Dear Editors,
Dupilumab is widely used to treat atopic dermatitis (AD) in both children and adults. We report a rare case of exacerbation of hand rash after dupilumab initiation in a child with AD, although treatment could be continued.
A 14-year-old boy, diagnosed with AD in early childhood, presented with erythroderma, accompanied by diffuse erythema, papules, and crusts (Eczema Area and Severity Index [EASI] score: 35.8; Investigator’s Global Assessment [IGA] score: 4). His hands were erythematous and partially cracked with lichenification (Figures 1a,b). Despite long-term treatment with topical corticosteroids at another clinic, such as fluocinolone acetonide, his symptoms persisted. Therefore, dupilumab was initiated at our hospital, while topical corticosteroid, betamethasone butyrate propionate, was continued once daily. Two weeks after starting dupilumab, the generalized rash had markedly improved; however, 4 weeks after the first dose, the rash worsened only on his hands, showing increased erythema of the finger pads (Figure 1c) and scaling with desquamation and pale erythema on the dorsal fingers (Figure 1d). Because dupilumab was suspected of inducing the exacerbation, it was temporarily interrupted, and betamethasone butyrate propionate was applied to the hands multiple times daily. After a one-week interruption, the erythema improved (Figures 1e,f), and dupilumab was restarted. At the onset of the hand rash, the scheduled biweekly dose of dupilumab was delayed by 1 week, resulting in a temporary one-week interruption of treatment (Figure 1i). No changes were made regarding the potency of the topical corticosteroid. In addition, other factors, such as the use of emollients, occlusive therapy, or avoidance measures (e.g., irritants or wet work), were not altered during this period. After dupilumab was restarted, no further exacerbation occurred, and the erythema resolved. The patient has since maintained remission of both the body and hands (Figures 1g,h) with ongoing dupilumab and topical corticosteroids.
FIGURE 1
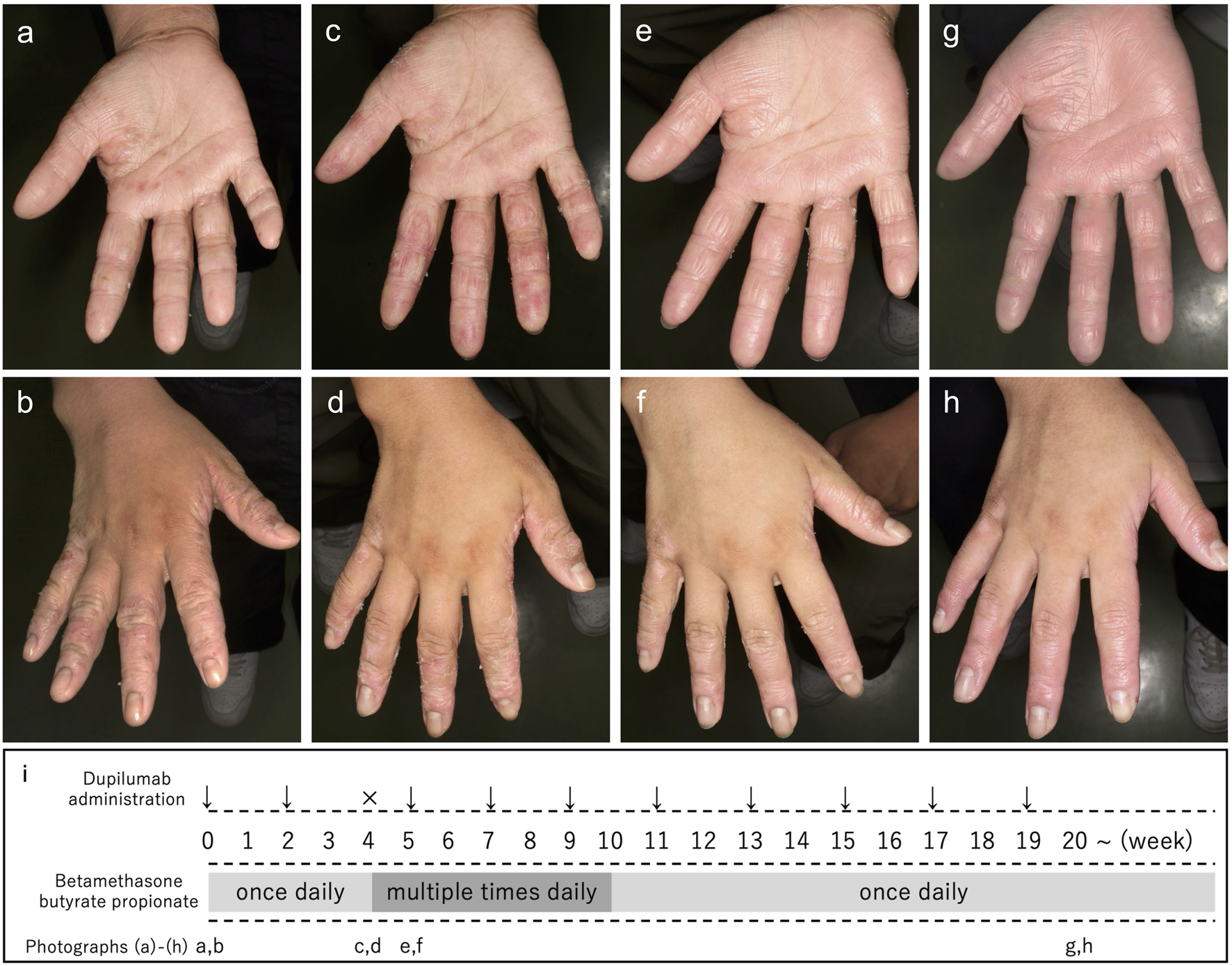
Clinical course and hand photographs of the present case. Before treatment with dupilumab, erythema and fissures were observed on the palm (a). Erythema, partially cracked skin, and lichenification were present on the dorsal aspects of the fingers (b). Four weeks after the first dose of dupilumab, the hand rash worsened, showing increased erythema of the finger pads (c) and scaling with noticeable desquamation on the dorsal fingers (d). After a temporary one-week interruption of dupilumab and an increased frequency of topical betamethasone butyrate propionate application to the hands (multiple times daily), the erythema