Abstract
We present a rare case of a 70-year-old male who developed multiple tense bullae with minimal inflammation after receiving CD19-directed chimeric antigen receptor (CAR)-T-cell therapy for diffuse large B-cell lymphoma. Although strong drug-induced lymphocyte stimulation test positivity was observed initially for multiple drugs, the reactions faded over time. Histopathology revealed subepidermal blistering with minimal inflammatory infiltration, including a mild perivascular infiltration of T lymphocytes, predominantly CD4+ cells rather than CD8+ cells. All of the previously-reported cases with CAR-T-related cutaneous eruptions involve marked inflammation. In contrast, this case may represent a unique blister formation with T-cell hyperreactivity associated with post-CAR-T immune reconstitution. A comparative analysis of previously published cases is provided.
Introduction
Chimeric antigen receptor (CAR)-T-cell therapy, particularly CD19-targeted agents such as tisagenlecleucel, has revolutionized and is increasingly used for the treatment of refractory B-cell malignancies [1, 2]. However, it carries a common risk of immune-mediated adverse events called cytokine release syndrome (CRS), presenting with a variety of symptoms ranging from mild, flu-like symptoms to severe life-threatening manifestations of the overshooting inflammatory response, which onset within a few days up to several weeks after infusion [3]. Besides rash and edema as cutaneous symptoms of CRS, patients undergoing CAR-T-cell therapy develop skin lesions such as secondary cutaneous malignancies, disseminated infection, and CAR-T-cell therapy-related ‘eruptions of lymphocyte recovery’ [4]. In addition, erythematous, purpuric and vesiculobullous lesions with substantial inflammation have been reported as cutaneous adverse events (AEs) of CAR-T-cell therapy [5–10].
Here we report an uncommon case of subepidermal bullous eruption with minimal inflammation, transient drug hypersensitivity and a favorable clinical course, potentially reflecting T-cell dysregulation due to post-CAR-T immune remodeling. We also compare our case with prior reports.
Case report
A 70-year-old male with diffuse large B-cell lymphoma was administered with tisagenlecleucel, a CD19-directed CAR-T therapy. He was prophylactically administered with sulfamethoxazole-trimethoprim, acyclovir, fluconazole, esomeprazole magnesium hydrate, and magnesium oxide. On 2 months post-treatment, he was consulted to our department for multiple tense bullae on the extremities, including a 4 × 5 cm blister on the right ankle, and facial erythema (Figures 1a,b). He claimed no subjective symptoms such as pruritus associated with the skin eruptions. No history of bullous skin was noted. The patient developed a high fever up to 39 °C from day 2 after infusion, which was considered grade 1 CRS. Tocilizumab was administered on days 3 and 4, and the fever resolved gradually, without any neurological complications. The CRP peaked at 17.43 mg/dL on day 4 and normalized by day 13. The bullous eruptions appeared on day 58, approximately 1 month after the resolution of obvious CRS. Blood tests revealed eosinophilia (11.5%), elevated LDH (271 U/L), and low IgG (294 mg/dL). CRP was not elevated and CD19+ B cells were undetectable. Anti-BP180 antibody was negative. Skin biopsy showed subepidermal blistering with red blood cell extravasation and minimal inflammatory cell infiltration (Figure 2). No deposition of IgG, IgA, or C3 was detected by direct immunofluorescence. Immunohistochemistry showed mild perivascular infiltration of T lymphocytes, predominantly CD4+ cells rather than CD8+ cells, with no CD19+ B cells. Small number of CD8+ T cells were also detected in the epidermis close to the blister.
