Abstract
During pregnancy, the fetal brain undergoes rapid development and is highly sensitive to environmental influences. Understanding the intricate processes that underlie fetal brain development will be critical for advancing maternal-fetal health and mitigating the risks associated with developmental brain disorders. Nonhuman primate (NHP) animal models provide a unique and highly translational platform for studying brain development during pregnancy due to the close anatomical, physiological, and behavioral resemblance of these animals to humans. Our review explores the use of NHP models in elucidating key milestones of prenatal brain maturation and the mechanisms that govern typical and atypical development. We further examine the impact of environmental insults on fetal brain development, including air pollution, infection, ionizing radiation, and exposure to toxicants, and highlight the ways in which these factors can disrupt brain development and neural circuitry, leading to long-term cognitive and behavioral deficits. Recent studies demonstrate that the baboon (Papio hamadryas) animal model provides a fruitful yet underused translational model for research related to environmental adverse effects on pregnancy. Lastly, we review the effects of drugs of abuse on the developing fetal brain, highlighting the underlying biological mechanisms identified through clinical and laboratory studies. A combined approach offers a comprehensive understanding of the vulnerabilities of the developing nervous system, informing new strategies for the treatment and prevention of neurodevelopmental disorders.
Introduction to non-human primate animal model and use of non-human primates in neurodevelopment research
A healthy pregnancy supports proper brain development and reduces the risk of neurodevelopmental disorders [1]. The similarities of pregnancy in non-human primates (NHPs) and humans make these animals valuable models for studying human pregnancy and fetal development [2, 3]. Both species experience a similar duration of gestation, with close physiological and hormonal parallels, such as the presence of a placenta that supports fetal growth and the regulation of maternal immune responses to prevent rejection of the fetus [2]. NHPs also undergo similar stages of embryonic and fetal development, including the formation of key brain structures and organ systems (Figure 1A) [3–5].
FIGURE 1
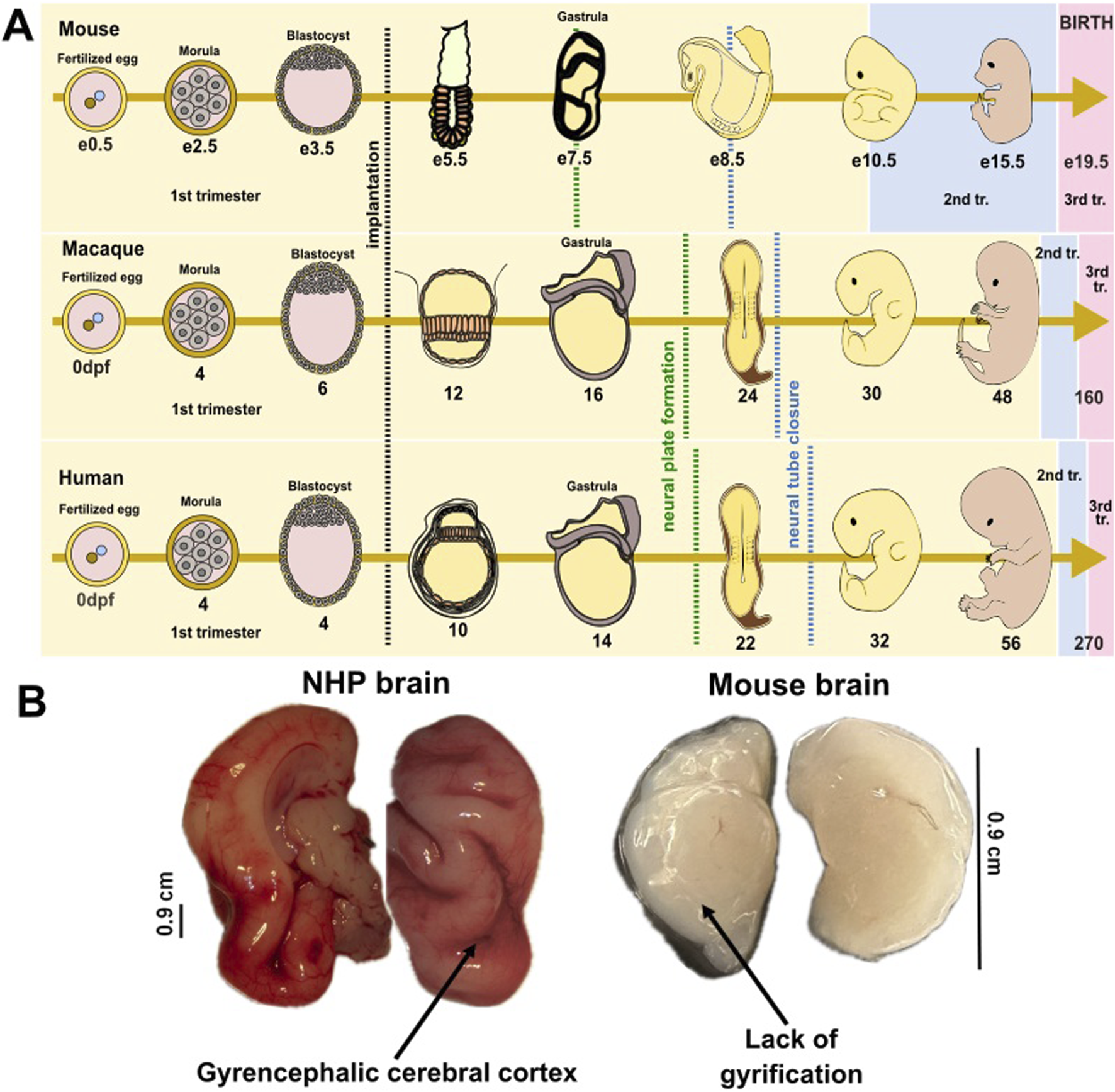
Embryonic and fetal development in mammalian species. (A) Schematic illustration of the embryonic development from fertilized egg to morula, blastocyst, and embryo in mice, non-human primates, and humans. While development milestones have not been detailed for baboons, macaque development is well-studied. Crucial developmental processes occur in a similar manner and timing between humans and primates, but the brain’s developmental trajectories show considerable differences between humans and rodents. The black dotted line depicts the period of implantation; the green dotted line depicts the period of neural tube formation; the blue dotted line depicts the period of neural tube closure; e- embryonic day (e0.5 refers to morning after mating, when vaginal plug was detected in mice); dpf- day post-fertilization (refers to number of days since the egg was fertilized). Trimesters of pregnancy depicted by colors: yellow-1st trimester; blue-2nd trimester; pink-3rd trimester. (B) Comparative image of fetal brain collected from a near-term (third trimester-equivalent of human pregnancy) NHP (baboon) and from a postnatal day 8 (third trimester-equivalent of human pregnancy) mouse.
The 1st, 2nd, and 3rd trimesters across species are shown in Figure 1 using colors (yellow, blue, pink, respectively) [6, 7]. Like humans, NHPs can be affected by environmental factors, infections, or stressors during pregnancy, providing insights into how these exposures influence fetal health and neurodevelopment [2, 4, 8]. These shared characteristics make non-human primates a critical model for investigating human pregnancy-related conditions, fetal development, and the impact of maternal health on offspring outcomes.
NHP have many similarities in developmental processes, physiology, neuroanatomy, reproduction, cognition, and social complexity with humans. [9–11]. As one of the species most closely related to humans, the baboon offers an excellent opportunity for comparative studies of neuronal maturation [12].
Baboons are estimated to share approximately 92% of their genomic sequence with humans [13, 14]. This genetic closeness might determine the similarities between the vascular and neuroanatomical patterns observed in baboon and human populations [15]. Research suggests that baboons exhibit a prolonged infancy and juvenile period, a long lifespan, and complex social behaviour, all of these parallel those of humans, thus rendering these animals ideal for the investigation of cerebral development and associated neurodevelopmental and psychiatric conditions [16]. Baboons and humans share a brain structural organization, including gyrencephalic brain structure, which is indicative of complex neural functions evolved in larger-brained primates [17].This anatomical feature is further illustrated in Figure 1B. Images presented in this figure compare the brains of a baboon and a mouse at similar developmental time-points (third trimester-equivalent of human pregnancy). Images highlight the presence of cortical gyrification in the baboon brain and the absence of such folding in the lissencephalic (smooth) mouse brain.
Brain imaging studies highlight that baboons have a high heritability of brain volume and cortical surface area and display a developmental trajectory in the corpus callosum that closely mirrors that of humans [16, 18] Additionally, both species exhibit similar ratios of grey matter to white matter, which is significant for understanding cognitive processes and potential neurodevelopmental disorders [13] (pre-print) [19, 20]. Importantly, the development of the central nervous system in both species has several similarities. In particular, maturation of the white and grey matter, including gyral formation, myelination, and cortical laminar development, shares strong temporary and structural similarities in baboon and human brains [21]. Baboons are highly suitable for neuroimaging studies due to their large brain size, the highest cerebral gyrification index among common monkeys used in laboratory studies, and the presence of all primary cortical structures homologous to those in humans. [22]. Changes in baboon corpus callosum throughout prenatal (fetal) and postnatal development parallel findings in human neurodevelopment, thereby underscoring the promise of baboons in preclinical models focusing on neurodevelopmental disorders [16]. Baboons and humans also share several key similarities in prenatal development of the cerebellum. Particularly, pronounced similarity in the increase in the thickness of the molecular layer of the cerebellum during the late gestational and early postnatal periods indicate comparable structural and functional development of cerebellar Purkinje cells in the cerebella of both human and baboon [23]. The developing cerebellum undergoes rapid and highly coordinated growth, making it especially vulnerable to various environmental factors affecting its numerous cellular processes.
The external granule cell layer (EGL) is a crucial area of cerebellar development, which, together with the rostral rhombic lip, gives rise to cerebellar granule neurons exclusively. Due to the vital role of the EGL in the differentiation and further migration of granule cell progenitors, changes in cell types may be one of the important mechanisms by which environmental insults induce cognitive and motor impairments [24, 25]. Developing EGL is composed of granule cell progenitors that express Pax6. Proliferating granule cell progenitors express Ki67. After initiating differentiation, they move to the inner EGL and express Tag1. All these cell types are crucial for estimating the development of the EGL in the cerebellum because they represent different cellular states and processes (Figure 2). The abundance of Ki67+ granule cell precursors in the EGL is critical for the production of granule neurons, responsible for motor control, motor learning, and potentially cognitive functions. [24, 25, 28]. The thickness of the Tag1+ layer shows the rate of early neuronal differentiation, indicating the cellular transition from proliferative precursors to migrating neurons. Pax6, Ki67, and Tag1 are markers for granule cell precursors, cell proliferation, and cell differentiation, respectively. Thus, quantification of these cell types may help to identify developmental abnormalities in the cerebellum caused by perinatal environmental insults.
FIGURE 2
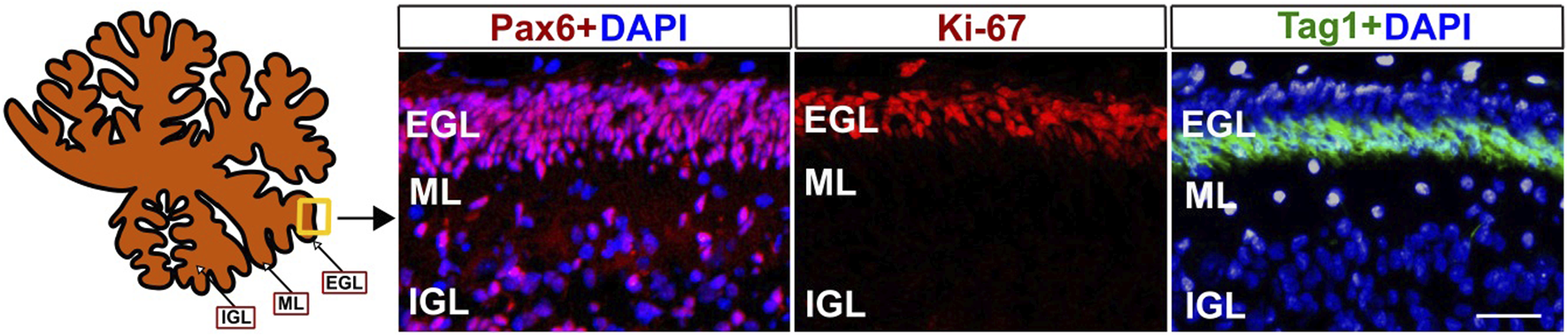
Markers of developing external granular layer (EGL) in baboon cerebellum (120 days of fetal development). Pax6 antibodies mark the progenitors of glutamatergic granule neurons, Ki-67 antibodies mark proliferating progenitor cells, and Tag1+ corresponds to differentiated cells in the inner EGL. Fixation, sectioning, and staining of the Baboon cerebella sections were performed as previously described [24, 26, 27]. Pax6, Ki67, and Taq1 antibodies were used as described in our previous papers [25, 27]. ML- Molecular layer, IGL- internal granular layer. DAPI was used to label cell nuclei. Scale bar - 25 µm.
In the developing cerebral cortex, human and non-human primates share an expanded subventricular zone (SVZ) relative to mice. Histological analysis demonstrated that the macaque rhombic lip shows significant similarities with the human rhombic lip, at least at early developmental stages [29]. Developing baboon and human brains exhibit a striking similarity in the temporal decrease in anisotropy relative to other neurodevelopmental stages such as cortical folding and white matter myelination [21]. Since several steps of cerebellar development occur during gestation in humans and baboons, as opposed to postnatally in rodents, the baboon model is particularly important for the study of neurodevelopmental disorders [23]. Particularly, the mechanisms of development of prenatal injury in baboons and humans, such as hippocampal atrophy, loss of cortical grey matter, and particular sensitivity of the subiculum of the hippocampus are highly similar [21].
