Abstract
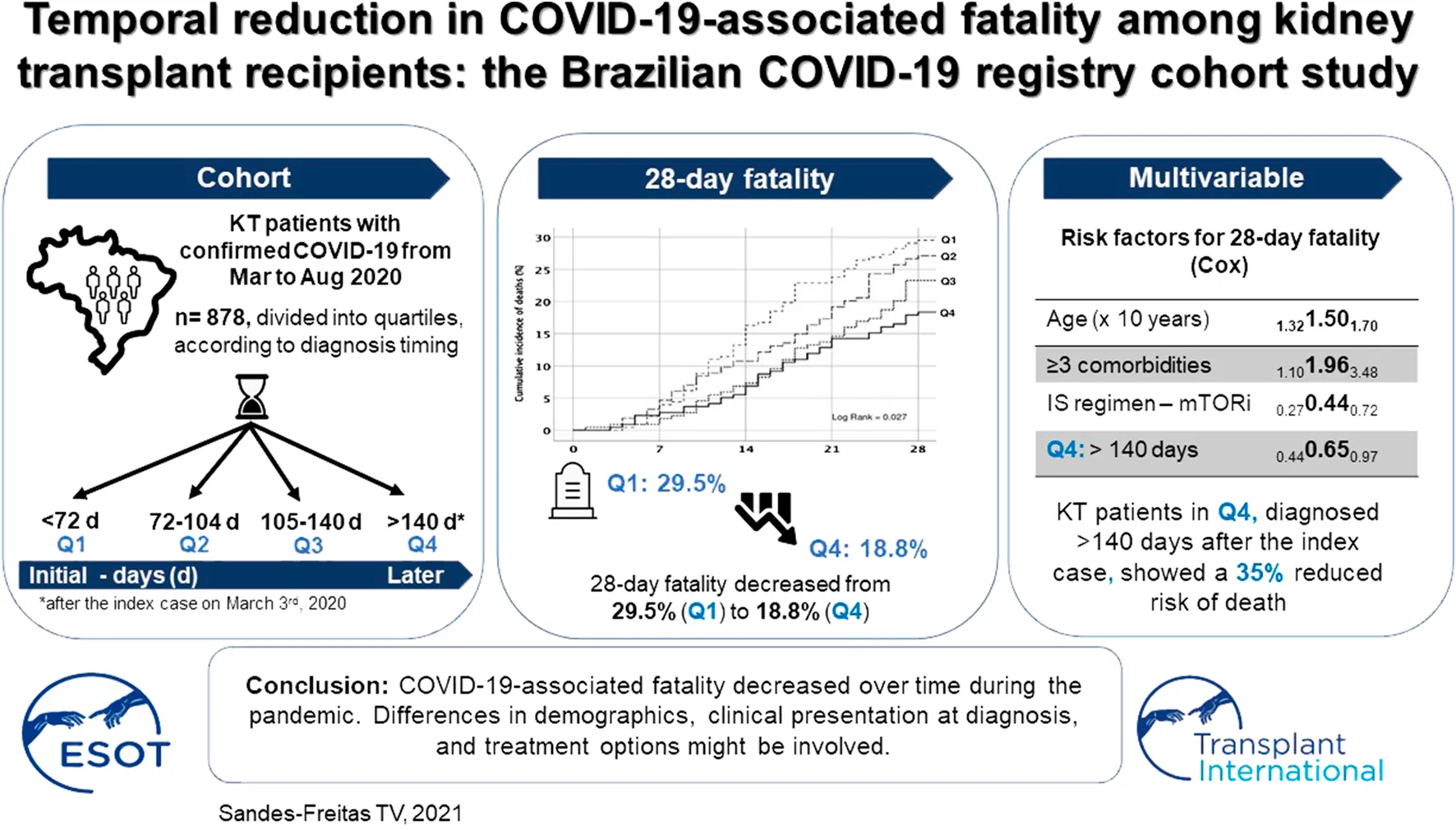
Data from the general population suggest that fatality rates declined during the course of the pandemic. This analysis, using data extracted from the Brazilian Kidney Transplant COVID-19 Registry, seeks to determine fatality rates over time since the index case on March 3rd, 2020. Data from hospitalized patients with RT-PCR positive SARS-CoV-2 infection from March to August 2020 (35 sites, 878 patients) were compared using trend tests according to quartiles (Q1: <72 days; Q2: 72–104 days; Q3: 105–140 days; Q4: >140 days after the index case). The 28-day fatality decreased from 29.5% (Q1) to 18.8% (Q4) (pfor-trend = 0.004). In multivariable analysis, patients diagnosed in Q4 showed a 35% reduced risk of death. The trend of reducing fatality was associated with a lower number of comorbidities (20.7–10.6%, pfor-trend = 0.002), younger age (55–53 years, pfor-trend = 0.062), and better baseline renal function (43.6–47.7 ml/min/1.73 m2, pfor-trend = 0.060), and were confirmed by multivariable analysis. The proportion of patients presenting dyspnea (pfor-trend = 0.001) and hypoxemia (pfor-trend < 0.001) at diagnosis, and requiring intensive care was also found reduced (pfor-trend = 0.038). Despite possible confounding variables and time-dependent sampling differences, we conclude that COVID-19-associated fatality decreased over time. Differences in demographics, clinical presentation, and treatment options might be involved.
Introduction
Over the past year, the coronavirus disease 2019 (COVID-19) global pandemic has been responsible for more than 126 million cases of severe acute respiratory syndrome worldwide and over 2.76 million deaths. With large numbers of COVID cases, Brazil has become an epicenter of the COVID-19 outbreak in the world (1, 2). Among many specific vulnerable groups affected by SARS-COV-2 infection, transplant immunocompromised recipients represent a recognized high-risk group for this infection (3).
