Abstract
Atopic dermatitis (AD) is a chronic inflammatory skin disease characterized by pruritus and impaired skin barrier function. Although difamilast, a topical phosphodiesterase-4 (PDE4) inhibitor, has demonstrated clinical efficacy in AD, its role in restoring barrier function remains incompletely understood. This study aimed to investigate the barrier-improving effects of topical difamilast in patients with AD and in an MC903-induced mouse model of AD-like dermatitis. We conducted a retrospective study involving seven Japanese adult patients with mild-to-moderate AD who received 1% difamilast ointment twice daily for 1 week. Transepidermal water loss (TEWL) was measured before and after treatment. Animal experiment was performed using MC903-induced AD-like dermatitis model, followed by treatment with difamilast or vehicle. Dermatitis scores, TEWL, histological changes, immunofluorescence for filaggrin and CD4, and mRNA expression of filaggrin, IL-4, IL-13 and TSLP were evaluated. Topical difamilast reduced TEWL in six out of seven AD patients. In the mouse model, difamilast markedly attenuated dermatitis severity and reduced TEWL. Histological analysis revealed suppression of epidermal thickening and inflammatory cell infiltration. Difamilast restored filaggrin expression and decreased mast cell and CD4+ T-cell infiltration in lesional skin. Quantitative PCR demonstrated that difamilast normalized the MC903-induced decrease in filaggrin and increase in IL-4 mRNA expression. Our findings suggest that difamilast improves skin barrier dysfunction in both human AD and an experimental AD-like mouse model. However, large-scale controlled studies are required to validate its barrier-improving efficacy in real-world clinical settings.
Introduction
Atopic dermatitis (AD) is a chronic eczematous skin disorder characterized by pruritus and impaired epidermal barrier function [1]. AD has a complex pathophysiology involving genetic predisposition, barrier dysfunction, and T cell–mediated type 2 inflammation. Among these factors, epidermal barrier dysfunction plays a central role in disease development. In AD patients, filaggrin expression is suppressed as a result of genetic polymorphisms and signaling to epidermal cells by interleukin (IL)-4 and IL-13 [2]. Recent advances in understanding the pathogenesis of AD have introduced new treatments into clinical practice.
Phosphodiesterases (PDEs) are enzymes that degrade cyclic nucleotides such as cAMP and cGMP, thereby regulating intracellular signaling pathways. By controlling the levels of these second messengers, PDEs play a crucial role in various physiological processes including inflammation [3]. Difamilast, a PDE4 inhibitor, is a topical agent used to treat AD across a wide age range, from children to adults. Clinical trials have revealed the efficacy and safety of difamilast [4]. Furthermore, in vitro studies have demonstrated that difamilast enhances filaggrin production in keratinocytes [5]. However, the effects of difamilast on improving barrier dysfunction in vivo have not yet been fully elucidated. To examine this point, we analyzed 7 Japanese adult patients with AD who were treated with difamilast. We also conducted studies using MC903-induced atopic dermatitis mouse model.
Methods
Human study
Patients with mild -to-moderate AD treated with difamilast from July 2023 to July 2024 at Gunma University Hospital, Japan, were included (Table 1). All patients fulfilled the criteria for AD according to Japanese guideline [1]. Seven Japanese patients (4 males, 3 females; mean age ±standard deviation, 29.9 ± 9.5 years) were completed the 1 week treatment without interruption at our hospital. All patients received topical application of 1% difamilast twice per day. Trans epidermal water loss (TEWL) was measured on the forearm before application and after 1 week using the DERMA-LAB TEWL probe (Cortex Technology). This retrospective cohort study was approved by the Institutional Review Board of Gunma University (HS2022-169) and was conducted according to the principles of the Declaration of Helsinki.
TABLE 1
| Case | Age | Sex | Severerity of AD | Past treatment | Measurement site | Before | After |
|---|---|---|---|---|---|---|---|
| #1 | 31 | M | Moderate | Moisturizer | Left forearm | 22.8 | 16 |
| #2 | 50 | M | Mild | TCS (very strong), moisturizer | Right forearm | 18.2 | 10.1 |
| #3 | 30 | M | Mild | Moisturizer | Left forearm | 16.9 | 7.7 |
| #4 | 26 | F | Moderate | TCS (very strong), moisturizer | Right forearm | 10.8 | 15.9 |
| #5 | 22 | M | Moderate | None | Right forearm | 20.8 | 13.1 |
| #6 | 22 | F | Mild | None | Right forearm | 22.6 | 12.3 |
| #7 | 28 | F | Moderate | Moisturizer | Left forearm | 20.6 | 12.7 |
Details of 7 patients with atopic dermatitis treated with difamilast.
