Abstract
Introduction:
It remains unclear which therapy contributes to atopic dermatitis (AD) remission and to what extent. We aimed to clarify which therapy contributes to the treatment of AD by investigating the time-to-remission and remission hazard ratios for each therapy using real-world data.
Methods:
This retrospective cohort study included 110 patients diagnosed with AD after their first visit to the Department of Dermatology at Fukuoka University Hospital between 2016 and 2022. The patients were categorized into six treatment groups: 1) topical treatment alone or topical treatment plus 2) ultraviolet light, 3) oral steroids, 4) oral cyclosporine, 5) dupilumab, and 6) oral Janus kinase inhibitors (JAKi). The topical therapy alone group served as the control, and the hazard ratios for remission (Investigator’s Global Assessment [IGA] 0/1) were calculated.
Results:
Forty patients achieved remission, while 70 did not (IGA ≥2) with the first treatment regimen. A multivariate Cox proportional hazards analysis adjusted for age, sex, and severity at the first visit (IGA) revealed that the hazard ratios for remission were 4.2 (95% confidence interval (C.I.): 1.28–13.83, p = 0.018) for the oral cyclosporine group, 5.05 (95% C.I.: 1.96–13, p = 0.001) for the dupilumab group, and 67.56 (95% C.I.: 12.28–371.68, p < .0001) for the oral JAKi group. The median time to remission was 3 months for JAKi, cyclosporine, and steroid was shorter than 6 months for dupilumab. No serious adverse events were observed.
Conclusion:
Oral therapy with small molecules requires a shorter duration to achieve remission. However, long-term safety and recurrence are important indicators.
Introduction
Atopic dermatitis (AD) is an inflammatory skin disease characterized by chronic eczema and pruritus, often associated with allergic diseases, such as asthma and allergic rhinitis, and is known to reduce patients’ quality of life [1, 2]. An average of 7.3% of the population is affected by AD; however, there are considerable differences in its prevalence between countries [3–6]. In Japan, the prevalence of AD in patients between 4 months and 30 years old is approximately 10% [4, 7, 8]. Severe cases of AD are also characterized by the development of erythroderma, which affects work capacity and quality of work life and decreases labor productivity [9, 10].
Recently, various new therapeutic agents have been introduced for the treatment of AD. In Japan, cyclosporine, an oral calcineurin inhibitor, was first used in 2008 for refractory AD of moderate or high severity; however, no new therapeutic agents have emerged in the following 9 years [6]. In 2017, dupilumab, an anti-interleukin (IL)-4 receptor antibody [11, 12], became available for the treatment of AD, followed by the Janus kinase (JAK) inhibitors, baricitinib in 2020 [13] and upadacitinib [14, 15] and abrocitinib [16] in 2021. Nemolizumab [17], an anti-IL-31 receptor antibody, was introduced in 2022, and anti-IL-13 and anti-OX40 antibodies will become available in the near future [18–20]. However, the abundance of new drugs makes it difficult for clinicians to decide which one to choose because there is yet to be sufficient real-world evidence regarding the effectiveness of these new systemic therapies. To clarify the effectiveness of these newly available therapeutic options, we investigated the proportion of patients and the time to achieve remission after the first systemic intervention.
Methods
Study design
This retrospective cohort study included patients younger than 65 years of age with AD who visited the Department of Dermatology, Fukuoka University Hospital, between 2016 and 2022. AD was diagnosed according to the Japanese Dermatological Association guidelines. The exclusion criteria were as follows: i) patients who had been followed up for less than 12 weeks at our hospital; ii) patients with mild symptoms (Investigator’s Global Assessment [IGA] value <2); iii) patients receiving two or more systemic therapies simultaneously (e.g., combined use of dupilumab and oral cyclosporine); and iv) patients treated in clinical trials (Figure 1).
