Abstract
Schnitzler syndrome (SchS) is a rare, chronic autoinflammatory disorder characterized by a persistent urticarial rash, intermittent fever, arthralgia, bone pain, and elevated systemic inflammatory biomarkers. Due to its diverse manifestations and overlap with other conditions such as hematologic malignancies, connective tissue diseases, and infections, SchS is often underdiagnosed or misdiagnosed, resulting in delayed appropriate treatment. This report delineates the first two documented cases of SchS in Iran. Both patients exhibited pruritic hives, fever, bone pain, and arthralgia. They were initially diagnosed with chronic idiopathic urticaria (CIU). The diagnosis of SchS was confirmed according to the Strasburg criteria, which included a chronic urticarial rash, monoclonal gammopathy, mild interstitial edema, and an interstitial neutrophilic infiltrate without vasculitis, as observed in the histopathological examination of the dermal lesions. Both patients received subcutaneous omalizumab, a disease-modifying antirheumatic drug (DMARD) such as methotrexate, antihistamines, and corticosteroid drugs. We describe their clinical presentations, diagnostic challenges, and therapeutic strategies, emphasizing the limitations encountered in resource-constrained settings, including restricted access to targeted biologic therapies such as interleukin-1 inhibitors. Our findings underscore the importance of heightened clinical vigilance and comprehensive evaluation of atypical urticarial syndromes to facilitate timely diagnosis and improve patient outcomes.
Introduction
Schnitzler syndrome (SchS) is an uncommon, chronic inflammatory disease characterized by urticarial rash, fever, arthralgia/arthritis, bone pain, and elevated acute phase reactants [1]. The immunological aspect appears to be the primary pathophysiology of SchS; however, it can be mistaken for other illnesses such as hematological malignancies, connective tissue disorders, certain infectious diseases, major depression, and peptic ulcer disease [2, 3]. Patients with SchS commonly receive medications such as corticosteroids, immunomodulators, or even cytotoxic drugs without FDA approval [2, 4]. Interleukin-1 inhibitors, such as anakinra, represent a novel category of biological therapies gaining acceptance, particularly in resistant cases [5]. However, limited availability and high costs restrict their use in resource-poor areas. In this manuscript, we report the first cases of SchS in Iran, who were diagnosed with chronic idiopathic urticaria (CIU) for many years. We discuss the clinical features, diagnostic process, and treatment challenges that ultimately led to a diagnosis of SchS.
Report of cases
Case 1
On 2 July 2023, a 57-year-old man was admitted to our hospital with recurrent pruritic hives on his lower extremities (Figure 1A) lasting 3 days and chronic arthralgia and bone pain. His medical history included food allergies for 20 years, chronic idiopathic urticaria (CIU) for 5 years, and his family history was unremarkable for autoimmune disorders. He experienced two to three relapses annually, which were managed with corticosteroids and antihistamines. He received two courses of subcutaneous omalizumab (Xolair, Genentech, US) at a dose of 15 mg every 10 days for 3 months in 2019 and 2020. However, his symptoms recurred 3 months after stopping the medication. Upon examination, his vital signs were normal. There were several pale-rose, slightly elevated papules and plaques, measuring 3–6 cm in diameter, on his lower extremities, particularly in his left groin (Figure 1A). He had no enlarged lymph nodes or hepatosplenomegaly. He had some bone pain and tenderness on vertebral percussion. Initial laboratory tests showed a WBC count of 4,100 cells per microliter (PMN = 68%, Lymph = 25%, Eos = 4%. Mono = 3%), an Hb level of 14.1 mg/dL, a Platelet count of 245 (× 109/L), and normal electrolytes, liver function, kidney function, and thyroid function tests, except for a CRP level of 18 mg/dL and a first-hour ESR of 48 mm/h. Additional tests, including HIV, hepatitis B and C, and stool examination, were normal. His serum IgM level increased more than threefold above the upper limit, to 780 mg/dL. The rheumatologic screening tests requested by rheumatology consultations, including ANA, anti-dsDNA, Indirect Immunofluorescence, and RF, revealed no notable findings. Likewise, serum and urine electrophoresis results ordered by hematology consults were also unremarkable.
