Abstract
In most vertebrates, hemoglobin’s primary function is to transport oxygen and carbon dioxide. Hemoglobin is also expressed in cells such as dopaminergic neurons and chondrocytes, as well as in organelles such as mitochondria. Depending on its location, hemoglobin subunits can interact with proteins involved in various functions, including anion exchange, nitric oxide synthesis, and ATP synthesis. These interactions suggest that hemoglobin has diverse regulatory roles beyond gas transport. During hypoxia and an excess of nitrite and protons, deoxygenated hemoglobin exhibits nitrite reductase activity and produces nitric oxide, a gaseous signaling molecule. Hemoglobin-derived nitric oxide is associated with vasodilation in mammals and the inhibition of mitochondrial respiration in cell cultures. This raises the question of whether hemoglobin functions as a gasoreceptor in these cells or organelles. The HIF1α/PHD2 pathway in mammals and cysteine oxidases in plants are largely responsible for sensing hypoxia, but the identity of oxygen gasoreceptors analogous to the mammalian nitric oxide gasoreceptor soluble guanylate cyclase and the plant ethylene gasoreceptor kinases remains unknown. Since the heme-based dual oxygen-binding and catalytic domain emerged earlier than the allosteric regions, I propose hemoglobin as an oxygen proto-gasoreceptor derivative. Furthermore, since hemoglobin interacts with and regulates proteins depending on its oxygen binding state, I propose that hemoglobin functions as an oxygen gasoreceptor in split-component signal transduction systems. Recognizing hemoglobin as a gasoreceptor will expand the emerging field of gasocrinology to encompass gases that were previously considered primarily metabolic substrates.
If there is a gasoreceptor for nitric oxide, is there a gasoreceptor for oxygen?
Gases are evolutionarily old signaling molecules and major regulators of cellular metabolism (Aono, 2017; Canfield, 2014). Embryo development is one of the crucial timepoints in which gas homeostasis in cells must be tightly regulated to prevent developmental disorders and embryonic lethality (Maltepe et al., 1997; Froehlich-Santino et al., 2014; Pan et al., 2024). It is conceivable that the diffusion of gases may also provide and regulate positional information and tissue scaling during embryogenesis (Čapek and Müller, 2019; Anbalagan, 2024a). For example, in a few model vertebrate and invertebrate embryos, exposure to an anoxic environment can lead to developmental arrest or suspended animation (Padilla and Roth, 2001; Teodoro and O’Farrell, 2003). Apart from dioxygen (O2), an environmentally-derived essential gaseous molecule for vertebrates, other gaseous molecules such as nitric oxide (NO), carbon monoxide (CO), hydrogen sulfide (H2S), methane (CH4), hydrogen cyanide (HCN), and ethylene (C2H4) are synthesized in mammalian cells (Carlew et al., 2020; Wang, 2002). The molecular machinery that synthesizes these gases is highly conserved throughout evolution. This suggests the potential importance of these molecules for signaling and maintaining cellular homeostasis throughout an animal’s life. Nevertheless, except for a few gaseous molecules, knowledge about the precise gasoreceptor-mediated signaling role of gases during embryo development is currently lacking both in animals and in plants (Liu et al., 2020; Sonnen and Janda, 2021; Zhang Y. et al., 2023; Corbineau, 2024).
Gasoreceptors and gasocrine signaling
Since the following sections focus primarily on direct gas-sensing mechanisms, I define gas sensor proteins as proteins that require gases (or their solutes) as substrates for enzyme activity. Gasoreceptors are proteins in which either the binding or lack of binding of a gaseous molecule (or its solute) as a ligand can trigger a cellular signal or response (Anbalagan, 2024a). Gasoreceptors can bind a gaseous molecule either via a metal cofactor or in a metal cofactor-independent manner. Gasoreceptors can be any gas binding-based allosterically-regulated protein with various functions, including enzyme, transcription factor, and ion channel activities (Aono, 2017; Anbalagan, 2024b). For instance, mammalian soluble guanylate cyclase (sGC) and the Escherichia coli Direct Oxygen Sensor (DosP) phosphodiesterase are NO and O2 gasoreceptors, respectively. While sGC is well known for its role in vasodilation in mammals, DosP regulates E. coli biofilm formation and motility (Ignarro and Freeman, 2017; Delgado-Nixon et al., 2000; Shimizu, 2013). Based on the classification of signal transduction systems (STSs) in prokaryotes, the sGC and DosP can potentially be described as a gasoreceptor that function in one-component STSs (Ulrich et al., 2005; Wuichet et al., 2010). However, the classification of sGC is controversial because the cGMP-generating catalytic site forms at the interface of heterodimer. Therefore, a more appropriate classification for sGC could may be as a co-component STS. Finally, diverse cellular signaling events mediated by gases acting as ligands for gasoreceptors are unified under the umbrella term “gasocrine signaling” (Anbalagan, 2024a).
Unanswered questions in acute O2 sensing mechanisms
To the best of my knowledge, the majority of the O2-sensing mechanisms in both plants and erythrocyte-containing vertebrates are largely based on O2 sensors (Hammarlund et al., 2020). These sensors can belong to major enzyme classes, such as dioxygenases, monooxygenases, and oxidases, all of which require O2 as a substrate. However, numerous O2-dependent enzymes (more than 200 enzymes in humans alone) can potentially be considered as O2 sensors. Depending on their cellular localization, these proteins could potentially perform O2-sensing roles in their respective cellular regions or organelles (Li et al., 2023). Amongst these enzymes, Prolyl hydroxylase domain proteins (PHDs) are well-known O2 sensor proteins in humans and mice. These enzymes require O2 for hydroxylase activity and regulate the Hypoxia-inducible factor 1-alpha/Prolyl Hydroxylase Domain Protein 2/von Hippel-Lindau (HIF1α/PHD2/VHL) pathway (Hammarlund et al., 2020; Li et al., 2023; Ratcliffe and Keeley, 2025). In plants, cysteine oxidases along with the N-degron pathway, and reactive oxygen species-mediated signaling are considered major mechanisms by which plants sense O2 (Hammarlund et al., 2020; Holdsworth and Gibbs, 2020).
However, the precise identity of vertebrate and plant O2 gasoreceptors similar to the mammalian NO gasoreceptor sGC or the E. coli O2 gasoreceptor phosphodiesterase (DosP) remains unclear. Even in chemoreceptors cells such as the glomus cells of the carotid body and pulmonary artery smooth muscle cells, the identity of O2 gasoreceptors are unknown (Weissmann et al., 2006; Moreno-Domínguez et al., 2020; Gao et al., 2022). In the carotid body, cytochrome c oxidase (complex IV) of the mitochondrial electron transport chain has been reported as an O2 sensor (Moreno-Domínguez et al., 2020; Peng et al., 2010; Kumar and Prabhakar, 2012; Peng et al., 2020. Nevertheless, it is unclear whether these sensors bind O2 and function as O2 gasoreceptors or respond to other accumulating molecules, such as reactive oxygen species, H2S, NO, or phosphatidic acid (Gao et al., 2022; Peng et al., 2023; Aragonés et al., 2001). In considering O2per se as a gaseous signaling molecule, I proposed it as an essential gasotransmitter, similar to “essential” amino acids (Anbalagan, 2024c).
In the main olfactory epithelium sensory neurons (type B cells) of mice, it has been reported that Soluble guanylate cyclase 1 subunit beta 2 (GUCY1B2) and Transient receptor potential cation channel subfamily C member 2 (TRPC2) mediate calcium influx responses under low O2 conditions. However, it is unclear whether these proteins act as O2 gasoreceptors (Bleymehl et al., 2016). Similar calcium influx-related responses to acute hypoxia have also been reported in astrocytes, which appear to function independently of peripheral chemoreceptor O2-sensing mechanisms. Specifically, in astrocytes isolated from the mice parafacial respiratory group and the retrotrapezoid nucleus, calcium influx is regulated by the differential accumulation of Transient receptor potential cation channel, subfamily A, member 1 (TRPA1) in the plasma membrane under hypoxic conditions (Uchiyama et al., 2020). PHD2 and Neural precursor cell-expressed developmentally downregulated gene 4-1 (NEDD4-1) E3 ubiquitin ligase-dependent trafficking of TRPA1 channels are implicated in this process; however, it is unclear whether these proteins perform the role as O2 gasoreceptors. Finally, the “hypoxia sensor” in astrocytes is considered to reside inside the mitochondria, thus suggesting a similar mechanism to that of glomus cells, in which mitochondrial cytochrome c oxidase has been reported as an O2 sensor protein, but not an O2 gasoreceptor (Moreno-Domínguez et al., 2020; Gao et al., 2022; Angelova et al., 2015). Overall, the identity of O2 gasoreceptors in vertebrates and plants remains unknown.
Oxygen gasoreceptors in one-component signal transduction system
O2 sensors such as E. coli DosP phosphodiesterase, Rhizobium meliloti FixL kinase, Caenorhabditis elegans GCY-35 soluble guanylate cyclase, and Leishmania major HemAc-Lm soluble adenylate cyclase, appear to be O2 gasoreceptors in one-component STS (Aono, 2017; Delgado-Nixon et al., 2000; Monson et al., 1992; Anbalagan, 2024b) (Figure 1A). These gasoreceptors contain both a heme-based O2-binding domain and a signaling domain that exhibits different enzyme activity in each organism, regulating diverse cellular responses. Thus, although “O2 sensors” that appear to function as O2 gasoreceptors have been reported in various other model organisms, it is surprising that knowledge of vertebrate and plant O2 sensing is based on O2 sensors and not O2 gasoreceptors (Hammarlund et al., 2020; Moreno-Domínguez et al., 2020; López-Barne et al., 2016). Then a few questions arise: Does O2 sensing occur only via O2 sensors? Are there vertebrate and plant O2 gasoreceptors that function as either one-, two-, or multi-component STSs? Or such O2 gasoreceptors already been identified but mislabeled, similar to the blind men and the elephant parable (The Blind Men and the Elephant, 1992; Anbalagan, 2023)?
