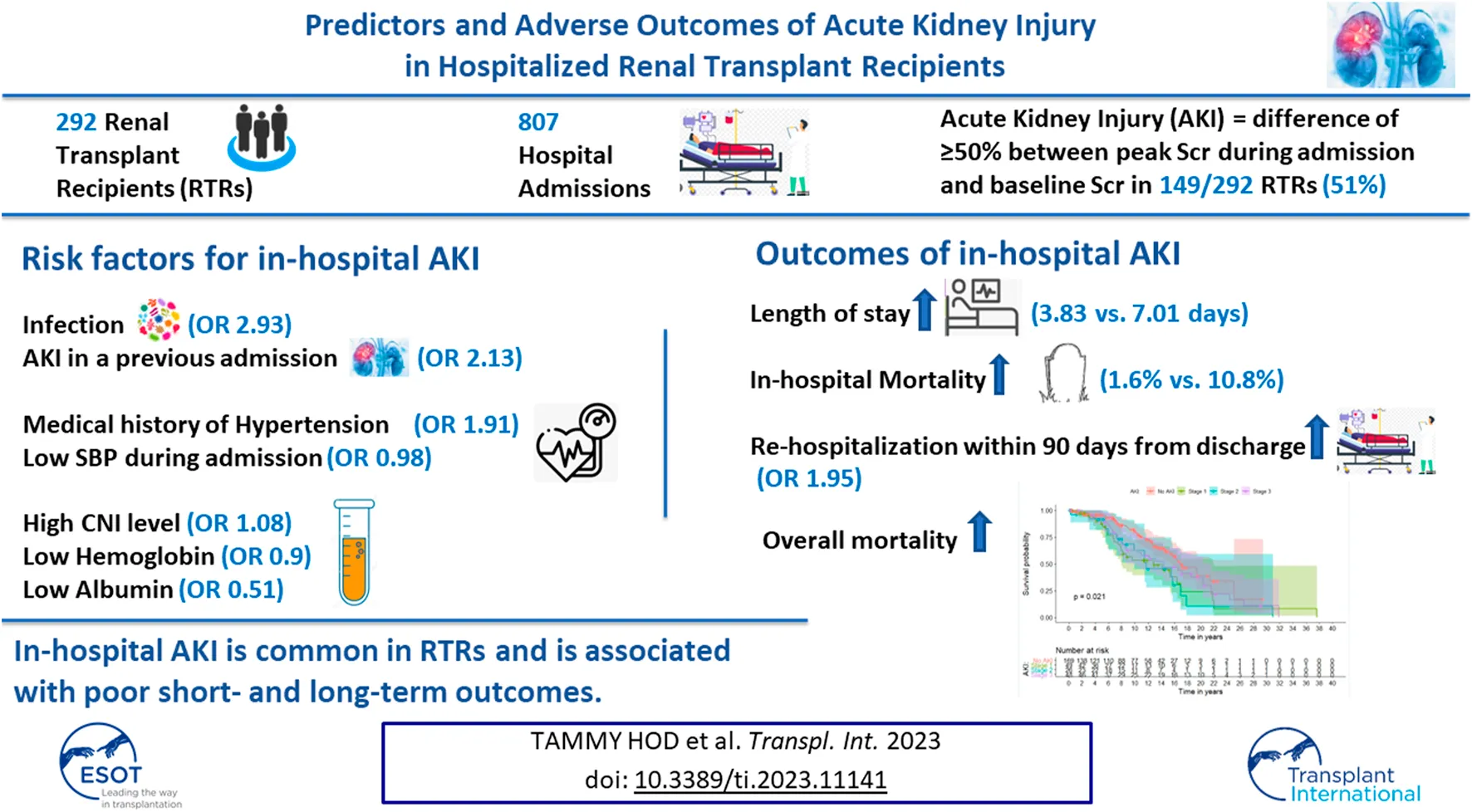
Abstract
Data about in-hospital AKI in RTRs is lacking. We conducted a retrospective study of 292 RTRs, with 807 hospital admissions, to reveal predictors and outcomes of AKI during admission. In-hospital AKI developed in 149 patients (51%). AKI in a previous admission was associated with a more than twofold increased risk of AKI in subsequent admissions (OR 2.13, p < 0.001). Other major significant predictors for in-hospital AKI included an infection as the major admission diagnosis (OR 2.93, p = 0.015), a medical history of hypertension (OR 1.91, p = 0.027), minimum systolic blood pressure (OR 0.98, p = 0.002), maximum tacrolimus trough level (OR 1.08, p = 0.005), hemoglobin level (OR 0.9, p = 0.016) and albumin level (OR 0.51, p = 0.025) during admission. Compared to admissions with no AKI, admissions with AKI were associated with longer length of stay (median time of 3.83 vs. 7.01 days, p < 0.001). In-hospital AKI was associated with higher rates of mortality during admission, almost doubled odds for rehospitalization within 90 days from discharge and increased the risk of overall mortality in multivariable mixed effect models. In-hospital AKI is common and is associated with poor short- and long-term outcomes. Strategies to prevent AKI during admission in RTRs should be implemented to reduce re-admission rates and improve patient survival.
Introduction
The prevalence of chronic kidney disease is increasing, accounting for more than 10% of hospital admissions in the adult population. The parallel increase in the rates of in-hospital acute kidney injury (AKI) may reach as much as 50% of intensive care unit (ICU) admissions (1, 2). The consequences of AKI during hospitalization are dismal (3, 4): Even modest changes in serum creatinine (Scr) (an increase >0.5 mg/dL) have been associated with a 6.5-fold increase in the odds of death and a 3.5-day increase in the length of stay (LOS) (5). Small changes in Scr have been also associated with increased mortality and prolonged hospitalizations in elderly patients admitted with congestive heart failure (6).
With the aim of preventing this serious complication, different studies have sought to establish predictors for AKI during hospitalization, both in the general population (7–11) and particularly for renal transplant recipients (RTRs). Unfortunately, however, information on predictors for AKI during hospitalization of RTRs is still lacking, although 11% of RTRs develop in-hospital AKI during the first three post-transplant years, which is associated with transplant failure and death (12). For RTRs, studies to date have focused mostly on delayed graft function, which is a form of AKI in the immediate peri-transplant period (13, 14).
RTRs constitute a unique population with an inherent increased risk vs. the general population for in-hospital AKI secondary to different etiologies related to subclinical and chronic rejection, higher risk of infections and immunosuppressive therapy. As a result, strategies to prevent or minimize the occurrence and consequences of AKI during hospitalization in this population would necessarily be more complex than those for the general population.
Renal allograft survival has improved significantly in the short term, with one-year graft survival rates reaching 98.4% (15). However, ensuring long-term graft survival still poses a very significant challenge in renal transplantation. For RTRs, a better understanding of the risk factors for AKI during admission would form the basis for developing preventive therapeutic measures aimed at reducing the rate of in-hospital AKI, resulting in improved long-term renal allograft survival.
In this study, we sought to pinpoint the risk factors for AKI during hospitalization of RTRs in a non-intensive care setting. In addition, we examined the implications of in-patient AKI for in-hospital mortality, duration of hospitalization, subsequent in-hospital AKI, re-hospitalizations, and overall mortality in this vulnerable population.
Materials and Methods
Study Population and Design
Clinical and biochemical parameters were collected retrospectively from the MdClone system, the data acquisition tool at Sheba Medical Center. Additional data was collected from clinical records, as needed. The study was approved by the local ethics committee (IRB approval number: SMC-70-5320). Data was collected for up to 12 admissions post “Renal Transplant” diagnosis for admission dates falling between July 2007 and November 2020.
The initial dataset included 1405 hospitalizations for 399 transplant recipients. We then excluded from the analysis admissions post graft loss (baseline eGFR<15 mL/min per 1.73 m2), post chronic dialysis initiation, and/or admissions ≤30 days from transplant to eliminate the effect of changes in immunosuppressive medications or in renal allograft function early post-transplant secondary to slow and/or delayed graft function, infections and early rejections. Hospitalizations with no Scr or only one Scr measured during admission were also excluded (Figure 1). The final study cohort included 292 RTRs who had undergone kidney transplantation between June 1982 and June 2000, with a total of 807 non-ICU admissions.
FIGURE 1
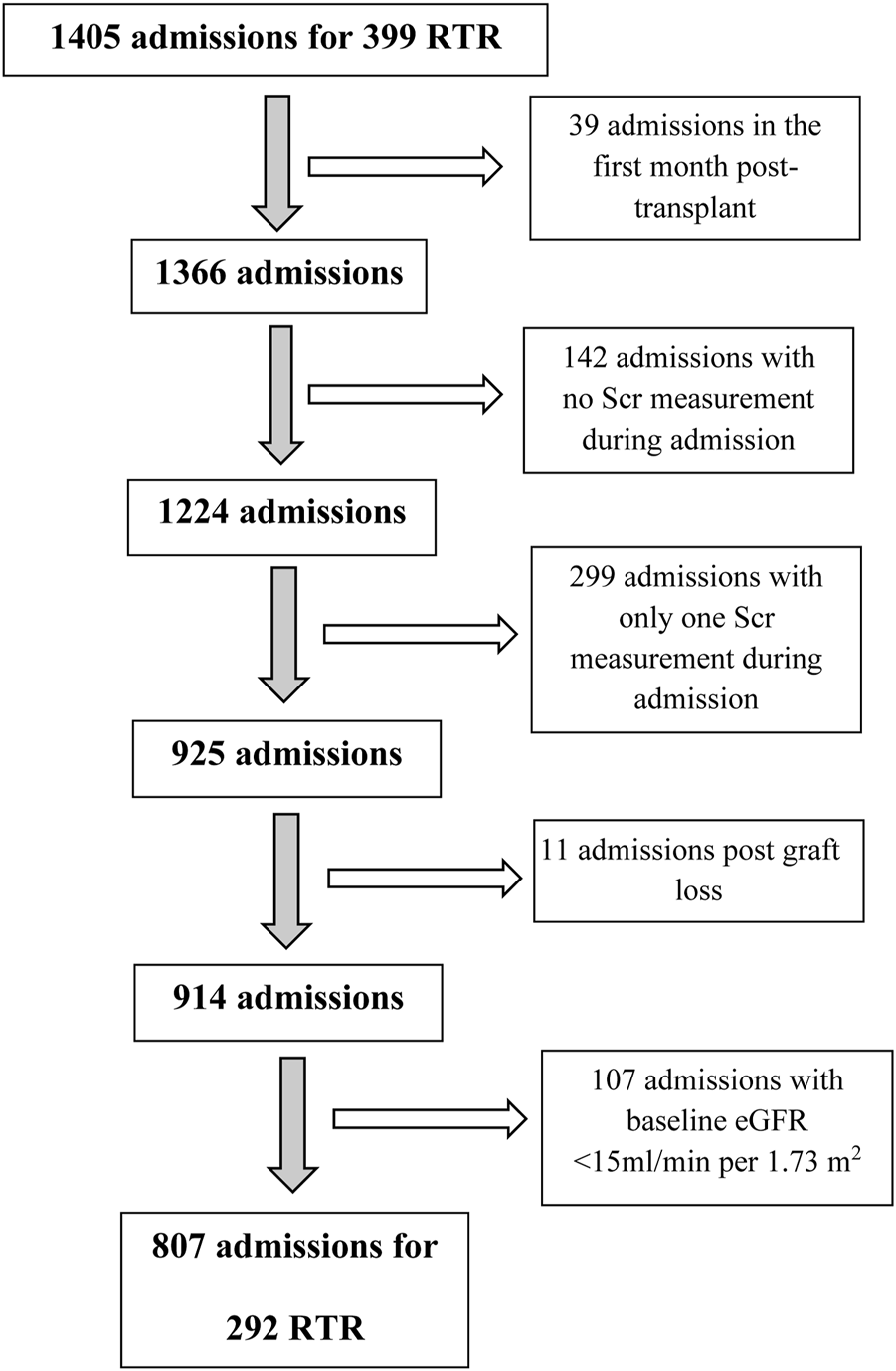
Consort diagram. RTR, renal transplant recipients; Scr, serum creatinine.
