRQOL using the KDQOL-SF™ was compared in KTRs who either received early postoperative basal insulin therapy or standard-of-care and in KTRs at risk of developing PTDM. Determinants of HRQOL outcomes were investigated using multivariable linear regression analysis. In total, 148 patients completed the KDQOL-SF at baseline. Standard-of-care or early basal insulin therapy after KT did not influence HRQOL. Overall, KT improved the mental (MCS) and physical component summary (PCS) scores at 6-month after KT, which remained stable during further follow-up visits. However, patients at high-risk for PTDM had significantly greater impairment in the PCS score (baseline, 24 months) without differences in MCS scores. In the multivariable regression analysis, allograft function and hemoglobin levels were associated with decreased MCS and PCS scores, respectively. A limitation of the study is the fact that only around 50% of the ITP-NODAT study patients participated in the HRQOL evaluation. Still, our data clearly show that early basal insulin therapy does not affect HRQOL after KT but is negatively influenced by classical clinical factors and PTDM-risk at 24 months after KT. The latter might be influenced by older age.
Introduction
Chronic kidney disease (CKD) has a major impact on both physical and mental health, especially in patients with advanced CKD including dialysis dependency [1–4]. Both reduced self-reported and objective physical function as well as mental health are associated with increased mortality rates in this patient population [5]. Kidney transplantation (KT) is considered the optimal and most cost-effective treatment in patients with advanced CKD with improved survival rates and clear benefits on quality of life (QOL) measures [6, 7].
Although clinically relevant improvements are substantive, a proportion of KTRs experience poor health-related quality of life (HRQOL) despite ongoing satisfactory allograft function [8, 9]. In addition, recent evidence suggests a decline in patients’ HRQOL in the long-term after KT [10]. Some clinical and psychological factors occurring in a substantial proportion of KTRs such as airflow limitation [11], gastrointestinal symptoms [12], side effects of immunosuppressive drugs [13], or anxiety [9] are well-documented to negatively affect HRQOL.
Post-transplant diabetes mellitus (PTDM) is a frequent complication associated with mortality in individuals after KT [14–16]. While the impact of PTDM on graft loss is debated [17], clear associations on cardiovascular (CV) outcomes, especially in patients with modifiable CV risk factors such as obesity are well known [18–21]. The appropriate management of PTDM remains challenging and has previously been reviewed [22, 23]. Neutral Protamin Hagedorn (NPH) insulin and insulin analogs to treat hyperglycemia are commonly used in the early post-transplant period, however, these require intensive blood glucose monitoring and patients’ adherence to avoid therapy-associated adverse events [24, 25].
According to the American Diabetes Association (ADA), HRQOL is a key measure, which should be integrated into the care of patients with diabetes mellitus (DM) in order to improve management and clinical outcomes [26]. Although the occurrence and challenges on treatment of PTDM in KTRs are well recognized, little is known about the impact of this complication on HRQOL of patients after KT. In line, many uncertainties on interventions improving HRQOL of KTRs remain unresolved. Thus, assessing modifiable risk factors and interventions to improve general health status and prevent decline in HRQOL are highly desired.
In this study, we aimed to investigate whether the application of early postoperative basal insulin therapy for the prevention of PTDM might affect HRQOL compared with standard care in KTR using long-term, protocoled HRQOL data obtained in the Insulin Therapy for the Prevention of New Onset Diabetes after Transplantation (ITP-NODAT) study [27]. Moreover, we evaluated the contributors of HRQOL in individuals at high-risk for developing PTDM after KT. Since certain KTRs might benefit from tailored basal insulin therapy, such data are important to allow for more individualized recommendations regarding PTDM prophylaxis strategies after KT.
Methods
Study Design
The detailed description of the original study protocol (ClinicalTrials.gov registration: NCT03507829) and the primary results have been published previously [27]. Briefly, the ITP-NODAT study was an investigator initiated, open label, prospective, randomized, multi-center clinical trial with an unblinded end-point evaluation to test the efficacy of early postoperative basal insulin therapy for the prevention of PTDM in KTRs. Four clinical transplant centers (Medical University of Vienna, Austria; Medical University of Graz, Austria; Hospital del Mar Barcelona, Spain; Charité Universitätsmedizin Berlin, Germany) participated in the study. Patients were randomized in a 1:1 ratio in each participating center prior to transplantation and stratified by first versus repeated kidney transplant. The 24-month follow-up was finalized in May 2020 and the primary results were published in 2021 [27].
Participants and Interventions
Detailed patient eligibility and the study interventions have been described previously [27]. In brief, n = 263 non-diabetic KTRs receiving standard immunosuppressive therapy (tacrolimus, mycophenolate, and steroids) were included in the study. After randomization, patients were divided into standard of care control and treatment groups. In the standard of care control group, once daily fasting plasma glucose monitoring was performed, and antihyperglycemic treatment was initiated according to the physician`s decision. In contrast, KTRs in the treatment group underwent regular capillary blood glucose monitoring (4-times daily) and received basal insulin therapy with intermediate acting (NPH) insulin (human insulin isophane, Humulin N [Eli Lilly]) combined with short-acting insulin (insulin lispro, Humalog [Eli Lilly]), if the afternoon (pre-supper) glucose values exceeded 140 mg/dL (7.8 mmol/L). Pre-specified dose adjustment schemes for insulin titration and application of antihyperglycemic medication for both study groups, as well as predefined schemes for immunosuppression, were applied in each participating center as described previously [27]. Predefined trial visits were performed at 3, 6, 12, and 24 months after KT.
Study Definitions
PTDM was defined as 2 h post oral glucose tolerance test ≥200 mg/dL (11.1 mmol/L) or hemoglobin A1c (HbA1c) ≥6.5% (48 mmol/mol) according to the ADA guideline criteria [
26]. Patients at high risk of developing PTDM after KT were defined using age, serum lipid levels, body mass index (BMI), family history of DM, and the history of polycystic kidney disease (PCKD) based on previously published literature data [
27]. Accordingly, patients fulfilling at least one of the following criteria at the time of transplantation were defined as part of the high-risk population:
1. Age ≥ 60 years
2. Age 45–59 plus one of the following criteria: triglycerides ≥200 mg/dL or triglycerides 150–200 mg/dL and BMI>27 or triglycerides 150–200 mg/dL and high-density lipoprotein (HDL) < 40 mg/dL (men) or triglycerides 150–200 mg/dL and HDL<50 mg/dL (women)
3. Family History of DM
4. Polycystic kidney disease (PCKD)
Assessment
HRQOL was evaluated using the kidney disease quality of life short form (KDQOL-SF™) [28]. The KDQOL-SF is a multidimensional patient reported outcome measurement. It is available in different languages including Spanish and German. The questionnaire was developed to assess the health-related disease burden of individuals with CKD and on dialysis with excellent psychometric properties (Cronbach’s alpha = 0.61–0.90). The multiple scales of the questionnaire include 43 disease-targeted items focusing on symptoms, effects of kidney disease, burden of kidney disease, work status, cognitive function, quality of social interaction, sexual function, sleep, social support, dialysis staff encouragement, and patient satisfaction. Additionally, the questionnaire includes the short form 36-health survey (SF-36™). The scoring of each scale ranges from zero to 100 and can be calculated if at least 50% of each scales’ items were completed by the participant. Higher scores reflect better self-reported QOL.
The KDQOL-SF measurements were self-reported and assessed at baseline, and during the trial visits at 6, 12, and 24-month follow-up. All assessments were carried out in parallel to collection of trial data in the parent trial at the respective study site.
Outcomes
In the original study, pre-specified primary and secondary endpoints were defined at month 12 and 24 post-transplant, respectively and were published previously [27]. The HRQOL was defined as secondary endpoint in the original study as the SF-36 mental component summary (MCS) and SF-36 physical component summary (PCS) scores derived from the KDQOL-SF at 6, 12, and 24 months after KT [27]. Exploratory outcomes include change in the KDQOL-SF subscales in the predefined study groups in the parent trial and in the low- and high-risk groups for PTDM at the same follow-up time points.