FIGURE 1
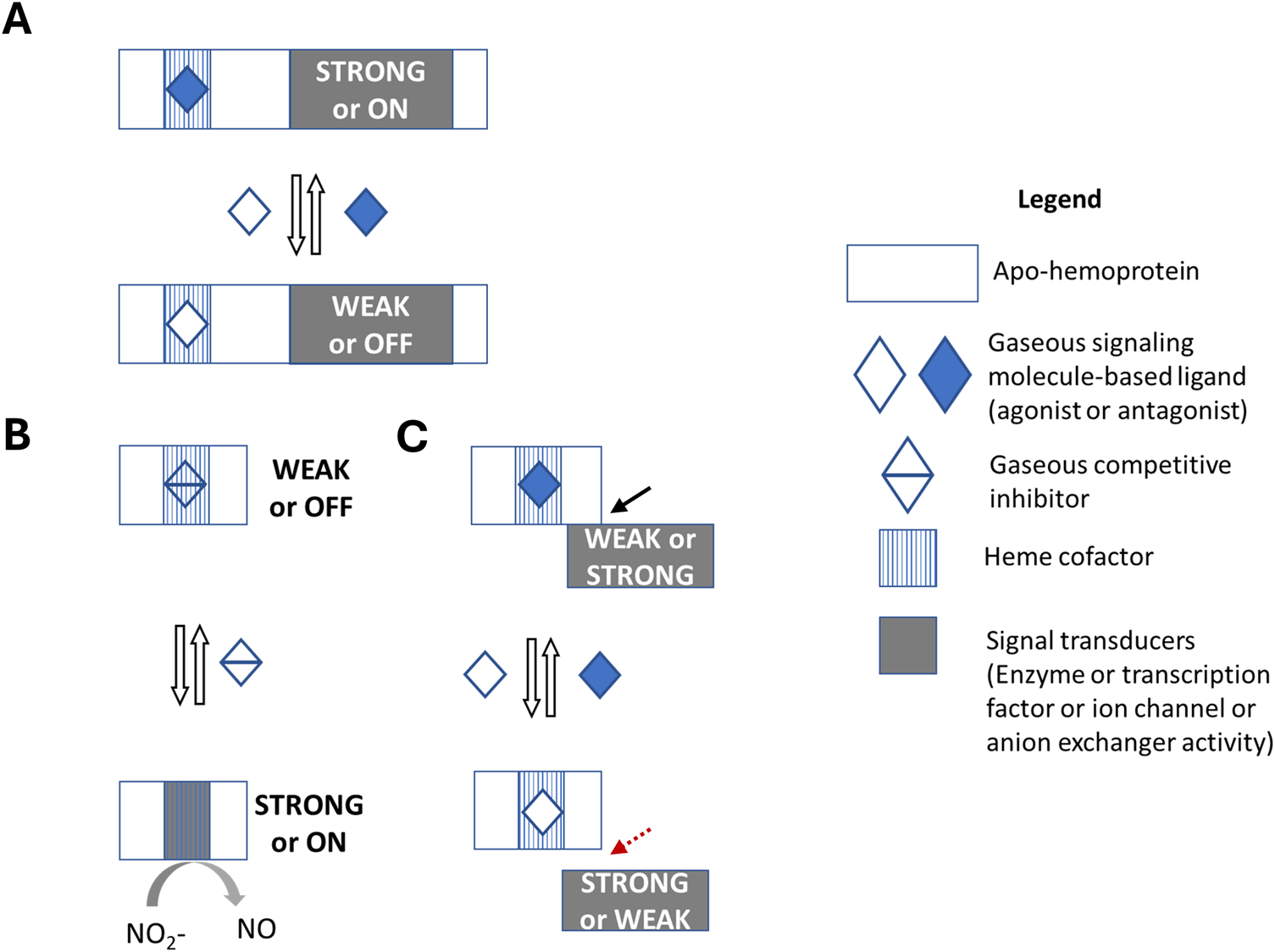
Gasoreceptors in signal transduction systems. Gasoreceptors can bind to gaseous molecules (solutes) in a metal cofactor-dependent or -independent manner. In this scheme, however, gaseous molecule-binding heme cofactor-based gasoreceptors in different signal transduction systems (STSs) are provided. The output domain of the signal transducer in STS can exhibit any type of activity, e.g., an enzyme, a transcription factor, an ion channel, an anion exchanger, etc. (A) A gasoreceptor in one-component STS contains two domains: an input domain that binds extracellular or intercellular gaseous molecule, and the second output domain that acts as a signal transducer. Binding of gaseous molecule to the input domain triggers a conformational change, which increases or decreases output domain activity. Examples of O2 gasoreceptors in one-component STS include the E. coli DosP phosphodiesterase and C. elegans GCY-35 soluble guanylate cyclase. (B) A proto-gasoreceptor in proto-component STS has one domain where both the input and output domains overlap in a mutually exclusive manner. Thus, the gaseous molecule binding event is competitive inhibition of the intrinsic signal transducer activity of the output domain. Deoxygenated hemoglobin is proposed as a proto-gasoreceptor derivative due to its nitrite reductase activity and the presence of additional allosteric sites. (C) A gasoreceptor in split-component STS contains two interacting proteins, one with the input domain and the other with the output domain. The activity of the signal transducer must be dependent on the gas-binding state of the gasoreceptor. Hemoglobin can be considered an O2 gasoreceptor and some of its signal transducers include the SLC4A1/BAND3 anion exchanger, deoxygenated hemoglobin with nitrite reductase activity in erythrocytes, eNOS in endothelial cells, and ATP synthase in mitochondria. The black line with an arrow and the red dashed line with an arrow indicate the formation and separation of the complex, respectively. Since additional allosteric regulators can control hemoglobin’s O2 binding state, as well as its nitrite reductase activity or interactions with proteins that act as signal transducers, hemoglobin can also be considered a receptor for co-ligands, such as protons and CO2 (not shown in the figure).
Androglobin - a candidate O2 gasoreceptor
Androglobin (ADGB) which is known to expressed in mammalian testis, female reproductive tract, lungs and brain is a promising candidate for an O2 gasoreceptor in one-component STS (Koay et al., 2021; Hoogewijs et al., 2012). The circular permuted globin domain of ADGB can bind O2 and NO and based on its binding kinetics, ADGB has been proposed to have an O2 sensing rather than transport role (Hoogewijs et al., 2012; Reeder et al., 2024). ADGB is necessary for sperm development in mice and ciliogenesis in cell cultures (Koay et al., 2021; Keppner et al., 2022). Mice ADGB-/- mutants exhibit defective maturation of elongating spermatids, abnormal sperm shape, defects in sperm microtubule and mitochondrial organization (Keppner et al., 2022). However, it is unclear whether ADGB’s protease activity (either autolytic or on its targets) is regulated by its O2-binding status. At least for one of its binding partner and protease activity target, Septin 10 (SEPT10), ADGB’s protease activity is unaffected by chronic hypoxia (24 h at 0.2% O2) in cell culture-based experiments. Finally, ADGB is not the only O2 binding protein that can be considered as a candidate O2 gasoreceptor.
Hemoglobin: a derivative of an ancestral proto-gasoreceptor
Nitrite reductase activity of deoxygenated hemoglobin
The role of archaeal, bacterial, and yeast protoglobins and flavohemoglobins was proposed to be that of enzymes such as nitrite reductase, NO dioxygenase, denitrosylase, and alkylhydroperoxide reductase, rather than that of gas transport (Zhu and Riggs, 1992; Durner et al., 1999; Bonamore et al., 2003; Corker and Poole, 2003; Freitas et al., 2004; Ascenzi et al., 2014). Similar enzymatic activity has also been reported in plant phytoglobins (Mukhi et al., 2017; Villar et al., 2020). Oxygenated hemoglobin is a NO dioxygenase, while deoxygenated hemoglobin (with 40%–60% oxyHb saturation) and deoxygenated form of its paralogs and orthologs also exhibit nitrite reductase activity. They use co-substrates, such as nitrite and protons, to generate NO, a gaseous signaling molecule and gasotransmitter with a well-known role in vasodilation, among other functions (Huang et al., 2005; Angelo et al., 2008). Given its use of nitrite and protons as a substrate, hemoglobin can be considered one of the nitrite and proton sensor proteins. However, O2 and CO are competitive inhibitors of deoxygenated hemoglobin’s nitrite reductase activity and resulting signaling response, thus warranting reconsideration of hemoglobin’s role as a gas-transporting protein.
Hemoglobin - a gas transporter protein in immobile cells?
Despite its nitrite reductase-dependent signaling role in vivo, hemoglobin and its paralogs are only referred to as a transporter or metabolic sensor, not an O2 gasoreceptor (Adepu et al., 2024). The well-known role of hemoglobin in transporting O2 and its low dissociation constant (KD) for O2 preclude its classification as an O2 gasoreceptor (Hammarlund et al., 2020). However, dissociation constants must be considered not from the perspective of in vitro studies but rather from the point of physiological O2 concentration in vivo, where hemoglobin can be present even in a transient manner. Moreover, reversible binding is not unique to gas-transporting proteins; it is also a feature of previously well-accepted gasoreceptors.
Both hemoglobin and the NO gasoreceptor sGC exhibit reversible binding of O2 and NO, respectively (Kharitonov et al., 1997; Winger et al., 2007). sGC binds NO with a relatively higher affinity (dissociation constant KD of 4.2 pM - 54 nM), but dissociates more freely due to its rapid dissociation kinetics. In contrast, O2 binds to hemoglobin with a relatively lower affinity (KD of 1–10 μM) and dissociates less freely (Winger et al., 2007; Unzai et al., 1998; Tsai et al., 2012). However, since the concentration of O2 in typical vertebrate tissues (5–50 μM) is higher than the concentration of NO (100pM-5 nM), hemoglobin’s relatively low KD for O2 should not exclude its consideration as an O2 gasoreceptor in a microenvironment-dependent manner (Hall and Garthwaite, 2009). Similar arguments can be made for CO (KD of 1–10 nM) and for other gases that can competitively inhibit hemoglobin (e.g., NO, H2S, HCN) (Yuan et al., 2022).