FIGURE 1
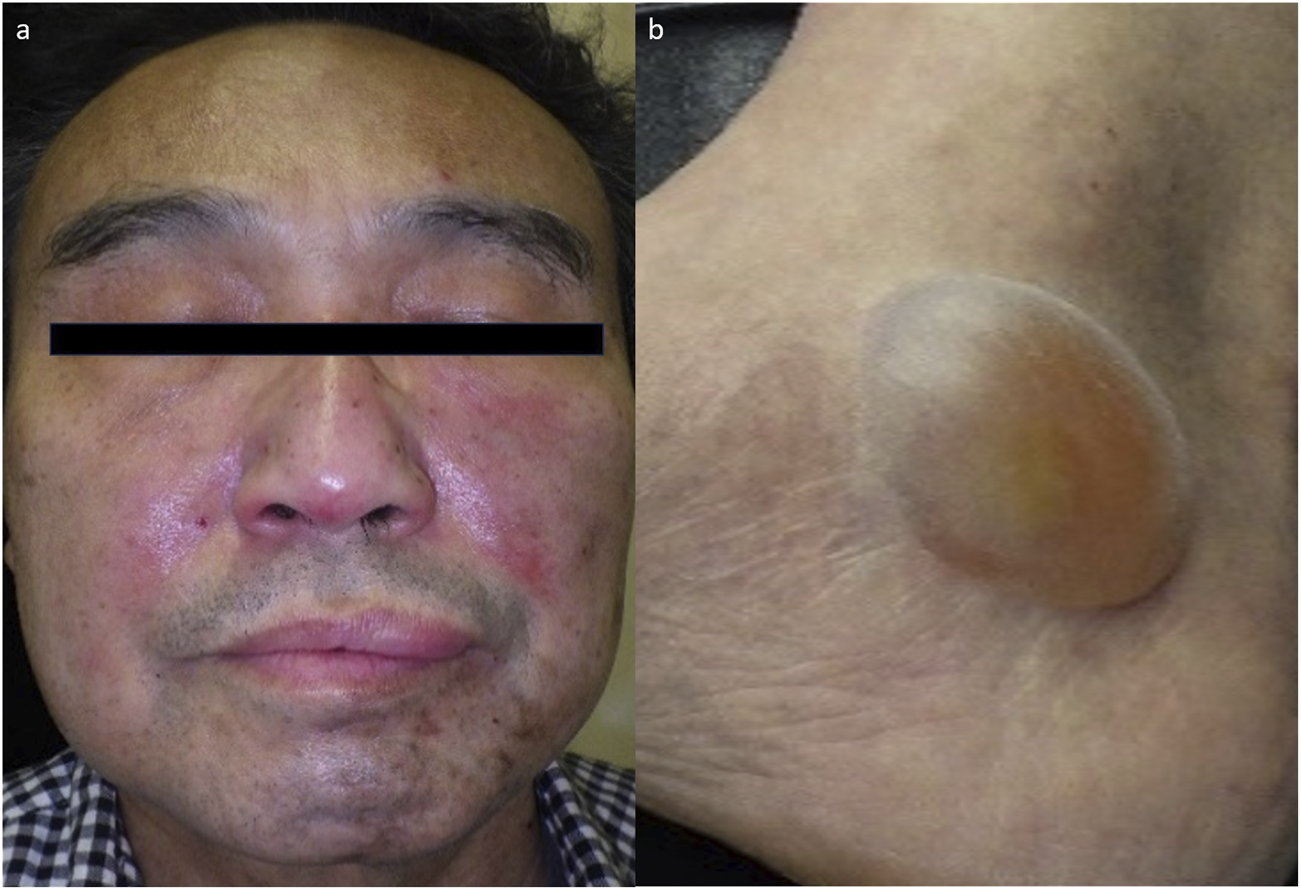
Clinical manifestations of the patient at the first visit (a) Erythematous skin rash on the face. (b) 4 cm × 5 cm large blister on the right ankle.
