Dear Editors,
Patient 1 was a 44-year-old woman who presented with intensely pruritic nodules and plaques on her limbs that had been present since childhood. She had also experienced nail dystrophy during adulthood. Patient 2, a 3-year-old boy, was the son of patient 1. He presented with papules and erythema on his extremities, accompanied by scratching behaviour. They had a family history of similar skin symptoms and/or nail dystrophy in other four individuals (Figure 1I). Physical examination of patient 1 revealed crusted plaques, nodules, and erosions, primarily located on the lower limbs and dorsum of the feet, with intense pruritus (Figures 1A,B). Histological examination of a nodule in the lower limb of patient 1 revealed hyperkeratosis and pseudo-horn cysts in the epidermis, with marked fibrosis and mild lymphocyte infiltration in the dermis (Figure 1J). Patient 2 presented with papules or erythema on his forehead, posterior neck, dorsum of hands, knees, and lower legs (Figures 1E,F). His mother had identified blisters or erosions that sometimes appeared on his fingers. The serum IgE level was elevated (2280 IU/mL) in patient 1. The genetic analysis identified that patients 1 and 2 carried the same heterozygous mutation, c.6900 + 4A>G, in the COL7A1 gene, which has been reported to cause dominant dystrophic epidermolysis bullosa (DEB) pruriginosa by skipping of exon 87 [1].
FIGURE 1
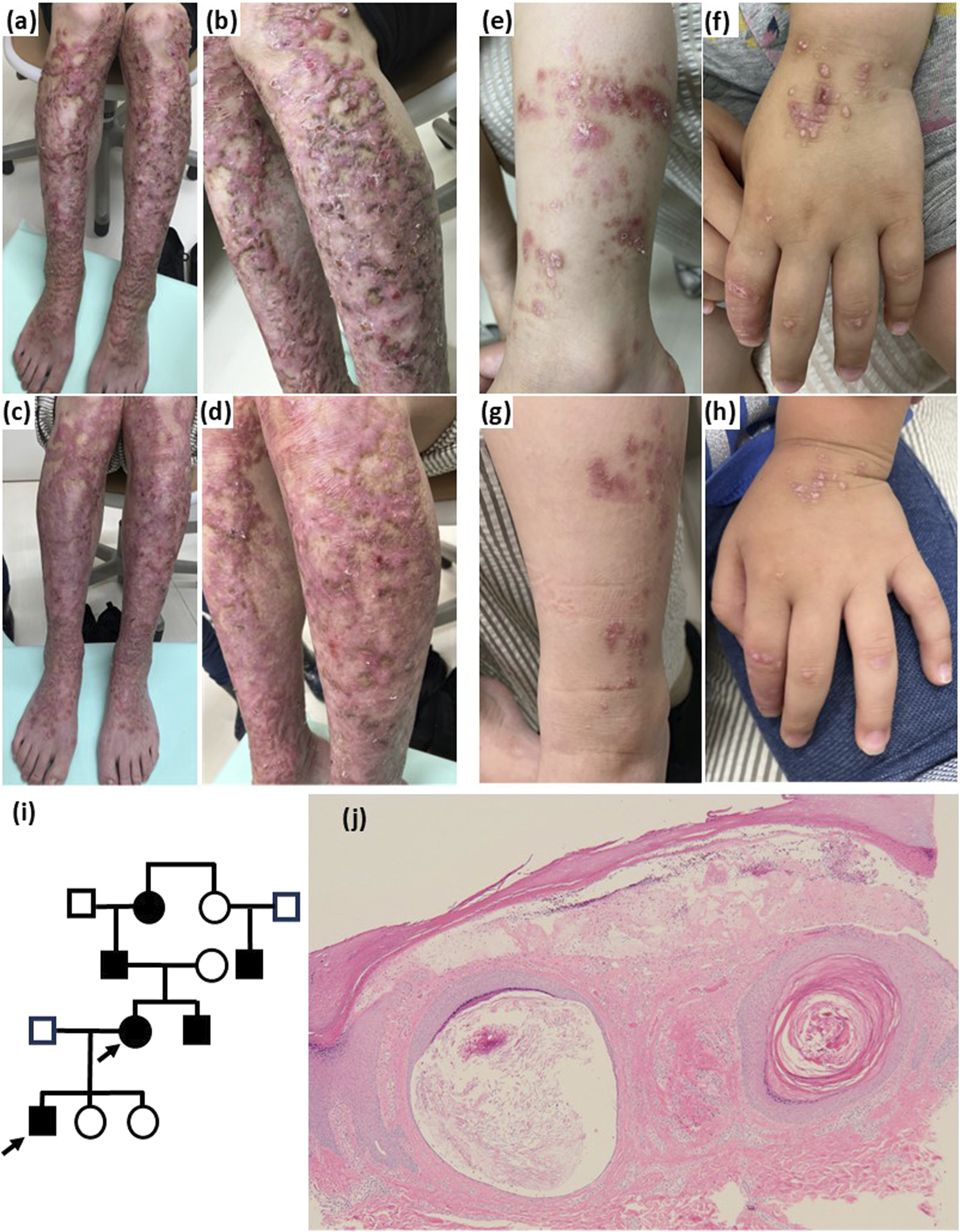
(A,B) Pruritic and scratched papules and nodules are observed over the patient 1’s limbs. Erythema with erosion indicated scar lesion. However, most of the lesions were keloidal because surface of elastic plaques was observed significantly elevated, accompanied with severe itching and with no episode of spontaneous