FIGURE 1
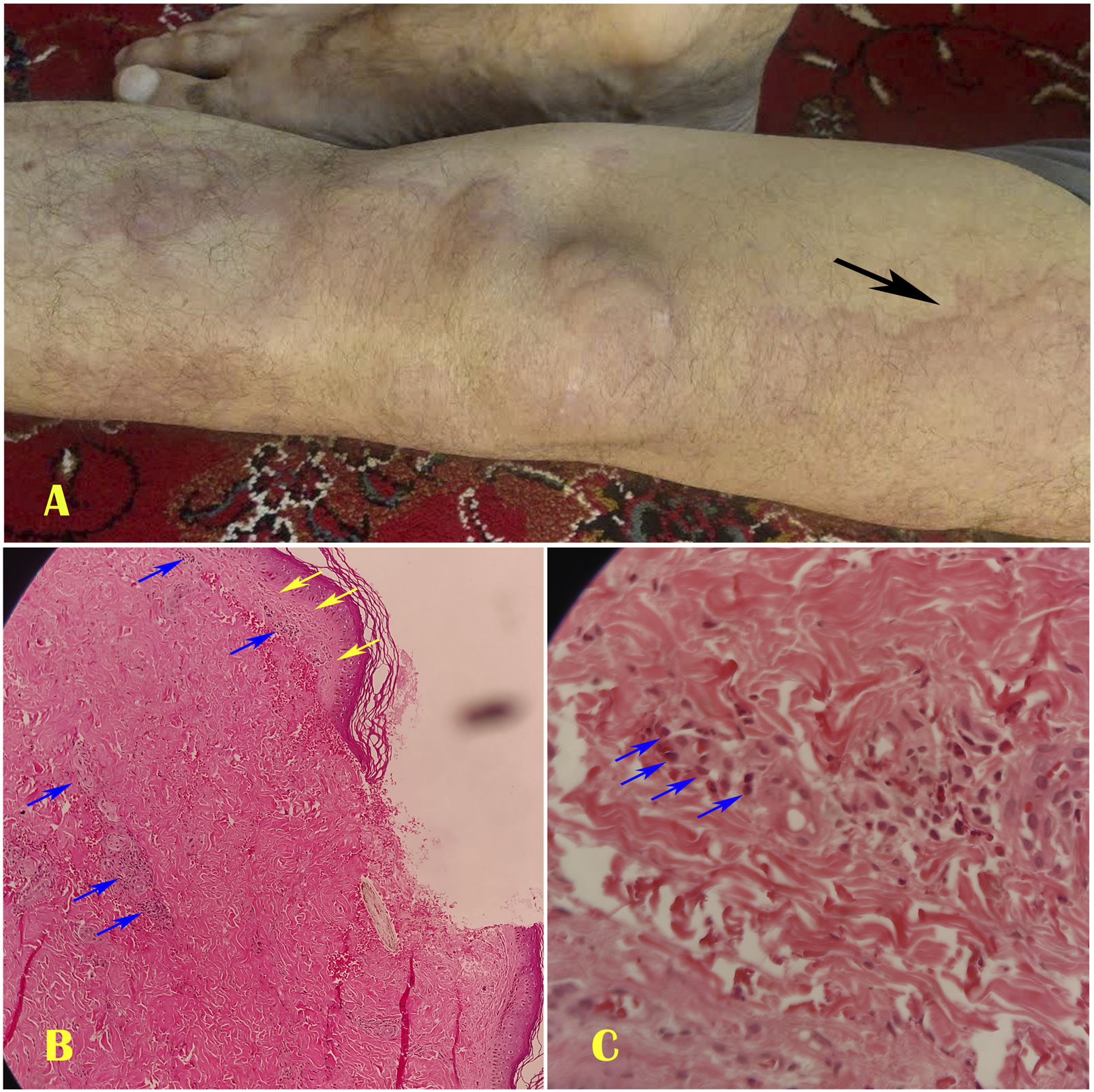
The first case of Schnitzler syndrome showed some giant pruritic urticarias on his left lower extremity (A). The histopathology of the dermal lesion revealed minimal interstitial edema (Yellow arrows) and a dense neutrophilic infiltrate (Blue arrows) surrounding tiny blood vessels at ×40 magnification (B) and ×100 magnification (C).