Primary Outcome
The primary outcome was AKI during admission, which was defined as a difference of ≥50% between peak Scr during admission and baseline Scr, according to the Kidney Disease Improving Global Outcomes (KDIGO) definition. AKI staging was based on the KDIGO definitions for SCr increases, i.e., a difference between peak Scr during admission and baseline Scr that was: Stage 1, ≥1.5–1.9 times the baseline Scr; Stage 2, ≥2–2.9 times the baseline Scr; and Stage 3, >3 times the baseline Scr or a peak Scr during admission ≥4.0 mg/dL or initiation of renal replacement therapy.
To determine baseline eGFR, we chose the minimum Scr in the 120 days to 1 day prior to admission. For admissions without Scr measurements in the 1- to 120-day period, we used minimum Scr during admission. To avoid misjudgment of baseline renal allograft function affected by frequent changes in Scr early post-transplant, we used minimum Scr during admission for baseline eGFR assessment in admissions of less than 150 days from transplant.
Data Extraction and Study Assessments
The following information was extracted from electronic patient records: age, gender, etiology of end stage renal disease (ESRD), dialysis pre-transplant (yes/no), transplant number, donor type, transplant date and relevant medical history, specifically hypertension, congestive heart failure (CHF), ischemic heart disease (IHD) and pre-transplant diabetes. All diagnoses during admissions were obtained from MDClone. After a manual review of in-patient diagnoses, the main hospitalization etiology was selected, and diagnoses were grouped into five categories: infectious disease, cardiovascular disease, disease of the gastrointestinal system, neoplasm, and all others.
The following biochemical parameters during hospitalization were retrieved in an automated fashion from MDClone: average and maximum tacrolimus trough level, average total white blood cell count, average and minimum absolute lymphocyte count, average hemoglobin, average albumin, maximum and minimum glucose, maximum globulins, and average C-reactive protein. The following additional clinical and biochemical information during admission was also retrieved from MDClone: average and minimum systolic and diastolic blood pressures, average weight and body mass index (BMI), fever, maximum pulse, and minimum oxygen saturation. Use and average dose administered during admission for the following medications was automatically obtained from MDClone: tacrolimus, cyclosporine, mycophenolic acid (MPA) (for 238 admissions, mycophenolate dose was converted to the equivalent MPA dose by dividing mycophenolate dose by 1.388) and steroids (steroid derivatives used during admission, such as hydrocortisone, dexamethasone and methylprednisolone, were converted to the equivalent prednisone dose). Other medications administered during admission were also recorded.
Statistical Analysis
All demographic, clinical and biochemical covariates of interest were tabulated and compared between patients for AKI during admission (yes/no) and between admissions (with and without AKI, and partitioned into AKI stage for AKI patients). Categorical variables were compared using the Chi-squared test (or Fisher’s test where the numbers were small), while continuous variables were first tested for normality using the Shapiro-Wilks Test (and for equality of variances), and were then compared using a t-test (or Anova) for normally distributed variables or a-parametric tests for non-normally distributed variables. An FDR [false discovery rate (Benjamini and Hochberg)] procedure was then carried out to correct for multiple comparisons.
For the primary outcome of AKI during admission, logistic mixed models were used, with RTR being a random effect, and other variables being fixed effects. Univariate models were considered first; variables that were significant (p = 0.05) and/or those with clinical importance were entered into multivariate models.
For the secondary outcomes, LOS was examined using a linear mixed model, with RTR as a random effect and the other variables being fixed effects. LOS (which is inherently right-tailed) was log-transformed to normalize it. Mortality during admission was modeled using the Cox proportional hazards model, which modeled the time from transplant to death or end of follow-up, taking into account the number of admissions per person. Overall mortality was modeled using Kaplan-Meier estimation, with AKI in different staging groups. Thereafter, the Cox proportional hazards model was used to model the time from transplant to death or end of follow-up. Readmission within 90 days was calculated. Logistic mixed models were used to predict the cause of readmission within 90 days, with RTR being a random effect, and other variables being mixed effects. For all secondary outcomes, univariate models were considered first. Significant variables and/or those with clinical importance were entered into multivariate models.
All statistical analyses were carried out using R-3.4.1 [R Core Team (2017). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/].
Results
Characteristics of the RTRs Cohort
A total of 292 RTRs comprised our study cohort (Table 1). Median transplant age was 54.9 (IQR, 46.2–64.2); 98 (33.6%) were females. Median time from transplant to first admission was 6.33 years (IQR, 2.5–11.8). Of the RTR cohort, 64 (21.9%), 39 (13.4%), 59 (20.2%) and 26 (8.9%) patients had ESRD secondary to diabetic nephropathy, autosomal dominant polycystic kidney disease (APCKD), glomerulonephritis and nephrosclerosis, respectively; 158 (54.1%) were on renal replacement therapy before the transplant; and 140 (47.9%) had a living donor renal transplant. Forty RTRs (13.7%) died during admission, and the overall mortality rate for the duration of the study was 41.1%. The total number of admissions ranged between 1 and 10.
TABLE 1
| Variable | Total cohort (n = 292) | Without AKI (n = 143) | With any AKI (n = 149) | p |
|---|---|---|---|---|
| RTR characteristics | ||||
| Transplant age, years [median (IQR)] | 54.98 (46.2, 64.2) | 55.53 (47.5, 64.51) | 54.21 (45.2, 64.2) | 0.89 |
| Female sex, n (%) | 98 (33.56) | 48 (33.57) | 50 (33.56) | 1 |
| Transplant to 1st admission, years [median (IQR)] | 6.33 (2.45, 11.81) | 6.7 (1.9, 11.2) | 6.3 (3.2, 12.2) | 0.35 |
| ESRD etiology, n (%) | ||||
| ADPCKD | 39 (13.36) | 28 (19.58) | 11 (7.38) | 0.07 |
| Diabetic nephropathy | 64 (21.92) | 31 (21.68) | 33 (22.15) | |
| Glomerulonephritis | 59 (20.21) | 26 (18.18) | 33 (22.15) | |
| Nephrosclerosis | 26 (8.9) | 12 (8.39) | 14 (9.4) | |
| Other | 69 (23.63) | 29 (20.28) | 40 (26.85) | |
| Unknown | 35 (11.99) | 17 (11.89) | 18 (12.08) | |
| Pre-transplant dialysis | ||||
| Yes | 158 (54.11) | 72 (50.35) | 86 (57.72) | 0.11 |
| No | 46 (15.75) | 29 (20.28) | 17 (11.41) | |
| Unknown | 88 (30.14) | 42 (29.37) | 46 (30.87) | |
| Transplant type, n (%) | ||||
| Kidney only | 280 (95.89) | 138 (96.5) | 142 (95.3) | 1 |
| Liver kidney | 4 (1.37) | 2 (1.4) | 2 (1.34) | |
| Heart kidney | 7 (2.4) | 3 (2.1) | 4 (2.68) | |
| Pancreas kidney | 1 (0.34) | 1 (0.67) | ||
| Transplant number, n (%) | ||||
| 1 | 262 (89.73) | 127 (88.81) | 135 (90.6) | 0.66 |
| 2 | 26 (8.9) | 13 (9.09) | 13 (8.72) | |
| 3 | 4 (1.37) | 3 (2.1) | 1 (0.67) | |
| Donor type, n (%) | ||||
| Living | 140 (47.9) | 82 (57.34) | 66 (44.3) | 0.08 |
| Deceased | 85 (29.11) | 36 (25.17) | 49 (32.89) | |
| Unknown | 59 (20.21) | 25 (17.48) | 34 (22.82) | |
| Number of admissions, n (%) | ||||
| 1 | 116 (39.73) | 86 (60.14) | 30 (20.13) | <0.001** |
| 2 | 55 (18.84) | 29 (20.28) | 26 (17.45) | |
| 3 | 33 (11.3) | 10 (6.99) | 23 (15.44) | |
| 4 | 31 (10.62) | 9 (6.29) | 22 (14.77) | |
| 5 | 22 (7.53) | 3 (2.1) | 19 (12.75) | |
| 6 | 15 (5.14) | 3 (2.1) | 12 (8.05) | |
| 7 | 7 (2.4) | 2 (1.4) | 5 (3.36) | |
| 8 | 9 (3.08) | 9 (6.04) | ||
| 9 | 3 (1.03) | 1 (0.7) | 2 (1.34) | |
| 10 | 1 (0.34) | 1 (0.67) | ||
| Number of AKI (per person), n (%) | ||||
| 0 | 143 (100) | |||
| 1 | 76 (26.03) | |||
| 2 | 30 (10.27) | |||
| 3 | 25 (8.56) | |||
| 4 | 11 (3.77) | |||
| 5 | 2 (0.68) | |||
| 6 | 4 (1.37) | |||
| 8 | 1 (0.34) | |||
| Death during admission, n (%) | 40 (13.7) | 5 (3.5) | 35 (23.49) | <0.001** |
| Overall mortality, n (%) | 120 (41.1) | 40 (27.97) | 80 (53.69) | <0.001** |
Demographic and clinical characteristics of RTRs, stratified by AKI during admission.
*p < 0.05; **p < 0.01.
ADPKD, autosomal dominant polycystic kidney disease; ESRD, end stage renal disease, RTR, renal transplant recipient. Bold values are the p values which are significant (<0.05 or <0.01).
Univariate Comparison of RTRs Without Any AKI vs. With Any AKI During Admission
Of the 292 RTRs, 149 (51%) had 1 to 8 AKI admission events. For patients with any AKI during admission, the number of admissions per person, the death rate during admission, and the overall mortality were all higher (p < 0.001). ESRD secondary to APCKD and renal living donor transplant were more common in RTRs without vs. with any AKI during admission as the difference between the groups approached statistical significance. All other comparisons of characteristics, including age, sex, time from transplant to first admission, transplant number and dialysis pretransplant, are shown in Table 1.
Characteristics of Total Admissions
Our cohort of 292 RTR had a total of 807 non-ICU admissions. Median age during admission was 66.75 (IQR 57.16–73.12); 266 (33%) were females. Median time from transplant to admission was 7.65 years (IQR 4.22–12.75). The most prevalent admission etiology [312 (38.7%) admissions] was an infection. Forty (4.96%) admissions resulted in death during admission. Median LOS was 4.65 days (IQR 2.67–9). For 302 (37.4%) admissions, patients were readmitted within 90 days (Table 2).
