Abstract
Forest soils are crucial carbon sinks, with the soil microbial community playing a key role in the stabilization of organic matter and harboring numerous ecosystems services. These ecosystems can be affected, among other factors, by the different tree species present in the forest canopy. This study is focused on forests soils located in Galicia (on the north-west of Spain). Different soil properties and microbial activity were analyzed in 54 forest plantations, with different plant covers: birch, chestnut, eucalyptus, walnut, pines, oak and shrublands. These forest soils have in general an acid pH and a high organic matter content, but a small amount of phosphorus. These properties are mainly related to the parent material and the overall climatic conditions of this region, namely the high rainfall scores. The soil under eucalyptus and birch plantations were the driest (13% and 14% on average respectively) vs. the moistest with 27% on average under shrublands. The results regarding microbial activity showed that soils under walnuts have the biggest respiration rates whereas the smallest were under eucalyptus but there were no differences regarding the β-glucosidase enzyme activity. These results show that the forest management, specifically, which tree species are cultivated, has an impact on the soil microbial respiration and should be considered when elaborating forestry exploitation plans, especially in the current scenario of climate change where the C amount that healthy forest soils will be able to fix become crucial.
Introduction
Forests all over the world are endangered by different factors such as fragmentation, landscape change, deforestation, pollution or inadequate management (Birdsey and Pan, 2015; Tin et al., 2018; Ruiz-Chután et al., 2025), having detrimental effects on biodiversity (Diaz-Martin and Karubian, 2021) or even on climate change (Delabre et al., 2020). These ecosystems are crucial carbon sinks (Pandey, 2002), both above and below-ground, even though the soil section is still sometimes excluded from the C pools estimations, despite the enormous amount of C stored in it (Robinson, 2007). The soil microbial community plays a key role in the stabilization of organic matter in the forest soil systems, even acting as a C sink in the case of fungi (Cairney, 2012), and harbors numerous ecosystems services (Delgado-Baquerizo et al., 2016).
The soils of Galicia are generally acidic as a result of a humid climate and a strongly subtractive system, which lead to intense leaching, and, frequently, an acidic parent material (Macías et al., 1982; Macías, 1986). Furthermore, vegetation plays an important role in determining the physicochemical and biological soil properties (Chandra et al., 2016; Waymouth et al., 2020; Sui et al., 2022). Therefore, it would be crucial to increase the understanding of the effect due to different tree species on soil properties in order to select the most appropriate plants for a given area.
Globally, forest structure has been subjected to enormous changes in the last decades, with a marked decrease in extension since 1990, whereas the area occupied by forest plantations has increased (Birdsey and Pan, 2015). The species used for the plantations differed depending on the specific pedoclimatic conditions, but in temperate regions natural broadleaved forests have been replaced by trees mainly belonging to the family Pinaceae (Sawada et al., 2021) or Eucalyptus (Tomé et al., 2021). Some authors have noted that natural forest stands have better control of the nutrient cycle and superior soil quality than forest plantations (Chandra et al., 2016; Sui et al., 2022). Soil degradation processes can be related to the conversion from natural to plantation forest (Widyati et al., 2022), involving C and N concentrations decrease (Liao et al., 2012) or modifications in both bacterial and fungal soil communities (Sawada et al., 2021; Wang Q. et al., 2021; Sui et al., 2022).
Several studies have been conducted to understand the interrelationship between tree species, soil microbiota, and the physicochemical properties of forest soils (Mahía et al., 2006; Álvarez et al., 2009; Chandra et al., 2016). The forest soil microbiome can be affected by the different forestry activities such as logging and clear-cutting (Hartmann et al., 2014; Chen et al., 2021), irrigation (Hartmann et al., 2017), fertilization (Addison et al., 2021) or canopy disturbance (van Nuland et al., 2020). Contrasting results have been published for other practices like thinning, with some authors describing a negative impact of this practice on microbial carbon use efficiency (Xue et al., 2023), meanwhile others did not detect and impact on the soil fungal community (Castaño et al., 2018).
The natural primary drivers of the soil microbiome in forest include soil composition and nutrient availability, plant community structure, microbial interactions within the soil, disturbances, succession, and temporal dynamics (Onet et al., 2025). One of the key factors that determines the soil microbial community composition and function of forest soil is the different tree species present in the forest canopy, since a high microhabitat specificity of bacterial communities interacting with forest type has been described (Rodríguez-Rodríguez et al., 2023).
The current study is focused on the analysis of the effect of different forest species on the soil microbial activity and soil properties of forests located in Galicia (on the north-west of Spain) with a temperate-oceanic climate, which favors that the forests in this region are very productive, and this has a clear impact in the tree species that grow on then naturally and the species planted with commercial purposes. We hypothesized that the variability on the different physicochemical and biological soil parameters will be determined by the different tree species, with the bigger differences between the native broadleaf species and the intensive plantations (pines or eucalyptus), in soils developed over the same parent material and under the same climatic conditions.
Materials and Methods
Study Area and Soil Sampling
The sampling area is located in Rianxo municipality (Galicia, north-west of Spain, Figure 1). The climatic classification applied to this region defines the bioclimate region as typical temperate, oceanic and superior thermo-temperate (Rodríguez-Guitián and Ramil-Rego, 2007). The average temperature in the study area in 2024 was 15.7 °C with an accumulated precipitation of 1774 L m2, according to a weather station located in the area (42.580074 | −8.804707 WGS84) belonging to Meteogalicia (Xunta de Galicia meteorological network). The parent material in this area is mainly granitic, and the soils are mostly classified as Leptosols and Umbrisols (WRB IUSS Working Group, 2022).
FIGURE 1
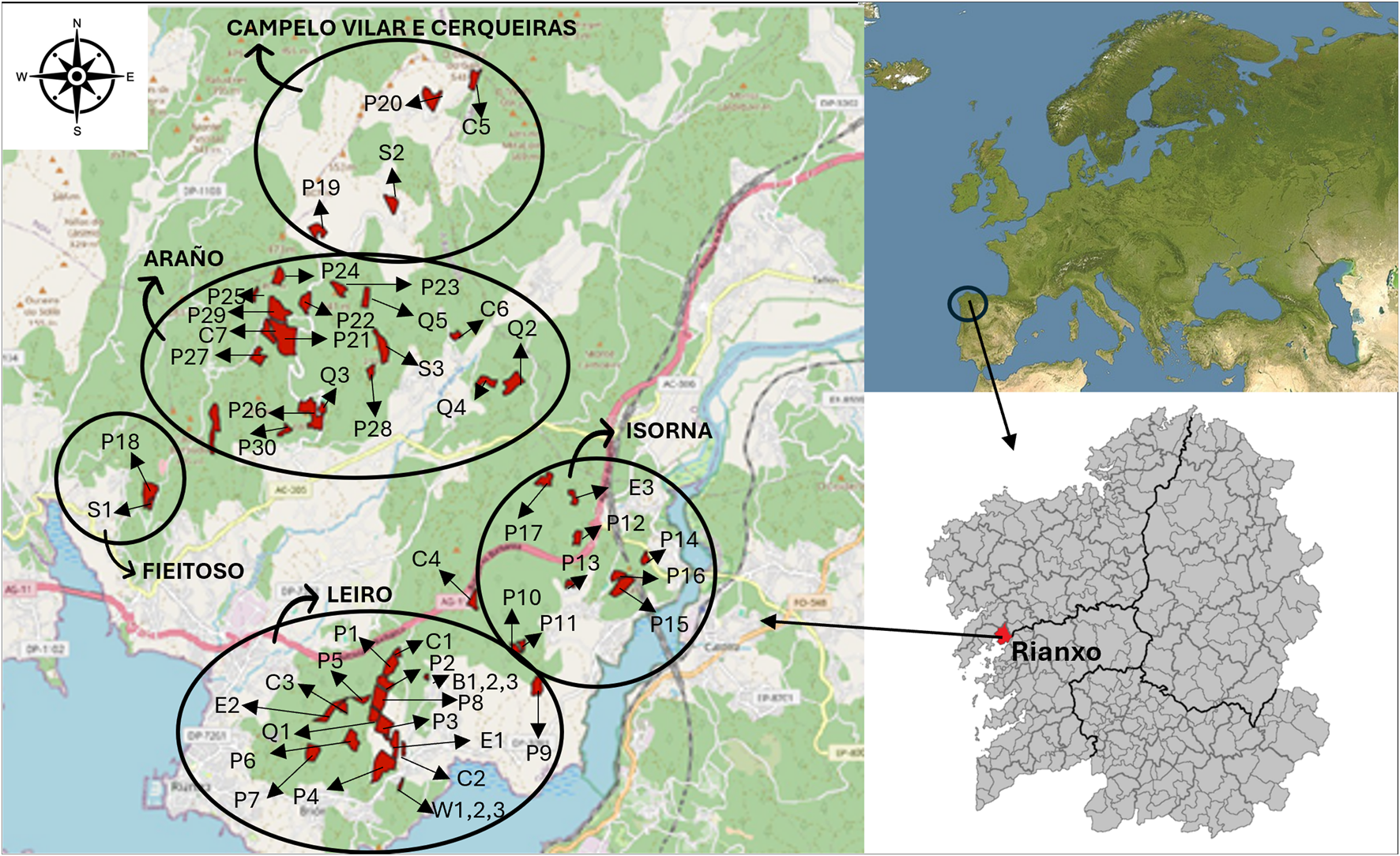
Location of the study area. The sampled plots are marked in red color in the left panel. The uppercase letters indicate the plot vegetation: P (pine), birch (B), walnut (W), chestnut (C), eucalyptus (E), oak (Q) and shrubland (S). The circles marked the different forest communities which are part of the study.
The type of property of these forests is communal, meaning that villagers are the group owners of specific areas of the forest usually near their village, which entails different challenges such as obtaining authorizations to access the property. The sampling was performed in collaboration with the forest owner associations in five different forest communities (Campelo Vilar e Cerqueiras, Araño, Fieitoso, Isorna and Leiro) within Rianxo municipality. Totally, 54 different forest plots were selected with different plantations established, 4 in Campelo Vilar e Cerqueira, 17 in Araño, 2 in Fieitoso, 9 in Isorna and 22 in Leiro (Figure 1). The number of replicates sampled differed between the different tree species, depending on what was available in the studied area and the agreement with the forest owners’ associations to sample, but the minimum number was three.