After local anesthesia, our dermatologist performed a punch biopsy of the right thigh lesion. The histopathological study of the dermal lesion revealed mild interstitial edema, with neutrophils clustered near the vasculature and within the interstitial space, without vasculitis (Figures 1B,C). We diagnosed SchS based on the Strasbourg criteria. Our dermatologist prescribed anakinra. However, due to limited access to the drug, we had to substitute it with prednisolone 0.5 mg/kg daily, colchicine 3 mg/d, and loratadine for 7 days to relieve his symptoms. We then tapered the prednisolone to mitigate its side effects. After 5 days, he was discharged on prednisolone 10 mg/day, colchicine 1 mg/day, methotrexate 7.5 mg/week, montelukast 10 mg daily, loratadine 5 mg daily, and calcium and vitamin D supplements. The patient adhered to the treatment regimen without experiencing a clinical relapse during the first 12 months of follow-up. Although bone pain persisted, it was less severe. One month after discharge, inflammation control tests (ESR and CRP) decreased to normal levels.
Case 2
A 50-year-old male patient with a history of major depressive disorder, CIU, arthralgia, and peptic ulcer disease spanning 5 years, presented with extensive, pruritic urticaria affecting the abdomen, groin, and back, accompanied by angioedema lasting for 2 days as of 19 March 2024. Additionally, he reported hypertension, non-alcoholic fatty liver disease, polyarthralgia, and generalized bone pain, predominantly in his knees and vertebral column, as well as peptic ulcer disease. He also experienced a respiratory viral infection 2 weeks prior. His mother had asthma and chronic sinusitis. The patient had been treated with long-term corticosteroids, antihistamines, and one course of Xolair over a three-month period in 2022; however, the urticaria recurred 4 months later. At presentation, he exhibited severe pruritic urticaria, arthralgia, mild shortness of breath, and neck pain. His vital signs included a temperature of 38.1 °C, a respiratory rate of 18 breaths per minute, and an oxygen saturation level of 93% on room air. Physical examination showed mild angioedema, large pale-rose urticaria, and palpable plaques with a diameter of 1–7 cm on his abdomen, groin, and back (Figures 2A–C), mild wheezing in both lungs on auscultation, and cervical spine tenderness (Figure 3). He also had a depressed mood. Initial laboratory results indicated a WBC count of 9,800 cells per milliliter (PMN = 72%, Lymph = 20%, Eos = 8%), a Hb level of 12.9 mg/dL, a Platelet count of 381 (× 109/L), ALT = 68, AST = 44 IU/mL, a CRP level of 18 mg/dL, and a first-hour ESR of 48 mm/h. Other laboratory parameters, including FBS = 115 mg/dL, TG = 186 mg/dL, and Cholesterol = 235 mg/dL. The serum electrolytes, BUN, Creatinine, thyroid function tests, HIV, HBV, HCV antibody, and stool examination for occult blood or parasitic infections were all normal. Furthermore, chest computed tomography revealed mild paratracheal opacity, and abdominal ultrasound showed no signs of lymphadenopathy or hepatosplenomegaly, except for a grade II fatty liver. Influenza and SARS-CoV-2 PCR tests were negative. The rheumatologic screening tests, including ANA, anti-dsDNA, RF, serum, and urine electrophoresis, revealed no notable findings. The serum immunotyping capillary electrophoresis test indicated monoclonal gammopathy of IgM (IgM = 648 mg/dL).