Although to date there is still no specific treatment for COVID-19, several pharmacological and non-pharmacological strategies have been explored to improve the clinical outcomes. Among these strategies, the following are noteworthy: 1) the use of prehospital pulse oximetry to early detect silent hypoxemia (4); 2) the important role of non-invasive mechanical ventilation often avoiding unnecessary early intubation (5); 3) prone position to improve oxygenation in intubated and non-intubated patients with COVID-19-related acute respiratory distress syndrome (6, 7); 4) anticoagulant treatment in patients with coagulopathy (8); and 5) corticosteroids in patients with severe disease (9).
Data from the general population suggest an improvement in survival rates during the pandemic, mainly among critically ill patients (10–13). Multicenter national studies have reported COVID-19-related fatality rates varying from 20.5 to 32% among hospitalized kidney transplant (KT) patients (14–18), but no study evaluated the impact of the timing on deaths in this population.
In this analysis of the multicenter national Brazilian registry of SARS-CoV-2 infection study, we aimed to assess fatality rates over the first 6 months of pandemic and to explore whether demographics, clinical profile, and in-hospital management of COVID-19 were associated with trends in the outcomes.
Materials and Methods
Study Design
This is an ongoing multicenter national Brazilian registry of SARS-CoV-2 infection among kidney transplant recipients (ClinicalTrials.gov: NCT04494776) (19). For this analysis, we extracted data of patients with COVID-19-related signs and symptoms and SARS-CoV-2 detected by reverse-transcription polymerase chain reaction (RT-PCR) of a respiratory sample, between 3rd March and 31st August 2020, who required hospitalization, totalizing 878 patients from 35 transplant centers of four Brazilian Regions (615 from the Southeast, 124 Northeast, 111 South, and 28 from the Midwest). Patients were followed for 3 months after the diagnosis or until death or graft loss, and the end-of-study data was 30th November 2020.
Variables
Patient age, gender, ethnicity, and body mass index were collected and included in the analysis. Comorbidities comprised the following conditions: hypertension, diabetes, cardiovascular, pulmonary, neurological or hepatic diseases, current or previous neoplasia, and autoimmune disease. The following clinical presentation parameters were also included in the analysis: fever and/or chills, cough, dyspnea, myalgia, diarrhea, headache, fatigue and or/asthenia, runny nose, and nausea and/or vomiting. Data related to KT such as donor source, end-stage kidney disease (ESKD) etiology, time after transplantation, baseline renal function, maintenance immunosuppressive (IS) drugs, steroid (ST) pulse therapy <3 months, use of rabbit antithymocyte globulin (rATG) <3 months were analyzed.
The following laboratory exams at admission were recorded: lymphocytes count, hemoglobin, platelets count, C-reactive protein, lactic dehydrogenase, aspartate transaminase; alanine transaminase; creatine phosphokinase, serum sodium, ferritin, serum creatinine. Chest radiography and/or computed tomography at admission were used to classify pulmonary abnormalities.
The following treatments available in the registry were analyzed: antibiotics, particularly azithromycin, high-dose steroids, prophylactic or therapeutic use of anticoagulants, and use of oseltamivir, ivermectin, and chloroquine or hydroxychloroquine.
The analysis of outcomes in COVID-19 transplant recipients across time was carried out considering fatality rates and the following variables: invasive mechanical ventilation, intensive care unit admission, and development of AKI with dialysis requirement.
Definitions
The COVID-19-associated fatality rate was defined as the percentage of deaths that occurred in patients with confirmed SARS-CoV-2 infection. Hospital admission criteria and the use of pharmacological and non-pharmacological treatments were at the discretion of each of the participating centers. The definition of “high-dose steroids” was at the center discretion, according to their local practices.
We considered as the index case the first KT patient diagnosed with COVID-19 and included in the Brazilian Kidney Transplant COVID-19 Registry (March 3rd, 2020). The sample was divided into quartiles, as demonstrated in Figure 1: Q1: patients diagnosed <72 days after the index case (n = 227); Q2: 72–104 days (n = 214); Q3: 105–140 days (n = 219); Q4: >140 days (n = 218).
FIGURE 1
Baseline serum creatinine (sCr) was defined as the last three available sCr measurements before COVID-19 infection. Glomerular filtration rate (eGFR) was estimated by the CKD-EPI formula. Delta sCr (Δ sCr) was the difference between admission and baseline sCr values. Acute kidney injury (AKI) was defined as a rise in sCr of ≥50% from its baseline value (20). Graft loss was defined as the return to long-term dialysis therapy or retransplantation.
Statistical Analysis
Categorical variables were presented as frequency and percentage. All continuous variables were non-normally distributed and were summarized as median and interquartile range (IQR). Trend analyses comparing data across the quartiles were performed using Cochran–Armitage test for categorical variables, and Jonckheere-Terpstra test for numerical variables. Survival curves were obtained using Kaplan-Meier method and compared using the log-rank test. Univariable and multivariable analyses to identify independent risk factors associated with death were performed using Cox regression, with center-based random effects (frailty model). Collinear variables, and those poorly associated with death in univariable analysis (p > 0.15) were excluded from the multivariable model. No variable exceeded 5% of missing values and Multiple Imputation by Chained Equation (MICE) was used to replace missing data values, as follows: 1) generating replacement values (“imputations”) for missing data and repeating this procedure 10 times, 2) analyzing the 10 imputed data sets, and 3) combining (pooling) the results using Rubin’s Rules (21). A significantly statistical difference was assumed when the p-value was less than 0.05. Statistical analysis was performed using the IBM SPSS 25 and R 4.0.2.
Results
Demographic Characteristics Across the Quartiles
The baseline demographic characteristics at COVID-19 diagnosis are shown in Table 1. Changes in patients’ clinical profile occurred over time, with a significant reduction in age, and in the percentage of patients with ≥3 comorbidities.
