Dear Editors,
Psoriasis affects a patient both physically and psychologically, thus also affecting their quality of life. This disease also causes the risk of systemic disorders including arthritis and cardiometabolic and gastrointestinal diseases. Treatment options range from conventional therapies, such as topical corticosteroid and vitamin D, to biological therapies. Biologics are superior to traditional systemic treatments such as oral cyclosporine and etretinate but the usage of biologics may be problematic due to the high costs and parenteral administration. Today, oral small molecules for psoriasis treatment have been developed. These include phosphodiesterase 4 (PDE4) and Janus kinase (JAK) inhibitors, in the form of apremilast and a selective tyrosinase kinase 2 (TYK2) inhibitor, deucravacitinib, respectively. Therefore, various drugs are available for severe psoriasis resistant to conventional therapies. In this regard, a case supportive of oral small molecules is reported here.
A 57-year-old Japanese male presented with widespread erythema of 35-year duration on the trunk and limbs (PASI = 17.9) (Figure 1A). He was previously diagnosed as having psoriasis vulgaris. Because intermittent application of topical corticosteroid and vitamin D had been ineffective, oral apremilast was introduced, starting with 10 mg/day and gradually increasing the dosage stepwise by 10 mg/day up to 60 mg/day. Three months later erythema began to regress (PASI = 5.3) (Figure 1B) and was well controlled for around 1 year. However, the erythema was exacerbated possibly because of loss of efficacy after 55 weeks of apremilast treatment (PASI = 13.6) (Figure 1C) without aggravating factors such as infections or stressful events, although he kept regularly applying the topical drug. Because of this, apremilast was discontinued and biologics were recommended to him. But he refused administration of biologics because of the high cost. Thereafter, only topical corticosteroid and vitamin D were again regularly continued for 3 years. Nonetheless, no clear effect was seen during this long period. Therefore, 6 mg/day of oral deucravacitinib was administrated for the persistent erythema (PASI = 16.4) (Figure 1D). For systemic screening, we