Dear Editors,
Down syndrome (DS) is a congenital chromosomal disorder resulting from the complete or partial trisomy of chromosome 21. It occurs in around one in every 700 live births and represents one of the most common chromosomal abnormalities. Beyond its characteristic developmental delay, DS is also associated with an increased risk of autoimmune disorders, such as autoimmune thyroid disease, celiac disease, and type 1 diabetes mellitus. Furthermore, several studies have reported that individuals with DS may exhibit a higher prevalence of cutaneous immune-mediated diseases, such as atopic dermatitis, alopecia areata, vitiligo, and psoriasis compared to the general population [1]. According to a systematic review of previous studies in 2022, the prevalence of psoriasis in populations with DS was reported to be 4.8% [1]. While the association between DS and psoriasis has yet to be fully elucidated, both conditions are thought to involve overlapping immunological dysregulation. In this report, we present two cases of severe childhood-onset psoriasis vulgaris in individuals with DS complicated by morbid obesity, whose cutaneous symptoms were markedly improved with risankizumab (RZB), an IL-23 inhibitor.
Patient 1 is a 32-year-old male with DS who developed skin lesions at around the age of nine and was diagnosed with psoriasis at 11 years old. He received topical therapy for this condition. Due to a worsening of cutaneous symptoms, cyclosporine was administered from the age of 19–25. At the age of 26, the treatment was switched to apremilast, resulting in clinical stabilization. However, he became resistant to apremilast at the age of 30, leading to his referral to our department. On clinical examination, scaly infiltrated erythematous plaques were observed on the trunk and extremities including the palms and soles (Figure 1A). He was considered to have psoriasis vulgaris complicated by palmoplantar psoriasis. The psoriasis area and severity index (PASI) score was calculated as 17.1. He had a body mass index (BMI) of 38.2, indicating morbid obesity. Laboratory investigations revealed mildly elevated liver enzymes: aspartate aminotransferase (AST) at 47 U/L and alanine aminotransferase (ALT) at 94 U/L. Abdominal computed tomography (CT) demonstrated fatty liver. Treatment with RZB was initiated, and the erythematous plaques including sole and palm lesions had resolved with residual post-inflammatory pigmentation at 16 weeks after starting the biologic (Figure 1B). The patient has been undergoing treatment for two and a half years and has not yet developed any adverse events.