Statistical Analysis
Patient characteristics were reported as absolute and relative frequencies for categorical data and for numerical data as means and standard deviation (SD) if normally distributed or medians (range) otherwise. Comparison between groups were done using t-tests, Mann-Whitney U tests, Chi-square, Wilcoxon signed-rank tests or Fisher’s exact tests as appropriate. Univariable and multivariable linear regression analyses were performed for physical and mental component scores at 6, 12, and 24 months after kidney transplantation. Treatment group, baseline PCS and MCS scores, risk group (for PTDM), renal function (eGFR), hemoglobin, inflammation (CRP), and glycemic control (HbA1c, OGTT) were included in the univariable analysis as covariates. All variables with a p-value <0.2 in the univariable analysis were included in the multivariable analysis. Beta coefficients were presented along with their 95% confidence interval (CI). A p-value of 0.05 or less was considered statistically significant. All statistical analyses were conducted using R version 4.2.
Results
Patient Characteristics in the Study Groups Stratified by Standard-of-Care and Treatment
The total study sample involved 73 and 75 participants (N = 148 in total, 56.3% overall response rate) in the standard of care and treatment groups, respectively. The low response rate resulted from patient incompliance, if they were unable to fill out the questionnaires or incomplete questionnaires. Baseline characteristics of the participants were balanced between the groups randomized to standard of care control or treatment groups. Participants in the treatment group had a higher proportion of polycystic kidney disease (PCKD) as primary kidney disease and tended to have a higher rate of DM in the family history, and had lower body weight as well as BMI. Patients’ characteristics at baseline in the whole cohort and study groups are summarized in Table 1.
TABLE 1
| Characteristic | Overall N = 148 | Control N = 73 | Treatment N = 75 | p-value |
|---|---|---|---|---|
| Female | 55 (37) | 25 (34) | 30 (40) | 0.5 |
| Age (years) | 49.9 (13.9) | 50.4 (14.5) | 49.4 (13.3) | 0.6 |
| Height (cm) | 169 (10) | 169 (10) | 170 (10) | 0.6 |
| Weight (kg) | 71.2 (63.5, 82.0) | 76.4 (66.0, 83.5) | 68.4 (63.0, 77.0) | 0.017 |
| BMI (kg/m2) | 25.5 (4.6) | 26.5 (5.2) | 24.6 (3.7) | 0.019 |
| Primary kidney disease | 0.054 | |||
| Glomerular | 59 (56) | 33 (66) | 26 (47) | |
| Vascular | 11 (10) | 5 (10) | 6 (11) | |
| Tubulointerstitial | 9 (9) | 5 (10) | 4 (7) | |
| PCKD | 23 (22) | 5 (10) | 18 (33) | |
| Other | 3 (3) | 2 (4) | 1 (2) | |
| Number of previous kidney allografts | 0.3 | |||
| 1 | 126 (85) | 60 (82) | 66 (88) | |
| 2 | 20 (14) | 12 (16) | 8 (11) | |
| 3 | 1 (1) | 0 (0) | 1 (1) | |
| 4 | 1 (1) | 1 (1) | 0 (0) | |
| Dialysis prior to KT | 134 (91) | 63 (86) | 71 (95) | 0.082 |
| Comorbidities | ||||
| Cardiovascular | 60 (41) | 31 (42) | 29 (39) | 0.6 |
| Respiratory | 11 (7) | 7 (10) | 4 (5) | 0.3 |
| Urinary | 14 (10) | 8 (11) | 6 (8) | 0.5 |
| Endocrinological | 18 (12) | 7 (10) | 11 (15) | 0.3 |
| Neurological | 3 (2) | 1 (1) | 2 (3) | >0.9 |
| Psychiatrical | 8 (5) | 1 (1) | 7 (9) | 0.063 |
| Other | 6 (4) | 4 (6) | 2 (3) | 0.4 |
| Laboratory results | ||||
| Hemoglobin (g/dL) | 11.9 (1.5) | 11.6 (1.5) | 12.1 (1.5) | 0.12 |
| Creatinine (mg/dL) | 7.4 (5.8, 9.5) | 7.1 (5.4, 9.2) | 7.7 (6.1, 9.5) | 0.2 |
| eGFR (ml/min/1.73m2) | 7.2 (5.3, 9.3) | 7.6 (5.6, 10.1) | 6.6 (4.8, 8.8) | 0.3 |
| CRP (mg/dL) | 2.0 (0.6, 6.7) | 1.6 (0.6, 5.8) | 2.0 (0.8, 6.8) | 0.5 |
| HbA1c (%) | 5.2 (4.8, 5.4) | 5.3 (4.8, 5.5) | 5.1 (4.9, 5.3) | 0.4 |
Baseline patient characteristics in the standard of care and treatment groups.
Statistically significant p-values in the analysis appear in bold (p < 0.05). Continuous variables are expressed as mean (SD) or median (minimum and maximum). Categorical variables are n (%).
Abbreviations: BMI, body mass index; CRP, C-Reactive Protein; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; KT, kidney transplantation; PCKD, polycystic kidney disease.
HRQOL Measures in the Study Groups Stratified by Standard of Care and Treatment
PCS and MCS scores were calculated from available and valid responses for 85 (57%) participants at baseline, 91 (61%) at 6-month, 67 (45%) at 12-month and 61 (41%) at 24-month follow-up. Missing data resulted mainly from patients lost to follow-up or incomplete questionnaires. In both, the standard of care control and the treatment groups, a significant increase in MCS [control: 54.9 (26.0, 63.2) vs. 49.6 (25.6, 67.7), p = 0.046 and treatment: 50.9 (24.3, 65.5) vs. 46.9 (24.6, 69.7), p = 0.004, respectively] and PCS [control: 51.6 (20.7, 62.0) vs. 42.5 (25.1, 60.7), p = 0.001 and treatment: 45.0 (23.0, 59.9) vs. 41.7 (23.2, 57.6) p = 0.015, respectively] scores were observed at 6-month as compared to baseline, which remained stable during the further follow-up visits (Figure 1).
FIGURE 1
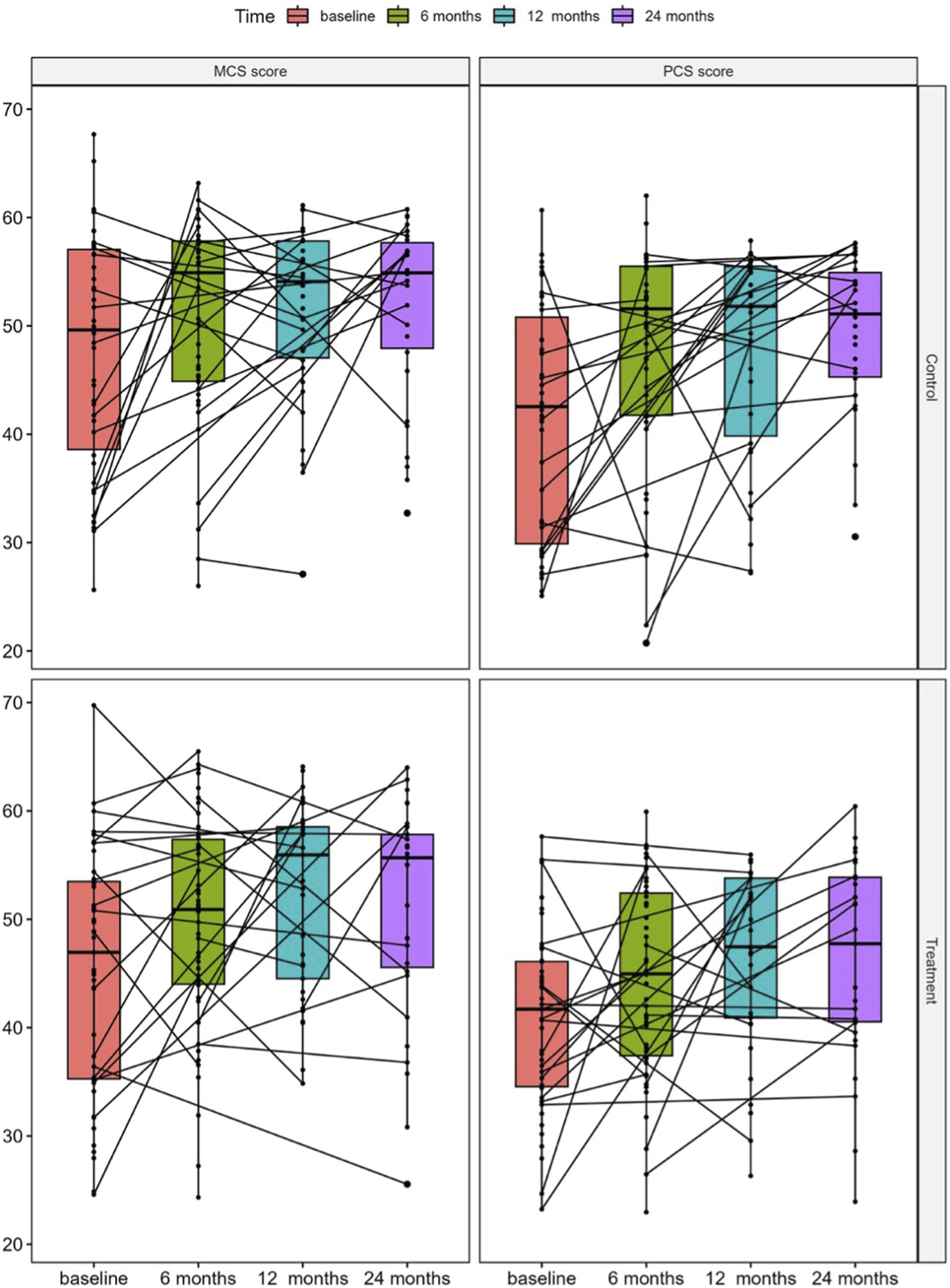
Median changes in KDQOL-SF SF-36 physical component summary (PCS) and mental component summary (MCS) scores in the study groups stratified by standard of care control and treatment at baseline and at 6, 12, and 24 months after KT.
