Abstract
Plant transcriptomes comprise nuclear and organellar (mitochondrial and plastid) transcripts expressed by nuclear and organellar genomes, respectively. They are spatiotemporally shaped during development. The aim of this review was to summarize the most relevant transcriptomic responses in various plant organs and tissues in the developmental context. The dynamicity of organ- or tissue-specific transcriptomic responses was discussed based on the complexity and diversity of the recently characterized plant genomes and transcriptomes. Data were taken from high-throughput studies on numerous species, including model, crop and medicinal plant species. Vascular element transcriptomes as well as the root, leaf, flower and seed transcriptomes were exhaustively characterized. Transcriptomic alterations within various tissue and organ-specific transcriptomes employed various gene classes depending on the species, a given organ/tissue and the developmental stage. The specificity of organ-specific transcriptomes related to the over-representation of certain gene families and a plethora of transcription factors was focused. In addition, transcriptomes of medicinal plant species were characterized. The perspectives of medicinal plant species to synthesize valuable secondary metabolites (including quinones, carotenoids, phytoestrogens, terpenoids, steroids, flavonoids, phenolic derivatives, polysaccharides, glycosides, anthocyanins and macrocyclic peptides) were described based on organ transcriptomic patterns. Future research should be broadened by investigation of transcriptomes from field grown plants. Also, the potential of biomedical plants should be better revealed by genetic engineering and genome editing in further biotechnological applications.
Introduction
Higher plants are characterized by the development of highly specialized tissues and the dominance of sporophyte over gametophyte (Kenrick and Crane, 1997; Forster et al., 2007). During life evolution, higher plants adapted to the environment in multiple aspects, including both developmental adaptations and adequate transcriptomic responses (Morris et al., 2018).
Complex plant transcriptomes, composed of RNA molecules of various origins, are shaped spatiotemporally during plant development. According to Imadi et al. (2015), plant transcriptome depends on an actively transcribed genome fraction, and which is required to respond to environmental cues in various tissues and organs. However, the transcriptomic dynamicity relies not only on the given tissue but also on sampling procedures. Tissue and organ-specific transcriptomes offer valuable information on the molecular processes that affect their functions, such as photosynthesis in leaves, or nutrient uptake in roots. Investigating tissue-specific transcriptomes, multiple specific genes and regulatory mechanisms that display unique attributes can be retrieved, allowing, for instance, agricultural improvements (Booth et al., 2022).
The development of high-throughput RNA-seq platforms with the elaborated meta-analyses and the subsequent decrease in sequencing costs, simplified plant transcriptomic studies. Currently they allow for the analysis of specific features of cells, tissues, or organs in various aspects (Rurek and Smolibowski, 2024). Notable factors that precisely regulate the transcriptional activity of a given biological system can also be characterized (Zhang, 2019; Tyagi et al., 2022). Recently, the relevance of transcriptomic responses from single cell or single nuclei RNA-seq (scRNA-seq and snRNA-seq, respectively) has been greatly expanded by organ-specific studies. They allow us to characterize numerous gene pathways and networks expressed in a cell-specific manner (Kortz et al., 2019; Kao et al., 2021; Picard et al., 2021; Lee et al., 2023). It should be noted that in the meantime various assays dedicated to single-cell sampling have been developed, including fluorescence-activated cell sorting (FACS) and laser-capture microdissection (LCM). LCM allows isolation of cells from tissues containing various cell types (Nelson et al., 2006; Galbraith, 2012; Gautam and Sarkar, 2015).
We have already presented dynamic transcriptomic replies to various stressors, including participation of specific gene families and transcription factors (TFs) (Rurek and Smolibowski, 2024). In the current review, the diversity of transcriptomic responses in various plant organs and tissues in development will be widely summarized. Focus will be made on data from high-throughput approaches, including various RNA-seq platforms, single cell/nuclei transcriptomics and methods employing expression slides. The transcriptomic patterns from model, crop as well as medicinal plant species will be discussed. It should be underlined that genomes of non-model plant species may be particularly complex and the transcriptomic data can bypass genomic gaps (Hirsch and Robin Buell, 2013). The dynamic transcriptomic patterns (including organellar responses) will be summarized based on studies on the biogenesis of vascular tissues, seeds, vegetative (including leaves and roots) as well as flowers. The potential of medicinal plant species for secondary metabolite synthesis will be also discussed based on valuable transcriptomic analyses. In general, data discussed in the current review would complement Rurek and Smolibowski (2024) study by summarizing organ- and tissue-specific transcriptomic responses across plant development.
The variety of higher plant genomes and transcriptomes contributes to the complexity of transcriptomic responsiveness
Before investigating a transcriptomic diversity across various plant organs and tissues, its dependance on the genomic level should be characterized first.
The plant genome contains all DNA molecules in the cell. It is composed of the nuclear and organellar genomes (plastome and mitogenome referred to chloroplast and mitochondrial DNA, respectively). Land plants contain the most conserved nuclear genomes by size, unlike plastids and mitochondria. For example, a carnivorous plant Genlisea aurea possess one of the smallest nuclear genomes (43.3 Mbps), while Paris japonica contains one of the largest ones (up to 150 Gbps) (Supplementary Table S1). Currently, the recurrent whole genome duplications (WGDs), depending on the polyploidy and the transposition frequency are thought to be responsible for the large-scale variation of nuclear genome size in land plants. Although duplication and alterations in chromosome numbers obviously explain the increasing genome size, transposon expansion results even in the greatest variation in the genome size. The relationship between genome size and transposon content is generally linear (Kress et al., 2022). The size of the nuclear genome of plants correlates also with the nutrient deficiency, when species with larger genomes are unable to dominate in ecosystems (Pellicer et al., 2018).
