Abstract
In recent years, cannabinoids and their derivatives have been tested for efficacy in epilepsy therapy and related disorders. Many of them may help alleviate ailments associated with seizures. An in-depth study of cannabinoid derivatives and the receptors on which they operate give us a chance for more effective use of these substances in epilepsy therapy. Many studies point to the beneficial synergy of cannabinoids with chemotherapeutics and the increase in effectiveness of the latter. As a result, both alternatives to drug treatment and support for the pharmacotherapy are being developed. In this review, we focused on compounds such as Δ9-THC, CBDV, Δ9-THCA, Δ9-THCV, H2CBD and their receptors as well as on CBD’s actions, and the enzymes, ion channels, and transporters engaged in the fundamental causes of epileptic seizures. Treating epilepsy and drug-resistant epilepsy are the two common medical uses of cannabinoids. We looked at approximately 150 current scientific articles from peer-reviewed journals to explore the molecular effects of cannabinoids in these applications. Our goal was to improve physician awareness of factors influencing treatment decisions and potential adverse reactions to minimize medical errors and optimize patient care.
Introduction
This review examines cannabinoid derivatives under worldwide research and their molecular targets, focusing on their therapeutic potential in epilepsy. Despite extensive research, many mechanisms underlying the therapeutic effects of cannabinoids remain incompletely understood are not fully appreciated in clinical practice. The primary objective of this review is to provide a comprehensive and critically appraised elucidation of these molecular mechanisms, with a particular emphasis on recent findings, to facilitate their informed and widespread application. Our integrated focus on molecular and clinical evidence provides a distinct contribution by bridging basic science with real-world patient outcomes.
We begin with Δ9-THC, one of the most widely recognized cannabinoids, and then extensively analyze Cannabidiol (CBD), acknowledging the wealth of ongoing and recently completed clinical trials that have led to its regulatory approval for specific epilepsy syndromes. We also explore less-known but promising compounds such as Cannabidivarin (CBDV), Δ9-Tetrahydrocannabinolic acid (Δ9-THCA), Δ9-Tetrahydrocannabivarin (Δ9-THCV), and the synthetic analog 8,9-dihydrocannabidiol (H2CBD). This review integrates the latest scientific developments, encompassing both molecular pharmacology and clinical outcomes, to offer a robust and up-to-date perspective on the role of cannabinoids in epilepsy.
Materials and methods
A comprehensive narrative review with systematic search elements was conducted to identify and critically appraise the relevant scientific literature on the molecular targets and therapeutic effects of cannabinoids in epilepsy.
Search strategy
The MEDLINE database via PubMed (United States National Library of Medicine) was systematically searched for articles published up to June 1st, 2025. The primary search strings used were:
1. (“cannabis” OR “cannabinoids” OR “cannabidiol”) AND “epilepsy” (in title/abstract)
2. (“THC” OR “CBD” OR “CBDV” OR “THCA” OR “THCV” OR “H2CBD”) AND (“epilepsy” OR “seizure”) AND (“mechanism” OR “target” OR “receptor” OR “enzyme” OR “channel” OR “transporter”)
3. (“cannabidiol” AND “epilepsy”) AND (“clinical trial” OR “meta-analysis” OR “adverse event”)
Inclusion and exclusion criteria
Titles and abstracts were initially screened for direct relevance to cannabinoids, epilepsy, and molecular mechanisms or clinical outcomes. Full-text articles were then retrieved for detailed assessment. Original research articles (in vitro, in vivo animal studies, and human clinical trials, including randomized controlled trials and observational studies) published in English were included. Review articles, commentaries, and editorials were excluded as primary data sources but were used to identify relevant primary research or existing meta-analyses. Studies not directly investigating molecular targets, therapeutic efficacy, or safety in the context of epilepsy were excluded.
Screening procedures and data extraction
Initial screening of titles and abstracts was performed by three independent reviewers, with any discrepancies resolved through discussion to reach consensus. Full-text articles of all potentially relevant studies were subsequently obtained and meticulously reviewed for their eligibility. Key data extracted included: specific cannabinoid(s) studied; identified molecular targets (receptors, enzymes, ion channels, transporters); proposed mechanisms of action; observed therapeutic or adverse effects; study design (in vitro, specific animal model, human clinical trial phase/type); and species (human, mouse, rat, pig). Information on clinical outcomes, such as seizure frequency reduction, responder rates, and specific adverse events, was extracted from clinical trials.
Study quality appraisal and evidence hierarchy
Given the diverse nature of the included studies (ranging from mechanistic in vitro experiments to multi-center clinical trials), a formal quantitative meta-analysis of molecular targets was not performed due to inherent heterogeneity. Instead, a rigorous qualitative critical appraisal was conducted. Evidence was hierarchically considered, prioritizing findings from well-designed human clinical trials (especially randomized, placebo-controlled trials and comprehensive meta-analyses) for clinical efficacy and safety. Mechanistic insights from in vivo animal models were considered highly relevant, while in vitro studies provided foundational understanding of molecular interactions.
Grey literature policy
We did not include grey literature in this review, as it has not undergone a peer-review process, ensuring that all cited sources meet scientific publication standards.
Objectives
To compile and critically evaluate the actions of known cannabis derivatives, specifically identifying which receptors/processes are responsible for these actions, comprehensively assessing the strength of the underlying molecular evidence, and integrating the most recent findings on efficacy and safety in epilepsy.
Δ9-THC
Δ9-Tetrahydrocannabidiol (Δ9-THC) is one of the best-known cannabinoids (Table 1). This substance is responsible for the psychotropic effects of marijuana. The best-known Δ9-THC targets are the cannabinoid receptors type 1 (CB1) and type 2 (CB2), for which it is a partial agonist (Pertwee et al., 2014). Numerous studies have shown that THC has an anticonvulsant effect or that it modulates the action of antiepileptic drugs (AEDs). However, it should be noted that studies have also been described in which THC had no effect on convulsions, was provocative or the effect was inconclusive (Gaston and Friedman, 2017). The expression of CB receptors was found both in epilepsy in humans and in animal models of epilepsy. Their activation, regardless of the type of transmitter, reduces the release of neurotransmitters, while epileptic activity may be the result of an imbalance between excitatory (E) and inhibitory (I) synaptic transmission (Alger, 2014). In studies in mice it was also shown that the lack of CB1 and CB2 receptors causes epilepsy, which also proves the role of the endocannabinoid system in the regulation of brain excitability (Rowley et al., 2017). The results of other studies suggest a synergistic role of CB signalling in the modulation of early epileptogenic changes and that correlates with CB1R, 5-HT2CR, and NMDAR functions (Di Maio et al., 2019). However, the results of studies on the action of anticonvulsant Δ9-THC are not conclusive. This may result from both the universal inhibitory effects of cannabinoid receptors (they inhibit the release of excitatory and inhibitory transmitters, which makes their total impact on neuronal circuits not easy to predict), as well as from Δ9-THC-pleiotropic effect (affecting various receptors and signalling systems) (Alger, 2014; Gaston and Friedman, 2017). There are also studies suggesting that activation of the endocannabinoid system may be neuroprotective and prevent neuronal damage caused by epileptic seizures.