TABLE 1
| Non-missing cases | Total | Q1 | Q2 | Q3 | Q4 | pfor-trend | |
|---|---|---|---|---|---|---|---|
| N = 878 | N = 227 | N = 214 | N = 219 | N = 218 | |||
| Age (years-old) | 878 | 54 (45–62) | 55 (46–64) | 54 (44–61) | 54 (45–61) | 53 (44–62) | 0.062 |
| Male gender | 878 | 535 (60.9) | 146 (64.3) | 131 (61.2) | 134 (61.2) | 124 (56.9) | 0.127 |
| Ethnicity | 878 | 0.204 | |||||
| Caucasian | 483 (55.0) | 111 (48.9) | 108 (50.5) | 125 (57.1) | 139 (63.8) | ||
| Mixed race | 255 (29.0) | 79 (34.8) | 68 (31.8) | 63 (28.8) | 45 (20.6) | ||
| Afro-Brazilian | 112 (12.8) | 28 (12.3) | 28 (13.1) | 24 (11.0) | 32 (14.7) | ||
| Asian | 14 (1.6) | 6 (2.6) | 3 (1.4) | 4 (1.8) | 1 (0.5) | ||
| Indian | 1 (0.1) | 0 (0) | 0 (0) | 1 (0.5) | 0 (0) | ||
| Not available | 13 (1.5) | 3 (1.3) | 7 (3.3) | 2 (0.9) | 1 (0.5) | ||
| BMI (kg/m2) | 842 | 26.5 (23.6–30.0) | 26.4 (23.3–29.5) | 26.0 (22.9–29.7) | 27.3 (24.4–30.9) | 26.8 (23.9–29.9) | 0.031 |
| Donor source | 878 | 0.084 | |||||
| KT - LD | 259 (29.5) | 79 (34.8) | 62 (29.0) | 67 (30.6) | 51 (23.4) | ||
| KT - DD | 601 (68.5) | 142 (62.6) | 151 (70.6) | 146 (66.7) | 162 (74.3) | ||
| Combined KTa | 18 (2.1) | 6 (0.7) | 1 (0.1) | 6 (0.7) | 5 (0.6) | ||
| ESKD etiology | 878 | 0.230 | |||||
| Unknown | 266 (30.3) | 57 (25.1) | 80 (37.4) | 69 (31.5) | 60 (27.5) | ||
| Diabetes | 174 (19.8) | 53 (23.3) | 41 (19.2) | 38 (17.4) | 42 (19.3) | ||
| Chronic GN | 151 (17.2) | 33 (14.5) | 30 (14.0) | 51 (23.3) | 37 (17.0) | ||
| Hypertension | 103 (11.7) | 34 (15.0) | 22 (10.3) | 20 (9.1) | 27 (12.4) | ||
| PKD | 73 (8.3) | 20 (8.8) | 14 (6.5) | 19 (8.7) | 20 (9.2) | ||
| Urological | 14 (1.6) | 4 (1.8) | 4 (1.9) | 3 (1.4) | 3 (1.4) | ||
| Other | 97 (11.0) | 26 (11.5) | 23 (10.7) | 19 (8.7) | 29 (13.3) | ||
| Time after KT (years) | 875 | 6.1 (2.2–11.2) | 6.9 (2.5–11.8) | 5.6 (2.1–10.3) | 6.1 (2.0–11.7) | 5.7 (2.5–11.2) | 0.541 |
| Comorbidities | 878 | ||||||
| Hypertension | 689 (78.5) | 179 (78.9) | 170 (79.4) | 175 (79.9) | 165 (75.7) | 0.471 | |
| Diabetes | 351 (40.0) | 101 (44.5) | 84 (39.3) | 89 (40.6) | 77 (35.2) | 0.075 | |
| Cardiovascular disease | 142 (16.2) | 49 (21.6) | 33 (23.2) | 32 (14.6) | 28 (12.8) | 0.014 | |
| Pulmonary disease | 30 (3.4) | 10 (4.4) | 7 (3.3) | 7 (3.2) | 6 (2.8) | 0.353 | |
| Neurological disease | 10 (1.1) | 5 (2.2) | 1 (0.5) | 1 (0.5) | 3 (1.4) | 0.416 | |
| Hepatic disease | 35 (4.0) | 8 (3.5) | 8 (3.7) | 8 (3.7) | 11 (5.0) | 0.449 | |
| Current or previous neoplasia | 59 (6.7) | 31 (13.7) | 14 (6.5) | 10 (4.6) | 4 (1.8) | <0.001 | |
| Autoimmune disease | 22 (2.5) | 11 (4.8) | 2 (0.9) | 6 (2.7) | 3 (1.4) | 0.062 | |
| No. of comorbidities | 878 | 0.002 | |||||
| None | 111 (12.6) | 23 (10.1) | 26 (12.1) | 31 (14.2) | 31 (14.2) | ||
| 1–2 | 644 (73.3) | 157 (69.2) | 161 (75.2) | 162 (74.0) | 164 (75.2) | ||
| 3 or more | 123 (14.0) | 47 (20.7) | 27 (12.6) | 26 (11.9) | 23 (10.6) | ||
| Maintenance IS drugs | 872 | ||||||
| CNI | 691 (79.2) | 170 (74.9) | 170 (79.8) | 180 (83.3) | 171 (79.2) | 0.172 | |
| MPA or AZA | 653 (74.9) | 163 (71.8) | 152 (71.4) | 167 (77.3) | 171 (79.2) | 0.033 | |
| mTORi | 135 (15.5) | 40 (17.9) | 42 (19.7) | 26 (12.2) | 267 (12.7) | 0.038 | |
| ST | 826 (94.7) | 212 (93.4) | 203 (94.9) | 202 (92.2) | 209 (95.9) | 0.496 | |
| RAAS blockade | 866 | 294 (33.9) | 74 (32.6) | 65 (30.4) | 76 (34.7) | 79 (36.2) | 0.787 |
| ST pulse therapy ≤3 months | 859 | 49 (5.7) | 11 (4.8) | 7 (3.3) | 12 (5.5) | 19 (8.7) | 0.460 |
| rATG ≤3 months | 844 | 30 (3.6) | 8 (3.5) | 6 (2.8) | 7 (3.2) | 9 (4.1) | 0.222 |
| eGFR (ml/min/1.73 m2) | 846 | 44.5 (28.7–60.9) | 43.6 (25.4–57.9) | 46.3 (30.0–61.1) | 40.9 (27.3–59.3) | 47.7 (31.9–66.7) | 0.060 |
Demographic characteristics of kidney transplanted patients at COVID-19 diagnosis across quartiles of time.