Baseline Patient Characteristics in the Low-, and High-Risk Groups
Within the study sample, 65% of the patients were defined as high-risk for developing PTDM. Detailed characteristics of the resampled study sample concerning the risk factors are provided in Table 2. The high-risk group included predominantly males (60%, n = 50), had a mean age of 56.4 years (SD: 12.5 years), 41% had glomerular disease as a primary kidney disease and had higher baseline HbA1c. Baseline patient characteristics were similar between high- and low-risk groups (Table 2).
TABLE 2
| Characteristic | High-risk N = 84 | Low-risk N = 45 | p-value |
|---|---|---|---|
| Female | 34 (40) | 16 (36) | 0.6 |
| Age (years) | 56.4 (12.5) | 39.7 (10.4) | <0.001 |
| Height (cm) | 168.4 (10.3) | 170.9 (10.4) | 0.2 |
| Weight (kg) | 75.0 (65.3, 84.7) | 70.0 (62.0, 79.0) | 0.062 |
| BMI (kg/m2) | 26.6 (4.8) | 23.8 (3.8) | 0.002 |
| Primary kidney disease | <0.001 | ||
| Glomerular | 26 (41) | 22 (71) | |
| Vascular | 7 (11) | 4 (13) | |
| Tubulointerstitial | 5 (8) | 4 (13) | |
| PCKD | 23 (37) | 0 (0) | |
| Other | 2 (3) | 1 (3) | |
| Number of previous kidney allografts | 0.9 | ||
| 1 | 71 (85) | 40 (89) | |
| 2 | 12 (14) | 5 (11) | |
| 3 | 0 (0) | 0 (0) | |
| 4 | 1 (1) | 0 (0) | |
| Dialysis prior to KT | 75 (89) | 40 (89) | >0.9 |
| Comorbidities | |||
| Cardiovascular | 38 (45) | 15 (33) | 0.2 |
| Respiratory | 9 (11) | 2 (4) | 0.3 |
| Urinary | 9 (11) | 4 (9) | >0.9 |
| Endocrinological | 13 (15) | 3 (7) | 0.15 |
| Neurological | 2 (2) | 1 (2) | >0.9 |
| Psychiatrical | 7 (8) | 0 (0) | 0.10 |
| Other | 2 (2) | 3 (7) | 0.3 |
| Laboratory results | |||
| Hemoglobin (g/dL) | 11.7 (1.5) | 12.0 (1.6) | 0.2 |
| Creatinine (mg/dL) | 7.1 (5.6, 9.3) | 7.8 (6.2, 9.7) | 0.3 |
| eGFR (ml/min/1.73m2) | 7.1 (5.1, 9.0) | 7.2 (5.5, 9.3) | 0.6 |
| CRP (mg/dL) | 2.0 (0.6, 10.0) | 1.7 (0.6, 3.4) | 0.3 |
| HbA1c (%) | 5.3 (5.0, 5.5) | 4.8 (4.6, 5.2) | <0.001 |
Baseline patient characteristics in the groups of low- and high-risk for post-transplant diabetes mellitus.
Statistically significant p-values in the analysis appear in bold (p < 0.05). Continuous variables are expressed as mean (SD) or median (minimum and maximum). Categorical variables are n (%).
Abbreviations: BMI, body mass index; CRP, C-Reactive Protein; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; KT, kidney transplantation; PCKD, polycystic kidney disease.
HRQOL Measures in the Groups of High- and Low-Risk for PTDM
Patients in the high-risk group had a significantly greater impairment in the PCS scores at baseline [35.9 (23.2, 60.7) vs. 46.1 (27.1, 57.6), p < 0.001] and 24 months after transplantation [46.5 (23.9, 60.4) vs. 53.9 (35.3, 57.7), p = 0.027] as shown in Figure 2. No significant differences in the MCS scores [baseline: 48.0 (24.6, 69.7) vs. 48.3 (29.1, 60.7), p = 0.591 and 24 months: 55.5 (25.5, 64.0) vs. 54.5 (37.0, 60.7), p = 0.539] were found.
FIGURE 2
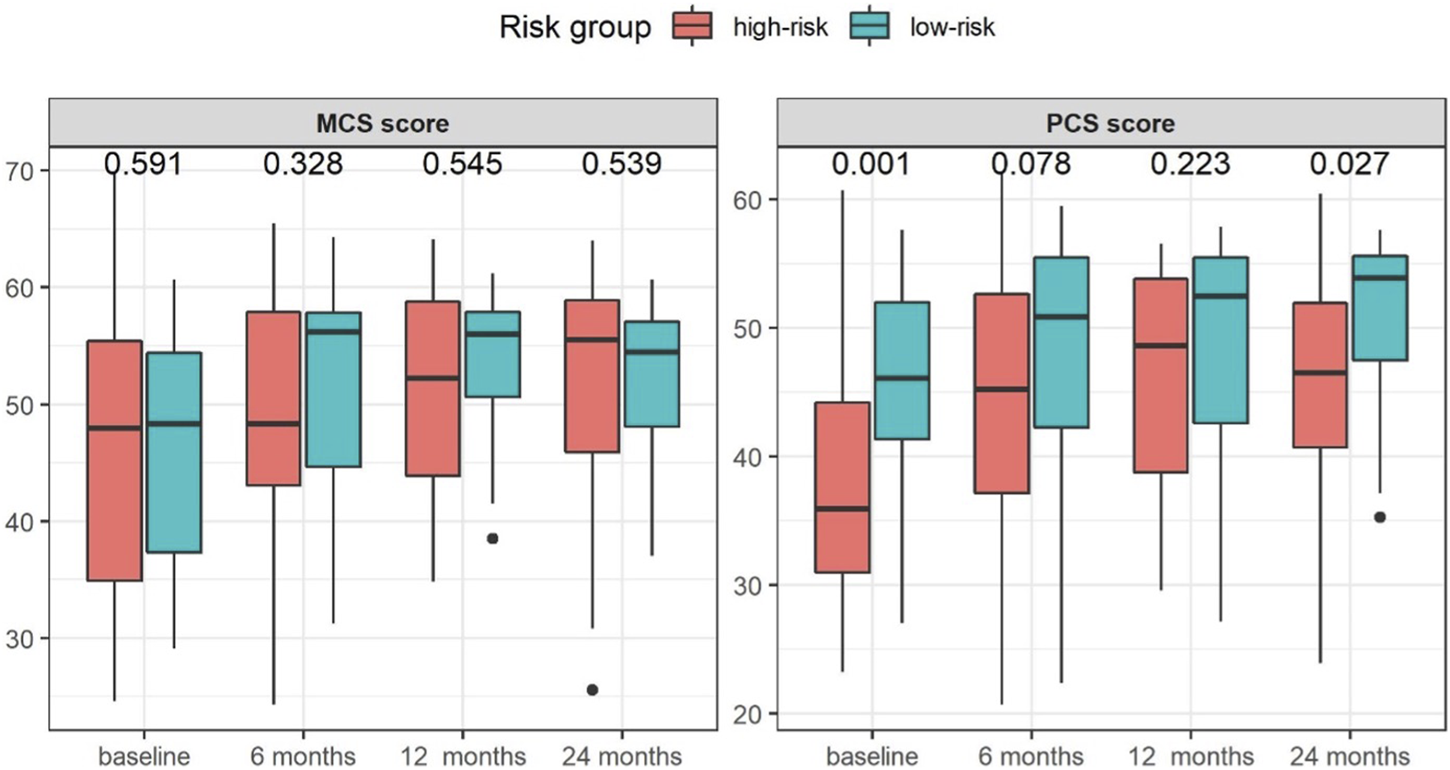
Median changes in KDQOL-SF SF-36 physical component summary (PCS) and mental component summary (MCS) scores in the study groups stratified by risk group for post-transplant diabetes mellitus at baseline and at 6, 12, and 24 months after KT.