Finally, in vertebrates with erythrocytes, the role of hemoglobin in gas transport largely depends on erythrocytes being passively carried in the bloodstream and O2 being delivered to cells due to the Bohr effect (Malte et al., 2021). In human and mice, the NO gasoreceptor sGC is expressed not only in “immobile” vascular smooth muscle cells. Relatively mobile cells, such as platelets, neutrophils, and macrophages, also express sGC (Ciuman et al., 2006; Makhoul et al., 2018). Similarly, hemoglobin is expressed not only in mobile cells, such as mature erythrocytes. Hemoglobin subunit expression has been reported in alveolar epithelial cells, endothelial cells, cortical neurons, A9 dopaminergic neurons in the substantia nigra, cortical and hippocampal astrocytes, oligodendrocytes, epidermal keratinocytes, and chondrocytes and even in organelles such as mitochondria and membraneless condensates (Hedy’s) (Bhaskaran et al., 2005; Biagioli et al., 2009; Richter et al., 2009; Shephard et al., 2014; Brunyanszki et al., 2015; Tahara et al., 2022; Zhang F. et al., 2023; Reed et al., 2025). These findings suggest that, similar to sGC, hemoglobin is expressed in various mobile and immobile cells. However, the precise reason hemoglobin is still accepted as an O2 transport protein in relatively immobile cells is unclear. Is it due to hemoglobin’s diffusion or intrinsic mobility within a cell (Kutchai and Staub, 1969; Richardson et al., 2020)? Or is it due to the interaction with other mobile proteins, entropy or the physical activity of immobile cells or animals? Therefore, hemoglobin, a multifunctional protein that transport gases, can also potentially act as a gasoreceptor.
Hemoglobin as a proto-gasoreceptor derivative
The receptors in one-component STSs are evolutionarily advanced. They have at least two protein domains that perform different functions: one binds the ligand, and the other signals (Ulrich et al., 2005). Since simple gases are evolutionarily ancient than peptides, proto-receptors for gases probably consisted of proteins or nucleic acids with fewer domains (Harold et al., 2023; Anbalagan, 2024b).
The concept of proto-receptors for peptides has been proposed before, but a clear definition has yet to be established (Root-Bernstein, 2005). I define a proto-receptor as an evolutionarily ancient minimalistic receptor type, in which the signaling and sensing domains completely overlap, and which lacks any additional sites for allosteric regulation. Signaling due to proto-receptors can be considered to function in a proto-component STS, that predates one- and split-component STSs. In simple terms, ancestral proteins lacking allosteric regulation can be considered as proto-receptors for the competitive inhibitors that bind and inhibit their activity. For both proto-cells and also modern cells, the absence of a signal can itself serve as a signal. As an analogy, consider a traffic signal that stopped working at a busy intersection. Drivers approaching the intersection would still interpret it as a message to proceed with caution at their own risk. Extending the concept of proto-receptors to gaseous ligands, I define a proto-gasoreceptor as a protein that becomes competitively inhibited by the binding of a gaseous molecule. This results in an absence of cellular signals or responses. O2 and hydrogen cyanide (HCN)-binding protein Campylobacter jejuni truncated hemoglobin P (Cj-trHbP) has been demonstrated to exhibit peroxidase-like activity in vitro (Lu et al., 2007; Bolli et al., 2008; Ascenzi and Pesce, 2017). Thus, Cj-trHbP can be considered as a candidate O2 and HCN proto-gasoreceptor in a proto-component STS.
However, the allosteric regulation of protons, CO2 and 2,3-Bisphosphoglycerate on mammalian hemoglobin evolved after the heme-containing globin domain had already emerged (Faggiano et al., 2022; Storz, 2025). Therefore, mammalian hemoglobin can be considered as a proto-gasoreceptor derivative (Vinogradov et al., 2006).
Exogenous nitrite–a bottleneck in hemoglobin’s gasoreceptor classification
In mature erythrocytes in humans, hemoglobin is abundant protein (at 5 mM), making up about 90%–95% of their cellular protein content (Pisciotta and Sullivan, 2008). Along with the erythrocytes, hemoglobin is cyclically carried to hypoxic capillaries, with erythrocyte residence time ranging from sub-second to a few seconds depending on tissue vascularization and blood flow rates (Pittman, 2013). Erythrocyte’s transit time will be further affected by the transit time of other cell types in the capillaries or the pathological state of the vasculature, such as lumen narrowing observed in Atherosclerosis (Wang and Popel, 1993; Hogg et al., 1994). These observation suggest that it could be relatively easier for hemoglobin to be deoxygenated not only in healthy capillaries in physiological conditions but also of vasculature in pathological conditions. In a low-O2 environment in vitro and under hypoxic conditions in vivo, deoxygenated hemoglobin in erythrocytes exhibits nitrite reductase activity, generating NO in a NO synthase-independent manner. This process has also been associated with vasodilation in human subjects, a canine model of hypotonic intravascular hemolysis, and the inhibition of mitochondrial respiration in cell cultures (Brooks, 1937; Nagababu et al., 2003; Basu et al., 2007; Minneci et al., 2008; Gladwin and Kim-Shapiro, 2008; Dent et al., 2021; Helms and Kim-Shapiro, 2013).
Apart from mature erythrocytes, the nitrite reductase activity of the hemoglobin α (HBA) subunit has also been demonstrated in the endothelium of mouse resistance arteries (Keller et al., 2022). Furthermore, endothelial cell-specific conditional Hba−/− mutant mice exhibited reductions in multiple parameters, including nitrite consumption, hypoxia-induced vasodilation, and exercise capacity. These results suggest a physiological role for HBA-derived NO under hypoxic conditions. Similarly, the nitrite reductase activity of myoglobin reduces myocardial infarction in mice via NO upon nitrite treatment; this response is lacking in myoglobin−/− mutants (Hendgen-Cotta et al., 2008).
However, the relatively low rate constant of human hemoglobin (1–10 M-1s-1), the local concentration of the co-substrate nitrite (200–300 nM in erythrocytes) and endogenous NO scavenging mechanisms, make it difficult to determine whether NO generated from deoxygenated hemoglobin acts as a signaling molecule in physiological or pathological conditions without an exogenous nitrite supply (Dent et al., 2021; Dejam et al., 2005; Wang and Kluger, 2016; Chamchoi et al., 2018).
Overall, at least in laboratory settings, the nitrite reductase activity of human and mouse hemoglobins is insufficient to label hemoglobin as a proto-gasoreceptor-like protein (Figure 1B). Nevertheless, in the case of cyanobacteria Synechocystis hemoglobin (SynHb), rice nonsymbiotic hemoglobin (nsHB1), and Arabidopsis thaliana phytoglobins (AHb1 and AHb2), which encounter relatively high nitrite concentration in vivo and exhibit nitrite reductase activity with relatively high rate constants, such hemoglobin orthologs are more promising candidates for O2 proto-gasoreceptor or as O2 proto-gasoreceptor derivates (Sturms et al., 2011; Tiso et al., 2012; Kumar et al., 2016). Vertebrates hemoglobin could perhaps act as an endogenous nitrite reductase with receptor-like signaling activity under conditions when nitrite concentration could be higher due to excess of environment-derived nitrite, dietary activities, nitrite enrichment responses, or the lack of homeostasis in the nitrite synthesis pathways (Washio and Takahashi, 2025; Sokal-Dembowska et al., 2025).
Hemoglobin as an oxygen gasoreceptor in split-component signal transduction systems
In addition to one-component STSs, there are also the examples of two-component STSs, in which the signal transduction occurs via the phosphorylation of a response regulator (Wuichet et al., 2010). These multi-component STSs have also been reported in microbial gas-sensing mechanisms (Lenz and Friedrich, 1998; Buhrke et al., 2004). However, limiting consideration to only one-, two-, or multi-component STS restricts the categories under which gasoreceptors can be classified. As an alternative, I propose the split-component STS.
Split-component signal transduction system
A split-component STS is a type of cellular signaling mechanism. It involves two proteins: one that constitutes a receptor or input domain, which binds or senses an environmental or intracellular stimulus, and another that constitutes a signal transducer with an output domain, which triggers a response. The ligand-binding status can influence the complex formation and activity of the signal transducer protein. In human and mice, the glucokinase regulator (GCKR, also known as GKRP or glucokinase regulatory protein) and glucokinase are examples of split-component STSs. The glucokinase regulator acts as a receptor for fructose 1-phosphate and fructose 6-phosphate, with glucokinase serving as its signal transducer (Beck and Miller, 2013; Anbalagan, 2025).
The presence of receptor in split-component STS for phosphorylated fructose in mammals raises the question of the identity of O2 gasoreceptors acting in split-component STSs (Figure 1C). In theory, any O2-binding protein that can physically interact with an enzyme and affect its activity could be considered an O2 gasoreceptor in split-component STSs. In this context, the major O2-binding proteins hemoglobin, cytoglobin, neuroglobin, and myoglobin could be considered potential O2 gasoreceptors and their interacting enzymes could be considered potential signal transducers, provided that the O2 binding state-dependent interaction with the enzyme promotes or inhibits the enzyme activity. As detailed in the following sections, hemoglobin interacts with various proteins in the erythrocytes, endothelial cells, and mitochondria. The precise reasons for the hemoglobin subunits’ interaction with proteins that exhibit diverse functions are unclear. Either hemoglobin “hitchhikes” across various proteins while transporting O2, or its transport role is regulated. Alternatively, hemoglobin may be an O2 gasoreceptor or signal transducer in split-component STS, with some of the interacting proteins acting as signal transducers and receptors, respectively.
Hemoglobin and SLC4A1 (anion exchanger)
The term “receptor-based signaling” is generally well accepted when a nuclear response is triggered as part of the ligand-receptor activation and signal transduction (Roberts and Kruchten, 2016). However, extracellular and intracellular receptors have been accepted in nuclei-less cells, such as mature erythrocytes and platelets (Tanneur et al., 2006; Vona et al., 2019; Jacobson et al., 2011; Balabin et al., 2018). This suggests that a nuclear response is unnecessary when considering a protein that is strongly allosteric regulated by ligand binding and can trigger a physiological cellular response.
However in mature erythrocytes, if we consider hemoglobin to be an O2 gasoreceptor in split-component STS, what is the identity of the signal transducers (Ellsworth et al., 2009)? Another question to consider is what can serve as a signal transducer in split-component STS? Can it only be enzymes, or could it also be other classes of proteins, such as membranal anion exchangers? Since, receptors in one-component STSs can be both ion channels (e.g., mammalian temperature-sensing TRPV ion channels) and enzymes (e.g., E. coli O2 gasoreceptor DosP phosphodiesterase), why not consider ion channels or anion exchangers as signal transducers (Figure 1C) (Dhaka et al., 2006).
