Abstract
Background:
Sweat plays a crucial role in maintaining skin homeostasis but is also considered a factor that exacerbates atopic dermatitis (AD). Patients with AD exhibit reduced sweat production and altered sweat composition, with impaired sweating function and psychological anxiety being implicated. However, few clinical studies with high-level evidence have investigated the effects of dupilumab treatment on sweating function and psychological anxiety.
Methods:
Seven patients with moderate-to-severe AD who received dupilumab treatment at Nagasaki University Hospital were evaluated at baseline, week 6, and week 24. Eczema severity was assessed using the Eczema Area and Severity Index (EASI) score and the Patients’ Oriented Eczema Measure (POEM). Sweating function was evaluated using the Quantitative Sudomotor Axon Reflex Test (QSART), and psychological anxiety was assessed using the State-Trait Anxiety Inventory (STAI).
Results:
EASI scores and POEM significantly improved at weeks 6 and 24. Sweat volume assessed by QSART increased at week 6 but showed a decreasing trend in some cases at week 24. Sweat latency shortened at week 6 but was prolonged again in some cases at week 24. State and trait anxiety scores of STAI decreased at week 6 but increased in some patients at week 24. Correlation analysis showed a negative correlation between sweat latency and state anxiety at baseline and a positive correlation between EASI and trait anxiety at week 6. No significant correlations were observed at week 24.
Conclusion:
Dupilumab improves skin symptoms in patients with AD and may temporarily enhance sweating function. It also affects psychological anxiety; however, its effects are inconsistent, suggesting individual variations in long-term changes.
Introduction
Sweat plays an essential role in maintaining skin homeostasis by contributing to thermoregulation, antimicrobial defense, skin hydration, and barrier formation [1]. At the same time, sweat is also known as a factor that exacerbates atopic dermatitis (AD) [2]. Although no studies have evaluated the effectiveness of instructing AD patients to avoid sweating for symptom improvement, self-reported questionnaire surveys have shown that many patients perceive sweating as a factor that worsens their symptoms [3]. Additionally, they report that post-sweat care positively impacts symptom improvement. The mechanisms underlying sweat-induced worsening of AD symptoms include decreased sweat production, sweat leakage into extraglandular tissues, and abnormal sweat composition [4–6]. In patients with more severe AD, alterations in sweat composition—such as increased glucose concentration, decreased pH, and reduced salt concentration—have been shown to delay skin barrier recovery [5]. These findings suggest that sweat contributes to the exacerbation of AD symptoms.
Previous studies have reported that many AD patients exhibit reduced sweat production and prolonged sweat latency (the time required to initiate sweating) [4, 7]. Some cases of sweating dysfunction in AD patients improve with AD treatment, suggesting that inflammation is involved in sweating abnormalities. However, since no correlation has been observed between AD severity and the degree of sweating dysfunction, it is considered that the severity of inflammation does not directly determine sweating abnormalities. Additionally, sweat latency has been reported to be positively correlated with trait anxiety [4], indicating a possible involvement of psychological anxiety in sweating dysfunction.
Although there have been reports on the effects of dupilumab treatment on sweating function and psychological anxiety in AD patients with sweating dysfunction, none of the studies have evaluated both factors together, and the evidence level remains relatively low. In this study, we conducted an open-label interventional study to assess the effects of dupilumab treatment on sweating function (sweat volume and sweat latency) and psychological anxiety in patients with AD and report our findings.
Materials and methods
Participants and accumulated clinical data
From July 2020 to July 2021, we conducted a study at the Department of Dermatology, Nagasaki University Hospital, enrolling seven patients with moderate to severe AD who received dupilumab treatment. Evaluations and assessments were performed at baseline (before treatment initiation), 6 weeks after initiation, and 24 weeks after initiation. The severity of eczema was assessed using the Eczema Area and Severity Index (EASI) and Patients’ Oriented Eczema Measure (POEM). Sweating function was evaluated using the Quantitative Sudomotor Axon Reflex Test (QSART), while psychological anxiety was assessed using the State-Trait Anxiety Inventory (STAI). STAI is a self-administered questionnaire that evaluates two aspects of anxiety: state anxiety, which fluctuates significantly depending on situational factors, and trait anxiety, which reflects a relatively stable personality characteristic. One patient’s STAI assessment was incomplete and excluded from the analysis. This study was conducted with the approval of the Ethics Committee of Nagasaki University Hospital (Ethics Committee Approval Number: jRCT1071200016) and with informed consent obtained from all participants.