AD, atopic dermatitis; M, Male; F, Female; TCS, topical corticosteroids; TEWL, transepidermal water loss.
Mouse
Animal experiments were approved by the Gunma University Animal Care and Experimentation Committee (24-062) and carried out in accordance with the approved guidelines. Eight-week-old female C57BL/6 mice were purchased from the SLC (Shizuoka, Japan). The mice were maintained in the Institute of Experimental Animal Research of Gunma University under specific pathogen-free conditions and were handled in accordance with the animal care guidelines of Gunma University.
Development of AD-like skin inflammation
AD-like skin inflammation was induced by topical application of commercially available calcipotriol (MC903; Tocris Bioscience, Bristol, UK), following previously described protocol [6]. The method for inducing AD-like skin inflammation using MC903 was adapted from our previous study [7]. Three days before initiating treatment, the dorsal fur of each mouse was shaved under anesthesia. Subsequently, 20 μL of MC903 (0.1 mM in ethanol) or an equivalent amount of ethanol (control) was applied topically once daily to a 1 cm2 area on the back for six consecutive days. The severity of dermatitis was evaluated based on a composite scoring system encompassing erythema, edema, dryness/scaling, and excoriation/erosion, with each parameter rated on a scale from 0 to 3, in accordance with previously described methods [7]. TEWL was measured on the back skin using the DERMA-LAB TEWL probe. To investigate the effect of difamilast on the development of AD-like skin inflammation in mice, 5 mg of difamilast or the same amount of vaseline as control were applied for 6 consecutive days.
Histological examination and immunostaining
Murine skins were removed, fixed with formalin, and embedded in paraffin. Paraffin-embedded murine skin sections (4 μm thick) were stained with hematoxylin and eosin (H&E) and toluidine blue. Immunofluorescence staining and subsequent analysis of paraffin-embedded tissue sections were conducted as described in previous study [8]. Rabbit anti-mouse filaggrin antibody (Ab) (905804; BioLegend) and rat anti-mouse CD4 Ab (#14-9766-82; Invitrogen) was used according to manufacturer’s protocol. Sections were stained with primary antibodies of interest, followed by Alexa Fluor 488–conjugated secondary antibodies. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI), and sections were mounted using ProLong Gold Antifade Reagent (Life Technologies). Fluorescence images were captured using an FV10i-DOC confocal laser-scanning microscope (Olympus). Positive cells stained with toluidine blue and CD4 in the dermis were counted in six randomly chosen microscopic fields using ImageJ software (version 1.52a).
Real-time reverse transcription polymerase chain reaction (RT-PCR)
To access the mRNA expression levels in the AD-like skin lesions, whole skin samples were corrected on day 6 following treatment. Total RNA was extracted using the RNeasy Mini Kits (Qiagen) and subsequently reverse-transcribed into cDNA with GoScript Reverse Transcription System, following the manufacturer’s instructions. Quantitative RT-PCR was carried out using the SYBR Green system (Applied Biosystems) on ABI 7300 real-time PCR platform (Life Technologies). Primers and SYBR probes were obtained from Sigma and TAKARA BIO INC. Expression of 18S rRNA was used as an internal reference, and relative gene expression levels were calculated using the comparative Ct method.
Statistics
P values were calculated using the Mann–Whitney U test for human data, and the unpaired Student’s t-test or one-way ANOVA followed by Tukey’s multiple comparisons test or Bonferroni’s post hoc test for mouse data, using GraphPad Prism software (Version 9). Error bars represent the standard error of the mean (SEM), and the number of experiments (n) is indicated in the figure legends.
Result
First, we measured TEWL to evaluate changes in skin barrier function before and after topical application of difamilast. Result showed that topical application of difamilast significantly decreased TEWL, with six out of seven patients showing a decrease and one patient experiencing worsening (Figures 1A,B).