TABLE 1
| Endocannabinoids | |
|---|---|
| N-arachidonoylethanolamine (anandamide, AEA) | 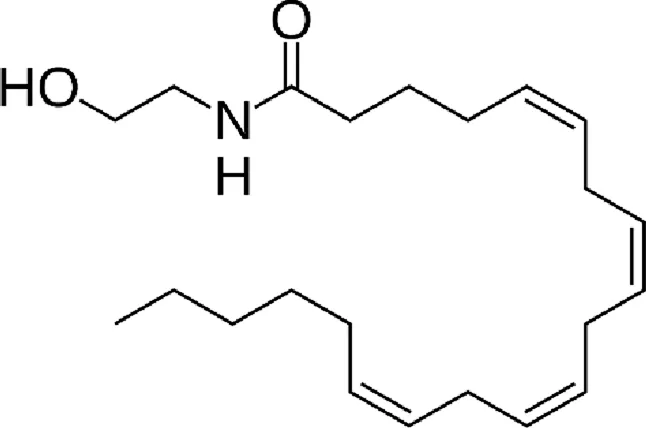 |
| 2-Arachidonoylglycerol (2-AG) | 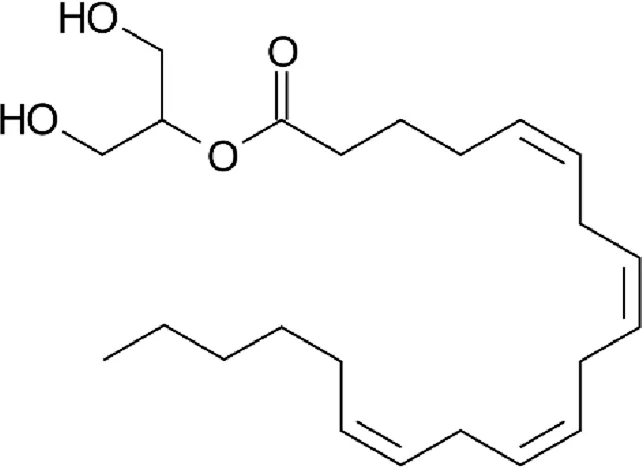 |
| Phytocannabinoids | |
|---|---|
| Tetrahydrocannabinol (THC) | 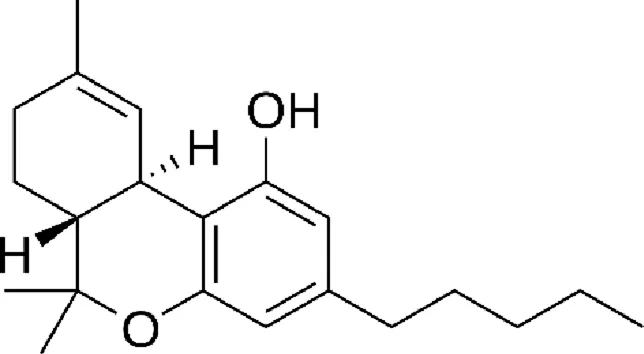 |
| Cannabidiol (CBD) | 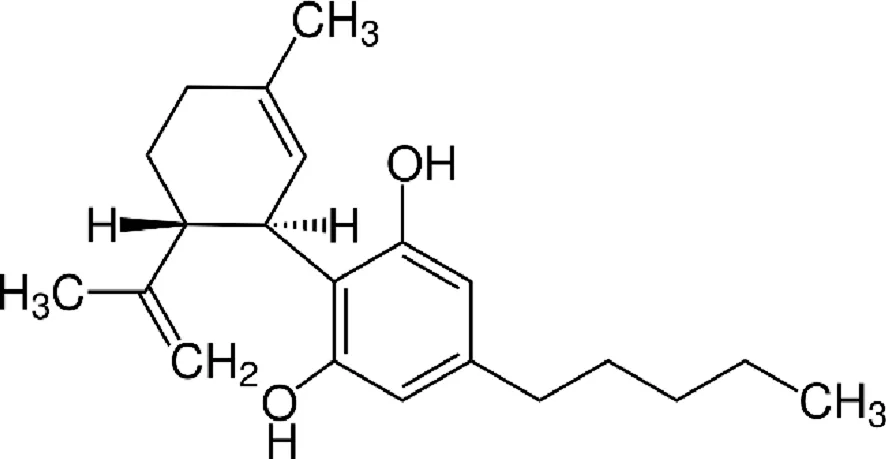 |
| Cannabidivarin (CBDV) | 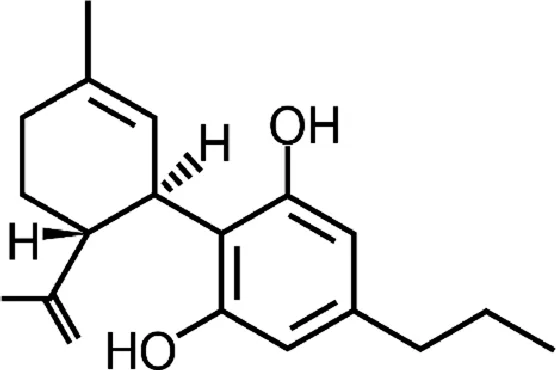 |
| Tetrahydrocannabinolic acid (THCA) | 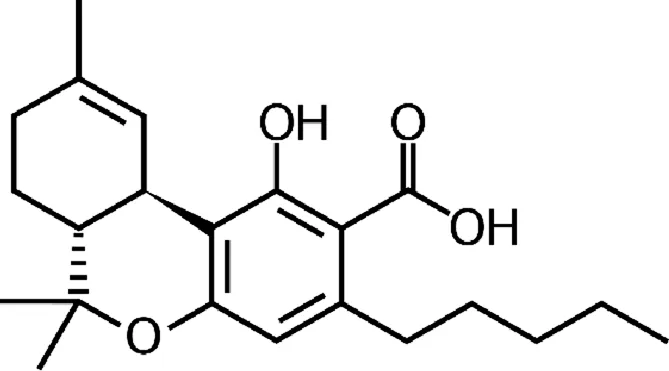 |
| Tetrahydrocannabivarin (THCV) | 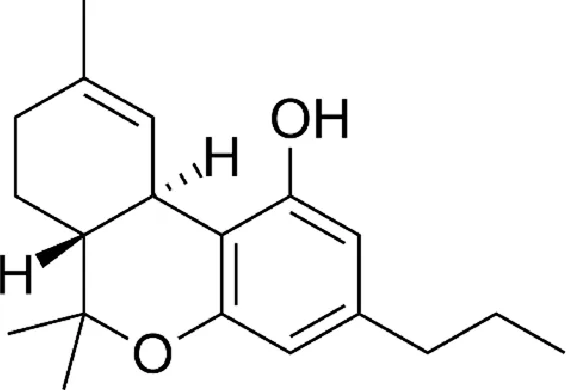 |
| Synthetic cannabinoids | |
|---|---|
| 8,9-dihydrocannabidiol (H2CBD) | 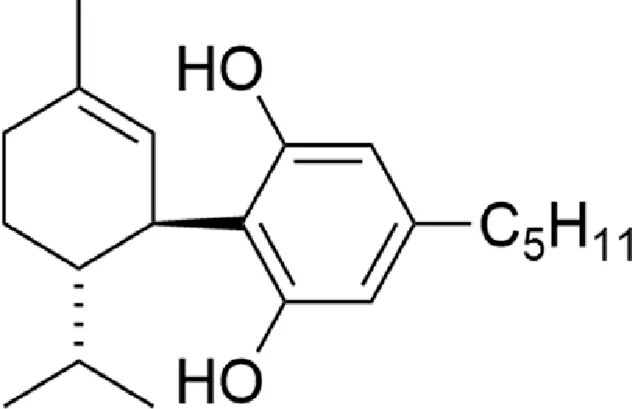 |
Chemical structure of the described cannabinoids.
In addition to the relatively well-described effect of THC on cannabinoid receptors, the following receptors are also mentioned in the literature: transient receptor potential (TRP) cation channels (TRPA1, TRPV2, TRPM8), 5-hydroxytryptamine receptor (5-HT3A), opioid receptors (μ, δ), orphan G-coupled protein GPR55 receptor, peroxisome proliferator-activated gamma receptor (PPARγ), β-adrenoreceptors and some ion channels, but the effects of their activation by THC are not fully understood. (Pertwee et al., 2014; Gaston and Friedman, 2017).