TABLE 2
| Variable | Total admissions (n = 807) | Admissions without AKI (n = 510) | Admissions with AKI (n = 297) | p a |
|---|---|---|---|---|
| RTR characteristics | ||||
| Admission age, years [median (IQR)] | 66.75 [57.2, 73.1] | 66.9 [58, 73.2] | 66.5 [56, 73] | 0.65 |
| Female sex, n (%) | 266 (33) | 169 (33.1) | 97 (32.7) | 0.95 |
| Transplant to admission, years [median (IQR)] | 7.65 [4.2, 12.8] | 7.6 [3.8, 12.6] | 7.7 [4.8, 13.5] | 0.43 |
| ESRD etiology, n (%) | ||||
| ADPCKD | 81 (10) | 63 (12.4) | 18 (6.1) | 0.06 |
| Diabetic nephropathy | 187 (23.2) | 119 (23.3) | 68 (22.9) | |
| Glomerulonephritis | 154 (19.1) | 102 (20.0) | 52 (17.5) | |
| Nephrosclerosis | 73 (9) | 40 (7.8) | 33 (11.1) | |
| Other | 212 (26.3) | 124 (24.3) | 88 (29.6) | |
| Unknown | 100 (12.4) | 62 (12.2) | 38 (12.8) | |
| Pre-transplant dialysis | ||||
| Yes | 449 (55.6) | 289 (56.7) | 160 (53.9) | 0.75 |
| No | 125 (15.5) | 79 (15.5) | 46 (15.5) | |
| Unknown | 233 (28.9) | 142 (27.8) | 91 (30.6) | |
| Transplant type, n (%) | ||||
| Kidney only | 775 (96) | 487 (95.5) | 288 (97.0) | 0.76 |
| Liver kidney | 7 (0.9) | 5 (1.0) | 2 (0.7) | |
| Heart kidney | 23 (2.8) | 17 (3.3) | 6 (2.0) | |
| Pancreas kidney | 2 (0.2) | 1 (0.2) | 1 (0.3) | |
| Transplant number, n (%) | ||||
| 1 | 735 (91.1) | 461 (90.4) | 274 (92.3) | 0.71 |
| 2 | 67 (8.3) | 45 (8.8) | 22 (7.4) | |
| 3 | 5 (0.6) | 4 (0.8) | 1 (0.3) | |
| Donor type, n (%) | ||||
| Living | 426 (52.8) | 267 (52.4) | 159 (53.5) | 0.96 |
| Deceased | 239 (29.6) | 152 (29.8) | 87 (29.3) | |
| Unknown | 142 (17.6) | 91 (17.8) | 51 (17.2) | |
| Medical history, n (%) | ||||
| Diabetes mellitus | 273 (33.8) | 169 (33.1) | 104 (35.0) | 0.7 |
| Hypertension | 496 (61.5) | 297 (58.2) | 199 (67.0) | 0.037* |
| IHD | 292 (36.2) | 164 (32.2) | 128 (43.1) | 0.006** |
| CHF | 170 (21.1) | 98 (19.2) | 72 (24.2) | 0.19 |
| Admission etiology, n (%) | ||||
| ID | 312 (38.7) | 170 (33.3) | 142 (47.8) | <0.001** |
| CV | 174 (21.6) | 132 (25.9) | 42 (14.1) | |
| GI | 64 (7.9) | 48 (9.4) | 16 (5.4) | |
| CA | 38 (4.7) | 23 (4.5) | 15 (5.1) | |
| Others | 219 (27.1) | 137 (26.9) | 82 (27.6) | |
| Vital signs and other clinical parameters during admission, [median (IQR)] | ||||
| Fever max, °C | 37.2 [36.9, 37.9] | 37.2 [36.9, 37.6] | 37.4 [37, 38.4] | <0.001** |
| Pulse max | 96 [84, 110] | 93 [81, 102] | 103 [89, 120] | <0.001** |
| Pulse min | 61 [54, 69] | 61 [55, 70] | 60 [53, 67] | 0.04* |
| Pulse average | 75.9 [68.1, 83.7] | 75 [67, 82.5] | 78 [70.1, 85.8] | <0.001** |
| SBP min mmHg | 103 [89, 117] | 108 [96, 120] | 95 [80, 108] | <0.001** |
| SBP average mmHg | 131.2 [118.8, 145.8] | 132.6 [121.2, 147.1] | 128.8 [115.5, 144.1] | 0.008* |
| DBP min mmHg | 54 [46, 62] | 56 [50, 63] | 50 [40, 59] | <0.001** |
| DBP average mmHg | 70.7 [65.2, 76.6] | 71 [66.4, 76.6] | 69.8 [63.1, 76.6] | 0.04* |
| O2 saturation min | 93 [89, 95] | 94 [90, 95] | 91 [85, 94] | <0.001** |
| Weight average | 75 [64.5, 87.4] | 75 [65, 90] | 73.8 [64, 85.1] | 0.34 |
| BMI average | 26.4 [23.1, 30.5] | 26.5 [23.4, 30.8] | 26.1 [22.8, 30.3] | 0.46 |
| Medications during admission | ||||
| Tacrolimus, n (%) | 386 (47.8) | 232 (45.5) | 154 (51.9) | 0.17 |
| Tacrolimus average dose mg [mean (SD)] | 1.49 (0.96) | 1.46 (0.92) | 1.53 (1.03) | 0.7 |
| Cyclosporine, n (%) | 97 (12.0) | 63 (12.4) | 34 (11.4) | 0.79 |
| Cyclosporine average dose, mg [mean (SD)] | 63.51 (30.15) | 60.60 (23.68) | 68.90 (39.30) | 0.3 |
| MPA, n (%) | 321 (39.8) | 212 (41.6) | 109 (36.7) | 0.28 |
| MPA average dose, mg [mean (SD)] | 157.8 (217.4) | 164.1 (218.7) | 146.9 (215.1) | 0.4 |
| Steroids, n (%) | 549 (68.0) | 333 (65.3) | 216 (72.7) | 0.06 |
| Steroids average dose, mg [mean (SD)] | 16.01 (33.13) | 12.27 (26.52) | 22.10 (41.01) | <0.001** |
| mTOR inhibitors, n (%) | 66 (8.2) | 50 (9.8) | 16 (5.4) | 0.06 |
| Azathioprine, n (%) | 45 (5.6) | 31 (6.1) | 14 (4.7) | 0.66 |
| Loop diuretics, n (%) | 339 (42) | 193 (37.8) | 146 (49.2) | 0.005* |
| Thiazides, n (%) | 44 (5.4) | 29 (5.7) | 15 (5.1) | 0.79 |
| Calcium channel blockers, n (%) | 314 (38.9) | 209 (41.0) | 105 (35.4) | 0.2 |
| Beta blockers, n (%) | 530 (65.7) | 338 (66.3) | 192 (64.6) | 0.75 |
| RAAS inhibitors, n (%) | 247 (30.6) | 176 (34.5) | 71 (23.9) | 0.005* |
| Aldosterone antagonists, n (%) | 37 (4.6) | 28 (5.5) | 9 (3.0) | 0.2 |
| Statins, n (%) | 387 (47.9) | 249 (48.8) | 138 (46.5) | 0.69 |
| NSAIDs, n (%) | 5 (0.6) | 2 (0.4) | 3 (1.0) | 0.48 |
| PPIs, n (%) | 550 (68.1) | 327 (64.1) | 223 (75.1) | 0.004* |
| Laboratory results during admission [median (IQR)] | ||||
| White blood cell average (K/μL) | 8.8 [6.5, 11.9] | 8.3 [6.2, 10.8] | 9.9 [7.3, 14.6] | <0.001** |
| Lymphocyte absolute average (K/μL) | 1.1 [0.7, 1.6] | 1.1 [0.8, 1.7] | 1.1 [0.7, 1.5] | 0.05 |
| Lymphocyte absolute min (K/μL) | 0.7 [0.4, 1.2] | 0.8 [0.5, 1.3] | 0.6 [0.3, 0.9] | <0.001** |
| Hemoglobin average (g/dL) | 10.5 [9.3, 12] | 11 [9.7, 12.3] | 9.9 [9, 10.98] | <0.001** |
| Hemoglobin min (g/dL) | 9.9 [8.3, 11.4] | 10.5 [8.9, 11.9] | 8.96 [7.66, 10.24] | <0.001** |
| Creatinine (mg/dL) | 1.4 [1.0, 1.96] | 1.3 [1.0, 1.8] | 1.63 [1.13, 2.37] | <0.001 |
| eGFR baseline (CKD-EPI)** | 59.9 [41.3, 80.98] | 61.2 [43.7, 81.1] | 58.18 [35.81, 80.34] | 0.34 |
| Glucose max (mg/dL) | 205 [137, 321.5] | 188 [130, 282] | 244 [152, 380] | <0.001** |
| Glucose min (mg/dL) | 86 [71, 106] | 90 [76, 112] | 78 [64, 93] | <0.001** |
| Albumin average (g/dL) | 3.2 [2.8, 3.6] | 3.4 [3.0, 3.8] | 2.9 [2.6, 3.3] | <0.001** |
| Albumin min (g/dL) | 2.9 [2.5, 3.3] | 3.1 [2.7, 3.5] | 2.6 [2.3, 3] | <0.001** |
| Globulins max (g/dL) | 2.9 [2.6, 3.3] | 2.9 [2.5, 3.2] | 3 [2.6, 3.6] | <0.001** |
| Globulins min (g/dL) | 2.5 [2.1, 2.9] | 2.5 [2.2, 2.9] | 2.5 [2, 2.9] | 0.47 |
| Tacrolimus trough level average (μg/L) | 5.5 [3.7, 8.2] | 5.1 [3.6, 7.9] | 6.0 [3.8, 8.6] | 0.25 |
| Tacrolimus trough level max (μg/L) | 6.2 [4, 9.6] | 5.7 [4.1, 8.5] | 7.1 [4.1, 10.9] | 0.028* |
| C-reactive protein average (mg/L) | 61.3 [19.1, 113.3] | 50.1 [14.8, 101.5] | 79.8 [36.1, 137.8] | <0.001** |
| Death during admission, n (%) | 40 (4.96) | 8 (1.6) | 32 (10.8) | <0.001** |
| LOS, days [median (IQR)] | 4.6 [2.7, 9.0] | 3.8 [2.1, 7.0] | 7.01 [3.62, 15.34] | <0.001** |
| Readmission in 90 days, n (%) | 302 (37.4) | 148 (29.0) | 154 (51.9) | <0.001** |
Demographic, clinical and biochemical characteristics of total admissions stratified by the presence or absence of AKI during admission.
After adjustment for multiple comparisons.
*p < 0.05; **p < 0.01.
***eGFR was calculated according to the following CKD-EPI formula: eGFR = 141* min (Scr/k, 1)α * max (Scr/k, 1)-1.209 * 0.993Age * 1.018 * 1.159 (if black) (where Scr - standardized serum creatinine; k = 0.7 if female, 0.9 if male; α = −0.329 if female, −0.411 if male; min = the minimum of Scr/k of 1; max = the maximum of Scr/k or 1).
ADPKD, autosomal dominant polycystic kidney disease; BMI, body mass index; CA, cancers; CHF, congestive heart failure; CV, cardiovascular disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; ESRD, end stage renal disease; GI, gastrointestinal; ID, infectious diseases; IHD, ischemic heart disease; LOS, length of stay; MPA, mycophenolic acid; NSAID, non-steroidal anti-inflammatory drug; RAAS, renin-angiotensin-aldosterone system; RTR, renal transplant recipients; SBP, systolic blood pressure. Bold values are the p values which are significant (<0.05 or <0.01).