In cerebrovascular system, both humans and baboons exhibit similarities in cerebrovascular morphology and responses to various physiological stimuli. Structurally, major cerebral arteries (anterior, middle, posterior and basilar) are already distinguishable in baboon fetuses by gestational day 120, corresponding to the end of the second trimester in humans (Figure 3A). One crucial aspect of cerebrovascular function is the development of myogenic tone. By gestational day 165, equivalent to the third trimester of human pregnancy, baboon fetal cerebral arteries are capable of exhibiting pressure-induced constriction in response to an elevated intraluminal pressure of 30 mmHg (Figure 3B). This indicates presence of a myogenic tone. Moreover, these cerebral arteries also respond robustly to depolarizing stimuli, such as 60 mM KCl, at both the second and third trimester-equivalents of human pregnancy (Figure 3C). Therefore, smooth muscle contractility is also being established during fetal baboon development. Together, these findings show that both the structural formation and functional regulation of cerebral arteries in baboons closely parallel those of humans.
FIGURE 3
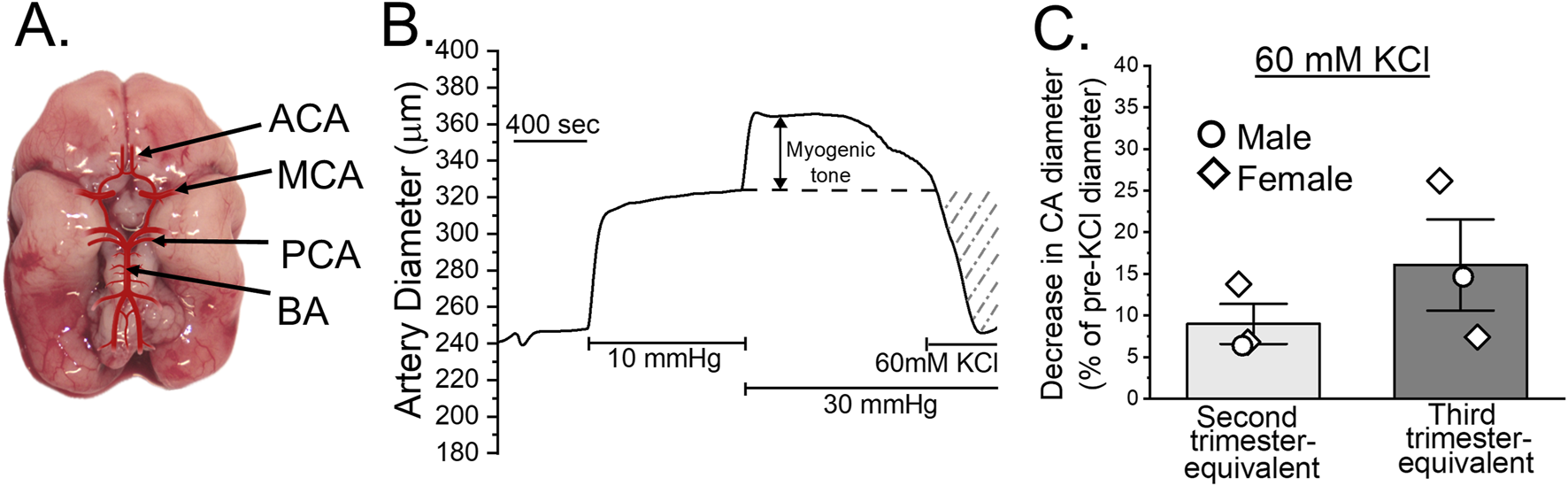
Cerebral arteries in the developing baboon brain. (A) Diagram of a fetal baboon brain at gestational day (GD) 120, illustrating the four major cerebral arteries: anterior cerebral artery (ACA), middle cerebral artery (MCA), posterior cerebral artery (PCA), and basilar artery (BA). (B) Representative trace showing changes in cerebral artery diameter over time. The artery was dissected out from a male fetus at GD 165, cannulated at both ends and pressurized in an ex vivo system. Following a 10-min incubation at 10 mmHg, pressure was increased to 30 mmHg and maintained to assess the development of myogenic tone, as described [30]. (C) KCl-induced constriction of fetal baboon cerebral arteries. The y-axis represents the percent change in diameter following exposure to 60 mM KCl compared to a diameter immediately prior to KCl application. Scatter box plots compare arterial responses between baboon fetal arteries collected at the end of the second versus third trimester-equivalent time-points. Circles denote arteries collected from male fetuses; diamonds denote arteries from female fetuses.
The mechanisms of brain cooling, regulation of blood flow, and adaptation to environmental changes also appear to be analogous in the two species, suggesting a conserved evolutionary pathway for maintaining brain health under duress [31, 32]. Both species possess a comparable vascular architecture, primarily relying on the internal carotid and vertebrobasilar arteries to supply blood to the brain [31, 33]. The anatomy of the carotid system artery, particularly of the internal carotid artery and thoracic aortic arch, and the degree of microvascular collaterals are similar in humans and baboons [34, 35]. The composition of the neurovascular unit, which includes neurons, astrocytes, and endothelial cells, is remarkably conserved across primate species, including humans [36, 37].This unit is vital for neurovascular coupling, ensuring that blood supply meets the metabolic demands of active neurons [37]. The brains of humans and NHPs share similar vascular organization, responses to ischemic episodes, and a cellular environment that supports angiogenesis [38, 39]. The parallels in gene expression and the responsiveness of astrocytes to injury further emphasize the similarities in angiogenic responses between humans and non-human primates [40]. For example, primate-specific responses to stroke, including the roles of astrocytes in modulating macrophage infiltration, illustrate how evolutionary adaptations have shaped the angiogenic process to maintain brain integrity post-injury [39, 40]. The presence of sophisticated inflammatory responses further supports these functional similarities observed in non-human primates and humans [39]. These pathological process such as inflammation, astrocyte activation, etc., accompany many neurodevelopmental disorders [41]. It should be emphasized that the mechanisms of cerebral vascularization and cortical development in NHPs, are markedly different from those seen in rodent models. Subtleties of primate brain vasculature, such as higher vascular density and complexity, suggest a more complex integration of angiogenic signals associated with cerebral growth and functionality, which has implications not only for development but also for the understanding of cognitive capacities unique to primates, thus making primate model superior to rodent model [42]. Non-human primates (NHPs) are characterized by more advanced vascular networks at birth, supporting prolonged development of the cerebral cortex, which includes postnatal neurogenesis and cortical gyrification. In contrast, at birth, rodents exhibit less developed vasculatures that mature postnatally. These differences emphasize the value of NHP models for studying human developmental brain disorders due to their closer vascular and cortical developmental timelines [42, 43].
NHPs demonstrate considerable similarity to humans in terms of cardiovascular physiology and thrombogenic mechanisms. Specifically, baboons offer a significant advantage in research due to the wide availability of cellular markers and advanced non-invasive imaging technologies compared to other large animal models [44]. Moreover, NHP models are used to evaluate the clinical efficacy of existing drugs and other therapeutic interventions. Despite rodents being the most used animal models in biomedical research, treatments shown to be effective in rodent preclinical trials often fail in clinical trials. This may be due to underlying differences between rodents and primates [39]. Thus, the anatomical, structural, and developmental parallels between NHPs and humans reinforce the significance of using baboons in research focused on brain development, genetics, and neurodevelopmental disorders. The similarities in cerebral injury patterns observed in baboon models of preterm birth and neonatal intensive care provide critical insights relevant to human infants that emphasize the high translational relevance of baboon models in understanding the onset and progression of neurodevelopmental disorders. These models allow for a more accurate understanding of how maternal health and environmental toxins can affect fetal neurodevelopment. Environmental insults, such as exposure to pollutants, toxins, viruses, and ionizing radiation, can have devastating consequences on fetal brain development, potentially leading to long-term cognitive, emotional, and behavioral impairments. Research into these environmental factors will help to identify critical windows of vulnerability during pregnancy, offering insights into prevention and intervention strategies to safeguard fetal neurodevelopment and long-term health.
The impact of environmental insults on pregnancy outcomes: studies with non-human primates address human pathology
Pregnancy is an intricate biological state that allows the fetus to grow and develop via crucial developmental processes. The course and outcome of pregnancy, fetal growth, and development are affected by numerous environmental factors, chemical, physical, and biological. Most importantly, the intake of alcohol, tobacco, and other drugs during pregnancy negatively affects both its course and outcome, as well as fetal growth and development, especially fetal neurodevelopment [45]. Chemical environmental insults that affect fetal development include air pollution, pesticides and herbicides, and heavy metals, the physical environmental insults include radiation and excess heat, and the biological environmental insults include infectious agents, e.g., viruses, bacteria, and parasites [46, 47]. Notably, maternal intake of alcohol, tobacco, cannabinoids, opioids, and other drugs represent modifiable lifestyle factors that can severely affect pregnancy course and outcome, fetal development and growth, and especially developmental trajectory of the brain.
Chemical pathogens
Heavy metals
Exposure to heavy metals in pregnancy can lead to severe adverse outcomes, such as miscarriage, stillbirth, preterm birth, and infants being small for gestational age (SGA) due to their ability to cross the blood-placental barrier [48].
Heavy metal exposure during pregnancy is a significant public health concern due to its potential to cross the placental barrier and adversely affect both maternal and fetal health. Several heavy metals have been implicated in negative pregnancy outcomes and long-term developmental issues in offspring. Arsenic exposure has been strongly associated with miscarriage, stillbirth, infant mortality, and intrauterine growth restriction [48–52]. These outcomes are thought to result from arsenic’s ability to induce oxidative stress, disrupt endocrine function, and impair placental development. Cadmium exposure has been linked to preterm birth and SGA infants [51, 53–55]. Cadmium may interfere with nutrient transport in the placenta and contribute to oxidative damage, which impairs fetal growth and development. Lead remains a critical concern due to its well-documented effects on pregnancy and fetal health. High maternal lead levels have been associated with miscarriage, low birth weight, impaired neurodevelopment, and disrupted bone formation (impaired osteogenesis) in the fetus [53, 56–60].
Mercury exposure during pregnancy has been shown to impair neurodevelopment, particularly affecting cognitive and motor functions in children exposed in utero [61]. Methylmercury, commonly found in contaminated seafood, readily crosses the placenta and accumulates in fetal tissues. Manganese isn’t always grouped with heavy metals like lead, mercury, or cadmium, yet it is often considered a heavy metal in discussions about environmental health risks and toxic exposure [62]. Exposure to manganese in excessive concentrations can lead to impaired neurodevelopment [63]. Elevated manganese exposure has been associated with deficits in cognitive function and motor skills in early childhood [64]. Copper deficiency has been previously described in savannah baboons (Papio cynocephalus). Copper deficiency can lead to anemia and developmental abnormalities in immature baboons [65]. Importantly, studies on pregnant baboons and their fetuses conducted by Dr. Schlabritz-Loutsevitch and her colleagues analyzed 40 elements using absorption spectrophotometry across multiple biological samples, including maternal and fetal blood, hair, nails, placenta, amniotic fluid, and fetal tissues [66]. Depending on an accumulating organ of the maternal or fetal organism as well as a transport mechanism, elements were found in different concentrations between mother and fetus and between different maternal and fetal organs (including placenta), showing both positive and negative correlation. This study revealed that the amount of these elements in baboons closely mirrored those observed in late-stage human pregnancies. It emphasizes that pregnant baboons serve as a valuable model for studying both normal maternal-fetal physiology and environmental toxicology. This research advances the medical field by providing a non-human primate model that closely parallels human pregnancy, thereby facilitating a better understanding of nutrient and toxin transfer during gestation.
Anesthetic-induced developmental neurotoxicity
Due to neurodevelopmental similarities to humans, nonhuman primate models offer a valuable translational tool for studying anesthesia-induced developmental neurotoxicity. NHP models allow researchers to investigate the effects of anesthetic exposure on brain development, revealing long-term behavioral and cognitive impairments. Recent studies in primates have demonstrated that early exposure to anesthetics can lead to an increase in anxiety-like and inhibition behaviors. Moreover, histopathological analysis of NHPs’ brains revealed that exposure to isoflurane during infancy led to increased astrogliosis 2 years after the exposure, indicating chronic astrocyte activation [67]. These studies offer critical insights into the mechanisms and long-term outcomes relevant to pediatric anesthesia safety.
Pesticides and herbicides
Exposure to pesticides and herbicides in pregnancy can lead to severe adverse outcomes, such as miscarriage, stillbirth, preterm birth, and birth defects [68, 69].
Importantly, even preconception exposure to certain pesticides has been associated with an increased risk of stillbirth. Specifically, pesticides linked to stillbirth risk during the preconception period include zeta-cypermethrin, organophosphates, malathion, cyfluthrin, and carbaryl [68, 69]. Similarly, exposure to certain pesticides during the first trimester of pregnancy has also been associated with stillbirth. These include fenpropathrin, permethrin, organophosphates, acephate, and formetanate hydrochloride [68, 69]. The toxicity mechanisms of these pesticides are largely unknown; however, the metabolites of permethrin and cypermethrin, which are also shared with zeta-cypermethrin, interact with cellular estrogen receptors, affecting women’s reproductive cycles, altering cycle lengths, and impacting the overall quality of the uterine environment during pre-implantation. Exposure to herbicides (glyphosate) is associated with shortened pregnancy lengths and reduced fetal growth [68–70]. Moreover, it affects neurodevelopment, as it was demonstrated in rats [69, 71]. A prolonged exposure to pesticides of Pigtail macaques (Macaca nemestrina) during pregnancy potentially caused a significantly increased level of infant mortality (more then 50%) compared to 30% infant mortality in the pigtail macaques groups habituating in areas not affected by pesticides [72]. Moreover, prenatal pesticides of baboons and chimpanzees caused congenital deformities, including cleft palate, as well as abnormal pigmentation and lowered fertility [73].