Trend analysis for categorical and continuous data were performed using Cochran–Armitage test and Jonckheere-Terpstra test, respectively. BMI, body mass index; KT, kidney transplant; LD, living donor; DD, deceased donor; CNI, calcineurin inhibitor; AZA, azathioprine; MPA, mycophenolate; mTORi, mammalian target of rapamycin inhibitor; RAAS, renin-angiotensin-aldosterone system; ST, steroids; rATG, rabbit antithymocyte globulin; ESKD, end-stage kidney disease; GN, glomerulonephritis; PKD, polycystic kidney disease; IS, immunosuppressive; eGFR, estimated glomerular filtration rate.
Bold values denote statistical significance at the p < 0.05 level.
Simultaneous pancreas-kidney = 8; simultaneous liver-kidney = 6; kidney after liver = 3; simultaneous heart-kidney = 1.
The Clinical Presentation Across the Quartiles
The analysis across quartiles showed a decrease in the proportion of patients with dyspnea and hypoxemia at diagnosis, whereas myalgia, diarrhea, and headache progressively increased. Although the time from the onset of COVID-19 symptoms to diagnosis remained stable over time (median 6 days; IQR 3–9), a longer time until hospitalization since symptoms onset was observed, increasing from Q1 (median 5 days, IQR 2–9) to Q4 (median 6 days, IQR 3–10) (pfor-trend = 0.005) (Figure 2).
FIGURE 2
Laboratory data and chest radiological findings at COVID-19 diagnosis are shown in Supplementary Table S1. An increase in the percentage of patients with normal chest radiological evaluation was observed from Q1 (2.1%) to Q4 (6.7%) (pfor-trend = 0.015).
Immunosuppression and Pharmacological Treatment Across the Quartiles
Complete immunosuppressive drug withdrawal decreased from Q1 to Q4 (from 43.6 to 30.3%, pfor-trend = 0.003), while no significant changes were observed in the percentage of patients submitted to withdrawal or reduction of the antiproliferative or calcineurin inhibitors agents, or no intervention on the immunosuppressive regimen (Figure 3A).
FIGURE 3
Regarding the pharmacological treatments, there was an increase in the use of antibiotics, high-dose steroids, prophylactic use of anticoagulants, and ivermectin, while the use of azithromycin, oseltamivir, chloroquine, or hydroxychloroquine decreased from Q1 to Q4 (Figure 3B).
The Outcomes Across the Quartiles
The 28-day fatality rate was 24.6% (n = 216), with a significant downward trend over time, from 29.5% in Q1 to 18.3% in Q4 (log rank = 0.027, pfor-trend = 0.004) (Figures 4A,B).
FIGURE 4
Causes of death within 28 days included septic shock (60.2%), acute respiratory failure (21.8%), cardiovascular or embolic event (5.1%), and in 13% the cause of death was not clearly defined nor registered. No difference in the distribution of the causes of death occurred from Q1 to Q4 (pfor-trend = 0.677). Although 69.5% of deaths occurred in the first 28 days, the median time from COVID-19 diagnosis to death increased from 17 days (Q1) to 25 days (Q4) (pfor-trend = 0.035). Within the 90-day follow-up, the overall fatality rate was 35.4% (n = 311), with a non-significant downward trend from 39.2 to 31.2% (Log-rank = 0.208, pfor-trend = 0.073) (Supplementary Figure S1). Causes of death within 90 days were similar to that described for 28 days.
No changes were observed in the percentage of patients receiving invasive mechanical ventilation. However, the time from the onset of symptoms to orotracheal intubation increased from 8 to 11 days in median (pfor-trend = <0.001), and fewer patients were admitted to intensive care units (ICU) over time (from 62.1 to 49.5%, pfor-trend = 0.038) (Figures 5A,B). No significant trend was observed in the percentage of patients requiring dialysis therapy (Figure 5C).
FIGURE 5
Fourteen (1.6%) patients lost the graft within the 90 days follow-up, most of them with advanced chronic kidney disease at the time of COVID-19 diagnosis (median baseline eGFR 16.9 ml/min/1.73 m2, IQR, 9.5–24.3) (Supplementary Table S2). Figure 5D shows the 28 and 90-day fatality rates in patients requiring dialysis therapy, ICU admission, and invasive mechanical ventilation.
Patients with COVID-19 diagnosis 140 days after the index case (Q4) showed a 35% reduction risk in 28-day mortality (HR 0.65, 95% CI 0.44–0.97, p = 0.037). Each month after March 3rd was associated with 10% reduction in the fatality (HR 0.90, 95% CI 0.82–0.99), p = 0.024). Age and presence of three or more comorbidities in addition to chronic kidney disease were also risk factors associated with increased risk of death, whereas the use of mTOR inhibitor and the increasing baseline glomerular filtration rate were associated with decreased risk of death (Table 2; Supplementary Table S3). The impact of timing on 90-day fatality was not clearly demonstrated (Supplementary Table S4).