Univariate Comparison of Admissions Without vs. With AKI During Admission
An AKI during admission was recorded for 297 of 807 (36.8%) admissions. In admissions with AKI vs. admissions without AKI, ESRD secondary to APCKD was less prevalent, while nephrosclerosis was more common (p = 0.06). RTRs with at least one AKI had higher rates of hypertension and IHD (67% and 43.1% compared to 58.2% and 32.2% of admissions without AKI, p = 0.037 and 0.006, respectively). The main admission diagnosis was an infection in 142 (47.8%) of admissions with AKI vs. 170 (33.3%) in admissions without AKI (p < 0.001). Significant differences in the various vital signs monitored during admission [maximum temperature, maximum, minimum and average pulse, minimum and average systolic blood pressure (SBP) and diastolic blood pressure (DBP), and O2 saturation] were observed between admissions with and without AKI (Table 2). Steroids use and average dose during admission were significantly higher in admissions with vs. without AKI [216 (72.2%) vs. 333 (65.3%), p = 0.06 (after adjustment for multiple comparisons) and 22.1 (SD 41.01) vs. 12.27 (SD 26.52) mg, p < 0.001 respectively]. The use of loop diuretics and of proton pump inhibitors was also significantly higher in admissions with vs. without AKI, as opposed to the use of renin-angiotensin-aldosterone system (RAAS) inhibitors, which was lower in admissions with vs. without in-hospital AKI. Laboratory results also differed significantly between admissions with and without AKI. Total white blood cell count, maximum glucose, maximum globulins and C-reactive protein levels were higher in admissions with vs. without AKI, whereas lymphocytes (absolute minimum), average and minimum hemoglobin, minimum glucose and average and minimum albumin were lower in admissions with vs. without AKI. Maximum tacrolimus 12-h trough level was significantly higher in admissions with vs. without AKI [7.1 (IQR 4.05–10.9) vs. 5.7 (4.05–8.5), p = 0.028]. Rates of death during admission and readmission within 90 days as well as LOS were significantly higher for those with vs. without AKI (Table 2).
Univariate Comparison of AKI Stages 1, 2 and 3 During Admission
Of 297 admissions with AKI during admission, 134 (45.1%), 70 (23.6%) and 93 (31.3%) presented with AKI Stages 1, 2 and 3, respectively. The rate of female RTRs fell as AKI progressed (39.6%, 31.4% and 23.7% in AKI stages 1, 2 and 3 respectively, p = 0.13). Time from transplant to admission increased from 6.95 (IQR 3.8–11.5) to 8.05 (IQR 4.65–12.3) to 8.7 years (IQR 5.9–16.5) as in-hospital AKI stage increased from 1 to 2 to 3, respectively (p = 0.04). Vital signs monitored during admission, such as maximum pulse, increased, whereas minimum SBP and minimum oxygen saturation decreased with worsening AKI stage. MPA use and average dose decreased, whereas average steroid dose increased with progression of AKI. Minimum glucose and average albumin decreased as in-hospital AKI progressed. LOS increased as AKI stage increased, but this was not statistically significant, possibly due to the low number of patients in each group. Rates of death during admission and readmission within 90 days increased as AKI worsened (Table 3).
TABLE 3
| Variable | Admissions with AKI (n = 297) | AKI stage 1 (n = 134) | AKI stage 2 (n = 70) | AKI stage 3 (n = 93) | p a |
|---|---|---|---|---|---|
| RTR characteristics | |||||
| Admission age, years, [median (IQR)] | 66.5 [55.98, 73] | 67.3 [58.6, 73.7] | 66.1 [55.5, 75.5] | 64.9 [53.4, 72] | 0.4 |
| Female sex, n (%) | 97 (32.7) | 53 (39.6) | 22 (31.4) | 22 (23.7) | 0.13 |
| Transplant to admission, years [median (IQR)] | 7.7 [4.77, 13.48] | 6.95 [3.8, 11.5] | 8.05 [4.65, 12.30] | 8.7 [5.9, 16.5] | 0.04* |
| ESRD etiology, n (%) | |||||
| ADPCKD | 18 (6.1) | 10 (7.5) | 5 (7.1) | 3 (3.2) | 0.29 |
| Diabetic nephropathy | 68 (22.9) | 37 (27.6) | 17 (24.3) | 14 (15.1) | |
| Glomerulonephritis | 52 (17.5) | 25 (18.7) | 9 (12.9) | 18 (19.4) | |
| Nephrosclerosis | 33 (11.1) | 18 (13.4) | 5 (7.1) | 10 (10.8) | |
| Other | 88 (29.6) | 29 (21.6) | 27 (38.6) | 32 (34.4) | |
| Unknown | 38 (12.8) | 15 (11.2) | 7 (10.0) | 16 (17.2) | |
| Pre-transplant dialysis | |||||
| Yes | 160 (53.9) | 77 (57.5) | 34 (48.6) | 49 (52.7) | 0.82 |
| No | 46 (15.5) | 18 (13.4) | 14 (20.0) | 14 (15.1) | |
| Unknown | 91 (30.6) | 39 (29.1) | 22 (31.4) | 30 (32.3) | |
| Transplant type, n (%) | |||||
| Kidney only | 288 (97.0) | 130 (97.0) | 68 (97.1) | 90 (96.8) | 0.86 |
| Liver kidney | 2 (0.7) | 1 (0.7) | 1 (1.4) | 0 (0.0) | |
| Heart kidney | 6 (2.0) | 2 (1.5) | 1 (1.4) | 3 (3.2) | |
| Pancreas kidney | 1 (0.3) | 1 (0.7) | 0 (0.0) | 0 (0.0) | |
| Transplant number, n (%) | |||||
| 1 | 274 (92.3) | 124 (92.5) | 67 (95.7) | 83 (89.2) | 0.6 |
| 2 | 22 (7.4) | 9 (6.7) | 3 (4.3) | 10 (10.8) | |
| 3 | 1 (0.3) | 1 (0.7) | 0 (0.0) | 0 (0.0) | |
| Donor type, n (%) | |||||
| Living | 159 (53.5) | 72 (53.7) | 36 (51.4) | 51 (54.8) | 0.55 |
| Deceased | 87 (29.3) | 45 (33.6) | 19 (27.1) | 23 (24.7) | |
| Unknown | 51 (17.2) | 17 (12.7) | 15 (21.4) | 19 (20.4) | |
| Medical history, n (%) | |||||
| Diabetes mellitus | 104 (35.0) | 49 (36.6) | 26 (37.1) | 29 (31.2) | 0.78 |
| Hypertension | 199 (67.0) | 90 (67.2) | 44 (62.9) | 65 (69.9) | 0.78 |
| IHD | 128 (43.1) | 54 (40.3) | 34 (48.6) | 40 (43.0) | 0.71 |
| CHF | 72 (24.2) | 26 (19.4) | 16 (22.9) | 30 (32.3) | 0.24 |
| Admission etiology, n (%) | |||||
| ID | 142 (47.8) | 68 (50.7) | 37 (52.9) | 37 (39.8) | 0.37 |
| CV | 42 (14.1) | 22 (16.4) | 5 (7.1) | 15 (16.1) | |
| GI | 16 (5.4) | 7 (5.2) | 6 (8.6) | 3 (3.2) | |
| CA | 15 (5.1) | 5 (3.7) | 2 (2.9) | 8 (8.6) | |
| Other | 82 (27.6) | 32 (23.9) | 20 (28.6) | 30 (32.3) | |
| Vital signs and other clinical parameters during admission, [median (IQR)] | |||||
| Fever max, °C | 37.4 [37, 38.40] | 37.3 [37, 38.4] | 37.4 [37, 38.25] | 37.5 [37.1, 38.5] | 0.49 |
| Pulse max | 103 [89, 120] | 98 [87, 111] | 103.5 [90.8, 128.5] | 110 [94, 130] | 0.01* |
| Pulse min | 60 [53, 67] | 60 [53.2, 67] | 59.5 [53, 65] | 61 [52, 68] | 0.8 |
| Pulse average | 78 [70, 85.84] | 76.6 [68, 84.6] | 77.9 [71.5, 84.6] | 79.2 [72.9, 87.1] | 0.24 |
| SBP min mmHg | 95 [80, 108] | 99 [86, 110.7] | 91.5 [72, 102.8] | 89 [70, 110] | 0.01* |
| SBP average mmHg | 128.8 [115.5, 144] | 130 [118.7, 146.4] | 128.4 [114.1, 137.5] | 126.5 [112, 145.8] | 0.04* |
| DBP min mmHg | 50 [40, 59]8 | 50 [45, 58] | 44 [39, 56.5]7 | 49 [36, 63] | 0.24 |
| DBP average mmHg | 69.8 [63.1, 76.6] | 70.5 [64.8, 75.3] | 68.7 [62.6, 75.1] | 70.6 [61.8, 81.1] | 0.6 |
| O2 saturation min | 91 [85, 94] | 93 [88, 95] | 91 [84.3, 93] | 90 [81, 94] | 0.04* |
| Weight average, kg | 73.8 [64, 85.1] | 72.9 [63.2, 85] | 72.7 [63.2, 91.2] | 76.4 [65, 85.6] | 0.78 |
| BMI average | 26.1 [22.8, 30.3] | 26.1 [23.3, 29.5] | 25.7 [22.1, 30.8] | 26.6 [22.8, 30.5] | 0.91 |
| Medications during admission | |||||
| Tacrolimus, n (%) | 154 (51.9) | 68 (50.7) | 47 (67.1) | 39 (41.9) | 0.03* |
| Tacrolimus average dose [mean (SD)] | 1.53 (1.03) | 1.49 (0.91) | 1.50 (0.97) | 1.62 (1.30) | 0.88 |
| Cyclosporine, n (%) | 34 (11.4) | 17 (12.4) | 7 (10.0) | 10 (10.8) | 0.94 |
| Cyclosporine average dose [mean (SD)] | 68.90 (39.30) | 71.81 (40.30) | 64.03 (41.24) | 67.35 (40.04) | 0.92 |
| MPA, n (%) | 109 (36.7) | 61 (45.5) | 26 (37.1) | 22 (23.7) | 0.02* |
| MPA average dose [mean (SD)] | 146.86 (215.11) | 186.40 (237.78) | 140.91 (192.53) | 94.35 (185.05) | 0.039* |
| Steroids, n (%) | 216 (72.7) | 92 (68.7) | 53 (75.7) | 71 (76.3) | 0.59 |
| Steroids average dose [mean (SD)] | 22.10 (41.01) | 14.47 (20.03) | 24.98 (38.47) | 30.72 (59.23) | 0.06 |
| mTOR inhibitors, n (%) | 16 (5.4) | 8 (6.0) | 3 (4.3) | 5 (5.4) | 0.91 |
| Azathioprine, n (%) | 14 (4.7) | 7 (5.2) | 3 (4.3) | 4 (4.3) | 0.93 |
| Loop diuretics, n (%) | 146 (49.2) | 61 (45.5) | 34 (48.6) | 51 (54.8) | 0.59 |
| Thiazides, n (%) | 15 (5.1) | 9 (6.7) | 1 (1.4) | 5 (5.4) | 0.47 |
| Calcium channel blockers, n (%) | 105 (35.4) | 41 (30.6) | 29 (41.4) | 35 (37.6) | 0.47 |
| Beta blockers, n (%) | 192 (64.6) | 83 (61.9) | 43 (61.4) | 66 (71.0) | 0.53 |
| RAAS inhibition, n (%) | 71 (23.9) | 35 (26.1) | 15 (21.4) | 21 (22.6) | 0.82 |
| Aldosterone antagonists, n (%) | 9 (3.0) | 4 (3.0) | 1 (1.4) | 4 (4.3) | 0.72 |
| Statins, n (%) | 138 (46.5) | 64 (47.8) | 35 (50.0) | 39 (41.9) | 0.72 |
| NSAIDs, n (%) | 3 (1.0) | 0 (0.0) | 0 (0.0) | 3 (3.2) | 0.16 |
| PPIs, n (%) | 223 (75.1) | 96 (71.6) | 54 (77.1) | 73 (78.5) | 0.65 |
| Laboratory results during admission [median (IQR)] | |||||
| White blood cell average (K/μL) | 9.92 [7.27, 14.57] | 9.5 [6.9, 14.1] | 11.9 [7.7, 17.1] | 10.2 [7.3, 13.5] | 0.42 |
| Lymphocyte absolute average (K/μL) | 1.09 [0.67, 1.52] | 1.1 [0.71, 1.5] | 1.1 [0.7, 1.6] | 1 [0.6, 1.35] | 0.42 |
| Lymphocyte absolute min (K/μL) | 0.58 [0.28, 0.94] | 0.6 [0.3, 1.1] | 0.65 [0.3, 0.96] | 0.5 [0.2, 0.8] | 0.24 |
| Hemoglobin average (g/dL) | 9.91 [9.00, 10.98] | 9.9 [8.98, 11] | 10.1 [9.2, 11.1] | 9.7 [8.8, 10.8] | 0.59 |
| Creatinine (mg/dL) | 1.63 [1.13, 2.37] | 1.4 [1.1, 1.9] | 1.4 [1.1, 2] | 2.5 [1.6, 3.5] | <0.001** |
| eGFR baseline (CKD-EPI)** | 58.2 [35.8, 80.3] | 59.6 [44, 77.8] | 72.1 [54.5, 88.7] | 34.3 [23.6, 72.6] | <0.001** |
| Glucose max (mg/dL) | 244 [152, 380] | 231 [152, 380] | 268 [150, 405] | 225 [168, 350] | 0.78 |
| Glucose min (mg/dL) | 78 [64, 93] | 81 [68, 103] | 77 [65.25, 90] | 74 [57, 85] | 0.018* |
| Albumin average (g/dL) | 2.9 [2.6, 3.3] | 3.1 [2.7, 3.35] | 2.8 [2.4, 3.1] | 2.7 [2.4, 3.8] | 0.003* |
| Globulins max (g/dL) | 3 [2.6, 3.6] | 3 [2.7, 3.6] | 3.2 [2.8, 3.5] | 3 [2.5, 3.6] | 0.61 |
| Tacrolimus trough level average (μg/L) | 5.97 [3.8, 8.6] | 6.5 [4.4, 9.1] | 5.5 [3.5, 7.1] | 4.95 [3.6, 8.1] | 0.11 |
| Tacrolimus trough level max (μg/L) | 7.1 [4.1, 10.9] | 8.1 [4.6, 11.2]7 | 6.2 [3.7, 8.5] | 6.95 [3.9, 11.7] | 0.39 |
| C-reactive protein average (mg/L) | 79.8 [36.1, 137.8] | 62.7 [31.7, 119] | 104.2 [42.9, 158.3] | 85.5 [43.7, 140.5] | 0.11 |
| Death during admission, n (%) | 32 (10.8) | 3 (2.2) | 9 (12.9) | 20 (21.5) | <0.001** |
| LOS, days, [median (IQR)] | 7 [3.6, 15.3] | 6.31 [3.28, 13.11] | 7.34 [4.3, 12.5] | 8.17 [3.62, 19.95] | 0.42 |
| Readmission in 90 days, n (%) | 154 (51.9) | 65 (48.5) | 31 (44.3) | 58 (62.4) | 0.042* |
Demographic, clinical and biochemical characteristics of patients admitted with AKI, stratified by AKI stage during admission.