FIGURE 1
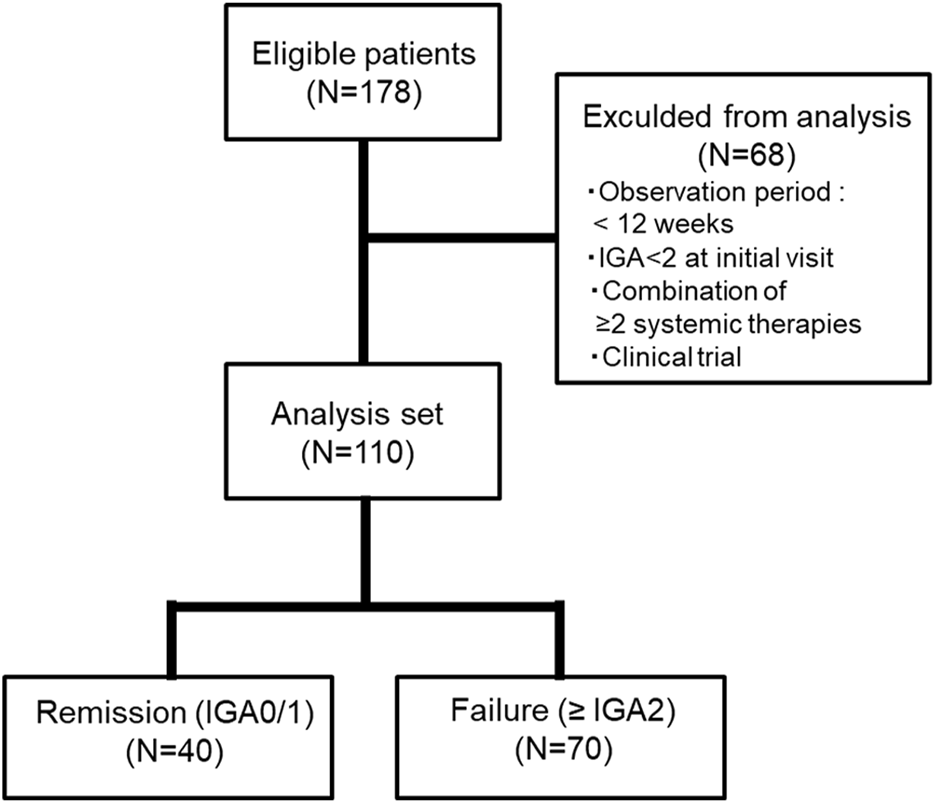
Analysis design and patient selection.
Treatment and comparison groups
The treatment initiated at the first visit for patients with AD included in this study was categorized into six groups, as follows: 1) topical treatment alone or topical treatments plus 2) ultraviolet light, 3) oral steroids, 4) oral cyclosporine, 5) dupilumab, and 6) oral JAK inhibitors. All six groups received topical treatment. For comparative analyses, group 1 served as the control.
Endpoints
Patients who experienced “remission” were those who initially had an IGA of 2 or higher and subsequently achieved an IGA of 0 or 1 (Figure 1). The endpoints of the study were the number of patients who achieved remission and the time needed to achieve remission with the first systemic treatment at our facility.
Follow-up methods
In this study, only the treatment initiated at the first visit was evaluated. If remission was achieved with the first treatment, the observation period was terminated, and the time (months) was recorded. However, if remission was not achieved, the observation period was terminated at 1) the time of switching to another treatment, 2) the time of quitting the hospital visit, or 3) the end of the observation period (31 December 2022) (Figure 2). Data on age, sex, severity of illness, treatment, duration of treatment, and serious adverse events were extracted from the medical records and tabulated.