Pinus was one of the most representative forest plantations in this area and 30 soil samples under this tree type were collected along the five forest communities (Figure 1). The pine stands had different ages: 4 Pinus pinaster stands located in Isorna were 40–45 years old; 10 P. pinaster and 2 Pinus radiata stands, dispersed through the sampling area, were 14–17 years old; and 14 samples, also dispersed, were recent P. pinaster plantations (2–4 years old) (Figure 1; Table 1). The non coniferous species were less represented in this area and the following forest plantations were sampled: 3 soil samples under 4-year old birch plantations (Betula spp) and 3 under 4-years old walnut plantations (Juglans regia), both located in Leiro; 7 soil samples under 1–17 years old chestnut plantations (Castanea x hybrida) located in Leiro, Campelo and Araño; 3 soil samples under 6–14 years old eucalyptus plantations (Eucalyptus globulus) located in Leiro and Isorna; 5 soil samples under 2–17 years old oak plantations (mixed of Quercus rubra and Quercus robur) located in Leiro and Araño; and 3 soil samples under shrublands (Ulex europaeus, Pteridium aquilinum, Erica cinerea, Pterospartum tridentatum, Callunavulgaris, Davoecia cantabrica and Cytisus scoparium) located in Fieitoso, Campelo and Araño (Figure 1; Table 2). Totally, 30 samples were collected under coniferous forest, 18 under deciduous forest, 3 under eucalyptus and 3 under shrubland at different altitudes (50–485 m above the sea level) (Tables 1, 2).
TABLE 1
| Forest community | Trees | Age (years) | Altitude (masl) | Parental rock | Soil depth (cm) | Wildfires (Years) | Management (last 5 years) |
|---|---|---|---|---|---|---|---|
| Leiro | P1 | 3 | 160 | Gneis | 50 | 2019 | Plantation |
| P2 | 4 | 180 | Gneis | 50 | 2019 | - | |
| P3 | 3 | 170 | Schists/Gneis | 50 | 2019 | Plantation | |
| P4 | 14 | 125 | Schists/Gneis | 100 | - | - | |
| P5 | 15 | 145 | Schists/Gneis | 50 | - | - | |
| P6 | 5 | 110 | Schists/Gneis | 20 | 2019 | - | |
| P7 | 4 | 90 | Granite | 50 | 2019 | - | |
| P8 | 3 | 180 | Granite | 50 | 2019 | Plantation | |
| P9 | 14 | 115 | Granite | 100 | - | - | |
| Isorna | P10 | 3 | 175 | Granite | 50 | 2019 | Plantation/Clearing |
| P11 | 3 | 140 | Granite | 50 | 2019 | Plantation/Clearing | |
| P12 | 3 | 105 | Granite | 100 | 2019 | Plantation/Clearing | |
| P13 | 40 | 50 | Granite | 150 | - | Clearing | |
| P14 | 45 | 65 | Granite | 150 | - | - | |
| P15 | 45 | 70 | Granite | 150 | - | - | |
| P16 | 45 | 80 | Granite | 150 | - | - | |
| P17 | 14 | 165 | Granite | 100 | - | - | |
| Fieitoso | P18 | 17 | 155 | Schists/Gneis | 100 | - | Clearing, acacia removing, extensive grazing |
| Campelo | P19 | 14 | 450 | Granite | 50 | - | Clearing, acacia removing |
| P20 | 2 | 485 | Granite | 50 | - | Plantation | |
| Araño | P21 | 17 | 290 | Granite | 100 | - | Clearing, trituration |
| P22 | 2 | 330 | Granite | 50 | - | Plantation | |
| P23 | 3 | 325 | Orthogneis | 50 | - | Plantation | |
| P24 | 3 | 480 | Granite | 50 | - | Plantation | |
| P25 | 3 | 445 | Granite | 50 | - | Clearing, trituration | |
| P26 | 17 | 160 | Granite | 150 | - | Plantation | |
| P27 | 17 | 315 | Granite | 100 | - | Clearing, trituration | |
| P28 | 17 | 150 | Granite | 150 | - | Clearing, trituration | |
| P29 | 17 | 360 | Granite | 100 | - | Clearing, trituration | |
| P30 | 17 | 145 | Granite | 150 | - | Clearing, trituration |
Environmental properties of the pine (P) plots.
TABLE 2
| Forest community | Trees | Age (years) | Altitude (masl) | Parental rock | Soil depth (cm) | Wildfires (Years) | Management (last 5 years) |
|---|---|---|---|---|---|---|---|
| Leiro | B1 | 4 | 85 | Granite | 50 | 2019 | Plantation |
| B2 | 4 | 85 | Granite | 50 | 2019 | Plantation | |
| B3 | 4 | 85 | Granite | 50 | 2019 | Plantation | |
| Leiro | W1 | 4 | 75 | Schists/Gneis | 50 | - | Clearing/Plantation |
| W2 | 4 | 75 | Schists/Gneis | 50 | - | Clearing/Plantation | |
| W3 | 4 | 75 | Schists/Gneis | 50 | - | Clearing/Plantation | |
| Leiro | C1 | 4 | 150 | Gneis | 50 | 2019 | Plantation |
| C2 | 5 | 85 | Gneis | 100 | 2018 | Plantation | |
| C3 | 3 | 110 | Schists/Gneis | 50 | 2019 | Plantation | |
| C4 | 4 | 125 | Granite | 50 | 2019 | Plantation | |
| Campelo | C5 | 14 | 455 | Granite | 100 | - | - |
| Araño | C6 | 1 | 125 | Granite | 100 | - | Clearing/plantation |
| C7 | 17 | 295 | Granite | 100 | - | Clearing, trituration | |
| Leiro | E1 | 6 | 130 | Granite | 50 | 2019 | - |
| E2 | 15 | 120 | Schists/Gneis | 50 | 2019 | - | |
| Isorna | E3 | 14 | 90 | Granite | 50 | 2019 | - |
| Leiro | Q1 | 17 | 160 | Schists/Gneis | 50 | 2007/2010/2016 | Plantation |
| Araño | Q2 | 2 | 190 | Granite | 50 | - | Eucalyptus removal |
| Q3 | 17 | 135 | Granite | 150 | - | Clearing, trituration | |
| Q4 | 2 | 140 | Granite | 100 | - | Eucalyptus removal | |
| Q5 | 17 | 195 | Orthogneis | 100 | - | Clearing | |
| Fieitoso | S1 | - | 150 | Schists/Gneis | 50 | - | Intensive grazing |
| Campelo | S2 | - | 375 | Granite | 50 | - | Clearing |
| Araño | S3 | - | 200 | Granite | 100 | - | Eucalyptus removal (2023) |
Environmental properties of the birch (B), walnut (W), chestnut (C), eucalyptus (E), oak (Q) and shrubland (S) plots.
The soil depth, estimated during the soil sampling, was 50–150 cm, being the deeper soils under the more mature forest stands (Tables 1, 2). This area has a history of wildfires, specifically the communities of Leiro and Isorna which had an important fire event in 2019. The management of the plots in the last 5 years is also shown in Tables 1, 2. In each of the 54 forest stands, 10 subsamples were collected in a zig-zag transect making a composite sample per plot, sampling the 0–20 cm depth soil layer with an auger in the summer of 2024. The samples were immediately transported to the laboratory, sieved (<2 mm) and hand homogenized (quartering method, 5 times). A sub-sample was stored at 4 °C for the microbial analysis, and the rest was dry at 40 °C for the analysis of the soil physicochemical properties.
Soil Physicochemical and Microbial Analyses
Soil physicochemical properties were analyzed using standard procedures (Guitián Ojea and Carballas, 1976; Tan, 1986). Soil texture was determined using the international method of Robison Pipette. Soil water content was determined by the gravimetric method, where soils were oven-dried at 105 °C until constant weight. The pH values were obtained in water (1:2.5), using a pH-meter CRISON, model 2001. Total C and N were determined by elemental analysis using LECO equipment, model TRUSPEC CHNS. Extractable P was measured in soil samples using the Olsen method (Olsen and Sommers, 1982). Soil exchange cations (Ca2+, Mg2+, Na+, K+, Al3+) were extracted using NH4Cl 1mol L−1 (Peech et al., 1947) in 1:10 solution. Ca2+, Mg2+ and Al were measured by atomic absorption spectrophotometry; Na+ and K+ were quantified using emission spectrophotometry. The sum of all these exchange cations was considered the effective cation exchange capacity (eCEC) (Kamprath, 1970).
Regarding microbial analysis, soil microbial activity was estimated by the respiration rate (CO2 production) by transferring 1 g of soil to a 20 mL glass vial, which was then sealed and incubated for 24 h at room temperature and without light. The CO2 concentration was determined using a gas chromatograph equipped with FID and using a standard of CO2. Additionally, the β-glucosidase soil enzyme activity, one enzyme from the C-cycle, was analyzed according to the method of Eivazi and Tabatabai (1988). Briefly, the released p-nitrophenol was determined colorimetrically after incubation of the soil with p-nitrophenyl β-D-glucopyranoside for 2 h at 37 °C.
Data Analysis
The effect of the different forest cover was analyzed by means of standard one-way ANOVA. In cases of significant F statistics, Tukey’s significant difference test was used to separate the means. For the properties with non-normal distribution Kruskal-Wallis test was used. The correlation between the different soil physicochemical properties and the microbial activity parameters was analyzed using Pearson correlation coefficients at the p < 0.05 level. The values corresponding to all the physicochemical and biological soil properties were subjected to a redundancy analysis (RDA) to elucidate the main differences between the different forest covers and explore the relationship with the environmental variables. The statistical analyses were performed using the R software package (R studio, version 4.1.0).
Results and Discussion
Soil Physicochemical Properties
The dominant soil texture in this area was sandy, with 76% of soils having a sandy loam texture, 22% had a loamy sand texture and 2% were sandy soils, in accordance with the granite material from which they originate (Macías et al., 1982). These forest soils have, on average, an acid pH (between 4.63 and 5.6), a high content of soil organic matter (SOM) (between 12.1% and 20.9%), with N concentrations ranging 0.41%–0.69% and C/N ratio between 14.8 and 17.6, but containing small phosphorus concentrations (between 4.0 and 8.4 mg P kg−1) (Table 3). The statistical analysis showed no significant differences for the physiochemical properties of these soils between the different forest species, but it was observed that the soils under oak, pine and shrublands had the most acidic pH, meanwhile soils under birch and eucalyptus showed the lower amounts of C, SOM and P (Table 3). The low available P content in all these forest soils can be related to the acid pH, since at these values P is retained by the variable charge soil components which are positively charged, as happens for most of the forest soils in Galicia (García-Rodeja and Gil-Sotres, 1997; Eimil-Fraga et al., 2014).
