Abstract
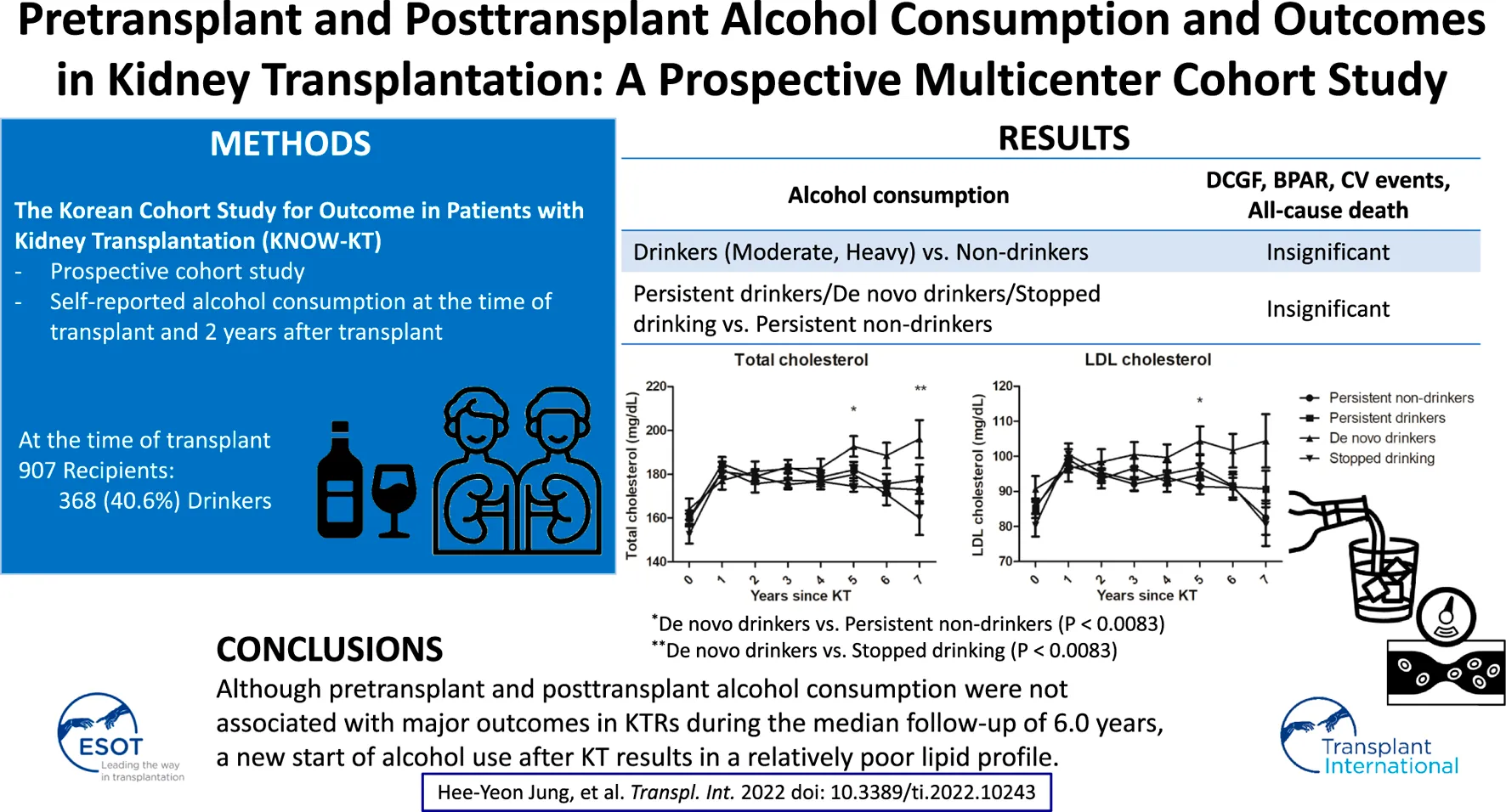
The impact of pretransplant and posttransplant alcohol consumption on outcomes in kidney transplant recipients (KTRs) is uncertain. Self-reported alcohol consumption was obtained at the time of transplant and 2 years after transplant in a prospective cohort study. Among 907 KTRs, 368 (40.6%) were drinkers at the time of transplant. Compared to non-drinkers, alcohol consumption did not affect the risk of death-censored graft failure (DCGF), biopsy-proven acute rejection (BPAR), cardiovascular events, or all-cause mortality. Compared to persistent non-drinkers, the development of DCGF, BPAR, cardiovascular events, all-cause mortality, or posttransplant diabetes mellitus was not affected by the alcohol consumption pattern (persistent, de novo, or stopped drinking) over time. However, de novo drinkers had a significantly higher total cholesterol (p < 0.001) and low-density lipoprotein cholesterol levels (p = 0.005) compared to persistent non-drinkers 5 years after transplant, and had significantly higher total cholesterol levels (p = 0.002) compared to the stopped drinking group 7 years after transplant, even after adjusting for the use of lipid-lowering agents, age, sex, and body mass index. Although pretransplant and posttransplant alcohol consumption were not associated with major outcomes in KTRs during the median follow-up of 6.0 years, a new start of alcohol use after KT results in a relatively poor lipid profile.
Clinical Trial Registration:clinicaltrials.gov, identifier NCT02042963.
Introduction
Though previous studies have reported that moderate alcohol consumption is associated with the improvement of some lipid profiles (1–3), as well as a reduced risk of cardiovascular events (4–6), including myocardial infarction, stroke, and heart failure, and mortality (7, 8) in the general population, recent evidence suggests that there is no safe level of moderate drinking in terms of mortality (9). However, robust evidence is lacking as to whether the potential protective effect of moderate alcohol use can be generalized to kidney transplant recipients (KTRs), or whether alcohol is an acceptable beverage for KTRs in terms of transplant outcomes. It is important to identify the effects of alcohol consumption in KTRs because transplant patients are on immunosuppressants; alcohol use may affect the metabolism of immunosuppressive agents and, thus, transplant outcomes. Alcohol metabolism by the cytochrome P450 enzyme system (CYP2E1) may be a potent enzyme inducer, and immunosuppressants are metabolized by CYP3A4; therefore, alcohol use may result in unexpected variation in immunosuppressant levels (10, 11). Moreover, KTRs have a large burden of cardiovascular complications, so it is necessary to determine the effects of alcohol consumption.