FIGURE 2
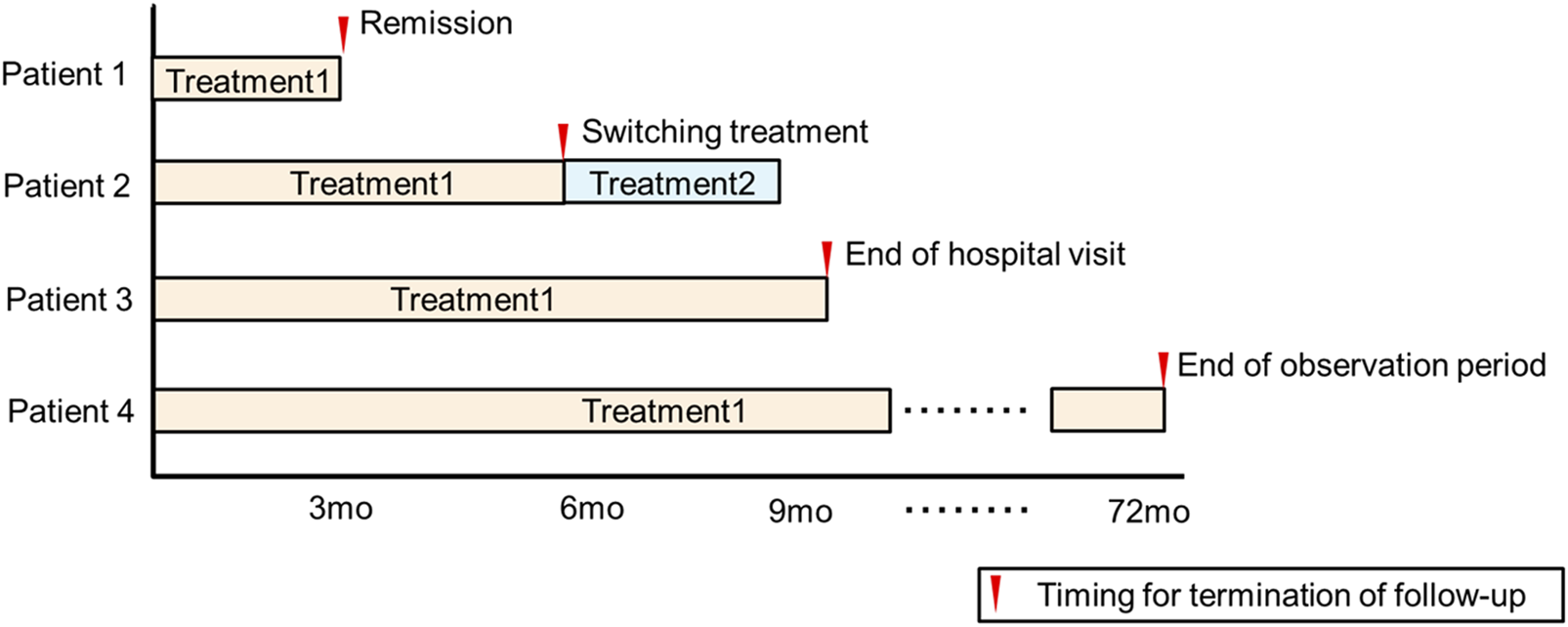
Follow-up methods for the study.
Statistical analysis
Patients’ background characteristics were compared between two groups (men versus [vs.] women, remission vs. failure) using the Mann–Whitney U test or unpaired t-test with Welch’s correction or Fisher exact test. Categorical data among the IGA groups, namely, IGA-2, IGA-3, and IGA-4, were analyzed using the chi-square test. The cumulative unsuccessful rate (IGA ≥2) of each treatment group was estimated using the Kaplan–Meier method. Differences in remission across the treatment groups were evaluated using Cox proportional hazards models, and hazard ratios, 95% confidence intervals (C.I.), and p-values are reported. In the multivariate analysis, age, sex, and the first IGA value were included in the Cox proportional hazards models. Statistical Analysis System (SAS) version 9.4 and GraphPad Prism version 5 were used to perform the statistical analysis. The significance level was set at p < 0.05.
Ethics
The study protocol was approved by the Institutional Review Board of the Fukuoka University School of Medicine (approval number: U21-08-004). This study complied with the Declaration of Helsinki and the Medical Ethics Guidelines for Research Involving Human Subjects.
Results
Patient demographic characteristics
A total of 178 first-time patients with AD were enrolled in the registry during the study period, and 110 patients (76 men and 34 women) were included in the analysis (Figure 1). The demographic characteristics of the patients are summarized in Table 1. There was no difference in the median age of men/women at their first visit (29.5 years for men and 31 years for women; p = 0.866, Mann–Whitney U test). The median severity at initial diagnosis (IGA) was slightly higher in men than in women (3.5 vs. 3.0), but the difference was not significant (p = 0.344, Mann–Whitney U test).
TABLE 1
| Total | Male | Female | p-value | |
|---|---|---|---|---|
| N | 110 | 76 | 34 | |
| Age (y) | ||||
| median | 30 | 29.5 | 31 | 0.866b |
| (Q1, Q3) | (20, 45) | (20, 46.5) | (22.5, 43.5) | |
| First IGAa | ||||
| IGA2, N | 12 | 6 | 6 | 0.316c |
| IGA3, N | 45 | 32 | 13 | |
| IGA4, N | 53 | 38 | 15 | |
| median | 3 | 3.5 | 3 | 0.344b |
| (Q1, Q3) | (3, 4) | (3, 4) | (3, 4) | |
Patient demographic data.
IGA:investigator’s Global Assessment Scale.