FIGURE 1
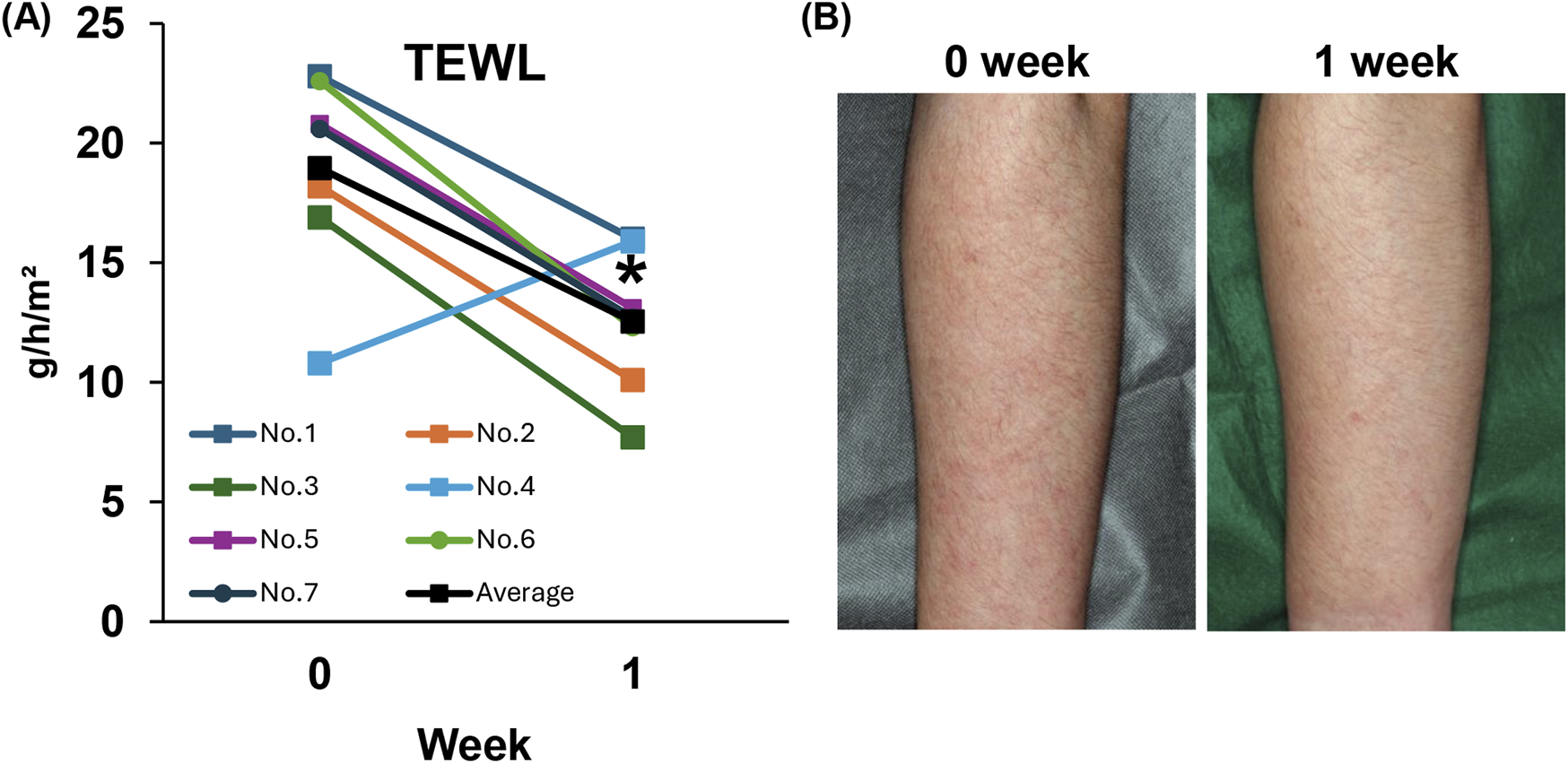
(A) Trans Epidermal Water Loss (TEWL) in patients with atopic dermatitis. n = 7. (B) Representative clinical skin manifestation at 0 week (before) and 1 week (after) in patients with AD treated with 1% difamilast. Statistical analysis was performed by Wilcoxon matched-pairs signed rank test.
We next investigated the suppressive effect of topical application of difamilast of MC903-induced AD-like dermatitis mouse model. The dermatitis score was significantly decreased by difamilast at day 6 compared with those in control mice (Figures 2A,B). In addition, the elevated TEWL value in MC903-treated mice was significantly reduced by difamilast on day 6 after treatment (Figure 2C).