CBD
Cannabidiol (CBD), like Δ9-THC, is a phytocannabinoid compound, but unlike it, has very low affinity for cannabinoid receptors. This lack of affinity for CB1 receptors results in a lack of psychoactivity. It also means that the antiepileptic effects of CBD cannot, as in the case of Δ9-THC, be explained by inhibition of transmitter secretion (McPartland et al., 2015). In studies on an animal model of epilepsy (maximum electrical shock), the efficacy of Δ9-THC, CBD and WIN 55,212-2 in the treatment of seizures has been demonstrated. Using the specific CB1 receptor antagonist (SR141716A), it was proven that the anticonvulsant effect of THC and WIN 55,212-2 is dependent on the CB1 receptor, whereas CBD is independent of it (Wallace et al., 2001). Although the exact CBD anticonvulsant mechanism remains unknown, many molecular targets have been identified in recent years and several potential anticonvulsant mechanisms have been proposed for this compound (Ib et al., 2015). A review of CBD molecular targets described in the literature can be found in Table 2. Due to their role in the cell, they can be divided into receptors, enzymes, ion channels and transporters. It is suggested that the vanilloid receptor from the group of transient potential channels (TRPV1) may participate in anticonvulsant CBD’s activity. TRPV1 is involved in the modulation of epileptic seizures. It is a non-selective channel characterized by significant permeability to calcium ions. Its activation leads to increased release of glutamate and concentration of calcium ions, which results in excitability of neurons (Nazıroglu, 2015). CBD is an agonist of TRPV1 channels, its action causes their desensitization and, as a consequence, normalization of intracellular calcium concentration (Vilela et al., 2017). Another possible mechanism of anticonvulsant CBD’s activity is associated with calcium type T ion channels (T-Type Ca2+). Calcium channels are involved in the regulation of neuronal excitability. Activation of these channels is associated with hyperpolarization of the neuronal cell membrane and leads to an increase in the concentration of calcium ions in the cell, which causes excitability. This mechanism is observed in epilepsy (Nelson et al., 2006). CBD blocks T-type calcium channels, which may be responsible for the antiepileptic effect, but there are no studies to confirm (Silvestro et al., 2019). Serotonin (5-HT) receptors may also be important, as they can polarize and depolarize neurons, thereby affecting their conductivity. However, the results of research on their role in epilepsy remain controversial (Gharedaghi et al., 2014). CBD is an agonist of 5-HT1A and 5-HT2A receptors (Russo et al., 2005). The role of these receptors in epilepsy remains unclear, although it is assumed that they may be a therapeutic target for CBD. Opioid (Theodore et al., 2012; Theodore et al., 2007) receptors (ORs) belong to the group of G-protein coupled receptors (GPCR) and are involved in the pathology of some neurological disorders, e.g., epilepsy (Snead, 1986). CBD is an allosteric modulator of μ and δ opioid receptors, which may contribute to the mechanism of its anticonvulsant activity, but this has not been definitively confirmed (Kathmann et al., 2006). CBD is an antagonist of GPR55, which belongs to the receptors involved in the modulation of synaptic transmission (Ryberg et al., 2007). It is an important therapeutic target for CBD, including the Dravet Syndrome (Kaplan et al., 2017). The impact of CBD on cytochrome P450 (CYP450) should also be discussed, although this does not affect the anticonvulsant effect. CBD inhibits hepatic metabolism (Jiang et al., 2013; Yamaori et al., 2011a; Yamaori et al., 2011b; Yamaori et al., 2012; Yamaori et al., 2010; Yamaori et al., 2015; Yamaori et al., 2013), which is important because this cytochrome is involved in the metabolism of some drugs used in epilepsy and can modify their action (Silvestro et al., 2019).
TABLE 2
| Molecular target | CBD function | Type of research | Reference |
|---|---|---|---|
| Receptors | |||
| CB1 receptor | No significant change | H in vitro H in vitro H in vitro | Ramer et al. (2013) Jenny et al. (2009) Ryberg et al. (2007) |
| Negative allosteric modulator | H in vitro | Laprairie et al. (2015) | |
| Activator | H in vitro | Sta et al. (2015) | |
| CB2 receptor | No significant change | H in vitro H in vitro H in vitro | Ramer et al. (2013) Jenny et al. (2009) Ryberg et al. (2007) |
| α1 glycine receptor | Activator | H in vitro | Ahrens et al. (2009) |
| α1β glycine receptor | Activator | H in vitro | Ahrens et al. (2009) |
| α3 glycine receptor | Suppresses inflammatory and neuropathic pain by targeting α3 GlyRs | M in vivo | Xiong et al. (2012) |
| GPR3 | Inverse agonist | H in vitro H in vitro | Laun and Song (2017) Laun et al. (2019) |
| GPR6 | Inverse agonist | H in vitro H in vitro | Laun and Song (2017) Laun et al. (2019) |
| GPR12 | Inverse agonist | H in vitro H in vitro | Laun et al. (2019) Brown et al. (2017) |
| GPR18 | Partial agonist/antagonist | M in vivo | McHugh (2012) |
| GPR55 | Antagonist | M in vivo M in vitro H in vitro | Li et al. (2013) Ryberg et al. (2007) |
| 5-HT1A | Agonist | H in vitro | Russo et al. (2005) |
| Activator | R in vivo | De Gregorio et al. (2019) | |
| Enhances cortical 5-HT/glutamate neurotransmission | M in vivo | Linge et al. (2016) | |
| 5-HT2A | Agonist | H in vitro | Russo et al. (2005) |
| nAChR α-7 | Inhibitor | H in vitro | Mahgoub et al. (2013) |
| Opioid receptor δ | Allosteric modulator | R in vitro | Kathmann et al. (2006) |
| Opioid receptor μ | Allosteric modulator | R in vitro | Kathmann et al. (2006) |
| PPARγ | Upregulation/Translocation of PPAR-γ to the nucleus PPAR-γ-dependent apoptotic cell death | H in vitro | Ramer et al. (2013) |
| Activator | M in vivo | Hegde et al. (2015) | |
| Receptor sigma-1 (σ1R) | Antagonist | M in vivo | Rodríguez-Muñoz et al. (2018) |
| NMDA receptor | Inhibitor | M in vivo | Rodríguez-Muñoz et al. (2018) |
| GABAA | Positive allosteric modulator | H in vitro | Bakas et al. (2017) |
| Dopamine D2High receptors | Partial agonist | R in vitro | Seeman (2016) |
| Enzymes | |||
| Acyltransferase acylo-CoA: cholesterol (ACAT) | Inhibitor | H in vitro | Cornicelli et al. (1981) |
| Arylalkylamine N-acetyltransferase (AANAT) | Inhibitor | R in vitro | Koch et al. (2006) |
| Catalase | Inhibitor | M in vitro | Usami et al. (2008) |
| Complex I | Inhibitor | P in vitro | Fišar et al. (2014) |
| Activator | R in vivo | Valvassori et al. (2013) | |
| Complex II | Inhibitor | P in vitro | Fišar et al. (2014) |
| Activator | R in vivo | Valvassori et al. (2013) | |
| Complex II–III | Activator | R in vivo | Valvassori et al. (2013) |
| Inhibitor | P in vitro | Singh et al. (2015) | |
| Complex IV | Activator | R in vivo | Valvassori et al. (2013) |
| Inhibitor | P in vitro P in vitro | Fišar et al. (2014) Singh et al. (2015) | |
| COX1 | Inhibitor | H in vitro | Ruhaak et al. (2011) |
| COX2 | No significant change | H in vitro | Massi et al. (2008) |
| Inhibitor | H in vitro | Ruhaak et al. (2011) | |
| CYP2C19 | Inhibitor | H in vitro | Jiang et al. (2013) |
| CYP2D6 | Inhibitor | H in vitro | Yamaori et al. (2011a) |
| CYP3A4 | Inhibitor | H in vitro | Yamaori et al. (2011b) |
| CYP3A5 | Inhibitor | H in vitro | Yamaori et al. (2011b) |
| CYP3A7 | Inhibitor | H in vitro | Yamaori et al. (2011b) |
| CYP2C9 | Inhibitor | H in vitro | Yamaori et al. (2012) |
| CYP1A1 | Inhibitor | H in vitro H in vitro | Yamaori et al. (2010) Yamaori et al. (2013) |
| Induction of expression | H in vitro | Yamaori et al. (2015) | |