Plastids and mitochondria originated from the endosymbiotic engulfing and maintenance of single-celled organisms in the early eukaryotic cell. In some taxa, serial endosymbiosis explains the complex origins of their plastids (Margulis, 1970; McFadden, 2001). The mitochondrial ancestors were probably γ-proteobacteria, while the plastid ones- cyanobacteria-similar organisms. Most organellar genes underwent an evolutionary transfer to the nucleus. Nowadays, organellar DNA insertions (nuclear-mitochondrial or nuclear-plastid insertions) can be also found in nuclear genomes (Rockwell et al., 2014). Genome-containing organelles thus exhibit a particularly complex pattern of their biogenesis, depending on concerted regulated expression of nuclear and organellar genes (Best et al., 2020a; Dobrogojski et al., 2020).
Land plant plastome is generally conserved in length and organization (120–180 kbps in size) (Provan et al., 2001; Asaf et al., 2017). The size of the Arabidopsis plastome (compared with nuclear genome of 119.1 Mbps) is 154,478 bps, when P. japonica, the owner of the largest land plant nuclear genome (150 Gbps), has a plastome of 155,957 bps only. This gives a difference of Arabidopsis and P. japonica plastomes of about 1,500 bps (Supplementary Table S1). Land plant plastomes encode a limited number of proteins involved in photosynthesis, transcription, translation and plastid signaling as well as diverse rRNA, tRNA and ncRNA molecules (Daniell et al., 2016; Rurek, 2016). Multiple nuclear factors regulate the expression of plastid genes (Barkan and Goldschmidt-Clermont, 2000).
Compared to plastome, land plant mitogenomes range from 208 kbps for white mustard (Brassica hirta), 366 kbps for Arabidopsis, up to 11.3 Mbps in size in Silene species (Supplementary Table S1), while the gene number (approx. 60 genes) remain relatively stable (Logan, 2006; Sloan D. B. et al., 2012a; Sloan D. et al., 2012b). However, the size of mitogenome copies can vary in the same species, for example, the potato (Solanum tuberosum) mitogenome contains few molecules ranging from 49,171 bps to 297,014 bps in size (Cho et al., 2017) (Supplementary Table S1). Higher plant mitogenomes are therefore particularly large. They display a complex structure (a mixture of circular, linear and concatemeric forms), which probably optimized seed germination in novel ecosystems in the Palaeophytic era. Interestingly, plant mitogenome structure and expression affect mitochondrial biogenesis under organogenesis (Best et al., 2020a).
Plant transcriptome is set of various RNA molecules spatiotemporally regulated. The transcriptome capacity extends the genome size. For instance, the capacity of maize (Zea mays) total transcriptome was estimated to 97 Mbps, which consists only 4% of the nuclear genome size (Schneider and Dekker, 2012). To date, Best et al. (2020b) have published maps of Arabidopsis and cauliflower (Brassica oleracea var. botrytis) mitochondrial transcriptomes. Arabidopsis and cauliflower mitogenomes encode 28 and 33 protein-coding genes, 3 and 3 rRNAs, 22 and 18 tRNAs, and cover approximately 85 and 35 ORFs of >100 amino acid residues, respectively. In addition to rRNAs, numerous tRNAs and ncRNA molecules (Rurek, 2016), plant mitogenomes encode OXPHOS proteins, ATP synthase subunits, mitoribosomal proteins, few proteins for cyt. c biogenesis and the twin-arginine translocation protein (Tat). At least 42 and 33 transcription units were found by RNA-seq of Arabidopsis and cauliflower mitogenomes, respectively. The expression of several Arabidopsis mitogenes leads to the formation of mono or bicistronic transcripts. Various open reading frames within the same polycistronic transcript are diversely expressed within post-transcriptional RNA processing (Cahoon et al., 2017; Best et al., 2020a).
To sum up, the diversity of plant genomes and transcriptomes belongs to important factors that contribute to the plasticity of transcriptomic dynamic response under development.
Transcriptomes of selected plant organs and tissues
The specialization of individual plant organs to perform specific functions is a consequence of differences in the gene expression patterns between those organs (Huang et al., 2016). Details on affected genes from developmental and organo-specific studies are given in Supplementary Table S2. Figures 1, 2 summarize the most relevant gene functional classes and the most notable TFs in diverse plant organs. Further details on tissue- and developmentally specific expression patterns within selected plant organs (roots, leaves, seeds) were shown in Figures 3, 4.