The Kidney Disease Improving Global Outcome (KDIGO) clinical practice guidelines do not provide specific guidance on alcohol consumption in KTRs (12). Surprisingly, relatively few studies have reported the effects of pretransplant (13, 14) or posttransplant (15, 16) alcohol use in KTRs, and these few have reported inconsistent results in terms of recipient mortality (13, 16). Furthermore, the impact of pretransplant and posttransplant alcohol consumption over time on major outcomes, including kidney graft survival, patient survival, biopsy-proven acute rejection (BPAR), cardiovascular events, kidney function, and glucose and lipid metabolism, has not been explored in KTRs in a prospective study design.
The present study was prospective multicenter longitudinal cohort study aiming to determine the association between pretransplant and posttransplant alcohol consumption and comprehensive outcomes in KTRs.
Methods
Study Participants
A total of 1,080 incident KTRs were enrolled from the Korean Cohort Study for Outcome in Patients with Kidney Transplantation (KNOW-KT) between 2012 and 2016 and followed up until 2020 (clinicaltrials.gov, identifier NCT02042963). After excluding 173 KTRs who had insufficient information on baseline alcohol consumption, 907 KTRs were included in this study. Among 598 KTRs with available alcohol information 2 years after transplant, 286 (47.8%) and 140 (23.4%) KTRs remained as persistent non-drinkers and persistent drinkers, respectively, and 71 (11.9%) KTRs became de novo drinkers and 101 (16.9%) KTRs stopped drinking (Figure 1).
FIGURE 1
Alcohol Consumption
Self-reported alcohol consumption was obtained from KTRs at the time of transplant and 2 years after transplant in a prospective multicenter longitudinal cohort study. Participants were asked how often they drank during the year prior to the transplant and how many drinks they drank at one time. KTRs were categorized as non-drinkers and drinkers based on baseline alcohol consumption, and alcohol consumption was categorized into two groups: moderate and heavy drinkers. The criteria for heavy drinking defined by the National Institute on Alcohol Abuse and Alcoholism (NIAAA) are as follows: for men, consuming more than four drinks on any day or more than 14 drinks per week; for women, consuming more than three drinks on any day or more than seven drinks per week (17). Pretransplant and posttransplant alcohol consumption over time was used to categorize KTRs as persistent non-drinker, persistent drinker, de novo drinker, and stopped drinking.
Outcomes
Outcomes included death-censored graft failure (DCGF), biopsy-proven acute rejection (BPAR), cardiovascular events, all-cause death, estimated glomerular filtration rate (eGFR), serum creatinine, posttransplant diabetes mellitus (PTDM), and lipid profiles. DCGF was defined as dialysis or new kidney transplant. BPAR was defined as biopsy-proven acute T cell-mediated rejection or acute antibody-medicated rejection. The Modification of Diet in Renal Disease (MDRD) study equation was used to calculate the eGFR. Cardiovascular events included myocardial infarction, unstable angina, percutaneous coronary intervention, coronary artery bypass grafting, and stroke.
Other Variables
Possible confounders for DCGF and BPAR were recipient age, donor age, recipient sex, donor sex, recipient body mass index (BMI) (18), diabetes, deceased-donor kidney transplantation (DDKT), re-transplantation, desensitization (direct crossmatch (+) plus donor-specific antibodies (+), direct crossmatch (-) plus donor-specific antibodies (+), or ABO-incompatible kidney transplantation), total number of human leukocyte antigen (HLA) mismatches, and antithymocyte globulin induction. Possible confounders for cardiovascular events and all-cause mortality were recipient age, recipient sex, recipient BMI, diabetes, hypertension, coronary artery disease, cerebrovascular disease, total cholesterol, high-density lipoprotein (HDL) cholesterol, DDKT (19), re-transplantation, desensitization, total number of HLA mismatches, antithymocyte globulin induction, use of cyclosporine or inhibitor of the mammalian target of rapamycin (sirolimus or everolimus), and steroid dose 1 year after transplantation. Possible confounders for PTDM included recipient age, recipient sex, recipient BMI, baseline HbA1c, total cholesterol, low-density lipoprotein (LDL) cholesterol, HDL cholesterol, triglycerides (TGs), re-transplantation, desensitization, total number of HLA mismatches, and antithymocyte globulin induction.
Statistical Analysis
Continuous variables were expressed as mean ± standard deviation or a median with the interquartile range (IQR). Intergroup differences were assessed by independent sample t-tests, chi-squared tests, and analysis of variance as appropriate. The Cox proportional hazards model was used to analyze the association between alcohol consumption and the development of DCGF, BPAR, cardiovascular events, or all-cause death. Logistic regression analysis was used to examine the association between alcohol consumption and the development of PTDM because posttransplant diabetes mellitus was recorded by annual follow-up after kidney transplantation and the exact date and year of occurrence could not be specified. A generalized linear mixed model with random slopes was used to determine the annual change in eGFR and serum creatinine by alcohol consumption group. Analysis of variance and the general linear model were used to determine between-group differences in the annual eGFR and lipid profiles, respectively. In the case of an overall F-test p < 0.05 when comparing the entire group, the comparison between the two groups was confirmed by Bonferroni’s post hoc method. The post hoc p-value adds six comparisons at the significance level of 0.05, so if the post hoc p-value was <0.0083, it was considered significant. When comparing outcomes between persistent non-drinkers, persistent drinkers, de novo drinkers, and KTRs who stopped drinking, events that occurred within 2 years posttransplant were excluded. Statistical analyses were performed using the SAS system for Windows, version 9.4 (SAS Institute Inc., Cary, NC, United States) and R (R Foundation for Statistical Computing, Vienna, Austria; www.r-project.org). p < 0.05 was considered significant.
Results
Baseline Characteristics
Table 1 shows the baseline characteristics according to baseline alcohol consumption. Among 907 eligible KTRs, 539 (59.4%) were non-drinkers and 368 (40.6%) were drinkers at the time of transplantation. Among the drinkers, 77.4% were moderate drinkers and 22.6% were heavy drinkers. Drinkers were significantly younger, tended to be male, had a higher proportion of diabetes and a lower proportion of coronary artery disease, were less likely to have received desensitization, and had lower total cholesterol levels compared to non-drinkers. We observed no significant differences in immunosuppressant types, doses, and drug concentrations at the time of discharge and 1 year after kidney transplantation between non-drinkers, moderate drinkers, and heavy drinkers.