Mann--Whitney test.
Chi Square.
Treatments contributing to the remission of atopic dermatitis
Of 110 patients, 40 achieved remission (Figure 1). Of the patients with IGA 2 (n = 12), 7 (58.3%) achieved remission. Of those with IGA 3 (n = 45) and 4 (n = 53), 26 (57.8%) and 7 (13.2%) achieved remission, respectively.
As shown in Table 2, 38 patients received topical therapy only at the first visit, 5 received ultraviolet light therapy, and 10 received oral steroids. Twenty-seven patients were administered oral cyclosporine, 25 were administered dupilumab, and 5 were administered JAK inhibitors (baricitinib [4 mg] in 2 patients, upadacitinib [15 mg] in 2 patients, and abrocitinib [100 mg] in one patient). Remission was achieved in 10/38 (26.3%) of the patients who were administered topical therapy alone, 1/5 (20.0%) of the patients who administered ultraviolet therapy, 3/10 (30.0%) of the patients who were administered oral steroids, 6/27 (22.2%) of the patients who were administered cyclosporine, 15/25 (60.0%) of those who were administered dupilumab, and 5/5 (100.0%) of the patients who were administered JAK inhibitors. The mean times to follow-up were 14.4 months for topical therapy alone, 6.7 months for ultraviolet therapy, 8.4 months for steroids, 6.2 months for cyclosporine, 10.3 months for dupilumab, and 4.2 months for JAK inhibitors (Table 2). The crude hazard ratios for remission were 0.73 (95% C.I.: 0.11–4.96, p = 0.7433) for the ultraviolet therapy group, 1.62 (95% C.I.: 0.34–7.67, p = 0.3162) for the oral steroid group, 1.14 (95% C.I.: 0.37–3.56, p = 0.5316) for the oral cyclosporine group, 2.57 (95% C.I.: 1.08–5.92, p = 0.0326) for the dupilumab group, and 60.85 (95% C.I.: 7.86–471.4, p < .0001) for the oral JAK inhibitor group (Table 2; Supplementary Figure S1). When factors strongly influencing remission, such as age, sex, and IGA at the first visit, were adjusted for the multivariate analysis, the hazard ratios for remission were 3.83 (95% C.I.: 0.37–39.76, p = 0.26) for the UV therapy group, 4.78 (95% C.I.: 1.09–20.94, p = 0.038) for the oral steroid group, 4.2 (95% C.I.: 1.28–13.83, p = 0.018) for the oral cyclosporine group, 5.05 (95% C.I.: 1.96–13, p = 0.001) for the dupilumab group, and 67.56 (95% C.I.: 12.28–371.68, p < .0001) for the oral JAK inhibitor group (Table 2). No serious adverse events leading to treatment discontinuation were observed during the observation period.
TABLE 2
| Topical treatment only | Ultraviolet therapy | Steroid | Cyclosporine | Dupilumab | JAK inhibitor | |
|---|---|---|---|---|---|---|
| Total, N | 38 | 5 | 10 | 27 | 25 | 5 |
| Remission, N | 10 | 1 | 3 | 6 | 15 | 5 |
| Follow-up time, mean (m) | 14.4 | 6.7 | 8.4 | 6.2 | 10.3 | 4.2 |
| Median time to remission (m)a | 4.5 | 3 | 3 | 3 | 6 | 3 |
| Crude hazard ratio | 1 | 0.73 | 1.62 | 1.14 | 2.57 | 60.85 |
| (95% C.I.)b | (reference) | (0.11-4.96) | (0.34-7.67) | (0.37-3.56) | (1.08-5.92) | (7.86-471.4) |
| p-valuec | - | 0.7433 | 0.3162 | 0.5316 | 0.0326 | <.0001 |
| Adjusted hazard ratiod | 1 | 3.83 | 4.78 | 4.2 | 5.05 | 67.56 |
| (95% C.I.)b | (reference) | (0.37-39.76) | (1.09-20.94) | (1.28-13.83) | (1.96-13) | (12.28-371.68) |
| p-valuee | - | 0.2602 | 0.0377 | 0.0182 | 0.0008 | <.0001 |
Time to remission and hazard ratios following treatment.
indicates only patients who achieved remission. The follow-up time of patients who did not achieve remission is not included.
C.I.: confidence interval.
Log-rank (Mantel-Cox) Test.