After adjustment for multiple comparisons.
*p < 0.05; **p < 0.01.
***eGFR was calculated according to the following CKD-EPI formula: eGFR = 141* min (Scr/k, 1)α * max (Scr/k, 1)-1.209 * 0.993Age * 1.018 * 1.159 (if black) (where Scr - standardized serum creatinine; k = 0.7 if female, 0.9 if male; α = −0.329 if female, −0.411 if male; min = the minimum of Scr/k of 1; max = the maximum of Scr/k or 1).
ADPKD, autosomal dominant polycystic kidney disease; BMI, body mass index; CA, cancers; CHF, congestive heart failure; CV, cardiovascular disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; ESRD, end stage renal disease; GI, gastrointestinal; ID, infectious diseases; IHD, ischemic heart disease; LOS, length of stay; MPA, mycophenolic acid; NSAID, non-steroidal anti-inflammatory drug; PPI, protein pump inhibitor; RAAS, renin-angiotensin-aldosterone system; RTR, renal transplant recipients; SBP, systolic blood pressure. Bold values are the p values which are significant (<0.05 or <0.01).
Multivariable Analysis for AKI During Admission in RTRs
A mixed-effect logistic model (including admission age, sex, time from transplant to admission, ESRD etiology, medical history of diabetes, hypertension, IHD, CHF and AKI in a previous admission, admission etiology, vital signs, medications during admission, maximum tacrolimus trough level and other laboratory results during admission) revealed that the odds for an AKI during admission increased by 93% (OR 1.93, 95% CI 1.13–3.32, p = 0.017) for AKI in a previous admission and by 91% (OR 1.91, 95% CI 1.07–3.41, p = 0.028) for a medical history of hypertension. In addition, for every increase in minimum SBP of 1 mm Hg, the odds for in-hospital AKI decreased by 2% (OR 0.98, 95% CI 0.97–0.99, p = 0.002). Tacrolimus maximum trough level and albumin level during admission were also found to be associated with AKI during admission (OR 1.08, 95% CI 1.02–1.13, p = 0.005 and OR 0.51, 95% CI 0.29–0.92, p = 0.025, respectively). When tacrolimus maximum trough level was excluded to increase the number of patients and admissions included in the analysis, the odds for in-hospital AKI was more than twofold higher in the case of AKI in a previous admission (OR 2.13, 95% CI 1.44–3.14, p < 0.001) and almost three times higher when the major diagnosis upon admission was an infection (OR 2.93, 95% CI 1.23–6.98, p = 0.015). For every increase in minimum hemoglobin of 1 g/dL, the odds for AKI during admission decreased by 10% (OR 0.9, 95% CI 0.82–0.98, p = 0.016). Minimum SBP and albumin level during admission were also found to be independent predictors for in-hospital AKI (Table 4).
TABLE 4
| Effect | Univariate logistic regression | Logistic regression (n = 193 patients, 385 admissions) | Logistic regression (n = 283 patients, 767 admissions) | |||||
|---|---|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | p | Odds ratio (95% CI) | p | Odds ratio (95% CI) | p | |||
| RTR characteristics | ||||||||
| Admission age, per 1 year increase | 0.99 (0.98, 1.01) | 0.85 | 0.99 (0.97, 1.02) | 0.45 | 0.99 (0.97, 1.01) | 0.21 | ||
| Female vs. male | 0.93 (0.59, 1.48) | 0.77 | 1.17 (0.67, 2.04) | 0.59 | 0.99 (0.65, 1.49) | 0.95 | ||
| Transplant to admission, years | 1.02 (0.99, 1.06) | 0.11 | 1.02 (0.97, 1.07) | 0.47 | 1.00 (0.97, 1.03) | 0.78 | ||
| ESRD etiology | ||||||||
| APCKD | 1 | 1 | 1 | |||||
| Diabetic nephropathy | 2.33 (1.00, 5.41) | 0.049* | 1.09 (0.34, 3.51) | 0.88 | 1.06 (0.43, 2.57) | 0.91 | ||
| Glomerulonephritis | 2.22 (0.94, 5.24) | 0.07 | 1.7 (0.53, 5.51) | 0.37 | 1.6 (0.7, 3.69) | 0.27 | ||
| Nephrosclerosis | 3.41 (1.26, 9.22) | 0.016* | 1.87 (0.52, 6.73) | 0.34 | 2.05 (0.77, 5.44) | 0.15 | ||
| Other | 2.89 (1.26, 6.59) | 0.012* | 1.41 (0.48, 4.15) | 0.53 | 1.72 (0.77, 3.82) | 0.19 | ||
| Unknown | 2.36 (0.93, 6.00) | 0.07 | 1.54 (0.43, 5.52) | 0.51 | 1.7 (0.68, 4.23) | 0.26 | ||
| Medical history | ||||||||
| Diabetes mellitus | 1.3 (0.82, 2.05) | 0.27 | ||||||
| Hypertension | 2.02 (1.27, 3.22) | 0.003* | 1.91 (1.07, 3.41) | 0.028* | 1.36 (0.88, 2.1) | 0.16 | ||
| IHD | 1.95 (1.25, 3.05) | 0.003* | 1.23 (0.67, 2.23) | 0.51 | 1.17 (0.75, 1.81) | 0.49 | ||
| CHF | 1.59 (0.98, 2.57) | 0.05 | 1.28 (0.64, 2.54) | 0.49 | 1.35 (0.81, 2.24) | 0.24 | ||
| Previous AKI | 2.88 (2.06, 4.03) | <0.001** | 1.93 (1.13, 3.32) | 0.017** | 2.13 (1.44, 3.14) | <0.001** | ||
| Admission etiology | ||||||||
| CA | 1 | 1 | 1 | |||||
| CV | 0.47 (0.19,1.14) | 0.1 | 1.03 (0.26, 4.02) | 0.97 | 1.5 (0.58, 3.9) | 0.4 | ||
| GI | 0.51 (0.18, 1.42) | 0.19 | 0.68 (0.16, 3.2.98) | 0.61 | 0.82 (0.28, 2.39) | 0.72 | ||
| ID | 1.34 (0.59, 3.05) | 0.49 | 1.9 (0.55, 6.61) | 0.31 | 2.93 (1.23, 6.98) | 0.015* | ||
| Others | 0.9 (0.39, 2.1) | 0.81 | 2.2 (0.6, 8.14) | 0.24 | 2.73 (1.11, 6.68) | 0.03* | ||
| Vital signs and other clinical parameters during admission | ||||||||
| Pulse max, per 1/min increase | 1.028 (1.02, 1.037) | <0.001** | 1.00 (0.99–1.02) | 0.64 | 1.01 (1.00, 1.02) | 0.03* | ||
| SBP min, per 1 mm Hg increase | 0.97 (0.96, 0.98) | <0.001** | 0.98 (0.97, 0.99) | 0.002* | 0.98 (0.97, 0.99) | <0.001** | ||
| DBP min, per 1 mm Hg increase | 0.95 (0.94, 0.97) | <0.001** | ||||||
| Sat O2 min per 1% increase | 0.97 (0.95, 0.99) | <0.001** | 1.00 (0.98, 1.02) | 0.95 | 1.01 (0.99, 1.02) | 0.55 | ||
| Medications during admission | ||||||||
| Steroids average dose, per 1 mg of prednisone increase | 1.011 (1.005, 1.016) | <0.001** | ||||||
| Loop diuretics use | 1.52 (1.04, 2.22) | 0.03* | 1.22 (0.66, 2.26) | 0.53 | 1.1 (0.71, 1.7) | 0.66 | ||
| PPI use | 1.83 (1.2, 2.77) | 0.0047** | 1.05 (0.56, 1.94) | 0.89 | 0.99 (0.64, 1.51) | 0.95 | ||
| Laboratory results during admission | ||||||||
| Lymphocyte absolute average per 1 K/μL increase | 0.94 (0.82, 1.07) | 0.36 | ||||||
| Lymphocyte absolute min per 1 K/μL increase | 0.76 (0.6, 0.95) | 0.015* | 1.02 (0.87, 1.19) | 0.79 | 1.0 (0.87, 1.15) | 0.99 | ||
| Hemoglobin average per 1g/dL increase | 0.74 (0.67, 0.82) | <0.001** | ||||||
| Hemoglobin min per 1g/dL increase | 0.75 (0.69, 0.81) | <0.001** | 0.95 (0.84, 1.08) | 0.41 | 0.9 (0.82, 0.98) | 0.016 | ||
| eGFR baseline (CKD-EPI)** per 1 mL/min increase | 0.999 (0.99, 1.01) | 0.8 | 1.01 (0.99, 1.02) | 0.44 | 1.00 (0.99, 1.01) | 0.42 | ||
| Glucose max per 1 mg/dL increase | 1.005 (1.00, 1.01) | <0.001** | 1.00 (1.00, 1.01) | 0.028 | 1.00 (1.00, 1.01) | <0.001** | ||
| Glucose min per 1 mg/dL increase | 0.98 (0.98, 0.99) | <0.001** | ||||||
| Albumin average per 1 g/dL increase | 0.59 (0.45, 0.8) | <0.001** | ||||||
| Albumin min per 1 g/dL increase | 0.2 (0.14, 0.28) | <0.001** | 0.51 (0.29, 0.92) | 0.025 | 0.42 (0.27, 0.64) | <0.001** | ||
| Globulins max per 1 g/dL increase | 1.87 (1.36, 2.56) | <0.001** | ||||||
| Globulins min per 1 g/dL increase | 0.72 (0.5, 1.04) | 0.08 | 1.02 (0.62, 1.69) | 0.94 | 1.05 (0.72, 1.51) | 0.81 | ||
| Tacrolimus trough level max per 1 μg/L increase | 1.065 (1.02, 1.11) | 0.0065* | 1.08 (1.02, 1.13) | 0.005* | ||||
Univariate and multivariate stepwise mixed effect logistic regression analysis for AKI during admission in RTRs.