TABLE 1
| Non-drinkers (n = 539) | Drinkers (n = 368) | p-value | Non-drinkers (n = 539) | Moderate drinkers (n = 285) | Heavy drinkersd (n = 83) | p-value | |
|---|---|---|---|---|---|---|---|
| Age, years | 46.5 ± 10.7 | 43.2 ± 11.7 | <0.001 | 46.5 ± 10.7c | 44.1 ± 11.3b | 40.4 ± 12.5a | <0.001 |
| Sex, male | 317 (58.8) | 269 (73.1) | <0.001 | 317 (58.8) | 205 (71.9) | 64 (77.1) | <0.001 |
| BMI, kg/m2 | 23.1 ± 3.6 | 22.7 ± 3.4 | 0.122 | 23.1 ± 3.6 | 22.7 ± 3.4 | 22.7 ± 3.4 | 0.303 |
| Diabetes | 147 (27.3) | 71 (19.3) | 0.006 | 147 (27.3) | 57 (20.0) | 14 (16.9) | 0.019 |
| Hypertension | 498 (92.4) | 336 (91.3) | 0.554 | 498 (92.4) | 262 (91.9) | 74 (89.2) | 0.601 |
| Coronary artery disease | 41 (8.0) | 15 (4.3) | 0.029 | 41 (8.0) | 12 (4.4) | 3 (3.8) | 0.090 |
| Cerebrovascular disease | 20 (3.9) | 7 (2.0) | 0.113 | 20 (3.9) | 6 (2.2) | 1 (1.3) | 0.261 |
| Donor type | |||||||
| Living | 447 (82.9) | 299 (81.3) | 0.515 | 447 (82.9) | 225 (79.0) | 74 (89.2) | 0.082 |
| Deceased | 92 (17.1) | 69 (18.8) | 92 (17.1) | 60 (21.1) | 9 (10.8) | ||
| Total number of HLA mismatches, median (IQR) | 3.0 (1.0–3.0) | 3.0 (2.0–3.5) | 0.319 | 3.0 (1.0–3.0) | 3.0 (2.0–3.0) | 3.0 (2.0–4.0) | 0.503 |
| Re-transplantation | 39 (7.2) | 21 (5.7) | 0.363 | 39 (7.2) | 18 (6.3) | 3 (3.6) | 0.452 |
| Desensitization | 154 (28.6) | 81 (22.0) | 0.027 | 154 (28.6) | 58 (20.4) | 23 (27.7) | 0.035 |
| Induction therapy | |||||||
| IL-2RB | 491 (91.1) | 338 (91.9) | 0.691 | 491 (91.1) | 263 (92.3) | 75 (90.4) | 0.795 |
| ATG | 48 (8.9) | 30 (8.2) | 48 (8.9) | 22 (7.7) | 8 (9.6) | ||
| Immunosuppressants at discharge | |||||||
| Tacrolimus | 511 (94.8) | 338 (91.9) | 0.074 | 511 (94.8) | 261 (91.6) | 77 (92.8) | 0.188 |
| Tacrolimus dose, mg/day | 5.0 (3.0–8.0) | 5.5 (4.0–8.0) | 0.434 | 5.0 (3.0–8.0) | 5.5 (4.0–8.0) | 6.0 (3.5–9.0) | 0.707 |
| Tacrolimus dose/kg | 0.10 ± 0.07 | 0.10 ± 0.06 | 0.711 | 0.10 ± 0.07 | 0.10 ± 0.07 | 0.10 ± 0.06 | 0.933 |
| Cyclosporine | 26 (4.8) | 26 (7.1) | 0.154 | 26 (4.8) | 20 (7.0) | 6 (7.2) | 0.361 |
| Cyclosporine dose, mg/day | 254.8 ± 79.7 | 257.7 ± 111.3 | 0.915 | 254.8 ± 79.7 | 266.3 ± 113.9 | 229.2 ± 106.6 | 0.712 |
| Cyclosporine dose/kg | 4.2 ± 1.6 | 4.2 ± 1.9 | 0.956 | 4.2 ± 1.6 | 4.4 ± 2.0 | 3.4 ± 1.5 | 0.466 |
| Sirolimus | 8 (3.3) | 16 (4.4) | 0.433 | 8 (3.3) | 13 (4.6) | 3 (3.6) | 0.678 |
| Everolimus | 6 (1.1) | 11 (3.0) | 0.041 | 6 (1.1) | 8 (2.8) | 3 (3.6) | 0.110 |
| Everolimus dose, mg/kg | 2.2 ± 0.7 | 1.9 ± 0.8 | 0.472 | 2.2 ± 0.7 | 1.6 ± 0.2 | 2.7 ± 1.3 | 0.063 |
| Everolimus dose/kg | 0.03 ± 0.01 | 0.03 ± 0.02 | 0.860 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.05 ± 0.03 | 0.249 |
| Steroid | 535 (99.3) | 366 (99.5) | 0.717 | 535 (99.3) | 283 (99.3) | 83 (100.0) | 1.000 |
| Steroid dose, mg/day | 16.0 (10.0–20.0) | 16.0 (20.0–24.0) | 0.054 | 16.0 (10.0–20.0) | 16.0 (10.0–24.0) | 16.0 (10.0–24.0) | 0.150 |
| Immunosuppressants 1 year posttransplant | |||||||
| Tacrolimus | 471 (87.4) | 313 (85.1) | 0.314 | 471 (87.4) | 243 (85.3) | 70 (84.3) | 0.589 |
| Tacrolimus dose, mg/day | 3.0 (2.0–5.0) | 3.0 (2.0–4.5) | 0.918 | 3.0 (2.0–5.0) | 3.0 (2.0–4.5) | 3.0 (2.0–5.0) | 0.726 |
| Tacrolimus dose/kg | 0.06 ± 0.04 | 0.06 ± 0.04 | 0.728 | 0.06 ± 0.04 | 0.06 ± 0.04 | 0.06 ± 0.04 | 0.908 |
| Tacrolimus trough levels, ng/ml | 6.2 ± 2.4 | 5.9 ± 2.2 | 0.089 | 6.2 ± 2.4 | 5.8 ± 2.1 | 6.1 ± 2.5 | 0.145 |
| Cyclosporine | 24 (4.5) | 19 (5.2) | 0.621 | 24 (4.5) | 15 (5.3) | 4 (4.8) | 0.873 |
| Cyclosporine dose, mg/day | 138.5 ± 74.8 | 125.0 ± 55.9 | 0.515 | 138.5 ± 74.8 | 116.7 ± 59.5 | 156.3 ± 23.9 | 0.799 |
| Cyclosporine dose/kg | 2.2 ± 1.4 | 2.0 ± 0.9 | 0.625 | 2.2 ± 1.4 | 1.9 ± 1.0 | 2.3 ± 0.4 | 0.536 |
| Cyclosporine trough levels, ng/ml | 103.3 ± 62.8 | 94.7 ± 42.5 | 0.615 | 103.3 ± 62.8 | 87.9 ± 41.5 | 120.5 ± 41.1 | 0.508 |
| Sirolimus | 28 (5.2) | 27 (7.3) | 0.184 | 28 (5.2) | 22 (7.2) | 5 (6.0) | 0.352 |
| Everolimus | 10 (1.9) | 7 (1.9) | 0.959 | 10 (1.9) | 4 (1.4) | 3 (3.6) | 0.425 |
| Steroid | 458 (85.0) | 316 (85.9) | 0.708 | 458 (85.0) | 247 (86.7) | 69 (83.1) | 0.676 |
| Steroid dose, mg/day | 5.0 (5.0–6.0) | 5.0 (5.0–10.0) | 0.056 | 5.0 (5.0–6.0) | 5.0 (5.0–10.0) | 5.0 (4.0–10.0) | 0.072 |
| Total cholesterol, mg/dl | 156.3 ± 41.1 | 150.7 ± 41.3 | 0.048 | 156.3 ± 41.1 | 152.0 ± 40.7 | 146.3 ± 43.2 | 0.076 |
| LDL cholesterol, mg/dl | 84.7 ± 31.4 | 81.1 ± 30.2 | 0.098 | 84.7 ± 31.4 | 82.2 ± 30.1 | 77.5 ± 30.6 | 0.127 |
| HDL cholesterol, mg/dl | 45.4 ± 16.7 | 46.1 ± 17.1 | 0.561 | 45.4 ± 16.7 | 46.2 ± 16.2 | 45.7 ± 19.9 | 0.821 |
| TGs, mg/dl | 124.2 ± 82.2 | 124.4 ± 89.8 | 0.969 | 124.2 ± 82.2 | 122.4 ± 89.5 | 131.3 ± 91.1 | 0.709 |
Baseline characteristics.