Adjusted for age, sex and first IGA, value.
Cox proportional-hazards model.
Discussion
Several novel and effective treatments have recently become available for patients with moderate-to-severe AD; however, few studies have compared and evaluated real-world data. Therefore, determining the best treatment for patients is challenging.
We compared six individual treatment groups for AD to obtain evidence from our retrospective cohort. Possible factors of bias that could affect the effectiveness, such as age [21], sex, and initial severity, were adjusted. A Cox proportional hazards analysis of five systemic therapies with the topical-alone group as the control showed that the hazard ratio for remission increased for all systemic therapies (Table 2). In particular, JAK inhibitors were associated with the highest hazard ratio for remission at 67.56 (p < .0001), as all five patients in this group achieved remission. However, a very limited number of patients used it as the first treatment. Dupilumab was highly effective in 15 of 25 patients who achieved remission and was associated with a multivariate hazard ratio for remission of 5.05 (p = 0.001) (Table 2). However, the median time to remission for the dupilumab group was 6 months, longer than that for the JAK inhibitors, cyclosporine and steroid (3 months) groups. This observation suggests that dermatologists should inform patients that steady treatment with dupilumab will help achieve remission; however, it takes longer than treatment with small molecules. This advice may help the patients maintain their motivation for treatment.
Many patients with AD are referred to our outpatient center because they are refractory to treatment at their primary dermatology clinic. We supported and educated these patients upon admission and instructed them on regular topical treatments [22]. Although regular topical treatment remains essential, our results illustrate that topical treatment alone is not sufficient to improve the condition, at least in certain populations. Therefore, the appropriate choice of systemic therapy is important.
Only five patients were treated with ultraviolet light therapy, and only one achieved remission. The hazard ratio for remission increased to 3.83, but this difference was not significant (p = 0.26). Oral steroids significantly increased the hazard ratio to 4.78 (p = 0.038); however, long-term management of AD with oral steroids is generally not recommended because of various side effects. Hence, oral steroids should be limited to a short period of treatment [4, 6, 23–25]. Dupilumab blocks IL-4 and IL-13 signaling and has been used for patients with an inadequate response to conventional therapy for both remission induction and maintenance [26–28]. It can cause [5, 11, 29] allergic conjunctivitis as an adverse reaction, but this is usually mild [12, 30, 31]. Newly developed JAK inhibitors, such as baricitinib, upadacitinib, and abrocitinib, are also used for moderate-to-severe or refractory AD [13, 14, 32]. We found that these JAK inhibitors induced AD remission in a shorter period than dupilumab (Table 2). The efficacy of JAK inhibitors is dose-dependent, and higher doses can provide better remission rates in refractory patients [33, 34]. However, JAK inhibitors often cause skin infections, such as acne and herpes viruses’ reactivation [13, 15, 16, 35]. A study of 112 Japanese moderate to severe AD patients (aged 12 years and older) reported that when treated with upadacitinib, herpes zoster (HZ) was more likely to develop in patients who had a history of HZ than those without a history of HZ. This result may be a class effect of JAK inhibitors, and attention to patients, especially with a history of HZ, may urge them earlier visits for treatment [35]. Furthermore, neutropenia, anemia, liver dysfunction, and renal dysfunction may rarely occur; therefore, regular blood tests are needed [33, 34, 36, 37]. The safety of JAK inhibitors may depend on the disease and age, but major adverse cardiovascular events (MACE), serious infections, malignancy, and thrombosis can occur in patients with rheumatoid arthritis (RA) [38, 39]. Although patients with RA are usually older than those with AD and have immunosuppressive conditions, the safety of JAK inhibitors in patients with AD should also be monitored in the long term. Long-term administration of cyclosporine has been reported to increase the risk of renal damage, malignancy [40, 41], and MACE [42]. We found that JAK inhibitors and cyclosporine have faster action; however, the risks of long-term administration should also be considered when choosing a therapeutic modality. In addition, our study did not assess the duration of remission or frequency of relapse. The Japanese Dermatological Association guidelines for AD do not recommend using JAK inhibitors for maintenance [6]. The total usefulness of the treatment should be determined on the basis of both long-term efficacy and safety; therefore, further studies are warranted.