FIGURE 1
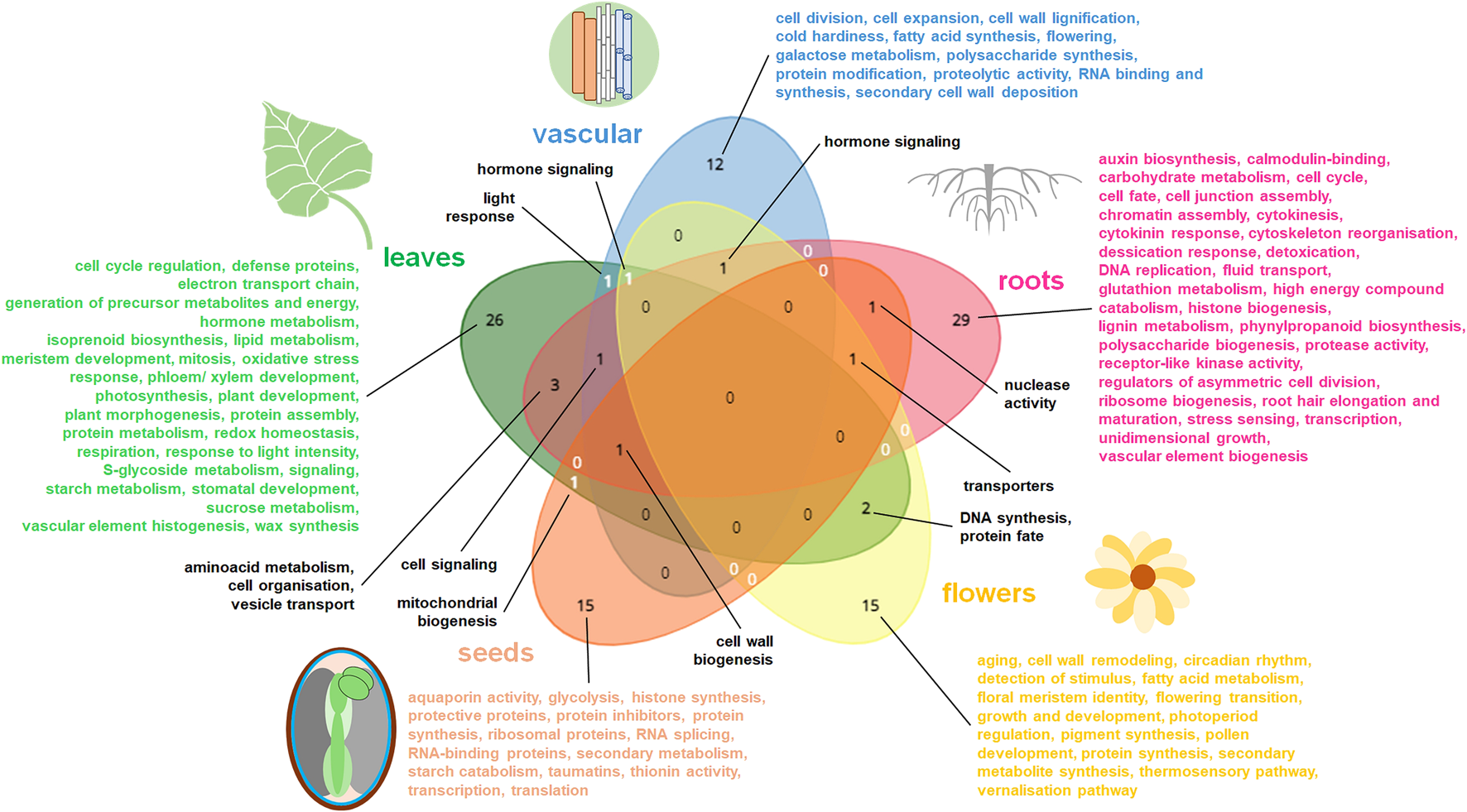
The summary of gene functions (GO: terms) for the most relevant, upregulated gene families in diverse plant organs. The most common and specific organo-specific transcriptomic replies from cited literature are shown. The data specific for distinct organs were presented in diverse colors on Venn diagram. Venn diagrams for five datasets (representing roots, vascular elements, leaves, flowers and seeds) were drawn using tool from https://www.biotools.fr/misc/venny.
FIGURE 2
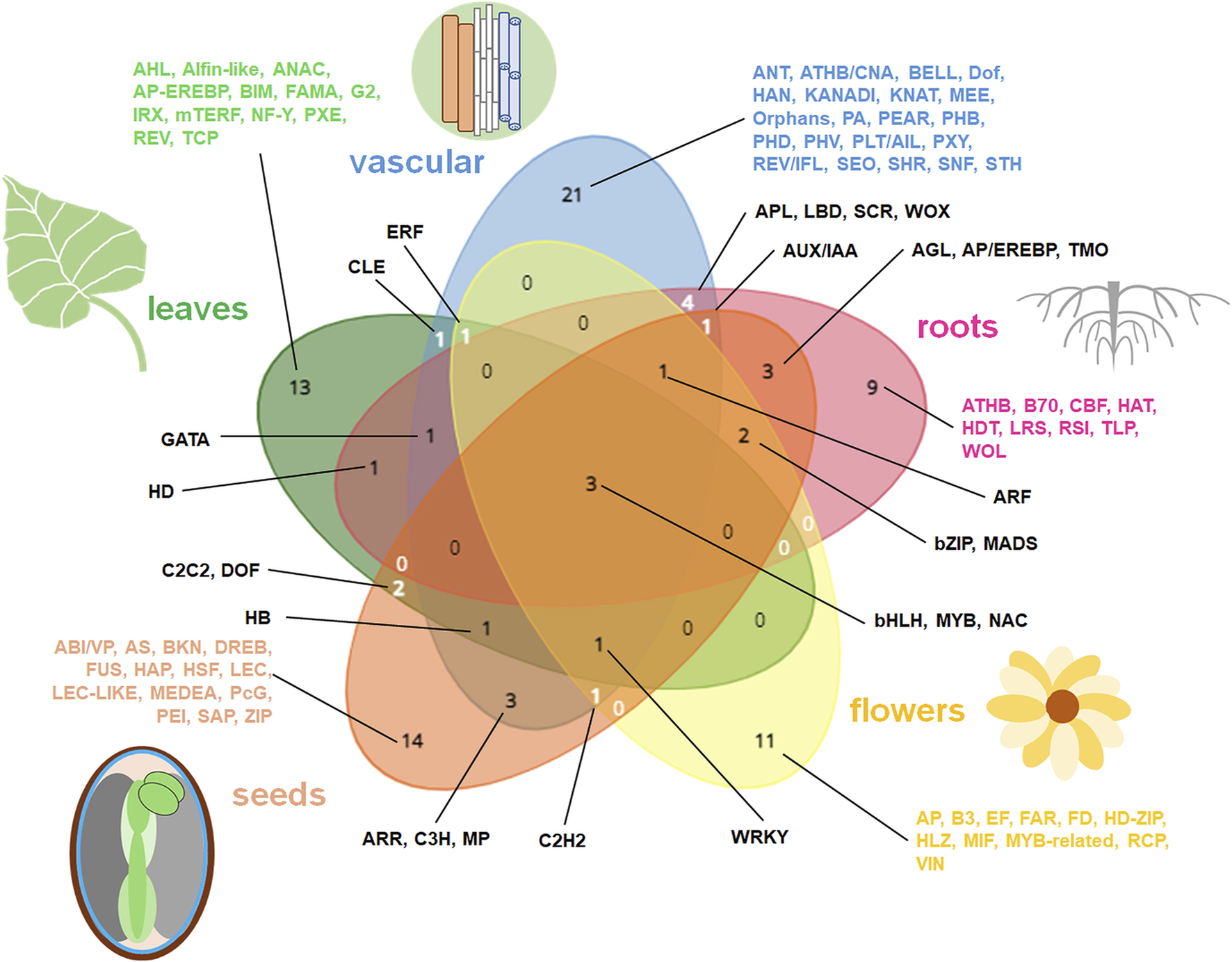
The summary of the most important TFs in diverse plant organs. The most common and specific organo-specific TFs from cited literature are shown. The data specific for distinct organs were presented in diverse colors on Venn diagram. Venn diagrams for five datasets (representing roots, vascular elements, leaves, flowers and seeds) were drawn using tool from https://www.biotools.fr/misc/venny.