FIGURE 2
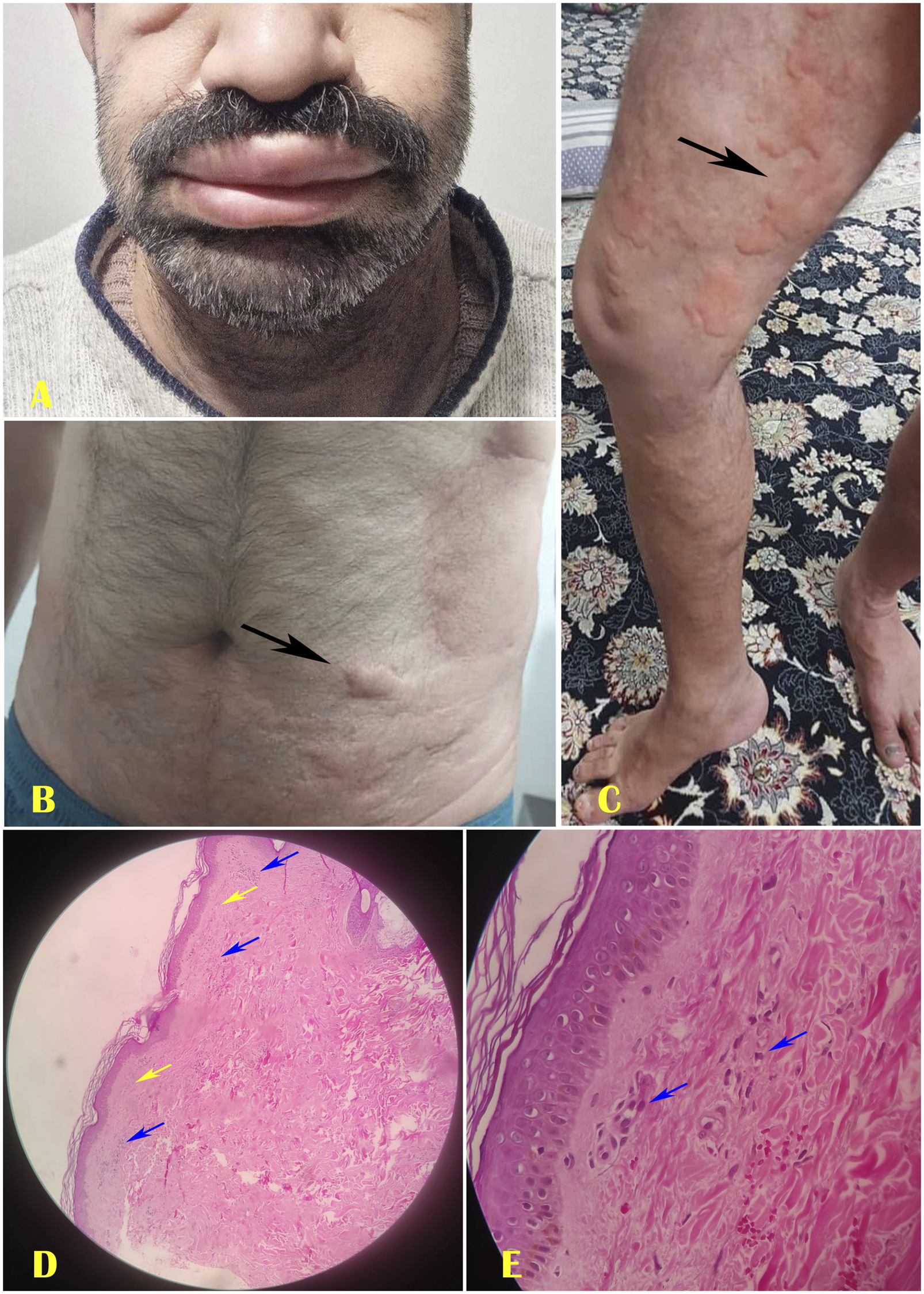
The second case of Schnitzler syndrome showed angioedema (A), Giant urticaria on the belly (B), and groin (C). The histopathology of the dermal lesion revealed superficial papillary edema (Yellow arrows) and a dense neutrophilic infiltrate (Blue arrows) surrounding tiny blood vessels at ×40 magnification (D) and ×100 magnification (E).