FIGURE 2
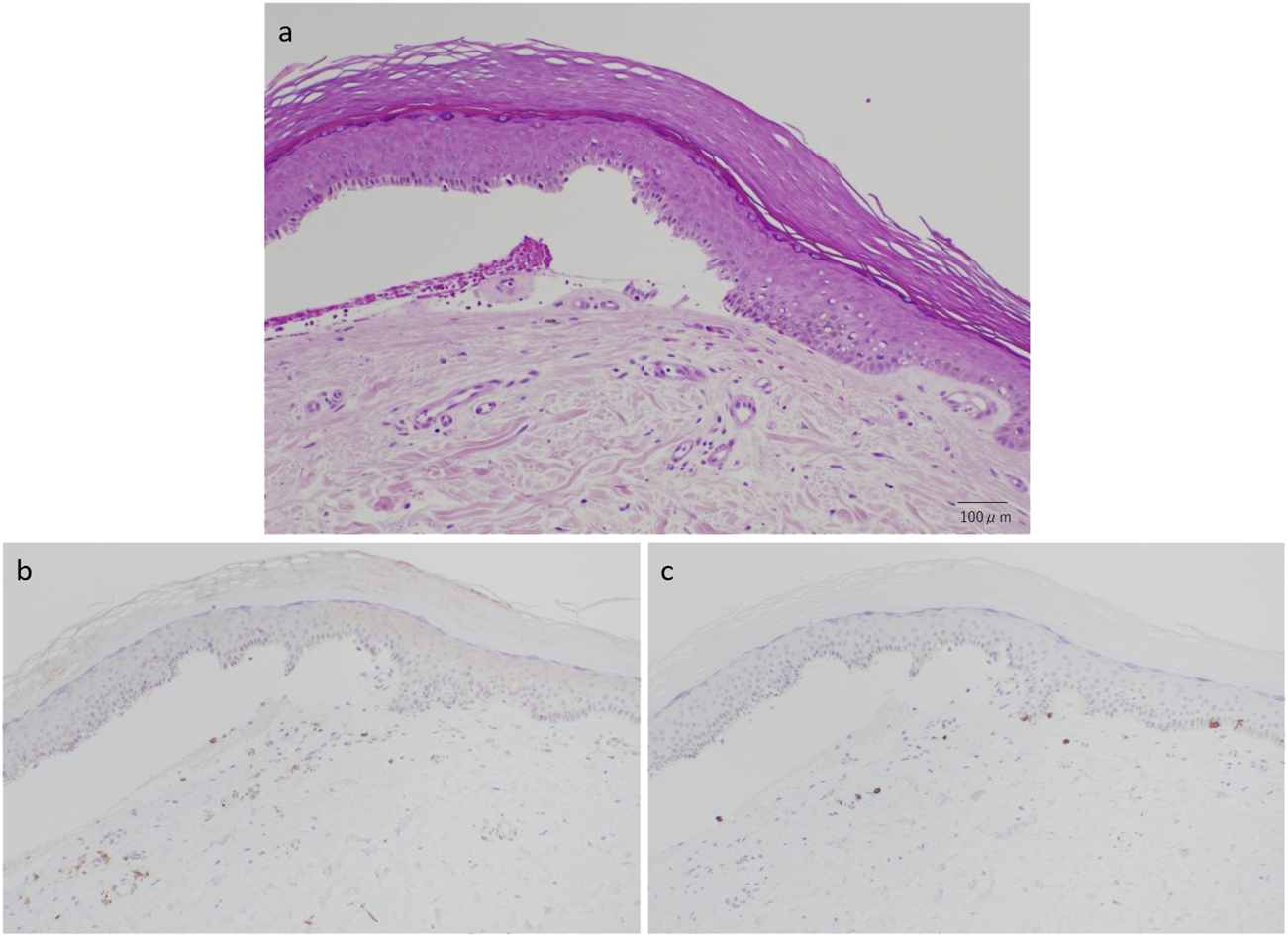
(a) Subepidermal blister formation with red blood cell extravasation and minimal inflammatory cell infiltration (hematoxylin and eosin stain, original magnification: ×200). (b) Immunohistochemical finding of predominant CD4+ cell infiltration (original magnification: ×100). (c) Immunohistochemical finding of CD8+ cell infiltration (original magnification: ×100).
Drug-induced lymphocyte stimulation tests (DLSTs), also referred to internationally as the lymphocyte transformation tests (LTTs), were strongly positive for all five drugs (sulfamethoxazole-trimethoprim, acyclovir, fluconazole, esomeprazole magnesium hydrate, and magnesium oxide) initially, but gradually diminished over time after discontinuation of all drugs (Table 1). While skin symptoms relapsed at 4 months after the first visit, anti-BP180 antibody again showed negative and DLST revealed positive for only sulfamethoxazole-trimethoprim and magnesium oxide. Closed patch testing was negative for sulfamethoxazole-trimethoprim on both post-lesional and non-lesional skin. Simple coverage of bullae with adhesive plasters and occasional topical betamethasone led to resolution without scarring or milia formation. No systemic immunosuppressants were administered and the facial erythema resolved without topical intervention. At 15-month follow-up, DLSTs turned completely negative and the patient remained in remission without significant recurrence. Drug use was initially restricted strictly but relieved after improvement.