TABLE 3
| Forest cover | pH | SOM (%) | N (%) | C/N | P (mg kg−1) | Na+ (cmol kg−1) | K+ (cmol kg−1) | Al3+ (cmol kg−1) | Sat Al3+ (%) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Birch | 4.92 | ± | 0.02 | 12.9 | ± | 0.4 | 0.45 | ± | 0.02 | 16.5 | ± | 0.2 | 6.0 | ± | 0.1 | 0.15 | ± | 0.03 | 0.29 | ± | 0.07 | 5.83 | ± | 0.62 | 63 | ± | 11 | ||
| Chesnut | 4.93 | ± | 0.16 | 15.5 | ± | 1.9 | 0.54 | ± | 0.06 | 17.0 | ± | 1.1 | 7.8 | ± | 1.5 | 0.19 | ± | 0.05 | 0.18 | ± | 0.02 | 6.31 | ± | 0.58 | 83 | ± | 2 | ||
| Eucalyptus | 4.98 | ± | 0.04 | 12.1 | ± | 2.3 | 0.41 | ± | 0.09 | 17.4 | ± | 1.8 | 4.0 | ± | 0.3 | 0.14 | ± | 0.03 | 0.25 | ± | 0.13 | 4.70 | ± | 1.49 | 72 | ± | 20 | ||
| Walnut | 5.06 | ± | 0.05 | 20.9 | ± | 0.4 | 0.69 | ± | 0.01 | 17.6 | ± | 0.1 | 7.6 | ± | 0.6 | 0.14 | ± | 0.02 | 0.22 | ± | 0.05 | 5.93 | ± | 0.25 | 75 | ± | 10 | ||
| Oak | 4.72 | ± | 0.10 | 15.4 | ± | 0.8 | 0.61 | ± | 0.03 | 14.8 | ± | 0.8 | 8.4 | ± | 1.5 | 0.15 | ± | 0.04 | 0.24 | ± | 0.05 | 7.43 | ± | 0.59 | 79 | ± | 7 | ||
| Shrubland | 4.63 | ± | 0.17 | 14.2 | ± | 0.8 | 0.54 | ± | 0.04 | 15.4 | ± | 1.6 | 7.2 | ± | 0.9 | 0.09 | ± | 0.01 | 0.11 | ± | 0.02 | 4.88 | ± | 0.91 | 90 | ± | 1 | ||
| Pine | 4.75 | ± | 0.05 | 15.1 | ± | 0.8 | 0.54 | ± | 0.03 | 16.3 | ± | 0.4 | 7.0 | ± | 0.8 | 0.14 | ± | 0.02 | 0.17 | ± | 0.01 | 6.58 | ± | 0.28 | 86 | ± | 1 | ||
Soil physicochemical properties under different types of forest cover. Average ± SE.
There are no significant differences between groups (p > 0.05), Krustal-Wallis test was used for pH and K and ANOVA test for the rest (n = 54).
These properties are mainly related to the climatic conditions of this region, namely the elevated precipitation since the humid climate favors the process of leaching and acidification, and are also due to the acidic nature of the parent material (Macías et al., 1982; Macías, 1986; García-Rodeja et al., 2023). There are numerous studies on the influence of different tree species on soil properties, but the results are inconsistent. Some authors point out that conifers acidify the soil and are therefore associated with degradation and podzolization processes, especially in cold regions (Matzner and Ulrich, 1983; Augusto et al., 1998; van Breemen et al., 2000, among others). More recent articles also describe a better quality of deciduous forest soils compared to coniferous forests and relate this to the quality of the leaf litter (Sui et al., 2022).
Conversely, in other areas, some authors indicate that vegetation may be a secondary factor influencing soil properties, subordinate to climate and parent material (Macías et al., 1982). Priha and Smolander (1999) state that it takes a long time for trees to cause changes in the soil, which could explain the lack of differences in the previously mentioned soil properties, since more than half of the forest stands analyzed in the present study were recent plantations (<6 years) (Tables 1, 2). To note that the soil solution is more sensitive than the solid phase for the detection of possible changes caused by anthropogenic activities (Álvarez et al., 2002; Álvarez et al., 2005). In this sense, these authors observed a significant effect of tree species on the pH of the liquid phase of Galician soils and on the most labile forms of aluminum (labile monomeric aluminum, and Al-F and Al-OH complexes), which were higher in soils under pine.
Soil moisture showed significant differences between some of the different forest plantations (Figure 2). Low soil water content was detected under eucalyptus and birch (13% and 14% on average, respectively) in contrast with higher values detected in the soils under shrubland (27%) (p = 0.042 and p = 0.035, respectively). The rest of the samples presented intermediate values, namely 19% for walnuts, 23% for oaks, 24% for chestnuts and 25% for pine (Figure 2). The ability of eucalyptus trees to extract a high amount of soil water has been described in the literature (Robinson et al., 2006), but other authors stated that eucalyptus water depletion is a myth and is more related to an inappropriate management of the plantation than to the trees themselves (Medeiros et al., 2025). The capacity of birch to decrease the water content of the topsoil has been well described, compared to coniferous trees (spruce) (Špulák et al., 2021), but in the mentioned study the stands were older than the ones in the current research.
FIGURE 2
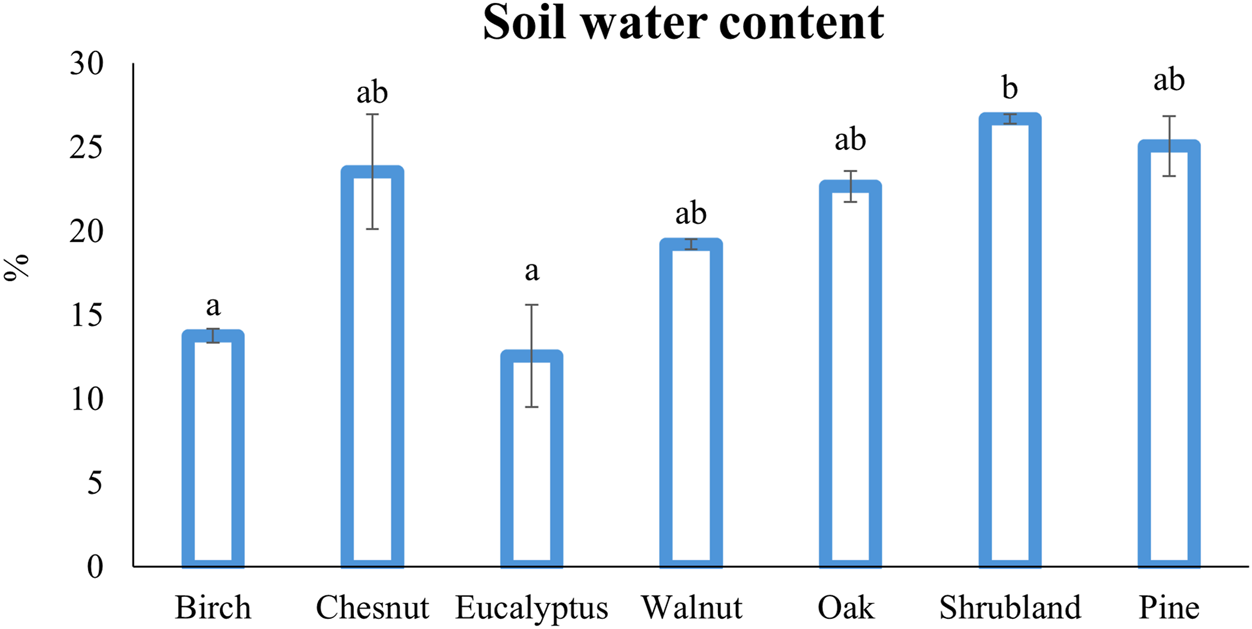
Soil water content (%) under the different type of forest cover. Different letters indicate significant differences, Kruskal-Wallis test (p < 0.05) (n = 54).
Both birch and eucalyptus stands were established over shallow soils (∼50 cm) that suffered the impact of a wildfire in 2019, whereas for the other species there were wildfire events in just some of the field replicates (pines, oaks and chestnuts) or a complete lack of these events in the case of walnut and shrubs (Table 2). If the disturbance of vegetation by fire is substantial enough, the resulting perturbations to soils can persist for years, resulting in lower water contents (Cooperdock et al., 2020). The soils under these trees showed the lower SOM content, 12.1% under eucalyptus and 12.9% under birch (Table 3) compared with the other species. The low water content of these samples might be related to the fire event, the soil management for the tree’s establishment or the low SOM content which would increase water retention under the other species (Rawls et al., 2003). Indeed, we found a significant positive correlation between soil water content and soil organic matter (r = 0.29, p < 0.05) (Figure 3).
FIGURE 3
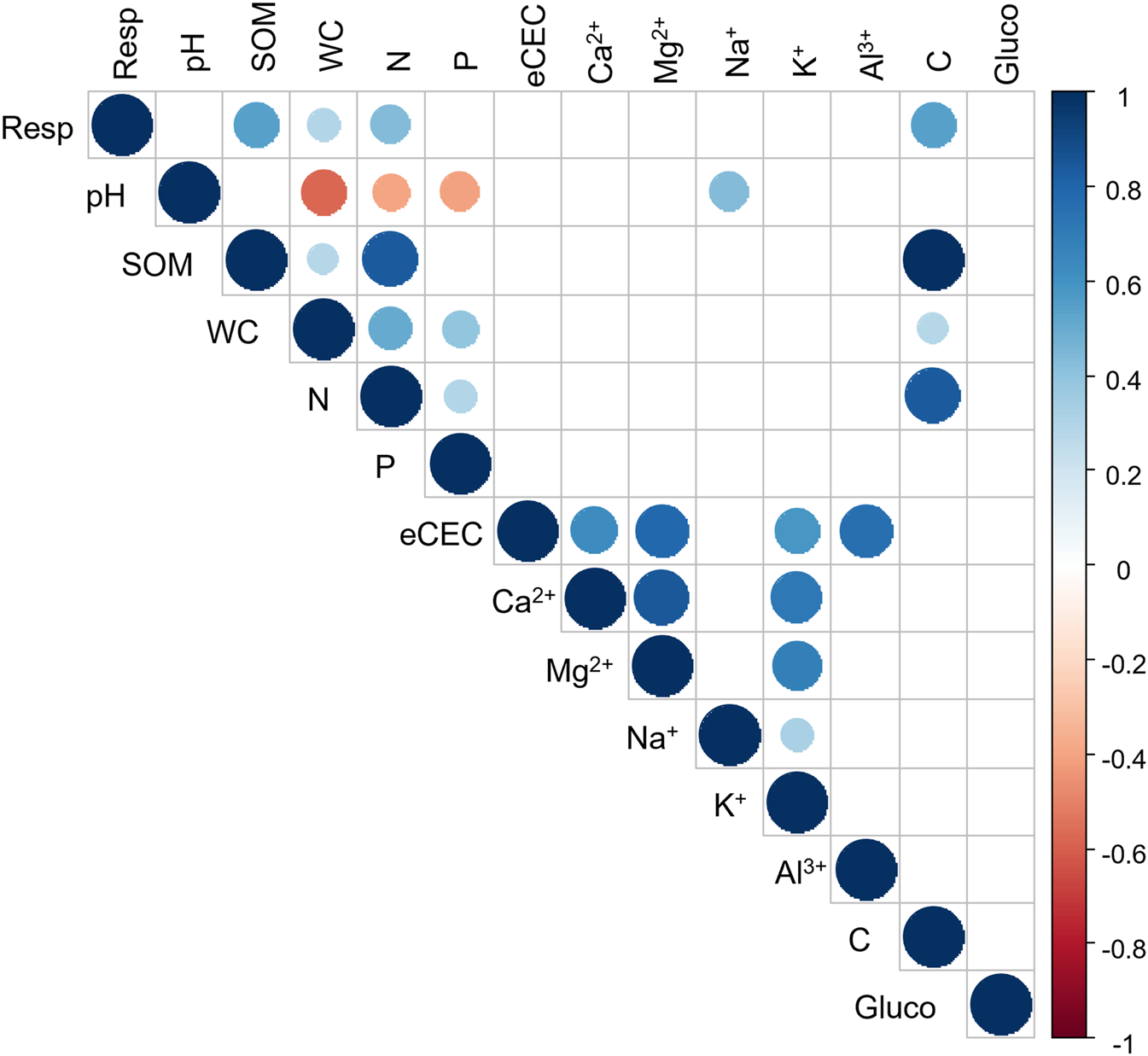
Pearson correlation of the different soil physicochemical properties. Blue dots mean significant positive correlations and red dots mean significant negative correlations, the size of the dot is proportional of the correlation coefficient (p < 0.05) (n = 54). Resp: respiration; SOM: soil organic matter; WC: water content; eCEC: effective cation exchange capacity; Gluco: β-glucosidase activity.