In erythrocytes, hemoglobin binds to solute carrier family 4 member 1 (SLC4A1), also known as band 3 anion exchanger (AE1), in a manner dependent on its O2 binding state. This affects the function of SLC4A1 in exchanging bicarbonate (HCO3−) for chloride (Cl−) ions in erythrocytes. This interaction can influence erythrocyte cell physiology including their acid-base balance, metabolic regulation, membrane stability, survival, and lifespan (Chu et al., 2016; Cendali et al., 2024; Jennings, 2021). Therefore, in mature erythrocytes, I propose hemoglobin as an O2 gasoreceptor and SLC4A1 as one of its signal transducers in split-component STS.
Oxygenated hemoglobin and deoxygenated hemoglobin
In mature erythrocytes, another potential signal transducer for hemoglobin in split-component STS is the deoxygenated hemoglobin itself. Heterogenous saturation within hemoglobin tetramers has been reported, and hemoglobin primarily exhibits nitrite reductase activity in its tetrameric form (Huang et al., 2005). The fact that peak nitrite reductase activity occurs at 40%–60% O2 saturation of hemoglobin suggests that the oxygenated hemoglobin may act as O2 gasoreceptors while the other bound deoxygenated hemoglobin that exhibit nitrite reductase activity could be signal transducer (Huang et al., 2005; Angelo et al., 2008). However, this proposal have the same bottleneck that I proposed earlier in the proto-gasoreceptor derivative section. Another alternative consideration is the role of oxygenated hemoglobin’s NO dioxygenase activity as a signal transducer, and deoxygenated hemoglobin as an O2 gasoreceptor in split-component STS. However, the nitrate-specific signaling mechanisms derived from hemoglobin are unclear (Gardner, 2012).
Hemoglobin and NO synthase
In endothelial cells, a potential signal transducer for hemoglobin in split-component STS is the endothelial NO synthase (eNOS or NOS3), which synthesizes NO. Hemoglobin α and β subunits can interact with endothelial eNOS in mice arterial endothelial cells (Straub et al., 2012; Marozkina et al., 2021). While these interactions seem to play an important role in NO signaling, it is still unknown whether the physical interaction between hemoglobin subunits and eNOS and the activity of eNOS depend on the O2 binding state (Straub et al., 2014).
Hemoglobin β and ATP synthase
In the mitochondria, a potential signal transducer for hemoglobin in split-component STS is the adenosine triphosphate (ATP) synthase (Shephard et al., 2014; Brunyanszki et al., 2015). The physical interaction between hemoglobin β and ATP synthase (ATP5A) has been observed in lysates from rat liver and Drosophila mitochondria (Ebanks et al., 2023). However, it is unclear whether the mitochondrial complex formation or dissociation in vivo can regulate ATP synthase activity depending on hemoglobin’s binding status to O2. Although the role of ATP synthase is well known for its synthesis in ATP, ATP synthase is also a negative regulator of the mitochondrial permeability transition pore, suggesting potential response in the mitochondria (Picard and Shirihai, 2022; Pekson et al., 2023).
Nevertheless, for mitochondrial ATP synthase to be considered a signal transducer, precise knowledge of the intracellular signaling pathways downstream of its products ATP, H2O and proton is required (if ATP synthase activity is the only enzyme activity exhibited by ATP synthase). ATP is well known as a substrate for numerous enzymes. Based on the rationale used for O2 sensors, many ATP-dependent enzymes can also be considered as ATP sensors (Li et al., 2023). However, most of this signaling will not be considered signaling due to ATP receptors unless the ATP sensor is also strongly allosterically regulated by ATP binding at a site distant from the catalytic site. Currently, only a few extracellular ATP-binding proteins, such as P2X ion channels and P2Y G-protein-coupled receptors, are accepted as ATP or purinergic receptors (Ledderose and Junger, 2020). It is unclear whether similar ATP receptors exist intracellularly in the cytoplasm or various organelles (Fountain et al., 2007). Likewise aquareceptors and proton receptor-mediated signaling pathways too must be considered (Anbalagan, 2024d). Once all these signaling pathways acting downstream of mitochondrial ATP synthase are identified, ATP synthase can be considered a signal transducer in split component-STS.
Hemoglobin as a receptor for co-ligands - protons and CO2
One major one potential pitfall of my proposal regarding hemoglobin as an O2 gasoreceptor is the need to distinguish between the roles of O2 binding versus hemoglobin’s strong allosteric regulators (protons and CO2), which can also favor the deoxyhemoglobin state (Kilmartin and Rossi-Bernardi, 1971; Perrella et al., 1975).
Receptors can exhibit multimodal ligand binding and duality in sensing either for biomolecules or for factors such as photons and temperature (Shen et al., 2011; Assadi-Porter et al., 2018). The majority of the vertebrate hemoglobin with nitrite reductase activity have additional residues that play a significant allosteric regulatory role. Some major allosteric regulators of hemoglobin include protons, CO2, bicarbonate, chloride, and organic phosphate anions (e.g., 2,3-bisphosphoglycerate) (Malte et al., 2021; Faggiano et al., 2022; Prange et al., 2001; Ta et al., 2024). As I mentioned earlier, the allosteric regulation by protons and CO2 evolved after the emergence of the core heme-binding globin domain responsible for O2 binding (Faggiano et al., 2022; Storz, 2025). Protonation and CO2 binding in hemoglobin occur at multiple residues, resulting in O2 release from hemoglobin (Bohr effect) (Perrella et al., 1975; Gros et al., 1981). Since these allosteric regulatory events must occur collectively to enable hemoglobin nitrite reductase activity, I propose that protons and CO2 act as co-ligands for hemoglobin’s gasoreceptor activity. To the best of my knowledge, no studies have tested hemoglobin nitrite reductase activity in wild-type or mutant hemoglobins lacking allosteric regulation by protons, CO2-binding, or other biomolecules. Likewise, these allosteric sites must be mutated to test the role of allosteric regulation in interacting with signal transducer-like proteins in split-component STSs. This would allow to determine whether hemoglobin is an O2 gasoreceptor or a receptor for multiple ligands, such as protons, CO2 and 2,3-bisphosphoglycerate.
Conclusion
Based on all the arguments presented in this manuscript, I propose hemoglobin as a microenvironment-dependent O2 proto-gasoreceptor derivative, as well as an O2 gasoreceptor in split-component STS. Similar to hemoglobin, all other O2-binding proteins must be considered as putative gasoreceptors, provided that their enzymatic activity, or that of an interacting signal transducer (e.g., enzymes, ion channels, or anion exchangers), is affected by O2 binding. A nuclear response is not necessary to consider O2-binding proteins as candidate O2 gasoreceptors that mediate gasocrine signaling, nor it is necessary for any other class of receptors or signaling in nuclei-less cells. O2 concentration and temperature can vary significantly across different tissues, and even within organelles. Therefore, if the dissociation constant (KD) values of O2 binding are used to exclude certain O2-binding proteins from being considered gasoreceptors, these values must be interpreted relative to the physiological O2 concentrations and temperatures at which the proteins function in vivo. This consideration applies even when O2 binding is affected transiently.
The identity of O2 gasoreceptors that act upstream of PHDs in hypoxia sensing is unknown. Amongst the numerous signaling molecules, NO is one of the signaling molecule that can act upstream of PHDs during hypoxia. It is tempting to speculate that either hemoglobin or its paralogs may serve as such O2 gasoreceptors acting upstream of HIF1α and PHD2 (Metzen et al., 2003; Berchner-Pfannsc et al., 2007). Genetic studies considering the role of hemoglobin or its paralogs as O2 gasoreceptors must also consider the possibility that the coding and non-coding nucleic acid sequences of these genes can be potential riboceptors, including gas-sensing riboceptors (Anbalagan, 2024b; Anbalagan, 2024e). Finally, as some of the heme-based gasoreceptors has been proposed to act as aquareceptors, due to the ability of hemoglobin to bind a water molecule at the O2-binding site, hemoglobin must also be considered as a proto-aquareceptor derivative or an aquareceptor in split-component STS (Bulone et al., 1993; Colombo et al., 1996; Shadrina et al., 2015; Anbalagan, 2024d).
The discovery of hormones and their functions, the molecular and structural characterization of their receptors and feedback loops, and their medical and diagnostic applications, eventually led to the field of endocrinology (Dittfeld et al., 2017). In the past, NO has been proposed as an endocrine hormone (Bahadoran et al., 2020). Now, a question arises: If the definitive experimental validation of O2 gasoreceptors leads to the acceptance of O2 as a gaseous signaling molecule and an exogenous, gas-based endocrine hormone? If so, might this lead to a new field of study called gasocrinology? I define gasocrinology as the study of gases, their sources and functions, and disorders affecting cells that exhibit gasocrine and gas-regulated physiological processes. The implications of gasocrinology will likely extend beyond the use of gases in anesthesia in clinics. It will also influence the reconsideration of mechanisms underlying cellular processes, such as metabolic pathways, ferroptosis and the Warburg effect. Additionally, gasocrinology will relate to the theory of consciousness, molecular mechanisms of neurodevelopmental disorders, such as autism spectrum disorder, and neurodegenerative diseases, such as Parkinson’s and Alzheimer’s. Furthermore, gasocrinology will extend to disorders and diseases due to hypoxia and cellular bioenergetics imbalances, and perhaps even to physiological cellular evolutionary mechanisms in a changing environment (Gao et al., 2023; Urbano, 2021; Tokarz and Blasiak, 2014; Wong et al., 2025; Thannickal, 2009).
Statements
Author contributions
SA: conceptualization, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The author is supported by National Science Centre grants (SONATA-BIS 2020/38/E/NZ3/00090 and SONATA 2021/43/D/NZ3/01798).