FIGURE 3
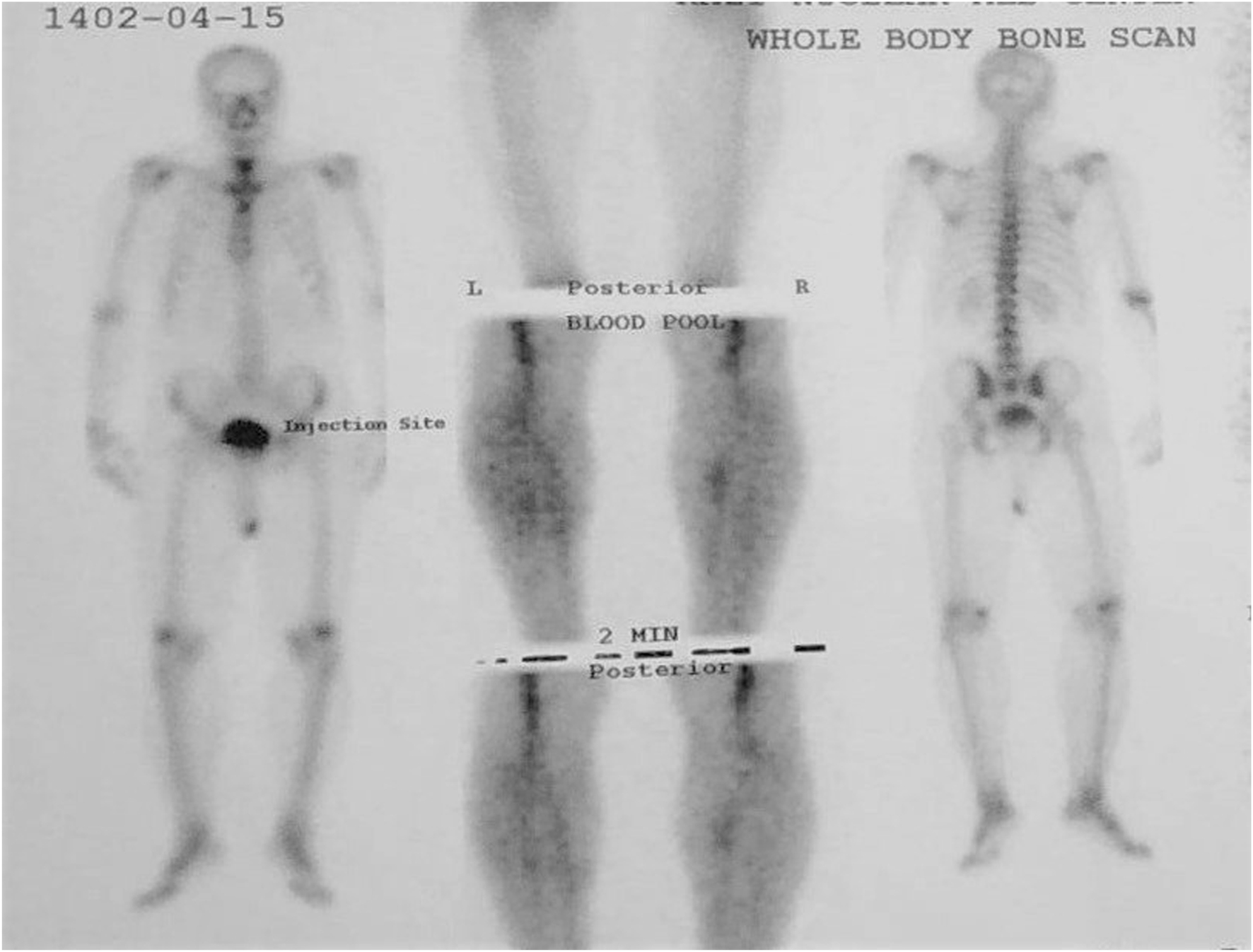
Whole body bone scan in patient 2 revealed degenerative changes of the spine and knees with mild lumbar scoliosis.
Our dermatologist performed a punch biopsy from the thigh lesion with local anesthesia. The histopathology examination revealed neutrophilic infiltrates and mild dermal papillary edema, with no clear vasculitis (Figures 2D,E). Based on the patient’s clinical presentation, laboratory findings, and histological characteristics of the dermal lesion, a diagnosis of SchS was established. Initial treatment consisted of intravenous antibiotics, prednisolone at a dosage of 0.5 mg/kg, tapered over 5 days, bronchodilators, colchicine at 3 mg daily, antihistamines, and consultations with both a rheumatologist and a hematologist. Additionally, our dermatologist recommended the use of anakinra; however, the patient was unable to procure this medication at the time of this report. On the fifth day, the patient was discharged with a prescribed medication regimen, as accepted by him, including oral prednisolone 10 mg daily, colchicine 1 mg daily, montelukast 10 mg daily, loratadine 5 mg daily, duloxetine 60 mg daily, as well as vitamin E, metformin 500 mg daily, calcium, and vitamin D supplements. The patient’s inflammatory biomarkers, including ESR and CRP, normalized 3 weeks after discharge, and the prednisolone dose was reduced to 5 mg/day, which was continued at this dose. During a six-month follow-up period post-discharge, the patient reported no recurrence.