Quantitative sudomotor axon reflex test (QSART)
QSART induces sweating by delivering acetylcholine into the skin via iontophoresis, which stimulates sweat glands both directly and through activation of postganglionic sympathetic nerve fibers. Consequently, sweating dysfunction detected by QSART indicates a dysfunction of postganglionic sympathetic nerves or sweat glands [1]. In this study, QSART was performed on the forearm of all participants. Based on previous studies [4], the normal range for sweat volume in the forearm was set at ≥0.5 mg/cm2/5 min. While there is no established normal range for sweat latency, shorter latency indicates a faster response to acetylcholine, whereas longer latency suggests a delayed response.
Statistical analysis
We examined changes in skin symptoms and sweat function, anxiety on seven consenting patients. Changes from baseline in EASI score and POEM at 6 and 24 weeks, respectively, were detected using paired-samples t-tests. Correlations between evaluation parameters were examined in the Pearson correlation coefficients and P-values at 0, 6, 24 weeks respectively.
Results
Baseline characteristics of participants
Table 1 summarizes the baseline characteristics of the participants. The male-to-female ratio was 2:1, with a predominance of male participants. The mean (±SD) values for each parameter were as follows: age, 33.78 ± 8.393 years; disease duration, 29.67 ± 7.314 years; EASI score, 24.7 ± 8.777; POEM score, 16.39 ± 6.382; TARC levels, 502 ± 10,136 pg/mL; total IgE levels, 5,000 ± 10,1336 IU/mL; state anxiety, 48.56 ± 11.74; trait anxiety, 45.33 ± 9.579. Regarding the QSART assessments, the axon reflex sweat volume was 0.7017 ± 0.5675 mg/5 min/cm2, and sweat latency was 71.5 ± 54.73 s.
TABLE 1
| Case no. | Gender | Age (years) | Disease duration (year) | Topical treatmenta | EASI | POEM | TARC (pg/mL) | IgE (IU/mL) | STAI | QSART | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| State anxiety | Trait anxiety | Sweat volume (mg/cm2/5 min) | Latency (sec) | |||||||||
| 1 | Male | 34 | 30 | Delgocitinib TSC | 28.0 | 12 | >30,000 | 13,500 | 37 | 46 | 0.387 | 130 |
| 2 | Female | 45 | 40 | ― | 26.5 | 26.5 | 3,153 | 2,749.9 | 65 | 60 | 0.53 | 0 |
| 3 | Male | 31 | 32 | TCS | 21.3 | 18 | 2,189 | 7,598.1 | 54 | 42 | 0.938 | 86 |
| 4 | Male | 24 | 20 | TCS | 27.2 | 24 | CNO | CNO | 55 | 33 | 0.074 | 300b |
| 5 | Male | 42 | 30 | TCS | 22 | 13 | 1,622 | CNO | 44 | 42 | 0.132 | 69 |
| 6 | Male | 43 | 40 | TCS Tacrolimus | 19.5 | 14 | 236 | 2,242.2 | 38 | 36 | 0.416 | 164 |
| 7 | Female | 26 | 25 | TCS Tacrolimus | 45 | 20 | 987 | 2,022.7 | 62 | 60 | 1.171 | 28 |
Baseline assessments, disease severity, and evaluation results of all.
CNO: consent not obtained.
All patients used moisturizer.
300 s were set as the testing time limit, during which no apparent sweat induction was observed.