| CYP1A2 | Inhibitor | H in vitro | Yamaori et al. (2010) |
| CYP1B1 | Inhibitor | H in vitro | Yamaori et al. (2010) |
| DAGL-α | No significant change | H/R in vitro | De Petrocellis et al. (2011) |
| Fatty-acid amide hydrolase (FAAH) | Inhibitor | H in vitro H/R in vitro | Bisogno et al. (2001) De Petrocellis et al. (2011) |
| Activator | H in vitro | Massi et al. (2008) | |
| Glutathione reductase | Inhibitor | M in vitro | Usami et al. (2008) |
| Indoleamine-2,3-dioxygenase (IDO) | Inhibitor | H in vitro | Jenny et al. (2009) |
| LOX-5 | Inhibitor | H in vitro | Takeda et al. (2009) |
| Activator | H in vitro | Massi et al. (2008) | |
| LOX-15 | Inhibitor | H in vitro | Takeda et al. (2009) |
| No significant change | H in vitro | Massi et al. (2008) | |
| NAD(P)H quinone reductase | Inhibitor | M in vitro | Usami et al. (2008) |
| Phospholipase A2 | Activator | H in vitro | Evans et al. (1987) |
| Progesterone 17α-hydroxylase | Inhibitor | R in vitro | Watanabe et al. (2005) |
| Aldose reductase | Inhibitor | H/P in vitro | Smeriglio et al. (2018) |
| Superoxide Dismutase (SOD) | Inhibitor | M in vitro | Usami et al. (2008) |
| Sphingomyelinase | Activator (especially Niemann-Pick’s cells) | H in vitro | Burstein et al. (1984) |
| Testosterone 6α-hydroxylase | Inhibitor | R in vitro | Watanabe et al. (2005) |
| Testosterone 16β-hydroxylase | Inhibitor | R in vitro | Watanabe et al. (2005) |
| Topoisomerase II | No significant change (oxidized CBD – inhibitor) | H in vitro | Wilson et al. (2018) |
| Ion channels | |||
| Cav3.1 T-type | Inhibitor | H in vitro | Ross et al. (2008) |
| Cav3.2 T-type | Inhibitor | H in vitro | Ross et al. (2008) |
| Cav3.3 T-type | Inhibitor | H in vitro | Ross et al. (2008) |
| TRPA1 | Activator | R in vitro R in vitro R in vitro | Qin et al. (2008) De Petrocellis et al. (2008) Iannotti et al. (2014) |
| Activator Desensitization | H/R in vitro | De Petrocellis et al. (2011) | |
| TRPV1 | Activator | H in vitro | Jenny et al. (2009) |
| Activator Desensitization | H/R in vitro | De Petrocellis et al. (2011) | |
| No significant change | R in vitro | Qin et al. (2008) | |
| Activator Desensitization | R in vitro | Iannotti et al. (2014) | |
| Activator | H in vitro R in vivo | Ligresti et al. (2006) De Gregorio et al. (2019) | |
| TRPV2 | Activator | R in vitro H in vitro H/R in vitro R in vitro | Qin et al. (2008) Nabissi et al. (2013) De Petrocellis et al. (2011) Iannotti et al. (2014) |
| TRPV3 | Activator | M in vivo | De Petrocellis et al. (2012) |
| TRPV4 | Activator | M in vivo | De Petrocellis et al. (2012) |
| TRPM8 | Inhibitor/No significant change | R in vitro | De Petrocellis et al. (2008) |
| Inhibitor | H/R in vitro | De Petrocellis et al. (2011) | |
| VDAC1 | Inhibitor | M in vitro | Rimmerman et al. (2013) |
| Sodium channels (Nav) | Inhibitor | H in vitro | Ghovanloo et al. (2018) |
| Voltage-gated potassium channel subunit Kv2.1 | Inhibitor | H in vitro | Ghovanloo et al. (2018) |
| Ca2+-activated K+ channels of large conductance (BKCa) | Activator | H in vitro M in situ | Bondarenko et al. (2018) |
| Transporters | |||
| ABCC1 | Inhibitor | H in vitro | Holland et al. (2008) |
| ABCG2 | Inhibitor | M in vitro | Holland et al. (2007) |
| Adenosine uptake (ENT-1) | Inhibitor | R/M in vivo M in vitro | Pandolfo et al. (2011) Carrier et al. (2006) |
| Anandamide uptake (AMT) | Inhibitor | H in vitro H/R in vitro | Jenny et al. (2009) De Petrocellis et al. (2011) |
| Dopamine uptake | Inhibitor | R/M in vivo | Pandolfo et al. (2011) |
| Glutamate uptake | Inhibitor | R/M in vivo | Pandolfo et al. (2011) |
| Mg2+-ATPase | Inhibitor | R in vitro | Gilbert et al. (1977) |
| Noradrenaline uptake | Inhibitor | R in vitro | Coyle and Snyder (1969) |
| Thymidine uptake | Inhibitor | M in vitro | Carrier et al. (2006) |
Molecular targets of cannabidiol (CBD) (H–human, M–mouse, R–rat, P–pig).
CBDV
Cannabidivarin (CBDV), also found in cannabis, is a CBD homolog with the sidechain shortened by 2 methylene bridges. Its anticonvulsant activity has been confirmed in preclinical studies in vitro and in vivo (animal model of epilepsy) (Hill et al., 2012; Hill TD. et al., 2013; Amada et al., 2013). The mechanism of anticonvulsant CBDV activity has not yet been explained, however it seems to be independent of cannabinoid receptors (CBDV, like CBD, has no psychoactive properties). The chemical similarity of CBDV to CBD suggests that these compounds may work similarly (Gaston and Friedman, 2017). CBDV also has agonistic activity at TRPA1, TRPV1 and TRPV2 receptors and antagonistic activity in TRPM8 (De Petrocellis et al., 2011).
Δ9-THCA
Delta-9-tetrahydrocannabinolic acid (Δ9-THCA) is a THC precursor that occurs in live cannabis. The decarboxylation of THCA to THC occurs under natural conditions in the storage and fermentation of cannabis and under the influence of temperature and light, while the in vivo metabolism of Δ9-THCA to Δ9-THC is limited due to its separate metabolic pathways (Moreno-Sanz, 2016). In vitro, Δ9-THCA effect on activation of TRPA1, TRPV2 and TRPV4 channels and blocking of TRPV1 and TRPM8 channels has been demonstrated (De Petrocellis et al., 2013). It also inhibits cyclooxygenase (COX 1, COX 2) and diacylglycerol lipase alpha (DLGα), which is an important enzyme in 2-AG biosynthesis (Cascio and Pertwee, 2014; Ruhaak et al., 2011; De Petrocellis et al., 2013). There is no evidence of the ability of THCA to penetrate the CNS after systemic administration or the effect of THCA on cannabinoid receptors (Moreno-Sanz, 2016). Despite online reports suggesting the antiepileptic activities of this compound, in fact, there is no evidence (Gaston and Friedman, 2017).
Δ9-THCV
Delta-9-tetrahydrocannabivarin (Δ9-THCV) is another cannabinoid found in cannabis. It has been shown to be a partial agonist of the CB1 and CB2 receptors (similar to Δ9-THC). In addition, it has activity on TRPA1, TRPV1-4 and GPR55 receptors (Cascio and Pertwee, 2014). A single study showed anticonvulsant efficacy of THCV in an animal model (Hill TD. et al., 2013).
H2CBD
The psychoactive properties of THC make the use of cannabinoids, in the treatment of diseases, some legal and social difficulties. In recent years, researchers have focused on CBD, which has no psychoactive effect. However, like other phytocannabinoids, it is a controlled substance in many countries, due to the ease of chemical conversion to THC. Due to these problems, a fully synthetic CBD analogue, 8.9-dihydrocannabidiol (H2CBD), was developed. This compound is produced from non-cannabis precursors and cannot be converted to THC (Mascal et al., 2019). In an animal model of epilepsy (pentylenetetrazole-induced seizures in rats), the effectiveness of H2CBD in reducing the number and severity of seizures has been shown to be comparable to CBD. The mechanism of H2CBD anticonvulsant action is unknown (Mascal et al., 2019).
Conclusion
Our review of the literature, integrating significant findings up to June 2025, has provided a comprehensive overview of the molecular targets underlying the therapeutic effects of cannabinoids, particularly CBD, in epilepsy. While a multitude of molecular targets have been elucidated through in vitro and animal models, the evidence clearly demonstrates the multi-faceted nature of cannabinoid action. Rigorous human clinical trials, especially randomized controlled trials and subsequent meta-analyses, have firmly established the clinical efficacy of CBD for specific drug-resistant epilepsies such as Dravet Syndrome, Lennox-Gastaut Syndrome, and Tuberous Sclerosis Complex.