FIGURE 1
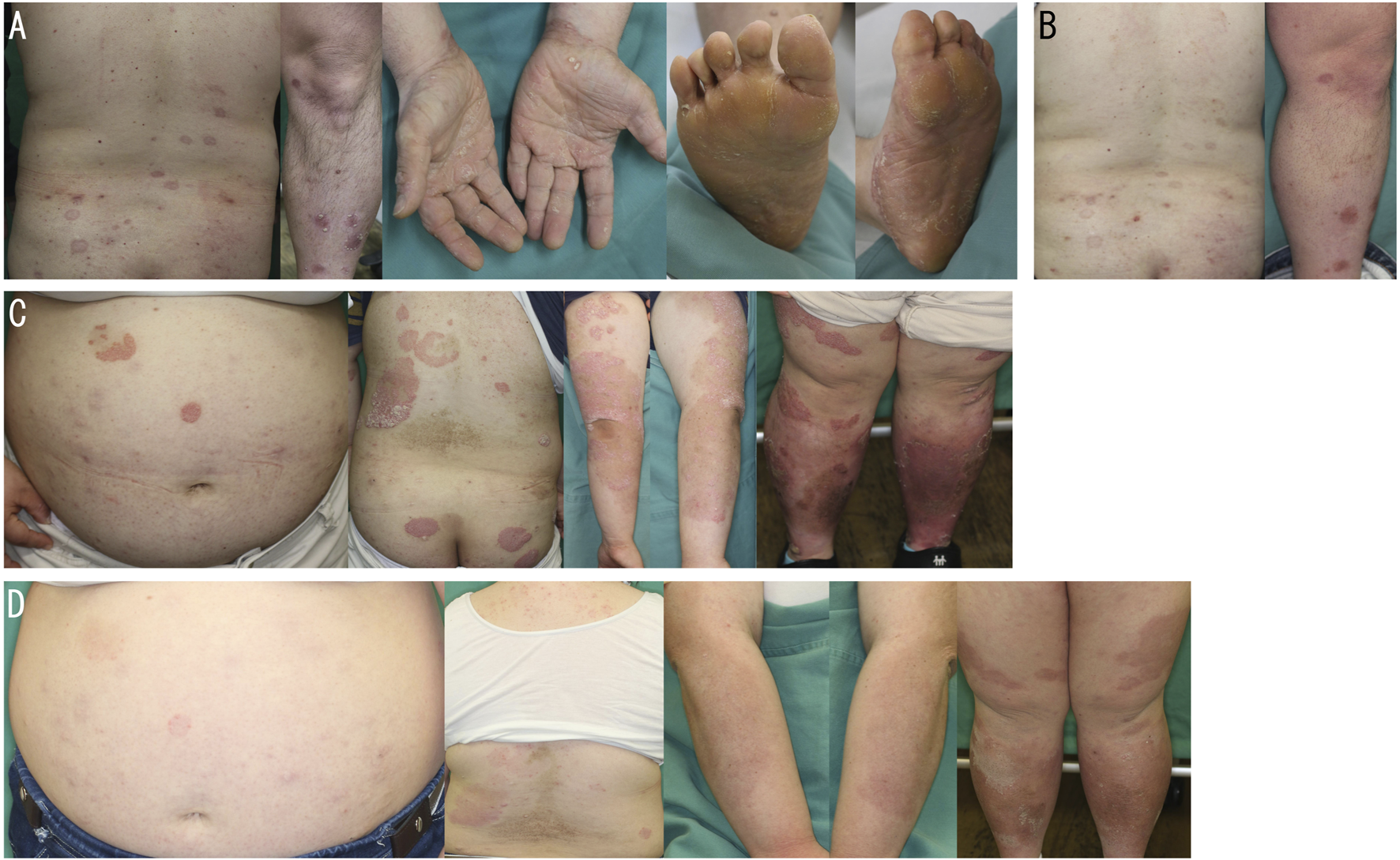
(A) Scaly infiltrated erythematous plaques on the back, left leg, palms and soles on initial examination in patient 1. (B) Improved back and left leg lesions 16 weeks after the administration of risankizumab in patient 1. (C,D) Scaly infiltrated erythematous plaques on the abdomen, back, and extremities before (C) and 16 weeks after (D) the administration of risankizumab in patient 2.
Patient 2 is a 28-year-old female with DS who was diagnosed with psoriasis at around the age of nine due to the development of skin lesions on her scalp. Topical therapy was initially used. Due to exacerbation of cutaneous symptoms, apremilast was started at the age of 21, leading to disease stabilization. However, the disease flared up again at around the age of 27, prompting a switch to biologic treatment. Clinical examination revealed hyperkeratotic erythematous plaques distributed on the trunk and extremities (Figure 1C). She was considered to have psoriasis vulgaris. The PASI score was 39.3. BMI was 38, indicating morbid obesity. Serum AST and ALT levels were 32 U/L and 63 U/L, respectively, and fatty liver was identified on abdominal CT. The hemoglobin A1c level was elevated at 7.5%, consistent with diabetes mellitus. RZB was started, and after 16 weeks, the erythematous plaques had resolved, leaving residual post-inflammatory pigmentation (Figure 1D). RZB is still being used for 9 months without any adverse events.
In both cases, psoriasis developed during childhood at around the age of nine. This is earlier than the typical age of onset for psoriasis vulgaris. Similar to psoriasis, in patients with DS, T helper type 1 (Th1) and Th17 subsets are enriched among non-regulatory CD4+ T cells, and the production of interferon-gamma (IFN-γ) from T cells and plasma levels of IL-17 and IL-22 are significantly higher compared to healthy controls [2]. Moreover, the trisomy of chromosome 21 is known to enhance sensitivity to IFN-γ, with the supernumerary copies of chromosome 21 conferring increased responsiveness to IFN-γ signaling [2]. Collectively, the abnormal immune dysregulation in DS similar to that in psoriasis may cause the early onset of the disease in our cases.