Changes in each evaluation parameter following dupilumab treatment
The EASI score showed a significant decrease at both 6 weeks and 24 weeks compared to baseline (p < 1 × 10−4, p = 3.7 × 10−4, respectively) (Figure 1A). The proportion of patients achieving EASI75 was 71% at both 6 weeks and 24 weeks (data not shown).
FIGURE 1
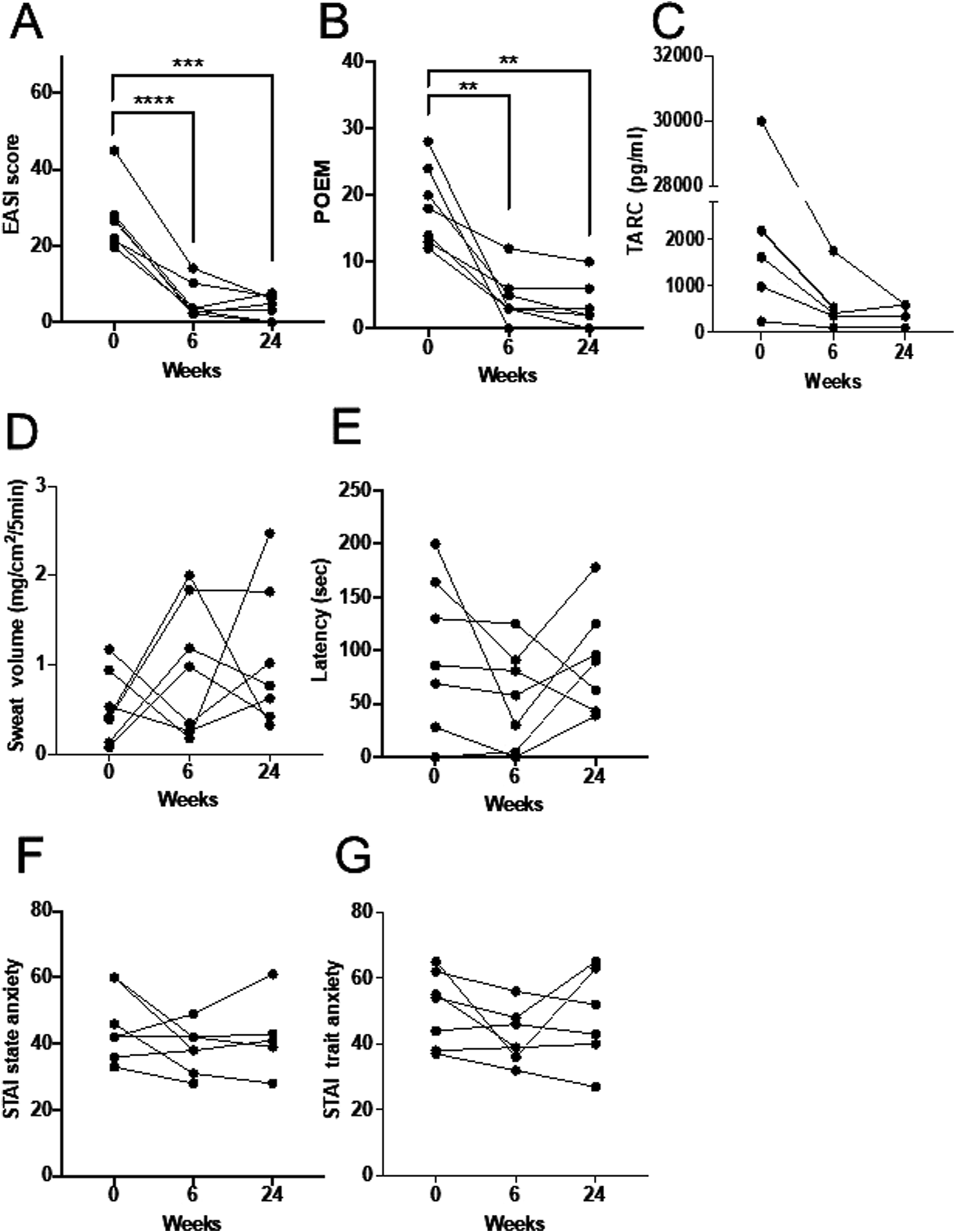
The longitudinal changes in each evaluation parameter. (A) Changes in EASI, indicating rash severity. (B) POEM. (C) TARC. (D) Changes in sweat volume measured by QSART. (E) Sweat latency measured by QSART. (F) State anxiety (STAI). (G) Trait anxiety (STAI).**p < 0.01, ***p < 0.001, ****p < 0.0001.