The PCS and MCS scores were comparable between high- and low-risk groups at baseline (Figure 3A), only showing a significant difference in the score of physical-role-functioning [0.0 (0.0, 100.0) vs. 50.0 (0.0, 100.0), p = 0.001] at this time-point. At 24 months after transplantation, significant differences in pain [77.5 (20.0, 100.0) vs. 100.0 (32.5, 100.0), p = 0.045], physical-role-functioning [50.0 (0.0, 100.0) vs. 100.0 (0.0, 100.0), p = 0.003], and emotional-role-functioning [100.0 (0.0, 100.0) vs. 100.0 (0.0, 100.0), p = 0.030] between the high-risk and the low-risk groups were found (Figure 3B).
FIGURE 3
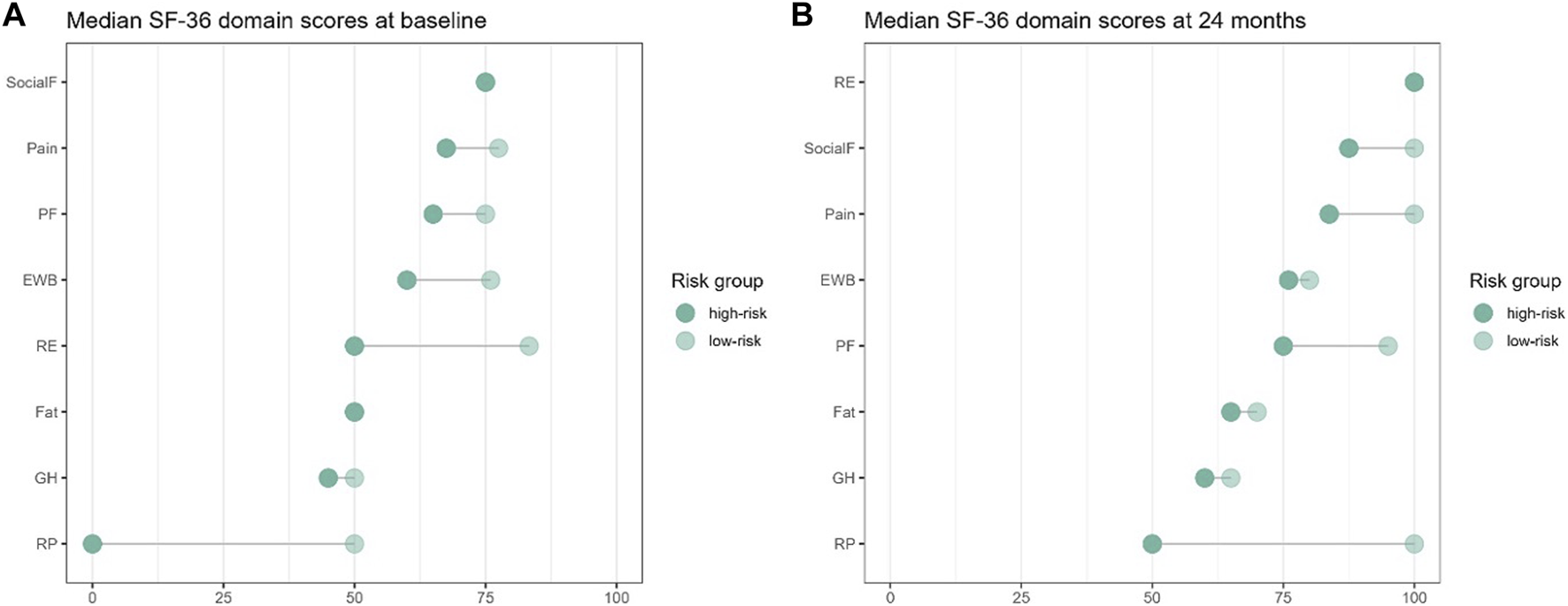
Changes of median KDQOL-SF SF-36 domain scores at (A) baseline (BL) and (B) 24-month for low-risk compared to high-risk for post-transplant diabetes mellitus.
Within the disease specific scores of the KDQOL-SF™, there were no significant differences between the scores of the symptom problem list [84.1 (43.2, 100.0) vs. 95.5 (56.8, 100.0), p = 0.064], effects of kidney disease [87.5 (53.1, 100.0) vs. 87.5 (37.5, 100.0), p = 0.453], burden of kidney disease [81.2 (6.2, 100.0) vs. 84.4 (25.0, 100.0), p = 0.946], work status [50.0 (0.0, 100.0) vs. 100.0 (0.0, 100.0), p = 0.076], cognitive function [86.7 (26.7, 100.0) vs. 93.3 (46.7, 100.0), p = 0.671], quality of social interaction [86.7 (46.7, 100.0) vs. 86.7 (53.3, 100.0), p = 0.586], sexual function [62.5 (0.0, 100.0) vs. 100.0 (12.5, 100.0), p = 0.236], sleep [63.8 (32.5, 100.0) vs. 74.2 (25.0, 92.5), p = 0.110], and overall health [80.0 (30.0, 100.0) vs. 80.0 (50.0, 100.0), p = 0.196] at 24 months.
Confounders of HRQOL Measures
In the univariable regression analysis, early postoperative insulin treatment was not significantly associated with the PCS or MCS scores at any timepoint but being in the low-risk group for developing PTDM was significantly related to a better PCS score at 24 months after transplantation (Beta: 5.1, 95% CI: 0.54–9.6, p = 0.029). Hemoglobin, renal function, CRP, baseline PCS and MCS scores, and OGTT were significantly associated with PCS and MCS scores at various timepoints (Table 3).