This study had some limitations. Firstly, it was conducted at a single institution, and the number of patients with AD was small. In particular, the number of patients who received JAK inhibitors, ultraviolet light therapy, and oral steroids as first-line treatment was limited. Secondly, the doses of cyclosporine and oral steroids were usually tapered, which may have affected our results. Finally, this study only assessed the time until remission with the initial treatment. AD waxes and wanes; therefore, a separate study regarding how long the remission lasts and how often the patient experiences a relapse is needed. Further studies are also required to determine the most beneficial treatments for these patients. We are planning to define recurrence in real-world clinical practice and assess recurrence in our facility in a future study. Additionally, we will perform a multicenter prospective study to further validate the results of systemic therapy in real-world clinical practice.
In conclusion, modern systemic therapies can remit AD in relatively short durations; however, biologics and small molecules exhibit different characteristics. Safety and recurrence should also be considered in the long term.
Statements
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Institutional Review Board of the Fukuoka University School of Medicine (approval number: U21-08-004). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
Conceptualization: ES and SI; methodology: HA; formal analysis: ES and HA; investigation: ES, KI, and MI; supervision: HA and SI; writing: ES; writing–review and editing: ES, KI, HA, and SI. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was partly supported by grants from the Program for Research Activities for Women Researchers at Fukuoka University (to ES). The funding body was not involved in the study design, collection, analysis, and interpretation of data, writing the report, or the decision to publish the article.
Acknowledgments
We thank the medical staff involved in the treatment of patients with atopic dermatitis at the Department of Dermatology, Fukuoka University Hospital. We also thank Editage (Cactus Communications Inc.) for editing a draft of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/jcia.2024.12974/full#supplementary-material
SUPPLEMENTARY FIGURE S1Kaplan–Meier curve of remission following treatment.
Abbreviations
AD, atopic dermatitis; IGA, Investigator’s Global Assessment; JAKi, janus kinase inhibitors; MACE, major adverse cardiovascular events; RA, rheumatoid arthritis.
References
1.
BeckLACorkMJAmagaiMDe BenedettoAKabashimaKHamiltonJDet alType 2 inflammation contributes to skin barrier dysfunction in atopic dermatitis. JID Innov (2022) 2(5):100131. 10.1016/j.xjidi.2022.100131
2.
MorizaneSSunagawaKNomuraHOuchidaM. Aberrant serine protease activities in atopic dermatitis. J Dermatol Sci (2022) 107(1):2–7. 10.1016/j.jdermsci.2022.06.004
3.
WilliamsHRobertsonCStewartAAit-KhaledNAnabwaniGAndersonRet alWorldwide variations in the prevalence of symptoms of atopic eczema in the international study of asthma and allergies in childhood. J Allergy Clin Immunol (1999) 103(1 Pt 1):125–38. 10.1016/s0091-6749(99)70536-1
4.
KatohNOhyaYIkedaMEbiharaTKatayamaISaekiHet alClinical practice guidelines for the management of atopic dermatitis 2018. J Dermatol (2019) 46(12):1053–101. 10.1111/1346-8138.15090
5.
BeckLAThaciDDeleuranMBlauveltABissonnetteRde Bruin-WellerMet alDupilumab provides favorable safety and sustained efficacy for up to 3 years in an open-label study of adults with moderate-to-severe atopic dermatitis. Am J Clin Dermatol (2020) 21(4):567–77. 10.1007/s40257-020-00527-x
6.
SaekiHOhyaYFurutaJArakawaKIchiyamaSKatsunumaTet alJapanese guidelines for the management of atopic dermatitis 2021. Jpn J Dermatol (2021) 131(13):2691–777. 10.14924/dermatol.131.2691
7.
SaekiHOisoNHonmaMOdajimaHIizukaHKawadaAet alComparison of prevalence of atopic dermatitis in Japanese elementary schoolchildren between 2001/2002 and 2007/2008. J Dermatol (2009) 36(9):512–4. 10.1111/j.1346-8138.2009.00687.x
8.
KatohNHiranoSKishimotoS. Prognostic factor of adult patients with atopic dermatitis. J Dermatol (2008) 35(8):477–83. 10.1111/j.1346-8138.2008.00507.x
9.
NakaharaTFujitaHArimaKTaguchiYMotoyamaSFurueMet alPerception gap between patients and physicians regarding disease burdenand treatment satisfactionin atopic dermatitis: findings from an on-line survey. Jpn J Dermatol (2018) 128(13):2843–55. 10.14924/dermatol.128.2843
10.