Physical pathogens
Ionizing radiation
Radiation exposure in pregnancy can lead to severe adverse outcomes, such as miscarriage, stillbirth, growth restriction, abnormal development, especially impaired neurodevelopment, malformations, and, most importantly, mutagenesis and carcinogenesis. The risk and type of such consequences depend largely on the stage of fetal development (dpf/embryonic day/gestational day) and the radiation dose. Both embryo and fetus are most sensitive to ionizing radiation at doses greater than 0.1 Gy (Gy). Even lower acute ionizing radiation doses can adversely affect both embryonal and fetal development. Dosage 0.05–0.5 Gy at 0–2 weeks post-conception may affect implantation of the embryo, and at 2–7 weeks post-conception may slightly affect organogenesis of the embryo. At early stages of fetal development (8–15 weeks), such dosage can cause growth restriction and lowered IQ in the future. Dosage higher than 0.5 Gy may cause miscarriage at any stage of embryonal and fetal development and even neonatal death at 38th postnatal week. Moreover, there is a significantly high risk of growth restriction, severe malformations, and lowered cognitive function. Higher doses of acute ionizing radiation prenatal exposure (1–5 Gy) are considered lethal [74–78].
Despite the rarity of environmental radiation exposure, pregnant women still frequently encounter various forms of ionizing (x-rays, computed tomography) and non-ionizing (ultrasound, magnetic resonance imaging) exposures in the form of medical imaging. Some medical procedures and imaging techniques exposing patients to low doses of radiation are generally considered safe. Yet, special considerations still must be made for their use in pregnancy to ensure the optimal development of the fetus and maternal health [79, 80]. The baboon animal model has been used to investigate biomarkers associated with radiation exposure [81]. The authors aimed to identify biomarkers that distinguish total-body irradiation and partial-body irradiation. Interestingly, the key biomarkers found included aspartate aminotransferase, LDH, urea, Flt3-ligand, iron, creatine kinase, absolute neutrophil count and neutrophil-to-lymphocyte ratio for the early period after the radiation exposure, C-reactive protein, and Flt3-ligand, platelet count, iron, hemoglobin, monocyte count, absolute neutrophil count and neutrophil-to-lymphocyte ratio for the acute radiation syndrome phase. In the study, biomarkers such as aspartate aminotransferase, LDH, Flt3-ligand, and neutrophil-to-lymphocyte ratio were identified as early indicators of tissue damage and hematopoietic stress caused by radiation. During the acute radiation syndrome phase, biomarkers like C-reactive protein, platelet count, and hemoglobin reflected systemic inflammation and hematopoietic impairment. These biomarkers help distinguish the extent of radiation exposure and the design of treatment protocols.
Thus, this data obtained using baboons as a clinically relevant animal model can be integrated into diagnostic and prognostic strategies to improve medical care for individuals exposed to ionizing radiation.
Air pollution
Air around the world is polluted by multiple sources. There is gaseous pollution with increased concentrations of greenhouse gases such as carbon dioxide (CO2), methane (CH4), nitrous oxide (N2O), ozone (O3), and fluorinated gases formed from the burning of fossil fuels. Particulate matter pollution is a mixture of various solids and aerosols, including particles of metals, dust, soil or dust particles, allergens, and numerous chemicals, both synthetic and natural. The diameter of particles being less than 10 μm (PM10) or, most importantly, less than 2.5 μm (PM2.5) is associated with adverse outcomes, depositing in the airways, lungs, or even entering the circulatory system. Critically important is indoor air pollution, which consists of particles of allergens such as mold spores and dust mites, smoke from cigarettes and burning stoves, and volatile chemicals originating in house cleaning products. Exposure to air pollution in pregnancy is associated with adverse pregnancy outcomes such as an increased risk of preterm birth, low birth weight (SGA), increased neonatal mortality, stillbirth, and miscarriage [82–84].
Excess heat
Excessive heat exposure during pregnancy can lead to adverse outcomes, including preterm birth, stillbirth, low birth weight, and congenital abnormalities (such as heart defects, neural tube defects, and ocular development defects) [85]. Using pregnant baboons as an NHP animal model it was demonstrated that maternal hyperthermia described as an absence of fever with body temperature 41–42°C caused a blood pressure drop and an elevation in heart rate in the fetus as well as severe acidosis (blood pH less than 7.2), hypoxia and hypercapnia (partial pressure of carbon dioxide in blood above 45 mmHg) [86, 87]. Moreover, excess heat exposure of the pregnant baboons causes an increased uterine contractility up to two-fold of the normal level, which may lead to a preterm birth [87, 88]. Even a single day of high heat can elevate the risk of pregnancy complications [85, 89–93].
Microbiology pathogens
Viral infection in pregnancy
The ubiquity of infectious diseases in pregnancy makes this biological insult a notable special consideration in healthcare. Viral infection in pregnancy can lead to increased maternal morbidity and mortality, miscarriage, stillbirth, intrauterine growth restriction (IUGR), and severe birth defects (e.g., microcephalia), congenital infection. Viruses can infect the decidua and placenta or directly infect the fetus [94, 95]. Table 1 represents studies describing the effects of various viruses on pregnancy and fetal development, including viruses such as CMV, Zika (ZIKV), and Rubella (RuV), which cause congenital infections and severe outcomes like miscarriage, stillbirth, and IUGR.
TABLE 1
| Virus | Abbreviation | Virus family | Route of infection, and mode of effect | Effect on pregnancy and fetus | References |
|---|---|---|---|---|---|
| Cytomegalovirus | CMV | Herpesviridae | Intrauterine transmission | Congenital infection, abnormal neurodevelopment, microcephaly, impaired development of hearing and vision, stillbirth, IUGR, preterm birth (human) Spontaneous abortions (macaque) |
[94–99] |
| Herpes simplex virus 1–2 | HSV-1 HSV-2 |
Herpesviridae | Infects decidua, placenta causing systemic and local changes | Miscarriage, stillbirth, abnormal neurodevelopment, congenital disease, preterm birth | [94–96, 100] |
| Human papilloma virus | HPV | Papillomaviri-dae | Infects placenta | Preterm birth, miscarriage, IUGR, stillbirth | [94, 101, 102] |
| Zika virus | ZIKV | Flaviviridae | Infects decidua, placenta causing systemic and local changes | Congenital infection, Severe developmental abnormalities: Fetal brain sequence (severe microcephalia, premature closure of fontanels, partial scull collapse), brain abnormalities, ocular abnormalities, IUGR with “femur-sparing” profile Miscarriage, preterm birth, stillbirth (human) Miscarriage (monkey) |
[94, 95, 100, 103] |
| Rubella virus (Rubivirus rubellae) | RuV | Matonaviridae | Transplacental infection | Congenital infection (rare), abnormal neurodevelopment Miscarriage, premature birth, IUGR, congenital rubella syndrome (triade: cataracts, congenital heart defects, deafness) (human) Spontaneous abortion, fetal lesions, IUGR (monkey) |
[94, 96, 104–106] |
| Influenza virus A | IAV | Orthomyxoviridae | Maternal systemic infection | Preterm birth, miscarriage, IUGR, birth defects such as cleft palate, neural tube defects, congenital heart defects (Human) Influenza infection during pregnancy affects neural development, reducing gray and white matter (monkey) |
[94, 107–109] |
| Severe acute respiratory syndrome coronavirus 2 | SARS-CoV-2 | Coronaviridae | Possible transplacental infection, maternal systemic infection | Congenital infection, stillbirth | [100, 110, 111] |
| Hepatitis A, B, C, and E viruses | HAV HBV HCV HEV |
Hepadnaviri-dae | HBV, HCV, HDV- maternal liver disease consequences HBV, HCV- mother-to-child transmission Transplacental infection |
HBV-preterm birth, low birth weight HAV-preterm birth HEV- severe fetal hepatitis, stillbirth, preterm birth, low-birth weight HCV-IUFGR, low birth weight, stillbirth, preterm birth (human) Premature delivery and fetal death (HEV) (macaque) |
[94, 100, 112–118] |
| Human Immunodeficiency Virus | HIV | Retroviridae | Mother-to-child transmission | Untreated maternal HIV-premature birth, SGA, low birth weight, stillbirth, abnormal neurodevelopment (human) Developmental delay (macaque) |
[100, 119–122] |
| Varicella zoster virus | VZV | Herpesviridae | Vertical transmission | Fetal varicella syndrome (cutaneous scars, limb defects, eye and brain abnormalities), IUGR | [96, 123–125] |
Adverse effects of viral infection on fetal development and pregnancy outcome in human population.
As was demonstrated in research conducted on baboons (Papio hamadryas), viral infection with herpesvirus papio 2 (HVP2) and cytomegalovirus (CMV) affects the placenta and causes placentitis, leading to adverse effects in pregnancy with a high prevalence in the baboon population up to 95% seropositivity [96]. Notably, the baboon model was successfully used for testing the placental transfer and fetal metabolism of antiretroviral drugs such as zidovudine (used in pregnancy to lower maternal-fetal HIV transmission). By using pregnant baboon dams, it was demonstrated that zidovudine and its glucuronide metabolite were able to cross the placenta, with evidence of fetal metabolism [126]. Moreover, baboons have been used in virology research to study the effects of the Zika virus infection on fetal development. Importantly, this study demonstrated that perinatal Zika virus infection can cause a significant fetal cerebral cortical injury resulting in fetal death in baboons, underscoring the baboon’s high value as an animal model of pregnancy and perinatal viral infection affecting fetal neurodevelopment [127].
Bacterial infection in pregnancy
Bacterial infection in pregnancy can cause fetal congenital disease, miscarriage, stillbirth, chorioamnionitis (inflammation of the fetal membranes), preterm birth, and low birth weight. Oral, sexually transmitted, or commensal bacterial infection in pregnancy can be transmitted vertically to the fetus and impact its development as well as pregnancy outcome. Bacterial vaginosis, caused by multiple bacteria, can also lead to premature labor and birth [128, 129]. Table 2 outlines the impact of various bacterial infections on fetal development and pregnancy. These infections are divided into categories: sexually transmitted infections (STIs), commensal bacteria, and those acquired through contaminated food or animal contact. This helps to emphasize the need for screening, early treatment, and preventive measures like food safety to mitigate negative outcomes on pregnancy. As was demonstrated by research with baboons (Papio hamadryas), bacterial infection with Ureaplasma urealyticum and Klebsiella spp. caused placentitis and intrauterine infection, leading to severe adverse effects on pregnancy, such as stillbirth and intrauterine growth restriction [96].
TABLE 2
| Type of infection | Bacteria | Effects on pregnancy and fetus | References |
|---|---|---|---|
| STI | Chlamydia trachomatis | Preterm birth, congenital infection (human) Births of weak, low-weight, and vitality-monkey calves were observed in infected macaques |
[96, 129–132] |
| Neisseria gonorrhoeae | Low birth weight, preterm birth | [129, 131] | |
| Treponema pallidum | Stillbirth, miscarriage, low birth weight | [96, 129] | |
| Trichomonas vaginalis | Preterm birth, low birth weight | [129, 131, 132] | |
| Ureaplasma urealyticum | Preterm birth, low birth weight | [96, 129, 132] | |
| Mycoplasma hominis | Preterm birth, low birth weight | [96, 129, 132] | |
| Commensal | E. coli (bacterial vaginosis) | Preterm birth, stillbirth | [96, 129] |
| Group B streptococcus | Preterm birth | [129, 133] | |
| Contaminated food consumption/contact with an infected animal | Listeria monocytogenes | Congenital disease, stillbirth, miscarriage (human) Stillbirth (monkey) |
[129, 134, 135] |
| Brucella spp | Miscarriage, preterm birth, chorioamnionitis | [129] |
Adverse effects of bacterial infection on fetal development and pregnancy outcome in human population.
Endoparasitic infection in pregnancy
Internal parasitic infection in pregnancy includes protozoan and helminth infection. Helminth infection, amebiasis can affect pregnancy outcome via systemic maternal adverse effects such as malnutrition and anemia. Infections such as malaria (caused by plasmodium) and amebiasis can cause preterm birth in humans [136, 137]. Notably, the protozoa Toxoplasma gondii causes toxoplasmosis, which has severe consequences on pregnancy and fetal development due to vertical transmission of the infection, causing severe developmental abnormalities of the neurodevelopment and both ocular and cardiac systems [96, 138].
The most important and severe biological environmental insults are separated into the acronym ToRCH, describing the most notable pathogens causing severe perinatal infection negatively affecting the fetus and pregnancy outcome. ToRCH stands for toxoplasmosis, other (Treponema pallidum, hepatitis viruses, HIV, varicella, parvovirus B19, enteroviruses, Zika virus, Dengue, MERS, SARS, SARS-CoV-2), rubella, cytomegalovirus, and herpes simplex virus [139–142]. ToRCH infections are severe, and some of these pathogens can be transmitted from mother to fetus and cause severe congenital abnormalities. In case the pathogen is unable to cross the placenta, it still may severely affect maternal health to the point of negative consequences for the fetus [143].
Psychoactive substances
Alcohol (ethyl alcohol)
Ethanol exposure during prenatal development has been extensively studied using NHP models, including rhesus and vervet monkeys and notably baboons [144, 145] due to their close genetic and neuroanatomical resemblance of humans and the similarities in the developmental processes during pregnancy. These studies have provided critical insights into how ethanol affects neurodevelopment, mirroring aspects of human fetal alcohol spectrum disorders (FASD) [146]. FASD includes a spectrum of physical, cognitive, behavioral and neurodevelopmental impairments. Notably, the developing brain is the most vulnerable to ethanol toxicity organ [147, 148].