TABLE 2
| N = 878 | Univariable HR (95%CI), p value | Multivariable HR (95%CI), p value |
|---|---|---|
| Age (×10 years-old) | 1.49 (1.31–1.69), <0.001 | 1.50 (1.32–1.70), <0.001 |
| Male gender | 0.76 (0.57–1.00), 0.050 | 0.76 (0.58–1.00), 0.051 |
| BMI (kg/m2) | 1.01 (0.98–1.04), 0.443 | — |
| Afro-Brazilian or mixed-race ethnicity | 0.92 (0.69–1.22), 0.568 | — |
| Living donor | 0.83 (0.57–1.19), 0.307 | — |
| Timer after KT (years) | 1.01 (0.98–1.03), 0.627 | — |
| Number of comorbidities | ||
| None | REF | REF |
| 1 or 2 | 1.27 (0.75–2.16), 0.370 | 1.34 (0.80–2.23), 0.260 |
| ≥3 | 1.81 (1.00–3.28), 0.050 | 1.96 (1.10–3.48), 0.022 |
| IS regimen – ST | 0.72 (0.42–1.25), 0.248 | — |
| IS regimen – CNI | 0.90 (0.49–1.65), 0.722 | — |
| IS regimen – MPA/AZA | 1.15 (0.63–2.08), 0.649 | — |
| IS regimen – mTORi | 0.44 (0.26–0.75), 0.003 | 0.44 (0.27–0.72), 0.001 |
| ST pulse therapy ≤3 months | 1.55 (0.68–3.57), 0.297 | — |
| rATG ≤3 months | 1.10 (0.39–3.05), 0.860 | — |
| RAS blockade | 1.22 (0.89–1.67), 0.209 | — |
| Baseline eGFR (×10 ml/min/1.73 m2) | 0.88 (0.82–0.94), <0.001 | 0.87 (0.82–0.93), <0.001 |
| Quartiles of time after index case | ||
| Q1: <72 days | REF | REF |
| Q2: 72–104 days | 1.03 (0.72–1.48), 0.863 | 1.04 (0.73–1.48), 0.843 |
| Q3: 105–140 days | 0.75 (0.52–1.10), 0.145 | 0.80 (0.55–1.15), 0.228 |
| Q4: >140 days | 0.60 (0.40–0.90), 0.014 | 0.65 (0.44–0.97), 0.037 |
Risk factors for 28-days fatality after COVID-19 infection in KT recipients.
BMI, body mass index; KT, kidney transplant; IS, immunosuppressive; ST, steroid; MPA, mycophenolate; AZA, azathioprine; CNI, calcineurin inhibitor; mTORi, mammalian target of rapamycin inhibitor; rATG, rabbit anti-thymocyte globulin; RAS, renin-angiotensin system; eGFR, estimated glomerular filtration rate; HR, hazard ratio; CI, confidence interval; REF, reference.
Bold values denote statistical significance at the p < 0.05 level.
Discussion
This national multicenter cohort suggests that COVID-19-associated fatality decreased over the first 6 months after the beginning of the pandemic. Changes in the demographic profile of infected patients, in the clinical presentation at diagnosis, and in pharmacological and non-pharmacological treatment options might explain this result.
The overall fatality rate was high and similar to that described in international published cohorts (15, 16, 18, 22). As a novelty, this cohort showed that the cumulative incidence of death within 28 days after diagnosis significantly decreased over time, and deaths occurred later. Changes in the demographic profile, mainly the reduction in the percentage of patients with multiple comorbid conditions, probably contributed to this finding, since the number of comorbidities was an independent risk factor for death (3). Despite the statistically significant trend for higher BMI over time, we believe that this finding is not clinically relevant. The reasons for the changes in the demographic profile over the months are not clear. The wide dissemination of the worst prognosis on the elderly, and patients with comorbidities might have resulted in intensification of protective measures in these individuals.
Other factors that might have impacted outcomes were the changes in the recommendations of the health care organizations, the higher availability of diagnostics tests, and the learning curve about disease diagnosis and management, leading to earlier and broader diagnosis, properly referred hospitalization, or better management of pharmacological and non-pharmacological interventions. In fact, the reduction in the percentage of patients with dyspnea, hypoxemia, and radiological chest findings suggest earlier demand for medical assistance, earlier clinical suspicion and diagnosis, and/or earlier hospitalization. The median time until intubation was prolonged by 3 days, suggesting improvements in the optimal use of non-invasive ventilation techniques. Unfortunately, we did not capture information about ventilatory management before invasive mechanical ventilation. Noteworthy, the interpretation of the downward trend in ICU admission must be cautious, since the availability of ICU beds is not uniform across the country’s centers and regions (2).
Interestingly, the improvement in the 90-day fatality was not evident. We believe that the 28-day mortality rate reflects disease severity, and prompt and proper diagnosis and treatment. In turn, 90-day mortality also seems to reflect intra-hospital care, such as preventing nosocomial infections, thromboembolic events, and other adverse events related to health care, malnutrition, and immobilization. Although these processes have probably also improved over the period, our study was not empowered to show this trend.
A clear change in the pharmacological supporting treatments was observed, which might also have impacted outcomes, mainly the higher use of high-dose steroids and anticoagulants (8, 9). The retrospective nature of a registry study, the absence of data on the onset of all interventions, and the diversity of COVID-19 management protocols in our continental country preclude any definitive conclusion about the efficacy of these strategies. We could not access information of patients who did not have access to medical care. The overwhelmed health system during the peaks of the pandemic could have hindered the arrival of more severe COVID-19 patients at the hospital, leading to deaths before hospitalization. In addition, despite the homogeneous number of patients in each quartile, groups have different duration, potentially hampering to capture the workload of periods with a higher incidence of cases and the effect of overwhelmed hospitals.
As another limitation, this study was limited to the first wave of the pandemic in Brazil, and reflected the pre-vaccination period. We do not have information on the viral genotype, which also might influence the clinical presentation and outcomes. However, at that time, the variants of concern leading to potential changes in the clinical profile and patients outcomes had not been identified yet (23). The imprecise definition of death cause in more than 10% of patients also impaired a better understanding of the reasons behind the reduction in fatality rates, as well as hampered the precise distinction between related and non-related COVID deaths.