regression. (C,D) Erythema and plaques became flattened and soft 1 year after dupilumab treatment was initiated in patient 1. (E,F) Scratched erythema and papules accompanied by pruritus are observed on the limbs and dorsal hands of patient 2. (G,H) Erythema was not changed after 2 months of dupilumab treatment in patient 2. Crusts were decreased and scratching behaviours were reduced by dupilumab treatment. (I) Family pedigree. The family comprised four affected individuals. One unknown case existed in addition to three confirmed cases. (J) Skin biopsy from patient 1 revealed hyperkeratosis and subepidermal cleavage. Milia and marked fibrosis are also observed (hematoxylin-eosin, original magnification, ×100).
Treatments with topical corticosteroids, topical Janus kinase (JAK) inhibitor (delgocitinib), oral antihistamine (fexofenadine), oral tokiinshi, which is a traditional Chinese medicine (Kampo), and oral cyclosporine 150 mg/day for 4 weeks were administered for patient 1, although the treatments showed a poor response. After obtaining informed consent from patient 1 for both patients, the mother was treated with a 600 mg loading dose of dupilumab, followed by 300 mg every 2 weeks. She noticed that the pruritus began to decrease within the first 3 weeks. Nodules and diffuse plaques in her lower limbs gradually flattened, with almost complete relief from itching (Figures 1C,D). Following the treatment plan for patient 1, we also treated patient 2 with 300 mg of dupilumab every 4 weeks to prevent scratching behaviours which induced new skin lesions. His parents noticed that the scratching behaviour gradually ceased within 4 weeks. Although papules and erythema remained unchanged, the crust and scratched plaques gradually disappeared (Figures 1G,H).