improved (e,f). Twenty weeks after dupilumab initiation, no further exacerbation of hand erythema was observed (g,h). The timeline of dupilumab administration and topical corticosteroid use is shown in (i). Week 0 indicates the initial visit; arrows indicate dupilumab administration, and × indicates temporary interruption of dupilumab. Dupilumab was administered as a subcutaneous injection at an initial dose of 600 mg, followed by a second dose of 300 mg 2 weeks later. The third dose was administered after a one-week interruption. Thereafter, dupilumab was administered every 2 weeks.
We considered two possible explanations for the transient worsening of the hand rash. The first was the concurrent development of dyshidrotic eczema. This condition can appear on the palms and soles during early improvement phases, especially after starting topical corticosteroids. Previous studies reported onset 4–12 days after initiating topical treatment [1]. However, our patient’s hand lesions mainly showed erythema without blisters or vesicles, making dyshidrotic eczema less likely. The second possibility is that suppression of the Th2 response by dupilumab caused an unopposed Th1 or Th17 response [2, 3]. Biological agents such as dupilumab can alter cytokine balance, disrupting the equilibrium among Th1, Th2, Th17, and Treg cells. This shift may lead to Th1- or Th17-dominant inflammation, contributing to contact dermatitis or psoriasis-like eruptions [4, 5]. Hand eruptions following dupilumab administration have been reported previously [5], with some differences in the distribution and morphological features compared with the present case. In their series [5], lesions involved the hands and the periocular area in case 1, the hands and the forehead in case 2, and the hands and feet in case 3, with some eruptions described as diffuse erythema. In contrast, the eruptions in our case were confined to the hands and were predominantly characterized by erythema of the finger pads accompanied by scaling and desquamation on the dorsal aspects of the fingers. Nevertheless, the hands were a common site of involvement across all cases, and the distribution and morphological features of the hand eruptions in cases 1 and 2 were similar to those observed in our patient. These observations suggest that site-specific immunological factors of the hands, such as differences in local cytokine profiles, may contribute to the observed variations in clinical presentation. Accordingly, pathogenic mechanisms related to psoriasis or contact dermatitis may have been latently present in the hands of this patient and unmasked by dupilumab treatment, although no apparent allergens or irritants were identified in the present case.