FIGURE 3
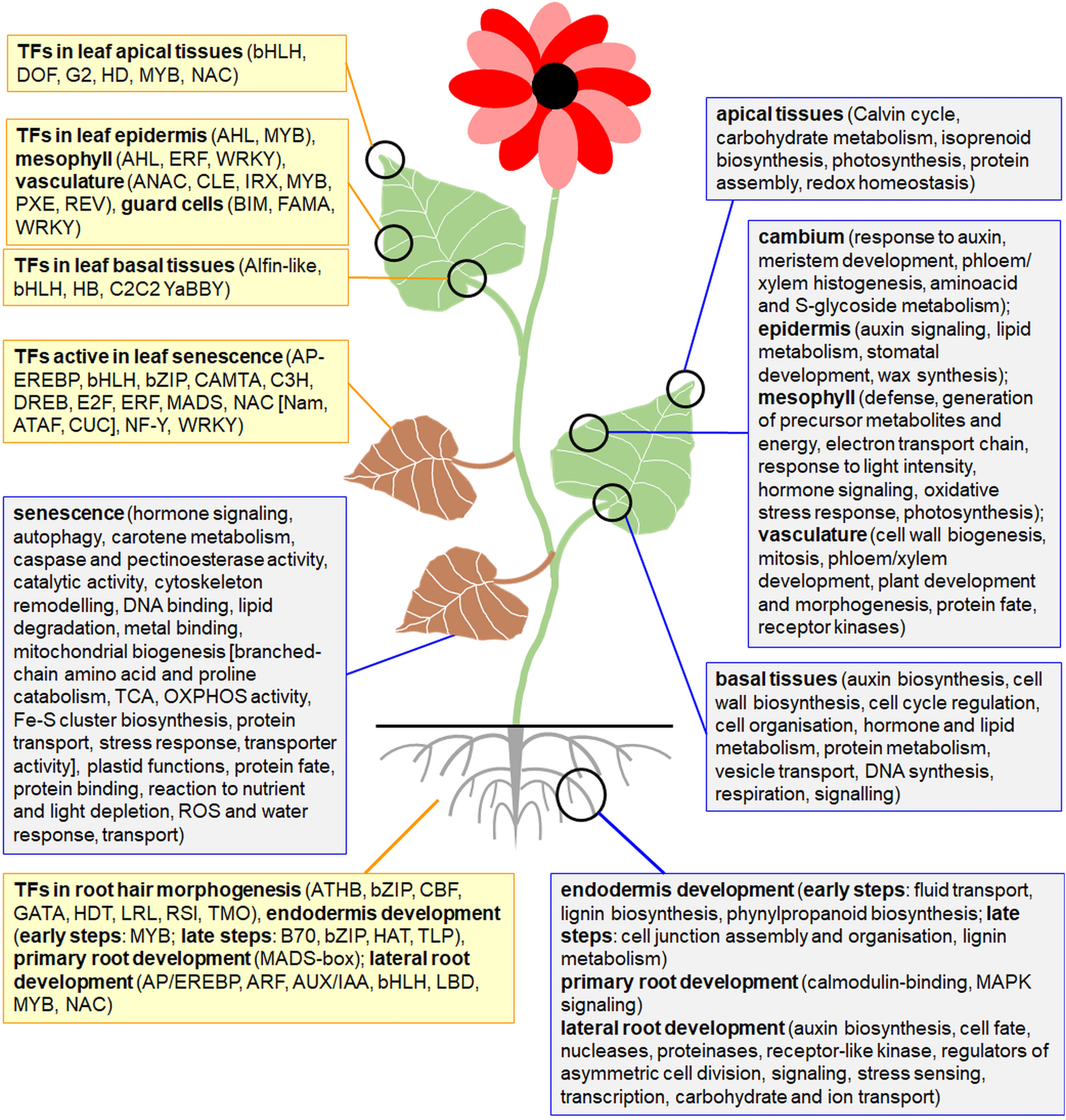
Tissue- and developmentally specific expression patterns in roots and leaves. The functional GO: terms for the most relevant, upregulated gene families and the most important TFs in various tissues of roots and leaves and across the diverse developmental stages were shown in separate boxes.