To closely mimic human FASD, researchers have developed NHP models where pregnant rhesus macaques voluntarily consume ethanol. These models take advantage of the similarities between humans and rhesus macaques in gestational length relative to brain development, as well as similarities in ethanol self-administration and metabolism [149–151]. Studies using this model have shown that a daily ethanol dose of 1.5 g/kg during the first trimester does not influence pregnancy success rates but does affect drinking behavior during the second month of pregnancy [149]. Subsequent research using this model aims to describe the effect of early-gestation ethanol exposure on anatomical and functional brain development at different gestational ages [149, 150, 152].
The timing of prenatal ethanol exposure plays a crucial role in determining its neurodevelopmental outcomes. In a study, rhesus monkeys were exposed to moderate amounts of ethanol (0.6 g/kg, voluntary alcohol consumption daily) during different gestational periods, namely, early gestation (gestational days 0–50), mid-to-late gestation (gestational days 50–135) or continuous exposure throughout gestation [151]. This exposure led to blood alcohol levels ranging from 20 mg to 50 mg/dL (∼4.3 mM–11 mM). The early gestation exposure to alcohol significantly reduced neurodevelopmental tests scores, including diminished infant orientation and motor maturity. In contrast, mid-to-late gestation exposure primarily affected motor maturity. These results obtained in non-human primates, underscored the heightened sensitivity of early gestational periods to the neurotoxic effects of ethanol [151]. During the first trimester of pregnancy, rhesus monkeys exposed to ethanol exhibited reduced placental blood flow, decreased overall growth, and impaired growth and development of the brain [153]. In baboons, fetal growth restriction has been documented following three episodes of maternal intragastric gavage with alcohol during the second trimester-equivalent of human pregnancy. Maternal blood alcohol levels were around 80 mg/dL, while the alcohol level in the amniotic fluid reached 63 mg/dL [30]. While 80 mg/dL (∼17 mM) represents the legal blood alcohol concentration limit for driving a motor vehicle in most of the United States, a concentration of 63 mg/dL (∼13.7 mM) marks the onset of noticeable behavioral impairment in humans [154, 155]. Near term, baboon progeny that was exposed to alcohol during the second trimester-equivalent had reduced circumferences of the abdomen and head without affecting femur length [145].
Besides growth curves, prenatal alcohol exposure has been documented to negatively impact neurogenesis and neurotransmitter systems in the developing brain. In vervet monkeys, exposure to ethanol, ranging from 13 mM to 29 mM, during the third trimester led to reduced numbers of hippocampal neurons and loss of hippocampal volume [156].
Moderate prenatal exposure of rhesus monkeys to alcohol, combined with serotonin transporter gene promoter (rh5-HTTLPR) polymorphism, altered the function of serotonin in the central nervous system. This exposure led to maternal blood alcohol levels of 20 mg–50 mg/dL (∼4.3 mM–11 mM). Monkeys carrying the short allele of the rh5-HTTLPR exhibited reduced cerebrospinal fluid levels of the serotonin metabolite 5-hydroxyindoleacetic acid (5-HIAA), suggesting a gene-environment interaction that may contribute to neurodevelopmental impairments associated with prenatal alcohol exposure [157].
Epigenetic mechanisms, including DNA methylation and histone modifications, have been implicated in the neurodevelopmental abnormalities that result from prenatal ethanol exposure. Temporal lobe samples from the brains of both humans and NHPS (macaque) with documented prenatal alcohol exposure exhibited significant decreases in global methylation of DNA and histones and increased histone acetylation, indicating that prenatal alcohol exposure can lead to widespread epigenetic changes that may underlie neurodevelopmental deficits [152].
Prenatal ethanol exposure also affects both gene expression and regulation by microRNAs (miRNAs) in the developing brain. During neurodevelopment, ethanol exposure disrupts miRNA expression, altering the regulation of genes which are critical for synaptic development, as well as proliferation, and migration of neuronal progenitors. Thus, ethanol-induced miRNA expression disruption results in structural and functional brain developmental defects [158]. In vervet monkeys, exposure to ethanol during the last 2 months of gestation led to reduced numbers of cortical and hippocampal neurons, accompanied by upregulation of miRNAs in the hippocampus [146]. It was significantly correlated with reduced expression of their predicted targets-messenger RNA (mRNA), which miRNA typically bind at the 3′ untranslated region, inducing mRNA degradation or inhibiting their translation. mRNAs are responsible for the biosynthesis of key proteins involved in developmental processes such as migration, differentiation, and proliferation. As it was observed in a previous study by Gillis et al. [150], mRNA was globally downregulated in vervet monkeys prenatally exposed to ethanol; thus, these results suggest that ethanol-induced upregulation of specific miRNAs may contribute to the downregulation of expression, potentially leading to neurodevelopmental impairments. Interestingly, these studies uncovered a previously unknown link between FASDs and the EFNB1 gene that encodes ephrin B1, which plays a crucial role in neurodevelopment and the development of craniofrontonasal syndrome. Considering the functions of EFNB1, this novel connection suggests a significant area for further investigation into the etiology and potential therapeutic targets of FASD [146, 150].
Computational models have been utilized to predict ethanol-induced neurodevelopmental toxicity across species. One such model applied mechanistic data from rodent studies to evaluate the sensitivity of primate species to ethanol-induced inhibition of neocortical neuronal proliferation. The model predicted that primates, including humans, are more sensitive to ethanol’s effects, with significant neuronal deficits occurring at lower blood ethanol concentrations compared to rodents. For example, the model predicted a significant decrease in neocortical neuronal number after prenatal ethanol exposure in a dose simulating a consumption of one standard drink within 1 hour in human. This increased sensitivity is attributed to the prolonged rapid growth period, compare to rodents, in the primate neuronal progenitor population highlighting the relevance of NHP models in assessing ethanol’s impact on human neurodevelopment [159].
The development of baboon model of FASD has provided valuable insights into the effects of prenatal alcohol exposure on primate development. Baboons (Papio spp.) are particularly suitable for FASD research because of their close genetic, anatomical, and physiological similarities to humans. Their complex brain structure, prolonged gestational period (approximately 6 months), and advanced social and cognitive behaviors make them an excellent model for studying the neurodevelopmental, behavioral, and physical consequences of alcohol exposure during pregnancy [160].
Approaches used in FASD studies using baboons as a large animal model typically involve administering ethanol to pregnant females through controlled dosing, which replicates human drinking or gastric infusion to maintain stable blood alcohol levels [30, 161]. One study reported administration of ethanol to baboon imitating a single binge drinking episode in an adult human. Ethanol was administered to the pregnant baboon dam via a gastro-nasal catheter at a dose of 3 gm of ethanol per kg of weight to approximate a blood-alcohol level concentration of ∼0.2%. This dose of alcohol is equivalent to the consumption of 6–8 alcoholic drinks in 2 hours by an adult human imitating a heavy binge-drinking episode [162]. The timing of fetal alcohol exposure is carefully managed to correspond to critical periods of human prenatal brain development, particularly during the first and second trimesters, when the brain is most vulnerable. Vasoactive properties of alcohol caused an increased placental permeability as well as an increased fetal brain perfusion making fetal brain more vulnerable to toxic insults [162].
In addition to its effects on neurodevelopment, prenatal alcohol exposure also compromises the fetal cerebral circulation [30, 145, 162–165]. Using the baboon model, a study from the Bukiya lab showed that moderate doses of ethanol (∼13.7 mM) administered during the second trimester of pregnancy can induce significant dilation of the middle cerebral artery, indicating disrupted regulation of vascular tone [30]. This period represents a critical window for cerebrovascular development [166, 167]. A subsequent study from the Bukiya lab implicated the endocannabinoid system in mediating this effect. Bukiya et al demonstrated that a pharmacological block of cannabinoid receptors CB1 and CB2 effectively reversed the ethanol-induced vasodilation [145]. Furthermore, proteomic profiling of cerebral arteries collected in the third trimester from baboon fetuses exposed to ethanol during mid-gestation revealed long-term molecular alterations, with the most prominent changes observed in mitochondrial and cytoskeletal proteins [144]. Such alterations may impair mitochondrial function, alter fetal cerebral artery contractility, and compromise vascular integrity. Collectively, these studies suggest that prenatal ethanol exposure disrupts both the structural and functional development of fetal cerebral arteries. However, the specific molecular mechanisms, receptor-specific contributions and long-term cerebrovascular consequences remain to be fully elucidated.
Advancements in neuroimaging and molecular biology will further enhance the understanding of the mechanisms by which alcohol disrupts fetal development in primates and may guide the development of targeted prevention and treatment strategies for FASD in humans [168] Owing to the long lifespan [169], baboons offer promising opportunities for longitudinal studies that can track the long-term effects of prenatal alcohol exposure and interventional studies to mitigate the impacts of FASD.
Cannabinoids
The legalization of marijuana is leading to an increased belief in the “safety” of the product. It led to a consequent growth of cannabis misuse in the population, including in pregnancy [170]. The most prevalent form of drug misuse during pregnancy is the maternal use of cannabinoids, with approximately 5% of pregnant women self-medicating with marijuana [171]. Cannabinoids, including tetrahydrocannabinol (THC) and cannabidiol (CBD), have been increasingly studied for their effects on neurodevelopment. NHP models provide critical insights into the long-term impact of cannabinoid exposure due to their close genetic and neuroanatomical similarity to humans [172, 173], and highlight the effects of perinatal maternal cannabinoid use, which may cause preterm birth and low birth weight [174].
The endocannabinoid system plays a crucial role in neurodevelopment, regulating synaptogenesis, neuronal differentiation, and circuit maturation [175]. Exogenous cannabinoids, including THC and CBD, can disrupt fetal endocannabinoid system signaling, potentially leading to long-term neurodevelopmental consequences [176, 177]. Given the ethical and methodological limitations of human studies, NHP models provide valuable insights into the effects of prenatal cannabinoid exposure on cognitive function and behavior [178].
Prenatal exposure to cannabinoids, particularly THC, has been linked to alterations in brain morphology [174] and functional connectivity in humans [179]. Chronic prenatal exposure to delta-9-tetrahydrocannabinol (THC) in a rhesus macaque model resulted in significant alterations in fetal brain development. MRI assessments revealed that THC exposure affected brain growth in both male and female fetuses compared to controls. Histological analysis at gestational day 155 indicated signs of brain dysregulation in the THC group. Additionally, two extracellular vesicle-associated microRNAs were identified in the cerebrospinal fluid of THC-exposed fetuses, with pathway analysis suggesting disrupted axonal guidance and netrin signaling [180].
Cannabinoids exert their effects primarily through CB1 and CB2 receptors, which are abundantly expressed in the developing brain. As shown in Figure 4, both CB1 and CB2 transcripts are detectable in the cerebellum of fetal baboons.
FIGURE 4
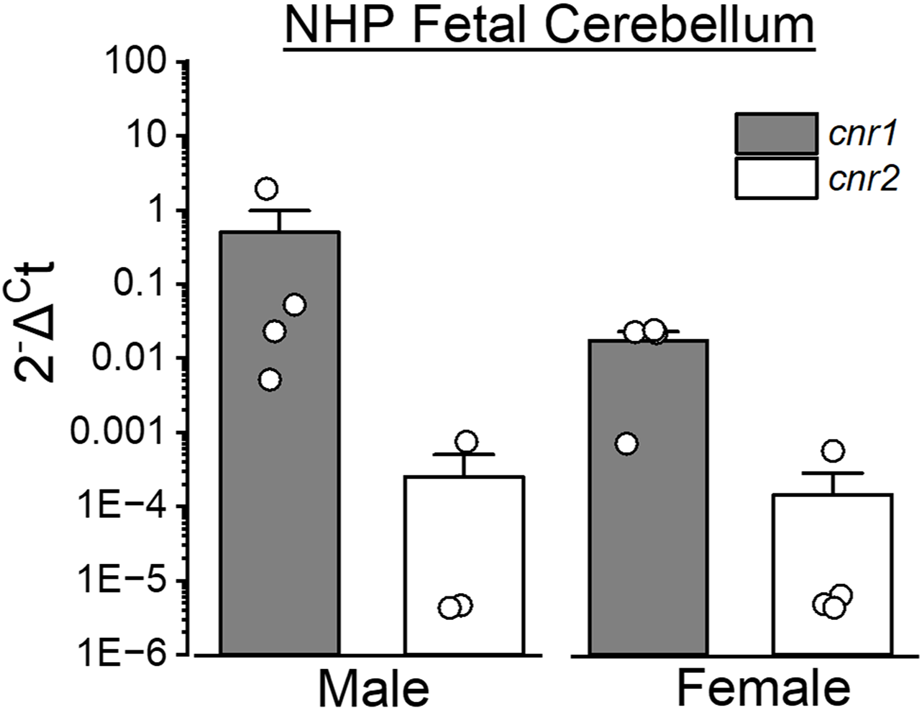
Expression of cannabinoid receptors-encoding genes in the fetal cerebellum of baboons. Cerebellar tissue was collected from male and female baboon fetuses at gestational day 120 (end of second trimester-equivalent of human pregnancy) to assess the expression level of cannabinoid receptor-encoding genes (Cnr1 and Cnr2 encoding CB1 and CB2 receptors, respectively). Quantitative PCR (qPCR) was performed using TaqMan Gene Expression Assays (ThermoFisher Scientific). The y-axis represents relative gene expression, where Ct is the cycle number at which fluorescence surpasses the detection threshold. Expression of Cnr1 and Cnr2 was normalized to β-actin (Actb) in each sample. Probes used: Cnr1 (Rh02787040_s1), Cnr2 (Rh02913156_m1) and Actb (Rh02621734_g1). qPCR procedures were performed using previously published protocols [181]. Sample sizes were n = 4, except for Cnr2 in male fetuses (n = 3). Each sample was collected from a separate baboon fetus.