FIGURE 2
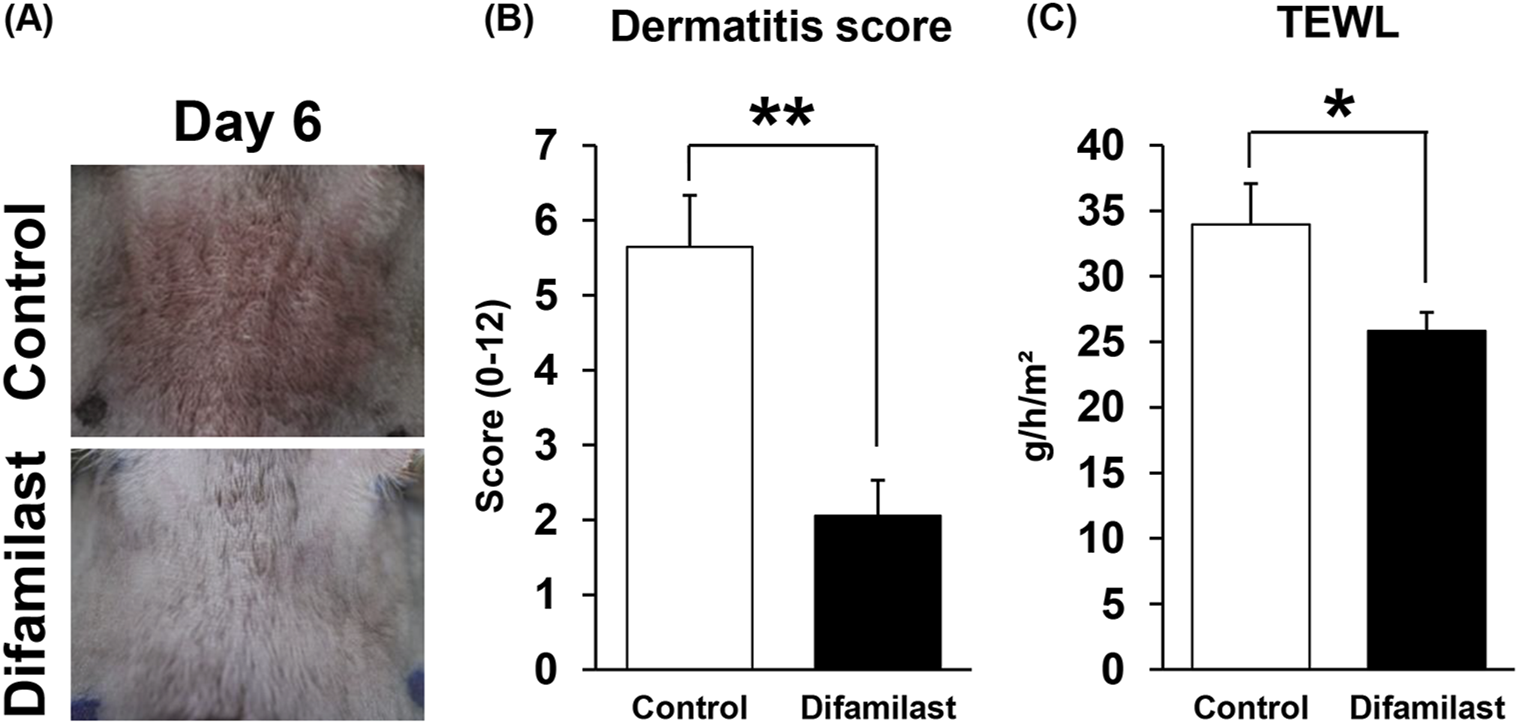
(A) Representative images of dermatitis skin in each groups. (B) Cumulative dermatitis score and (C) TEWL at day 6 in each group. n = 9 for each. Statistical analysis was performed by Mann Whitney test. Values represent mean ± standard error of the mean. *p < 0.05, **p < 0.01.
Next, we performed histological analyses using normal skin and MC903-treated dermatitis skin. Results showed that difamilast suppresses MC903-induced epidermal thickening and infiltration of inflammatory cells in the dermis (Figure 3A). Then, we examined the filaggrin expression in the lesional skin site by immunofluorescence staining. Results revealed that difamilast improved the impaired filaggrin expression caused by MC903 (Figure 3B). Next, we assessed the infiltration of inflammatory cells in the lesional skin. Toluidine blue staining showed that the increased number of mast cells in MC903-treated control mice significantly decreased in difamilast-treated mice (Figure 3C). Immunofluorescence revealed that the number of CD4+ T-cells was also significantly decreased in difamilast-treated group compared to that in control mice in MC903 treated mice (Figure 3D).