FIGURE 4
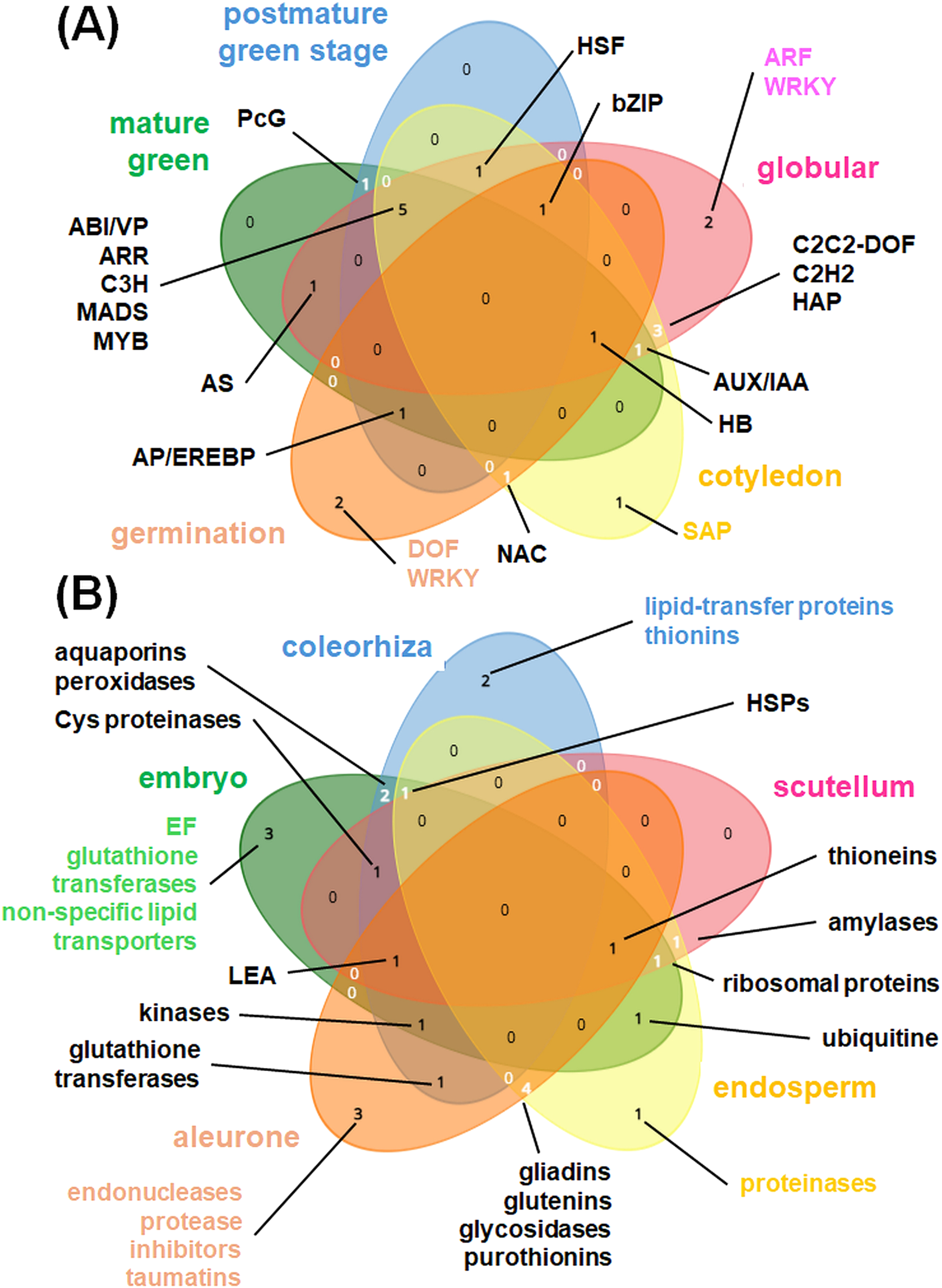
Tissue- and developmentally specific expression patterns in seeds. Venn diagrams showing (A) most abundant TFs active during various seed developmental stages (cotyledon, germination, globular, mature green, postmature green); (B) mostly enriched protein families coded by DEGs in coleorhiza, embryo, scutellum, endosperm as well as aleurone layer. Venn diagrams for five datasets at each time were drawn using tool from https://www.biotools.fr/misc/venny.
Vascular element transcriptomes
Vascular element transcriptomes belong to extensively studied tissue-specific transcriptomes. During the primary growth of stems and roots, procambium derived from apical meristems differentiates into xylem and phloem. Precursor xylem cells form xylem crumb cells or fibers. In the vascular bundle, xylem, procambium and phloem cells show dorsoventral polarization. Xylem is located on the dorsal (adaxial) side, phloem occurs on the ventral (axial) side, and procambium- between the phloem and xylem (Wang and Dixon, 2012; Růžička et al., 2015).
Cambium transcriptome is actively remodeled under dormancy, reactivation and activity stages. Multiple genes govern cambial cell division and expansion, and cell wall component biogenesis. Various MYB and NAC TFs regulate gene expression especially during lignin formation (Du et al., 2024). WOX and PXY genes for respective TFs belong also to important regulators of cambium biogenesis (Chen et al., 2021; Zhou et al., 2024; Sheng et al., 2025). Studying poplar P. tremula transcriptome Chen et al. (2021) characterized cambium marker genes. They were: MP gene for auxin-responsive TF, PIN1 for auxin efflux transporter, cytokinin-responsive transcription factor gene (ANT) and positive regulatory peptide of cambial activity (CLE47). Moreover, HD-ZIP III TFs (including PtrHB4, PtrHB7,and PtrHB8) and PagGRF15, a growth-regulating factor are also important for poplar cambium vascularization (Chen et al., 2021; Zhou et al., 2024). Overexpression of PagGRF15 results in the decreased plant height and internode number and by reduction of phloem and expansion of xylem. Those effects indicate that GRF15 belongs to positive regulators of xylem differentiation, which represses WOX and PXY gene activity and stimulates GID1.3 expression (Zhou et al., 2024). Multiple TF genes associated with phloem and cambium development in Citrus macrophylla trees were also active under Citrus tristeza virus infection (Khalilzadeh et al., 2022).