TABLE 3
| Physical component summary score | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariable | |||||||||
| N | m6 Beta, (95% CI) | p-value | N | m12 Beta, (95% CI) | p-value | N | m24 Beta, (95% CI) | p-value | |
| Group | 91 | 0.120 | 67 | 0.541 | 61 | 0.136 | |||
| Control | — | — | — | ||||||
| Treatment | −3.2 (−7.2, 0.84) | −1.4 (−5.8, 3.1) | −3.2 (−7.4, 1.0) | ||||||
| Risk group | 81 | 0.128 | 61 | 0.320 | 52 | 0.029 | |||
| High-risk | — | — | — | ||||||
| Low-risk | 3.6 (−1.1, 8.2) | 2.3 (−2.3, 7.0) | 5.1 (0.54, 9.6) | ||||||
| Baseline PCS | 48 | 0.42 (0.13, 0.71) | 0.006 | 43 | 0.24 (−0.04, 0.52) | 0.095 | 40 | 0.35 (0.05, 0.66) | 0.025 |
| HbA1c | 87 | 0.61 (−2.3, 3.5) | 0.677 | 62 | −1.5 (−5.1, 2.0) | 0.384 | 58 | −2.6 (−6.5, 1.4) | 0.194 |
| eGFR | 89 | 0.02 (−0.05, 0.09) | 0.599 | 67 | 0.18 (0.09, 0.28) | <0.001 | 61 | 0.18 (0.09, 0.28) | <0.001 |
| Hemoglobin | 86 | 1.6 (−0.54, 2.7) | 0.004 | 64 | −1.2 (−0.02, 2.4) | 0.046 | 59 | 2.0 (0.72, 3.2) | 0.003 |
| CRP | 85 | −0.1 (−0.18, −0.01) | 0.024 | 67 | −0.29 (−0.82, 0.25) | 0.291 | 61 | −0.26 (−0.54, 0.02) | 0.067 |
| oGTT | 91 | 0.02 (−0.01, 0.05) | 0.232 | 67 | −0.02 (−0.05, 0.02) | 0.314 | 61 | 0.00 (−0.08, 0.08) | 0.973 |
| Mental Component Summary Score | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariable | |||||||||
| N | m6 Beta, (95% CI) | p-value | N | m12 Beta, (95% CI) | p-value | N | m24 Beta, (95% CI) | p-value | |
| Group | 91 | 0.507 | 67 | 0.775 | 61 | 0.797 | |||
| Control | — | — | — | ||||||
| Treatment | −1.4 (−5.4, 2.7) | 0.59 (−3.5, 4.7) | −0.60 (−5.3, 4.1) | ||||||
| Risk group | 81 | 0.170 | 61 | 0.249 | 52 | 0.856 | |||
| High-risk | — | — | — | ||||||
| Low-risk | 3.2 (−1.4, 7.9) | 2.4 (−1.7, 6.6) | 0.45 (−4.5, 5.4) | ||||||
| Baseline MCS | 48 | 0.41 (0.16, 0.67) | 0.002 | 43 | 0.38 (0.16, 0.59) | 0.001 | 40 | 0.18 (−0.08, 0.44) | 0.178 |
| HbA1c | 87 | −0.29 (−3.2, 2.6) | 0.845 | 62 | 2.2 (−0.86, 5.2) | 0.158 | 58 | −0.85 (−4.9, 3.2) | 0.677 |
| eGFR | 89 | −0.03 (−0.04, 0.09) | 0.445 | 67 | 0.08 (−0.02, 0.17) | 0.107 | 61 | 0.12 (0.01, 0.23) | 0.034 |
| Hemoglobin | 86 | 1.2 (0.11, 2.3) | 0.032 | 64 | 0.91 (−0.22, 2.0) | 0.111 | 59 | 0.59 (−0.88, 2.1) | 0.427 |
| CRP | 85 | −0.05 (−0.13, 0.04) | 0.259 | 67 | −0.01 (−0.51, 0.49) | 0.968 | 61 | −0.10 (−0.42, 0.21) | 0.508 |
| oGTT | 91 | −0.03 (−0.07, 0.00) | 0.042 | 67 | 0.00 (−0.03, 0.04) | 0.884 | 61 | −0.01 (−0.10, 0.07) | 0.781 |
Univariable regression analyses of confounders for changes in KDQOL-SF™ physical and mental component scores at 6, 12, and 24 months after kidney transplantation.
Statistically significant p-values in the analysis appear in bold (p < 0.05). Only variables with a p-value <0.2 in the univariable analysis were carried on for multivariable analysis.
Numbers represent the beta coefficients and 95% confidence intervals in each group and variable, while N represents the number of patients with available data.
Abbreviations: BMI, body mass index; CRP, C-Reactive Protein; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; MCS, mental component score; PCKD, polycystic kidney disease; PCS, physical component score.
Neither the PCS nor the MCS scores were associated with early postoperative insulin treatment or risk-profile in our multivariable analysis (Table 4). However, our model showed a significant association of baseline PCS score at 6 months (Beta: 0.40, 95% CI: 0.04–0.76, p = 0.029) renal function at 12 months (Beta: 0.14, 95% CI: 0.02–0.27, p = 0.025), and hemoglobin at 24 months (Beta: 3.3, 95% CI: 1.50–5.10, p < 0.001) after transplantation with PCS score, while baseline MCS score (Beta: 0.32, 95% CI: 0.06–0.59, p = 0.017) and hemoglobin at 6 months (Beta: 2.6, 95% CI: 0.71–4.60, p = 0.009) as well as renal function at 24 months (Beta: 0.19, 95% CI: 0.05–0.32, p = 0.008) after KT were significantly associated with MCS score (Table 4).
TABLE 4
| Physical component summary score | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Multivariable | |||||||||
| N | m6 Beta, (95% CI) | p-value | N | m12 Beta, (95% CI) | p-value | N | m24 Beta, (95% CI) | p-value | |
| Group | 91 | 0.162 | 67 | 61 | 0.272 | ||||
| Control | — | — | — | ||||||
| Treatment | −4.5 (−11.0, 1.9) | −2.6 (−7.4, 2.2) | |||||||
| Risk group | 81 | 0.856 | 61 | 52 | 0.604 | ||||
| High-risk | — | — | — | ||||||
| Low-risk | −0.61 (−7.4, 6.2) | — | — | 1.9 (−5.4, 9.1) | |||||
| Baseline PCS | 48 | 0.40 (0.04, 0.76) | 0.029 | 43 | 0.16 (−0.12, 0.44) | 0.257 | 40 | 0.18 (−0.18, 0.54) | 0.318 |
| HbA1c | 87 | — | 62 | — | — | 58 | −4.0 (−9.0, 0.90) | 0.105 | |
| eGFR | 89 | — | 67 | 0.14 (0.02, 0.27) | 0.025 | 61 | 0.06 (−0.07, 0.20) | 0.360 | |
| Hemoglobin | 86 | 1.6 (−0.55, 3.7) | 0.139 | 64 | 0.31 (−1.2, 1.9) | 0.688 | 59 | 3.3 (1.5, 5.1) | <0.001 |
| CRP | 85 | 0.07 (−0.15–0.29) | 0.521 | 67 | — | — | 61 | 0.49 (−0.02, 1.0) | 0.060 |
| oGTT | 91 | — | 67 | — | — | 61 | — | — | |
| Mental Component Summary Score | |||||||||
| Multivariable | |||||||||
| N | m6 Beta, (95% CI) | p-value | N | m12 Beta, (95% CI) | p-value | N | m24 Beta, (95% CI) | p-value | |
| Group | 91 | — | 67 | — | 61 | — | |||
| Control | — | — | — | ||||||
| Treatment | — | ||||||||
| Risk group | 81 | 0.160 | 61 | — | 52 | — | |||
| High-risk | — | — | — | ||||||
| Low-risk | 4.2 (−1.7, 10) | — | — | ||||||
| Baseline MCS | 48 | 0.32 (0.06, 0.59) | 0.017 | 43 | 0.21 (−0.07, 0.49) | 0.142 | 40 | 0.07 (−0.19, 0.32) | 0.592 |
| HbA1c | 87 | — | — | 62 | 0.41 (−3.6, 4.5) | 0.836 | 58 | — | — |
| eGFR | 89 | — | — | 67 | 0.06 (−0.08, 0.20) | 0.401 | 61 | 0.19 (0.05, 0.32) | 0.008 |
| Hemoglobin | 86 | 2.6 (0.71, 4.6) | 0.009 | 64 | 0.60 (−1.3, 2.5) | 0.518 | 59 | — | — |
| CRP | 85 | — | — | 67 | — | — | 61 | — | — |
| oGTT | 91 | −0.03 (−0.07, 0.01) | 0.138 | 67 | — | — | 61 | — | — |
Multivariable regression analyses of confounders for changes in KDQOL-SF™ physical and mental component scores at 6, 12, and 24 months after kidney transplantation.
Statistically significant p-values in the analysis appear in bold (p < 0.05). Only variables with a p-value <0.2 in the univariable analysis were carried on for multivariable analysis.
Numbers represent the beta coefficients and 95% confidence intervals in each group and variable, while N represents the number of patients with available data.
Abbreviations: BMI, body mass index; CRP, C-Reactive Protein; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; MCS, mental component score; PCKD, polycystic kidney disease; PCS, physical component score.