Acknowledgments
This manuscript is dedicated to the memory of Leon Marchlewski, a pioneering scientist from Włocławek, Kingdom of Poland. The author thank Zofia Szweykowska-Kulinska (Institute of Molecular Biology and Biotechnology, Faculty of Biology, Adam Mickiewicz University, Poznan, Poland) for allowing him to attend her inspiring lectures on molecular evolution. The author also thank Agnieszka Chacinska (Past affiliation: Centre of New Technologies, University of Warsaw, Regenerative Mechanisms for Health - International Research Agendas Programme and current affiliation: International Institute of Molecular Machines and Mechanisms, Polish Academy of Science, Warsaw, Poland) for suggesting him to attend the 44th FEBS Congress meeting. The author also thanks José López Barneo (University of Seville, Spain) and James Imlay (University of Illinois, Urbana-Champaign, Illinois, USA) for e-discussions on oxygen sensing. This article was submitted as a preprint to Zenodo server https://doi.org/10.5281/zenodo.16724347.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used for the correction of this manuscript. The author used DeepL Write for English-language correction and takes full responsibility for the content of the manuscript.
Author disclaimer
The funding agency and institution S.A. is affiliated with was not involved in the contents of the manuscript.
Abbreviations
ADGB, Androglobin; ATP, Adenosine triphosphate; BAND3, Band 3 Anion Exchanger also known as Solute Carrier Family 4 Member 1 (Diego Blood Group); CO, Carbon monoxide; CO2, Carbon dioxide; DosP, Direct Oxygen Sensor DosP; eNOS, endothelial NO synthase or NOS3; HIF1α, Hypoxia-inducible factor 1-alpha; O2, Dioxygen; PHDs, Prolyl Hydroxylase Domain Proteins; sGC, Soluble guanylate cyclase; SLC4A1, Solute Carrier Family 4 Member 1 (Diego Blood Group); STS, Signal transduction system or signaling systems.
References
1
Adepu K. K. Anishkin A. Adams S. H. Chintapalli S. V. (2024). A versatile delivery vehicle for cellular oxygen and fuels or metabolic sensor? A review and perspective on the functions of myoglobin. Physiol. Rev.104, 1611–1642. 10.1152/physrev.00031.2023
2
Anbalagan S. (2023). “Blind men and an elephant”, the need for animals in research, drug safety studies, and understanding civilizational diseases. Animal Models Exp. Med.6, 627–633. 10.1002/ame2.12364
3
Anbalagan S. (2024a). Heme-based oxygen gasoreceptors. Am. J. Physiology-Endocrinology Metabolism326, E178–E181. 10.1152/ajpendo.00004.2024
4
Anbalagan S. (2024b). Gas-sensing riboceptors. RNA Biol.21, 758–763. 10.1080/15476286.2024.2379607
5
Anbalagan S. (2024c). Oxygen is an essential gasotransmitter directly sensed via protein gasoreceptors. Animal Models Exp. Med.7, 189–193. 10.1002/ame2.12400
6
Anbalagan S. (2024d). Akwareceptory na bazie hemu. Postepy Biochem.70, 420–423. 10.18388/pb.2021_551
7
Anbalagan S. (2024e). Temperature-sensing riboceptors. RNA Biol.21, 752–757. 10.1080/15476286.2024.2379118
8
Anbalagan S. (2025). Sugar-sensing swodkoreceptors and swodkocrine signaling. Animal Models Exp. Med.8, 944–961. 10.1002/ame2.70007
9
Angelo M. Hausladen A. Singel D. J. Stamler J. S. (2008). Interactions of NO with hemoglobin: from microbes to man. Methods Enzym.436, 131–168. 10.1016/S0076-6879(08)36008-X
10
Angelova P. R. Kasymov V. Christie I. Sheikhbahaei S. Turovsky E. Marina N. et al (2015). Functional oxygen sensitivity of astrocytes. J. Neurosci.35, 10460–10473. 10.1523/JNEUROSCI.0045-15.2015
11
Aono S. (2017). Gas sensing in cells. Royal Society of Chemistry.
12
Aragonés J. Jones D. R. Martı́n S. Juan M. A. S. Alfranca A. Vidal F. et al (2001). Evidence for the involvement of diacylglycerol kinase in the activation of hypoxia-inducible transcription factor 1 by low oxygen tension. J. Biol. Chem.276, 10548–10555. 10.1074/jbc.M006180200
13
Ascenzi P. Pesce A. (2017). Peroxynitrite scavenging by Campylobacter jejuni truncated hemoglobin P. J. Biol. Inorg. Chem.22, 1141–1150. 10.1007/s00775-017-1490-z
14
Ascenzi P. Leboffe L. Pesce A. Ciaccio C. Sbardella D. Bolognesi M. et al (2014). Nitrite-reductase and peroxynitrite isomerization activities of Methanosarcina acetivorans protoglobin. PLoS One9, e95391. 10.1371/journal.pone.0095391
15
Assadi-Porter F. M. Radek J. Rao H. Tonelli M. (2018). Multimodal ligand binding studies of human and mouse G-Coupled taste receptors to correlate their species-specific sweetness tasting properties. Molecules23, 2531. 10.3390/molecules23102531
16
Bahadoran Z. Carlström M. Mirmiran P. Ghasemi A. (2020). Nitric oxide: to be or not to be an endocrine hormone?Acta Physiol. (Oxf)229, e13443. 10.1111/apha.13443
17
Balabin F. A. Morozova D. S. Mayorov A. S. Martyanov A. A. Panteleev M. A. Sveshnikova A. N. (2018). Clusterization of inositol trisphosphate receptors determines the shape of the calcium oscillation peak in platelet cytosol. Mosc. Univ. Phys.73, 526–533. 10.3103/S0027134918050041
18
Basu S. Grubina R. Huang J. Conradie J. Huang Z. Jeffers A. et al (2007). Catalytic generation of N2O3 by the concerted nitrite reductase and anhydrase activity of hemoglobin. Nat. Chem. Biol.3, 785–794. 10.1038/nchembio.2007.46
19
Beck T. Miller B. G. (2013). Structural basis for regulation of human glucokinase by glucokinase regulatory protein. Biochemistry52, 6232–6239. 10.1021/bi400838t
20
Berchner-Pfannschmidt U. Yamac H. Trinidad B. Fandrey J. (2007). Nitric oxide modulates oxygen sensing by hypoxia-inducible factor 1-dependent induction of prolyl hydroxylase 2. J. Biol. Chem.282, 1788–1796. 10.1074/jbc.M607065200
21
Bhaskaran M. Chen H. Chen Z. Liu L. (2005). Hemoglobin is expressed in alveolar epithelial type II cells. Biochem. Biophysical Res. Commun.333, 1348–1352. 10.1016/j.bbrc.2005.06.042
22
Biagioli M. Pinto M. Cesselli D. Zaninello M. Lazarevic D. Roncaglia P. et al (2009). Unexpected expression of alpha- and beta-globin in mesencephalic dopaminergic neurons and glial cells. Proc. Natl. Acad. Sci. U. S. A.106, 15454–15459. 10.1073/pnas.0813216106
23
Bishop T. Ratcliffe P. J. (2020). Genetic basis of oxygen sensing in the carotid body: HIF2α and an isoform switch in cytochrome c oxidase subunit 4. Sci. Signal13, eaba1302. 10.1126/scisignal.aba1302
24
Bleymehl K. Pérez-Gómez A. Omura M. Moreno-Pérez A. Macías D. Bai Z. et al (2016). A sensor for low environmental oxygen in the mouse main olfactory epithelium. Neuron92, 1196–1203. 10.1016/j.neuron.2016.11.001
25
Bolli A. Ciaccio C. Coletta M. Nardini M. Bolognesi M. Pesce A. et al (2008). Ferrous Campylobacter jejuni truncated hemoglobin P displays an extremely high reactivity for cyanide - a comparative study. FEBS J.275, 633–645. 10.1111/j.1742-4658.2007.06223.x
26
Bonamore A. Gentili P. Ilari A. Schininà M. E. Boffi A. (2003). Escherichia coli flavohemoglobin is an efficient alkylhydroperoxide reductase. J. Biol. Chem.278, 22272–22277. 10.1074/jbc.M301285200
27
Brooks J. (1937). The action of nitrite on haemoglobin in the absence of oxygen. Proc. R. Soc. Lond. Ser. B - Biol. Sci.123, 368–382. 10.1098/rspb.1937.0057
28
Brunyanszki A. Erdelyi K. Szczesny B. Olah G. Salomao R. Herndon D. N. et al (2015). Upregulation and mitochondrial sequestration of hemoglobin occur in circulating leukocytes during critical illness, conferring a cytoprotective phenotype. Mol. Med.21, 666–675. 10.2119/molmed.2015.00187
29
Buhrke T. Lenz O. Porthun A. Friedrich B. (2004). The H2-sensing complex of Ralstonia eutropha: interaction between a regulatory [NiFe] hydrogenase and a histidine protein kinase. Mol. Microbiol.51, 1677–1689. 10.1111/j.1365-2958.2003.03933.x
30
Bulone D. San Biagio P. L. Palma-Vittorelli M. B. Palma M. U. (1993). The role of water in hemoglobin function and stability. Science259, 1335–1336. 10.1126/science.8446903
31
Canfield D. E. (2014). Oxygen: a four billion year history. Princeton, New Jersey, USA: Princeton University Press.