FIGURE 3
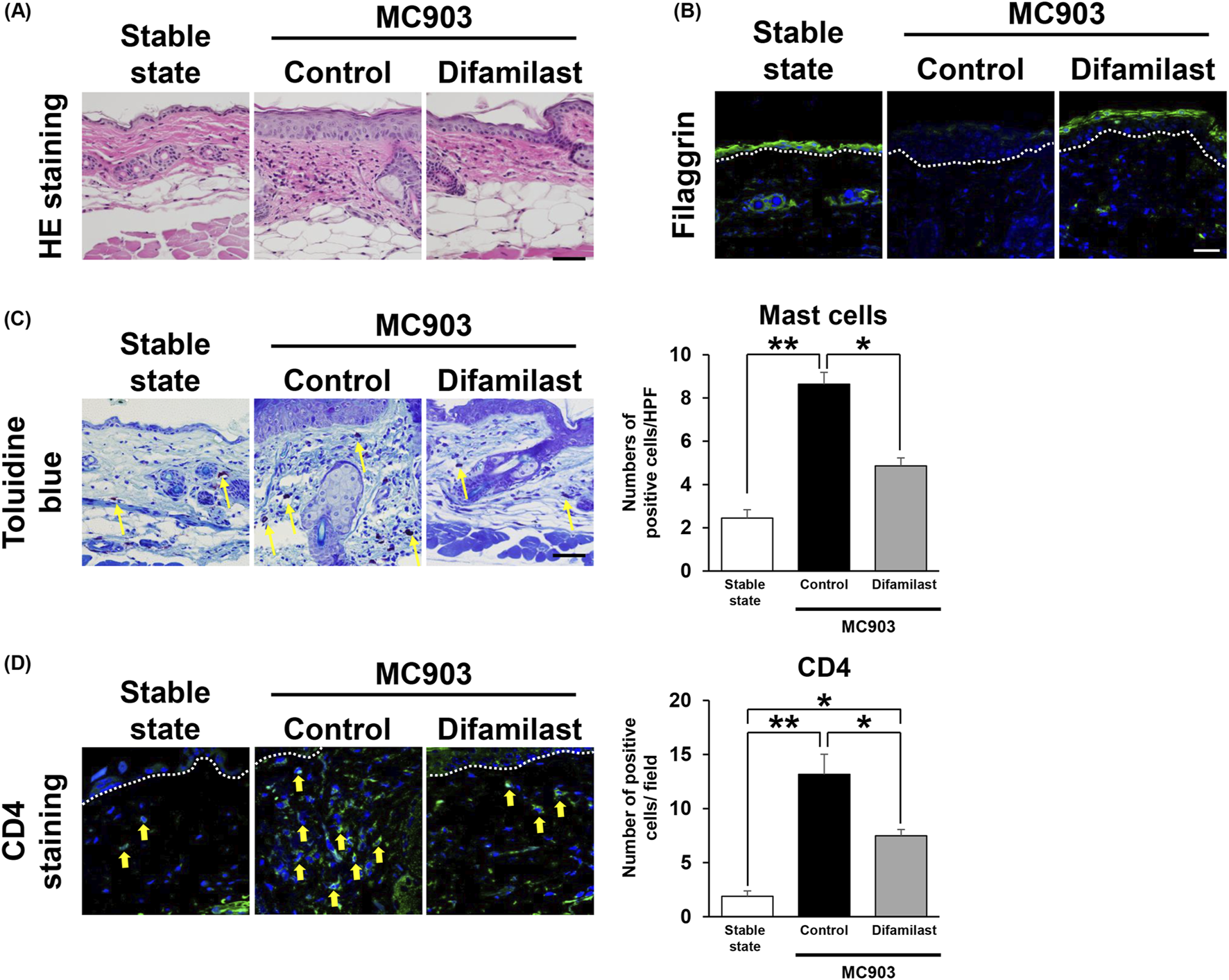
(A) Representative images of H&E staining of skin sections in stable state and at day 6 in mice. Scale bar = 50 μm. (B) Representative immunofluorescence staining image of filaggrin of skin in stable state and at day 6. Scale bar = 50 μm. (C) Representative images of toluidine blue staining of skin in stable state and at day 6. n = 3 for stable state and n = 5 for control and difamilast. Statistical analysis was performed by one-way ANOVA followed by Bonferroni’s post hoc test. Arrows indicate positive cells (yellow). (D) Representative immunofluorescence staining image of CD4 of skin in stable state and at day 6. Scale bar = 25 μm. n = 3 for stable state and n = 4 for control and difamilast group. Statistical analysis was performed by one-way ANOVA followed by Bonferroni’s multiple comparisons test. Arrows indicate positive cells (yellow).
Finally, we extracted RNA from the lesional skin and analyzed gene expression at the mRNA level using quantitative PCR. Result showed that mRNA expression of filaggrin and Interleukin (IL)-4 was elevated in MC903-treated control mice, but it was significantly reduced by difamilast (Figure 4). Increased mRNA expression of TSLP and IL-13 was observed in the MC903-treated groups compared with that in stable state; however, there was no significant difference between the control group and the difamilast-treated group in the MC903-treated model (Figure 4). These results suggest that difamilast may not protect keratinocytes from MC903-induced inflammation, and that its anti-inflammatory effect may be more closely associated with IL-4 rather than IL-13.