confirmed negativity for hepatitis B surface antigen, core antibody and hepatitis C antibody by blood tests, and tuberculosis infection by interferon-γ release assay and chest X-ray. Three months later the eruption clearly decreased (PASI = 2.7) (Figure 1E) and almost completely disappeared after 12-month therapy (PASI = 0.3) (Figure 1F). The remission was maintained for a further 12 months. During this clinical course, no side effect of apremilast or deucravacitinib was observed. Immunological safety concerns were checked especially after deucravacitinib administration by periodic blood tests and chest X-rays at 1, 3, 6, 12, 18, and 24 months of the treatment; no infection was found. Additionally, he did not feel any pruritus on the psoriatic lesions.
FIGURE 1
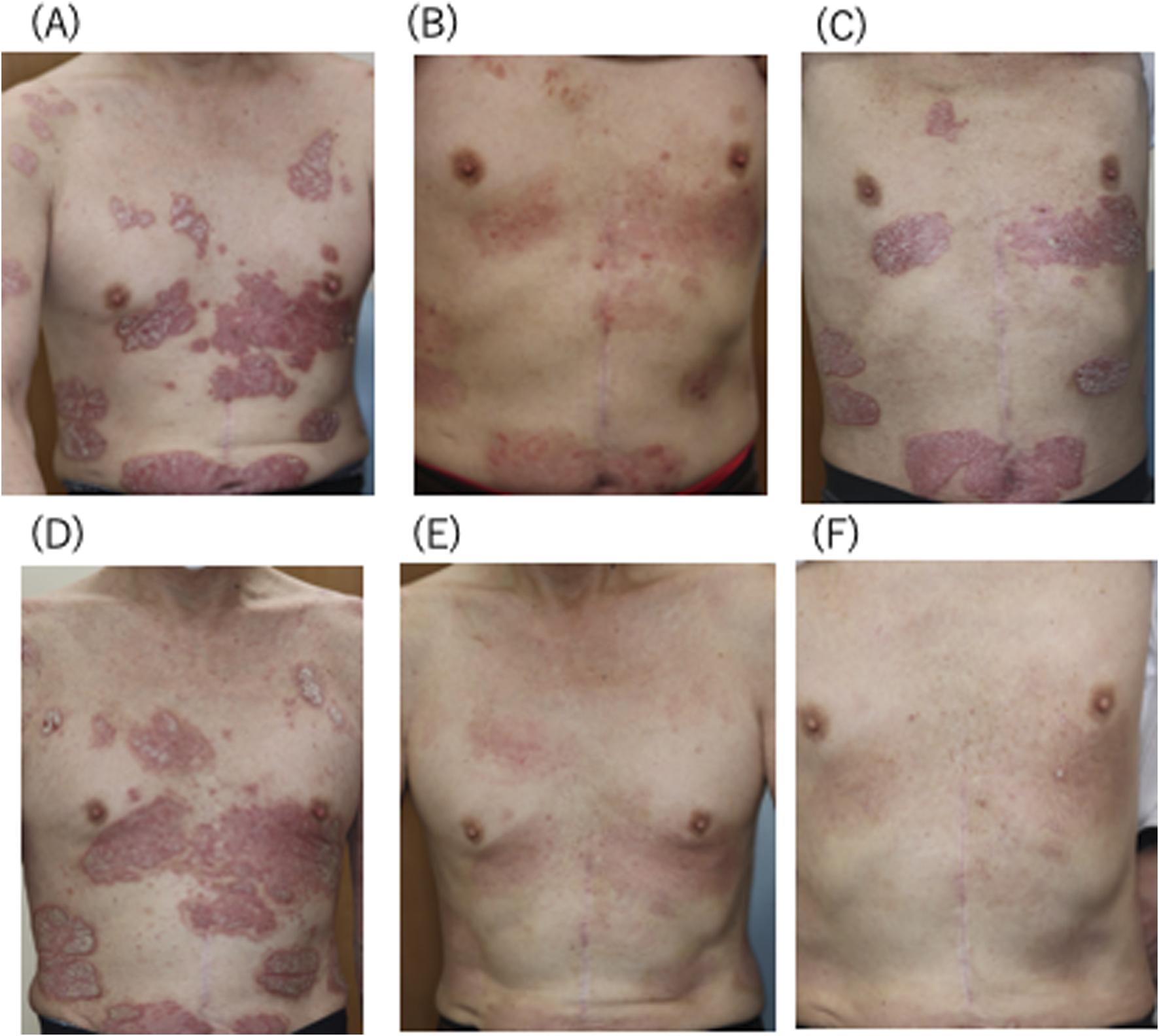
Clinical features of the patient. (A) Before apremilast administration, (B) after 3 months of apremilast treatment, (C) exacerbation after 55 weeks of apremilast treatment,(D) before deucravacitinib administration, (E) after 3 months of deucravacitinib treatment, (F) almost complete remission after 12 months of deucravacitinib treatment.
One real-world study of 281 patients treated with apremilast showed its 1-, 2-, 3-, and 4-year drug survival rates were 54%, 41%, 32%, and 30%, respectively, and loss of efficacy (27%) was highlighted as the most common reason for apremilast discontinuation [1]. Likewise, another group reported that secondary failure (37.5%) was the most common reason for the cessation [2]. The variability in response to apremilast suggests some contributing factors which could influence the drug effect. Our patient did not have comorbidities like metabolic or cardiac diseases, obesity, or smoking history. Further, the patient adhered well to treatment regimens including the topical drug. According to a Cox regression analysis of various factors and drug survival period [1], sex, duration of plaque psoriasis (<10 years vs. ≥10 years), presence of psoriatic arthritis, involvement of scalp lesions, involvement of palmoplantar lesions, involvement of nail lesions, having cardiometabolic comorbidities, and having a history of systemic treatment did not have any significant impact on drug survival.