It is also notable that a lower percentage of patients had their immunosuppressive regimen completely withdrawn over the study time. Despite plenty of in vitro studies suggesting the potential benefit of immunosuppressive drugs on the clinical outcomes of coronavirus infection (24–29), no clinical study supports robust conclusions. In the multivariable analysis, the use of mTOR inhibitors in the maintenance immunosuppressive regimen was associated with lower death risk. The reduction in SARS-CoV-2 replication after the inhibition of the Akt/mTOR/HIF-1 signaling pathway was previously demonstrated by a recently published in vitro study (29). However, no conclusion in this regard is feasible considering the limitation of the study design. Finally, despite the statistically significant linearly increasing trend through time, complex dynamics observed in some variables, such as the time between COVID-19 diagnosis and hospitalization, do not necessarily reflect clinically relevant changes.
Notwithstanding the above-mentioned limitations, inherent to registry data analysis, our study has important strengths: to the best of our knowledge, this is one of the largest multicenter national registers on COVID-19 in KT patients; the national representation is consistent with site activities and with COVID-19 incidence in the Brazilian States; a robust center-adjusted analysis was performed to minimize site-effect; and the selection of hospitalized patients only, excluding patients with mild COVID-19 forms, makes our sample more homogeneous as to the initial severity criterion.
In conclusion, this study suggests that the COVID-associated fatality in KT patients requiring hospitalization improved over the six first months of the pandemic. Prospective studies are of utmost needed to better understand the impact of each intervention on outcomes.
Capsule Sentence Summary
This multicenter national Brazilian study accessed the fatality rates of COVID-19 among kidney transplanted patients over the first 6 months after the beginning of the pandemic. Using trend analysis, we could observe a decrease in the fatality rates from March to August 2020. A center-adjusted analysis was performed to explore the reasons for the improvement in the outcomes. Differences in demographics, clinical presentation, and treatment options might be involved in this trend.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board (IRB) of the Hospital do Rim/Fundação Oswaldo Ramos, from where the study was coordinated and for the National Commission for Research Ethics (approval number 4.033.525). All participating centers also obtained local IRB approval before data collection. Informed consent or its exemption followed specific national legislations, the local IRB recommendations, and the guidelines of the Declaration of Helsinki. Patient records and information were anonymized and de-identified before the analysis.
Author contributions
Participated in research design, in the performance of the research, in the writing of the paper, and data analysis and analytic tools: TS-F, MC, LR-M, LA, LV, JM-P, HT. Participated in the performance of the research and in the reviewing of the paper: VG, CO, RE, PL, IC, TF, RF, KC, DS, GF, VS, RA, LD, AS, IN, LO, DC, RO.
Funding
This study was partially supported by Novartis Pharma Brazil, and it also received financial support from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES), Finance Code 88881.507066/2020-01, Edital 11/2020.
Acknowledgments
The authors thank Associação Brasileira de Transplantes de Órgãos (ABTO) for the support; Mônica Rika Nakamura for the assistance during regulatory process; and the Gerência de Ensino e Pesquisa (GEP)/Complexo Hospitalar da Universidade Federal do Ceará (CH-UFC), notedly Antonio Brazil Viana Junior, for enabling the use of the REDcap. Authors also thank all the patients who participated in the study and the health professionals who assisted them.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2022.10205/full#supplementary-material
Supplementary Figure S1Cumulative incidence of deaths of SARS-CoV-2-infected kidney transplant patients within 90 days.
Supplementary Table S1Laboratory tests and chest radiological findings of kidney transplanted patients at COVID-19 diagnosis across quartiles of time.
Supplementary Table S2Graft losses after COVID-19 diagnosis.
Supplementary Table S3Risk factors for 28-days fatality after COVID-19 infection in KT recipients.
Supplementary Table S4Risk factors for 90-days fatality after COVID-19 infection in KT recipients.
Abbreviations
AKI, Acute kidney injury; AUC-ROC, Area Under the Receiver Operating Curve; COVID-19, Coronavirus disease 2019; eGFR, Glomerular filtration rate; ESKD, End-stage kidney disease; GLMM, Generalized Linear Mixed Models; IQR, Interquartile range; IRB, Institutional Review Board; KT, Kidney transplant; rATG, Antithymocyte globulin; RT-PCR, Reverse-transcription polymerase chain reaction; sCr, Serum creatinine; ST, Steroid; Δ sCr, Delta serum creatinine.
The COVID-19-KT Brazil Study Group
Beyond the authors, the COVID-19-KT Brazil Study Group includes the following participants: Roger Kist8, Aline Lima Cunha Alcântara2, Maria Luiza de Mattos Brito Oliveira Sales3, Mario Abbud Filho9, Katia Cronenberge Sousa10, Roberto Ceratti Manfro11, Tomás Pereira Júnior12, Maria Eduarda Heinzen de Almeida Coelho13, Marilda Mazzali21, Marcos Vinicius de Sousa21, Juliana Bastos Campos14, Nicole Gomes Campos Rocha15, Tania Leme da Rocha Martinez17, Joao Egidio Romao Junior17, Maria Regina Teixeira Araújo17, Sibele Lessa Braga17, Marcos Alexandre Vieira16, Elen Almeida Romão22, Miguel Moysés Neto22, Juliana Aparecida Zanocco23, Auro Buffani Claudino23, Gustavo Guilherme Queiroz Arimatea19, Tereza Azevedo Matuck20, Alexandre Tortoza Bignelli24, Maria Ferneda Puerari24, José Hermógenes Rocco Suassuna25, Suzimar da Silveira Rioja25, Rafael Lage Madeira26, Sandra Simone Vilaça26, Carlos Alberto Chalabi Calazans27, Daniel Costa Chalabi Calazans27, Patricia Malafronte28, Luiz Antonio Miorin28, Larissa Guedes da Fonte Andrade29, Filipe Carrilho de Aguiar29, Fabiana Loss de Carvalho Contieri30, Karoline Sesiuk Martins30, Helady Sanders Pinheiro31, Emiliana Spadarotto Sertório31, André Barreto Pereira32, David Jose9; Barros Machado33, Carolina Maria Pozzi34, Leonardo Viliano Kroth34, Lauro Monteiro Vasconcellos Filho36, Rafael Fabio Maciel37, Amanda Maíra Damasceno Silva38, Ana Paula Maia Baptista39, Pedro Augusto Macedo de Souza40, Marcus Lasmar41, Luciana Tanajura Santamaria Saber42, Lilian Palma43.