32
Čapek D. Müller P. (2019). Positional information and tissue scaling during development and regeneration. Development146, dev177709. 10.1242/dev.177709
33
Carlew T. S. Allen C. J. Binder B. M. (2020). Ethylene receptors in nonplant species. Small Methods4, 1900266. 10.1002/smtd.201900266
34
Cendali F. I. Grier A. Lisk C. Dzieciatkowska M. Swindle D. Hay A. M. et al (2024). Got oxygen? the impact of band 3 on RBC function during exercise. Blood144, 3847. 10.1182/blood-2024-210637
35
Chamchoi A. Srihirun S. Paiboonsukwong K. Sriwantana T. Sathavorasmith P. Pattanapanyasat K. et al (2018). Decreased nitrite reductase activity of deoxyhemoglobin correlates with platelet activation in hemoglobin E/ß-thalassemia subjects. PLOS ONE13, e0203955. 10.1371/journal.pone.0203955
36
Chu H. McKenna M. M. Krump N. A. Zheng S. Mendelsohn L. Thein S. L. et al (2016). Reversible binding of hemoglobin to band 3 constitutes the molecular switch that mediates O2 regulation of erythrocyte properties. Blood128, 2708–2716. 10.1182/blood-2016-01-692079
37
Ciuman M. Siednienko J. Czyzyk R. Witwicka H. Kołosionek E. Kobiałka M. et al (2006). Cyclic GMP-dependent protein kinase and soluble guanylyl cyclase disappear in elicited rat neutrophils. Biochim. Biophys. Acta1760, 1618–1623. 10.1016/j.bbagen.2006.09.002
38
Colombo M. F. Bonilla-Rodriguez G. O. (1996). The water effect on allosteric regulation of hemoglobin probed in water/glucose and water/glycine solutions. J. Biol. Chem.271, 4895–4899. 10.1074/jbc.271.9.4895
39
Corbineau F. (2024). Ethylene, a signaling compound involved in seed germination and dormancy. Plants (Basel)13, 2674. 10.3390/plants13192674
40
Corker H. Poole R. K. (2003). Nitric oxide formation by escherichia coli: dependence on nitrite reductase, the no-sensing regulator fnr, and flavohemoglobin hmp *. J. Biol. Chem.278, 31584–31592. 10.1074/jbc.M303282200
41
Dejam A. Hunter C. J. Pelletier M. M. Hsu L. L. Machado R. F. Shiva S. et al (2005). Erythrocytes are the major intravascular storage sites of nitrite in human blood. Blood106, 734–739. 10.1182/blood-2005-02-0567
42
Delgado-Nixon V. M. Gonzalez G. Gilles-Gonzalez M. A. (2000). Dos, a heme-binding PAS protein from Escherichia coli, is a direct oxygen sensor. Biochemistry39, 2685–2691. 10.1021/bi991911s
43
Dent M. R. DeMartino A. W. Tejero J. Gladwin M. T. (2021). Endogenous hemoprotein-dependent signaling pathways of nitric oxide and nitrite. Inorg. Chem.60, 15918–15940. 10.1021/acs.inorgchem.1c01048
44
Dhaka A. Viswanath V. Patapoutian A. (2006). Trp ion channels and temperature sensation. Annu. Rev. Neurosci.29, 135–161. 10.1146/annurev.neuro.29.051605.112958
45
Dittfeld A. Gwizdek K. Brończyk-Puzoń A. (2017). History of endocrinology in the world and in Poland. Pediatr. Endocrinol. Diabetes Metab.23, 146–151. 10.18544/PEDM-23.03.0086
46
Durner J. Gow A. J. Stamler J. S. Glazebrook J. (1999). Ancient origins of nitric oxide signaling in biological systems. Proc. Natl. Acad. Sci. U. S. A.96, 14206–14207. 10.1073/pnas.96.25.14206
47
Ebanks B. Katyal G. Taylor C. Dowle A. Papetti C. Lucassen M. et al (2023). Mitochondrial haemoglobin is upregulated with hypoxia in skeletal muscle and has a conserved interaction with ATP synthase and inhibitory factor 1. Cells12, 912. 10.3390/cells12060912
48
Ellsworth M. L. Ellis C. G. Goldman D. Stephenson A. H. Dietrich H. H. Sprague R. S. (2009). Erythrocytes: oxygen sensors and modulators of vascular tone. Physiol. (Bethesda)24, 107–116. 10.1152/physiol.00038.2008
49
Faggiano S. Ronda L. Bruno S. Abbruzzetti S. Viappiani C. Bettati S. et al (2022). From hemoglobin allostery to hemoglobin-based oxygen carriers. Mol. Aspects Med.84, 101050. 10.1016/j.mam.2021.101050
50
Fountain S. J. Parkinson K. Young M. T. Cao L. Thompson C. R. L. North R. A. (2007). An intracellular P2X receptor required for osmoregulation in Dictyostelium discoideum. Nature448, 200–203. 10.1038/nature05926
51
Freitas T. A. K. Hou S. Dioum E. M. Saito J. A. Newhouse J. Gonzalez G. et al (2004). Ancestral hemoglobins in archaea. Proc. Natl. Acad. Sci. U. S. A.101, 6675–6680. 10.1073/pnas.0308657101
52
Froehlich-Santino W. Londono Tobon A. Cleveland S. Torres A. Phillips J. Cohen B. et al (2014). Prenatal and perinatal risk factors in a twin study of autism spectrum disorders. J. Psychiatr. Res.54, 100–108. 10.1016/j.jpsychires.2014.03.019
53
Gao L. Ortega-Sáenz P. Moreno-Domínguez A. López-Barneo J. (2022). Mitochondrial redox signaling in O2-Sensing chemoreceptor cells. Antioxid. Redox Signal37, 274–289. 10.1089/ars.2021.0255
54
Gao X. Lu K. Li C. (2023). Emerging relationship between hydrogen sulfide and ferroptosis: a literature review. Acta Biochim. Pol.70, 735–744. 10.18388/abp.2020_6756
55
Gardner P. R. (2012). Hemoglobin: a nitric-oxide dioxygenase. Sci. (Cairo)2012, 1–34. 10.6064/2012/683729
56
Gladwin M. T. Kim-Shapiro D. B. (2008). The functional nitrite reductase activity of the heme-globins. Blood112, 2636–2647. 10.1182/blood-2008-01-115261
57
Gros G. Rollema H. S. Forster R. E. (1981). The carbamate equilibrium of alpha- and epsilon-amino groups of human hemoglobin at 37 degrees C. J. Biol. Chem.256, 5471–5480. 10.1016/s0021-9258(19)69225-2
58
Hall C. N. Garthwaite J. (2009). What is the real physiological NO concentration in vivo?Nitric Oxide21, 92–103. 10.1016/j.niox.2009.07.002
59
Hammarlund E. U. Flashman E. Mohlin S. Licausi F. (2020). Oxygen-sensing mechanisms across eukaryotic kingdoms and their roles in complex multicellularity. Science370, eaba3512. 10.1126/science.aba3512
60
Harold S. E. Warf S. L. Shields G. C. (2023). Prebiotic dimer and trimer peptide formation in gas-phase atmospheric nanoclusters of water. Phys. Chem. Chem. Phys.25, 28517–28532. 10.1039/D3CP02915H
61
Helms C. Kim-Shapiro D. B. (2013). Hemoglobin-mediated nitric oxide signaling. Free Radic. Biol. Med.61, 464–472. 10.1016/j.freeradbiomed.2013.04.028
62
Hendgen-Cotta U. B. Merx M. W. Shiva S. Schmitz J. Becher S. Klare J. P. et al (2008). Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury. Proc. Natl. Acad. Sci. U. S. A.105, 10256–10261. 10.1073/pnas.0801336105
63
Hogg J. C. Coxson H. O. Brumwell M. L. Beyers N. Doerschuk C. M. MacNee W. et al (1994). Erythrocyte and polymorphonuclear cell transit time and concentration in human pulmonary capillaries. J. Appl. Physiology77, 1795–1800. 10.1152/jappl.1994.77.4.1795
64
Holdsworth M. J. Gibbs D. J. (2020). Comparative biology of oxygen sensing in plants and animals. Curr. Biol.30, R362–R369. 10.1016/j.cub.2020.03.021
65
Hoogewijs D. Ebner B. Germani F. Hoffmann F. G. Fabrizius A. Moens L. et al (2012). Androglobin: a chimeric globin in metazoans that is preferentially expressed in mammalian testes. Mol. Biol. Evol.29, 1105–1114. 10.1093/molbev/msr246
66
Horst B. G. Yokom A. L. Rosenberg D. J. Morris K. L. Hammel M. Hurley J. H. et al (2019). Allosteric activation of the nitric oxide receptor soluble guanylate cyclase mapped by cryo-electron microscopy. Elife8, e50634. 10.7554/eLife.50634
67
Huang Z. Shiva S. Kim-Shapiro D. B. Patel R. P. Ringwood L. A. Irby C. E. et al (2005). Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J. Clin. Invest115, 2099–2107. 10.1172/JCI24650
68
Ignarro L. J. Freeman B. (2017). Nitric oxide: biology and pathobiology. Academic Press.