These clinical advancements underscore that the anticonvulsant activity of phytocannabinoids like CBD, Δ9-THC, Δ8-THC, and Δ9-THCB, is not attributable to a single receptor interaction but rather to a complex modulation of numerous physiological pathways. The concept of the “entourage effect,” suggesting a synergistic interplay of active and inactive botanical molecules in whole plant extracts, warrants further rigorous scientific validation in controlled human trials, as current evidence remains largely observational or preclinical.
Recent years have seen the identification of further molecular targets, including various serotonin receptor subtypes, glycine receptors, α2 adrenergic receptors, voltage-gated calcium channels (VGCCs), and acetylcholine receptors, adding to the complexity of cannabinoid pharmacology. While these interactions suggest broader therapeutic potential, the precise contribution of each target to the overall beneficial effect in epilepsy, and particularly whether cannabinoids exert beneficial effects solely through these newly identified targets, remains a key focus of ongoing research. Furthermore, novel compounds like Δ9-THCB and Δ9-THCP, recently isolated and showing high affinity for CB1 receptors and potent cannabimimetic activity, represent promising tools for future investigations into the pathophysiology and treatment of epilepsy. The continuous elucidation of these molecular targets, coupled with robust clinical translation, will pave the way for more targeted and effective cannabinoid-based therapies.
Discussion
The therapeutic promise of cannabinoids, particularly cannabidiol (CBD), in the treatment of epilepsy has been substantially confirmed in recent years, leading to its regulatory approval for specific severe forms of epilepsy. While the existing literature provides compelling evidence for the anticonvulsant properties of CBD, several critical aspects warrant in-depth discussion and ongoing scientific scrutiny.
Efficacy and safety: Clinical outcomes and adverse events
The efficacy of CBD in significantly reducing seizure frequency has been unequivocally demonstrated in multiple large-scale, randomized, placebo-controlled clinical trials, particularly for treatment-resistant epilepsies such as Dravet Syndrome, Lennox-Gastaut Syndrome, and Tuberous Sclerosis Complex (Wu et al., 2022; Gastrop, 2022). Typical seizure reduction rates for CBD range from 30% to over 50% in responder populations, with some patients achieving complete seizure freedom. However, the variability in response among patients remains a critical challenge, highlighting the need for personalized treatment approaches. Factors such as genetic variations (e.g., SCN1A mutations in Dravet syndrome), specific epilepsy syndromes, concomitant antiepileptic medications, and individual pharmacokinetic profiles can profoundly influence CBD’s effectiveness.
While CBD is generally well-tolerated, adverse events (AEs) are common and require careful monitoring, especially in pediatric and polypharmacy patients. The most frequently reported AEs in clinical trials include somnolence, decreased appetite, diarrhea, fatigue, and elevated liver transaminases (ALT and AST) (Pauli et al., 2020). Liver enzyme elevations, often transient and dose-dependent, are particularly noteworthy when CBD is co-administered with valproate or clobazam, due to known CYP450 interactions. This necessitates regular liver function monitoring, as acknowledged in the literature. Long-term safety data are still accumulating, emphasizing the need for ongoing post-marketing surveillance and dedicated research into the sustained effects of CBD on organ systems and brain development in vulnerable populations.
Mechanisms of action: Disentangling complexity and divergence
The precise mechanisms by which CBD exerts its anticonvulsant effects are complex and polypharmacological, involving interactions with multiple molecular targets as detailed in Table 2. CBD’s engagement with TRPV1, T-type calcium channels (Cav3.1, 3.2, 3.3), 5-HT1A receptors, GPR55, and adenosine uptake (via ENT-1) collectively contribute to its broad therapeutic profile. However, it is crucial to distinguish between hypothetical mechanisms identified in in vitro or animal models and those definitively established in human epilepsy. For instance, while CBD’s blockade of T-type calcium channels is robustly shown in cell-based assays (Ross et al., 2008), its clinical relevance as the primary anticonvulsant pathway in humans remains to be fully elucidated. Conversely, the antagonism of GPR55 and modulation of intracellular calcium via TRPV1 desensitization represent more strongly supported mechanisms directly relevant to neuronal hyperexcitability (Vilela et al., 2017; Kaplan et al., 2017).
The existence of divergent research findings across different groups, particularly in preclinical studies (e.g., conflicting reports on THC’s pro-vs. anticonvulsant effects (Gaston and Friedman, 2017)), underscores the importance of experimental rigor. These discrepancies can often be attributed to variations in cannabinoid purity, formulation, dosing regimens, specific animal models of epilepsy, species differences, and the experimental conditions of in vitro assays. A critical appraisal of these factors is essential when interpreting and comparing results, as emphasized in our methodology. The polypharmacology of CBD, while beneficial in terms of broad therapeutic potential, also complicates the prediction of drug interactions and a complete understanding of its side effect profile. Further research using advanced techniques, such as optogenetics, chemogenetics, and in vivo electrophysiology, is needed to delineate the primary pathways and identify robust biomarkers predictive of patient response.
Regulatory and ethical considerations
The varying legal status of CBD globally, despite its FDA/EMA approval for specific epilepsies, continues to impact its accessibility and the conduct of large-scale clinical trials. Regulatory hurdles and the lingering stigma associated with cannabis-derived products can impede both scientific research and clinical integration. Ethical considerations are particularly salient in pediatric epilepsy, where the long-term effects of chronic CBD administration on brain development, cognitive function, and endocrine systems are still under investigation. Balancing the demonstrated clinical benefits against these potential long-term risks, especially in vulnerable pediatric populations, requires ongoing vigilance and robust pharmacovigilance programs. The distinction between pharmaceutical-grade CBD and unregulated CBD products is also a critical regulatory and safety concern, as the latter may contain inconsistent CBD concentrations, impurities, or undeclared cannabinoids.
In conclusion, while CBD now stands as a recognized and effective treatment for specific forms of epilepsy, a deeper, integrated understanding of its comprehensive mechanisms, validated efficacy across diverse populations, and long-term safety profile remains essential. Continued collaborative efforts among scientists, clinicians, and policymakers, coupled with stringent critical appraisal of evidence, will be key to unlocking the full and safe potential of cannabinoids in epilepsy therapy.