FIGURE 4
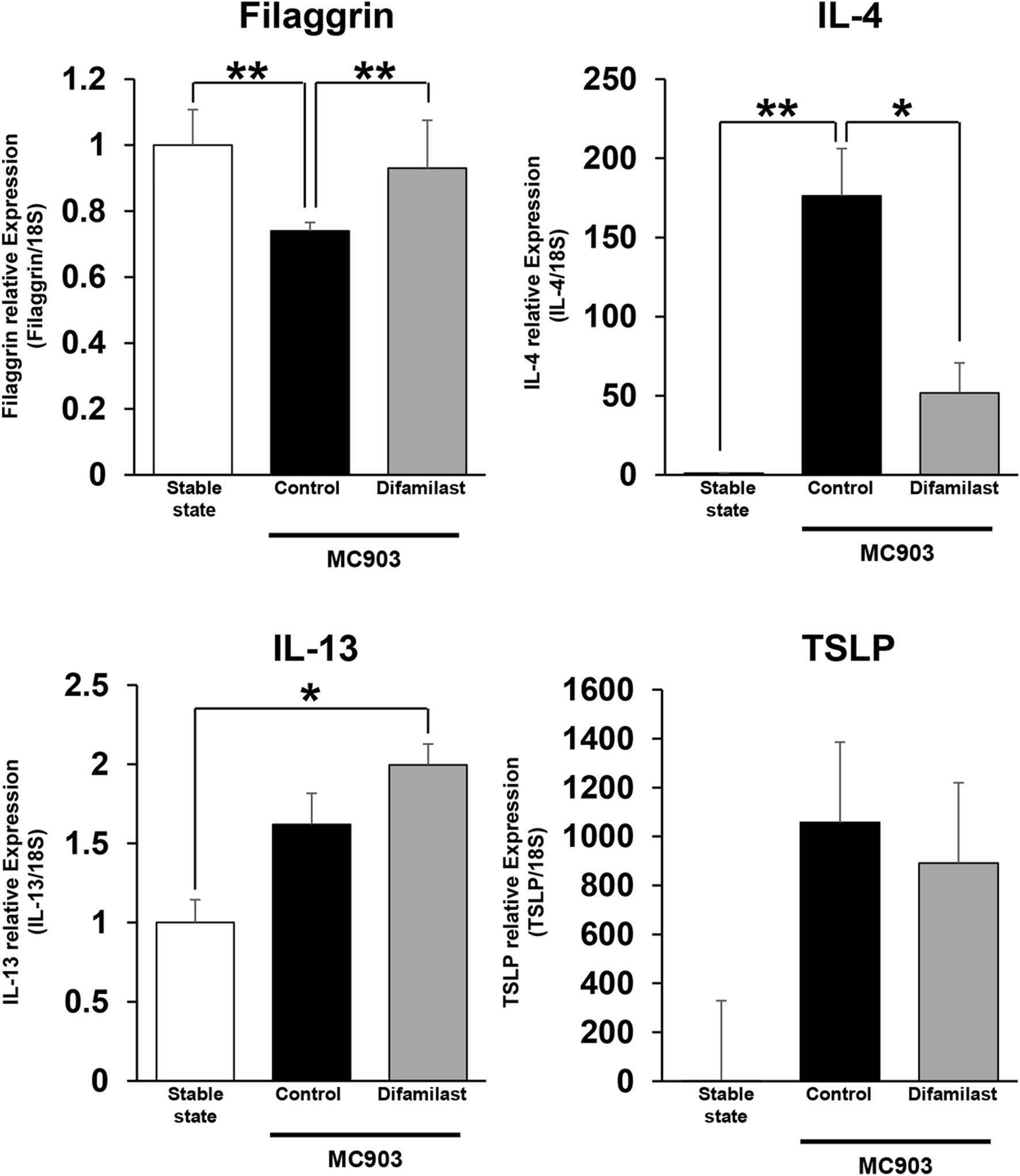
mRNA levels of filaggrin, IL-4, IL-13 and TSLP in the stable state and lesional skin on day 6. n = 3-5 for filaggrin, IL-4, TSLP, and IL-13 in each group. Statistical analysis was performed by one-way ANOVA followed by Tukey’s multiple comparisons test.