Tachyphylaxis of another PDE4-selective inhibitor, roflumilast, for chronic obstructive pulmonary disease is known to be caused by PDE4B upregulation [3]. The phosphorylation of NF-κB p65 subunit by PKA-Cβ is required for the PDE4B induction. Moreover, Ser276 of p65 is critical for mediating the PKA-Cβ−induced p65 phosphorylation, suggesting this negative feedback mechanism is influenced by genetic predispositions related to the p65 gene. Likewise, the secondary failure of apremilast may involve genetic polymorphism of signaling molecules mediating the PDE4 pathway.
In the case presented here, apremilast initially showed sufficient efficacy but around 1 year later this effect declined. On the contrary, deucravacitinib showed excellent effect, maintaining remission for a further 12 months. Indeed, the recent 52-week, randomized, double-blinded study [4] showed superiority of deucravacitinib versus apremilast in patients with moderate to severe psoriasis. Additionally, Hagino et al [5] reported that deucravacitinib efficiently decreased PASI and DLQI scores for 52 weeks in psoriasis patients both with and without prior apremilast or biologics and, further, apremilast-experienced patients showed higher achievement rates of PASI 100 or absolute PASI ≤1 later than week 24 compared with apremilast-naive patients. Their findings suggest broader efficacy by deucravacitinib regardless of prior treatments and a possible cooperative effect of prior apremilast for deucravacitinib. Putting these findings together with the observation of this patient, it is suggested that deucravacitinib is a potential candidate drug for psoriasis refractory to apremilast. In clinical practice, considering the immunosuppressive action of drugs, although apremilast may be preferred to a TYK2 inhibitor such as deucravacitinib endowed with immunosuppressive properties, it should be kept in mind that the negative feedback of PDE4 inhibition by apremilast can occur. Further, when the efficiency of apremilast starts to decrease, deucravacitinib should be one of the next choices for treatment.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SI drafted the manuscript and all authors listed have made a substantial and direct contribution to the work and approved it for publication. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
References
1.
KishimotoMKomineMKamiyaKSugaiJKuwaharaAOhtsukiM. Four-year drug survival of apremilast in patients with psoriasis. J Dermatol (2023) 50(7):960–3. 10.1111/1346-8138.16766
2.
OhataCOhyamaBKuwaharaFKatayamaENakamaT. Real-world data on the efficacy and safety of apremilast in Japanese patients with plaque psoriasis. J Dermatolog Treat (2019) 30(4):383–6. 10.1080/09546634.2018.1525480
3.
Suzuki-MiyataSMiyataMLeeBCXuHKaiHYanCet alCross-talk between PKA-Cβ and p65 mediates synergistic induction of PDE4B by roflumilast and NTHi. Proc Natl Acad Sci (2015) 112(14):E1800–9. 10.1073/pnas.1418716112
4.
ArmstrongAWGooderhamMWarrenRBPappKAStroberBThaçiDet alDeucravacitinib versus placebo and apremilast in moderate to severe plaque psoriasis: efficacy and safety results from the 52-week, randomized, double-blinded, placebo-controlled phase 3 POETYK PSO-1 trial. J Am Acad Dermatol (2023) 88(1):29–39. 10.1016/j.jaad.2022.07.002
5.
HaginoTSaekiHFujimotoEKandaN. Long-term real-world effectiveness of deucravacitinib in psoriasis: a 52-week prospective study stratified by prior apremilast or biologic therapy. J Dermatol (2025) 2:634–41. 10.1111/1346-8138.17665
Summary
Keywords
psoriasis vulgaris, deucravacitinib, apremilast, secondary failure, PDE4
Citation
Inui S, Nakagawa Y and Fujimoto M (2025) Successful treatment by deucravacitinib of psoriasis refractory to apremilast. J. Cutan. Immunol. Allergy 8:14639. doi: 10.3389/jcia.2025.14639
Received
18 March 2025
Accepted
21 April 2025
Published
04 June 2025
Volume
8 - 2025
Updates
Copyright
© 2025 Inui, Nakagawa and Fujimoto.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shigeki Inui, inui@inuihifuka.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.