21Hospital de Clínicas da Universidade de Campinas-UNICAMP, Campinas, SP, Brazil; 22Divisão de Nefrologia, Faculdade de Medicina de Ribeirão Preto da Universidade de São Paulo (FMRP-USP), Ribeirão Preto, SP, Brazil; 23Hospital Santa Marcelina, São Paulo, SP, Brazil; 24Hospital Universitário Cajuru, Curitiba, PR, Brazil; 25Hospital Universitário Pedro Ernesto, Rio de Janeiro, RJ, Brazil; 26Hospital Felício Rocho, Belo Horizonte, BH, Brazil; 27Hospital Marcio Cunha, Ipatinga, MG, Brazil; 28Santa Casa de Misericórdia de São Paulo, São Paulo, SP, Brazil; 29Hospital das Clínicas da UFPE Universidade Federal de Pernambuco, Recife, PE, Brazil; 30Hospital do Rocio, Campo Largo, PR, Brazil; 31Hospital Universitário da Universidade Federal de Juiz de Fora, Juiz de Fora, MG, Brazil; 32Hospital Marieta Konder Bornhausen, Itajai, SC, Brazil; 33Hospital Alemão Osvaldo Cruz, São Paulo, SP, Brazil; 34Hospital Evangélico, Curitiba, PR, Brazil; 35Hospital São Lucas da PUCRS, Porto Alegre, RS, Brazil; 36Hospital Meridional, Cariacica, ES, Brazil; 37Hospital Nossa Senhora das Neves, João Pessoa, PB, Brazil; 38Hospital Antonio Targino, Campina Grande, PB, Brazil; 39Hospital São Rafael, Salvador, BA, Brazil; 40Santa Casa de Misericórdia de Belo Horizonte, Belo Horizonte, MG, Brazil; 41Hospital Universitário Ciências Médicas, Belo Horizonte, MG, Brazil; 42Santa Casa de Misericórdia de Ribeirão Preto, Ribeirão Preto, SP, Brazil; 43Centro Médico de Campinas, Campinas, SP, Brazil.
References
1.
World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available at: https://covid19.who.int/ (Accessed March 28, 2021).
2.
RanzaniOTBastosLSLGelliJGMMarchesiJFBaiãoFHamacherSet alCharacterisation of the First 250000 Hospital Admissions for COVID -19 in Brazil: A Retrospective Analysis of Nationwide Data. Lancet Respir Med (2021). 9(4):407–18. 10.1016/S2213-2600(20)30560-9
3.
WilliamsonEJWalkerAJBhaskaranKBaconSBatesCMortonCEet alFactors Associated with COVID-19-Related Death Using OpenSAFELY. Nature (2020). 584(7821):430–6. 10.1038/s41586-020-2521-4
4.
JouffroyRJostDPrunetBPrehospital Pulse Oximetry: a Red Flag for Early Detection of Silent Hypoxemia in COVID-19 Patients. Crit Care (2020). 24(1):313. 10.1186/s13054-020-03036-9
5.
WalkerJDollySNgLPrior-OngMSabapathyKThe Role of CPAP as a Potential Bridge to Invasive Ventilation and as a Ceiling-of-Care for Patients Hospitalized with Covid-19-An Observational Study. PLoS One (2020). 15(12):e0244857. 10.1371/journal.pone.0244857
6.
AlhazzaniWMøllerMHArabiYMLoebMGongMNFanEet alSurviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med (2020). 46(5):854–87. 10.1007/s00134-020-06022-5
7.
SztajnbokJMaselli-SchoueriJHCunha de Resende BrasilLMFarias de SousaLCordeiroCMSansão BorgesLMet alProne Positioning to Improve Oxygenation and Relieve Respiratory Symptoms in Awake, Spontaneously Breathing Non-intubated Patients with COVID-19 Pneumonia. Respir Med Case Rep (2020). 30:101096. 10.1016/j.rmcr.2020.101096
8.
TangNBaiHChenXGongJLiDSunZAnticoagulant Treatment is Associated with Decreased Mortality in Severe Coronavirus Disease 2019 Patients with Coagulopathy. J Thromb Haemost (2020). 18(5):1094–9. 10.1111/jth.14817
9.
HorbyPLimWSEmbersonJRMafhamMBellJLLinsellLet alDexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N Engl J Med (2020). 84(8):693–704. 10.1056/NEJMoa2021436
10.
HorwitzLIJonesSACerfolioRJFrancoisFGrecoJRudyBet alTrends in COVID-19 Risk-Adjusted Mortality Rates. J Hosp Med (2021). 16(2):90–2. 10.12788/jhm.3552
11.
AuldSCCaridi-ScheibleMRobichauxCCoopersmithCMMurphyDJ, Emory COVID-19 Quality and Clinical Research Collaborative. Declines in Mortality over Time for Critically Ill Adults with Coronavirus Disease 2019. Crit Care Med (2020). 48(12):e1382–e1384. 10.1097/ccm.0000000000004687
12.
DennisJMMcGovernAPVollmerSJMateenBAImproving Survival of Critical Care Patients With Coronavirus Disease 2019 in England: A National Cohort Study, March to June 2020*. Crit Care Med (2021). 49(2):209–14. 10.1097/ccm.0000000000004747
13.
Garcia-VidalCCózar-LlistóAMeiraFDueñasGPuerta-AlcaldePCillonizCet alTrends in Mortality of Hospitalised COVID-19 Patients: A Single centre Observational Cohort Study from Spain. Lancet Reg Health Eur (2021). 3:100041. 10.1016/j.lanepe.2021.100041
14.
CravediPMothiSSAzziYHaverlyMFaroukSSPérez‐SáezMJet alCOVID‐19 and Kidney Transplantation: Results from the TANGO International Transplant Consortium. Am J Transpl (2020). 20(11):3140–8. 10.1111/ajt.16185
15.