TABLE 1
| 1 month after the onset of skin eruptions | 5 months after the onset of skin eruptions | 15 months after the onset of skin eruptions | ||
|---|---|---|---|---|
| Positivity by DLST | Sulfamethoxazole-trimethoprim | ++ (626, 489%) | + (171, 234%) | - (95, 163%) |
| Aciclovir | ++ (618, 482%) | - (108, 147%) | n.d. | |
| Fluconazole | ++ (763, 596%) | - (124, 169%) | n.d. | |
| Esomeprazome magnesium hydrate | ++ (450, 351%) | - (127, 173%) | n.d. | |
| Magnesium oxide | ++ (1007, 786%) | + (150, 205%) | n.d. | |
| Unstimulated control | (128, 100%) | (73, 100%) | (58, 100%) | |
| Ratio in blood lymphocytes | CD19+ B cells | 0% | 0% | 0% |
| CD8+ T cells | 64.1% | 44.1% | 29.5% | |
Summary of the results of DLSTs and lymphocyte ratio during the disease course.
Results of DLSTs are shown by 3H-thymidine incorporation (cpm) and its ratio to unstimulated control (Stimulation Index: SI, %). SI levels more than 180 and 300 are shown as + and ++, respectively, with bold letters.
Discussion
We have reported a case of subepidermal bullous eruption with minimal inflammation, who also showed transient multiple drug hypersensitivity and a favorable clinical course. Although a sulfamethoxazole-trimethoprim-induced non-scarring bullous drug eruption had been reported [11], multiple positivity on DLST and negative closed patch test reduce the possibility of the allergic drug eruption. Along with the bullous drug eruption, autoimmune bullous disease and the side effect of CAR-T-cell therapy should be considered for differential diagnosis. As autoimmune bullous disease can be denied by negative direct immunofluorescence, we consider that this case represents the side effect of CAR-T-cell therapy. However, current knowledge on the pathogenesis of dermatologic bullous change associated with CAR-T-cell therapy is limited. Our case differs from so far reported cutaneous AEs of CAR-T-cell therapy developing bullous eruptions, which all exhibit intense inflammation and/or necrosis (Table 2). Although CRS commonly occurs after CAR-T-cell therapy and can present with cutaneous manifestations, in this case, initial CRS was grade 1 and resolved within 2 weeks after infusion. As the bullous eruptions developed about 1 month later, late-onset CRS, rather than direct contribution of the initial CRS, may be involved. However, it has not been clarified by a measurement of cytokine levels. The patient initially achieved disease control, but residual FDG uptake on PET imaging suggested possible early relapse, indicating that mild CRS in this case might predict temporal remission. This may be consistent with previous studies, suggesting that the severity of CRS may correlate with the efficacy of CAR-T-cell therapy, as robust immune activation could reflect higher CAR-T expansion and anti-tumor activity [2, 3]. Further studies are needed to clarify these associations. Although the overall inflammatory infiltrate was minimal, immunohistochemistry revealed a mild predominance of CD4+ over CD8+ T cells. This suggests that limited T-cell activation might have contributed to the pathogenesis, despite the lack of massive inflammation. Given the delayed timing of eruption after CAR-T infusion, we hypothesize that localized T-cell reactivity, possibly associated with immune reconstitution, may have played a role. This may explain the initial DLST hyperresponsiveness, which normalized over time, in accordance with the change of CD8+ T cell ratio in blood lymphocytes (Table 1). In this context, our case is considered to be another example of CAR-T-cell therapy-related ‘eruptions of lymphocyte recovery’ [4].