As a partial agonist at CB1 receptors, THC disrupts the tightly regulated balance of excitatory and inhibitory neurotransmission, leading to long-term synaptic deficits [182, 183]. Additionally, epigenetic modifications, including DNA methylation and histone modifications, have been observed after prenatal cannabinoid exposure, suggesting that effects may persist in the next-generation (intergenerational effect) or even across multiple generations (transgenerational effect) [184, 185]. Cannabis exposure during critical developmental windows can disrupt epigenetic processes, leading to heritable changes in genes and molecular pathways. These alterations are linked to psychiatric diseases like autism spectrum disorder, attention-deficit/hyperactivity disorder (ADHD), schizophrenia, and addiction. Functionally, prenatal cannabinoid exposure has been associated with deficits in executive function, aggression, increased impulsivity, altered social behaviors, and other psychiatric disorders [186, 187]. Thus, increased cannabis use, especially during brain development, has been associated with a rise in mental health issues among adolescents and young adults in the U.S. [187]. Primate models provide critical insights into the neurodevelopmental impact of cannabinoid exposure, highlighting structural, functional, and behavioral consequences. Under-utilization of NHPs, including baboons, in research may impede further progress in elucidating dose-dependent effects, the role of genetic predisposition, and potential interventions to mitigate adverse outcomes of developmental exposure to cannabis.
Tobacco
The impact on fetal neurodevelopment of tobacco exposure during prenatal and perinatal periods has been extensively studied in NHPs, particularly rhesus monkeys [188]. Rodents such as rats and mice are altricial species, so their brain development at birth corresponds to fetal stages of human development; therefore, the concentrations of nicotine may not be correlated with those observed in typical human exposure scenarios [189].
Studies involving rhesus monkeys exposed to tobacco both as direct nicotine exposure and as environmental tobacco smoke (ETS) during the perinatal period have demonstrated significant alterations in brain development. Perinatal exposure to ETS in rhesus monkeys resulted in selective upregulation of nicotinic acetylcholine receptors in the brainstem and cerebral cortex. This change was selective, with no effects on m [2]-muscarinic or beta-adrenergic receptors. The upregulation of nicotinic receptors suggests chronic nicotine stimulation, a hallmark of nicotine-induced neuroteratogenesis, indicating that perinatal ETS exposes the fetus and neonate to nicotine levels that can alter brain development [189, 190].
ETS (also known as involuntary, secondary, or passive smoking) causes multiple adverse effects on exposed subjects. In particular, prenatal ETS exposure leads to region-specific neurodevelopmental damage in primate brains. The neurotoxicity and neuroteratogenic effects of nicotine exposure disrupt the formation, survival, and differentiation of brain cells, leading to apoptosis and reduced cell size in the forebrain, midbrain, and hindbrain. These lead, in turn, to structural deficits, impaired synaptic function, and behavioral abnormalities in rats [191, 192]. These findings underscore the vulnerability of the developing primate brain to nicotine and highlight the potential for long-term neurodevelopmental impairments [193].
Bruin et al have examined the enduring effects of fetal and neonatal nicotine exposure on postnatal health, emphasizing the increased risk for neurodevelopmental disorders such as ADHD, anxiety, and depression. These outcomes are linked to alterations in brain regions like the prefrontal cortex, and hippocampus, and changes in neurotransmitter systems, including nicotinic acetylcholine receptors [194].
The parallels between findings in NHP studies and human epidemiological data are striking. Prenatal tobacco exposure in humans is associated with an increased risk of behavioral disorders in children and adolescents, not only ADHD, but oppositional defiant disorder, and conduct disorder. Studies in NHPs have been crucial in elucidating the detrimental effects of tobacco exposure on neurodevelopment. They pave the way to the development of new strategies of a neurodevelopmental disorders treatment and prevention.
Discussion, concluding remarks and future directions
Despite the scientific advantages of using NHPs, their enrollment into research entails significant ethical and logistical challenges. Ethical considerations are paramount in primate research, necessitating strict adherence to welfare regulations and a clear justification for the use of NHPs [168]. Moreover, many NHPs (including baboons) have long lifespans and require complex care, making these studies expensive and resource-intensive. Despite these challenges, we believe that the use of NHPs in research is beneficial. NPH is a unique translation model for human diseases and conditions. NHPs have a complex brain, allowing them to perform sophisticated behavioral, visual, and electrophysiological studies for elucidating mechanisms and new treatment strategies of human neurodevelopmental disorders such as ADHD, autism spectrum disorders, etc. Environmental insults adversely affecting pregnancy and fetal development, especially neurodevelopment, are ubiquitous and numerous, and, as demonstrated in previous research [160, 195], the NHP animal model provided by the baboon Papio hamadryas is a great translational model for the research of pregnancy, placental, and fetal development. The insights gained from NHP studies are crucial for advancing preventive and therapeutic strategies to mitigate the impact of environmental exposures on human development.
Statements
Author contributions
II and AB conceived the idea of review. II and ST wrote the review, performed experiments (when described), and prepared illustrations. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported in part by R01 grant AA029673 (AB) from the National Institute on Alcohol Abuse and Alcoholism.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Abbreviations
ADHD, attention-deficit/hyperactivity disorder; CBD, cannabidiol; EGL, external granular layer; ETS, environmental tobacco smoke; FASD, fetal alcohol spectrum disorders; GCP, granule cell precursor; IGL, internal granular layer; IUGR, intrauterine growth restriction; ML, medial layer; NHP, non-human primate; PM, particulate matter; SGA, small for gestational age; THC, tetrahydrocannabinol.
References
1.
Doi M Usui N Shimada S . Prenatal environment and neurodevelopmental disorders. Front Endocrinol (Lausanne) (2022) 13,860110. 10.3389/fendo.2022.860110
2.
Ryan AM Bauman MD . Primate models as a translational tool for understanding prenatal origins of neurodevelopmental disorders associated with maternal infection. Biol Psychiatry Cogn Neurosci Neuroimaging (2022) 7(5),510–23. 10.1016/j.bpsc.2022.02.012
3.
Stouffer RL Woodruff TK . Nonhuman primates, a vital model for basic and applied research on female reproduction, prenatal development, and women's health. Ilar j (2017) 58(2),281–94. 10.1093/ilar/ilx027
4.
Vijayan KKV De Paris K . Nonhuman primate models of pediatric viral diseases. Front Cell Infect Microbiol (2024) 14,1493885. 10.3389/fcimb.2024.1493885
5.
Nakamura T Fujiwara K Saitou M Tsukiyama T . Non-human Primates as a model for human development. Stem Cell Rep (2021) 16(5),1093–103. 10.1016/j.stemcr.2021.03.021
6.
Tarantal AF Noctor SC Hartigan-O'Connor DJ . Nonhuman Primates in translational research. Annu Rev Anim Biosci (2022) 10,441–68. 10.1146/annurev-animal-021419-083813
7.
Patten AR Fontaine CJ Christie BR . A comparison of the different animal models of fetal alcohol spectrum disorders and their use in studying complex behaviors. Front Pediatr (2014) 2,93. 10.3389/fped.2014.00093
8.
Li M Brokaw A Furuta AM Coler B Obregon-Perko V Chahroudi A et al Non-human primate models to investigate mechanisms of infection-associated fetal and pediatric injury, teratogenesis and stillbirth. Front Genet (2021) 12,680342. 10.3389/fgene.2021.680342
9.
Atkinson EG Rogers J Mahaney MC Cox LA Cheverud JM . Cortical folding of the primate brain, an interdisciplinary examination of the genetic architecture, modularity, and evolvability of a significant neurological trait in pedigreed baboons (genus papio). Genetics (2015) 200(2),651–65. 10.1534/genetics.114.173443
10.
Franke K Clarke GD Dahnke R Gaser C Kuo AH Li C et al Premature brain aging in baboons resulting from moderate fetal undernutrition. Front Aging Neurosci (2017) 9,92. 10.3389/fnagi.2017.00092
11.
VandeBerg JW-BS Tardif S . The baboon in biomedical research. Springer (2009).
12.
Ek CJ Nathanielsz P Li C Mallard C . Transcriptomal changes and functional annotation of the developing non-human primate choroid plexus. Front Neurosci (2015) 9,82. 10.3389/fnins.2015.00082
13.
Rhodes C . Predictive functions of H3K27me3 and H4K20me3 in primate hippocampal stem and progenitor cells. (2025).
14.
Li X Trivedi U Brejnrod AD Vestergaard G Mortensen MS Bertelsen MF et al The microbiome of captive hamadryas baboons. Anim Microbiome (2020) 2(1),25. 10.1186/s42523-020-00040-w
15.
Naidoo-Variawa S Hey-Cunningham AJ Lehnert W Kench PL Kassiou M Banati R et al High-resolution imaging of the large non-human primate brain using microPET, a feasibility study. Phys Med Biol (2007) 52(22),6627–38. 10.1088/0031-9155/52/22/005
16.
Phillips KA Kochunov P . Tracking development of the corpus callosum in fetal and early postnatal baboons using magnetic resonance imaging. Open Neuroimag J (2011) 5,179–85. 10.2174/1874440001105010179
17.
Amiez C Sallet J Hopkins WD Meguerditchian A Hadj-Bouziane F Ben Hamed S et al Sulcal organization in the medial frontal cortex provides insights into primate brain evolution. Nat Commun (2019) 10(1),3437. 10.1038/s41467-019-11347-x
18.
Rogers J Kochunov P Lancaster J Shelledy W Glahn D Blangero J et al Heritability of brain volume, surface area and shape, an MRI study in an extended pedigree of baboons. Hum Brain Mapp (2007) 28(6),576–83. 10.1002/hbm.20407
19.
Kochunov P Glahn DC Fox PT Lancaster JL Saleem K Shelledy W et al Genetics of primary cerebral gyrification, heritability of length, depth and area of primary sulci in an extended pedigree of papio baboons. Neuroimage (2010) 53(3),1126–34. 10.1016/j.neuroimage.2009.12.045
20.
Rogers J Kochunov P Zilles K Shelledy W Lancaster J Thompson P et al On the genetic architecture of cortical folding and brain volume in Primates. Neuroimage (2010) 53(3),1103–8. 10.1016/j.neuroimage.2010.02.020
21.
Dieni S Inder T Yoder B Briscoe T Camm E Egan G et al The pattern of cerebral injury in a primate model of preterm birth and neonatal intensive care. J Neuropathol Exp Neurol (2004) 63(12),1297–309. 10.1093/jnen/63.12.1297
22.
Kochunov P Castro C Davis D Dudley D Brewer J Zhang Y et al Mapping primary gyrogenesis during fetal development in primate brains, high-resolution in utero structural MRI of fetal brain development in pregnant baboons. Front Neurosci (2010) 4,20. 10.3389/fnins.2010.00020
23.
Barron T Kim JH . Preterm birth impedes structural and functional development of cerebellar purkinje cells in the developing Baboon cerebellum. Brain Sci (2020) 10(12),897. 10.3390/brainsci10120897
24.
Iskusnykh IY Buddington RK Chizhikov VV . Preterm birth disrupts cerebellar development by affecting granule cell proliferation program and bergmann glia. Exp Neurol (2018) 306,209–21. 10.1016/j.expneurol.2018.05.015
25.
Iskusnykh IY Fattakhov N Buddington RK Chizhikov VV . Intrauterine growth restriction compromises cerebellar development by affecting radial migration of granule cells via the JamC/Pard3a molecular pathway. Exp Neurol (2021) 336,113537. 10.1016/j.expneurol.2020.113537
26.
Iskusnykh IY Fattakhov N Li Y Bihannic L Kirchner MK Steshina EY et al Lmx1a is a master regulator of the cortical hem. Elife (2023) 12,e84095. 10.7554/eLife.84095
27.
Chizhikov D Buddington RK Iskusnykh IY . Effects of phosphatidylserine source of docosahexaenoic acid on cerebellar development in preterm pigs. Brain Sci (2020) 10(8),475. 10.3390/brainsci10080475
28.
D’Angelo E . Handbook of the cerebellum and cerebellar disorders. In, MantoMSchmahmannJDRossiFGruolDLKoibuchiN, editors. Cerebellar granule cell. Dordrecht, Springer (2013).
29.
Haldipur P Aldinger KA Bernardo S Deng M Timms AE Overman LM et al Spatiotemporal expansion of primary progenitor zones in the developing human cerebellum. Science (2019) 366(6464),454–60. 10.1126/science.aax7526
30.
Seleverstov O Tobiasz A Jackson JS Sullivan R Ma D Sullivan JP et al Maternal alcohol exposure during mid-pregnancy dilates fetal cerebral arteries via endocannabinoid receptors. Alcohol (2017) 61,51–61. 10.1016/j.alcohol.2017.01.014
31.
Lake AR Van Niekerk IJ Le Roux CG Trevor-Jones TR De Wet PD . Angiology of the brain of the baboon Papio ursinus, the vervet monkey cercopithecus pygerithrus, and the bushbaby Galago senegalensis. Am J Anat (1990) 187(3),277–86. 10.1002/aja.1001870307
32.
Maloney SK Mitchell D Mitchell G Fuller A . Absence of selective brain cooling in unrestrained baboons exposed to heat. Am J Physiol Regul Integr Comp Physiol (2007) 292(5),R2059–2067. 10.1152/ajpregu.00809.2006
33.
Swindler DWD . An atlas of primate gross anatomy, Baboon, chimpanzee, and man. Seattle, Univ. of Washington Press (1973).
34.