In stems of poplar hybrid lines (Populus alba x P. glandulosa), rectangle-shaped procambium-like cells develop from procambium meristem and produce phloem cells, whereas fusiform-shaped cells (inside the cambium zone) are produced by fusiform metacambium meristem and differentiate into xylem. Meristem identity markers enriched in those cells included mainly genes for TFs, e.g., ANT, CLE, WOX, SCR and SHR. Phloem and xylem identity genes covered also genes for important TFs and receptor kinases, like CLE, SMXL, BAM, Dof, APL and SEO for phloem, and WOX, PXY, ATHB and VND genes in case of xylem. Genes for the secondary cell wall biosynthesis were also highly enriched (Du et al., 2023). In poplar stems, Li et al. (2023) used WOX4 and PXY genes as cambium markers and XCP1 and XCP2 genes as vessel element markers. ACL5 was xylem precursor marker and SEOR1 and CLE41 genes were phloem markers. In addition, genes for xylem (ACL5, MP, LBD4, NPY2, BSK6, ARR4, LAC6) and phloem precursor branch development (CLE41, BAM3, KNAT1, PA, MEE32, GH9B13, PER73) encode important developmental regulatory proteins. Furthermore, transcriptomes of Eucalyptus urograndis vascular cambium at four developmental stages were investigated by Liu et al. (2023). Differentially expressed genes (DEGs) for various proteins, including expansins, kinesins, cyclins, lignification enzymes and various TFs were highly upregulated.
Seasonal variations in transcriptomes of meristem cambium and derivative cells of Japanese cedar (Cryptomeria japonica) under development were also studied (Mishima et al., 2014). Multiple DEGs involved in xylem formation, e.g., genes associated with cell wall biogenesis (including PAL, 4CL, C4H, HCT, CCOAOMT and CCR for lignification), carbohydrate metabolism and cold hardiness, were upregulated in spring (Supplementary Table S2). The activation of cell division and the induction of cell cycle genes are thus correlated in early stages of xylogenesis. Various TFs active in C. japonica cambium cells covered C3H, NAC, PHD, AP2-EREB, HB, bHLH, SNF2, WRKY, C2H2, MYB and Orphans proteins (Mishima et al., 2014). WOX and PXY gene co-expression associated with the June peak of vascular system development in Camellia chekiangoleosa stems (Sheng et al., 2025).
Phloem elements are frequently enriched in highly upregulated DEGs for RNA polymerases subunits, enzymes of galactose metabolism, polysaccharide and fibrous element synthesis. In contrast, upregulated genes involved in fatty acid synthesis are expressed mostly in xylem (Weber, 2002; Chen et al., 2014). In phloem-associated cells of potato (Solanum tuberosum) petioles, genes for PPR proteins, kinase receptors, UDP-glucuronic acid decarboxylase were uniquely expressed (Supplementary Table S2) (Lin et al., 2015). Multiple TFs, including AUX/IAA, ERF, bZIP, BELL, HD, NAC and WRKY regulated gene expression in phloem-associated cells of potato leaf petioles and plant stems. Mutations in REV/IFL1, PHB and PHV genes have been shown to affect the organization of vascular tissues that contain phloem, which surrounds xylem. However, when KANADI genes (e.g., KAN1, KAN2, KAN3) are mutated, xylem surrounds phloem (Eshed et al., 2001; Dinneny and Yanofsky, 2004; Eshed et al., 2004). HD-ZIP III (e.g., ATHB8, ATHB15/CNA, PHV, PHB, REV/IFL1 TFs) and KANADI TFs act antagonistically on the xylem and phloem patterning, respectively. Other genes, coding for APL and MYB family TFs, are also necessary for the phloem specification (Bonke et al., 2003). Using scRNA-seq, Roszak et al. (2021) studied participation of PEAR proteins which mediate early asymmetric divisions during phloem cell biogenesis and in laterally adjacent procambium cells. They promote further activity of APL gene for the phloem regulator protein as well as protophloem, metaphloem and procambium differentiation. Gradient of PLT/AIL and HAN TFs affects differentiation of protophloem sieve elements by repressing APL transcription.
Root and leaf transcriptomes
Generally responsive DEGs and marker genes
Roots store high-energy compounds, e.g., complex carbohydrates, while leaves are photosynthetically active (Mason et al., 2020; Schmidt-Rohr, 2021). Those functions count for main differences in root and leaf transcriptomes (Figures 1, 2). For instance, in sugarcane (Saccharum spp. hybrids) and Atractylodes lancea transcriptomes, more DEGs for photosynthetic proteins (including Rubisco and chlorophyll a-b binding proteins, indicating for plastid relevance) and for signal transduction were expressed in leaves. Leaf transcriptomes are enriched in genes coding photosynthetic proteins, cell redox homeostasis and hormone signaling pathways (Figure 1) (Xanthopoulou et al., 2021). Among proteins upregulated in leaves, most of mTERF TFs are organelle targeted proteins. In contrast, root transcriptomes of both species were enriched with transcripts for polysaccharide biogenesis, amino acid metabolism, catabolism of high energy compounds and hormonal signaling (Supplementary Table S2) (Huang et al., 2016; Mason et al., 2022). Interestingly, leaf and root transcriptomes from the same cell layers possess similar features, with some exceptions. A set of 40 marker genes for 13 leaf cell types and at least 15 markers for 5 root cell types included also some genes for plastid (Rubisco small subunits, chlorophyll a/b binding protein) and mitochondrial (formate dehydrogenase) proteins. RBCS4 and Lhcb1.1 genes were expressed in mesophyll cells of diverse origin. WOX4 and CDKB2 genes were active in vascular initial cells and leaf primordia cells (Wang et al., 2021).
In recent years, detailed analysis of root and leaf transcriptomes often employed scRNA-seq (Efroni et al., 2016; Denyer et al., 2019; Shulse et al., 2019; Liu H. et al., 2021; Wang et al., 2021; Tenorio Berrío et al., 2022; Guo et al., 2025).