TABLE 2
| Case | Age/Sex | Disease onset after CAR-T cell therapy | Location | Cutaneous findings | Histopathology | Treatment and disease course | Reference |
|---|---|---|---|---|---|---|---|
| 1 | 76/M | 7 days | Trunk, head, neck, upper and lower extremities | Nonblanching purpuric erythematous rash, flaccid bullae, ulcer | Epidermal pallor, minimal lymphohistiocytic inflammation and red-cell extravasation, necrosis and granulation | Remained with granulation | [5] |
| 2 | 68/M | 10 months | Neck, arms | Tense fluid-filled bullae with underlying ecchymosis | Marked spongiosis with a prominent eosinophilic infiltrate in the underlying dermis as well as along the dermoepidermal junction | Resolved with prednisone, doxycycline, nicotinamide, and topical clobetasol within 6 weeks | [6] |
| 3 | 69/M | 5 days | Head, chest, back, bilateral upper and lower extremities | Diffuse maculopapular rash followed by tense bullae on upper extremities with fingertip cyanosis | Papillary edema, subepidermal bullae, perivascular lymphocytic infiltrates in the dermis | Resolved with tocilizumab at day 9 and 13 and methylprednisolone on day 11 | [7] |
| 4 | 63/M | 8 days | Head, bilateral extremities | Tense bullae, mucosal erosion, pruriginous and painful maculopapular rash | Subepidermal bullae with necrosis of the epidermis and perivascular moderately-intense predominantly-lymphocytic infiltrate in the dermis | Resolved with methylprednisolone and intravenous immunoglobulin in 1 month | [8] |
| 5 | 20/F | 6 days | Malar region, upper and lower limbs | Erythematous itchy rash, erythematous bullous lesions with brownish hyperpigment and macules | Not performed | Systemic steroids and intravenous immunoglobulin, complete remission at 2 months | [9] |
| 6 | 69/M | 5 days | Back, bilateral upper and lower extremities | Erythematous macular eruption progressing to vesiculation with bullae | Vacuolar interface alteration with full-thickness epidermal necrosis and subepidermal clefting | Significant improvement with systemic steroids | [9] |
| Our case | 70/M | 1 month | Lower and upper extremities, face | Erythematous rash on the face and multiple tense bullae on the extremities | Subepdermal bullae with minimal inflammatory cell infiltration | Resolution without scarring or milia formation by simple coverage of bullae with adhesive plasters and occasional topical betamethasone, relapse after 4 months and then no recurrence until 15 months after the first cutaneous manifestation |
Comparison of published cases with bullous eruptions after CAR-T-cell therapy.
While trauma and diabetic bullae were clinically excluded, the localized nature of bullae, including a solitary large blister over the medial malleolus, suggests participation of any site-specific factors. Although fixed drug eruptions (FDEs), especially non-pigmented bullous FDEs, were suspected in this case, patch testing was negative for the most suspected drug, sulfamethoxazole-trimethoprim, even on the lesional skin. Regarding sulfamethoxazole-trimethoprim, a patient was reported who rapidly developed nonscarring sublamina densa blisters within hours after the intravenous administration [11]. In our case, histological analysis revealed that some basal cells still attached to the dermis after the blister formation, suggesting that the blister is not formed sublamina densa (Figure 1b). Although we did not perform a formal drug provocation test, suspected drugs were re-administered after symptom improvement, but importantly, no recurrence of the skin lesions was observed following re-exposure. This clinical course indirectly supports the notion that the skin lesions were unlikely to be caused by the suspected drugs.
This report contributes to the understanding of atypical cutaneous AEs of CAR-T-cell therapy. Since not all bullous eruptions post-CAR-T represent drug allergies or autoimmune disease, a careful work-up including histopathology, immunostaining, and DLST interpretation in temporal context is crucial. This case highlights a rare presentation of post-CAR-T subepidermal blistering with minimal inflammation and transient DLST positivity. Lack of early immunophenotyping, cytokine measurements and inability to perform drug rechallenges are listed as limitations. We advocate for heightened clinical awareness of immune reconstitution–related cutaneous reactions.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This study was approved by the Ethics Committee of Hyogo Medical University. Written informed consent was obtained from the patient for the publication of this case report.