Kroma G Li J Wey H Gupton T Jr PR Leland M et al Similarity of the baboon carotid vasculature to humans, anatomical analysis of the baboon carotid and anterior cerebral circulation. J Vasc Interv Radiol (2014) 25(3),206. 10.1016/j.jvir.2013.12.555
35.
Huang J Mocco J Choudhri TF Poisik A Popilskis SJ Emerson R et al A modified transorbital baboon model of reperfused stroke. Stroke (2000) 31(12),3054–63. 10.1161/01.str.31.12.3054
36.
Phillips KA Bales KL Capitanio JP Conley A Czoty PW t Hart BA et al Why primate models matter. Am J Primatol (2014) 76(9),801–27. 10.1002/ajp.22281
37.
Lecrux C Hamel E . The neurovascular unit in brain function and disease. Acta Physiol (Oxf) (2011) 203(1),47–59. 10.1111/j.1748-1716.2011.02256.x
38.
Freret T Bouet V Toutain J Saulnier R Pro-Sistiaga P Bihel E et al Intraluminal thread model of focal stroke in the non-human primate. J Cereb Blood Flow Metab (2008) 28(4),786–96. 10.1038/sj.jcbfm.9600575
39.
Higo N . Non-human primate models to explore the adaptive mechanisms after stroke. Front Syst Neurosci (2021) 15,760311. 10.3389/fnsys.2021.760311
40.
Boghdadi A . Primate-specific response of astrocytes to stroke limits peripheral macrophage infiltration. (2025).
41.
Monet MC Quan N . Complex neuroimmune involvement in neurodevelopment, a mini-review. J Inflamm Res (2023) 16,2979–91. 10.2147/JIR.S410562
42.
Fonta C Imbert M . Vascularization in the primate visual cortex during development. Cereb Cortex (2002) 12(2),199–211. 10.1093/cercor/12.2.199
43.
Zhang R Quan H Wang Y Luo F . Neurogenesis in Primates versus rodents and the value of non-human primate models. Natl Sci Rev (2023) 10(11),nwad248. 10.1093/nsr/nwad248
44.
Swartz DD Andreadis ST . Animal models for vascular tissue-engineering. Curr Opin Biotechnol (2013) 24(5),916–25. 10.1016/j.copbio.2013.05.005
45.
Gil'denskiol'd RS Baĭkov BK Iudina TV Koval'chuk VK Stepanov LG . Materials to be used in the hygienic forecast of air pollution during the development of heat energy at the kansk-achinsk fuel and energy complex. Gig Sanit (1987)(5) 9–12.
46.
Koppe JG Bartonova A Bolte G Bistrup ML Busby C Butter M et al Exposure to multiple environmental agents and their effect. Acta Paediatr Suppl (2006) 95(453),106–13. 10.1080/08035320600886646
47.
Delbès G Hales BF Robaire B . Toxicants and human sperm chromatin integrity. Mol Hum Reprod (2010) 16(1),14–22. 10.1093/molehr/gap087
48.
Wai KM Mar O Kosaka S Umemura M Watanabe C . Prenatal heavy metal exposure and adverse birth outcomes in Myanmar, a birth-cohort study. Int J Environ Res Public Health (2017) 14(11),1339. 10.3390/ijerph14111339
49.
Vahter ME . Interactions between arsenic-induced toxicity and nutrition in early life. J Nutr (2007) 137(12),2798–804. 10.1093/jn/137.12.2798
50.
Laine JE Bailey KA Rubio-Andrade M Olshan AF Smeester L Drobná Z et al Maternal arsenic exposure, arsenic methylation efficiency, and birth outcomes in the biomarkers of exposure to ARsenic (BEAR) pregnancy cohort in Mexico. Environ Health Perspect (2015) 123(2),186–92. 10.1289/ehp.1307476
51.
Kippler M Wagatsuma Y Rahman A Nermell B Persson L Raqib R et al Environmental exposure to arsenic and cadmium during pregnancy and fetal size, a longitudinal study in rural Bangladesh. Reprod Toxicol (2012) 34(4),504–11. 10.1016/j.reprotox.2012.08.002
52.
Yang CY Chang CC Tsai SS Chuang HY Ho CK Wu TN . Arsenic in drinking water and adverse pregnancy outcome in an arseniasis-endemic area in northeastern Taiwan. Environ Res (2003) 91(1),29–34. 10.1016/s0013-9351(02)00015-4
53.
Al-Saleh I Shinwari N Mashhour A Rabah A . Birth outcome measures and maternal exposure to heavy metals (lead, cadmium and Mercury) in Saudi Arabian population. Int J Hyg Environ Health (2014) 217(2-3),205–18. 10.1016/j.ijheh.2013.04.009
54.
Sun H Chen W Wang D Jin Y Chen X Xu Y . The effects of prenatal exposure to low-level cadmium, lead and selenium on birth outcomes. Chemosphere (2014) 108,33–9. 10.1016/j.chemosphere.2014.02.080
55.
Huang K Li H Zhang B Zheng T Li Y Zhou A et al Prenatal cadmium exposure and preterm low birth weight in China. J Expo Sci Environ Epidemiol (2017) 27(5),491–6. 10.1038/jes.2016.41
56.
Potula V Kaye W . Report from the CDC. Is lead exposure a risk factor for bone loss?J Womens Health (Larchmt) (2005) 14(6),461–4. 10.1089/jwh.2005.14.461
57.
Stasenko S Bradford EM Piasek M Henson MC Varnai VM Jurasović J et al Metals in human placenta, focus on the effects of cadmium on steroid hormones and leptin. J Appl Toxicol (2010) 30(3),242–53. 10.1002/jat.1490
58.
Zhu M Fitzgerald EF Gelberg KH Lin S Druschel CM . Maternal low-level lead exposure and fetal growth. Environ Health Perspect (2010) 118(10),1471–5. 10.1289/ehp.0901561
59.
Gundacker C Forsthuber M Szigeti T Kakucs R Mustieles V Fernandez MF et al Lead (pb) and neurodevelopment, a review on exposure and biomarkers of effect (BDNF, HDL) and susceptibility. Int J Hyg Environ Health (2021) 238,113855. 10.1016/j.ijheh.2021.113855
60.
Guo X Jiang S Xu J Tian Y Ouyang F Yu X et al Effects of single and combined exposure to lead and stress during pregnancy on offspring neurodevelopment. Dev Cogn Neurosci (2022) 56,101124. 10.1016/j.dcn.2022.101124
61.
Dack K Fell M Taylor CM Havdahl A Lewis SJ . Prenatal mercury exposure and neurodevelopment up to the age of 5 years, a systematic review. Int J Environ Res Public Health (2022) 19(4),1976. 10.3390/ijerph19041976
62.
Wu R Yao F Li X Shi C Zang X Shu X et al Manganese pollution and its remediation, a review of biological removal and promising combination strategies. Microorganisms (2022) 10(12),2411. 10.3390/microorganisms10122411
63.
Heng YY Asad I Coleman B Menard L Benki-Nugent S Hussein Were F et al Heavy metals and neurodevelopment of children in low and middle-income countries, a systematic review. PLoS One (2022) 17(3),e0265536. 10.1371/journal.pone.0265536
64.
Zoni S Lucchini RG . Manganese exposure, cognitive, motor and behavioral effects on children, a review of recent findings. Curr Opin Pediatr (2013) 25(2),255–60. 10.1097/MOP.0b013e32835e906b
65.
Markham AC Gesquiere LR Bellenger JP Alberts SC Altmann J . White monkey syndrome and presumptive copper deficiency in wild Savannah baboons. Am J Primatol (2011) 73(11),1160–8. 10.1002/ajp.20983
66.
Schlabritz-Loutsevitch NE Hubbard GB Dammann MJ Jenkins SL Frost PA McDonald TJ et al Normal concentrations of essential and toxic elements in pregnant baboons and fetuses (papio species). J Med Primatol (2004) 33(3),152–62. 10.1111/j.1600-0684.2004.00066.x
67.
Neudecker V Xu J Thomas MA Penberthy KK Kang E Berg DA et al An update on preclinical research in anesthetic-induced developmental neurotoxicity in nonhuman primate and rodent models. J Neurosurg Anesthesiol (2023) 35(1),104–13. 10.1097/ANA.0000000000000885
68.
Parvez S Gerona RR Proctor C Friesen M Ashby JL Reiter JL et al Glyphosate exposure in pregnancy and shortened gestational length, a prospective Indiana birth cohort study. Environ Health (2018) 17(1),23. 10.1186/s12940-018-0367-0
69.
Furlong MA Paul KC Parra KL Fournier AJ Ellsworth PC Cockburn MG et al Preconception and first trimester exposure to pesticides and associations with stillbirth. Am J Epidemiol (2025) 194(1),44–55. 10.1093/aje/kwae198
70.
Gerona RR Reiter JL Zakharevich I Proctor C Ying J Mesnage R et al Glyphosate exposure in early pregnancy and reduced fetal growth, a prospective observational study of high-risk pregnancies. Environ Health (2022) 21(1),95. 10.1186/s12940-022-00906-3
71.
Coullery R Pacchioni AM Rosso SB . Exposure to glyphosate during pregnancy induces neurobehavioral alterations and downregulation of Wnt5a-CaMKII pathway. Reprod Toxicol (2020) 96,390–8. 10.1016/j.reprotox.2020.08.006
72.
Holzner A Mohd Rameli NIA Ruppert N Widdig A . Agricultural habitat use affects infant survivorship in an endangered macaque species. Curr Biol (2024) 34(2),410–6.e4. 10.1016/j.cub.2023.12.002
73.
Krief S Berny P Gumisiriza F Gross R Demeneix B Fini JB et al Agricultural expansion as risk to endangered wildlife, pesticide exposure in wild chimpanzees and baboons displaying facial dysplasia. Sci Total Environ (2017) 598,647–56. 10.1016/j.scitotenv.2017.04.113
74.
Doll R Wakeford R . Risk of childhood cancer from fetal irradiation. Br J Radiol (1997) 70,130–9. 10.1259/bjr.70.830.9135438
75.
Russell JG . Pregnancy and ionising radiation. Bmj (1992) 305(6863),1172–3. 10.1136/bmj.305.6863.1172
76.
Frangione B Hinton P Villeneuve PJ . Low-dose ionizing radiation and adverse birth outcomes, a systematic review and meta-analysis. Int Arch Occup Environ Health (2023) 96(1),77–92. 10.1007/s00420-022-01911-2
77.
Mainprize JG Yaffe MJ Chawla T Glanc P . Effects of ionizing radiation exposure during pregnancy. Abdom Radiol (Ny) (2023) 48(5),1564–78. 10.1007/s00261-023-03861-w
78.
Williams PM Fletcher S . Health effects of prenatal radiation exposure. Am Fam Physician (2010) 82(5),488–93.
79.
Mattsson S Leide-Svegborn S Andersson M . X-ray and molecular imaging during pregnancy and breastfeeding-when should we be worried?Radiat Prot Dosimetry (2021) 195(3-4),339–48. 10.1093/rpd/ncab041
80.
Anderson A Singh J Bove R . Neuroimaging and radiation exposure in pregnancy. Handb Clin Neurol (2020) 171,179–91. 10.1016/B978-0-444-64239-4.00009-6
81.
Valente M Denis J Grenier N Arvers P Foucher B Desangles F et al Revisiting biomarkers of total-body and partial-body exposure in a baboon model of irradiation. PLoS One (2015) 10(7),e0132194. 10.1371/journal.pone.0132194
82.
deSouza PN Dey S Mwenda KM Kim R Subramanian SV Kinney PL . Robust relationship between ambient air pollution and infant mortality in India. Sci Total Environ (2022) 815,152755. 10.1016/j.scitotenv.2021.152755
83.
Aguilera J Konvinse K Lee A Maecker H Prunicki M Mahalingaiah S et al Air pollution and pregnancy. Semin Perinatol (2023) 47(8),151838. 10.1016/j.semperi.2023.151838
84.
Rani P Dhok A . Effects of pollution on pregnancy and infants. Cureus (2023) 15(1),e33906. 10.7759/cureus.33906
85.
Konkel L . Taking the heat, potential fetal health effects of hot temperatures. Environ Health Perspect (2019) 127(10),102002. 10.1289/EHP6221
86.
Bach V Libert JP . Hyperthermia and heat stress as risk factors for sudden infant death syndrome, a narrative review. Front Pediatr (2022) 10,816136. 10.3389/fped.2022.816136
87.
Morishima HO Glaser B Niemann WH James LS . Increased uterine activity and fetal deterioration during maternal hyperthermia. Am J Obstet Gynecol (1975) 121(4),531–8. 10.1016/0002-9378(75)90087-3
88.
Samuels L Nakstad B Roos N Bonell A Chersich M Havenith G et al Physiological mechanisms of the impact of heat during pregnancy and the clinical implications, review of the evidence from an expert group meeting. Int J Biometeorol (2022) 66(8),1505–13. 10.1007/s00484-022-02301-6
89.
Zhang Y Yu C Wang L . Temperature exposure during pregnancy and birth outcomes, an updated systematic review of epidemiological evidence. Environ Pollut (2017) 225,700–12. 10.1016/j.envpol.2017.02.066
90.
Zhang W Spero TL Nolte CG Garcia VC Lin Z Romitti PA et al Projected changes in maternal heat exposure during early pregnancy and the associated congenital heart defect burden in the United States. J Am Heart Assoc (2019) 8(3),e010995. 10.1161/JAHA.118.010995
91.
Auger N Fraser WD Arbour L Bilodeau-Bertrand M Kosatsky T . Elevated ambient temperatures and risk of neural tube defects. Occup Environ Med (2017) 74(5),315–20. 10.1136/oemed-2016-103956
92.