Post hoc by Bonferroni’s method (a < b < c). dThe criteria for heavy drinking defined by the National Institute on Alcohol Abuse and Alcoholism are as follows: for men, consuming more than 4 drinks on any day or more than 14 drinks per week; for women, consuming more than 3 drinks on any day or more than 7 drinks per week.
Values are given as the mean ± standard deviation or n (%) unless otherwise noted.
ATG, antithymocyte globulin; BMI, body mass index; HDL, high-density lipoprotein; HLA, human leukocyte antigen; IL-2RB, interleukin-2 receptor blocker; LDL, low-density lipoprotein; TGs, triglycerides.
Alcohol Consumption and Major Outcomes
During a median follow-up of 6.0 (IQR 4.9–7.0), 5.9 (IQR 4.7–7.0), 6.0 (IQR 5.0–7.0), and 6.1 (IQR 5.1–7.0) years, 46 DCGFs, 102 BPARs, 36 cardiovascular events, and 21 all-cause deaths occurred, respectively. Multivariate Cox regression analysis demonstrated no significant differences in the risk of DCGF, BPAR, cardiovascular events, or all-cause death between non-drinkers and drinkers (Table 2). Comparing non-drinkers, moderate drinkers, and heavy drinkers also showed consistent results. No significant differences in the risk of DCGF, BPAR, cardiovascular events, or all-cause death were observed between persistent non-drinkers and persistent drinkers, between persistent non-drinkers and de novo drinkers, or between persistent non-drinkers and KTRs who stopped drinking (Table 3).
TABLE 2
| DCGF | BPAR | Cardiovascular events | All-cause death | |||||
|---|---|---|---|---|---|---|---|---|
| Alcohol consumptiona | aHRb (95% CI) | p-value | aHRb (95% CI) | p-value | aHRc (95% CI) | p-value | aHRc (95% CI) | p-value |
| Drinker vs. Non-drinker | 0.95 (0.52–1.75) | 0.875 | 1.03 (0.68–1.54) | 0.898 | 0.54 (0.22–1.31) | 0.713 | 1.39 (0.43–4.43) | 0.581 |
| Moderate drinker vs. Non-drinker | 0.87 (0.44–1.70) | 0.680 | 1.06 (0.68–1.64) | 0.805 | 0.56 (0.22–1.45) | 0.233 | 1.57 (0.49–5.02) | 0.444 |
| Heavy drinker vs. Non-drinker | 1.37 (0.51–3.69) | 0.533 | 1.05 (0.51–2.17) | 0.896 | 0.42 (0.05–3.28) | 0.410 | 0.00 | 0.999 |
| Heavy drinker vs. Moderate drinker | 1.30 (0.43–3.90) | 0.641 | 0.94 (0.44–2.04) | 0.884 | 0.99 (0.10–10.03) | 0.991 | 0.00 | 0.997 |
Adjusted hazard ratios (aHRs) for death-censored graft failure (DCGF), biopsy-proven acute rejection (BPAR), cardiovascular events, and all-cause death based on pretransplant alcohol consumption.
The criteria for heavy drinking defined by the National Institute on Alcohol Abuse and Alcoholism are as follows: for men, consuming more than 4 drinks on any day or more than 14 drinks per week; for women, consuming more than 3 drinks on any day or more than 7 drinks per week.
Adjusted for recipient age, donor age, recipient sex, donor sex, recipient body mass index, diabetes, deceased-donor kidney transplantation, re-transplantation, desensitization, total number of human leukocyte antigen mismatches, and antithymocyte globulin induction.
Adjusted for recipient age, recipient sex, recipient body mass index, diabetes, hypertension, coronary artery disease, cerebrovascular disease, total cholesterol, high-density lipoprotein cholesterol, deceased-donor kidney transplantation, re-transplantation, desensitization, total number of human leukocyte antigen mismatches, antithymocyte globulin induction, use of cyclosporine, sirolimus, or everolimus 1 year posttransplant, and steroid dose 1 year posttransplant.
CI, confidence interval.
TABLE 3
| DCGF | BPAR | Cardiovascular events | All-cause death | |||||
|---|---|---|---|---|---|---|---|---|
| aHRa (95% CI) | p-value | aHRa (95% CI) | p-value | aHRb (95% CI) | p-value | aHRb (95% CI) | p-value | |
| Persistent non-drinkers | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | ||||
| Persistent drinkers | 0.56 (0.18–71.74) | 0.315 | 0.72 (0.12–4.20) | 0.711 | 0.00 | 0.996 | 1.07 (0.09–13.25) | 0.960 |
| De novo drinkers | 0.59 (0.13–2.64) | 0.488 | 0.87 (0.09–8.31) | 0.900 | 3.95 (0.69–22.47) | 0.122 | 1.69 (0.12–24.56) | 0.700 |
| Stopped drinking | 0.42 (0.09–1.86) | 0.251 | 1.24 (0.22–7.20) | 0.808 | 0.00 | 0.997 | 2.39 (0.17–33.16) | 0.515 |
Adjusted hazard ratios (aHRs) for death-censored graft failure (DCGF), biopsy-proven acute rejection (BPAR), cardiovascular events, and all-cause death based on pretransplant and posttransplant alcohol consumption.