The bigger soil moisture in the soils under shrubland might be related to the afforestation process in terms of tree water consumption (Herron et al., 2022; Farley et al., 2004), being the differences in tree physiology, plantation design and management and the forestry operations the factors that affect the most to forest hydrology (van Dijk and Keenan, 2007). The permanent soil cover in the shrubland and the shrub roots (Gao et al., 2021) favor the high-water content in those soils. The lack of previous fire events in these plots (Table 2) might be another positive factor for the biggest soil water content.
On the other hand, the soils under shrubs showed the lowest effective cation exchange capacity, meanwhile soils under birch, oak and walnut had the highest values (Figure 4A). Conversely, other authors detected bigger eCEC scores under pine compared with oak trees in acid forest soils (Gruba and Mulder, 2015). The highest eCEC values coincide with the higher levelsof organic matter and/or higher pH (Table 3), which favors the increase of the negative charges in variable charge components (Gruba and Mulder, 2015). There were no differences regarding the concentration of Mg2+, Ca2+, Na+, K+ and Al3+ (Table 3; Figures 4B,C) among the different plantations.
FIGURE 4
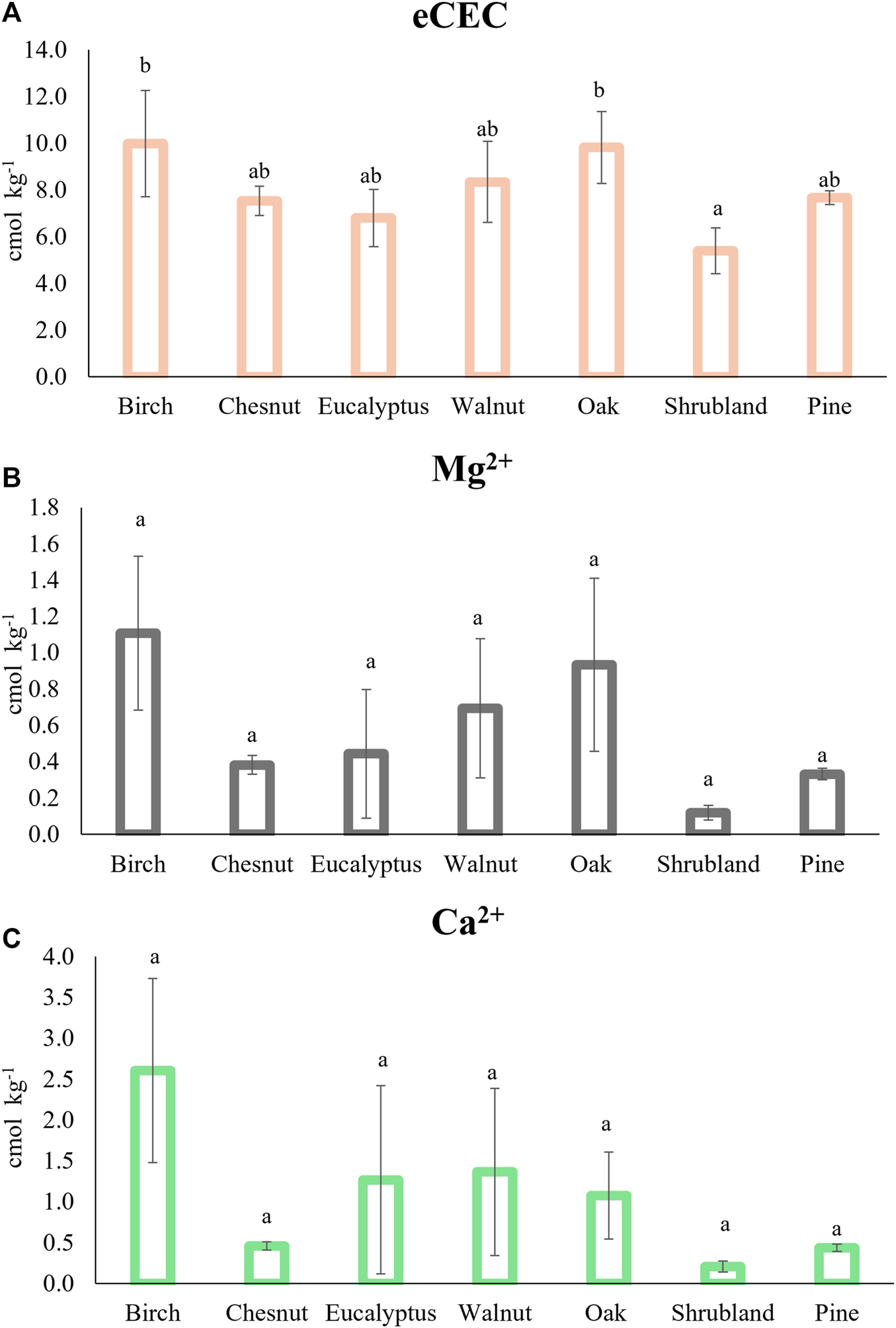
Effective cation exchange capacity (eCEC) (A), exchangeable Mg2+(B) and Ca2+(C) under the different the different type of forest cover. Different letters indicate significant differences (p < 0.05) Krustal-Wallis test was used for Mg2+ and Ca2+ and ANOVA test for eCEC (n = 54).
The amount of Al3+ (on average between 4.7 and 7.43 cmol kg−1) was quite high, which is common in these forest granitic soils (Macías et al., 1982). These Al3+ concentrations represented on average between 63% and 90% saturation of the exchange complex (Table 3), with practically all samples having values > 60% Al3+ saturation, indicative of alic soils, that can limit plant growth (Kochian et al., 2004; Gupta et al., 2013). The percentage of Al3+ saturation was higher under shrublands (90%), pines (86%) and chestnut (83%), even though this difference was not significant. In addition, an elevated Al3+ saturation under pine has been previously described (Álvarez et al., 2002).
However, despite the high Al3+ saturation of these forest soils, timber production in Galicia is high in the context of the temperate-humid region (Corbelle-Rico and Tubío-Sánchez, 2018). This may be related to different causes (species adaptation to these soils, mycorrhization, etc.), but with respect to Al3+, it has been shown that organic matter plays an important role in reducing the toxicity of this element, complexing Al3+ in the form of polymers or monomers (Álvarez et al., 2002; Álvarez et al., 2005; Eimil-Fraga et al., 2015; Eimil-Fraga et al., 2016). In addition, anions such as sulfate or fluoride can also bind to Al (Al-SO4, Al-F), decreasing its toxicity (Álvarez et al., 2005; Eimil-Fraga et al., 2016).
It was also observed that soils under chestnut, shrubland and pines had the lowest concentration of Mg2+ and Ca2+ (Figures 4B,C), even though these differences were not significant. To cope with the Al toxicity some species demand large quantities of Ca2+ and Mg2+ to reach high levels of productivity (Rocha et al., 2019). In fact, the Ca/Al ratio in soils, leaves and roots has been used as an index to evaluate the toxicity of this element (Cronan and Grigal, 1995; Álvarez et al., 2005; Eimil-Fraga et al., 2016). Kinraide (2003) and Kinraide et al. (2004) referred that the addition of Mg2+ to the external medium relieved Al toxicity in many plants. We detected a correlation between the exchangeable Ca2+ and Mg2+ in our samples with r = 0.85, p < 0.05 (Figure 3). The same trend was described in base-poor soils by other authors (Rocha et al., 2019). The lower concentrations of Ca2+ and Mg2+ in the soil under chestnuts, shrubland and pines could be a result of the major requirements of these plants to tolerate these high Al concentrations (Table 3).
Soil Microbial Activity
The results regarding microbial activity showed that soil under broadleaf species (birch, chestnut, walnut and oaks) have bigger respiration rates than soil under pines, shrublands and especially under eucalyptus (Figure 5B). The only significant differences, when divided at the species level, were between the soil respiration under eucalyptus and walnuts, with average values of 537 ± 211 ppm CO2 g−1 and 1440 ± 97 ppm CO2 g−1, respectively, with the soil respiration under the rest of the tree species being between those values (Figure 5A).
FIGURE 5
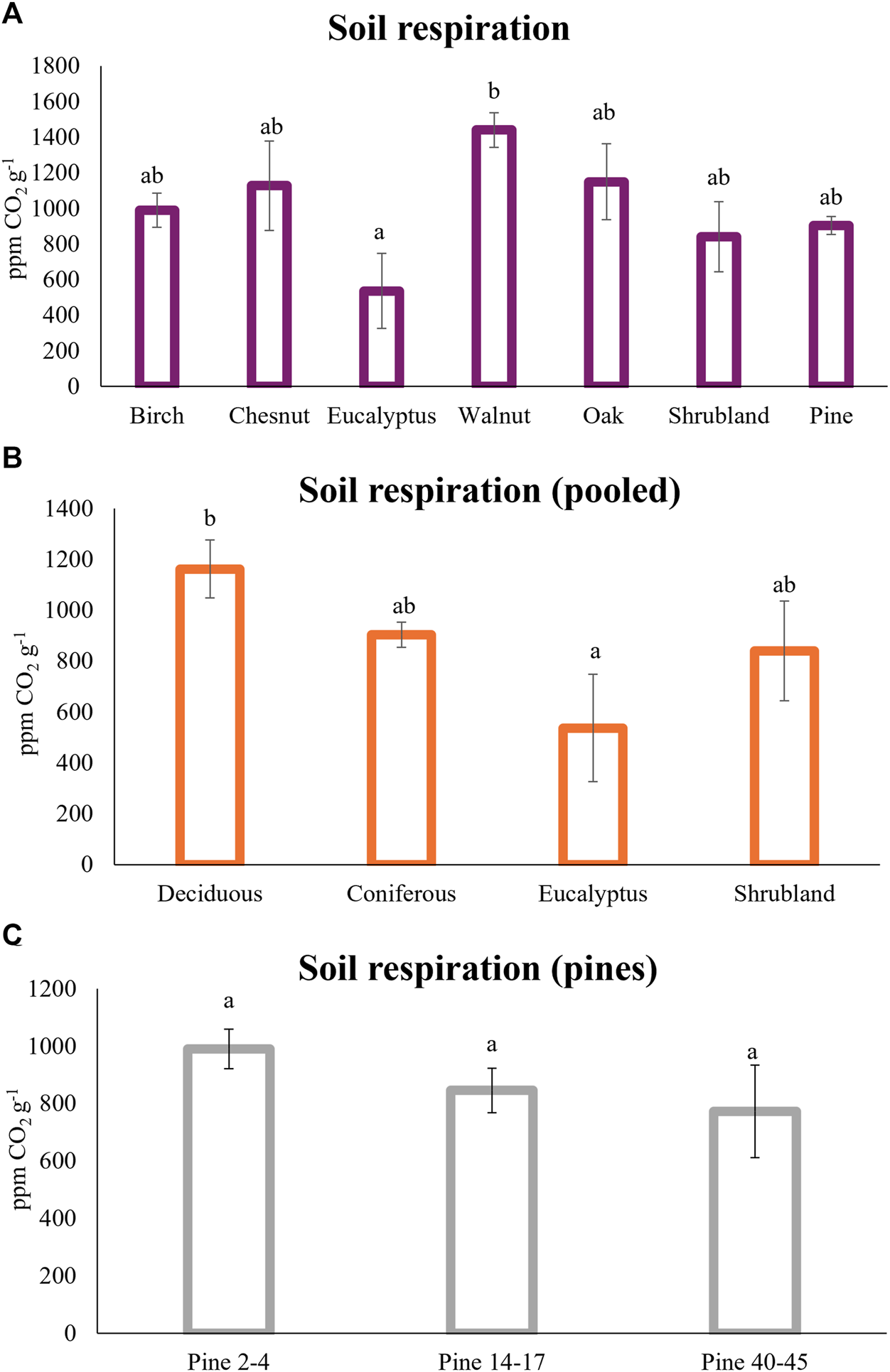
Soil respiration (ppm CO2 g−1) under the different tree plantations (A); with the deciduous species pool together (oak, birch, walnut and chestnut) (B); and under pine plantations with different ages (C). Different letters indicate significant differences, ANOVA test (p < 0.05).