*p < 0.05; **p < 0.01.
***eGFR was calculated according to the following CKD-EPI formula: eGFR = 141* min (Scr/k, 1)α * max (Scr/k, 1)-1.209 * 0.993Age * 1.018 * 1.159 (if black) (where Scr - standardized serum creatinine; k = 0.7 if female, 0.9 if male; α = −0.329 if female, −0.411 if male; min = the minimum of Scr/k of 1; max = the maximum of Scr/k or 1).
ADPKD, autosomal dominant polycystic kidney disease; CA, cancers; CHF, congestive heart failure; CV, cardiovascular disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; ESRD, end stage renal disease, GI, gastrointestinal; ID, infectious diseases; IHD, ischemic heart disease; PPI, protein pump inhibitor; RTR, renal transplant recipients; SBP, systolic blood pressure. Bold values are the p values which are significant (<0.05 or <0.01).
Outcomes of AKI During Admission in RTRs
We examined four outcomes of AKI during admission: readmission within 90 days, mortality during admission, overall mortality, and LOS.
Readmission in 90 Days
In a mixed effect logistic regression analysis, in-hospital AKI increased the odds for readmission within 90 days by 95% (OR 1.95, 95% CI 1.35–2.81, p < 0.001). For every increase in minimum hemoglobin of 1 g/dL, the odds for readmission in 90 days decreased by 8% (OR 0.92, 95% CI 0.85–0.99, p = 0.02; Table 5).
TABLE 5
| Effect | Odds ratio (95% CI) | p |
|---|---|---|
| Admission age | 1.01 (0.99–1.03) | 0.17 |
| Gender, F vs. M | 0.93 (0.62–1.38) | 0.72 |
| Hypertension, yes vs. no | 1.34 (0.91–1.97) | 0.14 |
| In-hospital AKI, yes vs.no | 1.95 (1.35–2.81) | <0.001** |
| SBP min (for every increase of 1 mm Hg) | 1.0 (0.99–1.01) | 0.93 |
| Albumin min per 1g/dL increase | 0.76 (0.53–1.1) | 0.15 |
| Glucose max per 1 mg/dL increase | 1.0 (0.99–1.0) | 0.94 |
| Hemoglobin min per 1 g/dL increase | 0.92 (0.85–0.99) | 0.02* |
Multivariate mixed effect logistic regression analysis for readmission within 90 Days in RTRs.
*p < 0.05; **p < 0.01.
RTR, renal transplant recipients; SBP, systolic blood pressure. Bold values are the p values which are significant (<0.05 or <0.01).
Mortality During Admission
In a mixed effect logistic regression analysis, admission age, AKI stage 3 vs. no AKI, minimum SBP and minimum albumin during admission were found to be independent predictors for in-hospital mortality. The odds of mortality during admission were four times higher in RTRs with AKI stage 3 vs. RTRs with no AKI during admission (OR 4.0, 95% CI 1.38–11.6, p = 0.01; Table 6).
TABLE 6
| Effect | Odds ratio (95% CI) | p |
|---|---|---|
| Admission age (for every increase in 1 year) | 1.05 (1.01–1.09) | 0.01* |
| Gender, F vs. M | 0.88 (0.35–2.18) | 0.77 |
| Reference- no AKI during admission | ||
| AKI stage 1 | 0.65 (0.16–2.67) | 0.55 |
| AKI stage 2 | 2.76 (0.86–9.98) | 0.09 |
| AKI stage 3 | 4.00 (1.38–11.6) | 0.01* |
| SBP min (for every increase of 1 mm Hg) | 0.97 (0.95–0.98) | <0.001** |
| Albumin min per 1 g/dL increase | 0.21 (0.08–0.55) | 0.001** |
| Glucose max per 1 mg/dL increase | 1.0 (0.99–1.0) | 0.56 |
| Hemoglobin min per 1 g/dL increase | 0.99 (0.85–1.17) | 0.94 |
Multivariate mixed effect logistic regression analysis for mortality during admission in RTRs.
*p < 0.05; **p < 0.01.
RTR, renal transplant recipients; SBP, systolic blood pressure. Bold values are the p values which are significant (<0.05 or <0.01).
Overall Mortality
Figure 2 shows Kaplan–Meier curves for time to death according to the presence and severity of AKI in the last admission for each patient. In a multivariable Cox proportional hazards regression model for long-term mortality, transplant age, diabetic nephropathy vs. all other ESRD etiologies, and presence of AKI vs. no AKI in any admission were associated with a 1.08-fold (95% CI 1.06–1.1), 1.95-fold (95% CI 1.27–2.99) and 1.51-fold (95% CI 1.01–2.25) increased risk of death, respectively (Table 7).
FIGURE 2
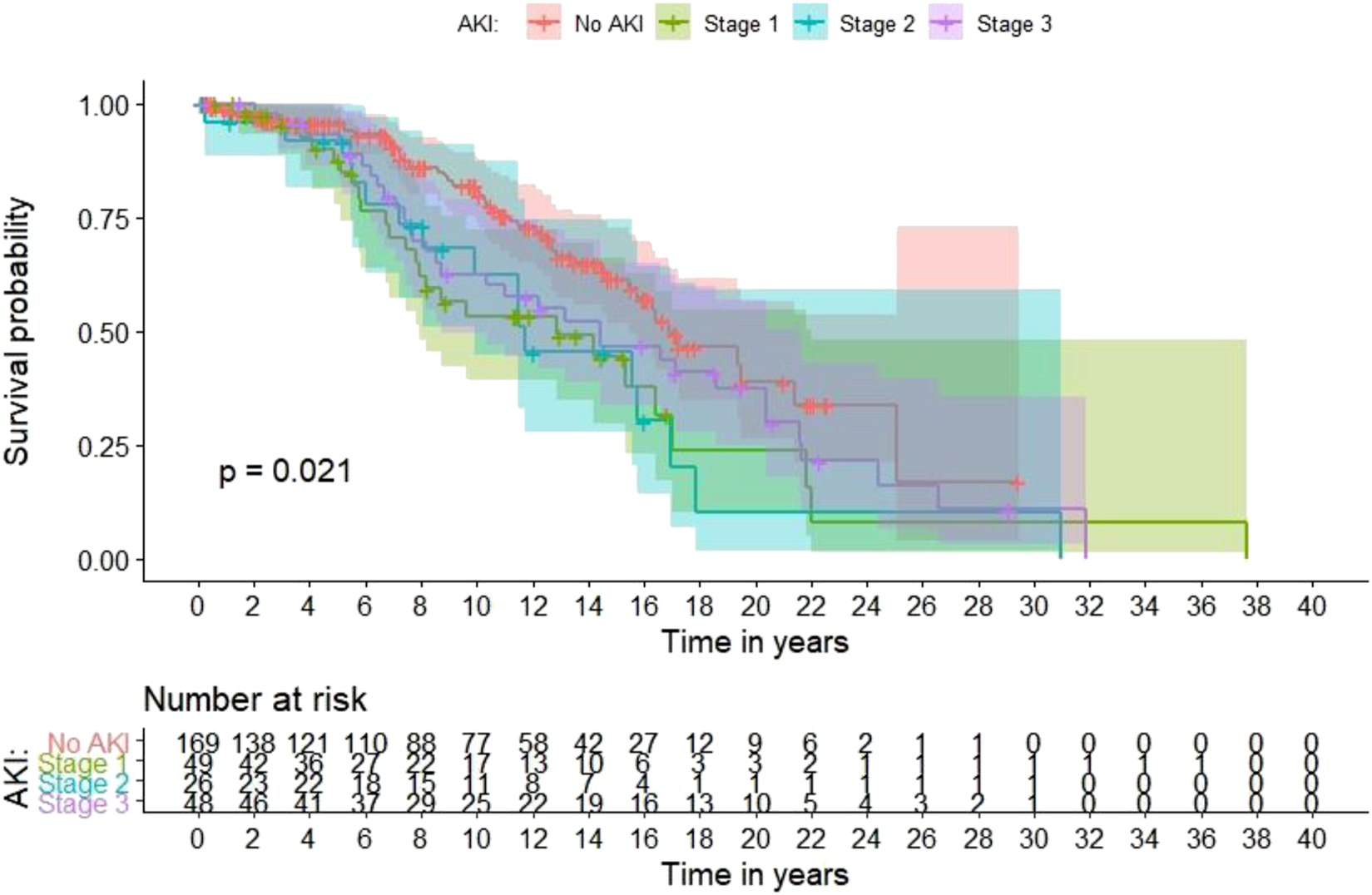
Long-term mortality based on the occurrence and severity of in-hospital AKI in the last admission for each patient.
TABLE 7
| Effect | Hazard ratio (95% CI) | p |
|---|---|---|
| Transplant age | 1.08 (1.06–1.1) | <0.001** |
| Gender, F vs. M | 0.93 (0.61–1.4) | 0.72 |
| Diabetic nephropathy vs. all other ESRD etiologies | 1.95 (1.27–2.99) | 0.002** |
| AKI, ever vs. never | 1.51 (1.01–2.25) | 0.04* |
Multivariate cox regression hazard model for overall mortality in RTRs.
*p < 0.05;
**p < 0.01.
RTR, renal transplant recipients; ESRD, end stage renal disease. Bold values are the p values which are significant (<0.05 or <0.01).
Length of Stay
Figure 3 shows a box-plot diagram for in-hospital LOS according to the presence and severity of in-hospital AKI. In a multivariable linear mixed model for LOS, a major admission diagnosis of a cancer significantly prolonged the LOS compared to all other admission etiologies. For every 1 mm Hg increase in minimum SBP, LOS was shortened by 1% (0.99, 95% CI 0.98–0.99, p < 0.001). Use of a calcineurin inhibitor (tacrolimus or cyclosporine) during admission increased the LOS by 41% (1.41, 95% CI 1.26–1.58, p < 0.001). Loop diuretic use, minimum hemoglobin, maximum glucose and minimum albumin during admission were independently associated with LOS. AKI during admission was not found to be an independent predictor for hospital LOS (Table 8).