69
Jacobson K. A. Deflorian F. Mishra S. Costanzi S. (2011). Pharmacochemistry of the platelet purinergic receptors. Purinergic Signal.7, 305–324. 10.1007/s11302-011-9216-0
70
Jennings M. L. (2021). Cell physiology and molecular mechanism of anion transport by erythrocyte band 3/AE1. Am. J. Physiology-Cell Physiology321, C1028–C1059. 10.1152/ajpcell.00275.2021
71
Keller T. C. S. Lechauve C. Keller A. S. Broseghini-Filho G. B. Butcher J. T. Askew Page H. R. et al (2022). Endothelial alpha globin is a nitrite reductase. Nat. Commun.13, 6405. 10.1038/s41467-022-34154-3
72
Keppner A. Correia M. Santambrogio S. Koay T. W. Maric D. Osterhof C. et al (2022). Androglobin, a chimeric mammalian globin, is required for male fertility. eLife11, e72374. 10.7554/eLife.72374
73
Kharitonov V. G. Russwurm M. Magde D. Sharma V. S. Koesling D. (1997). Dissociation of nitric oxide from soluble guanylate cyclase. Biochem. Biophysical Res. Commun.239, 284–286. 10.1006/bbrc.1997.7470
74
Kilmartin J. V. Rossi-Bernardi L. (1971). The binding of carbon dioxide by horse haemoglobin. Biochem. J.124, 31–45. 10.1042/bj1240031
75
Koay T. W. Osterhof C. Orlando I. M. C. Keppner A. Andre D. Yousefian S. et al (2021). Androglobin gene expression patterns and FOXJ1-dependent regulation indicate its functional association with ciliogenesis. J. Biol. Chem.296, 100291. 10.1016/j.jbc.2021.100291
76
Kumar P. Prabhakar N. R. (2012). Peripheral chemoreceptors: function and plasticity of the carotid body. Compr. Physiol.2, 141–219. 10.1002/cphy.c100069
77
Kumar N. Astegno A. Chen J. Giorgetti A. Dominici P. (2016). Residues in the distal heme pocket of arabidopsis non-symbiotic hemoglobins: implication for nitrite reductase activity. Int. J. Mol. Sci.17, 640. 10.3390/ijms17050640
78
Kutchai H. Staub N. C. (1969). Steady-state, hemoglobin-facilitated O2 transport in human erythrocytes. J. General Physiology53, 576–589. 10.1085/jgp.53.5.576
79
Ledderose C. Junger W. G. (2020). Mitochondria synergize with P2 receptors to regulate human T cell function. Front. Immunol.11, 549889. 10.3389/fimmu.2020.549889
80
Lenz O. Friedrich B. (1998). A novel multicomponent regulatory system mediates H2 sensing in Alcaligenes eutrophus. Proc. Natl. Acad. Sci. U. S. A.95, 12474–12479. 10.1073/pnas.95.21.12474
81
Li L. Shen S. Bickler P. Jacobson M. P. Wu L. F. Altschuler S. J. (2023). Searching for molecular hypoxia sensors among oxygen-dependent enzymes. Elife12, e87705. 10.7554/eLife.87705
82
Liu C. Wajih N. Liu X. Basu S. Janes J. Marvel M. et al (2015). Mechanisms of human erythrocytic bioactivation of nitrite. J. Biol. Chem.290, 1281–1294. 10.1074/jbc.M114.609222
83
Liu T. Mukosera G. T. Blood A. B. (2020). The role of gasotransmitters in neonatal physiology. Nitric Oxide95, 29–44. 10.1016/j.niox.2019.12.002
84
López-Barneo J. González-Rodríguez P. Gao L. Fernández-Agüera M. C. Pardal R. Ortega-Sáenz P. (2016). Oxygen sensing by the carotid body: mechanisms and role in adaptation to hypoxia. Am. J. Physiology-Cell Physiology310, C629–C642. 10.1152/ajpcell.00265.2015
85
Lu C. Egawa T. Wainwright L. M. Poole R. K. Yeh S.-R. (2007). Structural and functional properties of a truncated hemoglobin from a food-borne pathogen Campylobacter jejuni. J. Biol. Chem.282, 13627–13636. 10.1074/jbc.M609397200
86
Makhoul S. Walter E. Pagel O. Walter U. Sickmann A. Gambaryan S. et al (2018). Effects of the NO/soluble guanylate cyclase/cGMP system on the functions of human platelets. Nitric Oxide76, 71–80. 10.1016/j.niox.2018.03.008
87
Malte H. Lykkeboe G. Wang T. (2021). The magnitude of the Bohr effect profoundly influences the shape and position of the blood oxygen equilibrium curve. Comp. Biochem. Physiology Part A Mol. and Integr. Physiology254, 110880. 10.1016/j.cbpa.2020.110880
88
Maltepe E. Schmidt J. V. Baunoch D. Bradfield C. A. Simon M. C. (1997). Abnormal angiogenesis and responses to glucose and oxygen deprivation in mice lacking the protein ARNT. Nature386, 403–407. 10.1038/386403a0
89
Marozkina N. Smith L. Zhao Y. Zein J. Chmiel J. F. Kim J. et al (2021). Somatic cell hemoglobin modulates nitrogen oxide metabolism in the human airway epithelium. Sci. Rep.11, 15498. 10.1038/s41598-021-94782-5
90
Metzen E. Zhou J. Jelkmann W. Fandrey J. Brüne B. (2003). Nitric oxide impairs normoxic degradation of HIF-1α by inhibition of prolyl hydroxylases. Mol. Biol. Cell14, 3470–3481. 10.1091/mbc.E02-12-0791
91
Minneci P. C. Deans K. J. Shiva S. Zhi H. Banks S. M. Kern S. et al (2008). Nitrite reductase activity of hemoglobin as a systemic nitric oxide generator mechanism to detoxify plasma hemoglobin produced during hemolysis. Am. J. Physiology-Heart Circulatory Physiology295, H743–H754. 10.1152/ajpheart.00151.2008
92
Minning D. M. Gow A. J. Bonaventura J. Braun R. Dewhirst M. Goldberg D. E. et al (1999). Ascaris haemoglobin is a nitric oxide-activated “deoxygenase.”. Nature401, 497–502. 10.1038/46822
93
Monson E. K. Weinstein M. Ditta G. S. Helinski D. R. (1992). The FixL protein of Rhizobium meliloti can be separated into a heme-binding oxygen-sensing domain and a functional C-terminal kinase domain. Proc. Natl. Acad. Sci. U. S. A.89, 4280–4284. 10.1073/pnas.89.10.4280
94
Moreno-Domínguez A. Ortega-Sáenz P. Gao L. Colinas O. García-Flores P. Bonilla-Henao V. et al (2020). Acute O2 sensing through HIF2α-dependent expression of atypical cytochrome oxidase subunits in arterial chemoreceptors. Sci. Signal13, eaay9452. 10.1126/scisignal.aay9452
95
Mukhi N. Kundu S. Kaur J. (2017). NO dioxygenase- and peroxidase-like activity of Arabidopsis phytoglobin 3 and its role in Sclerotinia sclerotiorum defense. Nitric Oxide68, 150–162. 10.1016/j.niox.2017.03.004
96
Nagababu E. Ramasamy S. Abernethy D. R. Rifkind J. M. (2003). Active nitric oxide produced in the red cell under hypoxic conditions by deoxyhemoglobin-mediated nitrite reduction. J. Biol. Chem.278, 46349–46356. 10.1074/jbc.M307572200
97
Padilla P. A. Roth M. B. (2001). Oxygen deprivation causes suspended animation in the zebrafish embryo. Proc. Natl. Acad. Sci. U. S. A.98, 7331–7335. 10.1073/pnas.131213198
98
Pan Y. Zhang Y. Zheng W.-Y. Zhu M.-Z. Li H.-Y. Ouyang W.-J. et al (2024). Intermittent hypobaric hypoxia ameliorates autistic-like phenotypes in mice. J. Neurosci.44, e1665232023. 10.1523/JNEUROSCI.1665-23.2023
99
Pekson R. Liang F. G. Axelrod J. L. Lee J. Qin D. Wittig A. J. H. et al (2023). The mitochondrial ATP synthase is a negative regulator of the mitochondrial permeability transition pore. Proc. Natl. Acad. Sci.120, e2303713120. 10.1073/pnas.2303713120
100
Peng Y.-J. Nanduri J. Raghuraman G. Souvannakitti D. Gadalla M. M. Kumar G. K. et al (2010). H2S mediates O2 sensing in the carotid body. Proc. Natl. Acad. Sci. U. S. A.107, 10719–10724. 10.1073/pnas.1005866107
101
Peng Y.-J. Gridina A. Wang B. Nanduri J. Fox A. P. Prabhakar N. R. (2020). Olfactory receptor 78 participates in carotid body response to a wide range of low O2 levels but not severe hypoxia. J. Neurophysiology123, 1886–1895. 10.1152/jn.00075.2020
102
Peng Y.-J. Wang N. Nanduri J. Chen X. Storm D. Prabhakar N. (2023). Adenylate cyclase 3 and cyclic nucleotide-gated channel alpha 2 are downstream signaling to H2S/Olfr78 to carotid body responses to hypoxia. Physiology38, 5731746. 10.1152/physiol.2023.38.S1.5731746
103
Perrella M. Bresciani D. Rossi-Bernardi L. (1975). The binding of CO2 to human hemoglobin. J. Biol. Chem.250, 5413–5418. 10.1016/s0021-9258(19)41197-6
104
Picard M. Shirihai O. S. (2022). Mitochondrial signal transduction. Cell Metab.34, 1620–1653. 10.1016/j.cmet.2022.10.008
105
Pisciotta J. M. Sullivan D. (2008). Hemozoin: oil versus water. Parasitol. Int.57, 89–96. 10.1016/j.parint.2007.09.009
106
Pittman R. N. (2013). Oxygen transport in the microcirculation and its regulation. Microcirculation20, 117–137. 10.1111/micc.12017
107
Prange H. D. Shoemaker J. L. Westen E. A. Horstkotte D. G. Pinshow B. (2001). Physiological consequences of oxygen-dependent chloride binding to hemoglobin. J. Appl. Physiology91, 33–38. 10.1152/jappl.2001.91.1.33
108
Ratcliffe P. J. Keeley T. P. (2025). Making sense of oxygen sensing.
109
Reed E. C. Kim J. D. Case A. J. (2025). Non-canonical hemoglobin: an updated review on its ubiquitous expression. Redox Biol.82, 103602. 10.1016/j.redox.2025.103602
110
Reeder B. J. Deganutti G. Ukeri J. Atanasio S. Svistunenko D. A. Ronchetti C. et al (2024). The circularly permuted globin domain of androglobin exhibits atypical heme stabilization and nitric oxide interaction. Chem. Sci.15, 6738–6751. 10.1039/d4sc00953c
111
Richardson S. L. Hulikova A. Proven M. Hipkiss R. Akanni M. Roy N. B. A. et al (2020). Single-cell O2 exchange imaging shows that cytoplasmic diffusion is a dominant barrier to efficient gas transport in red blood cells. Proc. Natl. Acad. Sci. U. S. A.117, 10067–10078. 10.1073/pnas.1916641117
112
Richter F. Meurers B. H. Zhu C. Medvedeva V. P. Chesselet M.-F. (2009). Neurons express hemoglobin alpha- and beta-chains in rat and human brains. J. Comp. Neurology515, 538–547. 10.1002/cne.22062
113
Roberts M. S. Kruchten A. E. (2016). Receptor biology. Weinheim, Germany: John Wiley and Sons.
114
Root-Bernstein R. S. (2005). Peptide self-aggregation and peptide complementarity as bases for the evolution of peptide receptors: a review. J. Mol. Recognit.18, 40–49. 10.1002/jmr.690
115
Shadrina M. S. Peslherbe G. H. English A. M. (2015). O2 and water migration pathways between the solvent and heme pockets of hemoglobin with open and closed conformations of the distal HisE7. Biochemistry54, 5279–5289. 10.1021/acs.biochem.5b00369
116
Shen W. L. Kwon Y. Adegbola A. A. Luo J. Chess A. Montell C. (2011). Function of rhodopsin in temperature discrimination in drosophila. Science331, 1333–1336. 10.1126/science.1198904
117
Shephard F. Greville-Heygate O. Marsh O. Anderson S. Chakrabarti L. (2014). A mitochondrial location for haemoglobins—Dynamic distribution in ageing and Parkinson’s disease. Mitochondrion14, 64–72. 10.1016/j.mito.2013.12.001
118
Shimizu T. (2013). The heme-based oxygen-sensor phosphodiesterase Ec DOS (DosP): structure-function relationships. Biosens. (Basel)3, 211–237. 10.3390/bios3020211
119
Sokal-Dembowska A. Jarmakiewicz-Czaja S. Tabarkiewicz J. Filip R. (2025). Nitrate and nitrite in the diet: protective and harmful effects in health and disease. Curr. Nutr. Rep.14 (89), 89. 10.1007/s13668-025-00678-5
120
Sonnen K. F. Janda C. Y. (2021). Signalling dynamics in embryonic development. Biochem. J.478, 4045–4070. 10.1042/BCJ20210043
121
Storz J. F. (2025). Hemoglobin: insights into protein structure, function, and evolution. Oxford University Press.