Statements
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
References
1
AhrensJ.DemirR.LeuwerM.de la RocheJ.KrampflK.FoadiN.et al (2009). The nonpsychotropic cannabinoid cannabidiol modulates and directly activates alpha-1 and alpha-1-Beta glycine receptor function. Pharmacology83 (4), 217–222. 10.1159/000201556
2
AlgerB. E. (2014). Seizing an opportunity for the endocannabinoid system. Epilepsy Curr.14 (5), 272–276. 10.5698/1535-7597-14.5.272
3
AmadaN.YamasakiY.WilliamsC. M.WhalleyB. J. (2013). Cannabidivarin (CBDV) suppresses pentylenetetrazole (PTZ)-induced increases in epilepsy-related gene expression. Peer J.1, e214. 10.7717/peerj.214
4
BakasT.van NieuwenhuijzenP. S.DevenishS. O.McGregorI. S.ArnoldJ. C.ChebibM. (2017). The direct actions of cannabidiol and 2-arachidonoyl glycerol at GABAA receptors. Pharmacol. Res.119, 358–370. 10.1016/j.phrs.2017.02.022
5
BisognoT.HanusL.De PetrocellisL.TchilibonS.PondeD. E.BrandiI.et al (2001). Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol.134 (4), 845–852. 10.1038/sj.bjp.0704327
6
BondarenkoA.PanasiukO.DrachukK.MontecuccoF.BrandtK. J.MachF. (2018). The quest for endothelial atypical cannabinoid receptor: BKCa channels act as cellular sensors for cannabinoids in in vitro and in situ endothelial cells. Vasc. Pharmacol.102, 44–55. 10.1016/j.vph.2018.01.004
7
BrownK. J.LaunA. S.SongZ. H. (2017). Cannabidiol, a novel inverse agonist for GPR12. Biochem. Biophysical Res. Commun.493 (1), 451–454. 10.1016/j.bbrc.2017.09.001
8
BursteinS.HunterS. A.RenzulliL. (1984). Stimulation of sphingomyelin hydrolysis by cannabidiol in fibroblasts from a Niemann-Pick patient. Biochem. Biophysical Res. Commun.121 (1), 168–173. 10.1016/0006-291x(84)90702-2
9
CarrierE. J.AuchampachJ. A.HillardC. J. (2006). Inhibition of an equilibrative nucleoside transporter by cannabidiol: A mechanism of cannabinoid immunosuppression. Proc. Natl. Acad. Sci. U. S. A.103 (20), 7895–7900. 10.1073/pnas.0511232103
10
CascioM. G.PertweeR. G. (2014). Known pharmacological actions of nine nonpsychotropic phytocannabinoids. Br. J. Pharmacol.171 (3), S3–S22. 10.1093/acprof:oso/9780199662685.003.0006
11
CornicelliJ. A.GilmanS. R.KromB. A.KottkeB. A. (1981). Cannabinoids impair the formation of cholesteryl ester in cultured human cells. Arteriosclerosis1 (6), 449–454. 10.1161/01.ATV.1.6.449
12
CoyleJ. T.SnyderS. H. (1969). Catecholamine uptake by synaptosomes in homogenates of rat brain: stereospecificity in different areas. J. Pharmacol. Exp. Ther.170 (2), 221–231. 10.1016/S0022-3565(25)28416-6
13
De GregorioD.McLaughlinR. J.PosaL.Ochoa-SanchezR.EnnsJ.Lopez-CanulM.et al (2019). Cannabidiol modulates serotonergic transmission and reverses both allodynia and anxiety-like behavior in a model of neuropathic pain. Pain160 (1), 136–150. 10.1097/j.pain.0000000000001386
14
De PetrocellisL.VellaniV.Schiano-MorielloA.MariniP.MagheriniP. C.OrlandoP.et al (2008). Plant-derived cannabinoids modulate the activity of transient receptor potential channels of ankyrin type-1 and melastatin type-8. J. Pharmacol. Exp. Ther.325 (3), 1007–1015. 10.1124/jpet.107.134809
15
De PetrocellisL.LigrestiA.MorielloA. S.AllaràM.BisognoT.PetrosinoS.et al (2011). Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol.163 (7), 1479–1494. 10.1111/j.1476-5381.2010.01166.x
16
De PetrocellisL.OrlandoP.MorielloA. S.AvielloG.StottC.IzzoA. A.et al (2012). Cannabinoid actions at TRPV channels: Effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation. Acta Physiol.204 (2), 255–266. 10.1111/j.1748-1716.2011.02338.x
17
De PetrocellisL.LigrestiA.Schiano MorielloA.IappelliM.VerdeR.StottC. G.et al (2013). Non-THC cannabinoids inhibit prostate carcinoma growth in vitro and in vivo: Pro-apoptotic effects and underlying mechanisms. Br. J. Pharmacol.168 (1), 79–102. 10.1111/j.1476-5381.2012.02027.x
18
Di MaioR.ColangeliR.Di GiovanniG. (2019). WIN 55,212-2 reverted pilocarpine-induced status epilepticus early changes of the interaction among 5-HT2C/NMDA/CB1 receptors in the rat Hippocampus. ACS Chem. Neurosci.10 (7), 3296–3306. 10.1021/acschemneuro.9b00080
19
EvansA. T.FormukongE.EvansF. J. (1987). Activation of phospholipase A2 by cannabinoids. Lack of correlation with CNS effects. FEBS Lett.211 (2), 119–122. 10.1016/0014-5793(87)81420-5
20
FišarZ.SinghN.HroudováJ. (2014). Cannabinoid-induced changes in respiration of brain mitochondria. Toxicol. Lett.231 (1), 62–71. 10.1016/j.toxlet.2014.09.002
21
GastonT. E.FriedmanD. (2017). Pharmacology of cannabinoids in the treatment of epilepsy. Epilepsy and Behav.70, 313–318. 10.1016/j.yebeh.2016.11.016
22
GastropR. (2022). Cannabidiol for refractory Lennox-Gastaut syndrome: a phase 3, randomized, placebo-controlled trial. N. Engl. J. Med.386 (17), 1605–1614. 10.1016/S0140-6736(18)30136-3
23
GharedaghiM. H.SeyedabadiM.GhiaJ. E.DehpourA. R.RahimianR. (2014). The role of different serotonin receptor subtypes in seizure susceptibility. Exp. Brain Res.232 (2), 347–367. 10.1007/s00221-013-3757-0
24
GhovanlooM. R.ShuartN. G.MezeyovaJ.DeanR. A.RubenP. C.GoodchildS. J. (2018). Inhibitory effects of cannabidiol on voltage-dependent sodium currents. J. Biol. Chem.293 (43), 16546–16558. 10.1074/jbc.RA118.004929
25
GilbertJ. C.PertweeR. G.WyllieM. G. (1977). Effects of δ9‐tetrahydrocannabinol and cannabidiol on a mg2+‐atpase of synaptic vesicles prepared from rat cerebral cortex. Br. J. Pharmacol.59 (4), 599–601. 10.1111/j.1476-5381.1977.tb07727.x
26
HegdeV. L.SinghU. P.NagarkattiP. S.NagarkattiM. (2015). Critical role of mast cells and peroxisome proliferator-activated receptor γ in the induction of myeloid-derived suppressor cells by marijuana cannabidiol in vivo. J. Immunol.194 (11), 5211–5222. 10.4049/jimmunol.1401844
27
HillA. J.MercierM. S.HillT. D.GlynS. E.JonesN. A.YamasakiY.et al (2012). Cannabidivarin is anticonvulsant in mouse and rat. Br. J. Pharmacol.167 (8), 1629–1642. 10.1111/j.1476-5381.2012.02207.x
28
HillT. D.CascioM. G.RomanoB.DuncanM.PertweeR. G.WilliamsC. M.et al (2013). Cannabidivarin‐rich cannabis extracts are anticonvulsant in mouse and rat via a CB1 receptor‐independent mechanism. Br. J. Pharmacol.170 (3), 679–692. 10.1111/bph.12321
29
HollandM. L.LauD. T. T.AllenJ. D.ArnoldJ. C. (2007). The multidrug transporter ABCG2 (BCRP) is inhibited by plant-derived cannabinoids. Br. J. Pharmacol.152 (5), 815–824. 10.1038/sj.bjp.0707467
30
HollandM. L.AllenJ. D.ArnoldJ. C. (2008). Interaction of plant cannabinoids with the multidrug transporter ABCC1 (MRP1). Eur. J. Pharmacol.591 (1-3), 128–131. 10.1016/j.ejphar.2008.06.079
31
IannottiF. A.HillC. L.LeoA.AlhusainiA.SoubraneC.MazzarellaE.et al (2014). Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: Potential for the treatment of neuronal hyperexcitability. ACS Chem. Neurosci.5 (11), 1131–1141. 10.1021/cn5000524