The POEM score also showed a significant decrease in all cases at 6 weeks and 24 weeks compared to baseline (p = 3.4 × 10−3, p = 3.0 × 10−3, respectively) (Figure 1B).
Serum TARC levels did not show a statistically significant change at either 6 weeks or 24 weeks, including cases where blood samples were not obtained due to lack of consent (Figure 1C).
Regarding sweat volume, four participants had sweat volumes below the normal threshold of 0.5 mg/cm2/5 min at baseline. All of these participants exhibited an increase in sweat volume at 6 weeks, but the values declined again at 24 weeks (Figure 1D). In contrast, the remaining three participants, whose baseline sweat volume was ≥0.5 mg/cm2/5 min, showed a temporary decrease at 6 weeks, followed by an increase at 24 weeks, returning to levels comparable to or exceeding baseline.
Regarding sweat latency, six participants exhibited shorter latency at 6 weeks; however, three of these participants showed prolonged latency again at 24 weeks (Figure 1E).
There was no consistent trend in changes in sweating function according to the season of evaluation (Supplementary Figure S1).
For STAI state anxiety, five participants showed a reduction at 6 weeks, but three of them exhibited an increase again at 24 weeks, while one case was missing (Figure 1F). STAI trait anxiety decreased in five participants at 6 weeks, but two of them showed an increase again at 24 weeks (Figure 1G).
Correlations between evaluation parameters
Table 2 presents the Pearson correlation coefficients and p-values between evaluation parameters at 0, 6, and 24 weeks.
TABLE 2
| 0 week | EASI | Sweat volume | Latency | STAI tr |
|---|---|---|---|---|
| STAI st | r = 0.6621 p = 0.0737 | r = 0.5810 p = 0.1309 | r = -0.8370 p = 0.0095 | r = 0.6077 p = 0.11 |
| STAI tr | r = 0.6238 p = 0.0984 | r = 0.3320 p = 0.4217 | r = −0.3924 p = 0.3363 | |
| Latency | r = −0.2725 p = 0.5138 | r = −0.5606 p = 0.1483 | ||
| Sweat volume | r = 0.4687 p = 0.2414 |
| 6 weeks | EASI | Sweat volume | Latency | STAI tr |
|---|---|---|---|---|
| STAI st | r = 0.6118 p = 0.1070 | r = −0.5168 p = 0.2350 | r = −0.4532 p = 0.3071 | r = 0.7015 p = 0.0525 |
| STAI tr | r = 0.8048 p = 0.016 | r = −0.5463 p = 0.2045 | r = −04532 p = 0.3071 | |
| Latency | r = −0.2738 p = 0.5525 | r = 0.7030 p = 0.078 | ||
| Sweat volume | r = −0.5600 p = 0.1911 |
| 24 weeks | EASI | Sweat volume | Latency | STAI tr |
|---|---|---|---|---|
| STAI st | r = 0.0345 p = 0.9415 | r = 0.4227 p = 0.3447 | r = −0.2165 p = 0.6410 | r = 0.8099 p = 0.0508 |
| STAI tr | r = −0.3822 p = 0.4546 | r = 0.1431 p = 0.7868 | r = −0.3106 p = 0.5491 | |
| Latency | r = −0.6006 p = 0.1154 | r = −0.6857 p = 0.0605 |
A table evaluating the correlation between various assessment values at weeks 0, 6, and 24 of treatment with dupilumab.