Discussion
In this analysis of data from a randomized controlled trial [27], the overall HRQOL of the study population remained stable during the follow-up period but increased significantly in the PCS after KT. In addition, KTRs at high risk of developing PTDM had a significantly greater impairment in physical functioning at baseline and 24 months after KT. Most importantly, early postoperative insulin therapy was not associated with worse HRQOL measures, but kidney allograft function and associated anemia were independent predictors of reduced PCS and MCS scores at certain time points during the 2 years follow-up after KT.
The risk of hypoglycemic complications in PTDM patients was found to be comparable to other types of DM [29], and basal insulin treatment can negatively affect HRQOL by inducing symptomatic albeit mild hypoglycemia [30]. In the ITP-NODAT trial [27], the postoperative basal insulin therapy was initiated early with a preventive intention requiring a stringent risk-benefit assessment. Early basal insulin treatment did show significantly higher hypoglycemic events in the treatment group, but mainly within the first 3 months of post-operative treatment. Some patients escalated their insulin dosage without clear indication resulting in seriously low blood sugar levels and suggesting an insecurity with the handling of insulin at the beginning of the treatment. However, with ongoing treatment no further hypoglycemic events were registered [27]. In accordance, the results of our analysis indicate no negative effect of early basal insulin therapy on the HRQOL of the patients. These data are of critical significance since we need a personalized glucose-lowering therapy for different patient cohorts according to their risk profile without negatively affecting HRQOL.
A possible explanation for why early basal insulin therapy does not affect HRQOL in KT recipients despite having similar diabetes and hypoglycemic associated complications may be a stronger positive effect of KT itself [29, 30]. Basal insulin therapy and the accompanied fear of hypoglycemic events is known to negatively affect the HRQOL of non-transplant diabetes patients. While these fears may also appear in KT recipients, the overall positive effects of transplantation including cessation of hemodialysis, increased physical functioning, and decreased effects and burden of kidney disease may outweigh the negative effects of early basal insulin therapy in post-operative KT patients [9].
To further explore the effects of early postoperative insulin therapy on HRQOL, we resampled the study participants according to their risk of developing PTDM using the factors age and clinical predisposition for metabolic dysfunction in addition to laboratory markers. Metabolic dysfunction may be the result of an unfavorable lifestyle or a genetic predisposition including a positive family history of DM. In line, PCKD as a systemic genetic disorder resulting in a progressive growth of cysts not only in the kidneys, but also in the liver, seminal ducts, and/or pancreas are at high-risk to develop PTDM [31, 32]. Nevertheless, metabolic dysfunction and overweight are considered as a distinct pathological entity, the metabolic syndrome, which itself affects the HRQOL of patients [33, 34]. The resulting two groups of high versus low-risk for the development of PTDM showed significant differences in their physical health (SF-36 PCS) 24 months after KT, while there were no differences in mental health (MCS) at any timepoint. Interestingly, at 6 and 12 months after transplantation, both groups showed increased and comparable HRQOL scores, suggesting a valuable benefit of KT during the first year for all patients. Positive changes in HRQOL observed early after KT are particularly driven by increased physical activity, reduced symptom burden or improvements in social functioning, among others [10]. In the long-term course after KT, a significant impairment of physical health, which is associated with a pronounced muscular weakness resulting in a lower physical activity has been—comparable to our results—proven before [9].
The cause of muscular weakness in KTR is multifactorial. Older patients experience geriatric syndromes like sarcopenia, which itself, has a complex pathophysiology including reduced physical activity, systemic inflammation, and neuropathic changes leading to a denervation of muscles [35]. However, in younger patients, the metabolic syndrome and obesity are known to induce a loss of muscular strength relative to their body mass, which is again linked to systemic inflammation and reduced physical activity [35, 36]. Pro-inflammatory cytokines such as tumor-necrosis factor alpha and interleukin 6, are known to stimulate muscle protein degradation and reduce muscle protein synthesis. Pro-inflammatory states have all been described in sarcopenia [37], metabolic syndrome, obesity [38], and PCKD [32], which define the high-risk PTDM group. Accordingly, CRP levels tended to increase in the high-risk group in both the uni- and multivariable model 24 months after KT. The resulting sarcopenia together with the pre-existing metabolic syndrome might potentially explain our findings of reduced physical health 24 months after KT in the high-risk PTDM group. A potential strategy to prevent muscle weakness, sarcopenia, and metabolic risk factors in KT recipients could be controlled physical exercise. Recent randomized controlled trials investigating the effect of a 10–12 weeks training program of either resistance or combined resistance and aerobic exercise compared to no training in post KT showed notable improvements in functional performance, body composition, muscular strength, renal function, fatigue, and HRQOL [39–41]. Promoting physical activity in KT recipients may therefore prevent the observed vanishing effect of KT in high-risk PTDM patients.
The high-risk group also had a greater disease specific symptom burden, pain, and impaired emotional-role-functioning 24 months after KT compared to the low-risk PTDM group, with comparable values between groups at 6 and 12 months, suggesting again a slowly vanishing effect of KT on HRQOL in the high-risk PTDM group. We also found a statistical association of renal function (eGFR) and hemoglobin levels to the HRQOL 24 months after KT independent from early insulin treatment and PTDM-risk in the multivariable analysis. Hemoglobin levels were associated with the physical health domain (PCS score), while renal function was related to the mental health domain (MCS score) of the SF-36. The symptom burden of KTRs influencing HRQOL is multifactorial including decreased renal function, anemia, depressive symptoms, anxiety, and elevated BMI, as well as treatment-specific encompassing side effects and complications of immunosuppressive therapy [42, 43]. Both severely impaired renal function and anemia lead to uremic symptoms, fatigue, and breathlessness [43, 44], while immunosuppressive agents and metabolic syndrome can cause peripheral neuropathy, thereby enhancing pain and reducing mobility [45]. Particularly calcineurin-inhibitors may induce a disabling pain-syndrome in 5%–15% of KTRs within the first year after KT [46]. Psychological symptoms constitute a burden to patients per se but also enhance the perception of physical symptoms in a multidimensional way [47], together potentially creating a vicious cycle amplifying the total symptom burden after KT. The high-risk PTDM group of our study cohort were older and displayed a higher BMI, both associated with more depressive symptoms [33]. Additionally, metabolic dysregulation decreases renal function of the graft in the long-term [48], causing fears of graft rejection and anxiety further enhancing depressive symptoms [49, 50].
Our study has certain limitations including a considerable proportion of missing data within the HRQOL questionnaires, especially towards the end of the study period which might lead to underestimation to detect lower effect sizes. This is mainly explained by patients lost to follow-up or incompliance with completing the questionnaires. Furthermore, this analysis lacks socioeconomic data as well as comparison of different ethnicities since Caucasians were in the majority included in the ITP-NODAT study [27]. Sociodemographic differences may especially affect the definition of metabolic risk profiles limiting our results mainly to Caucasians living in Europe. In addition, the measurement instrument is generic and might miss to capture important diabetes-specific aspects. Nevertheless, the strengths of this analysis are its multicenter randomized design comprising detailed clinical data and the long-term follow-up of the study participants providing unique data on determinants of HRQOL on the long-term after KT.
Taken together, early postoperative insulin therapy, which might be reasonable in selected patient groups, is not compromising the HRQOL. Although KT substantially improves the HRQOL of CKD patients 1 year after transplantation, patients in the high-risk PTDM group experience a significant impairment in HRQOL in the long course after KT. The HRQOL of KTR is significantly dependent on graft function and anemia. Given the complex relationship between manageable risk factors, physical and psychological symptom burden, and nephrological treatment, a multidisciplinary post-transplant care should be considered to meet the multidimensional needs of KTR, especially within high-risk PTDM populations.
Statements
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The study was undertaken with independent external monitoring in accordance with International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use-Good Clinical Practice principles, and the Declaration of Helsinki. Written informed consent was obtained from all patients after approval from the institutional review board at each participating center.