32
Ibeas BihC.ChenT.NunnA. V.BazelotM.DallasM.WhalleyB. J. (2015). Molecular targets of cannabidiol in neurological disorders. Neurotherapeutics12 (4), 699–730. 10.1007/s13311-015-0377-3
33
JennyM.SanterE.PirichE.SchennachH.FuchsD. (2009). Δ9-Tetrahydrocannabinol and cannabidiol modulate mitogen-induced tryptophan degradation and neopterin formation in peripheral blood mononuclear cells in vitro. J. Neuroimmunol.207 (1-2), 75–82. 10.1016/j.jneuroim.2008.12.004
34
JiangR.YamaoriS.OkamotoY.YamamotoI.WatanabeK. (2013). Cannabidiol is a potent inhibitor of the catalytic activity of cytochrome P450 2C19. Drug Metabolism Pharmacokinet.28 (4), 332–338. 10.2133/dmpk.DMPK-12-RG-129
35
KaplanJ. S.StellaN.CatterallW. A.WestenbroekR. E. (2017). Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. Proc. Natl. Acad. Sci.114 (42), 11229–11234. 10.1073/pnas.1711351114
36
KathmannM.FlauK.RedmerA.TränkleC.SchlickerE. (2006). Cannabidiol is an allosteric modulator at mu- and delta-opioid receptors. Naunyn-Schmiedeberg's Archives Pharmacol.372 (5), 354–361. 10.1007/s00210-006-0033-x
37
KochM.DehghaniF.HabazettlI.SchomerusC.KorfH. W. (2006). Cannabinoids attenuate norepinephrine-induced melatonin biosynthesis in the rat pineal gland by reducing arylalkylamine N-acetyltransferase activity without involvement of cannabinoid receptors. J. Neurochem.98 (1), 267–278. 10.1111/j.1471-4159.2006.03873.x
38
LaprairieR. B.BagherA. M.KellyM. E.Denovan‐WrightE. M. (2015). Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol.172 (20), 4790–4805. 10.1111/bph.13250
39
LaunA. S.SongZ. H. (2017). GPR3 and GPR6, novel molecular targets for cannabidiol. Biochem. Biophysical Res. Commun.490 (1), 17–21. 10.1016/j.bbrc.2017.05.165
40
LaunA. S.ShraderS. H.BrownK. J.SongZ. H. (2019). GPR3, GPR6, and GPR12 as novel molecular targets: Their biological functions and interaction with cannabidiol. Acta Pharmacol. Sin.40 (3), 300–308. 10.1038/s41401-018-0031-9
41
LiK.FichnaJ.SchichoR.SaurD.BashashatiM.MackieK.et al (2013). A role for O-1602 and G protein-coupled receptor GPR55 in the control of colonic motility in mice. Neuropharmacology71, 255–263. 10.1016/j.neuropharm.2013.03.029
42
LigrestiA.MorielloA. S.StarowiczK.MatiasI.PisantiS.De PetrocellisL.et al (2006). Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J. Pharmacol. Exp. Ther.318 (3), 1375–1387. 10.1124/jpet.106.105247
43
LingeR.Jiménez-SánchezL.CampaL.Pilar-CuéllarF.VidalR.PazosA.et al (2016). Cannabidiol induces rapid-acting antidepressant-like effects and enhances cortical 5-HT/glutamate neurotransmission: Role of 5-HT1A receptors. Neuropharmacology103, 16–26. 10.1016/j.neuropharm.2015.12.017
44
MahgoubM.Keun-HangS. Y.SydorenkoV.AshoorA.KabbaniN.Al KuryL.et al (2013). Effects of cannabidiol on the function of α7-nicotinic acetylcholine receptors. Eur. J. Pharmacol.720 (1-3), 310–319. 10.1016/j.ejphar.2013.10.011
45
MascalM.HafeziN.WangD.HuY.SerraG.DallasM. L.et al (2019). Synthetic, non-intoxicating 8,9-dihydrocannabidiol for the mitigation of seizures. Sci Rep.11, 7778. 10.1038/s41598-019-44056-y
46
MassiP.ValentiM.VaccaniA.GasperiV.PerlettiG.MarrasE.et al (2008). 5-Lipoxygenase and anandamide hydrolase (FAAH) mediate the antitumor activity of cannabidiol, a non-psychoactive cannabinoid. J. Neurochem.104 (4), 1091–1100. 10.1111/j.1471-4159.2007.05073.x
47
McHughD. (2012). GPR18 in microglia: Implications for the CNS and endocannabinoid system signalling. Br. J. Pharmacol.167 (8), 1575–1582. 10.1111/j.1476-5381.2012.02019.x
48
McPartlandJ. M.DuncanM.Di MarzoV.PertweeR. G. (2015). Are cannabidiol and Δ(9)-tetrahydrocannabivarin negative modulators of the endocannabinoid system? A systematic review. Br. J. Pharmacol.172 (3), 737–753. 10.1111/bph.12944
49
Moreno-SanzG. (2016). Can you pass the acid test? Critical review and novel therapeutic perspectives of Δ9-tetrahydrocannabinolic acid A. Cannabis Cannabinoid Res.1 (1), 124–130. 10.1089/can.2016.0008
50
NabissiM.MorelliM. B.SantoniM.SantoniG. (2013). Triggering of the TRPV2 channel by cannabidiol sensitizes glioblastoma cells to cytotoxic chemotherapeutic agents. Carcinogenesis34 (1), 48–57. 10.1093/carcin/bgs328
51
NazırogluM. (2015). TRPV1 channel: a potential drug target for treating epilepsy. Curr. Neuropharmacol.13 (2), 239–247. 10.2174/1570159X13666150216222543
52
NelsonM. T.TodorovicS. M.Perez-ReyesE. (2006). The role of T-type calcium channels in epilepsy and pain. Curr. Pharm. Des.12 (18), 2189–2197. 10.2174/138161206777585184
53
PandolfoP.SilveirinhaV.Santos-RodriguesA. d.VenanceL.LedentC.TakahashiR. N.et al (2011). Cannabinoids inhibit the synaptic uptake of adenosine and dopamine in the rat and mouse striatum. Eur. J. Pharmacol.655 (1-3), 38–45. 10.1016/j.ejphar.2011.01.013
54
PertweeR. G.CascioM. G. (2014). “Known pharmacological actions of delta-9-tetrahydrocannabinol and of four other chemical constituents of cannabis that activate cannabinoid receptors,” in Handbook of cannabis. Editor PertweeR. G. (Oxford: Oxford University Press), 115. 10.1093/acprof:oso/9780199662685.003.0006
55
PauliC. S.ConroyM.Vanden HeuvelB. D.ParkS. H. (2020). Cannabidiol drugs clinical trial outcomes and adverse effects. Front Pharmacol.11, 63. 10.3389/fphar.2020.00063
56
QinN.NeeperM. P.LiuY.HutchinsonT. L.LubinM. L.FloresC. M. (2008). TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J. Neurosci.28 (24), 6231–6238. 10.1523/JNEUROSCI.0504-08.2008
57
RamerR.HeinemannK.MerkordJ.RohdeH.SalamonA.LinnebacherM.et al (2013). COX-2 and PPAR-γ confer cannabidiol-induced apoptosis of human lung cancer cells. Mol. Cancer Ther.12 (1), 69–82. 10.1158/1535-7163.MCT-12-0335
58
RimmermanN.Ben-HailD.PoratZ.JuknatA.KozelaE.DanielsM. P.et al (2013). Direct modulation of the outer mitochondrial membrane channel, voltage-dependent anion channel 1 (VDAC1) by cannabidiol: A novel mechanism for cannabinoid-induced cell death. Cell Death Dis.4 (12), e949. 10.1038/cddis.2013.471
59
Rodríguez-MuñozM.OnettiY.Cortés-MonteroE.GarzónJ.Sánchez-BlázquezP. (2018). Cannabidiol enhances morphine antinociception, diminishes NMDA-mediated seizures and reduces stroke damage via the sigma 1 receptor. Mol. Brain11 (1), 51. 10.1186/s13041-018-0395-2
60
RossH. R.NapierI.ConnorM. (2008). Inhibition of recombinant human T-type calcium channels by Δ9-tetrahydrocannabinol and cannabidiol. J. Biol. Chem.283 (23), 16124–16134. 10.1074/jbc.M707104200
61
RowleyS.SunX.LimaI. V.TavenierA.de OliveiraA. C. P.DeyS. K.et al (2017). Cannabinoid receptor 1/2 double-knockout mice develop epilepsy. Epilepsia58 (8), e162–e166. 10.1111/epi.13930
62
