Dear Editors,
Immune checkpoint inhibitors (ICIs) have dramatically changed cancer treatment, progressing from conventional chemotherapies that directly kill tumor cells to novel immunological therapies that enhance T-cell-mediated immunity. However, ICIs frequently provoke immune-related adverse events (irAEs) which attack not only malignancies but also benign cells and tissues including the skin. Cutaneous irAEs are generally mild. Severe cutaneous irAEs, such as Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), are quite rare but life-threatening complications. Here, we report a fatal case of TEN that developed during treatment with nivolumab, an anti-PD-1 antibody, used in combination with chemotherapies.
An 85-year-old woman who was suffering from Stage IV gastric cancer with peritoneal carcinomatosis received her second course of monthly nivolumab plus SOX (S-1 and oxaliplatin) therapy. On day 20 of the second-course injection of nivolumab, she developed acute liver failure, which was diagnosed as an irAE and was treated with steroid pulse therapy (methylprednisolone (mPSL) 1 g/day intravenous injection, 3 days). Oral prednisolone (PSL) was initiated at 50 mg/day (1 mg/kg/day) and gradually tapered to 35 mg/day (0.7 mg/kg/day). On day 48, she complained of pain in her lips, genital area, and back, which progressed to erosions and blistering. On day 50, she was referred to our dermatology department. Physical examination revealed fever, extensive dusky-red erythema, and flaccid blisters covering approximately 49% of her body surface area (Figure 1A), along with erosions on the lips, oral cavity, and genital area (Figure 1B). Laboratory tests showed mild elevation of alanine aminotransferase (ALT, 39 U/L; <23 U/L), blood urea nitrogen (BUN, 30 mg/dL; <20 mg/dL), and blood glucose (Glu, 150 mg/dL; <109 mg/dL) and slightly elevated anti-desmoglein 3 antibodies (39.8 U/mL; <20 U/L). Histopathological examination revealed subepidermal blisters accompanied by epidermal necrotic changes with extensive keratinocyte necrosis and mild lymphocytic inflammatory infiltration in the dermis (Figure 1C). TEN was diagnosed based on clinical presentation, histopathology, and the exclusion of other etiologies. Her CRISTEN score, which is a clinical risk prediction model of SJS/TEN, was 7 points, indicating a mortality rate of 66.7% [1]. Mycoplasma pneumoniae infection or any other infectious etiology of TEN were ruled out. Prior medications, including furosemide, lansoprazole, edoxaban, bisoprolol, magnesium oxide, sulfamethoxazole-trimethoprim, and alendronate, which had been administered 2 weeks ago or longer, were discontinued. Steroid pulse therapy was initiated, followed by intravenous PSL at 75 mg/day (1.5 mg/kg/day). However, the necrotic lesions continued to expand (Figure 1D). On day 57, a second course of steroid pulse therapy was administered, followed by a combination of intravenous immunoglobulin (IVIG) therapy (400 mg/kg/day, 5 days) and intravenous PSL at 75 mg/day. Despite these interventions, the patient showed no improvement and passed away on day 67 (Figure 1E).