Author contributions
BO, MHu, AB, and KE contributed to the design of the analysis of the original study, interpretation of data, and wrote the first draft of the manuscript. AB performed the statistical analysis. MHe, ES, AK, MK, HH-G, GE, FB, AF, and MJP-S contributed to the data collection and reviewing of the manuscript. MHe, JP, KB, and AR contributed to the design and writing of the protocol of the parent study and reviewed the manuscript. KE is the principal investigator of the ITP-NODAT study, gave final approval of the published version of the manuscript and is the guarantor of this work and as such had full access to all the data in the study. All authors contributed to the article and approved the submitted version.
Funding
The authors declare that the ITP-NODAT study received funding from the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases in the form of grant R01DK092475, issued to University of Michigan, for which the Medical University of Vienna held a subcontract (#3002300292). The ITP-NODAT study received additional support from Astellas Pharma and Eli Lilly in the form of contracts with the Medical University of Vienna. None of the funders were involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1.
Webster AC Nagler EV Morton RL Masson P . Chronic Kidney Disease. Lancet (2017) 389(10075):1238–52. 10.1016/S0140-6736(16)32064-5
2.
Cox KJ Parshall MB Hernandez SHA Parvez SZ Unruh ML . Symptoms Among Patients Receiving In-Center Hemodialysis: A Qualitative Study. Hemodial Int (2017) 21(4):524–33. 10.1111/hdi.12521
3.
Wyld M Morton RL Hayen A Howard K Webster AC . A Systematic Review and Meta-Analysis of Utility-Based Quality of Life in Chronic Kidney Disease Treatments. Plos Med (2012) 9(9):e1001307. 10.1371/journal.pmed.1001307
4.
Legrand K Speyer E Stengel B Frimat L Ngueyon Sime W Massy ZA et al Perceived Health and Quality of Life in Patients With CKD, Including Those With Kidney Failure: Findings From National Surveys in France. Am J Kidney Dis (2020) 75(6):868–78. 10.1053/j.ajkd.2019.08.026
5.
MacKinnon HJ Wilkinson TJ Clarke AL Gould DW O'Sullivan TF Xenophontos S et al The Association of Physical Function and Physical Activity With All-Cause Mortality and Adverse Clinical Outcomes in Nondialysis Chronic Kidney Disease: A Systematic Review. Ther Adv Chronic Dis (2018) 9(11):209–26. 10.1177/2040622318785575
6.
Hariharan S Israni AK Danovitch G . Long-Term Survival After Kidney Transplantation. N Engl J Med (2021) 385(8):729–43. 10.1056/NEJMra2014530
7.
Tonelli M Wiebe N Knoll G Bello A Browne S Jadhav D et al Systematic Review: Kidney Transplantation Compared With Dialysis in Clinically Relevant Outcomes. Am J Transpl (2011) 11(10):2093–109. 10.1111/j.1600-6143.2011.03686.x
8.
Griva K Davenport A Newman SP . Health-Related Quality of Life and Long-Term Survival and Graft Failure in Kidney Transplantation: A 12-Year Follow-Up Study. Transplantation (2013) 95(5):740–9. 10.1097/TP.0b013e31827d9772
9.
Villeneuve C Laroche ML Essig M Merville P Kamar N Coubret A et al Evolution and Determinants of Health-Related Quality-Of-Life in Kidney Transplant Patients Over the First 3 Years After Transplantation. Transplantation (2016) 100(3):640–7. 10.1097/TP.0000000000000846
10.
Wang Y Hemmelder MH Bos WJW Snoep JD de Vries APJ Dekker FW et al Mapping Health-Related Quality of Life After Kidney Transplantation by Group Comparisons: A Systematic Review. Nephrol Dial Transpl (2021) 36(12):2327–39. 10.1093/ndt/gfab232
11.
Knobbe TJ Kremer D Eisenga MF van Londen M Gomes-Neto AW Douwes RM et al Airflow Limitation, Fatigue, and Health-Related Quality of Life in Kidney Transplant Recipients. Clin J Am Soc Nephrol (2021) 16(11):1686–94. 10.2215/CJN.06600521
12.
Chan S Cao C Pascoe EM Johnson DW Shah A Holtmann GA et al Patient-Reported Gastrointestinal Symptoms and the Association With Quality of Life Following Kidney Transplantation. Kidney Int Rep (2021) 6(1):138–45. 10.1016/j.ekir.2020.10.013
13.
Madariaga ML Spencer PJ Shanmugarajah K Crisalli KA Chang DC Markmann JF et al Effect of Tolerance Versus Chronic Immunosuppression Protocols on the Quality of Life of Kidney Transplant Recipients. JCI Insight (2016) 1(8):e87019. 10.1172/jci.insight.87019
14.
Eide IA Halden TA Hartmann A Asberg A Dahle DO Reisaeter AV et al Mortality Risk in Post-Transplantation Diabetes Mellitus Based on Glucose and HbA1c Diagnostic Criteria. Transpl Int (2016) 29(5):568–78. 10.1111/tri.12757
15.
Kasiske BL Snyder JJ Gilbertson D Matas AJ . Diabetes Mellitus After Kidney Transplantation in the United States. Am J Transpl (2003) 3(2):178–85. 10.1034/j.1600-6143.2003.00010.x
16.
Shivaswamy V Boerner B Larsen J . Post-Transplant Diabetes Mellitus: Causes, Treatment, and Impact on Outcomes. Endocr Rev (2016) 37(1):37–61. 10.1210/er.2015-1084
17.
Jenssen T Hartmann A . Post-Transplant Diabetes Mellitus in Patients With Solid Organ Transplants. Nat Rev Endocrinol (2019) 15(3):172–88. 10.1038/s41574-018-0137-7
18.
Cosio FG Kudva Y van der Velde M Larson TS Textor SC Griffin MD et al New Onset Hyperglycemia and Diabetes are Associated With Increased Cardiovascular Risk After Kidney Transplantation. Kidney Int (2005) 67(6):2415–21. 10.1111/j.1523-1755.2005.00349.x
19.
Hjelmesaeth J Hartmann A Leivestad T Holdaas H Sagedal S Olstad M et al The Impact of Early-Diagnosed New-Onset Post-Transplantation Diabetes Mellitus on Survival and Major Cardiac Events. Kidney Int (2006) 69(3):588–95. 10.1038/sj.ki.5000116
20.
Wauters RP Cosio FG Suarez Fernandez ML Kudva Y Shah P Torres VE . Cardiovascular Consequences of New-Onset Hyperglycemia After Kidney Transplantation. Transplantation (2012) 94(4):377–82. 10.1097/TP.0b013e3182584831
21.
Weiner DE Park M Tighiouart H Joseph AA Carpenter MA Goyal N et al Albuminuria and Allograft Failure, Cardiovascular Disease Events, and All-Cause Death in Stable Kidney Transplant Recipients: A Cohort Analysis of the FAVORIT Trial. Am J Kidney Dis (2019) 73(1):51–61. 10.1053/j.ajkd.2018.05.015
22.
Hecking M Sharif A Eller K Jenssen T . Management of Post-Transplant Diabetes: Immunosuppression, Early Prevention, and Novel Antidiabetics. Transpl Int (2021) 34(1):27–48. 10.1111/tri.13783
23.
Montero N Oliveras L Soler MJ Cruzado JM . Management of Post-Transplant Diabetes Mellitus: An Opportunity for Novel Therapeutics. Clin Kidney J (2022) 15(1):5–13. 10.1093/ckj/sfab131
24.
Chakkera HA Knowler WC Devarapalli Y Weil EJ Heilman RL Dueck A et al Relationship Between Inpatient Hyperglycemia and Insulin Treatment After Kidney Transplantation and Future New Onset Diabetes Mellitus. Clin J Am Soc Nephrol (2010) 5(9):1669–75. 10.2215/CJN.09481209
25.
Chakkera HA Weil EJ Castro J Heilman RL Reddy KS Mazur MJ et al Hyperglycemia During the Immediate Period After Kidney Transplantation. Clin J Am Soc Nephrol (2009) 4(4):853–9. 10.2215/CJN.05471008
26.
American Diabetes Association Professional Practice Committee. 1. Improving Care and Promoting Health in Populations: Standards of Medical Care in Diabetes-2022. Diabetes Care (2022) 45(1):S8–S16. 10.2337/dc22-S001
27.