RuhaakL. R.FelthJ.KarlssonP. C.RafterJ. J.VerpoorteR.BohlinL. (2011). Evaluation of the cyclooxygenase inhibiting effects of six major cannabinoids isolated from Cannabis sativa. Biol. Pharm. Bull.34 (5), 774–778. 10.1248/bpb.34.774
63
RussoE. B.BurnettA.HallB.ParkerK. K. (2005). Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem. Res.30 (8), 1037–1043. 10.1007/s11064-005-6978-1
64
RybergE.LarssonN.SjögrenS.HjorthS.HermanssonN. O.LeonovaJ.et al (2007). The orphan receptor GPR55 is a novel cannabinoid receptor. Br. J. Pharmacol.152 (7), 1092–1101. 10.1038/sj.bjp.0707460
65
SeemanP. (2016). Cannabidiol is a partial agonist at dopamine D2High receptors, predicting its antipsychotic clinical dose. Transl. Psychiatry6 (10), e920. 10.1038/tp.2016.195
66
SilvestroS.MammanaS.CavalliE.BramantiP.MazzonE. (2019). Use of cannabidiol in the treatment of epilepsy: efficacy and security in clinical trials. Molecules24 (8), 1459. 10.3390/molecules24081459
67
SinghN.HroudováJ.FišarZ. (2015). Cannabinoid-induced changes in the activity of electron transport Chain complexes of brain mitochondria. J. Mol. Neurosci.56 (4), 926–931. 10.1007/s12031-015-0545-2
68
SmeriglioA.GiofrèS. V.GalatiE. M.MonforteM. T.CiceroN.D'AngeloV.et al (2018). Inhibition of aldose reductase activity by Cannabis sativa chemotypes extracts with high content of cannabidiol or cannabigerol. Fitoterapia127, 101–108. 10.1016/j.fitote.2018.02.002
69
SneadO. C. (1986). Opiate-induced seizures: a study of μ and δ specific mechanisms. Exp. Neurol.93 (2), 348–358. 10.1016/0014-4886(86)90195-0
70
StanleyC. P.HindW. H.TufarelliC.O'SullivanS. E. (2015). Cannabidiol causes endothelium-dependent vasorelaxation of human mesenteric arteries via CB1 activation. Cardiovasc Res.107 (4), 568–578. 10.1093/cvr/cvv179
71
TakedaS.UsamiN.YamamotoI.WatanabeK. (2009). Cannabidiol-2',6'-dimethyl ether, a cannabidiol derivative, is a highly potent and selective 15-lipoxygenase inhibitor. Drug Metabolism Dispos.37 (8), 1733–1737. 10.1124/dmd.109.026930
72
TheodoreW. H.HaslerG.GiovacchiniG.KelleyK.Reeves‐TyerP.HerscovitchP.et al (2007). Reduced hippocampal 5HT1A PET receptor binding and depression in temporal lobe epilepsy. Epilepsia48 (8), 1526–1530. 10.1111/j.1528-1167.2007.01089.x
73
TheodoreW. H.WiggsE. A.MartinezA. R.DustinI. H.KhanO. I.AppelS.et al (2012). Serotonin 1A receptors, depression, and memory in temporal lobe epilepsy. Epilepsia53 (1), 129–133. 10.1111/j.1528-1167.2011.03309.x
74
UsamiN.YamamotoI.WatanabeK. (2008). Generation of reactive oxygen species during mouse hepatic microsomal metabolism of cannabidiol and cannabidiol hydroxy-quinone. Life Sci.83 (21-22), 717–724. 10.1016/j.lfs.2008.09.011
75
ValvassoriS. S.BavarescoD. V.ScainiG.VarelaR. B.StreckE. L.ChagasM. H.et al (2013). Acute and chronic administration of cannabidiol increases mitochondrial complex and creatine kinase activity in the rat brain. Rev. Bras. Psiquiatr.35 (4), 380–386. 10.1590/1516-4446-2012-0886
76
VilelaL. R.LimaI. V.KunschE. B.PintoH. P. P.de MirandaA. S.VieiraE. L. M.et al (2017). Anticonvulsant effect of cannabidiol in the pentylenetetrazole model: pharmacological mechanisms, electroencephalographic profile, and brain cytokine levels. Epilepsy and Behav.75, 29–35. 10.1016/j.yebeh.2017.07.014
77
WallaceM. J.WileyJ. L.MartinB. R.DeLorenzoR. J. (2001). Assessment of the role of CB1 receptors in cannabinoid anticonvulsant effects. Eur. J. Pharmacol.428 (1), 51–57. 10.1016/S0014-2999(01)01243-2
78
WatanabeK.MotoyaE.MatsuzawaN.FunahashiT.KimuraT.MatsunagaT.et al (2005). Marijuana extracts possess the effects like the endocrine disrupting chemicals. Toxicology206 (3), 471–478. 10.1016/j.tox.2004.08.005
79
WilsonJ. T.FiefC. A.JacksonK. D.MercerS. L.DeweeseJ. E. (2018). HU-331 and oxidized cannabidiol act as inhibitors of human topoisomerase IIα and β. Chem. Res. Toxicol.31 (2), 137–144. 10.1021/acs.chemrestox.7b00302
80
WuJ.ZhangL.ZhouX.WangJ.ZhengX.HuH.et al (2022). Efficacy and safety of adjunctive antiseizure medications for dravet syndrome: A systematic review and network meta-analysis. Epilepsy Res.12, 980937. 10.3389/fphar.2022.980937
81
XiongW.CuiT.ChengK.YangF.ChenS. R.WillenbringD.et al (2012). Cannabinoids suppress inflammatory and neuropathic pain by targeting α3 glycine receptors. J. Exp. Med.209 (6), 1121–1134. 10.1084/jem.20120242
82
YamaoriS.KushiharaM.YamamotoI.WatanabeK. (2010). Characterization of major phytocannabinoids, cannabidiol and cannabinol, as isoform-selective and potent inhibitors of human CYP1 enzymes. Biochem. Pharmacol.79 (11), 1691–1698. 10.1016/j.bcp.2010.01.028
83
YamaoriS.OkamotoY.YamamotoI.WatanabeK. (2011a). Cannabidiol, a major phytocannabinoid, as a potent atypical inhibitor for CYP2D6. Drug Metabolism Dispos.39 (11), 2049–2056. 10.1124/dmd.111.041384
84
YamaoriS.EbisawaJ.OkushimaY.YamamotoI.WatanabeK. (2011b). Potent inhibition of human cytochrome P450 3A isoforms by cannabidiol: Role of phenolic hydroxyl groups in the resorcinol moiety. Life Sci.88 (15-16), 730–736. 10.1016/j.lfs.2011.02.017
85
YamaoriS.KoedaK.KushiharaM.HadaY.YamamotoI.WatanabeK. (2012). Comparison in the in vitro inhibitory effects of major phytocannabinoids and polycyclic aromatic hydrocarbons contained in marijuana smoke on cytochrome P450 2C9 activity. Drug Metabolism Pharmacokinet.27 (3), 294–300. 10.2133/dmpk.DMPK-11-RG-107
86
YamaoriS.OkushimaY.MasudaK.KushiharaM.KatsuT.NarimatsuS.et al (2013). Structural requirements for potent direct inhibition of human cytochrome P450 1A1 by cannabidiol: Role of pentylresorcinol moiety. Biol. Pharm. Bull.36 (7), 1197–1203. 10.1248/bpb.b13-00183
87
YamaoriS.KinugasaY.JiangR.TakedaS.YamamotoI.WatanabeK. (2015). Cannabidiol induces expression of human cytochrome P450 1A1 that is possibly mediated through aryl hydrocarbon receptor signaling in HepG2 cells. Life Sci.136, 87–93. 10.1016/j.lfs.2015.07.007
Summary
Keywords
cannabinoids, epilepsy, Δ9-THC, CBDV, Δ9-THCA, CBD
Citation
Marciniak S, Wasyluk W and Wojtak A (2025) Molecular targets of cannabinoids and their derivatives in epilepsy – a review with focus on CBD. Acta Biochim. Pol. 72:15251. doi: 10.3389/abp.2025.15251
Received
10 July 2025
Accepted
12 September 2025
Published
30 September 2025
Volume
72 - 2025
Edited by
Grzegorz Wegrzyn, University of Gdansk, Poland
Reviewed by
Joanna Kanabus, Institute of Agricultural and Food Biotechnology – State Research Institute, Poland
Marek Roszko, Prof. Wacław Dąbrowski Institute of Agriculture and Food Biotechnology, Poland
Updates
Copyright
© 2025 Marciniak, Wasyluk and Wojtak.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sebastian Marciniak, sebastian.marciniak@umlub.pl
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.