Light gray shading indicates a statistical trend, while dark gray shading and bold values represents a statistically significant difference. r denotes the Pearson correlation coefficient. STAI tr: STAI trait anxiety, STAI st: STAI state anxiety, EASI: eczema area and severity index, Latency: Time to sweat onset in QSART: The time (in seconds) from the start of stimulation to the onset of sweating. Sweat volume: The amount of sweat excreted per square centimeter during the 5-minute measurement period in QSART (mg/cm2/5 min).
At baseline (0 weeks), a significant negative correlation was observed between sweat latency and STAI state anxiety. A positive correlation trend was noted between STAI state anxiety and EASI score (p = 0.0737), but no other significant correlations were found.
At 6 weeks, the correlations observed at 0 weeks were no longer significant. However, a significant positive correlation was detected between EASI score and STAI trait anxiety. Additionally, a positive correlation trend was noted between STAI trait anxiety and STAI state anxiety, as well as between sweat volume and sweat latency (p = 0.0525, p = 0.016, respectively).
At 24 weeks, no statistically significant correlations were found between the evaluated parameters. However, a positive correlation trend was observed between state anxiety and trait anxiety (p = 0.0508), while sweat volume and sweat latency showed a negative correlation trend (p = 0.0605).
No evaluation parameters demonstrated a consistent correlation across all time points. After dupilumab administration, state anxiety and trait anxiety showed a positive correlation trend. Additionally, the correlation between sweat volume and latency changed over time: there was no correlation at baseline, a positive correlation at 6 weeks, and a negative correlation at 24 weeks.
Discussion
In patients with AD, changes in sweat composition and decreased sweating function have been reported [1]. Possible causes include obstruction of sweat pores by horny plugs, leakage of sweat outside the sweat glands [1], autonomic nerve dysfunction, and reduced postganglionic axon reflex response [8]. Dupilumab, a human monoclonal antibody targeting the IL-4/IL-13 receptor, has been recommended as a treatment option for moderate-to-severe AD in clinical guidelines since 2018. Mizukawa et al. [9] evaluated the sweating function of seven AD patients treated with dupilumab using a quantitative sweat measurement method and reported that, at 16 weeks, the average number of sweat droplets recovered to a level close to that of healthy controls. Furthermore, their report suggested that the improvement in sweating dysfunction did not necessarily correlate with clinical symptoms, indicating that the recovery of sweating function was not merely a result of improved skin symptoms.
In our study, skin symptoms improved in all cases by week 6 and remained improved at week 24. However, the changes in sweating volume and latency did not necessarily correlate with the changes in skin symptoms. Previous studies have reported that sweating function in AD patients improved to a level comparable to that of healthy individuals. However, our analysis did not show a consistent trend in the longitudinal changes of sweating volume and sweating latency. This discrepancy may be due to differences in the methods used for sweat induction and measurement—Mizukawa et al. assessed sweat function using skin replicas after thermally induced sweating, whereas we quantitatively measured axon reflex sweating induced by acetylcholine.
In our QSART measurements, four cases exhibited decreased sweating at baseline, with sweat volume below the reference threshold of 0.5 mg/cm2/5 min. All four cases showed an increase in sweat volume and a shortening of sweating latency at 6 weeks (Figures 1D,E). However, by week 24, sweat volume in these four cases had declined, with two cases falling below the reference threshold, and sweating latency was prolonged. Sweating latency reflects the reactivity of the postganglionic axon reflex induced by acetylcholine. These findings suggest that abnormal responsiveness to acetylcholine is a factor in the QSART-measured reduction in sweat volume in AD. Dupilumab may temporarily improve this acetylcholine responsiveness, but its effect diminishes after 6 months of treatment.
These changes were not related to the longitudinal changes in AD severity, implying that AD patients have an intrinsic abnormality in acetylcholine responsiveness, possibly due to underlying neurological dysfunction, including autonomic nervous system involvement. Our study also found a significant negative correlation between baseline state anxiety and sweating latency, and a trend toward a positive correlation between EASI and sweating latency (Table 2).