Schwaiger E Krenn S Kurnikowski A Bergfeld L Perez-Saez MJ Frey A et al Early Postoperative Basal Insulin Therapy Versus Standard of Care for the Prevention of Diabetes Mellitus After Kidney Transplantation: A Multicenter Randomized Trial. J Am Soc Nephrol (2021) 32(8):2083–98. 10.1681/ASN.2021010127
28.
Hays RD Kallich JD Mapes DL Coons SJ Carter WB . Development of the Kidney Disease Quality of Life (KDQOL) Instrument. Qual Life Res (1994) 3(5):329–38. 10.1007/BF00451725
29.
Burroughs TE Swindle J Takemoto S Lentine KL Machnicki G Irish WD et al Diabetic Complications Associated With New-Onset Diabetes Mellitus in Renal Transplant Recipients. Transplantation (2007) 83(8):1027–34. 10.1097/01.tp.0000259617.21741.95
30.
Rossi MC Nicolucci A Ozzello A Gentile S Aglialoro A Chiambretti A et al Impact of Severe and Symptomatic Hypoglycemia on Quality of Life and Fear of Hypoglycemia in Type 1 and Type 2 Diabetes. Results of the Hypos-1 Observational Study. Nutr Metab Cardiovasc Dis (2019) 29(7):736–43. 10.1016/j.numecd.2019.04.009
31.
Nowak KL Hopp K . Metabolic Reprogramming in Autosomal Dominant Polycystic Kidney Disease: Evidence and Therapeutic Potential. Clin J Am Soc Nephrol (2020) 15(4):577–84. 10.2215/CJN.13291019
32.
Steele C Nowak K . Obesity, Weight Loss, Lifestyle Interventions, and Autosomal Dominant Polycystic Kidney Disease. Kidney Dial (2022) 2(1):106–22. 10.3390/kidneydial2010013
33.
Limon VM Lee M Gonzalez B Choh AC Czerwinski SA . The Impact of Metabolic Syndrome on Mental Health-Related Quality of Life and Depressive Symptoms. Qual Life Res (2020) 29(8):2063–72. 10.1007/s11136-020-02479-5
34.
Okosun IS Annor F Esuneh F Okoegwale EE . Metabolic Syndrome and Impaired Health-Related Quality of Life and in Non-Hispanic White, Non-Hispanic Blacks and Mexican-American Adults. Diabetes Metab Syndr (2013) 7(3):154–60. 10.1016/j.dsx.2013.06.007
35.
Tomlinson DJ Erskine RM Morse CI Winwood K Onambele-Pearson G . The Impact of Obesity on Skeletal Muscle Strength and Structure Through Adolescence to Old Age. Biogerontology (2016) 17(3):467–83. 10.1007/s10522-015-9626-4
36.
Stenholm S Alley D Bandinelli S Griswold ME Koskinen S Rantanen T et al The Effect of Obesity Combined With Low Muscle Strength on Decline in Mobility in Older Persons: Results From the InCHIANTI Study. Int J Obes (Lond) (2009) 33(6):635–44. 10.1038/ijo.2009.62
37.
Bano G Trevisan C Carraro S Solmi M Luchini C Stubbs B et al Inflammation and Sarcopenia: A Systematic Review and Meta-Analysis. Maturitas (2017) 96:10–5. 10.1016/j.maturitas.2016.11.006
38.
Reddy P Lent-Schochet D Ramakrishnan N McLaughlin M Jialal I . Metabolic Syndrome Is an Inflammatory Disorder: A Conspiracy Between Adipose Tissue and Phagocytes. Clin Chim Acta (2019) 496:35–44. 10.1016/j.cca.2019.06.019
39.
Hernandez Sanchez S Carrero JJ Morales JS Ruiz JR . Effects of a Resistance Training Program in Kidney Transplant Recipients: A Randomized Controlled Trial. Scand J Med Sci Sports (2021) 31(2):473–9. 10.1111/sms.13853
40.
Lima PS de Campos AS de Faria Neto O Ferreira TCA Amorim CEN Stone WJ et al Effects of Combined Resistance Plus Aerobic Training on Body Composition, Muscle Strength, Aerobic Capacity, and Renal Function in Kidney Transplantation Subjects. J Strength Cond Res (2021) 35(11):3243–50. 10.1519/JSC.0000000000003274
41.
Senthil Kumar TG Soundararajan P Maiya AG Ravi A . Effects of Graded Exercise Training on Functional Capacity, Muscle Strength, and Fatigue After Renal Transplantation: A Randomized Controlled Trial. Saudi J Kidney Dis Transpl (2020) 31(1):100–8. 10.4103/1319-2442.279929
42.
Jhamb M Abdel-Kader K Yabes J Wang Y Weisbord SD Unruh M et al Comparison of Fatigue, Pain, and Depression in Patients With Advanced Kidney Disease and Cancer-Symptom Burden and Clusters. J Pain Symptom Manage (2019) 57(3):566–75.e3. 10.1016/j.jpainsymman.2018.12.006
43.
Dano S Pokarowski M Liao B Tang E Ekundayo O Li V et al Evaluating Symptom Burden in Kidney Transplant Recipients: Validation of the Revised Edmonton Symptom Assessment System for Kidney Transplant Recipients - A Single-Center, Cross-Sectional Study. Transpl Int (2020) 33(4):423–36. 10.1111/tri.13572
44.
Afshar M Rebollo-Mesa I Murphy E Murtagh FE Mamode N . Symptom Burden and Associated Factors in Renal Transplant Patients in the U.K. J Pain Symptom Manage (2012) 44(2):229–38. 10.1016/j.jpainsymman.2011.08.005
45.
Mour G Wu C . Neurologic Complications After Kidney Transplantation. Semin Nephrol (2015) 35(4):323–34. 10.1016/j.semnephrol.2015.06.004
46.
Grotz WH Breitenfeldt MK Braune SW Allmann KH Krause TM Rump JA et al Calcineurin-Inhibitor Induced Pain Syndrome (CIPS): A Severe Disabling Complication After Organ Transplantation. Transpl Int (2001) 14(1):16–23. 10.1007/s001470000285
47.
Trivedi MH . The Link Between Depression and Physical Symptoms. Prim Care Companion J Clin Psychiatry (2004) 6(1):12–6.
48.
Anagnostis P Paschou SA Spartalis E Sarno G De Rosa P Muscogiuri G . Metabolic Complications and Kidney Transplantation: Focus on Glycaemia and Dyslipidaemia. Curr Vasc Pharmacol (2020) 18(3):273–81. 10.2174/1570161117666190619143005
49.
Baines LS Joseph JT Jindal RM . Emotional Issues After Kidney Transplantation: A Prospective Psychotherapeutic Study. Clin Transpl (2002) 16(6):455–60. 10.1034/j.1399-0012.2002.02080.x
50.
Huang Y Tilea A Gillespie B Shahinian V Banerjee T Grubbs V et al Understanding Trends in Kidney Function 1 Year After Kidney Transplant in the United States. J Am Soc Nephrol (2017) 28(8):2498–510. 10.1681/ASN.2016050543
Summary
Keywords
kidney transplantation, HRQOL, insulin, PTDM, clinical study
Citation
Odler B, Huemer M, Schwaiger E, Borenich A, Kurnikowski A, Krall M, Hafner-Giessauf H, Eleftheriadis G, Bachmann F, Faura A, José Pérez-Sáez M, Pascual J, Budde K, Rosenkranz AR, Hecking M and Eller K (2023) Influence of Early Postoperative Basal Insulin Treatment and Post-Transplant Diabetes Mellitus Risk on Health-Related Quality of Life in Kidney Transplant Recipients—An Analysis of Data From a Randomized Controlled Trial. Transpl Int 36:11370. doi: 10.3389/ti.2023.11370
Received
18 March 2023
Accepted
17 July 2023
Published
03 August 2023
Volume
36 - 2023
Updates
Copyright
© 2023 Odler, Huemer, Schwaiger, Borenich, Kurnikowski, Krall, Hafner-Giessauf, Eleftheriadis, Bachmann, Faura, José Pérez-Sáez, Pascual, Budde, Rosenkranz, Hecking and Eller.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kathrin Eller, kathrin.eller@medunigraz.at
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.