Adjusted for recipient age, donor age, recipient sex, donor sex, recipient body mass index, diabetes, deceased-donor kidney transplantation, re-transplantation, desensitization, total number of human leukocyte antigen mismatches, and antithymocyte globulin induction.
Adjusted for recipient age, recipient sex, recipient body mass index, diabetes, hypertension, coronary artery disease, cerebrovascular disease, total cholesterol, high-density lipoprotein cholesterol, deceased-donor kidney transplantation, re-transplantation, desensitization, total number of human leukocyte antigen mismatches, and antithymocyte globulin induction, use of cyclosporine, sirolimus, or everolimus 1 year posttransplant, and steroid dose 1 year posttransplant.
CI, confidence interval.
Table 4 shows the annual changes in eGFR and serum creatinine according to pretransplant alcohol consumption. Compared to non-drinkers, no significant annual changes in eGFR and serum creatinine were observed in moderate drinkers and heavy drinkers, or when taking all drinkers. No significant differences in annual eGFR were observed between persistent non-drinkers, persistent drinkers, de novo drinkers, and KTRs who stopped drinking (Figure 2).
TABLE 4
| Alcohol consumption group | eGFR, ml/min/1.73 m2/yr (95%CI) | p-value | sCr, mg/dl (95% CI) | p-value |
|---|---|---|---|---|
| Non-drinker | 0.21 (−0.12–0.55) | Ref | −0.01 (−0.03–0.00) | Ref |
| Drinker | −0.19 (−0.63–0.24) | 0.389 | 0.01 (−0.01–0.02) | 0.392 |
| Moderate drinker | −0.13 (−0.47–0.21) | 0.465 | 0.00 (−0.01–0.02) | 0.925 |
| Heavy drinkera | −0.09 (−0.47–0.29) | 0.655 | 0.01 (−0.01–0.03) | 0.277 |
Annual change in the estimated glomerular filtration rate (eGFR) and serum creatinine (sCr) levels according to pretransplant alcohol consumption.
The criteria for heavy drinking defined by the National Institute on Alcohol Abuse and Alcoholism are as follows: for men, consuming more than 4 drinks on any day or more than 14 drinks per week; for women, consuming more than 3 drinks on any day or more than 7 drinks per week.
CI, confidence interval.
FIGURE 2
Alcohol Consumption, PTDM, and Lipid Profiles
Compared to the group of persistent non-drinkers, persistent drinkers, de novo drinkers, and KTRs who stopped drinking were not significantly associated with the development of PTDM (Table 5). Figure 3 shows the results of the general linear model for the relationships between alcohol consumption over time and total cholesterol, LDL cholesterol, HDL cholesterol, and TGs after adjusting for the use of lipid-lowering agents, age, sex, and BMI. 5 years after transplant, there were significant differences between the groups in total cholesterol levels (p = 0.007) and LDL cholesterol levels (p = 0.044). In particular, the total cholesterol levels (192.7 ± 4.7 mg/dl vs. 174.5 ± 2.5 mg/dl, p < 0.001) and LDL cholesterol levels (104.4 ± 4.1 mg/dl vs. 91.4 ± 2.2 mg/dl, p = 0.005) were significantly higher in de novo drinkers than in persistent non-drinkers. 7 years after transplant, there was a significant difference between the groups in total cholesterol levels (p = 0.022). In particular, the total cholesterol levels were significantly higher in de novo drinkers than in the group that stopped drinking (196.1 ± 8.6 mg/dl vs. 160.0 ± 7.7 mg/dl, p = 0.002).
TABLE 5
| aORa (95% CI) | p-value | |
|---|---|---|
| Persistent non-drinkers | 1.00 (Ref) | |
| Persistent drinkers | 0.92 (0.35–2.43) | 0.679 |
| De novo drinkers | 0.71 (0.20–2.50) | 0.384 |
| Stopped drinking | 2.02 (0.60–6.82) | 0.166 |
Adjusted odds ratios (aORs) for posttransplant diabetes mellitus among kidney transplant recipients without pretransplant diabetes mellitus.
Adjusted for recipient age, recipient sex, recipient body mass index, baseline HbA1c, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, re-transplantation, desensitization, total number of human leukocyte antigen mismatches, and antithymocyte globulin induction.
CI, confidence interval.
FIGURE 3
Discussion
In this prospective longitudinal cohort study, pretransplant alcohol consumption did not affect the risk of major outcomes, including DCGF, BPAR, cardiovascular events, or all-cause mortality, or annual changes in eGFR over a median follow-up of 6.0 years. The risk of major outcomes was not different according to the amount and frequency of alcohol consumption. Considering posttransplant alcohol consumption, compared to persistent non-drinkers, the development of DCGF, BPAR, cardiovascular events, all-cause mortality, or PTDM was not affected by the alcohol consumption pattern over time, including persistent drinking, de novo drinking, and stopped drinking. However, de novo drinkers had significantly higher total cholesterol and LDL cholesterol levels compared to persistent non-drinkers 5 years after transplant, and had significantly higher total cholesterol levels compared to the group that stopped drinking 7 years after transplant, even after adjusting for the use of lipid-lowering agents, age, sex, and BMI.
The prevalence of alcohol consumption at the time of transplantation (40.6%) in our study was relatively lower than the posttransplant alcohol consumption in previous kidney transplant studies (52%–52.8%) (15, 16). Compared to the prevalence of current drinkers in the general population [80%–100% in South Korean men and 60%–79.9% in South Korean women (9), 60% of Koreans drank at least once a month according to the Korea National Health and Nutrition Examination Survey 2013 (20)], considerably lower alcohol drinking rates in KTRs may reflect that the patients themselves are refraining from drinking for medical reasons or upon the advice of physicians. As for the effects of alcohol consumption on patient survival, prior studies have reported conflicting results depending on alcohol consumption before or after transplantation. One retrospective study including 425 KTRs with alcohol dependence before transplantation and 60,532 KTRs who did not use alcohol reported that pretransplant alcohol dependency is a risk factor for graft failure and patient death (13). A retrospective study of more than one million patients with kidney failure also presented that abuse of alcohol, tobacco, or drugs is associated with graft failure, but the effect of alcohol use alone was not reported (14). However, another prospective study including 600 KTRs demonstrated that moderate alcohol consumption (10–30 g/day) posttransplant is associated with a reduced risk of mortality in KTRs (16). In contrast to the results from previous studies, neither pretransplant not posttransplant alcohol use was associated with graft failure and recipient death in our study. Previous studies have not clearly identified the frequency of alcohol consumption; the various results may be due to differences in the distribution of the frequency of alcohol among heavy drinkers. Although we adjusted for considerable risk factors associated with graft failure and mortality in this study, differences in other traditional risk factors, such as smoking may affect the results.