The soil under walnuts showed the biggest amount of SOM (20.9%) meanwhile the soils under eucalyptus had the lowest values (12.1%) (Table 3). Even though these average values were not significantly different, soil respiration was positively correlated with soil organic matter (r = 0.54), total nitrogen (r = 0.43) and total C (r = 0.54) (Figure 3). On the other hand, walnut leaf litter has a big amount of phenolic compounds (Mungai and Motavalli, 2006) which can inhibit Gram-positive bacteria but do not affect fungi (Pereira et al., 2007), which are the main responsible of respiration on forest soils (Fransson, 2012).
The walnut plots did not suffer wildfires recently, whereas all the eucalyptus plots were burnt in 2019, therefore the smaller values in respiration for the latter could be a consequence of the wildfire event 5 years ago. However, recent metanalysis studies reveal that generally after 5 years total soil respiration recovers to pre-fire values (Zhou et al., 2023) and specifically for temperate forests the recovery time will be around 3 years (Gui et al., 2023). In general, microbial activity changes can be transitory, and their values can reach pre-fire ones, but diversity changes seem to be maintained at a longer time (Barreiro and Raviña, 2021).
When the values of soil respiration under de deciduous trees were pooled together (Figure 5B), it was observed that the respiration under eucalyptus was significantly lower than under the deciduous forests, but the coniferous values were between them. These results are in agreement with other authors (Raich and Potter, 1995; Hibbard et al., 2005) that did not find statistical differences in soil respiration between coniferous and deciduous forests. The litter input provided by the deciduous trees had a strong positive impact on soil respiration (Zhang et al., 2020). Similarly, soil respiration could increase under deciduous tree species, compared with evergreen tree species, due the bigger root exudation rates and annual root exudate carbon fluxes of the deciduous trees (Wang Y. et al., 2021).
The forest plantations with lower soil water content were birch and eucalyptus, and correlations between soil respiration and soil water content were detected (r = 029, p < 0.05) (Figure 3). However the soil respiration was lower under the eucalyptus plantation, meanwhile the values under birch were in the same order as the rest of the broadleaf species (Figure 5A). The dependency on the soil water content of soil respiration under eucalyptus has been described (Epron et al., 2004), and these authors also found a correlation of the soil respiration with both leaf and total aboveground litter in eucalyptus plantations but this was not analyzed in the current study. Other authors have described a modification in the microbial community structure within eucalyptus plantations, specifically a decrease in the fungal dominance (Behera and Sahani, 2003), which could explain the lower soil respiration rates under this forest plantation (Figure 5A).
For the pine plantations, soil respiration was also compared between stands of different ages, but no differences were observed (Figure 5C), even though the average values tended to decrease with the tree longevity, from 991 ± 69 ppm CO2 g−1 for the 2–4 years pine stand to 774 ± 162 ppm CO2 g−1 for the older pine stands (40–45 years). A similar decrease with age stands under pine was detected by Zhao et al. (2016).
In the analysis of the β-glucosidase enzyme activity no differences were detected for the soils under the different forest plantations, with average values between 60 ± 5 µg p-nitrophenol g−1 h−1 for soils under walnuts and 31 ± 15 µg p-nitrophenol g−1 h−1 for soils under shrublands. No differences were found neither when the values for the deciduous trees were pooled together for the distinct age stands in the case on the pine plantations (Figures 6A–C). Other studies described bigger β-glucosidase values under pines compared with oak (Błońska and Lasota, 2017).
FIGURE 6
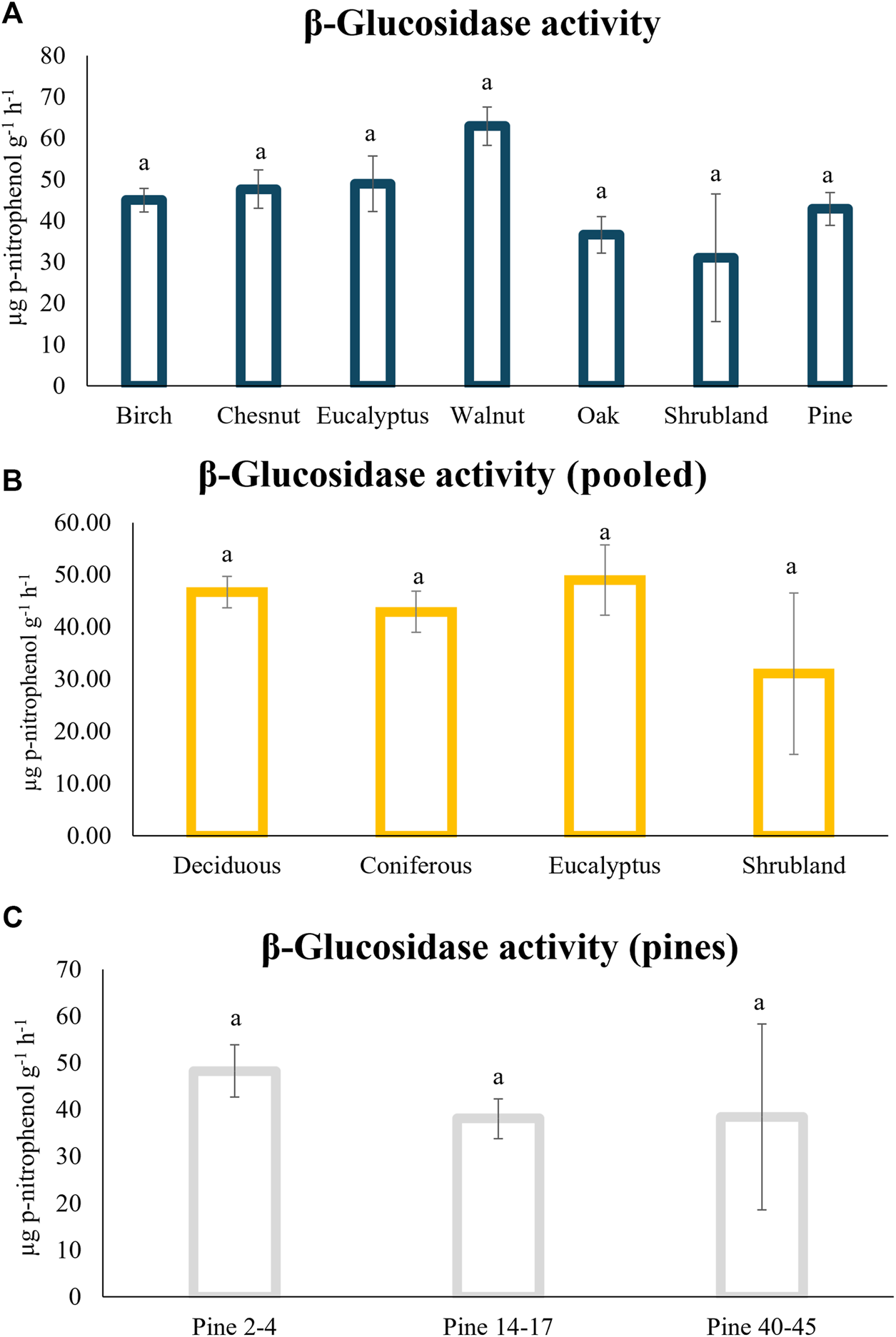
β-Glucosidase soil enzyme activity under the different tree plantations (A) with the deciduous species pool together (oak, birch, walnut and chestnut) (B); and under pine plantations with different ages (C). Different letters indicate significant differences, ANOVA test (p < 0.05).
Within our pine plantations, the young stands (2–4 years old) showed slightly higher average activity (48 ± 6 µg p-nitrophenol g−1 h−1) than the older stands (38 ± 4 and 38 ± 20 µg p-nitrophenol g−1 h−1 for 14–17 and 40–45 years old stands respectively), even though this difference was not significant (Figure 6C). Wang et al. (2019), using plantations within the same age range also found bigger β-glucosidase activity in the younger stands (3–6 years old) compared with stands that were 12–18 years old. However, these authors detected an increase in this enzyme activity in the soils under the oldest tree stands that was not found in our samples.
The role of this enzyme is key in the cellulose degradation (Zang et al., 2018), since β-glucosidases complete the final step of cellulose hydrolysis by converting cellobiose to glucose. This enzymatic activity is key in the C-cycling and can be used as a soil quality indicator (Stott et al., 2010). This decomposition process is stand-specific with a particularly negative effect in the case of evergreen tree litter (Joly et al., 2017) that was not detected in our study.
Soil enzyme activity in general, including β-glucosidase activity, usually correlated with soil pH, soil organic carbon and total nitrogen and edaphic properties, had higher importance in explaining enzyme variability than climate or stand properties (Oliveira et al., 2025). The limited variability observed in most of the edaphic properties within our samples (Table 3) might explain the lack of differences between the various forest stands (Figure 6A) or the non-significant correlation with such properties (Figure 3).