FIGURE 1
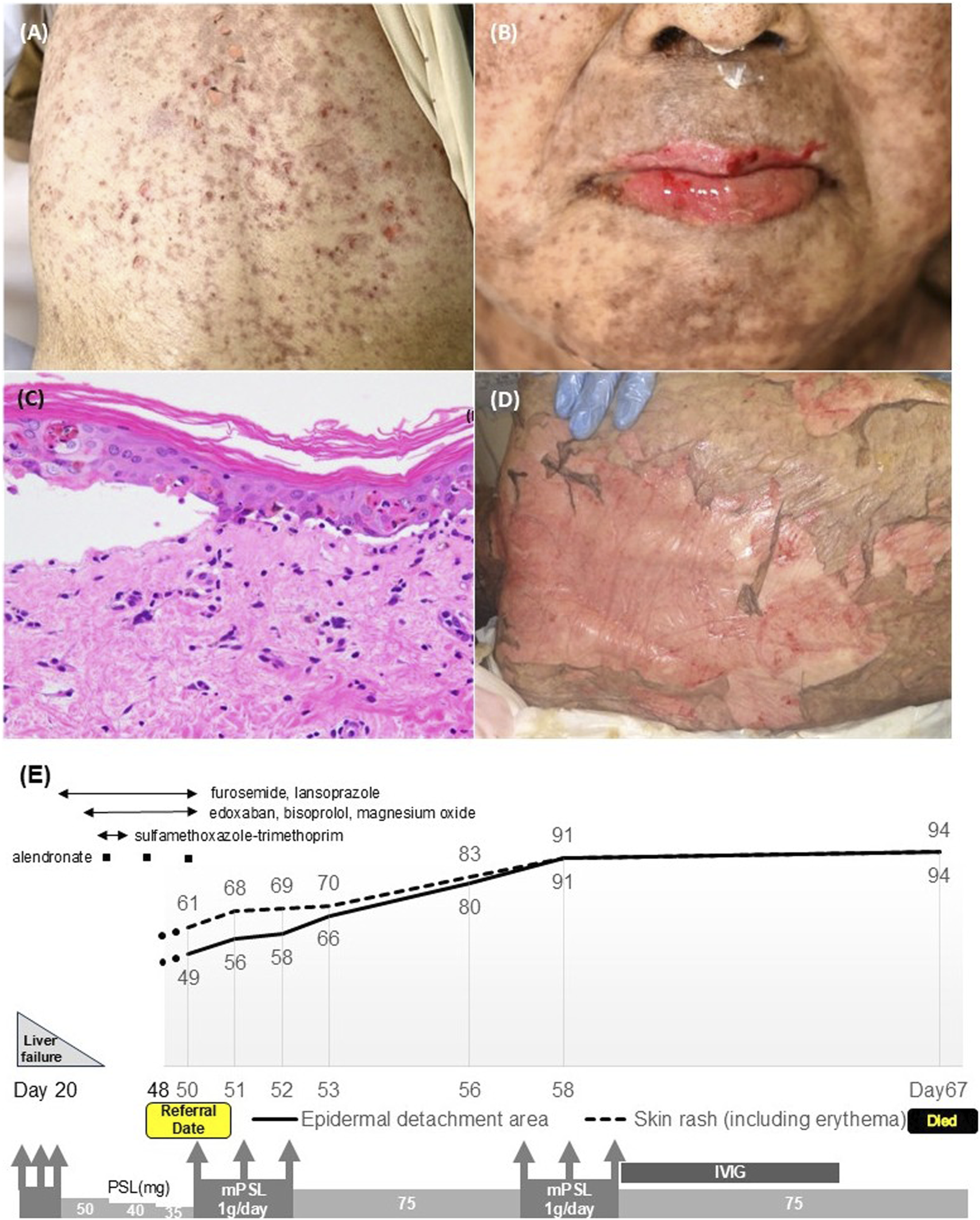
Physical examination of the ICI-associated TEN showed extensive dirty-red, dusky-red and flaccid blisters on the back skin (A) and severe erosions on the lips (B). Histopathological examination revealed extensive keratinocyte necrosis in the epidermis and mild lymphocytic inflammatory infiltration in the dermis [(C), hematoxylin and eosin staining; ×400]. Necrotic skin lesions were refractory to treatment and spread throughout the whole body (D). Clinical course of the case of ICI-associated TEN. Days refer to the day of receiving the second course of monthly nivolumab plus SOX (S-1 and oxaliplatin) therapy. The body surface area of TEN (%) is shown in the line graph (E).
Although cutaneous irAEs frequently develop during treatment with ICI, severe and fatal irAE lesions such as SJS and TEN are uncommon. In a recently published narrative review, a comprehensive search of SJS/TEN case reports associated with ICI identified only 95 cases up to 2022 [2]. In addition, it is often challenging to conclude ICIs as the causative agent in SJS/TEN cases. One of the reasons for this difficulty is the increase of ICIs’ combination therapies with chemotherapy. Among cases diagnosed as SJS/TEN, combination therapies with ICIs and anticancer agents accounted for 34% [3]. There are no reports that specifically compare the incidence of SJS/TEN among combination therapies with ICIs and anticancer agents, ICI monotherapy, and anticancer agent monotherapy. The second reason is that, unlike typical drug eruption mechanisms associated with type IV hypersensitivity, the cutaneous irAEs including ICI-associated SJS/TEN show variability in days to onset. ICI-associated SJS/TEN most often occurs within 4 weeks of ICIs initiation (51.7%); however, the onset timing ranges widely from 0 to approximately 52 weeks [2]. In our case, although it is difficult to definitely conclude ICI as the causative agent, the fact that the onset following 11 weeks after the first administration of ICI suggests that ICI could have induced TEN.