The influence of alcohol consumption on BPAR and kidney allograft function in KTRs is still not clearly defined. Although low adherence to immunosuppressive agents has been associated with heavy drinking and dependence (21, 22), pretransplant and posttransplant alcohol consumption did not increase the risk of BPAR in KTRs in this study. This could be explained by the fact that the proportion of heavy-frequent drinkers was not high. With regard to the association between alcohol consumption and kidney function in the general population, previous studies have reported inconsistent results. No adverse outcome or protective effect of moderate alcohol consumption on kidney function has been shown in general population studies, but a decreased risk of the development of chronic kidney disease has been reported (23–26). However, other studies reported that a daily alcohol intake of 30 g or more is an independent risk factor for the development of albuminuria (27), 2 units of alcohol per day or more increases the risk of kidney failure (28), and that alcohol use has an adverse impact on kidney function (29–31). The lack of an significant association between pretransplant and posttransplant alcohol consumption and the changes in the annual kidney function in this study may also be related to the lower proportion of heavy-frequent drinkers or other stronger immunological and demographic factors than alcohol itself.
The protective effect of moderate alcohol consumption on cardiovascular disease in the general population was previously assumed to be due to alcohol-associated increases in HDL cholesterol and apolipoprotein A1 levels (32, 33), increased insulin sensitivity (34, 35), and reduced platelet aggregation (36). One kidney transplant study reported that moderate alcohol consumption (10–30 g/day) is associated with a low prevalence of PTDM (16). In contrast to our expectations and the results from previous studies, no association was found between pretransplant and posttransplant alcohol consumption and PTDM, and de novo drinkers had higher total cholesterol and LDL cholesterol levels than persistent non-drinkers or the stopped drinking group, even after adjusting for several related factors. Although it is difficult to determine the exact mechanism underlying this result, we cannot completely rule out the possibility that relatively higher lipid profiles in de novo drinkers are related to other unhealthy life style patterns that develop after transplantation, as well as the effects of alcohol itself.
In this study, we found significant differences in total cholesterol levels and LDL cholesterol levels between de novo drinkers and non-drinkers, but we found no significant differences between persistent drinkers and non-drinkers. This is probably due to the difference between the two groups in the amount of alcohol consumed each year after kidney transplantation. Changes in the alcohol consumption patterns of persistent drinkers were confirmed; initially, 23.6% were heavy drinkers, but this decreased to 13.6% in the second year after kidney transplantation. To clarify this, information on the amount of alcohol consumed each year after kidney transplantation will be needed in both groups. Unfortunately, in this prospective study, information on the amount of alcohol consumed each year after kidney transplantation was not obtained, so it is difficult to fully explain this with current data alone.
This study has some limitations. First, the information on alcohol use relied on self-reporting, which is susceptible to inaccurate recall or a desire to give socially acceptable answers, ultimately underestimating alcohol consumption (37–39). Second, no information was obtained regarding the type of alcohol consumed by participants. Third, because alcohol consumption was investigated based on the prior year at the time of transplant, it is possible that remote former drinkers were classified as non-drinkers. Fourth, the response rate to alcohol consumption 2 years after transplantation was 65.9%, which was not very high. Therefore, the distribution of groupings over time with alcohol consumption may not accurately reflect changes in the actual alcohol consumption pattern. Fifth, considering racial and ethnic differences in alcohol metabolism (40), the results of the present study have limited generalizability because this study included only an Asian kidney transplant population. Finally, although pretransplant and posttransplant alcohol consumption were not associated with major outcomes, including DCGF, BPAR, cardiovascular events, and all-cause death in KTRs, this study did not confirm the long-term safety of alcohol consumption in terms of other alcohol-related medical problems, such as alcohol use disorder, liver disease, or cancer (9).
Nevertheless, this study has definite strengths. Few alcohol-related research studies have been conducted in kidney transplant populations compared to the general population, and all of them have used cross-sectional alcohol consumption information. Our results were obtained from a prospective multicenter study including consecutive incident KTRs. Furthermore, this study explored both pretransplant and posttransplant alcohol use, including the amount and frequency, for the first time to evaluate the impact on adverse outcomes, which extended our knowledge. Lastly, the number of participants was considerable and the median follow-up duration considerably long.
In conclusion, although pretransplant and posttransplant alcohol consumption is not associated with major outcomes in KTRs, a new start of alcohol use after kidney transplantation results in a relatively poor lipid profile. As dyslipidemia can be associated with cardiovascular events and mortality in the long-term, the results of this study should be kept in mind when monitoring KTRs to optimize long-term transplant outcomes. Furthermore, this study did not confirm the long-term safety of alcohol in terms of other alcohol-related medical problems, such as alcohol use disorder, liver disease, or cancer, and assessment of the effects of alcohol consumption on KTRs should proceed with caution. Larger and longer-term studies will be needed to develop firm guidelines on alcohol use by KTRs.
Statements
Data availability statement
The datasets generated and analysed during the current study are not readily available because the data was collected for specific research purposes. Requests to access the datasets should be directed to So Hyeon Park, js041571@yuhs.ac.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Committee of each participating center approved the KNOW-KT study protocol [Chonbuk National University Hospital; Gachon University Gil Medical Center; Keimyung University Dongsan Hospital; Korea University Anam Hospital; Kyungpook National University Hospital; Samsung Medical Center, Seoul; Seoul National University Hospital; Yonsei University, Severance Hospital (in alphabetical order)] (41). The patients/participants provided their written informed consent to participate in this study.
Author contributions
Conceptualization, H-YJ; Methodology, H-YJ and YJ; Formal analysis, H-YJ and YJ; Investigation, H-YJ, KHH, JBP, M-GK, SL, SH, HR, JY, J-HC, S-HP, Y-LK, and C-DK; Data curation, H-YJ, KHH, JBP, M-GK, SL, SH, HR, JY, and CA; Funding acquisition, CA; Writing—original draft preparation, H-YJ; Writing—review and editing, H-YJ and C-DK; Approval of final manuscript, H-YJ, YJ, KHH, JBP, M-GK, SL, SH, HR, JY, CA, J-HC, S-HP, Y-LK, and C-DK.