The values of this activity were lower than others described under oak and eucalyptus in the same region and with similar physicochemical properties (Lombao et al., 2015), but the referred soil had double the amount of moisture compared to the soil of the present study. In this case, the sampling was performed in summer, where a marked decrease in precipitation occurs every year, leading to a decrease in the water content, which might be the responsible for the lower enzyme activity detected in our samples. This suggest that β-glucosidase was sensitive to changing soil moisture regimes, as reported by other authors (Zhang et al., 2011). However, other studies in coniferous forest soils detected an impact of seasonality in the β-glucosidase gene pool, but not in the activity itself (Pathan et al., 2017). Different studies that identify differences between the soil β-glucosidase activity under different forest stands (Salazar et al., 2011) usually refers to adult trees, meanwhile in our study most of the trees are <17 years old.
Figure 7 represents the redundancy analysis (RDA) of the whole data set. Despite of the differences described for water content, eCEC and soil respiration between different forest plantations, when all the physiochemical and biological properties were analyzed together in the RDA analysis no structural differences were detected. The permutation test for the RDA reduced model showed that the age of the stand was the only significant factor (p < 0.05) affecting the soil properties, meanwhile wildfire events were marginally significant (p = 0.056). For the other environmental factors considered, community where the forest belongs, altitude, tree species, parental rock, and soil depth had no significant effect on the analyzed soil properties (p > 0.05). The soil depth and parental rock were correlated as the deeper soils developed generally over granitic rocks; as well as the altitude and the community, since some communities were located at higher altitudes than others (Figure 7; Tables 1, 2).
FIGURE 7
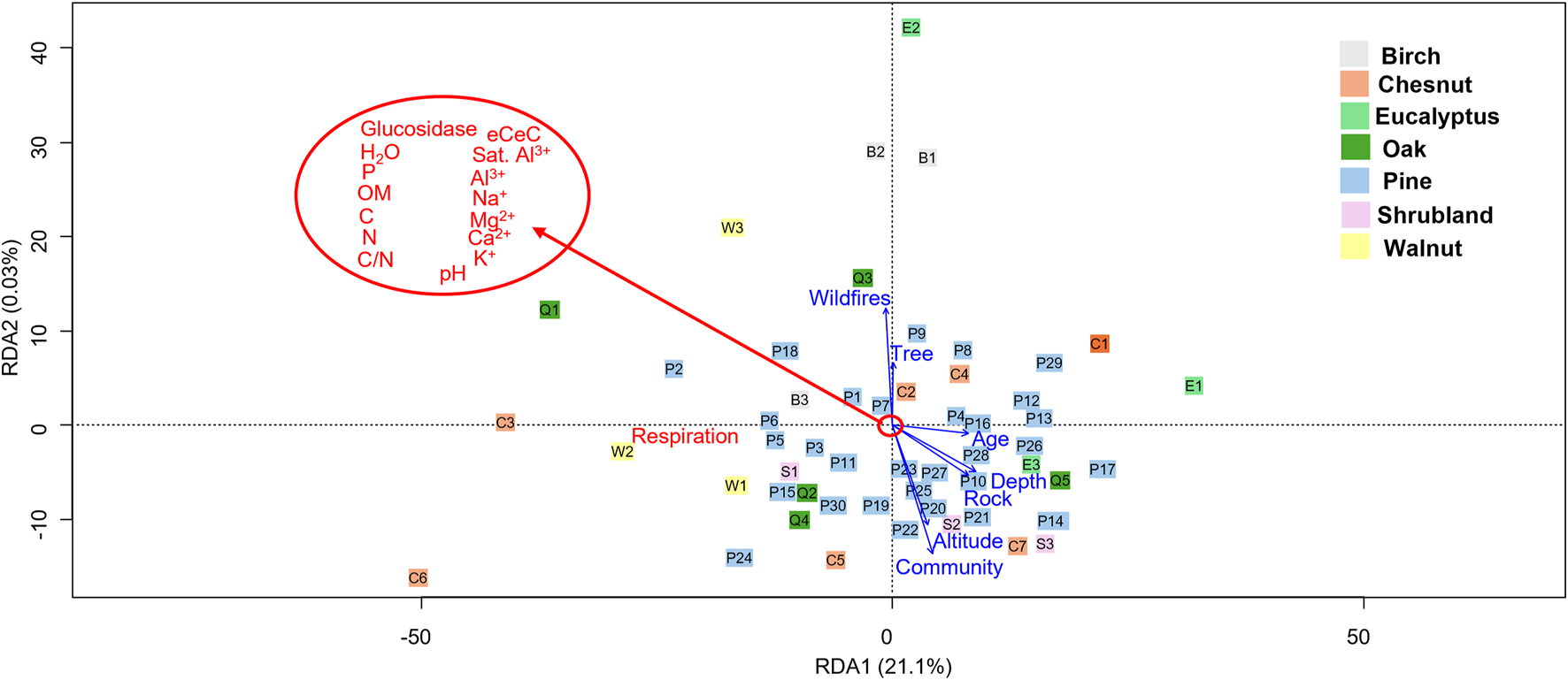
Redundancy analysis (RDA) of physicochemical and biological soil properties of the 54 soils under different forest covers. The uppercase letters indicate the plot vegetation: P (pine), birch (B), walnut (W), chestnut (C), eucalyptus (E), oak (Q) and shrubland (S).
The first component, which explained 21.1% of the variability, was related to the soil respiration, bigger in the soil samples with the high amount of SOM (Figure 7), in agreement with what has been previously published about the relation of CO2 efflux and carbon pools in roots and soils (Zhou et al., 2013). The rest of the soil properties did not have a clear impact on the distribution of the different samples in the RDA analysis. The influence of the stand age might be hindering the effect of the forest type, which has legacy effects in defining soil community composition (Rodríguez-Rodríguez et al., 2023). Forest age and structure have a noticeable effect on the soil nutrients and metals that tend to accumulate in soil rather than the litter (Lucas-Borja et al., 2019).
Regarding the soil microbial activity, younger stands have lower soil enzymatic activities and respiration, related with soil moisture, litterfall, soil organic matter and water holding capacity, compared with older stands (Lucas-Borja et al., 2016). However clear correlations between the age of the stand and the soil respiration and β-glucosidase activity were not detected in our study, probably due to the young age of most of the stands analyzed. Our hypothesis regarding the influence of the tree species in the soil properties was not fulfilled, most likely due to the different age of the stands which acted as a confounding factor.
Conclusion
The results of the current research indicate that tree species do not significantly affect soil pH, SOM, C, N, P or exchangeable cations, likely due to the analyses being restricted to the solid soil phase and the different ages of the plantations.
In contrast, three species influenced soil moisture and microbial respiration, with soils under eucalyptus exhibiting the lowest values for both parameters. These findings are critical for informing forestry management plans, particularly under current climate change scenarios, where productive forests serve as significant C sinks, yet management practices can markedly alter soil C pools.
Considering the entire spectrum of soil properties, stand age and historical wildfire events emerged as primary drivers of the observed soil variability. Further research is needed to elucidate species impact on soil properties and to guide forest management under specific edapho-climatic conditions. Integrating microbial community analysis with functional assessments, alongside evaluating long-term wildfire and management impacts is essential in the context of climate change.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
AB and AM-G participated on software, data curation, writing-Original draft preparation, visualization, and investigation. RC-D participated in software, data curation, visualization and investigation. MD-R, AN-D, and MF-S participated in conceptualization, methodology, writing-Original draft preparation, visualization, supervision, and validation. EÁ-R participated in visualization, supervision, validation, writing-reviewing and editing. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Laboratorio Ecosocial do Barbanza has the support of Fundación Biodiversidad from Ministerio para la Transición Ecológica y el Reto Demográfico (MITECO) within the Plan de Recuperación, Transformación y Resiliencia (PRTR), funded by European Union – NextGenerationEU.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
References
1
AddisonS. L.SmaillS. J.GarrettL. G.WakelinS. A. (2021). Fertiliser Use Has Multi-Decadal Effects on Microbial Diversity and Functionality of Forest Soils. Appl. Soil Ecol.163, 103964. 10.1016/j.apsoil.2021.103964
2
ÁlvarezE.MonterrosoC.MarcosM. F. (2002). Aluminium Fractionation in Galician (NW Spain) Forest Soils as Related to Vegetation and Parent Material. For. Ecol. Manag.166, 193–206. 10.1016/S0378-1127(01)00658-2
3
ÁlvarezE.Fernández-MarcosM. L.MonterrosoC.Fernández-SanjurjoM. J. (2005). Application of Aluminium Toxicity Indices to Soils Under Various Forest Species. For. Ecol. Manag.211, 227–239. 10.1016/j.foreco.2005.02.044
4
ÁlvarezE.TorradoV. T.Fernández-MarcosM. L.Díaz-RaviñaM. (2009). Microbial Biomass and Activity in a Forest Soil Under Different Tree Species. Electron. J. Food, Agric. Chem.8, 878–887.
5
AugustoL.BonnaudP.RangerJ. (1998). Impact of Tree Species on Forest Soil Acidification. For. Ecol. Manag.105, 67–78. 10.1016/S0378-1127(97)00270-3
6
BarreiroA.RaviñaD. (2021). Fire Impacts on Soil Microorganisms: Mass, Activity, and Diversity. Curr. Opin. Environ. Sci. and Health22, 100264. 10.1016/j.coesh.2021.100264
7
BeheraN.SahaniU. (2003). Soil Microbial Biomass and Activity in Response to Eucalyptus Plantation and Natural Regeneration on Tropical Soil. For. Ecol. Manag.174, 1–11. 10.1016/S0378-1127(02)00057-9
8
BirdseyR.PanY. (2015). Trends in Management of the World's Forests and Impacts on Carbon Stocks. For. Ecol. Manag.355, 83–90. 10.1016/j.foreco.2015.04.031
9
BłońskaE.LasotaJ. (2017). “β-Glucosidase Activity of Forest Soil as an Indicator of Soil Carbon Accumulation,” in Soil Biological Communities and Ecosystem Resilience (Springer International Publishing), 253–263.