Author contributions
NW drafted the manuscript and managed data collection and clinical documentation. NK supervised the study, contributed to data interpretation, and revised the manuscript critically for intellectual content. SY provided clinical oversight and contributed to the hematologic aspects of the case. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
References
1.
BlümPKayserS. Chimeric antigen receptor (CAR) T-cell therapy in hematologic malignancies: clinical implications and limitations. Cancers (Basel) (2024) 16:1599. 10.3390/cancers16081599
2.
BrudnoJNKochenderferJN. Recent advances in CAR T-cell toxicity: mechanisms, manifestations and management. Blood Rev (2019) 34:45–55. 10.1016/j.blre.2018.11.002
3.
Shimabukuro-VornhagenAGödelPSubkleweMStemmlerHJSchlößerHASchlaakMet alCytokine release syndrome. J Immunother Cancer (2018) 6:56. 10.1186/s40425-018-0343-9
4.
RubinCBElenitsasRTaylorLLaceySFKulikovskayaIGuptaMet alEvaluating the skin in patients undergoing chimeric antigen receptor modified T-cell therapy. J Am Acad Dermatol (2016) 75:1054–7. 10.1016/j.jaad.2016.06.062
5.
KhodaFGhadiriSPalmerTWarburtonK. A constellation of cutaneous reactions to chimeric antigen receptor T-cell therapy. Clin Exp Dermatol (2024) 50:152–3. 10.1093/ced/llae311
6.
NusbaumKBDulmageBChoiJNJaglowskiSMKormanAM. Cutaneous manifestations of chimeric antigen receptor T-cell therapy: an introduction for dermatologists. J Am Acad Dermatol (2022) 87:597–604. 10.1016/j.jaad.2021.07.017
7.
HuYZhengWQiaoJLuoYShiJYuJet alBullous and exanthematous lesions associated with chimeric antigen receptor T-cell therapy in a patient with diffuse large B-cell lymphoma. JAMA Dermatol (2025) 156(9):1026–8. 10.1001/jamadermatol.2020.0636
8.
FiorilloGTosoFCorteseAPiscazziFBressanABramantiSet alCutaneous toxicity of CAR T-cell therapy: case report of a bullous life-threatening reaction. J Eur Acad Dermatol Venereol (2023) 37:e729–e731. 10.1111/jdv.18892
9.
MasucciCPepeSLa RoccaUZullinoVDe ProprisMSBarberiWet alCase report: severe cutaneous adverse event associated with checkpoint inhibition in the setting of CAR T-cell therapy: beyond CRS. Case Report: Severe Cutaneous Adverse Event Associated Checkpoint Inhibition Setting CAR T-cell Therapy: Beyond CRS Front Oncol (2023) 13:1171031. 10.3389/fonc.2023.1171031
10.
StorgardRDuszaSShouvalRScordoMMarkovaA. Dermatologic adverse events associated with chimeric antigen receptor T-cell therapy: a pharmacovigilance analysis of the FDA reporting system. Transpl Cell Ther (2024) 30:1035.e1–1035.e7. 10.1016/j.jtct.2024.06.024
11.
RoholtNSLapiereJCTraczykTSchanLWoodleyPT. Non scarring sublamina densa bullous drug eruption. J Am Acad Dermatol (1995) 32:367–71. 10.1016/0190-9622(95)90406-9
Summary
Keywords
CAR-T-cell therapy, CRS, bullous eruption, DLST, drug hypersensitivity
Citation
Watanabe N, Kanazawa N and Yoshihara S (2025) Case Report: Blister formation with transient drug hypersensitivity following CAR-T-cell therapy. J. Cutan. Immunol. Allergy 8:15280. doi: 10.3389/jcia.2025.15280
Received
16 July 2025
Accepted
26 September 2025
Published
08 October 2025
Volume
8 - 2025
Updates
Copyright
© 2025 Watanabe, Kanazawa and Yoshihara.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nobuo Kanazawa, nkanazaw@hyo-med.ac.jp
ORCID: Natsu Watanabe, orcid.org/0009-0003-3065-2659; Nobuo Kanazawa, orcid.org/0000-0003-3000-9711; Satoshi Yoshihara, orcid.org/0000-0002-8537-2422
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.