122
Straub A. C. Lohman A. W. Billaud M. Johnstone S. R. Dwyer S. T. Lee M. Y. et al (2012). Endothelial cell expression of haemoglobin α regulates nitric oxide signalling. Nature491, 473–477. 10.1038/nature11626
123
Straub A. C. Butcher J. T. Billaud M. Mutchler S. M. Artamonov M. V. Nguyen A. T. et al (2014). Hemoglobin α/eNOS coupling at myoendothelial junctions is required for nitric oxide scavenging during vasoconstriction. Arteriosclerosis, Thrombosis, Vasc. Biol.34, 2594–2600. 10.1161/ATVBAHA.114.303974
124
Sturms R. DiSpirito A. A. Hargrove M. S. (2011). Plant and cyanobacterial hemoglobins reduce nitrite to nitric oxide under anoxic conditions. Biochemistry50, 3873–3878. 10.1021/bi2004312
125
Takahashi K. Lee Y. Fago A. Bautista N. M. Storz J. F. Kawamoto A. et al (2024). The unique allosteric property of crocodilian haemoglobin elucidated by cryo-EM. Nat. Commun.15, 6505. 10.1038/s41467-024-49947-x
126
Tahara U. Matsui T. Atsugi T. Fukuda K. Kubo A. Amagai M. (2022). 532 unexpected expression of hemoglobin α as an endogenous antioxidant in epidermal keratinocytes. J. Investigative Dermatology142, S272. 10.1016/j.jid.2022.09.547
127
Tanneur V. Duranton C. Brand V. B. Sandu C. D. Akkaya C. Kasinathan R. S. et al (2006). Purinoceptors are involved in the induction of an osmolyte permeability in malaria-infected and oxidized human erythrocytes. FASEB J.20, 133–135. 10.1096/fj.04-3371fje
128
Teodoro R. O. O’Farrell P. H. (2003). Nitric oxide-induced suspended animation promotes survival during hypoxia. EMBO J.22, 580–587. 10.1093/emboj/cdg070
129
Thannickal V. J. (2009). Oxygen in the evolution of complex life and the price we pay. Am. J. Respir. Cell Mol. Biol.40, 507–510. 10.1165/rcmb.2008-0360PS
130
The Blind Men and the Elephant (1992). The blind men and the elephant. Scholastic Incorporated.
131
Tiso M. Tejero J. Kenney C. Frizzell S. Gladwin M. T. (2012). Nitrite reductase activity of nonsymbiotic hemoglobins from Arabidopsis thaliana. Biochemistry51, 5285–5292. 10.1021/bi300570v
132
Tokarz P. Blasiak J. (2014). Role of mitochondria in carcinogenesis. Acta Biochim. Pol.61, 671–678. 10.18388/abp.2014_1829
133
Tsai A.-L. Berka V. Martin E. Olson J. S. (2012). A “sliding scale rule” for selectivity among NO, CO, and O2 by heme protein sensors. Biochemistry51, 172–186. 10.1021/bi2015629
134
Uchiyama M. Nakao A. Kurita Y. Fukushi I. Takeda K. Numata T. et al (2020). O2-Dependent protein internalization underlies astrocytic sensing of acute hypoxia by restricting multimodal TRPA1 channel responses. Curr. Biol.30, 3378–3396.e7. 10.1016/j.cub.2020.06.047
135
Ulrich L. E. Koonin E. V. Zhulin I. B. (2005). One-component systems dominate signal transduction in prokaryotes. Trends Microbiol.13, 52–56. 10.1016/j.tim.2004.12.006
136
Unzai S. Eich R. Shibayama N. Olson J. S. Morimoto H. (1998). Rate constants for O2 and CO binding to the alpha and beta subunits within the R and T states of human hemoglobin. J. Biol. Chem.273, 23150–23159. 10.1074/jbc.273.36.23150
137
Urbano A. M. (2021). Otto Warburg: the journey towards the seminal discovery of tumor cell bioenergetic reprogramming. Biochimica Biophysica Acta (BBA) - Mol. Basis Dis.1867, 165965. 10.1016/j.bbadis.2020.165965
138
Villar I. Larrainzar E. Milazzo L. Pérez-Rontomé C. Rubio M. C. Smulevich G. et al (2020). A plant gene encoding one-heme and two-heme hemoglobins with extreme reactivities toward diatomic gases and nitrite. Front. Plant Sci.11, 600336. 10.3389/fpls.2020.600336
139
Vinogradov S. N. Hoogewijs D. Bailly X. Arredondo-Peter R. Gough J. Dewilde S. et al (2006). A phylogenomic profile of globins. BMC Evol. Biol.6, 31. 10.1186/1471-2148-6-31
140
Vona R. Gambardella L. Ortona E. Santulli M. Malorni W. Carè A. et al (2019). Functional estrogen receptors of red blood cells. Do they influence intracellular signaling? | cell physiol Biochem. Cell. physiology Biochem. Int. J. Exp. Cell. physiology, Biochem. Pharmacol.53, 186–199. 10.33594/000000129
141
Wang R. (2002). Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter?FASEB J.16, 1792–1798. 10.1096/fj.02-0211hyp
142
Wang A. Kluger R. (2016). Enhanced nitrite reductase activity and its correlation with oxygen affinity in hemoglobin bis-tetramers. Biochemistry55, 4688–4696. 10.1021/acs.biochem.6b00542
143
Wang C.-H. Popel A. S. (1993). Effect of red blood cell shape on oxygen transport in capillaries. Math. Biosci.116, 89–110. 10.1016/0025-5564(93)90062-f
144
Wang S. Yu X. Lin Z. Zhang S. Xue L. Xue Q. et al (2017). Hemoglobins likely function as peroxidase in blood clam Tegillarca granosa hemocytes. J. Immunol. Res.2017, 1–10. 10.1155/2017/7125084
145
Wang S. Huang Y. Liu S. Lin Z. Zhang Y. Bao Y. (2021). Hemoglobins from Scapharca subcrenata (Bivalvia: arcidae) likely play an bactericidal role through their peroxidase activity. Comp. Biochem. Physiology Part B Biochem. Mol. Biol.253, 110545. 10.1016/j.cbpb.2020.110545
146
Washio J. Takahashi N. (2025). Nitrite production from nitrate in the oral microbiome and its contribution to oral and systemic health. Adv. Exp. Med. Biol.1472, 89–101. 10.1007/978-3-031-79146-8_6
147
Weissmann N. Dietrich A. Fuchs B. Kalwa H. Ay M. Dumitrascu R. et al (2006). Classical transient receptor potential channel 6 (TRPC6) is essential for hypoxic pulmonary vasoconstriction and alveolar gas exchange. Proc. Natl. Acad. Sci. U. S. A.103, 19093–19098. 10.1073/pnas.0606728103
148
Winger J. A. Derbyshire E. R. Marletta M. A. (2007). Dissociation of nitric oxide from soluble guanylate cyclase and heme-nitric oxide/oxygen binding domain constructs. J. Biol. Chem.282, 897–907. 10.1074/jbc.M606327200
149
Wong W. Bravo P. Yunker P. J. Ratcliff W. C. Burnetti A. J. (2025). Oxygen-binding proteins aid oxygen diffusion to enhance fitness of a yeast model of multicellularity. PLOS Biol.23, e3002975. 10.1371/journal.pbio.3002975
150
Wuichet K. Cantwell B. J. Zhulin I. B. (2010). Evolution and phyletic distribution of two-component signal transduction systems. Curr. Opin. Microbiol.13, 219–225. 10.1016/j.mib.2009.12.011
151
Yuan Z. De La Cruz L. K. Yang X. Wang B. (2022). Carbon monoxide signaling: examining its engagement with various molecular targets in the context of binding affinity, concentration, and biologic response. Pharmacol. Rev.74, 825–875. 10.1124/pharmrev.121.000564
152
Zhang Y Y. Wang R. Wang X. Zhao C. Shen H. Yang L. (2023). Nitric oxide regulates seed germination by integrating multiple signalling pathways. Int. J. Mol. Sci.24, 9052. 10.3390/ijms24109052
153
Zhang F. Zhang B. Wang Y. Jiang R. Liu J. Wei Y. et al (2023). An extra-erythrocyte role of haemoglobin body in chondrocyte hypoxia adaption. Nature622, 834–841. 10.1038/s41586-023-06611-6
154
Zhao Y. Brandish P. E. Ballou D. P. Marletta M. A. (1999). A molecular basis for nitric oxide sensing by soluble guanylate cyclase. Proc. Natl. Acad. Sci. U. S. A.96, 14753–14758. 10.1073/pnas.96.26.14753
155
Zhu H. Riggs A. F. (1992). Yeast flavohemoglobin is an ancient protein related to globins and a reductase family. Proc. Natl. Acad. Sci. U. S. A.89, 5015–5019. 10.1073/pnas.89.11.5015
Summary
Keywords
oxygen sensing, oxygen receptor, gasoreceptor, proto-receptor, split-component signal transduction system, gasotransmitter, gasocrine signaling, gasocrinology
Citation
Anbalagan S (2025) Hemoglobin as an oxygen gasoreceptor. Acta Biochim. Pol. 72:15546. doi: 10.3389/abp.2025.15546
Received
05 September 2025
Accepted
08 October 2025
Published
17 December 2025
Volume
72 - 2025
Edited by
Grzegorz Wegrzyn, University of Gdansk, Poland
Reviewed by
Akito Nakao, Gunma University, Japan
Emily Reed, Texas A&M University, United States
Updates
Copyright
© 2025 Anbalagan.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Savani Anbalagan, savani.anbalagan@amu.edu.pl
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.