10
CairneyJ. W. G. (2012). Extramatrical Mycelia of Ectomycorrhizal Fungi as Moderators of Carbon Dynamics in Forest Soil. Soil Biol. Biochem.47, 198–208. 10.1016/j.soilbio.2011.12.029
11
CastañoC.AldayJ. G.LindahlB. D.Martínez de AragónJ.de-MiguelS.ColinasC.et al (2018). Lack of Thinning Effects Over Inter-Annual Changes in Soil Fungal Community and Diversity in a Mediterranean Pine Forest. For. Ecol. Manag.424, 420–427. 10.1016/j.foreco.2018.05.004
12
ChandraL. R.GuptaS.PandeV.SinghN. (2016). Impact of Forest Vegetation on Soil Characteristics: A Correlation Between Soil Biological and Physico-Chemical Properties. 3 Biotech.6, 188–12. 10.1007/s13205-016-0510-y
13
ChenJ.ChazdonR. L.SwensonN. G.XuH.LuoT. (2021). Drivers of Soil Microbial Community Assembly During Recovery from Selective Logging and Clear-Cutting. J. Appl. Ecol.58, 2231–2242. 10.1111/1365-2664.13976
14
CooperdockS. C.HawkesC. V.XuD. R.BreeckerD. O. (2020). Soil Water Content and Soil Respiration Rates Are Reduced for Years Following Wildfire in a Hot and Dry Climate. Glob. Biogeochem. Cycles34, e2020GB006699. 10.1029/2020GB006699
15
Corbelle-RicoE. J.Tubío-SánchezM. J. (2018). Productivism and Abandonment: The Two Sides of Forest Transition in Galicia (Spain), 1966-2009. Rev. Bosque39, 457–467. 10.4067/S0717-92002018000300457
16
CronanC. S.GrigalD. F. (1995). Use of calcium/aluminum Ratios as Indicators of Stress in Forest Ecosystems. J. Environ. Qual.24, 209–226. 10.2134/jeq1995.00472425002400020002x
17
DelabreI.BoydE.BrockhausM.CartonW.KrauseT.NewellP.et al (2020). Unearthing the Myths of Global Sustainable Forest Governance. Glob. Sustain.3, e16. 10.1017/sus.2020.11
18
Delgado-BaquerizoM.MaestreF. T.ReichP. B.JeffriesT. C.GaitanJ. J.EncinarD.et al (2016). Microbial Diversity Drives Multifunctionality in Terrestrial Ecosystems. Nat. Commun.7, 10541. 10.1038/ncomms10541
19
Diaz-MartinZ.KarubianJ. (2021). Forest Cover at Landscape Scales Increases Male and Female Gametic Diversity of Palm Seedlings. Mol. Ecol.30, 4353–4367. 10.1111/mec.16060
20
Eimil-FragaC.Rodríguez-SoalleiroR.Sánchez-RodríguezF.Pérez-CruzadoC.Álvarez-RodríguezE. (2014). Significance of Bedrock as a Site Factor Determining Nutritional Status and Growth of Maritime Pine. For. Ecol. Manag.331, 19–24. 10.1016/j.foreco.2014.07.024
21
Eimil-FragaC.Álvarez-RodríguezE.Rodríguez-SoalleiroR.Fernández-SanjurjoM. J. (2015). Influence of Parent Material on the Aluminium Fractions in Acidic Soils Under Pinus pinaster in Galicia (NW Spain). Geoderma255, 50–57. 10.1016/j.geoderma.2015.04.026
22
Eimil‐FragaC.Fernández‐SanjurjoM. J.Rodríguez‐SoalleiroR.Álvarez‐RodríguezE. (2016). Aluminium Toxicity Risk for Pinus pinaster in Acid Soils (Galicia, NW Spain). Land Degrad. and Dev.27, 1731–1739. 10.1002/ldr.2539
23
EivaziF.TabatabaiM. A. (1988). Glucosidases and Galactosidases in Soils. Soil Biol. Biochem.20, 601–606. 10.1016/0038-0717(88)90141-1
24
EpronD.NouvellonY.RoupsardO.MouvondyW.MabialaA.Saint-AndréL.et al (2004). Spatial and Temporal Variations of Soil Respiration in a Eucalyptus Plantation in Congo. For. Ecol. Manag.202, 149–160. 10.1016/j.foreco.2004.07.019
25
FarleyK. A.KellyE. F.HofstedeR. G. M. (2004). Soil Organic Carbon and Water Retention After Conversion of Grasslands to Pine Plantations in the Ecuadorian Andes. Ecosystems7, 729–739. 10.1007/s10021-004-0047-5
26
FranssonP. (2012). Elevated CO2 Impacts Ectomycorrhiza-Mediated Forest Soil Carbon Flow: Fungal Biomass Production, Respiration and Exudation. Fungal Ecol.5, 85–98. 10.1016/j.funeco.2011.10.001
27
GaoZ.HuX.LiX. Y. (2021). Changes in Soil Water Retention and Content During Shrub Encroachment Process in Inner Mongolia, Northern China. Catena206, 105528. 10.1016/j.catena.2021.105528
28
García-RodejaI.Gil-SotresF. (1997). Prediction of Parameters Describing Phosphorus-Desorption Kinetics in Soils of Galicia (Northwest Spain). J. Environ. Qual.26, 1363–1369. 10.2134/jeq1997.00472425002600050023x
29
García-RodejaE.Nóvoa-MuñozJ. C.Pontevedra-PombalX. (2023). “Soils of Galicia,” in The Environment in Galicia: A Book of Images. Galician Environment Through Images (Cham: Springer International Publishing), 109–135.
30
GrubaP.MulderJ. (2015). Tree Species Affect Cation Exchange Capacity (CEC) and Cation Binding Properties of Organic Matter in Acid Forest Soils. Sci. Total Environ.511, 655–662. 10.1016/j.scitotenv.2015.01.013
31
GuiH.WangJ.HuM.ZhouZ.WanS. (2023). Impacts of Fire on Soil Respiration and Its Components: A Global meta-analysis. Agric. For. Meteorology336, 109496. 10.1016/j.agrformet.2023.109496
32
Guitián OjeaF.CarballasT. (1976). “Técnicas De Análisis De Suelos,” in Pico Sacro.
33
GuptaN.GauravS. S.KumarA. (2013). Molecular Basis of Aluminum Toxicity in Plants: A Review. Am. J. Plant Sci.4, 21–37. 10.4236/ajps.2013.412A3004
34
HartmannM.NiklausP. A.ZimmermannS.SchmutzS.KremerJ.AbarenkovK.et al (2014). Resistance and Resilience of the Forest Soil Microbiome to Logging-Associated Compaction. ISME J.8, 226–244. 10.1038/ismej.2013.141
35
HartmannM.BrunnerI.HagedornF.BardgettR. D.StierliB.HerzogC.et al (2017). A Decade of Irrigation Transforms the Soil Microbiome of a Semi-Arid Pine Forest. Mol. Ecol.26, 1190–1206. 10.1111/mec.13995
36
HerronN.DavisR.JonesR. (2002). The Effects of Large-Scale Afforestation and Climate Change on Water Allocation in the Macquarie River Catchment, NSW, Australia. J. Environ. Manag.65, 369–381. 10.1006/jema.2002.0562
37
HibbardK. A.LawB. E.ReichsteinM.SulzmanJ. (2005). An Analysis of Soil Respiration Across Northern Hemisphere Temperate Ecosystems. Biogeochemistry73, 29–70. 10.1007/s10533-004-2946-0
38
JolyF. X.MilcuA.Scherer-LorenzenM.JeanL. K.BussottiF.DawudS. M.et al (2017). Tree Species Diversity Affects Decomposition Through Modified Micro-Environmental Conditions Across European Forests. New Phytol.214, 1281–1293. 10.1111/nph.14452
39
KamprathE. (1970). Exchangeable Aluminum as a Criterion for Liming Leached Mineral Soils. Soil Sci. Soc. Am. J.34, 252–254. 10.2136/sssaj1970.03615995003400020022x
40
KinraideT. B. (2003). Toxicity Factors in Acidic Forest Soils: Attempts to Evaluate Separately the Toxic Effects of Excessive Al3+ and H+ and Insufficient Ca2+ and Mg2+ upon Root Elongation. Eur. J. Soil Sci.54, 323–333. 10.1046/j.1365-2389.2003.00538.x
41
KinraideT. B.PedlerJ. F.ParkerD. R. (2004). Relative Effectiveness of Calcium and Magnesium in the Alleviation of Rhizotoxicity in Wheat Induced by Copper, Zinc, Aluminum, Sodium, and Low Ph. Plant Soil259, 201–208. 10.1023/b:plso.0000020972.18777.99
42
KochianL. V.HoekengaO. A.PinerosM. A. (2004). How Do Crop Plants Tolerate Acid Soils? Mechanisms of Aluminium Tolerance and Phosphorous Efficiency. Annu. Rev. Plant Biol.55, 459–493. 10.1146/annurev.arplant.55.031903.141655
43
LiaoC.LuoY.FangC.ChenJ.LiB. (2012). The Effects of Plantation Practice on Soil Properties Based on the Comparison Between Natural and Planted Forests: A Meta‐Analysis. Glob. Ecol. Biogeogr.21, 318–327. 10.1111/j.1466-8238.2011.00690.x
44
LombaoA.BarreiroA.CarballasT.FontúrbelM. T.MartínA.VegaJ. A.et al (2015). Changes in Soil Properties After a Wildfire in Fragas Do Eume Natural Park (Galicia, NW Spain). Catena135, 409–418. 10.1016/j.catena.2014.08.007
45
Lucas-BorjaM. E.HedoJ.CerdáA.Candel-PérezD.ViñeglaB. (2016). Unravelling the Importance of Forest Age Stand and Forest Structure Driving Microbiological Soil Properties, Enzymatic Activities and Soil Nutrients Content in Mediterranean Spanish Black Pine (Pinus nigra Ar. Ssp. Salzmannii) Forest. Sci. Total Environ.562, 145–154. 10.1016/j.scitotenv.2016.03.160
46
Lucas-BorjaM. E.Hedo de SantiagoJ.YangY.ShenY.Candel-PérezD. (2019). Nutrient, Metal Contents and Microbiological Properties of Litter and Soil Along a Tree Age Gradient in Mediterranean Forest Ecosystems. Sci. Total Environ.650, 749–758. 10.1016/j.scitotenv.2018.09.079
47
MacíasF. (1986). Materias Orixinais E Solos De Galiza. Cuad. Seminario De Sargadelos. n°47, 47–79.
48
MacíasF.CalvoR. M.GarcíaC.García-RodejaE.SilvaB. (1982). “El Material Original: Su Formación E Influencia En Las Propiedades De Los Suelos De Galicia,” in Anales De Edafología Y Agrobiología, 1747–1768.
49
MahíaJ.Pérez-VenturaL.CabaneiroA.Díaz-RaviñaM. (2006). Soil Microbial Biomass Under Pine Forests of the Northern Spain: Influence of Stand Age, Site Quality and Parent Material. Investig. Agrar. Sist. Recur. For.15, 152–159.
50
MatznerE.UlrichB. (1983). “The Turnover of Protons by Mineralization and Ion Uptake in a Beech (Fagus silvatica) and a Norway Spruce Ecosystem,” in Effects of Accumulation of Air Pollutants in Forest Ecosystems: Proceedings of a Workshop Held at Göttingen, West Germany, May 16-18 (Dordrecht: Springer Netherlands), 93–103.
51
MedeirosP. L. D.PimentaA. S.MirandaN. D. O.MeloR. R. D.AmorimJ. D. S.AzevedoTKBD (2025). The Myth that Eucalyptus Trees Deplete Soil water-a Review. Forests16, 423. 10.3390/f16030423
52
MungaiN. W.MotavalliP. P. (2006). Litter Quality Effects on Soil Carbon and Nitrogen Dynamics in Temperate Alley Cropping Systems. Appl. Soil Ecol.31, 32–42. 10.1016/j.apsoil.2005.04.009
53
OliveiraF. C. C.BaconA. R.FoxT. R.JokelaE. J.KaneM. B.MartinT. A.et al (2025). Do Soil Enzymes Respond to Silvicultural Management?For. Ecol. Manag.585, 122651. 10.1016/j.foreco.2025.122651
54
OlsenS. R.SommersL. E. (1982). “Phosphorus,” in Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties. Editor PageA. L. (Madison, WI: Agronomy Monographs), 9.