Van Zutphen AR Lin S Fletcher BA Hwang SA . A population-based case-control study of extreme summer temperature and birth defects. Environ Health Perspect (2012) 120(10),1443–9. 10.1289/ehp.1104671
93.
Sun S Weinberger KR Spangler KR Eliot MN Braun JM Wellenius GA . Ambient temperature and preterm birth, a retrospective study of 32 million US singleton births. Environ Int (2019) 126,7–13. 10.1016/j.envint.2019.02.023
94.
Racicot K Mor G . Risks associated with viral infections during pregnancy. J Clin Invest (2017) 127(5),1591–9. 10.1172/JCI87490
95.
Yu W Hu X Cao B . Viral infections during pregnancy, the big challenge threatening maternal and fetal health. Matern Fetal Med (2022) 4(1),72–86. 10.1097/FM9.0000000000000133
96.
Schlabritz-Loutsevitch NE Moore CM Lopez-Alvarenga JC Dunn BG Dudley D Hubbard GB . The baboon model (Papio hamadryas) of fetal loss, maternal weight, age, reproductive history and pregnancy outcome. J Med Primatol (2008) 37(6),337–45. 10.1111/j.1600-0684.2008.00297.x
97.
Canfield D Gabby L Vaziri Fard E Gyamfi-Bannerman C . Cytomegalovirus in pregnancy. Obstet Gynecol Clin North Am (2023) 50(2),263–77. 10.1016/j.ogc.2023.02.002
98.
Krstanović F Britt WJ Jonjić S Brizić I . Cytomegalovirus infection and inflammation in developing brain. Viruses (2021) 13(6),1078. 10.3390/v13061078
99.
Andrews EJ . Spontaneous abortions in Macaca mulatta. Lab Anim Sci (1971) 21(6),964.
100.
Auriti C De Rose DU Santisi A Martini L Piersigilli F Bersani I et al Pregnancy and viral infections, mechanisms of fetal damage, diagnosis and prevention of neonatal adverse outcomes from cytomegalovirus to SARS-CoV-2 and zika virus. Biochim Biophys Acta Mol Basis Dis (2021) 1867(10),166198. 10.1016/j.bbadis.2021.166198
101.
Sehnal B Halaška MJ Vlk R Drochýtek V Pichlík T Hruda M et al Human papillomavirus infection (HPV) and pregnancy. Epidemiol Mikrobiol Imunol (2024) 73(1),37–50. 10.61568/emi/11-6254/20240123/136241
102.
Chilaka VN Navti OB Al BM Ahmed B Konje JC . Human papillomavirus (HPV) in pregnancy - an update. Eur J Obstet Gynecol Reprod Biol (2021) 264,340–8. 10.1016/j.ejogrb.2021.07.053
103.
Imbeloni AA de Alcantara BN Coutinho LN de Azevedo Scalercio SRR Carneiro LA Oliveira KG et al Prenatal disorders and congenital zika syndrome in squirrel monkeys. Sci Rep (2021) 11(1),2698. 10.1038/s41598-021-82028-3
104.
Leung AKC Hon KL Leong KF . Rubella (German measles) revisited. Hong Kong Med J (2019) 25(2),134–41. 10.12809/hkmj187785
105.
Mawson AR Croft AM . Rubella virus infection, the congenital rubella syndrome, and the link to autism. Int J Environ Res Public Health (2019) 16(19),3543. 10.3390/ijerph16193543
106.
Delahunt CS Rieser N . Rubella-induced embryopathies in monkeys. Am J Obstet Gynecol (1967) 99(4),580–8. 10.1016/0002-9378(67)90309-2
107.
Oseghale O Vlahos R O'Leary JJ Brooks RD Brooks DA Liong S et al Influenza virus infection during pregnancy as a trigger of acute and chronic complications. Viruses (2022) 14(12),2729. 10.3390/v14122729
108.
Liong S Oseghale O To EE Brassington K Erlich JR Luong R et al Influenza A virus causes maternal and fetal pathology via innate and adaptive vascular inflammation in mice. Proc Natl Acad Sci U S A (2020) 117(40),24964–73. 10.1073/pnas.2006905117
109.
Short SJ Lubach GR Karasin AI Olsen CW Styner M Knickmeyer RC et al Maternal influenza infection during pregnancy impacts postnatal brain development in the rhesus monkey. Biol Psychiatry (2010) 67(10),965–73. 10.1016/j.biopsych.2009.11.026
110.
De Luca D Vauloup-Fellous C Benachi A Vivanti A . Transmission of SARS-CoV-2 from mother to fetus or neonate, what to know and what to do?Semin Fetal Neonatal Med (2023) 28(1),101429. 10.1016/j.siny.2023.101429
111.
Schwartz DA Mulkey SB Roberts DJ . SARS-CoV-2 placentitis, stillbirth, and maternal COVID-19 vaccination, clinical-pathologic correlations. Am J Obstet Gynecol (2023) 228(3),261–9. 10.1016/j.ajog.2022.10.001
112.
Terrault NA Levy MT Cheung KW Jourdain G . Viral hepatitis and pregnancy. Nat Rev Gastroenterol Hepatol (2021) 18(2),117–30. 10.1038/s41575-020-00361-w
113.
Tagkou NM Kondylis G Cholongitas E . Pregnancy and viral hepatitis, current concepts. Curr Pharm Des (2021) 27(36),3775–85. 10.2174/1381612826666201210113841
114.
Khuroo MS . Hepatitis E and pregnancy, an unholy alliance unmasked from kashmir, India. Viruses (2021) 13(7),1329. 10.3390/v13071329
115.
Bergløv A Hallager S Weis N . Hepatitis E during pregnancy, maternal and foetal case-fatality rates and adverse outcomes-A systematic review. J Viral Hepat (2019) 26(11),1240–8. 10.1111/jvh.13129
116.
Page CM Hughes BL Rhee EHJ Kuller JA . Hepatitis C in pregnancy, review of current knowledge and updated recommendations for management. Obstet Gynecol Surv (2017) 72(6),347–55. 10.1097/OGX.0000000000000442
117.
Chaudhry SA Koren G . Hepatitis A infection during pregnancy. Can Fam Physician (2015) 61(11),963–4.
118.
Yu W Hao X Li Y Yang C Li Y He Z et al Vertical transmission of hepatitis E virus in pregnant rhesus macaques. Sci Rep (2020) 10(1),17517. 10.1038/s41598-020-74461-7
119.
Muller WJ . Treatment of perinatal viral infections to improve neurologic outcomes. Pediatr Res (2017) 81(1-2),162–9. 10.1038/pr.2016.191
120.
Silasi M Cardenas I Kwon JY Racicot K Aldo P Mor G . Viral infections during pregnancy. Am J Reprod Immunol (2015) 73(3),199–213. 10.1111/aji.12355
121.
Pfeifer C Bunders MJ . Maternal HIV infection alters the immune balance in the mother and fetus; implications for pregnancy outcome and infant health. Curr Opin HIV AIDS (2016) 11(2),138–45. 10.1097/COH.0000000000000239
122.
Worlein JM Leigh J Larsen K Kinman L Schmidt A Ochs H et al Cognitive and motor deficits associated with HIV-2(287) infection in infant pigtailed macaques, a nonhuman primate model of pediatric neuro-AIDS. J Neurovirol (2005) 11(1),34–45. 10.1080/13550280590901732
123.
Nanthakumar MP Sood A Ahmed M Gupta J . Varicella zoster in pregnancy. Eur J Obstet Gynecol Reprod Biol (2021) 258,283–7. 10.1016/j.ejogrb.2021.01.009
124.
Ramachandra S Metta AK Haneef NS Kodali S . Fetal varicella syndrome. Indian J Dermatol Venereol Leprol (2010) 76(6),724. 10.4103/0378-6323.72475
125.
Lamont RF Sobel JD Carrington D Mazaki-Tovi S Kusanovic JP Vaisbuch E et al Varicella-zoster virus (chickenpox) infection in pregnancy. Bjog (2011) 118(10),1155–62. 10.1111/j.1471-0528.2011.02983.x
126.
Garland M Szeto HH Daniel SS Tropper PJ Myers MM Stark RI . Placental transfer and fetal metabolism of zidovudine in the baboon. Pediatr Res (1998) 44(1),47–53. 10.1203/00006450-199807000-00008
127.
Gurung S Reuter N Preno A Dubaut J Nadeau H Hyatt K et al Zika virus infection at mid-gestation results in fetal cerebral cortical injury and fetal death in the olive baboon. Plos Pathog (2019) 15(1),e1007507. 10.1371/journal.ppat.1007507
128.
Mendling W Martius J Hoyme UB . S1-Guideline on bacterial vaginosis in gynecology and obstetrics, long version - AWMF guideline, registration no. 015/028, July 2013 langfassung - awmf-register nr. 015/028, Juli 2013. Geburtshilfe Frauenheilkd (2014) 74(1),51–4. 10.1055/s-0033-1360230
129.
Kumar M Saadaoui M Al Khodor S . Infections and pregnancy, effects on maternal and child health. Front Cell Infect Microbiol (2022) 12,873253. 10.3389/fcimb.2022.873253
130.
Angelova M Kovachev E Tsankova V Koleva I Mangarova S . Role and importance of Chlamydia Trachomatis in pregnant patients. Open Access Maced J Med Sci (2016) 4(3),410–2. 10.3889/oamjms.2016.077
131.
Filho AC Marcos C Colnago JM Miranda AEB Duarte JN Peruchi LS . Sexually transmitted infections with Chlamydia trachomatis, Neisseria gonorrhoeae, Mycoplasma genitalium, and Trichomonas vaginalis in pregnant women as detected by molecular testing. Indian J Sex Transm Dis AIDS (2023) 44(2),139–42. 10.4103/ijstd.ijstd_119_22
132.
Kebu TI Dzhikidze EK Chikobava MG Arshba IM . Spontaneous urogenital infection in macaques. Zh Mikrobiol Epidemiol Immunobiol (2006)(7) 91–4.
133.
Silva ALM Silva ECO Botelho RM Tenorio LPG Marques ALX Rodrigues I et al Uvaol Prevents Group B streptococcus-induced trophoblast cells inflammation and possible endothelial dysfunction. Front Physiol (2021) 12,766382. 10.3389/fphys.2021.766382
134.
Egal ES Ardeshir A Mariano FV Gondak RO Montalli VA dos Santos HT et al Contribution of endemic listeriosis to spontaneous abortion and stillbirth in a large outdoor-housed colony of rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci (2015) 54(4),399–404.
135.
McClure HM Strozier LM . Perinatal listeric septicemia in a Celebese black ape. J Am Vet Med Assoc (1975) 167(7),637–8. 10.2460/javma.1975.167.07.637
136.
Mahande AM Mahande MJ . Prevalence of parasitic infections and associations with pregnancy complications and outcomes in northern Tanzania, a registry-based cross-sectional study. BMC Infect Dis (2016) 16,78. 10.1186/s12879-016-1413-6
137.
Petersen E . Protozoan and helminth infections in pregnancy. Short-term and long-term implications of transmission of infection from mother to foetus. Parasitology (2007) 134(Pt 13),1855–62. 10.1017/S0031182007000182
138.
Paquet C Yudin MH, Society of Obstetricians and Gynaecologists of Canada. Toxoplasmosis in pregnancy, prevention, screening, and treatment. J Obstet Gynaecol Can (2013) 35(1),78–81. 10.1016/s1701-2163(15)31053-7
139.
Fitzpatrick D Holmes NE Hui L . A systematic review of maternal TORCH serology as a screen for suspected fetal infection. Prenat Diagn (2022) 42(1),87–96. 10.1002/pd.6073
140.
Neu N Duchon J Zachariah P . TORCH infections. Clin Perinatol (2015) 42(1), 77–103. 10.1016/j.clp.2014.11.001
141.
Awadalla M Liu A . TORCH infections. Pediatr Ann (2023) 52(11),e400–e406. 10.3928/19382359-20230908-01
142.
Mate A Reyes-Goya C Santana-Garrido Á Sobrevia L Vázquez CM . Impact of maternal nutrition in viral infections during pregnancy. Biochim Biophys Acta Mol Basis Dis (2021) 1867(11),166231. 10.1016/j.bbadis.2021.166231
143.
Lorea CF Pressman K Schuler-Faccini L . Infections during pregnancy, an ongoing threat. Semin Perinatol (2025) 49(4),152075. 10.1016/j.semperi.2025.152075
144.
Bisen S Kakhniashvili D Johnson DL Bukiya AN . Proteomic analysis of Baboon cerebral artery reveals potential pathways of damage by prenatal alcohol exposure. Mol Cell Proteomics (2019) 18(2),294–307. 10.1074/mcp.RA118.001047
145.
Tobiasz AM Duncan JR Bursac Z Sullivan RD Tate DL Dopico AM et al The effect of prenatal alcohol exposure on fetal growth and cardiovascular parameters in a baboon model of pregnancy. Reprod Sci (2018) 25(7),1116–23. 10.1177/1933719117734317
146.
Gillis RF Palmour RM . miRNA expression analysis of the hippocampus in a vervet monkey model of fetal alcohol spectrum disorder reveals a potential role in global mRNA downregulation. Brain Sci (2023) 13(6),934. 10.3390/brainsci13060934
147.
Lange S Shield K Rehm J Anagnostou E Popova S . Fetal alcohol spectrum disorder, neurodevelopmentally and behaviorally indistinguishable from other neurodevelopmental disorders. BMC Psychiatry (2019) 19(1),322. 10.1186/s12888-019-2289-y
148.
Jacobson SW Hoyme HE Carter RC Dodge NC Molteno CD Meintjes EM et al Evolution of the physical phenotype of fetal alcohol spectrum disorders from childhood through adolescence. Alcohol Clin Exp Res (2021) 45(2),395–408. 10.1111/acer.14534
149.