Overall, the hand rash was considered a transient immune-mediated reaction, potentially induced by dupilumab that did not require permanent discontinuation. Although this adverse event is not described in the product labeling, recognizing its transient nature allowed continuous dupilumab therapy without permanent discontinuation. Because neither a skin biopsy nor an immunological evaluation was performed, the exact mechanism of the hand rash remains unclear. Moreover, it is possible that the temporary interruption of dupilumab may not have been necessary. Indeed, in the cases reported by Kim et al. [5], interruption of dupilumab was not described, suggesting that improvement of similar eruptions may occur even with continued treatment.
There are some limitations to this report. AD is characterized by a fluctuating clinical course, with spontaneous exacerbations and remissions frequently occurring, particularly during the early phase of treatment. Furthermore, from a pharmacodynamic perspective, given the gradual onset of dupilumab’s therapeutic effects and its long half-life, the observed exacerbation may have occurred before the drug’s efficacy became apparent, rather than representing an adverse reaction. The subsequent improvement after a one-week dose delay is unlikely to reflect a rapid change in pharmacological activity; therefore, causal interpretation based solely on temporal associations should be made with caution. In this case, other factors—such as emollient use, topical therapy, or lifestyle-related factors (e.g., exposure to irritants or wet work)—were not altered during the exacerbation. Moreover, marked improvement of the generalized skin eruption—excluding the hands—was observed approximately 2 weeks after the first administration of dupilumab, suggesting that clinical responses to biologic therapy may vary across anatomical sites and among individuals. Although a spontaneous flare cannot be excluded, the possibility that dupilumab contributed to the exacerbation was also considered. To establish a causal relationship between dupilumab administration and the observed skin exacerbation, a small case series or additional supportive evidence would be required.
Nonetheless, exacerbation of hand rash following dupilumab initiation appears to be uncommon in pediatric patients with AD, and this case highlights a potential but manageable reaction. The finding that dupilumab therapy could be continued offers valuable information for the management of similar cases.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Informed consent was obtained from the individual(s) for the publication of images or data included in this article.
Author contributions
KK: Data curation; Investigation; Visualization; Writing – original draft; Writing – review and editing. TS: Conceptualization; Investigation; Project administration; Supervision; Visualization; Writing – review and editing. YH: Supervision; Writing – review and editing. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declared that financial support was not received for this work and/or its publication.
Conflict of interest
TS and YH have received lecture fees from Sanofi K.K.
The remaining author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was used in the creation of this manuscript. Generative AI was used solely to assist with English language editing and grammatical refinement. All content was thoroughly reviewed, revised, and approved by the authors, who take full responsibility for the accuracy and integrity of the manuscript.
References
1.
NorrisPGLeveneGM. Pompholyx occurring during hospital admission for treatment of atopic dermatitis. Clin Exp Dermatol (1986) 12(3):189–90. 10.1111/j.1365-2230.1987.tb01892.x
2.
MirzaFNWangARamachandranSMDamskyWCohenJM. Dupilumab-induced phenotype switch from atopic dermatitis to psoriasis is characterized by de novo interleukin-17A expression: a case report. Br J Dermatol (2021) 185(2):432–4. 10.1111/bjd.20064
3.
NapolitanoMGaiazzoGFabbrociniGBalatoADi CaprioRScalaEet alIncreased expression of IL-23A in lesional skin of atopic dermatitis patients with psoriasiform reaction during treatment. Br J Dermatol (2021) 184(2):341–3. 10.1111/bjd.19459
4.
KychyginaACassagneMTauberMGaliacySPaulCFourniePet alDupilumab-associated adverse events during treatment of allergic diseases. Clin Rev Allergy Immunol (2022) 62(4):519–33. 10.1007/s12016-022-08934-0
5.
KimYJLeeMYWonCH. Acral erythema arising in patients with atopic dermatitis after dupilumab therapy: a case report of 3 patients. Asia Pac Allergy (2022) 12(1):e1. 10.5415/apallergy.2022.12.e1
Summary
Keywords
atopic dermatitis, dupilumab, exacerbation, hand rash, Th2
Citation
Kono K, Sakai T and Hatano Y (2026) Exacerbation of hand rash after dupilumab initiation in a child with atopic dermatitis who was able to continue treatment. J. Cutan. Immunol. Allergy 9:16078. doi: 10.3389/jcia.2026.16078
Received
17 December 2025
Revised
21 January 2026
Accepted
23 January 2026
Published
05 February 2026
Volume
9 - 2026
Updates
Copyright
© 2026 Kono, Sakai and Hatano.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takashi Sakai, t-sakai@oita-u.ac.jp
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.