Funding
This research was supported by the Research Program funded by the Korea Centers for Disease Control and Prevention (Nos. 2012E3301100, 2013E3301600, 2013E3301601, 2013E3301602, 2016E3300200, 2016E3300201, 2016E3300202, 2019E320100, and 2019E320101). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
BMI, body mass index; BPAR, biopsy-proven acute rejection; CI, confidence interval; DCGF, death-censored graft failure; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; HLA, human leukocyte antigen; HR, hazard ratio; IQR, interquartile range; KTR, kidney transplant recipient; LDL, low-density lipoprotein; PTDM, posttransplant diabetes mellitus; TGs, triglycerides.
References
1.
ParkHKimK. Relationship Between Alcohol Consumption and Serum Lipid Levels in Elderly Korean Men. Arch Gerontol Geriatr (2012) 55:226–30. 10.1016/j.archger.2011.08.014
2.
TabaraYUeshimaHTakashimaNHisamatsuTFujiyoshiAZaidMet alMendelian Randomization Analysis in Three Japanese Populations Supports a Causal Role of Alcohol Consumption in Lowering Low-Density Lipid Cholesterol Levels and Particle Numbers. Atherosclerosis (2016) 254:242–8. 10.1016/j.atherosclerosis.2016.08.021
3.
WakabayashiI. Difference in Sensitivities of Blood HDL Cholesterol and LDL Cholesterol Levels to Alcohol in Middle-Aged Japanese Men. Alcohol (2018) 67:45–50. 10.1016/j.alcohol.2017.08.011
4.
LeongDPSmythATeoKKMcKeeMRangarajanSPaisPet alPatterns of Alcohol Consumption and Myocardial Infarction Risk: Observations from 52 Countries in the INTERHEART Case-Control Study. Circulation (2014) 130:390–8. 10.1161/circulationaha.113.007627
5.
ChristensenAINordestgaardBGTolstrupJS. Alcohol Intake and Risk of Ischemic and Haemorrhagic Stroke: Results from a Mendelian Randomisation Study. J Stroke (2018) 20:218–27. 10.5853/jos.2017.01466
6.
DjousséLGazianoJM. Alcohol Consumption and Heart Failure: A Systematic Review. Curr Atheroscler Rep (2008) 10:117–20. 10.1007/s11883-008-0017-z
7.
XiBVeerankiSPZhaoMMaCYanYMiJ. Relationship of Alcohol Consumption to All-Cause, Cardiovascular, and Cancer-Related Mortality in U.S. Adults. J Am Coll Cardiol (2017) 70:913–22. 10.1016/j.jacc.2017.06.054
8.
WoodAMKaptogeSButterworthASWilleitPWarnakulaSBoltonTet alRisk Thresholds for Alcohol Consumption: Combined Analysis of Individual-Participant Data for 599 912 Current Drinkers in 83 Prospective Studies. Lancet (2018) 391:1513–23. 10.1016/S0140-6736(18)30134-X
9.
GriswoldMGFullmanNHawleyCArianNZimsenSRTymesonHDet alAlcohol Use and Burden for 195 Countries and Territories, 1990-2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet (2018) 392:1015–35. 10.1016/S0140-6736(18)31310-2
10.
IwasakiK. Metabolism of Tacrolimus (FK506) and Recent Topics in Clinical Pharmacokinetics. Drug Metab Pharmacokinet (2007) 22:328–35. 10.2133/dmpk.22.328
11.
ParkerRArmstrongMJCorbettCDayEJNeubergerJM. Alcohol and Substance Abuse in Solid-Organ Transplant Recipients. Transplantation (2013) 96:1015–24. 10.1097/tp.0b013e31829f7579
12.
Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. Special Issue: KDIGO Clinical Practice Guideline for the Care of Kidney Transplant Recipients. Am J Transpl (2009) 9(Suppl. 3):S1–S155. 10.1111/j.1600-6143.2009.02834.x
13.
GueyeASChelamcharlaMBairdBCNguyenCTangHBarenbaumALet alThe Association between Recipient Alcohol Dependency and Long-Term Graft and Recipient Survival. Nephrol Dial Transplant (2007) 22:891–8. 10.1093/ndt/gfl689
14.
SandhuGSKhattakMWoodwardRSHantoDWPavlakisMDimitriNet alImpact of Substance Abuse on Access to Renal Transplantation. Transplantation (2011) 91:86–93. 10.1097/tp.0b013e3181fc8903
15.
FierzKSteigerJDenhaerynckKDobbelsFBockADe GeestS. Prevalence, Severity and Correlates of Alcohol Use in Adult Renal Transplant Recipients. Clin Transpl (2006) 20:171–8. 10.1111/j.1399-0012.2005.00460.x
16.
ZelleDMAgarwalPKRamirezJLPvan der HeideJJHCorpeleijnEGansROBet alAlcohol Consumption, New Onset of Diabetes After Transplantation, and All-Cause Mortality in Renal Transplant Recipients. Transplantation (2011) 92:203–9. 10.1097/tp.0b013e318222ca10
17.
NIAAA. Drinking Levels Defined (2021). Available at: https://www.niaaa.nih.gov/alcohol-health/overview-alcohol consumption/moderate-binge-drinking (Accessed October 29, 2021).
18.
CurranSPFamureOLiYKimSJ. Increased Recipient Body Mass Index Is Associated with Acute Rejection and Other Adverse Outcomes After Kidney Transplantation. Transplantation (2014) 97:64–70. 10.1097/tp.0b013e3182a688a4
19.
DevinePACourtneyAEMaxwellAP. Cardiovascular Risk in Renal Transplant Recipients. J Nephrol (2019) 32:389–99. 10.1007/s40620-018-0549-4
20.
Ministry of Health and Welfare, Korea Centers for Disease Control and Prevention. Korea Health Statistics 2013: Korea National Health and Nutrition Examination Survey (KNHANES VI-1). Cheongju: Korea Centers for Disease Control and Prevention (2014).
21.
ShapiroPAWilliamsDLForayATGelmanISWukichNSciaccaR. Psychosocial Evaluation and Prediction of Compliance Problems and Morbidity After Heart Transplantation. Transplantation (1995) 60:1462–6. 10.1097/00007890-199560120-00016
22.
BeresfordTPSchwartzJWilsonDMerionRLuceyMR. The Short-Term Psychological Health of Alcoholic and Non-Alcoholic Liver Transplant Recipients. Alcohol. Clin Exp Res (1992) 16:996–1000. 10.1111/j.1530-0277.1992.tb01908.x
23.