CaillardSAnglicheauDMatignonMDurrbachAGrezeCFrimatLet alAn Initial Report from the French SOT COVID Registry Suggests High Mortality Due to COVID-19 in Recipients of Kidney Transplants. Kidney Int (2020). 98(6):1549–58. 10.1016/j.kint.2020.08.005
16.
FavàACucchiariDMonteroNToapantaNCentellasFJVila‐SantandreuAet alClinical Characteristics and Risk Factors for Severe COVID‐19 in Hospitalized Kidney Transplant Recipients: A Multicentric Cohort Study. Am J Transpl (2020). 20(11):3030–41. 10.1111/ajt.16246
17.
HilbrandsLBDuivenvoordenRVartPFranssenCFMHemmelderMHJagerKJet alCOVID-19-Related Mortality in Kidney Transplant and Dialysis Patients: Results of the ERACODA Collaboration. Nephrol Dial Transpl (2020). 35(11):1973–83. 10.1093/ndt/gfaa261
18.
KatesOSHaydelBMFlormanSSRanaMMChaudhryZSRameshMSet alCOVID-19 in Solid Organ Transplant: A Multi-Center Cohort Study. Clin Infect Dis (2020). 73(11):e4090–e4099. 10.1093/cid/ciaa1097
19.
Requião-MouraLRSandes-FreitasTVd.VianaLACristelliMPAndradeLGMd.GarciaVDet alHigh Mortality Among Kidney Transplant Recipients Diagnosed with Coronavirus Disease 2019: Results from the Brazilian Multicenter Cohort Study. PLoS One (2021). 16(7):e0254822. 10.1371/journal.pone.0254822
20.
KhwajaAKDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron (2012). 120(4):c179–c184. 10.1159/000339789
21.
RubinDB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley & Sons (1987).
22.
CravediPSurajSMAzziYHaverlyMFaroukSPerez-SaezMJet alCOVID-19 and Kidney Transplantation: Results from the TANGO International Transplant Consortium. Am J Transpl (2020). 20(11):3140–8. 10.1111/ajt.16185
23.
TegallyHWilkinsonEGiovanettiMIranzadehAFonsecaVGiandhariJet alEmergence of a SARS-CoV-2 Variant of Concern with Mutations in Spike Glycoprotein. Nature (2021). 592:438–43. 10.1038/s41586-021-03402-9
24.
ChenXChouC-YChangG-GThiopurine Analogue Inhibitors of Severe Acute Respiratory Syndrome-Coronavirus Papain-Like Protease, a Deubiquitinating and deISGylating Enzyme. Antivir Chem Chemother (2009). 19(4):151–6. 10.1177/095632020901900402
25.
HartBJDyallJPostnikovaEZhouHKindrachukJJohnsonRFet alInterferon-β and Mycophenolic Acid Are Potent Inhibitors of Middle East Respiratory Syndrome Coronavirus in Cell-Based Assays. J Gen Virol (2014). 95(Pt 3):571–7. 10.1099/vir.0.061911-0
26.
Carbajo-LozoyaJMüllerMAKalliesSThielVDrostenCvon BrunnAReplication of Human Coronaviruses SARS-CoV, HCoV-NL63 and HCoV-229E is Inhibited by the Drug FK506. Virus Res (2012). 165(1):112–7. 10.1016/j.virusres.2012.02.002
27.
Carbajo-LozoyaJMa-LauerYMaleševićMTheuerkornMKahlertVPrellEet alHuman Coronavirus NL63 Replication is Cyclophilin A-Dependent and Inhibited by Non-immunosuppressive Cyclosporine A-Derivatives Including Alisporivir. Virus Res (2014). 184:44–53. 10.1016/j.virusres.2014.02.010
28.
PfefferleSSchöpfJKöglMFriedelCCMüllerMACarbajo-LozoyaJet alThe SARS-Coronavirus-Host Interactome: Identification of Cyclophilins as Target for Pan-Coronavirus Inhibitors. PLoS Pathog (2011). 7(10):e1002331. 10.1371/journal.ppat.1002331
29.
AppelbergSGuptaSSvensson AkusjärviSAmbikanATMikaeloffFSacconEet alDysregulation in Akt/mTOR/HIF-1 Signaling Identified by Proteo-Transcriptomics of SARS-CoV-2 Infected Cells. Emerg Microbes Infect (2020). 9(1):1748–60. 10.1080/22221751.2020.1799723
Summary
Keywords
Sars-CoV-2, Covid-19, kidney transplant, coronavirus, renal transplantation
Citation
Sandes-Freitas TV, Cristelli MP, Requião-Moura LR, Modelli de Andrade LG, Viana LA, Garcia VD, de Oliveira CMC, Esmeraldo RM, de Lima PR, Charpiot IMMF, Ferreira TCA, Franco RF, Costa KMAH, Simão DR, Ferreira GF, Santana VBBM, Almeida RAMB, Deboni LM, Saldanha ALR, Noronha IL, Oliveira LC, Carvalho DDBM, Oriá RB, Medina-Pestana JO and Tedesco-Silva Junior H (2022) Temporal Reduction in COVID-19-Associated Fatality Among Kidney Transplant Recipients: The Brazilian COVID-19 Registry Cohort Study. Transpl Int 35:10205. doi: 10.3389/ti.2022.10205
Received
09 November 2021
Accepted
05 January 2022
Published
01 February 2022
Volume
35 - 2022
Updates
Copyright
© 2022 Sandes-Freitas, Cristelli, Requião-Moura, Modelli de Andrade, Viana, Garcia, de Oliveira, Esmeraldo, de Lima, Charpiot, Ferreira, Franco, Costa, Simão, Ferreira, Santana, Almeida, Deboni, Saldanha, Noronha, Oliveira, Carvalho, Oriá, Medina-Pestana and Tedesco-Silva Junior.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tainá Veras de Sandes-Freitas, taina.sandes@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.