Discussion
In this study, we investigated the effects of barrier function improvement in human atopic dermatitis and the MC903 topical application-induced model. In double-blind vehicle-controlled study, topical application of difamilast ointment 1% showed significantly higher value of improvement of investigator global assessment score than with the vehicle at week 4 (38.46% vs. 12.64%, respectively, P < 0.0001). Moreover, significant improvements of least-square (LS) mean percent change in overall EASI score from the baseline was observed at week 1. These rapid improvements support our result of improvement of TEWL at week 1. PDE4 is involved in various cells, including epidermal cells, fibroblasts, and inflammatory cells, difamilast is thought to exert multiple effects [9]. Recent report has identified a novel mechanism by which difamilast regulates the expression of filaggrin and loricrin in human keratinocytes via cAMP-responsive element binding protein (CREB) and keratinocyte proline-rich protein (KPRP) axis [5]. The disruption of the skin barrier by harmful substances results in the increased expression of alarmins, including TSLP, IL-25, IL-33, and type 2 chemokines. These alarmins activate ILC2s and Th2 cells, initiating type 2 inflammatory responses [10]. Previous study revealed that elevated TSLP expression was observed from Day 2 after topical application of MC903 [6]. In our study, TSLP expression and dermatitis scores were assessed only on Day 6, which may be insufficient for a comprehensive evaluation. To fully clarify the inhibitory effect of difamilast on TSLP production from MC903-induced epidermal cells, it is necessary to evaluate multiple time points.
Results showed that difamilast treatment decreased the number of infiltrating CD4+ T-cells. IL-4 is well known to induce barrier dysfunction in atopic dermatitis, and previous studies have shown that PDE4 inhibitors reduce IL-4 production in PBMCs derived from patients with atopic dermatitis in vitro [11]. IL-13 also is known to play a key role in inducing barrier dysfunction in AD. IL-13 is produced by both CD4+ T-cells and ILC2 in lesional skin site. The lack of reduction in IL-13 expression following difamilast application may be due to the absence of an inhibitory effect of difamilast on IL-13 production or a limited impact on ILC2 activity. However, further evaluation is necessary to clarify this point. Mast cell activation and migration are primarily regulated by stem cell factor (SCF), while chemokines such as CCL2, CXCL8, and CXCL12 have also been shown to play contributory roles [12, 13]. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, has been shown to restore CCL2 expression to normal levels in a DNCB-induced murine model of atopic dermatitis [14]. Cilomilast, a phosphodiesterase-4 (PDE4) selective inhibitor, has suppressive effects on RV16-induced ICAM-1 and CXCL8 expression [15]. These results indicate that PDE4 inhibitors might suppressed the infiltration of mast cells via suppressing those cytokines and chemokines. These combined effects suggest that difamilast may improve barrier dysfunction in vivo in both humans and mice.
There are several limitations in this study. It was very small numbers of samples, short durations, non-blinded, and non-controlled designs in human experiments. With regard to animal studies, we have not examined models other than the MC903-induced model. It should also be taken into account that there are substantial differences in transdermal drug absorption rates between humans and mice. Therefore, further research is needed to elucidate the mechanisms by which difamilast regulates barrier dysfunction in AD in vivo.
In conclusion, our results demonstrate the potential of difamilast to improve barrier dysfunction in AD. However, further investigation is required for long-term evaluation and control study for human AD.
Statements
Data availability statement
The original contributions presented in the study are publicly available. This data can be found here: https://figshare.com/articles/figure/Data_for_manuscript_/30112927.
Ethics statement
The studies involving humans were approved by Institutional Review Board of Gunma University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. The animal study was approved by Gunma University Animal Care and Experimentation Committee. The study was conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
AUc and S-IM conducted the design. AUc, BT, and KK carried out the studies and analysis of the data. AU wrote the manuscript with support from MI, AUe, MS, and KR. S-IM confirmed the final version of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to thank members of the department of dermatology in Gunma University Hospital for their assistance.
Conflict of interest
S-IM serves as Editor-in-Chief of the Journal of Cutaneous Immunology and Allergy.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. During the preparation of this work, the authors used ChatGPT 4o in order to assist with grammar correction.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/jcia.2026.16328.
References
1.
KatohNOhyaYIkedaMEbiharaTKatayamaISaekiHet alJapanese guidelines for atopic dermatitis 2020. Allergol Int (2020) 69(3):356–69. 10.1016/j.alit.2020.02.006
2.
FurueMChibaTTsujiGUlziiDKido-NakaharaMNakaharaTet alAtopic dermatitis: immune deviation, barrier dysfunction, IgE autoreactivity and new therapies. Allergol Int (2017) 66(3):398–403. 10.1016/j.alit.2016.12.002
3.