ICI-associated SJS/TEN, like those associated with other drug-induced and infectious diseases, is sometimes fatal. Of the 52 cases of ICI-associated SJS/TEN, the mortality rate for SJS was 16% and that for TEN was 47.8% [2]. The risk of severe cases of ICI-associated SJS/TEN is gradually becoming clearer. Mucosal involvement is a risk factor predicting severe cases, which was reported in 65% of ICI-related SJS/TEN cases and was significantly linked to extensive cases and fatal outcomes [3]. CRISTEN, a novel clinical risk score for general SJS/TEN, identifies older age (>65 years) as a risk factor to develop fatal cases [1]. On the other hand, a systematic review revealed that poor outcomes are related with young ages in severe ICI-associated TEN (≥30% BSA) [3]. Systemic corticosteroids remain the most used treatment for ICI-related SJS/TEN, often combined with adjunct therapies such as IVIG, cyclosporine, and TNF-α inhibitors [2]. A network meta-analysis revealed that corticosteroid/IVIG combination therapy was the only treatment with a mortality benefit compared to control for SJS/TEN [3].
In our case, extensive epidermal detachment was already present at initial presentation, likely indicating delayed treatment and contributing to the poor outcome. Although plasmapheresis was one of the key treatments for TEN, we prioritized treatment with corticosteroids and IVIG to suppress the disease course due to the severe skin erosion on the vascular access site required for plasmapheresis. As mentioned above, there is a constant risk of developing SJS/TEN, not only immediately after initiation but also at any time during ICI therapy. If skin rashes appear during ICI therapy, it is essential to refer the patient to an expert dermatologist as soon as possible. Estimation of high-risk patients, early recognition of severe cases, and standard treatment strategies are crucial for improving patient outcomes of ICI-related SJS/TEN.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Ethical approval was not required for the studies involving humans because this is the single case report, and the patient passed away. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements because this is the single case report, and the patient passed away. Written informed consent was not obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article because this is the single case report, and the patient passed away.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
References
1.
HamaNSunagaYOchiaiHKokazeAWatanabeHKurosawaMet alDevelopment and validation of a novel score to predict mortality in stevens-johnson syndrome and toxic epidermal necrolysis: CRISTEN. J Allergy Clin Immunol Pract (2023) 11(10):3161–8.e2. 10.1016/j.jaip.2023.07.001
2.
SatohTKNeulingerMMStadlerPCAokiRFrenchLE. Immune checkpoint inhibitor-induced epidermal necrolysis: a narrative review evaluating demographics, clinical features, and culprit medications. The J Dermatol (2024) 51(1):3–11. 10.1111/1346-8138.17039
3.
BrayERLinRRLiJNElgartGWElmanSAMaderalAD. Immune checkpoint inhibitor associated epidermal necrosis, beyond SJS and TEN: a review of 98 cases. Arch Dermatol Res (2024) 316(6 233):233. 10.1007/s00403-024-03061-6
Summary
Keywords
toxic epidermal necrolysis, Stevens-Johnson syndrome, immune checkpoint inhibitors, immune-related adverse events, drug eruption
Citation
Seki C, Koike Y, Higuchi M, Ikenaga E, Waseda T, Ichiki M, Ehara D, Takenaka M, Kusano K, Sonoda Y and Murota H (2025) Fatal toxic epidermal necrolysis associated with an immune checkpoint inhibitor. J. Cutan. Immunol. Allergy 8:14584. doi: 10.3389/jcia.2025.14584
Received
04 March 2025
Accepted
22 May 2025
Published
30 May 2025
Volume
8 - 2025
Updates
Copyright
© 2025 Seki, Koike, Higuchi, Ikenaga, Waseda, Ichiki, Ehara, Takenaka, Kusano, Sonoda and Murota.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuta Koike, y-koike@nagasaki-u.ac.jp
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.