BosmaALOuwerkerkWGunalMHyseniAMArentsBWMGerbensLAAet alWork ability and quality of working life in atopic dermatitis patients treated with dupilumab. J Dermatol (2021) 48(9):1305–14. 10.1111/1346-8138.15939
11.
SimpsonELBieberTGuttman-YasskyEBeckLABlauveltACorkMJet alTwo phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med (2016) 375(24):2335–48. 10.1056/NEJMoa1610020
12.
SimpsonELAkinladeBArdeleanuM. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med (2017) 376(11):1090–1. 10.1056/NEJMc1700366
13.
ReichKKabashimaKPerisKSilverbergJIEichenfieldLFBieberTet alEfficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol (2020) 156(12):1333–43. 10.1001/jamadermatol.2020.3260
14.
Guttman-YasskyETeixeiraHDSimpsonELPappKAPanganALBlauveltAet alOnce-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure up 1 and Measure up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet (2021) 397(10290):2151–68. 10.1016/S0140-6736(21)00588-2
15.
ReichKTeixeiraHDde Bruin-WellerMBieberTSoongWKabashimaKet alSafety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (2021) 397(10290):2169–81. 10.1016/S0140-6736(21)00589-4
16.
SimpsonELSinclairRFormanSWollenbergAAschoffRCorkMet alEfficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet (2020) 396(10246):255–66. 10.1016/S0140-6736(20)30732-7
17.
KabashimaKMatsumuraTKomazakiHKawashimaMNemolizumabJPSG. Trial of nemolizumab and topical agents for atopic dermatitis with pruritus. N Engl J Med (2020) 383(2):141–50. 10.1056/NEJMoa1917006
18.
WollenbergABlauveltAGuttman-YasskyEWormMLyndeCLacourJPet alTralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol (2021) 184(3):437–49. 10.1111/bjd.19574
19.
LytvynYGooderhamM. Targeting interleukin 13 for the treatment of atopic dermatitis. Pharmaceutics (2023) 15(2):568. 10.3390/pharmaceutics15020568
20.
Guttman-YasskyESimpsonELReichKKabashimaKIgawaKSuzukiTet alAn anti-OX40 antibody to treat moderate-to-severe atopic dermatitis: a multicentre, double-blind, placebo-controlled phase 2b study. Lancet (2023) 401(10372):204–14. 10.1016/S0140-6736(22)02037-2
21.
KimJPChaoLXSimpsonELSilverbergJI. Persistence of atopic dermatitis (AD): a systematic review and meta-analysis. J Am Acad Dermatol (2016) 75(4):681–7. 10.1016/j.jaad.2016.05.028
22.
KanekoSSumikawaYDekioIMoritaEKakamuT. Questionnaire-based study on the skill of medical personnel with regard to providing directives to outpatients with atopic dermatitis. Nishinihon J Dermatol (2011) 73(6):614–8. 10.2336/nishinihonhifu.73.614
23.
KulthananKTuchindaPNitiyaromRChunharasAChantaphakulHAunhachokeKet alClinical practice guidelines for the diagnosis and management of atopic dermatitis. Asian Pac J Allergy Immunol (2021) 39(3):145–55. 10.12932/AP-010221-1050
24.
SaekiHNakaharaTTanakaAKabashimaKSugayaMMurotaHet alClinical practice guidelines for the management of atopic dermatitis 2016. J Dermatol (2016) 43(10):1117–45. 10.1111/1346-8138.13392
25.
KatayamaIAiharaMOhyaYSaekiHShimojoNShojiSet alJapanese guidelines for atopic dermatitis 2017. Allergol Int (2017) 66(2):230–47. 10.1016/j.alit.2016.12.003
26.
MiyanoKTsunemiY. Current treatments for atopic dermatitis in Japan. J Dermatol (2021) 48(2):140–51. 10.1111/1346-8138.15730
27.
ThibodeauxQSmithMPLyKBeckKLiaoWBhutaniT. A review of dupilumab in the treatment of atopic diseases. Hum Vaccin Immunother (2019) 15(9):2129–39. 10.1080/21645515.2019.1582403
28.
KatoAKamataMItoMUchidaHNagataMFukayaSet alHigher baseline serum lactate dehydrogenase level is associated with poor effectiveness of dupilumab in the long term in patients with atopic dermatitis. J Dermatol (2020) 47(9):1013–9. 10.1111/1346-8138.15464
29.