There are no guidelines for choosing biologics in cases of psoriasis complicated by DS. Considering the immunologic abnormalities in DS as described above, any biologics can be considered for such patients. Although anti-TNF-α antibodies have been frequently used in the literature, a recent report presented cases of psoriasis complicated by DS successfully treated with ustekinumab, secukinumab, ixekizumab, or risankizumab [3]. Similarly, risankizumab exerted its effect sufficiently in our cases. However, more research is needed to decide which biologics are suitable for psoriasis patients with DS.
In addition, interestingly, both of our cases showed severe obesity. They had been prone to obesity since childhood, and marked obesity became apparent several years after the onset of psoriasis. Both children and adults with DS have been reported to exhibit a higher prevalence of obesity compared to the general population [4]. Obesity has been recognized as a risk factor for the development of psoriasis and is further associated with increased severity of cutaneous symptoms, the development of joint manifestations, and reduced response to biologics. Furthermore, obesity increases the production of IFN-γ within adipose tissue, which induce differentiation of macrophages toward the pro-inflammatory M1 phenotype and contributes to the sustained production of inflammatory cytokines [5]. In addition, IFN-γ impairs adipocyte function and promotes the development of insulin resistance. In light of the above, DS is characterized by chronic systemic inflammation, and when compounded by obesity-related adipose tissue inflammation, it may augment the risk of psoriasis development and promote increased severity. Thus, obesity due to DS may also contribute to the early development and repeated exacerbation of psoriasis, as well as to the complications of metabolic comorbidities, such as fatty liver and/or diabetes mellitus, in our cases.
In conclusion, we present clinically intriguing cases that provide insights into the potential interplay among DS, psoriasis, and obesity, suggesting that systemic inflammation may influence each of these conditions.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YH, TM, and HW wrote the manuscript. All authors contributed to determining the treatment plan. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
References
1.
LamMLuJDElhadadLSibbaldCAlhusayenR. Common dermatologic disorders in Down syndrome: systematic review. JMIR Dermatol (2022) 5:e33391. 10.2196/33391
2.
ArayaPWaughKASullivanKDNúñezNGRoselliESmithKPet alTrisomy 21 dysregulates T cell lineages toward an autoimmunity-prone state associated with interferon hyperactivity. Proc Natl Acad Sci USA (2019) 116:24231–41. 10.1073/pnas.1908129116
3.
TancrediVLicataGCalabreseGBuononatoDDe RosaAArgenzianoGet alDown syndrome and biological treatments in dermatology: efficacy and safety in our real-life experience and review of literature. Australas J Dermatol (2023) 64:285–8. 10.1111/ajd.14006
4.
PtomeyLTWalpitageDLMohseniMGilletteMLDDavisAMForsethBet alWeight status and associated comorbidities in children and adults with Down syndrome, autism spectrum disorder and intellectual and developmental disabilities. J Intellect Disabil Res (2020) 64:725–37. 10.1111/jir.12767
5.
HuangLYChiuCJHsingCHHsuYH. Interferon Family cytokines in obesity and insulin sensitivity. Cells (2022) 11:4041. 10.3390/cells11244041
Summary
Keywords
psoriasis, Down syndrome, obesity, metabolic comorbidities, rinsankizumab
Citation
Hara Y, Miyagaki T, Okano T, Kadono T and Watabe H (2025) Two cases of severe childhood-onset psoriasis successfully treated with risankizumab in patients with Down syndrome and morbid obesity. J. Cutan. Immunol. Allergy 8:15043. doi: 10.3389/jcia.2025.15043
Received
06 June 2025
Accepted
07 August 2025
Published
14 August 2025
Volume
8 - 2025
Updates
Copyright
© 2025 Hara, Miyagaki, Okano, Kadono and Watabe.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tomomitsu Miyagaki, asahikari1979@gmail.com; Hidenori Watabe, watabe0424@yahoo.co.jp
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.