FIGURE 3
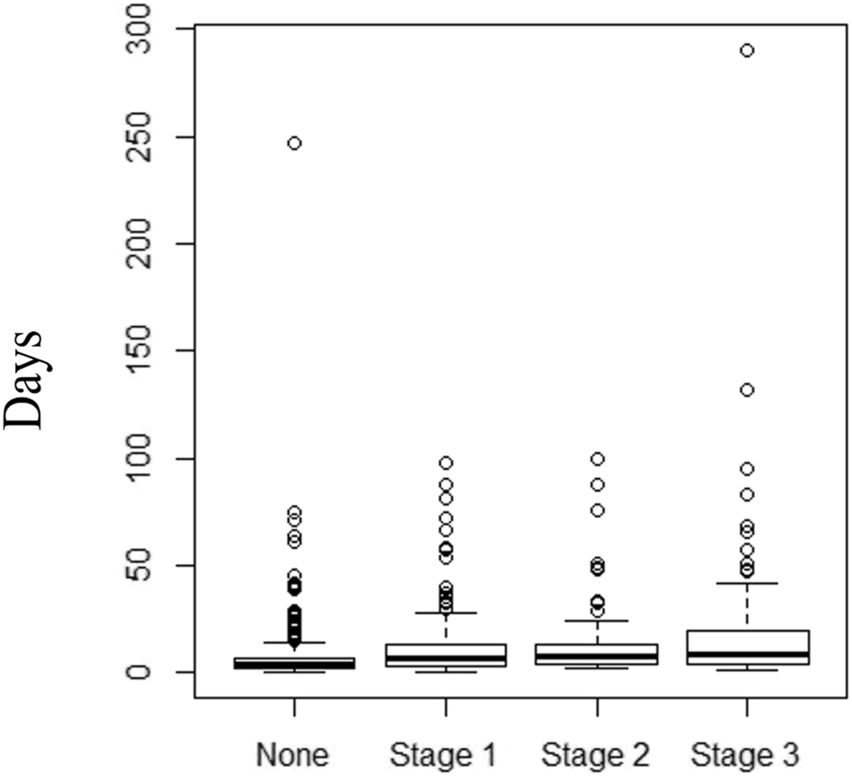
Box-plot diagram for in-hospital length of stay (days) in RTR with no AKI, and AKI stages 1,2 and 3.
TABLE 8
| Effect | Hazard ratio (95% CI) | p |
|---|---|---|
| Admission age (for every increase in 1 year) | 0.99 (0.99–1.00) | 0.003* |
| Gender, F vs. M | 0.93 (0.81–1.06) | 0.25 |
| Diabetic nephropathy vs. all other ESRD etiologies | 1.13 (0.96–1.33) | 0.13 |
| IHD, yes vs. no | 1.1 (0.97–1.26) | 0.13 |
| AKI during admission yes vs. no | 1.04 (0.92–1.18) | 0.5 |
| Major admission diagnosis | ||
| CA | References | |
| CV | 0.58 (0.44–0.76) | <0.001** |
| GI | 0.57 (0.42–0.77) | <0.001** |
| ID | 0.69 (0.53–0.89) | 0.005* |
| SBP min (for every increase in 1 mm Hg) | 0.99 (0.98–0.99) | <0.001** |
| Loop diuretics use | 1.27 (1.12–1.43) | <0.001** |
| PPI use | 1.05 (0.93–1.20) | 0.4 |
| CNI use | 1.41 (1.26–1.58) | <0.001** |
| Hemoglobin min per 1 g/dL increase | 0.97 (0.94–0.99) | 0.01* |
| Glucose max per 1 mg/dL increase | 1.00 (1.00–1.00) | <0.001 |
| Albumin min per 1 g/dL increase | 0.65 (0.57–0.73) | <0.001** |
Multivariate linear mixed model for LOS during admission in RTRs.
*p < 0.05; **p < 0.01.
CA, cancers; CNI, calcineurin inhibitors; CV, cardiovascular disease; GI, gastrointestinal disease; ID, infectious diseases; IHD, ischemic heart disease; PPI, protein pump inhibitor; RTR, renal transplant recipients; SBP, systolic blood pressure. Bold values are the p values which are significant (<0.05 or <0.01).
Discussion
In this study of 292 RTRs, with a total of 807 non-ICU admissions, we found a 51% rate (149/292) of any AKI over multiple hospital admissions. AKI during admission was observed in 36.8% (297/807) of total admissions. Of 297 admissions with AKI, stages 1, 2 and 3 were recorded for 134 (45.1%), 70 (23.6%) and 93 (31.3%) admissions, respectively. Multivariable mixed effect models for AKI during admission revealed that an AKI in a previous admission doubled the odds for AKI in the subsequent admission. The odds for AKI during an admission were almost three times higher with major diagnosis of infectious etiology during admission. In addition, a medical history of hypertension, minimum SBP, minimum hemoglobin, albumin and tacrolimus maximum trough level were significantly associated with AKI during admission. AKI during admission was associated with adverse events, i.e., for patients who developed AKI, LOS and mortality during admission rose and rates of readmission within 90 days increased with worsening outcomes as AKI severity increased. The overall mortality risk was also higher in RTRs with any AKI vs. no AKI during admission.
The overall incidence of AKI developing 3 months or later after kidney transplantation, excluding RTRs with deceased donor transplants and recipients of second or third transplants, was 20.4% (16). In seeking to compare this finding with values in the literature, we found that there are only very few studies dealing with the subject. In pediatric kidney transplant recipients, the incidence of AKI was 37% over a study period of 12 years (17). A very much lower value – 3,066 of 27,232 transplant recipients (11.3%) – was reported in the only study focused on in-hospital AKI (4181 hospitalizations) during the first three post-transplant years. In that study, AKI was identified by the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM), which has limited sensitivity, and therefore the overall incidence of AKI was probably underestimated (12). Based on Scr levels pre and post admission, we detected AKI in a median time from transplant of 7.7 years (IQR 4.77–13.48) in 149/292 (51%) of RTRs, with a total of 513 admissions (1-10 admissions per person). Our analysis provides a more accurate assessment of the higher incidence of in-hospital AKI in RTRs compared to the non-transplant population, in which AKI occurs in 4%–20% of hospitalized patients (16, 18, 19). Similarly to our findings, a higher rate of AKI following cardiac surgery (46% vs. 28%) was observed in RTRs compared with non-RTRs (2).
In a study of 11,683 patients developing in-hospital AKI, 2954 (25%) were re-hospitalized with recurrent AKI within 12 months of discharge (20), with each episode of recurrence conferring an increased risk for progression to chronic kidney disease (21). Analysis of a large database of about 150,000 patients revealed that approximately 20% were readmitted with AKI and about 10% were seen in an emergency room within 30 days of discharge (22). We found the readmission rate within 90 days to be 51.9% vs. 29% in admissions without in-hospital AKI (p < 0.001). Moreover, the severity of AKI also affected the 90-day readmission rate, which reached 62.4% in stage 3 as opposed to 48.5% in stage 1 AKI. Our study is the first to show the negative effects of in-hospital AKI on the readmission rate and subsequent AKI events in RTRs.
It is not surprising that a major diagnosis of an infection was associated with in-hospital AKI; for example, in a study conducted in Italy the incidence of in-hospital AKI was 31.7% during the COVID-19 pandemic compared to 25.9% during the pre-COVID-19 period (23). RTRs are prone to infections and complications of infections, given the immunosuppressive agents they receive to prevent rejection. Infections are commonly complicated by AKI secondary to sepsis associated with hemodynamic instability, volume depletion, and the nephrotoxicity of antibiotics, among other factors. A medical history of hypertension, associated with oxidative stress and endothelial dysfunction (24), was also found to be an independent predictor for in-hospital AKI in our population, as previously described in patients with AKI following surgical resection of malignant pleural mesothelioma (25).
Given the large number and extensive variety of the components of our dataset (different vital signs, clinical parameters, medications and laboratory results during admission) that were retrieved as possible confounders, we were able to demonstrate associations of SBP, hemoglobin, albumin and maximum tacrolimus trough level with in-hospital AKI. CNI nephrotoxicity, a well-known complication of CNI use (26, 27), remains the leading cause of renal failure after transplantation of a non-renal organ (28, 29). Similar abnormalities have been found when CNIs are used in other settings, for example, in patients with psoriasis (30). The pathophysiology of acute CNI nephrotoxicity is related to profound alterations in renal vascular resistance and blood flow in the afferent and efferent arterioles and even a reduced diameter of the afferent arterioles (27). In line with our findings, higher vs. lower preoperative CNI trough levels (73% vs. 36%) have been associated with higher rates of AKI following cardiac surgery in RTRs (2).
The pathophysiology of low SBP and a low hemoglobin level associated AKI is related to the reduction in perfusion pressure and oxygenation, leading to ischemic injury. Renal ischemia-reperfusion injury in kidney transplantation leads to AKI, delayed graft function, and even graft loss (31). Kidney transplantation involves implantation of denervated kidneys, with impairment of blood flow autoregulation, rendering the renal allograft highly susceptible to ischemic injury and subsequent inflammation and cell death. A reduced nephron mass in RTRs may also increase their susceptibility to ischemic injury. Furthermore, renal allograft ischemia may exacerbate immune-related mechanisms of allograft injury, as manifested by the effect of cold and warm ischemia times on graft function and rejection (32).
AKI is common in RTRs and confers a high risk for graft failure and death (12, 17, 33). In populations other than RTRs, AKI has been associated with increased LOS and higher mortality (25, 34). We are the first to describe the association of in-hospital AKI in RTRs with increased LOS and mortality during admission. Our study is probably underpowered to detect an association between in-hospital mortality and milder AKI, found in studies of non-transplant patients (25). In addition, we found a strong association of AKI with overall mortality over a period of more than 30 years.
Several limitations should be mentioned, including the retrospective study design. In addition, minimum Scr during admission used as baseline Scr in recipients with no Scr within 120 days prior to admission or within 150 days from transplant may not reflect baseline Scr as it could be elevated due to AKI prior to admission, there is no information about the exact timing of maximum Scr during admission, rejection, use of erythropoietin stimulating agents, admissions to other hospitals, transplant loss, renal replacement therapy or recovery from AKI, mortality (death with a functioning graft) and death-censored graft loss. Urine output criteria were not used. In addition, the use of serum creatinine levels to estimate GFR has limitations in assessing kidney function. The strengths of this study include the large size of the cohort, creatinine-based definitions to capture index and recurrent AKI, and the power to examine multiple potential confounders. Nonetheless, we cannot exclude potential residual confounding as in-hospital AKI may be a surrogate for disease severity.
In conclusion, in-hospital AKI in RTRs is an independent risk factor associated with poor short- and long-term outcomes. RTRs with an AKI during admission should be followed up closely, with specific monitoring after discharge to reduce the risk of rehospitalization and death. Efforts should be made to identify patients at high risk for AKI, to develop strategies to prevent AKI during admission, and to minimize adverse outcomes. RTRs should be closely monitored during admission to prevent hypotension, anemia, and hypoalbuminemia. The association of CNI with in-hospital AKI further emphasizes the importance of individualized tailoring of immunosuppressive therapy based on rejection vs. infection risk to prevent complications associated with over immunosuppression, including infections, which are independently associated with in-hospital AKI and AKI itself.
Statements
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The study was approved by the local ethics committee in Sheba medical center (IRB approval number: SMC-70-5320). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
TH: Conception and design, data acquisition, data interpretation, writing, revising; BO: Data analysis; NS: Data acquisition; LL: Data interpretation; GS: Conception and design; PB: Data acquisition; KC-H: Data interpretation; EM: Revising; EG: Revising; EZ: Conception and design, data interpretation; MS: conception and design, data interpretation, revising. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors gratefully acknowledge the invaluable contribution of Dr. Suhrut S. Waikar, Head of the Nephrology Department at Boston Medical Center, Boston, Massachusetts for critical reading the paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
BMI, body mass index; CHF, congestive heart failure; CI, confidence interval; CNI, calcineurin inhibitor; DBP, diastolic blood pressure; DM, diabetes; eGFR, estimated glomerular filtration rate; ESRD, end stage renal disease; HTN, hypertension; ICU, intensive care unit; IHD, ischemic heart disease; KDIGO, Kidney Disease Improving Global Outcomes; LOS, length of stay; MPA, mycophenolic acid; OR, odds ratio; RTR, renal transplant recipients; SBP, systolic blood pressure; Scr, serum creatinine; SD, standard deviation.