Kijima et al. [4] reported a significant positive correlation between trait anxiety and sweating latency in both lesional and non-lesional areas in AD patients, but no correlation between SCORAD and sweating latency [4]. Moreover, their study showed a positive correlation between state anxiety and sweating latency in lesional areas (r = 0.3628, p = 0.097), which contradicts our findings. This discrepancy may be attributed to differences in sample size. While our study aimed to track the longitudinal changes in sweating function under dupilumab treatment, future studies should carefully consider the impact of sample size when conducting similar investigations.
Several reports have discussed the relationship between acetylcholine and anxiety. It has been suggested that dysregulation of nicotinic acetylcholine receptors is involved in depression [10], that endogenous acetylcholine levels are elevated in patients with depression, leading to reduced nicotinic receptor activity [11], and that antimuscarinic drugs exert antidepressant effects [12]. It is also known that the skin of AD patients accumulates high concentrations of acetylcholine [13], which may contribute to the link between sweating dysfunction and anxiety.
In our clinical study, dupilumab initially improved sweating function at 6 weeks, but this improvement reverted by week 24. However, there is currently no clear scientific evidence to explain this phenomenon. Seasonal variations may have contributed to this effect. Although sweating volume and latency were analyzed by baseline season (Supplementary Figure S1), no significant trends were identified. IL-4 is a cytokine with neuroimmune activity that directly affects nerves [14]. Peripheral nerves exposed to IL-4 may experience some form of axon reflex impairment, leading to decreased sweating function. Alternatively, a study has reported that long-term dupilumab treatment results in the accumulation of IL-13 in the skin of AD patients. IL-13 receptor, which is composed of IL-13Rα1 and the IL-4 receptor α chain, has been reported to be expressed in sweat glands [15]. If dupilumab initially inhibits IL-13, leading to improved sweating function, but prolonged administration shifts the skin environment to an IL-13-dominant state, causing sweating function to decline again, IL-13 could be considered a key cytokine in sweating inhibition. Nevertheless, these hypotheses remain speculative. Now that anti-IL-13 biologics are available for clinical use, evaluating sweating function in patients treated with anti-IL-13 agents may provide answers.
The major limitations of our study include its small sample size, single-group open-label design, and missing data, which limit the statistical robustness of our interpretations. However, our study is significant in that we evaluated the effects of dupilumab on sweating function over 24 weeks, demonstrated the dissociation between clinical severity and sweating function, and revealed that sweating function exhibited unexpected temporal changes. These findings provide valuable data that can be utilized in future clinical studies assessing the recovery of sweating function in AD patients.
If the relationship between sweat function and physiological and psychological effects during dupilumab treatment can be demonstrated in future case series, it may be possible to influence clinical decision-making.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of Nagasaki University Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MM and HM conducted the design. MM carried out the experiment. MM, YK, and HM participated interpretation of the studies and analysis of the data. MM wrote the manuscript with support from YK, MT, and HM. All authors reviewed the manuscript. YK confirmed the final version of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. HM declares that this study received funding from Sanofi Corporation. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/jcia.2025.14517/full#supplementary-material
References
1.
MurotaHYamagaKOnoEKatayamaI. Sweat in the pathogenesis of atopic dermatitis. Allergol Int (2018) 67(4):455–9. 10.1016/j.alit.2018.06.003
2.
MurotaHYamagaKOnoEMurayamaNYokozekiHKatayamaI. Why does sweat lead to the development of itch in atopic dermatitis?Exp Dermatol (2019) 28(12):1416–21. 10.1111/exd.13981
3.
KanekoSMurotaHMurataSKatayamaIMoritaE. Usefulness of sweat management for patients with adult atopic dermatitis, regardless of sweat allergy: a pilot study. Biomed Res Int (2017) 2017:8746745. 10.1155/2017/8746745
4.