Discussion
Schnitzler syndrome (SchS) is a rare, chronic autoinflammatory disorder, typically diagnosed utilizing the Strausburg criteria, which comprise obligate criteria such as a chronic urticarial rash and a monoclonal gammopathy of either IgM or IgG type, in addition to at least two minor criteria—recurrent fever, bone pain or abnormal bone remodeling, elevated C-reactive protein (CRP) or leukocytosis, and a neutrophilic infiltrate observed in a skin biopsy [1]. The rarity of the syndrome, its heterogeneous presentation, and overlap with other autoimmune, infectious, and hematologic conditions often lead to delayed or missed diagnoses [2]. The present cases highlight both classic features and diagnostic challenges unique to resource-limited settings. We report the first cases of SchS from Iran, based on our literature review. While SchS is most often characterized by neutrophilic urticarial dermatosis (a perivascular neutrophilic infiltrate without fibrinoid necrosis or significant dermal edema), true vasculitis is rare but has been reported in atypical presentations [6]. Distinguishing SchS lesions from urticarial vasculitis and other dermatoses is crucial for accurate diagnosis. Immunofluorescence may occasionally reveal vascular IgM deposition in SchS. The cornerstone of SchS management is interleukin-1 (IL-1) blockade, most successfully with anakinra, which often induces rapid and sustained symptom remission. However, access to such targeted therapies is limited in many settings due to cost or drug availability, as our patients have encountered. Other treatments, including corticosteroids, colchicine, methotrexate, and additional biologic agents, may provide partial relief of symptoms; however, they are typically less effective and are associated with potential long-term adverse effects [7, 8]. We believe that, in the absence of access to anakinra, a combination therapy involving a low-dose corticosteroid and an immunomodulatory agent, as is often the case in many rheumatological diseases, a mast cell degranulation stabilizer, such as ketotifen or loratadine, a leukotriene receptor antagonist, such as montelukast, and management of comorbidities such as underlying infections and mood disorders in patients, may be associated with satisfactory outcomes, as evidenced in both of our patients.
Practitioners often overlook infectious diseases in the differential diagnosis of SchS, especially for certain microorganisms with atypical presentations. Acute urticaria, bone pain, arthralgia, and fever are prevalent symptoms in viral infections such as acute hepatitis B and C, parvovirus B19, EBV, and, recently, SARS-CoV-2; bacterial infections such as Streptococcus spp., Mycoplasma pneumoniae, Yersinia spp., and H. pylori; and parasitic infections such as Blastocystis hominis, Giardia lamblia, and Strongyloides stercoralis [2, 9]. So, we evaluated both of our patients for these probable infectious diseases.
Despite the introduction of omalizumab for the treatment of CIU for a decade, the lack of striking efficacy in SchS, such as our patients, made this drug unacceptable over an extended period [4]. As a result, there has been a tendency to prescribe novel biological therapies, such as anakinra and canakinumab [10, 11]. The primary concerns regarding the prescription of these medications in resource-limited areas are their limited availability and high expenses. Furthermore, treating underlying conditions such as infectious diseases, dyspepsia, environmental allergens, and mood disorders may be effective in prolonging the symptom-free period.
SchS is a lifelong illness with a risk of progression to lymphoproliferative disorders in approximately 15% of patients, particularly Waldenström macroglobulinemia and IgM myeloma [12]. Regular monitoring is essential for early detection of hematological transformation. Although fatal outcomes are uncommon, the disease significantly impacts quality of life if not promptly recognized and treated with effective agents. Early recognition of SchS in patients with chronic urticaria unresponsive to standard therapies is critical, especially when accompanied by systemic inflammatory features and monoclonal gammopathy. A multidisciplinary approach and periodic surveillance for lymphoproliferative evolution are recommended [12]. When access to IL-1 inhibitors is limited, clinicians should optimize available therapies and advocate for improved access to targeted treatment.
Conclusion
We report the initial cases of SchS in Iran. Clinicians should consider this disease when diagnosing patients with chronic, recurrent urticaria and should perform histopathology to confirm the diagnosis. Additionally, we suggest conducting meta-analyses to assess the effectiveness of current treatments in conjunction with the management of psychosocial factors, in comparison to new biological therapies, particularly in resource-limited settings. This strategy can guide decision-making to improve patient health while considering economic limitations.