References
1.
Cruz DN de Cal M Garzotto F Perazella MA Lentini P Corradi V et al Plasma Neutrophil Gelatinase-Associated Lipocalin Is an Early Biomarker for Acute Kidney Injury in an Adult ICU Population. Intensive Care Medicine. Mar (2010) 36(3):444–51. 10.1007/s00134-009-1711-1
2.
Hundemer GL Srivastava A Jacob KA Acute Kidney Injury in Renal Transplant Recipients Undergoing Cardiac Surgery. Nephrology, Dialysis, Transplantation: Official Publication Eur Dial Transpl Assoc - Eur Ren Assoc (2021) 36(1):185–96. 10.1093/ndt/gfaa063
3.
Lassnigg A Schmidlin D Mouhieddine M Bachmann LM Druml W Bauer P et al Minimal Changes of Serum Creatinine Predict Prognosis in Patients after Cardiothoracic Surgery: a Prospective Cohort Study. J Am Soc Nephrol : JASN. Jun (2004) 15(6):1597–605. 10.1097/01.asn.0000130340.93930.dd
4.
Loef BG Epema AH Smilde TD Henning RH Ebels T Navis G et al Immediate Postoperative Renal Function Deterioration in Cardiac Surgical Patients Predicts In-Hospital Mortality and Long-Term Survival. J Am Soc Nephrol : JASN. Jan (2005) 16(1):195–200. 10.1681/ASN.2003100875
5.
Chertow GM Burdick E Honour M Bonventre JV Bates DW . Acute Kidney Injury, Mortality, Length of Stay, and Costs in Hospitalized Patients. J Am Soc Nephrol : JASN (2005) 16(11):3365–70. 10.1681/ASN.2004090740
6.
Smith GL Vaccarino V Kosiborod M Lichtman JH Cheng S Watnick SG et al Worsening Renal Function: what Is a Clinically Meaningful Change in Creatinine during Hospitalization with Heart Failure? J Card Fail Feb (2003) 9(1):13–25. 10.1054/jcaf.2003.3
7.
Hoste EA Lameire NH Vanholder RC Benoit DD Decruyenaere JM Colardyn FA . Acute Renal Failure in Patients with Sepsis in a Surgical ICU: Predictive Factors, Incidence, Comorbidity, and Outcome. J Am Soc Nephrol : JASN (2003) 14(4):1022–30. 10.1097/01.asn.0000059863.48590.e9
8.
Malhotra R Kashani KB Macedo E Kim J Bouchard J Wynn S et al A Risk Prediction Score for Acute Kidney Injury in the Intensive Care Unit. Nephrology, Dialysis, Transplantation : Official Publication Eur Dial Transpl Assoc - Eur Ren Assoc (2017) 32(5):814–22. 10.1093/ndt/gfx026
9.
Chawla LS Abell L Mazhari R Egan M Kadambi N Burke HB et al Identifying Critically Ill Patients at High Risk for Developing Acute Renal Failure: a Pilot Study. Kidney International Nov (2005) 68(5):2274–80. 10.1111/j.1523-1755.2005.00686.x
10.
Tomasev N Glorot X Rae JW Zielinski M Askham H Saraiva A et al A Clinically Applicable Approach to Continuous Prediction of Future Acute Kidney Injury. Nat Aug (2019) 572(7767):116–9. 10.1038/s41586-019-1390-1
11.
Park S Cho H Park S Lee S Kim K Yoon HJ et al Simple Postoperative AKI Risk (SPARK) Classification before Noncardiac Surgery: A Prediction Index Development Study with External Validation. J Am Soc Nephrol : JASN. Jan (2019) 30(1):170–81. 10.1681/ASN.2018070757
12.
Mehrotra A Rose C Pannu N Gill J Tonelli M Gill JS . Incidence and Consequences of Acute Kidney Injury in Kidney Transplant Recipients. Am J kidney Dis : official J Natl Kidney Found (2012) 59(4):558–65. 10.1053/j.ajkd.2011.11.034
13.
Perico N Cattaneo D Sayegh MH Remuzzi G . Delayed Graft Function in Kidney Transplantation. Lancet (2004) 364(9447):1814–27. 10.1016/S0140-6736(04)17406-0
14.
Siedlecki A Irish W Brennan DC . Delayed Graft Function in the Kidney Transplant. Am J Transplant : official J Am Soc Transplant Am Soc Transpl Surgeons. Nov (2011) 11(11):2279–96. 10.1111/j.1600-6143.2011.03754.x
15.
Shimizu T Kato S Kijima Y Kano K Horiuchi T Iida S et al Japanese Single-Center Experience of Kidney Transplant: Experience in a Japanese Private Hospital. Transplant Proc (2022) 54:282–5. 10.1016/j.transproceed.2021.09.074
16.
Waikar SS Liu KD Chertow GM . Diagnosis, Epidemiology and Outcomes of Acute Kidney Injury. Clin J Am Soc Nephrol : CJASN. May (2008) 3(3):844–61. 10.2215/CJN.05191107
17.
Alkandari O Nguyen L Hebert D Langlois V Jawa NA Parekh RS et al Acute Kidney Injury in Children with Kidney Transplantation. Clin J Am Soc Nephrol : CJASN. Nov 7 (2018) 13(11):1721–9. 10.2215/CJN.02440218
18.
Ricci Z Cruz D Ronco C . The RIFLE Criteria and Mortality in Acute Kidney Injury: A Systematic Review. Kidney International Mar (2008) 73(5):538–46. 10.1038/sj.ki.5002743
19.
Ishani A Xue JL Himmelfarb J Eggers PW Kimmel PL Molitoris BA et al Acute Kidney Injury Increases Risk of ESRD Among Elderly. J Am Soc Nephrol : JASN. Jan (2009) 20(1):223–8. 10.1681/ASN.2007080837
20.
Siew ED Parr SK Abdel-Kader K Eden SK Peterson JF Bansal N et al Predictors of Recurrent AKI. J Am Soc Nephrol : JASN. Apr (2016) 27(4):1190–200. 10.1681/ASN.2014121218
21.
Thakar CV Christianson A Himmelfarb J Leonard AC . Acute Kidney Injury Episodes and Chronic Kidney Disease Risk in Diabetes Mellitus. Clin J Am Soc Nephrol : CJASN. Nov (2011) 6(11):2567–72. 10.2215/CJN.01120211
22.
Silver SA Harel Z McArthur E Nash DM Acedillo R Kitchlu A et al 30-Day Readmissions after an Acute Kidney Injury Hospitalization. Am J Med (2017) 130(2):163–72. 10.1016/j.amjmed.2016.09.016
23.
Esposito P Russo E Picciotto D Cappadona F Battaglia Y Traverso GB et al Changes of Acute Kidney Injury Epidemiology during the COVID-19 Pandemic: A Retrospective Cohort Study. J Clin Med (2022) 11(12):3349. 10.3390/jcm11123349
24.
Xu D Hu YH Gou X Li FY Yang XYC Li YM et al Oxidative Stress and Antioxidative Therapy in Pulmonary Arterial Hypertension. Molecules (2022) 27(12):3724. 10.3390/molecules27123724
25.
Hod T Freedberg KJ Motwani SS Chen M Frendl G Leaf DE et al Acute Kidney Injury after Cytoreductive Surgery and Hyperthermic Intraoperative Cisplatin Chemotherapy for Malignant Pleural Mesothelioma. J Thorac Cardiovasc Surg (2021) 161(4):1510–8. 10.1016/j.jtcvs.2020.05.033
26.
de Mattos AM Olyaei AJ Bennett WM . Nephrotoxicity of Immunosuppressive Drugs: Long-Term Consequences and Challenges for the Future. Am J kidney Dis : official J Natl Kidney Found Feb (2000) 35(2):333–46. 10.1016/s0272-6386(00)70348-9
27.
Naesens M Kuypers DR Sarwal M . Calcineurin Inhibitor Nephrotoxicity. Clin J Am Soc Nephrol : CJASN. Feb (2009) 4(2):481–508. 10.2215/CJN.04800908
28.
Ojo AO Held PJ Port FK Wolfe RA Leichtman AB Young EW et al Chronic Renal Failure after Transplantation of a Nonrenal Organ. New Engl J Med (2003) 349(10):931–40. 10.1056/NEJMoa021744
29.
Kubal C Cockwell P Gunson B Jesky M Hanvesakul R Dronavalli V et al Chronic Kidney Disease after Nonrenal Solid Organ Transplantation: a Histological Assessment and Utility of Chronic Allograft Damage index Scoring. Transplant Feb 27 (2012) 93(4):406–11. 10.1097/TP.0b013e318240e984
30.
Young EW Ellis CN Messana JM Johnson KJ Leichtman AB Mihatsch MJ et al A Prospective Study of Renal Structure and Function in Psoriasis Patients Treated with Cyclosporin. Kidney Int (1994) 46(4):1216–22. 10.1038/ki.1994.387
31.
Wei X Deng W Dong Z Xie Z Zhang J Wang R et al Identification of Subtypes and a Delayed Graft Function Predictive Signature Based on Ferroptosis in Renal Ischemia-Reperfusion Injury. Front Cel Dev Biol (2022) 10:800650. 10.3389/fcell.2022.800650
32.
Oweira H Ramouz A Ghamarnejad O Khajeh E Ali-Hasan-Al-Saegh S Nikbakhsh R et al Risk Factors of Rejection in Renal Transplant Recipients: A Narrative Review. Journal Of Clinical Medicine. Mar (2022)(5) 11. 10.3390/jcm11051392
33.
Nakamura M Seki G Iwadoh K Nakajima I Fuchinoue S Fujita T et al Acute Kidney Injury as Defined by the RIFLE Criteria Is a Risk Factor for Kidney Transplant Graft Failure. Clin Transplant (2012) 26(4):520–8. 10.1111/j.1399-0012.2011.01546.x
34.
Fidalgo P Ahmed M Meyer SR Lien D Weinkauf J Cardoso FS et al Incidence and Outcomes of Acute Kidney Injury Following Orthotopic Lung Transplantation: a Population-Based Cohort Study. Nephrology, Dialysis, Transplantation : Official Publication Eur Dial Transpl Assoc - Eur Ren Assoc (2014) 29(9):1702–9. 10.1093/ndt/gfu226
Summary
Keywords
acute kidney injury, calcineurin inhibitors, readmission, renal transplant recipients, mortality abbreviations
Citation
Hod T, Oberman B, Scott N, Levy L, Shlomai G, Beckerman P, Cohen-Hagai K, Mor E, Grossman E, Zimlichman E and Shashar M (2023) Predictors and Adverse Outcomes of Acute Kidney Injury in Hospitalized Renal Transplant Recipients. Transpl Int 36:11141. doi: 10.3389/ti.2023.11141
Received
18 December 2022
Accepted
27 February 2023
Published
09 March 2023
Volume
36 - 2023
Updates
Copyright
© 2023 Hod, Oberman, Scott, Levy, Shlomai, Beckerman, Cohen-Hagai, Mor, Grossman, Zimlichman and Shashar.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tammy Hod, Tamar.Hod@sheba.health.gov.il
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.