BondarevADAttwoodMMJonssonJChubarevVNTarasovVVLiuWet alRecent developments of phosphodiesterase inhibitors: clinical trials, emerging indications and novel molecules. Front Pharmacol (2022) 13:1057083. 10.3389/fphar.2022.1057083
4.
SaekiHItoKYokotaDTsubouchiH. Difamilast ointment in adult patients with atopic dermatitis: a phase 3 randomized, double-blind, vehicle-controlled trial. J Am Acad Dermatol (2022) 86(3):607–14. 10.1016/j.jaad.2021.10.027
5.
TsujiGHashimoto-HachiyaAYumineATakemuraMKido-NakaharaMItoTet alPDE4 inhibition by difamilast regulates filaggrin and loricrin expression via keratinocyte proline-rich protein in human keratinocytes. J Dermatol Sci (2023) 110(2):61–8. 10.1016/j.jdermsci.2023.04.007
6.
LiMHenerPZhangZKatoSMetzgerDChambonP. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci U S A (2006) 103(31):11736–41. 10.1073/pnas.0604575103
7.
NasanbatBUchiyamaAAmaliaSNInoueYYokoyamaYOginoSet alKaempferol therapy improved MC903 induced-atopic dermatitis in a mouse by suppressing TSLP, oxidative stress, and type 2 inflammation. J Dermatol Sci (2023) 111(3):93–100. 10.1016/j.jdermsci.2023.06.008
8.
UchiyamaAYamadaKPereraBOginoSYokoyamaYTakeuchiYet alProtective effect of MFG-E8 after cutaneous ischemia-reperfusion injury. J Invest Dermatol (2015) 135(4):1157–65. 10.1038/jid.2014.515
9.
Guttman-YasskyEHanifinJMBoguniewiczMWollenbergABissonnetteRPurohitVet alThe role of phosphodiesterase 4 in the pathophysiology of atopic dermatitis and the perspective for its inhibition. Exp Dermatol (2019) 28(1):3–10. 10.1111/exd.13808
10.
OgulurIMitamuraYYaziciDPatYArdicliSType 2 immunity in allergic diseases. Cell Mol Immunol (2025) 22(3):211–42. 10.1038/s41423-025-01261-2
11.
HanifinJMChanSCChengJBTofteSJHendersonWRKirbyDSet alType 4 phosphodiesterase inhibitors have clinical and in vitro anti-inflammatory effects in atopic dermatitis. J Invest Dermatol (1996) 107(1):51–6. 10.1111/1523-1747.ep12297888
12.
CambierSGouwyMProostP. The chemokines CXCL8 and CXCL12: molecular and functional properties, role in disease and efforts towards pharmacological intervention. Cell Mol Immunol (2023) 20(3):217–51. 10.1038/s41423-023-00974-6
13.
CollingtonSJHallgrenJPeaseJEJonesTGRollinsBJWestwickJet alThe role of the CCL2/CCR2 axis in mouse mast cell migration in vitro and in vivo. J Immunol (2010) 184(11):6114–23. 10.4049/jimmunol.0904177
14.
SchaferPHAdamsMHoranGTruzziFMarconiAPincelliC. Apremilast normalizes gene expression of inflammatory mediators in human keratinocytes and reduces antigen-induced atopic dermatitis in mice. Drugs R D (2019) 19(4):329–38. 10.1007/s40268-019-00284-1
15.
ZhuJEdwardsMRMessageSDStanciuLAJohnstonSLJefferyPK. Cilomilast modulates rhinovirus-induced airway epithelial ICAM-1 expression and IL-6, CXCL8 and CCL5 production. Pharmaceuticals (Basel) (2024) 17(11):1554. 10.3390/ph17111554
Summary
Keywords
retrospective study, atopic dermatitis, Japanese, difamilast, barrier dysfunction
Citation
Uchiyama A, Taivanbat B, Kosaka K, Ishikawa M, Uehara A, Shimaoka M, Ryuzaki K and Motegi S-I (2025) The possible effectiveness of difamilast in improving barrier dysfunction in patients with atopic dermatitis and in mouse model. J. Cutan. Immunol. Allergy 8:15151. doi: 10.3389/jcia.2025.15151
Received
23 June 2025
Accepted
29 August 2025
Published
22 September 2025
Corrected
20 February 2026
Volume
8 - 2025
Updates
Copyright
© 2025 Uchiyama, Taivanbat, Kosaka, Ishikawa, Uehara, Shimaoka, Ryuzaki and Motegi.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Akihiko Uchiyama, akihiko1016@gunma-u.ac.jp
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.