Limitations
Our main limitation was the unavailability of immunohistochemical staining for the patients’ dermal lesions and the lack of access to interleukin-1 inhibitor drugs, which are considered the most effective treatment options for Schnitzler syndrome in our patients.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Aja University of Medical Sciences. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
MA participated in the diagnosis and treatment of patients, as well as reviewed the literature, and wrote the primary and final drafts. SS-M participated in the diagnosis and treatment of patients, reviewing the literature and contributing to the primary and final drafts. SM participated in the review of the literature, data collection, image preparation, and writing the primary draft. MA participated in the review of the literature, data collection, and writing the primary draft. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Abbreviations
SchS, Schnitzler syndrome; CIU, Chronic idiopathic urticaria; WBC, White blood cell; PMN, Poly morpho nuclear; ESR: Erythrocyte sedimentation rate; CRP: C-Reactive protein; HIV, Human Immunodeficiency Virus.
References
1.
SokumbiODrageLAPetersMS. Clinical and histopathologic review of Schnitzler syndrome: the Mayo Clinic experience (1972-2011). J Am Acad Dermatol (2012) 67(6):1289–95. 10.1016/j.jaad.2012.04.027
2.
de KoningHD. Schnitzler's syndrome: lessons from 281 cases. Clin Transl Allergy (2014) 4:41. 10.1186/2045-7022-4-41
3.
De BartolomeisFSavoiaAAitellaESacerdotiCParlatoAPalmieriCet alUrticaria by neurogenic switching of gastroesophageal chemical-infective inflammation: a phenomenon that should always be evaluated in suspected multiple drug hypersensitivity. Clin Translational Allergy (2014) 4(3):P26. 10.1186/2045-7022-4-s3-p26
4.
PaulinoMCostaC. The challenge of omalizumab-refractory chronic spontaneous urticaria and the relevance of suspecting Schnitzler syndrome without monoclonal gammopathy. Revista Portuguesa de Imunoalergologia. (2023) 31(3):227–32. 10.32932/rpia.2023.08.119
5.
syndromeS. Prompt response to treatment with anakinra. J Am Acad Dermatol (2015) 72(5):AB151. 10.1016/j.jaad.2015.02.620
6.
TannebergerOBüchnerSZimmerliLU. Schnitzler's syndrome with urticaria vasculitis. Internist (Berl) (2007) 48(12):1432–5. 10.1007/s00108-007-1956-0
7.
SchusterCKränkeBAbererEArbabESturmGAbererW. Schnitzler syndrome: response to anakinra in two cases and a review of the literature. Int J Dermatol (2009) 48(11):1190–4. 10.1111/j.1365-4632.2009.04151.x
8.
syndromeS. A diagnostic and therapeutic challenge. J Am Acad Dermatol (2013) 68(4):AB141. 10.1016/j.jaad.2012.12.584
9.
WediBRaapUWieczorekDKappA. Urticaria and infections. Allergy Asthma Clin Immunol (2009) 5(1):10. 10.1186/1710-1492-5-10
10.
EilingEMöllerMKreiselmaierIBraschJSchwarzT. Schnitzler syndrome: treatment failure to rituximab but response to anakinra. J Am Acad Dermatol (2007) 57(2):361–4. 10.1016/j.jaad.2007.03.036
11.
GorodetskiyVRSaluginaSOFedorovES. Increasing the interval of canakinumab administration effectively supports the remission of Schnitzler's syndrome. Case Rep Rheumatol (2018) 2018:5416907. 10.1155/2018/5416907
12.
SinghGGoswamiKTrehanSKachhadiaMPFarooqAPuriPet alSchnitzler-like syndrome presenting with IgG Kappa monoclonal gammopathy: a case report and review of diagnostic and therapeutic challenges. Cureus (2024) 16(7):e64440. 10.7759/cureus.64440
Summary
Keywords
case report, Schnitzler syndrome, therapeutic challenges, chronic urticaria with gammopathy, anakinra
Citation
Aminianfar M, Soleiman-Meigooni S, Mohammadi S and Aminianfar M (2025) Case Report: First reports of Schnitzler syndrome in Iran: clinical presentation and therapeutic challenges. J. Cutan. Immunol. Allergy 8:15322. doi: 10.3389/jcia.2025.15322
Received
24 July 2025
Accepted
03 September 2025
Published
11 September 2025
Volume
8 - 2025
Updates
Copyright
© 2025 Aminianfar, Soleiman-Meigooni, Mohammadi and Aminianfar.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Saeed Soleiman-Meigooni, dr.saeed.meigooni@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.