Jimenez VA Wang X Newman N Walter NAR Gonzales S Lo JO et al Detecting neurodevelopmental effects of early-gestation ethanol exposure, a nonhuman primate model of ethanol drinking during pregnancy. Alcohol Clin Exp Res (2019) 43(2),250–61. 10.1111/acer.13938
150.
Gillis RF Palmour RM . mRNA expression analysis of the hippocampus in a vervet monkey model of fetal alcohol spectrum disorder. J Neurodev Disord (2022) 14(1),21. 10.1186/s11689-022-09427-z
151.
Schneider ML Moore CF Becker EF . Timing of moderate alcohol exposure during pregnancy and neonatal outcome in rhesus monkeys (Macaca mulatta). Alcohol Clin Exp Res (2001) 25(8),1238–45. 10.1097/00000374-200108000-00021
152.
Jarmasz JS Stirton H Basalah D Davie JR Clarren SK Astley SJ et al Global DNA methylation and histone posttranslational modifications in Human and Nonhuman Primate Brain in Association with prenatal alcohol exposure. Alcohol Clin Exp Res (2019) 43(6),1145–62. 10.1111/acer.14052
153.
Lo JO Schabel MC Roberts VH Wang X Lewandowski KS Grant KA et al First trimester alcohol exposure alters placental perfusion and fetal oxygen availability affecting fetal growth and development in a non-human primate model. Am J Obstet Gynecol (2017) 216(3),302.e301–302. 10.1016/j.ajog.2017.01.016
154.
Heatley MK Crane J . The blood alcohol concentration at post-mortem in 175 fatal cases of alcohol intoxication. Med Sci L (1990) 30(2),101–5. 10.1177/002580249003000203
155.
Zador PL Krawchuk SA Voas RB . Alcohol-related relative risk of driver fatalities and driver involvement in fatal crashes in relation to driver age and gender, an update using 1996 data. J Stud Alcohol (2000) 61(3),387–95. 10.15288/jsa.2000.61.387
156.
Burke MW Ptito M Ervin FR Palmour RM . Hippocampal neuron populations are reduced in vervet monkeys with fetal alcohol exposure. Dev Psychobiol (2015) 57(4),470–85. 10.1002/dev.21311
157.
Schneider ML Moore CF Barr CS Larson JA Kraemer GW . Moderate prenatal alcohol exposure and serotonin genotype interact to alter CNS serotonin function in rhesus monkey offspring. Alcohol Clin Exp Res (2011) 35(5),912–20. 10.1111/j.1530-0277.2010.01421.x
158.
Sushma DA Kanchan S Jha G Mishra S Sharma D Rath SK et al Alcohol induced impairment/abnormalities in brain, role of MicroRNAs. Neurotoxicology (2021) 87,11–23. 10.1016/j.neuro.2021.08.013
159.
Gohlke JM Griffith WC Faustman EM . Computational models of ethanol-induced neurodevelopmental toxicity across species, implications for risk assessment. Birth Defects Res B Dev Reprod Toxicol (2008) 83(1),1–11. 10.1002/bdrb.20137
160.
Bukiya AN . Fetal cerebral artery mitochondrion as target of prenatal alcohol exposure. Int J Environ Res Public Health (2019) 16(9),1586. 10.3390/ijerph16091586
161.
Weerts EM Goodwin AK Kaminski BJ Hienz RD . Environmental cues, alcohol seeking, and consumption in baboons, effects of response requirement and duration of alcohol abstinence. Alcohol Clin Exp Res (2006) 30(12),2026–36. 10.1111/j.1530-0277.2006.00249.x
162.
Kochunov P Castro C Davis DM Dudley D Wey HY Purdy D et al Fetal brain during a binge drinking episode, a dynamic susceptibility contrast MRI fetal brain perfusion study. Neuroreport (2010) 21(10),716–21. 10.1097/WNR.0b013e32833b5047
163.
Parnell SE Ramadoss J Delp MD Ramsey MW Chen WJ West JR et al Chronic ethanol increases fetal cerebral blood flow specific to the ethanol-sensitive cerebellum under normoxaemic, hypercapnic and acidaemic conditions, ovine model. Exp Physiol (2007) 92(5),933–43. 10.1113/expphysiol.2007.038091
164.
Bake S Tingling JD Miranda RC . Ethanol exposure during pregnancy persistently attenuates cranially directed blood flow in the developing fetus, evidence from ultrasound imaging in a murine second trimester equivalent model. Alcohol Clin Exp Res (2012) 36(5),748–58. 10.1111/j.1530-0277.2011.01676.x
165.
Bukiya AN Dopico AM . Fetal cerebral circulation as target of maternal alcohol consumption. Alcohol Clin Exp Res (2018) 42(6),1006–18. 10.1111/acer.13755
166.
Menshawi K Mohr JP Gutierrez J . A functional perspective on the embryology and anatomy of the cerebral blood supply. J Stroke (2015) 17(2),144–58. 10.5853/jos.2015.17.2.144
167.
Anderson RH Bamforth SD . Morphogenesis of the Mammalian aortic arch arteries. Front Cell Dev Biol (2022) 10,892900. 10.3389/fcell.2022.892900
168.
Meombe Mbolle A Thapa S Bukiya AN Jiang H . High-resolution imaging in studies of alcohol effect on prenatal development. Adv Drug Alcohol Res (2023) 3,10790. 10.3389/adar.2023.10790
169.
Bronikowski AM Alberts SC Altmann J Packer C Carey KD Tatar M . The aging baboon, comparative demography in a non-human primate. Proc Natl Acad Sci U S A (2002) 99(14),9591–5. 10.1073/pnas.142675599
170.
Metz TD Borgelt LM . Marijuana use in pregnancy and while breastfeeding. Obstet Gynecol (2018) 132(5),1198–210. 10.1097/AOG.0000000000002878
171.
Andrade C . Maternal Cannabis use during pregnancy and maternal and neonatal adverse outcomes. J Clin Psychiatry (2024) 85(4),24f15611. 10.4088/JCP.24f15611
172.
Shorey-Kendrick LE Roberts VHJ D'Mello RJ Sullivan EL Murphy SK McCarty OJT et al Prenatal delta-9-tetrahydrocannabinol exposure is associated with changes in rhesus macaque DNA methylation enriched for autism genes. Clin Epigenetics (2023) 15(1),104. 10.1186/s13148-023-01519-4
173.
National Academies of Sciences E. Medicine, division on E, life S, health, medicine D, Institute for Laboratory Animal R, board on Health Sciences P, Committee on the state of the S, future needs for nonhuman primate model S. The national academies collection, reports funded by national institutes of health. In, YostOCDowneyARamosKS, editors. Nonhuman primate models in biomedical research, state of the science and future needs. Washington (DC), National Academies Press (US) Copyright 2023 by the National Academy of Sciences. All rights reserved. (2023).
174.
Mulligan MK Hamre KM . Influence of prenatal cannabinoid exposure on early development and beyond. Adv Drug Alcohol Res (2023) 3,10981. 10.3389/adar.2023.10981
175.
Díaz-Alonso J Aguado T Wu CS Palazuelos J Hofmann C Garcez P et al The CB(1) cannabinoid receptor drives corticospinal motor neuron differentiation through the Ctip2/Satb2 transcriptional regulation axis. J Neurosci (2012) 32(47),16651–65. 10.1523/JNEUROSCI.0681-12.2012
176.
Richardson KA Hester AK McLemore GL . Prenatal cannabis exposure - the “first hit” to the endocannabinoid system. Neurotoxicol Teratol (2016) 58,5–14. 10.1016/j.ntt.2016.08.003
177.
Hurd YL Manzoni OJ Pletnikov MV Lee FS Bhattacharyya S Melis M . Cannabis and the developing brain, insights into its long-lasting effects. J Neurosci (2019) 39(42),8250–8. 10.1523/JNEUROSCI.1165-19.2019
178.
Olyaei AF Campbell LR Roberts VHJ Lo JO . Animal models evaluating the impact of prenatal exposure to tobacco and marijuana. Clin Obstet Gynecol (2022) 65(2),334–46. 10.1097/GRF.0000000000000693
179.
Grewen K Salzwedel AP Gao W . Functional connectivity disruption in neonates with prenatal marijuana exposure. Front Hum Neurosci (2015) 9,601. 10.3389/fnhum.2015.00601
180.
Ryan KS Karpf JA Chan CN Hagen OL McFarland TJ Urian JW et al Prenatal delta-9-tetrahydrocannabinol exposure alters fetal neurodevelopment in rhesus macaques. Sci Rep (2024) 14(1),5808. 10.1038/s41598-024-56386-7
181.
Schneider EH Fitzgerald AC Ponnapula SS Dopico AM Bukiya AN . Differential distribution of cholesterol pools across arteries under high-cholesterol diet. Biochim Biophys Acta Mol Cell Biol Lipids (2022) 1867(12),159235. 10.1016/j.bbalip.2022.159235
182.
Lu HC Mackie K . Review of the endocannabinoid system. Biol Psychiatry Cogn Neurosci Neuroimaging (2021) 6(6),607–15. 10.1016/j.bpsc.2020.07.016
183.
Hoffman AF Lupica CR . Synaptic targets of Δ9-tetrahydrocannabinol in the central nervous system. Cold Spring Harb Perspect Med (2013) 3(8),a012237. 10.1101/cshperspect.a012237
184.
Smith A Kaufman F Sandy MS Cardenas A . Cannabis exposure during critical windows of development, epigenetic and molecular pathways implicated in neuropsychiatric disease. Curr Environ Health Rep (2020) 7(3),325–42. 10.1007/s40572-020-00275-4
185.
Reece AS Hulse GK . Impacts of cannabinoid epigenetics on human development, reflections on murphy et. Al. Cannabinoid exposure and altered DNA methylation in rat and human sperm' epigenetics. Epigenetics (2019) 14(11),1041–56. 10.1080/15592294.2019.1633868
186.
Keim SA Fried P Yeates KO Boone KM Vrantsidis DM Dean A et al Prenatal cannabis exposure and executive function and aggressive behavior at age 5 years. JAMA Pediatr (2024) 178(12),1316–25. 10.1001/jamapediatrics.2024.4352
187.
Wu CS Jew CP Lu HC . Lasting impacts of prenatal cannabis exposure and the role of endogenous cannabinoids in the developing brain. Future Neurol (2011) 6(4),459–80. 10.2217/fnl.11.27
188.
Slotkin TA Seidler FJ Spindel ER . Prenatal nicotine exposure in rhesus monkeys compromises development of brainstem and cardiac monoamine pathways involved in perinatal adaptation and sudden infant death syndrome, amelioration by vitamin C. Neurotoxicol Teratol (2011) 33(3),431–4. 10.1016/j.ntt.2011.02.001
189.
Slotkin TA Pinkerton KE Seidler FJ . Perinatal environmental tobacco smoke exposure in rhesus monkeys, critical periods and regional selectivity for effects on brain cell development and lipid peroxidation. Environ Health Perspect (2006) 114(1),34–9. 10.1289/ehp.8286
190.
Slotkin TA Pinkerton KE Auman JT Qiao D Seidler FJ . Perinatal exposure to environmental tobacco smoke upregulates nicotinic cholinergic receptors in monkey brain. Brain Res Dev Brain Res (2002) 133(2),175–9. 10.1016/s0165-3806(02)00281-x
191.
Roy TS Andrews JE Seidler FJ Slotkin TA . Nicotine evokes cell death in embryonic rat brain during neurulation. J Pharmacol Exp Ther (1998) 287(3),1136–44. 10.1016/s0022-3565(24)37911-x
192.
Smith AM Dwoskin LP Pauly JR . Early exposure to nicotine during critical periods of brain development, mechanisms and consequences. J Pediatr Biochem (2010) 1(2),125–41. 10.3233/JPB-2010-0012
193.
Slotkin TA Seidler FJ Qiao D Aldridge JE Tate CA Cousins MM et al Effects of prenatal nicotine exposure on primate brain development and attempted amelioration with supplemental choline or vitamin C, neurotransmitter receptors, cell signaling and cell development biomarkers in fetal brain regions of rhesus monkeys. Neuropsychopharmacology (2005) 30(1),129–44. 10.1038/sj.npp.1300544
194.
Bruin JE Gerstein HC Holloway AC . Long-term consequences of fetal and neonatal nicotine exposure, a critical review. Toxicol Sci (2010) 116(2),364–74. 10.1093/toxsci/kfq103
195.
North K Tobiasz A Sullivan RD Bursac Z Duncan J Sullivan JP et al Prenatal alcohol exposure, anesthesia, and fetal loss in baboon model of pregnancy. J Drug Alcohol Res (2018) 7,236064. 10.3389/adar.2022.10818
Summary
Keywords
in utero , drugs of abuse, alcohol, environmental insult, developmental exposure, neurogenesis, cerebral artery, cerebrovascular development
Citation
Iskusnykh IY, Thapa S, Chizhikov VV and Bukiya AN (2025) Prenatal environmental exposures and brain development: studies with baboons and other nonhuman primates. Adv. Drug Alcohol Res. 5:14858. doi: 10.3389/adar.2025.14858
Received
05 May 2025
Accepted
22 July 2025
Published
11 August 2025
Volume
5 - 2025
Edited by
Emmanuel Onaivi, William Paterson University, United States
Reviewed by
Sean Farris, University of Pittsburgh, United States
Viola Neudecker, Columbia University, United States
Updates
Copyright
© 2025 Iskusnykh, Thapa, Chizhikov and Bukiya.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Igor Y. Iskusnykh, iiskusny@uthsc.edu; Anna N. Bukiya, abukiya@uthsc.edu
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.