Root transcriptomes
High-resolution expression atlas of Arabidopsis roots allowed for the fine resolution of marker genes by defining unique clusters for all major cell types. Expression of some nuclear genes was specific to those clusters. Markers specific to columella covered ATL6 for RING-type E3 ubiquitin related enzyme, PLT2/PLT3 for patterning root cells, COBL2 for glycosylphosphatidylinositol-anchored protein and NCED2 for 9-cis-epoxycarotenoid dioxygenase (all in columella). SHR gene for SCARECROW-like TF was expressed in endodermis, and APL gene for FE protein - in phloem and pericycle. In addition, COBL9 gene was active in trichoblasts and GL2 (coding HD protein) in atrichoblasts. Expression profiles of 239 TFs were distinctive, including various TFs regulating root hair biogenesis (Denyer et al., 2019). In general, almost 800 genes encoding proteins for cell junction biogenesis, polysaccharide synthesis, stress response, transport and protective functions were active for the endodermis biogenesis in Arabidopsis; diverse TFs regulated early and late stages of this process (Figure 3) (Shulse et al., 2019).
Known roots tissue markers are GL2 and WER for MYB-like TF in non-hair cells, SCR in endodermis, AGL42 and WOX5 (for HB protein) in quiescent center cells, APL, MYB46, SUC2 (encoding sucrose transporter) and WOL for two-component signal transducer in stele cells and COBL9 for hair cells. Shulse et al. (2019) proposed a set of 17 additional nuclear genetic markers for diverse cell subpopulations. Markers for the lateral root organogenesis included PASPA3, BFN1, SMB and RCP1 genes for proteinase family protein, bifunctional nuclease, NAC family TF and the maltose transporter, respectively (Figure 3) (Kortz et al., 2019).
Genes expressed in primary and lateral roots of Summer squash (Cucurbita pepo) highly overlapped. However, ACS gene for stress-sensing and signaling and genes for Cu and nitrate transporters belong to the lateral root-specific genes and genes for calmodulin-binding proteins and for MAPK signaling- for primary root specific genes (Xanthopoulou et al., 2021). The primary root development is also controlled by multiple factors, including MADS-box proteins that repress root growth and control meristem features, cell division rate and the length of elongated cells (Figure 3) (Castañón-Suárez et al., 2024).
The pattern of root regeneration after root tip excision followed embryonic patterning and was not driven by the initiation program of lateral root biogenesis. In fact, the transcriptome of regenerating cells prior to stem cell activation resembled that of embryonic root progenitor cells. The activity of endodermal SCR promoter appeared important for regeneration of new endodermis and lateral root caps (Efroni et al., 2016).
Some genes for plant root development were edited with a very promising effects. For instance, ARG gene for arginase which targets NO synthase was knocked out by Wang et al. (2017). Those attempts resulted in the increase of lateral root number and total root surface which improved root development by more efficient water and nutrient uptake from the soil. RNA virus-mediated delivery of sgRNA along with the cytokinin biosynthesis gene, isopentenyl transferase to potato (Solanum tuberosum) axillary meristems was carried out by Liu et al. (2024). In result, abundant gene-edited shoots displayed normal phenotype. This approach overcame challenges in virus-induced gene editing strategy to dicot crop species.
Transcriptomes of leaf tissues and senescent leaves
In Arabidopsis leaf transcriptome, a number of tissue-specific genes decreased from the leaf vasculature to the epidermis and mesophyll, indicating their various sensitivity to transcriptional responsiveness. Multiple nuclear genes specific to biogenesis of epidermis (KCS, GPAT, CER, LACS, MYB16, MYB30 genes for various enzymes and genes for MYB TFs), mesophyll (genes for ERF and WRKY TFs) and vasculature (IRX, CLE, PXE and REV genes as well as genes for ANAC and MYB TFs) were characterized (Berkowitz et al., 2021). Various classes of DEGs were upregulated in distinct zones of maize (Zea mays) leaves, for instance respiratory, cell wall biogenesis, and auxin and brassinosteroid signaling genes in basal tissues, while photosynthetic and sucrose transporter genes were expressed mainly in leaf tips (Figure 3).
Furthermore, genetic markers for transcriptomes of distinct cells from Arabidopsis leaves were proposed by Tenorio Berrío et al. (2022). The expression pattern of PHYTOCYSTATIN1 (CYS1) and genes for plastid proteins delineated all vasculature- and mesophyll-derived (mostly photosynthetic) cells, respectively. In addition, nuclear genes: EXTENSIN-LIKE PROTEIN (ELP) and GERMIN3 (GER3) were enriched in mesophyll, PROLINE-RICH PROTEIN4 for a cell-wall protein–in epidermis and WINDHOSE1 - in vasculature. Interestingly, flavonols and anthocyanins were synthesized mostly at the adaxial leaf side. Genes for glucosinolate metabolism enzymes differentiated diverse vasculature-derived cells. Transcriptomes of bundle sheath (genes for photosynthetic, respiratory and transport proteins) and mesophyll cells (genes for PSII, translation, secondary metabolism, and vesicle transport proteins) were also compared (Li et al., 2010; Tenorio Berrío et al., 2022).
As concerns mesophyll biogenesis, palisade cells are able to differentiate into spongy cells, while epidermal cells originate earlier than the primordial ones. Ectopically expressed AHL23, a nuclear TF belonging to proteins important for mesophyll and epidermis development, alleviated peanut (Arachis hypogaea) leaf growth. Five highly expressed marker genes for each of eight cell clusters coded, among others, ribosomal subunits (RPS, RPL), plastid (NDHJ, psbB) and mitochondrial (COX3) proteins (Liu H. et al., 2021). Such results highlight the relevance of expression patterns of genes for organellar proteins in leaf development. For instance, mutations in PPR446 gene for chloroplast protein with 11 PPR domains or silencing of PPR466 expression affected leaf development (the appearance of albino phenotype; Zhao et al., 2021). Some conserved TFs were particularly highly accumulated in guard cells (Figure 3) (Lee et al., 2023).