KijimaAMurotaHMatsuiSTakahashiAKimuraAKitabaSet alAbnormal axon reflex-mediated sweating correlates with high state of anxiety in atopic dermatitis. Allergol Int (2012) 61(3):469–73. 10.2332/allergolint.12-OA-0429
5.
OnoEMurotaHMoriYYoshiokaYNomuraYMunetsuguTet alSweat glucose and GLUT2 expression in atopic dermatitis: implication for clinical manifestation and treatment. PLoS One (2018) 13(4):e0195960. 10.1371/journal.pone.0195960
6.
YamagaKMurotaHTamuraAMiyataHOhmiMKikutaJet alClaudin-3 loss causes leakage of sweat from the sweat gland to contribute to the pathogenesis of atopic dermatitis. J Invest Dermatol (2018) 138(6):1279–87. 10.1016/j.jid.2017.11.040
7.
TakahashiAMurotaHMatsuiSKijimaAKitabaSLeeJBet alDecreased sudomotor function is involved in the formation of atopic eczema in the cubital fossa. Allergol Int (2013) 62(4):473–8. 10.2332/allergolint.13-OA-0547
8.
HendricksAJVaughnARClarkAKYosipovitchGShiVY. Sweat mechanisms and dysfunctions in atopic dermatitis. J Dermatol Sci (2018) 89(2):105–11. 10.1016/j.jdermsci.2017.11.005
9.
MizukawaYSatoYOhyamaMShioharaT. Restoration of sweating disturbance in atopic dermatitis treated with dupilumab. J Dermatol Sci (2020) 100(1):79–81. 10.1016/j.jdermsci.2020.08.007
10.
AndersonKRCaoWLeeHSCrenshawMAPalumboTBFisher-PerezEet alA novel anxiety-associated SNP identified in LYNX2 (LYPD1) is associated with decreased protein binding to nicotinic acetylcholine receptors. Front Behav Neurosci (2024) 18:1347543. 10.3389/fnbeh.2024.1347543
11.
SaricicekAEsterlisIMaloneyKH. Persistent beta2*-nicotinic acetylcholinergic receptor dysfunction in major depressive disorder. Am J Psychiatry (2012) 169(8):851–9. 10.1176/appi.ajp.2012.11101546
12.
FureyMLDrevetsWC. Antidepressant efficacy of the antimuscarinic drug scopolamine: a randomized, placebo-controlled clinical trial. Arch Gen Psychiatry (2006) 63(10):1121–9. 10.1001/archpsyc.63.10.1121
13.
WesslerIReinheimerTKilbingerHBittingerFKirkpatrickCJSalogaJet alIncreased acetylcholine levels in skin biopsies of patients with atopic dermatitis. Life Sci (2003) 72(18-19):2169–72. 10.1016/s0024-3205(03)00079-1
14.
KimBRothenbergMESunXBachertCArtisDZaheerRet alNeuroimmune interplay during type 2 inflammation: symptoms, mechanisms, and therapeutic targets in atopic diseases. J Allergy Clin Immunol (2024) 153(4):879–93. 10.1016/j.jaci.2023.08.017
15.
AkaiwaMYuBUmeshita-SuyamaRTeradaNSutoHKogaTet alLocalization of human interleukin 13 receptor in non-haematopoietic cells. Cytokine (2001) 13(2):75–84. 10.1006/cyto.2000.0814
Summary
Keywords
dupilumab, atopic dermatitis, anxiety, sweat function, QSART
Citation
Matsumoto M, Koike Y, Takenaka M and Murota H (2025) An open-label interventional clinical study evaluating sweating function in dupilumab treatment for atopic dermatitis. J. Cutan. Immunol. Allergy 8:14517. doi: 10.3389/jcia.2025.14517
Received
20 February 2025
Accepted
23 May 2025
Published
10 June 2025
Volume
8 - 2025
Updates
Copyright
© 2025 Matsumoto, Koike, Takenaka and Murota.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuta Koike, y-koike@nagasaki-u.ac.jp
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.