Dystrophic epidermolysis bullosa (DEB) is caused by mutations in the COL7A1 gene, which encodes type VII collagen, leading to skin fragility and itch-induced lichenification. Epidermolysis bullosa pruriginosa (EBP) is a rare phenotype of DEB characterised by mildly fragile skin, trauma-induced lichenified or nodular lesions, and scarring accompanied by intense pruritus [2] and splice site mutations resulting in the in-frame exon 87 skipping have been reported repeatedly [1]. A number of case reports have recently been published that present the regression of pruritus and skin lesions in DEB following dupilumab treatment [2]. Darbord et al. reported that T helper type 2 (Th2) cell-mediated immunity, including mast cell infiltration, elevated IgE levels, and an expanded Th2 subset without atopic dermatitis, is the pathogenesis of pruritus in patients with EBP who have responded to dupilumab [2]. Furthermore, single-cell transcriptomic analyses have reported that glycolytically active GATA3+ Th2 cells, which reside in the skin affected by dominant DEB, induce pruritus in the lesional skin of patients with DEB [3]. In the two cases that shared the same mutations, the clinical response was clearer in patient 1 than in patient 2, although the treatment was sufficiently effective in patient 2. Considering the responses to dupilumab in our cases, dupilumab may have contributed to the reduction in the number of keloidal skin lesions in patient 1 by reducing itching and mechanical scratching in addition to reducing levels of Th2-related inflammation in both patients. Our cases unexpectedly clarified the predominance of Dupilumab in treatment for senior patients of EBP with chronic keloidal skin lesions which tend to be recognized as irreversible before. Recently, dupilumab has been reported to be a promising treatment not only for patients with prurigo nodularis [4], but also for keloid lesions in a patient with a history of atopic dermatitis. Diaz A et al. reported dysregulation of interleukin (IL)-4 and IL-13 was identified in lesional and/or non-lesional skin in patients with keloid disease compared with healthy controls. In their ex vivo study in 2020, increased expression of IL-4/13 in fibroblasts promoted transforming growth factor-β signaling and led to fibrosis in patients with keloids. Overall, our cases suggest that dupilumab may reduce activity of the IL-4/13 pathway, which leads to the regression of abnormal collagen proliferation in senior patients with EBP. These results indicate that dupilumab may be particularly beneficial in preventing inflammation, fibrosis. Although chronic scar lesions and DEB are possible independent risk factors of squamous cell carcinoma (SCC), we believe the risk of developing secondary SCC may partly decreased by dupilumab treatment. We recently identified diverse treatment options, including repurposing medicines, such as biologics and oral JAK inhibitors. Although the use of oral JAK inhibitors may bring a better clinical improvement in itch numeric rating score and in the result of transcriptomic analysis in lesional skin of EBP [5], these treatments may exacerbate symptoms either by promoting secondary bacterial infections. Therefore, ours and recent case reports suggest that dupilumab could be a viable option for controlling symptoms of EBP with minimal adverse effects. Although dupilumab may be an off-label therapeutic option, its safety profile in patients remains to be further evaluated.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Juntendo University Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by- product of routine care or industry. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
MK wrote the manuscript. AI contributed to confirm genetic diagnosis. YS supervised the treatment of the patient. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
References
1.
SaitoMMasunagaTIshikoA. A novel de novo splice-site mutation in the COL7A1 gene in dominant dystrophic epidermolysis bullosa (DDEB): specific exon skipping could be a prognostic factor for DDEB pruriginosa. Clin Exp Dermatol (2009) 34:e934–6. 10.1111/j.1365-2230.2009.03254.x
2.
DarbordDHickmanGPirononNBarbieuxCBonnet-des-ClaustresMTiteuxMet alDystrophic epidermolysis bullosa pruriginosa: a new case series of a rare phenotype unveils skewed Th2 immunity. J Eur Acad Dermatol Venereol (2022) 36:133–43. 10.1111/jdv.17671
3.
AalaWJFHouPCHongYKLinYCLeeYRTuWTet alDominant dystrophic epidermolysis bullosa is associated with glycolytically active GATA3+ T helper 2 cells which may contribute to pruritus in lesional skin. Br J Dermatol (2024) 191:252–60. 10.1093/bjd/ljae110
4.
YosipovitchGMollanazarNStanderSKwatraSGKimBSLawsEet alDupilumab in patients with prurigo nodularis: two randomized, double-blind, placebo-controlled phase 3 trials. Nat Med (2023) 29:1180–90. 10.1038/s41591-023-02320-9
5.
HouPCAalaWJrTuWTMcGrathJAHsuCK. Real-world experience of using dupilumab and JAK inhibitors to manage pruritus in epidermolysis bullosa pruriginosa. Skin Health Dis (2024) 4:e445. 10.1002/ski2.445
Summary
Keywords
dupilumab, epidermolysis bullosa pruriginosa, dystrophic epidermolysis bullosa, keloid, COL7A1
Citation
Kaga M, Suga Y and Ishiko A (2025) Successful treatment of familial cases of epidermolysis bullosa pruriginosa with dupilumab. J. Cutan. Immunol. Allergy 8:14677. doi: 10.3389/jcia.2025.14677
Received
26 March 2025
Accepted
15 April 2025
Published
28 April 2025
Volume
8 - 2025
Updates
Copyright
© 2025 Kaga, Suga and Ishiko.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maya Kaga, mkaga@juntendo.ac.jp
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.