55
OnetA.GrenniP.OnetC.StoianV.CrisanV. (2025). Forest Soil Microbiomes: A Review of Key Research From 2003 to 2023. Forests16, 148. 10.3390/f16010148
56
PandeyD. N. (2002). Global Climate Change and Carbon Management in Multifunctional Forests. Curr. Sci.83, 593–602.
57
PathanS. I.ŽifčákováL.CeccheriniM. T.PantaniO. L.VětrovskýT.BaldrianP. (2017). Seasonal Variation and Distribution of Total and Active Microbial Community of β-glucosidase Encoding Genes in Coniferous Forest Soil. Soil Biol. Biochem.105, 71–80. 10.1016/j.soilbio.2016.11.003
58
PeechL.AlexanderL. T.DeanL. A. (1947). Methods of Soil Analysis for Soil-Fertility Investigations. Washington D.C. USA: USDA Cir. N°, 757.
59
PereiraJ. A.OliveiraI.SousaA.ValentãoP.AndradeP. B.FerreiraICFRet al (2007). Walnut (Juglans regia L.) Leaves: Phenolic Compounds, Antibacterial Activity and Antioxidant Potential of Different Cultivars. Food Chem. Toxicol.45, 2287–2295. 10.1016/j.fct.2007.06.004
60
PrihaO.SmolanderA. (1999). Nitrogen Transformations in Soil Under Pinus sylvestris, Picea Abies and Betula pendula at Two Forest Sites. Soil Biol. Biochem.31, 965–977. 10.1016/S0038-0717(99)00006-1
61
RaichJ. W.PotterC. S. (1995). Global Patterns of Carbon Dioxide Emissions from Soils. Glob. Biogeochem. Cycles9, 23–36. 10.1029/94GB02723
62
RawlsW. J.PachepskyY. A. RitchieJ. C.SobeckiT. M.BloodworthH. (2003). Effect of soil organic carbon on soil water retention. Geoderma, 116(1–2), 61–76.
63
RobinsonD. (2007). Implications of a Large Global Root Biomass for Carbon Sink Estimates and for Soil Carbon Dynamics. Proc. R. Soc. B Biol. Sci.274, 2753–2759. 10.1098/rspb.2007.1012
64
RobinsonN.HarperR. J.SmettemK. R. J. (2006). Soil Water Depletion by Eucalyptus spp. Integrated into Dryland Agricultural Systems. Plant Soil286, 141–151. 10.1007/s11104-006-9032-4
65
RochaJ. H. T.du ToitB.GonçalvesJLDM (2019). Ca2+ and Mg2+ Nutrition and Its Application in Eucalyptus and Pinus Plantations. For. Ecol. Manag.442, 63–78. 10.1016/j.foreco.2019.03.062
66
Rodríguez GuitiánM. A.Ramil RegoP. (2007). Clasificaciones Climáticas Aplicadas a Galicia: Revisión Desde Una Perspectiva Biogeográfica.
67
Rodríguez-RodríguezJ. C.FentonN. J.BergeronY.KembelS. W. (2023). Soil and Tree Phyllosphere Microbial Communities Differ Between Coniferous and Broadleaf Deciduous Boreal Forests. Plant Soil488, 233–253. 10.1007/s11104-023-05959-y
68
Ruiz-ChutánJ. A.KalousováM.LojkaB.Colocho-HernándezS.Prado-CórdovaJ. P.MontesL.et al (2025). Impacts of Habitat Fragmentation on the Genetic Diversity of the Endangered Guatemalan Fir (Abies Guatemalensis Rehder). Genetica153, 8. 10.1007/s10709-024-00225-0
69
SalazarS.SánchezL. E.ÁlvarezJ.ValverdeA.GalindoP.IgualJ. M.et al (2011). Correlation Among Soil Enzyme Activities Under Different Forest System Management Practices. Ecol. Eng.37, 1123–1131. 10.1016/j.ecoleng.2011.02.007
70
SawadaK.InagakiY.SugiharaS.FunakawaS.RitzK.ToyotaK. (2021). Impacts of Conversion From Natural Forest to Cedar Plantation on the Structure and Diversity of Root-Associated and Soil Microbial Communities. Appl. Soil Ecol.167, 104027. 10.1016/j.apsoil.2021.104027
71
ŠpulákO.ŠachF.KacálekD. (2021). Topsoil Moisture Depletion and Recharge Below Young Norway Spruce, White Birch, and Treeless Gaps at a Mountain-Summit Site. Forests12, 828. 10.3390/f12070828
72
StottD. E.AndrewsS. S.LiebigM. A.WienholdB. J.KarlenD. L. (2010). Evaluation of β-Glucosidase Activity as a Soil Quality Indicator for the Soil Management Assessment Framework. Soil Sci. Soc. Am. J.74, 107–119. 10.2136/sssaj2009.0029
73
SuiX.ZengX.LiM.WengX.FreyB.YangL.et al (2022). Influence of Different Vegetation Types on Soil Physicochemical Parameters and Fungal Communities. Microorganisms10, 829. 10.3390/microorganisms10040829
74
TanK. H. (1986). Soil Sampling, Preparation, and Analysis. New York, USA: Marcel Dekker.
75
TinH. S.PalanivelooK.AnilikJ.VickneswaranM.TashiroY.VairappanC. S.et al (2018). Impact of Land-Use Change on Vertical Soil Bacterial Communities in Sabah. Microb. Ecol.75, 459–467. 10.1007/s00248-017-1043-6
76
ToméM.AlmeidaM. H.BarreiroS.BrancoM. R.DeusE.PintoG.et al (2021). Opportunities and Challenges of Eucalyptus Plantations in Europe: The Iberian Peninsula Experience. Eur. J. For. Res.140, 489–510. 10.1007/s10342-021-01358-z
77
van BreemenN.LundströmU. S.JongmansA. G. (2000). Do Plants Drive Podzolization Via Rock-Eating Mycorrhizal Fungi?Geoderma94, 163–171. 10.1016/S0016-7061(99)00050-6
78
van DijkAIJMKeenanR. J. (2007). Planted Forests and Water in Perspective. For. Ecol. Manag.251, 1–9. 10.1016/j.foreco.2007.06.010
79
van NulandM. E.SmithD. P.BhatnagarJ. M.StefanskiA.HobbieS. E.ReichP. B.et al (2020). Warming and Disturbance Alter Soil Microbiome Diversity and Function in a Northern Forest Ecotone. FEMS Microbiol. Ecol.96, fiaa108. 10.1093/FEMSEC/FIAA108
80
WangC.XueL.DongY.HouL.WeiY.ChenJ.et al (2019). Contrasting Effects of Chinese Fir Plantations of Different Stand Ages on Soil Enzyme Activities and Microbial Communities. Forests10, 11. 10.3390/f10010011
81
WangQ.XiaoJ.DingJ.ZouT.ZhangZ.LiuQ.et al (2021). Differences in Root Exudate Inputs and Rhizosphere Effects on Soil N Transformation Between Deciduous and Evergreen Trees. Plant Soil458, 277–289. 10.1007/s11104-019-04156-0
82
WangY.ChenL.XiangW.OuyangS.ZhangT.ZhangX.et al (2021). Forest Conversion to Plantations: A Meta‐Analysis of Consequences for Soil and Microbial Properties and Functions. Glob. Change Biol.27, 5643–5656. 10.1111/gcb.15835
83
WaymouthV.MillerR. E.EdeF.BissettA.AponteC. (2020). Variation in Soil Microbial Communities: Elucidating Relationships with Vegetation and Soil Properties, and Testing Sampling Effectiveness. Plant Ecol.221, 837–851. 10.1007/s11258-020-01029-w
84
WidyatiE.NuroniahH. S.TataH. L.MindawatiN.LisnawatiY.DarwoA. L.et al (2022). Soil Degradation due to Conversion from Natural to Plantation Forests in Indonesia. Forests13, 1913. 10.3390/f13111913
85
WRB IUSS Working Group (2022). World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. 4th ed. Vienna, Austria: International Union of Soil Sciences.
86
XueW.ZhangW.ChenY. (2023). Heavy Thinning Temporally Reduced Soil Carbon Storage by Intensifying Soil Microbial Phosphorus Limitation. Plant Soil484, 33–48. 10.1007/s11104-022-05782-x
87
ZangX.LiuM.FanY.XuJ.LiH. (2018). The Structural and Functional Contributions of β-Glucosidase-Producing Microbial Communities to Cellulose Degradation in Composting. Biotechnol. Biofuels11, 51. 10.1186/s13068-018-1045-8
88
ZhangY.ChenL.WuZ.SunC. (2011). Kinetic Parameters of Soil β-Glucosidase Response to Environmental Temperature and Moisture Regimes. Rev. Bras. Ciência do Solo35, 1285–1291. 10.1590/S0100-06832011000400022
89
ZhangY.ZouJ.MengD.DangS.ZhouJ.OsborneB.et al (2020). Effect of Soil Microorganisms and Labile C Availability on Soil Respiration in Response to Litter Inputs in Forest Ecosystems: A Meta-Analysis. Ecol. Evol.10, 13602–13612. 10.1002/ece3.6965
90
ZhaoX.LiF.ZhangW.AiZ.ShenH.LiuX.et al (2016). Soil Respiration at Different Stand Ages (5, 10, and 20/30 Years) in Coniferous (Pinus tabulaeformis Carrière) and Deciduous (Populus davidiana Dode) Plantations in a Sandstorm Source Area. Forests7, 153. 10.3390/f7080153
91
ZhouZ.ZhangZ.ZhaT.LuoZ.ZhengJ.SunO. J. (2013). Predicting Soil Respiration Using Carbon Stock in Roots, Litter and Soil Organic Matter in Forests of Loess Plateau in China. Soil Biol. Biochem.57, 135–143. 10.1016/j.soilbio.2012.08.010
92
ZhouL.LiuS.GuY.WuL.HuH. W.HeJ. Z. (2023). Fire Decreases Soil Respiration and Its Components in Terrestrial Ecosystems. Funct. Ecol.37, 3124–3135. 10.1111/1365-2435.14443
Summary
Keywords
forestry management, forest soils, soil respiration, β-glucosidase, soil C
Citation
Barreiro A, Míguez-González A, Cela-Dablanca R, Díaz-Raviña M, Núñez-Delgado A, Fernández-Sanjurjo MJ and Álvarez-Rodríguez E (2025) Effect of Different Tree Plantations on the Chemical Properties and Microbial Activity in Galician Forests Soils. Span. J. Soil Sci. 15:14988. doi: 10.3389/sjss.2025.14988
Received
29 May 2025
Accepted
14 October 2025
Published
24 October 2025
Volume
15 - 2025
Edited by
Isabel Miralles Mellado, University of Almeria, Spain
Updates
Copyright
© 2025 Barreiro, Míguez-González, Cela-Dablanca, Díaz-Raviña, Núñez-Delgado, Fernández-Sanjurjo and Álvarez-Rodríguez.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: A. Barreiro, ana.barreiro.bujan@usc.es
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.