SchaeffnerESKurthTde JongPEGlynnRJBuringJEGazianoJM. Alcohol Consumption and the Risk of Renal Dysfunction in Apparently Healthy Men. Arch Intern Med (2005) 165:1048–53. 10.1001/archinte.165.9.1048
24.
KnightELStampferMJRimmEBHankinsonSECurhanGC. Moderate Alcohol Intake and Renal Function Decline in Women: A Prospective Study. Nephrol Dial Transplant (2003) 18:1549–54. 10.1093/ndt/gfg228
25.
YamagataKIshidaKSairenchiTTakahashiHOhbaSShiigaiTet alRisk Factors for Chronic Kidney Disease in a Community-Based Population: A 10-year Follow-Up Study. Kidney Int (2007) 71:159–66. 10.1038/sj.ki.5002017
26.
KoningSHGansevoortRTMukamalKJRimmEBBakkerSJLJoostenMM. Alcohol Consumption Is Inversely Associated with the Risk of Developing Chronic Kidney Disease. Kidney Int (2015) 87:1009–16. 10.1038/ki.2014.414
27.
WhiteSLPolkinghorneKRCassAShawJEAtkinsRCChadbanSJ. Alcohol Consumption and 5-year Onset of Chronic Kidney Disease: The AusDiab Study. Nephrol Dial Transplant (2009) 24:2464–72. 10.1093/ndt/gfp114
28.
PernegerTVWheltonPKPuddeyIBKlagMJ. Risk of End-Stage Renal Disease Associated with Alcohol Consumption. Am J Epidemiol (1999) 150:1275–81. 10.1093/oxfordjournals.aje.a009958
29.
MenonVKatzRMukamalKKestenbaumBde BoerIHSiscovickDSet alAlcohol Consumption and Kidney Function Decline in the Elderly: Alcohol and Kidney Disease. Nephrol Dial Transplant (2010) 25:3301–7. 10.1093/ndt/gfq188
30.
BundyJDBazzanoLAXieDCohanJDolataJFinkJCet alSelf-Reported Tobacco, Alcohol, and Illicit Drug Use and Progression of Chronic Kidney Disease. Clin J Am Soc Nephrol (2018) 13:993–1001. 10.2215/cjn.11121017
31.
JooYSKohHNamKHLeeSKimJLeeCet alAlcohol Consumption and Progression of Chronic Kidney Disease: Results from the Korean Cohort Study for Outcome in Patients with Chronic Kidney Disease. Mayo Clinic Proc (2020) 95:293–305. 10.1016/j.mayocp.2019.06.014
32.
GazianoJMBuringJEBreslowJLGoldhaberSZRosnerBVanDenburghMet alModerate Alcohol Intake, Increased Levels of High-Density Lipoprotein and its Subfractions, and Decreased Risk of Myocardial Infarction. N Engl J Med (1993) 329:1829–34. 10.1056/nejm199312163292501
33.
ChungB-HDoranSLiangPOsterlundLChoBSOsterRAet alAlcohol-Mediated Enhancement of Postprandial Lipemia: A Contributing Factor to an Increase in Plasma HDL and a Decrease in Risk of Cardiovascular Disease. Am J Clin Nutr (2003) 78:391–9. 10.1093/ajcn/78.3.391
34.
DaviesMJBaerDJJuddJTBrownEDCampbellWSTaylorPR. Effects of Moderate Alcohol Intake on Fasting Insulin and Glucose Concentrations and Insulin Sensitivity in Postmenopausal Women: A Randomized Controlled Trial. JAMA (2002) 287:2559–62. 10.1001/jama.287.19.2559
35.
KiechlSWilleitJPoeweWEggerGOberhollenzerFMuggeoMet alInsulin Sensitivity and Regular Alcohol Consumption: Large, Prospective, Cross Sectional Population Study (Bruneck Study). BMJ (1996) 313:1040–4. 10.1136/bmj.313.7064.1040
36.
RenaudSCBeswickADFehilyAMSharpDSElwoodPC. Alcohol and Platelet Aggregation: the Caerphilly Prospective Heart Disease Study. Am J Clin Nutr (1992) 55:1012–7. 10.1093/ajcn/55.5.1012
37.
GilliganCAndersonKGLaddBOYongYMDavidM. Inaccuracies in Survey Reporting of Alcohol Consumption. BMC Public Health (2019) 19:1639. 10.1186/s12889-019-7987-3
38.
GualAÁngel ArbesúJZarcoJBalcells-OliveróMd. l. MLópez-PelayoHMiquelLet alRisky Drinkers Underestimate Their Own Alcohol Consumption. Alcohol Alcohol (2017) 52:516–7. 10.1093/alcalc/agx029
39.
LivingstonMCallinanS. Underreporting in Alcohol Surveys: Whose Drinking Is Underestimated?J Stud Alcohol Drugs (2015) 76:158–64. 10.15288/jsad.2015.76.158
40.
EdenbergHJ. The Genetics of Alcohol Metabolism: Role of Alcohol Dehydrogenase and Aldehyde Dehydrogenase Variants. Alcohol Res Health (2007) 30:5–13.
41.
YangJLeeJHuhKHParkJBChoJ-HLeeSet alKNOW-KT (Korean Cohort Study for Outcome in Patients with Kidney Transplantation: A 9-year Longitudinal Cohort Study): Study Rationale and Methodology. BMC Nephrol (2014) 15:77. 10.1186/1471-2369-15-77
Summary
Keywords
kidney transplantation, alcohol, all-cause mortality, biopsy-proven acute rejection, cardiovascular events, death-censored graft failure, low-density lipoprotein cholesterol, total cholesterol
Citation
Jung H-Y, Jeon Y, Huh KH, Park JB, Kim M-G, Lee S, Han S, Ro H, Yang J, Ahn C, Cho J-H, Park S-H, Kim Y-L and Kim C-D (2022) Pretransplant and Posttransplant Alcohol Consumption and Outcomes in Kidney Transplantation: A Prospective Multicenter Cohort Study. Transpl Int 35:10243. doi: 10.3389/ti.2022.10243
Received
16 November 2021
Accepted
06 April 2022
Published
30 May 2022
Volume
35 - 2022
Updates
Copyright
© 2022 Jung, Jeon, Huh, Park, Kim, Lee, Han, Ro, Yang, Ahn, Cho, Park, Kim and Kim.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hee-Yeon Jung, hy-jung@knu.ac.kr; Chan-Duck Kim, drcdkim@knu.ac.kr
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.