GriffithsCde Bruin-WellerMDeleuranMFargnoliMCStaumont-SalleDHongCHet alDupilumab in adults with moderate-to-severe atopic dermatitis and prior use of systemic non-steroidal immunosuppressants: analysis of four phase 3 trials. Dermatol Ther (Heidelb) (2021) 11(4):1357–72. 10.1007/s13555-021-00558-0
30.
SibbaldC. Long-term safety of dupilumab in children. Br J Dermatol (2021) 184(5):792–3. 10.1111/bjd.19613
31.
PappKAHongCHLansangMPTurchinIAdamDNBeeckerJRet alPractical management of patients with atopic dermatitis on dupilumab. Dermatol Ther (Heidelb) (2021) 11(5):1805–28. 10.1007/s13555-021-00586-w
32.
FerreiraSGuttman-YasskyETorresT. Selective jak1 inhibitors for the treatment of atopic dermatitis: focus on upadacitinib and abrocitinib. Am J Clin Dermatol (2020) 21(6):783–98. 10.1007/s40257-020-00548-6
33.
BieberTSimpsonELSilverbergJIThaciDPaulCPinkAEet alAbrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med (2021) 384(12):1101–12. 10.1056/NEJMoa2019380
34.
BlauveltATeixeiraHDSimpsonELCostanzoADe Bruin-WellerMBarbarotSet alEfficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol (2021) 157(9):1047–55. 10.1001/jamadermatol.2021.3023
35.
HaginoTSaekiHFujimotoEKandaN. Background factors predicting the occurrence of herpes zoster in atopic dermatitis patients treated with upadacitinib. J Dermatol (2023) 50(10):1301–12. 10.1111/1346-8138.16879
36.
BurmesterGRWinthropKBlancoRNashPGoupillePAzevedoVFet alSafety profile of upadacitinib up to 3 years in psoriatic arthritis: an integrated analysis of two pivotal phase 3 trials. Rheumatol Ther (2022) 9(2):521–39. 10.1007/s40744-021-00410-z
37.
DeodharAVan den BoschFPoddubnyyDMaksymowychWPvan der HeijdeDKimTHet alUpadacitinib for the treatment of active non-radiographic axial spondyloarthritis (SELECT-AXIS 2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (2022) 400(10349):369–79. 10.1016/S0140-6736(22)01212-0
38.
YtterbergSRBhattDLMikulsTRKochGGFleischmannRRivasJLet alCardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med (2022) 386(4):316–26. 10.1056/NEJMoa2109927
39.
WollenhauptJLeeEBCurtisJRSilverfieldJTerryKSomaKet alSafety and efficacy of tofacitinib for up to 9.5 years in the treatment of rheumatoid arthritis: final results of a global, open-label, long-term extension study. Arthritis Res Ther (2019) 21(1):89. 10.1186/s13075-019-1866-2
40.
PatelRVClarkLNLebwohlMWeinbergJM. Treatments for psoriasis and the risk of malignancy. J Am Acad Dermatol (2009) 60(6):1001–17. 10.1016/j.jaad.2008.12.031
41.
PaulCFHoVCMcGeownCChristophersESchmidtmannBGuillaumeJCet alRisk of malignancies in psoriasis patients treated with cyclosporine: a 5 y cohort study. J Invest Dermatol (2003) 120(2):211–6. 10.1046/j.1523-1747.2003.12040.x
42.
ChenYJLiuSCLaiKLTangKTLinCHChenYMet alFactors associated with risk of major adverse cardiovascular events in patients with rheumatoid arthritis: a nationwide, population-based, case-control study. Ther Adv Musculoskelet Dis (2021) 13:1759720X211030809. 10.1177/1759720X211030809
Summary
Keywords
cyclosporine, atopic dermatitis, dupilumab, JAK inhibitor, steroid
Citation
Sato E, Arima H, Ito K, Iwata M and Imafuku S (2024) Comparative effectiveness of treatments on time to remission in atopic dermatitis: real-world insights. J. Cutan. Immunol. Allergy 7:12974. doi: 10.3389/jcia.2024.12974
Received
12 March 2024
Accepted
29 May 2024
Published
10 June 2024
Volume
7 - 2024
Updates
Copyright
© 2024 Sato, Arima, Ito, Iwata and Imafuku.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emi Sato, emsato@fukuoka-u.ac.jp
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.