The transcriptome of senescent Arabidopsis leaves became distinct from that of mature leaves (Breeze et al., 2011). During leaf senescence, genes for jasmonic acid (JA) and ethylene signaling, stress response, caspase and pectinoesterase activity, lipid degradation, cytoskeleton, metal binding and transport were upregulated while DEGs related to chlorophyll biogenesis, photosynthesis, cytokinin signaling, ribosome biogenesis, amino acid metabolism and cell cycle were downregulated. Some genes coding for plastid proteins (PSBQ and PSBP subunits of PSII, and CAROTENOID cleavage dioxygenase4) are also involved in carotenoid degradation in senescent leaves (Gonzalez-Jorge et al., 2013). Leaf senescence is also regulated by multiple TFs (Figure 3) (Breeze et al., 2011). Chrobok et al. (2016) performed the meta-analysis of Breeze et al. (2011) data and identified more than 1,000 genes for mitochondrial proteins active in leaves especially at the early senescence stages. The mitochondrial cluster was particularly enriched in genes for OXPHOS proteins, subunits of main mitochondrial importin, some transporters, auxin signaling, reaction to nutrient and light depletion, plastid functions, stress response, protein fate. As senescence progressed, genes for plastid proteins displayed similar patterns to the mitochondrial ones. The late stages of leaf senescence were enriched in genes active in branched amino acid and proline metabolism, which represented ancestral mitochondrial functions (Figure 3). Interestingly, chloroplasts size, but not quantity considerably declined, and mitochondria quantity decrease under leaf senescence. However, mitochondrial integrity as well as ATP production was substantially preserved during this process. Overall, Chrobok et al. (2016) highlights the relevance of mitochondrial metabolism to support the aminoacid and fatty acid catabolism in the senescence of Arabidopsis leaves.
Guo et al. (2025) generated Arabidopsis transcriptomic atlas from spatiotemporal snRNA-seq and proposed molecular markers to quantify cells. Thousands of senescence-associated genes (SAGs; especially at the late stages) were analyzed. Investigated markers covered early (SAG13) and late (SAG12) senescence genes as well as genes associated with Rubisco activity (RBCS1A) and chlorophyll catabolism (NYE2).
Transcriptomes of generative organs
Flower morphology is species-specific. Flowering depends on pollination strategy, photoperiod, vernalization, phytohormone activity, thermosensing and aging-associated processes (Melzer et al., 2008; Kim et al., 2009; Mutasa-Gottgens and Hedden, 2009; Srikanth and Schmid, 2011; Liu et al., 2016). Despite the differences between flower organogenesis, the transition from the vegetative to the generative phase is tightly controlled (Huang et al., 2013).
In Annona squamosa transcriptome, upregulated genes for vernalization and photoperiod induction covered phytochrome (PHY) and cryptochrome (CRY), as well as early flowering (EF1, EF3) genes and FIE and VIN3 genes (Liu et al., 2016). Regarding TFs important for flowering transition, the activity of MADS-box genes (e.g., AGL, FUL, SOC) and MADS proteins (including SOC1 and FUL) is notable (Figures 1, 2). MADS-box proteins, including VRN1, FUL2 and FUL3 play a role in the differentiation of the upper spikelet ridge (formed by the inflorescence meristem) and HD-ZIP III TF (WPS1-like protein) controls spikelet pairing in wheat (Triticum aestivum) (Li et al., 2019; Zhang et al., 2022). Other genes affecting flower morphology and size (by promoting petal expansion) belong to AUX/IAA family with expression repressed by ethylene (Jia et al., 2022; Wang et al., 2024b).
In early flowering stages, hormone signaling-related and stress-responsive genes are expressed. Genes related to floral organ development are upregulated generally in flower determination and maturation stages (Figure 1) (Jiao et al., 2019). Disturbances in the expression pattern of those genes contributed to delayed flower formation, despite the unaffected photoperiod (Melzer et al., 2008). In common walnut (Juglans regia) under flower bud development various genes for DNA replication and flavonoid synthesis were stage-specifically expressed. The circadian rhythm plant pathway dominated at the initial stages of apical meristem transformation into pistil primordium and covered genes homologous to Arabidopsis circadian clock genes LHY, PRR, FKF and GI (Ma et al., 2021). At the petal formation stage, genes coding for enzymes for carotenoids or anthocyanins, were significantly upregulated in diverse Achimenes species (Roberts and Roalson, 2017). Some flower buds go through dormancy phase; Prudencio et al. (2021) studied early and late flowering genes in endodormant and ecodormant almond (Prunus dulcis) flower buds related with proteins for carbohydrate metabolism and cell wall remodeling (endoglucosidases, glucanases, galactosyltransferases), transmembrane transport (aquaporins, sugar transporters), lipoxygenases, hormonal signaling (ABA biosynthesis), pollen development as well as MADS-box and HLZ proteins (Supplementary Table S2).
Flower petal colorization is an important step, where expression of multiple genes for various pigment (e.g., flavonoid) synthesis is turned on. It depends on the activity of regulatory proteins, including mini zinc-finger protein (MIF1), as was shown for Gentiana triflora flowers. In faint-blue plants, MIF1 gene is differentially expressed and determines gentian color intensity (Tasaki et al., 2022). Strikingly, enhanced expression of some flavonoid biogenesis genes resulted in decreased anthocyanin pigmentation in Chrysanthemum flowers under thermal stress (Shi et al., 2022). In Lysimachia arvensis with blue- and orange-petaled flowers, two F3′5′H and DFR genes for key enzymes for petal colorization (flavonoid 3′,5′-hydroxylase and dihydroflavonol 4-reductase, respectively) were differentially expressed (Supplementary Table S2) (Sánchez-Cabrera et al., 2021).
Aberrations in flower development (including anthers) are present in cytoplasmic male sterile (CMS) plants. For instance, mitogenomes of maintainer and Ogura CMS line of cabbage contains 4 specific orfs, including orf138a and orf154a, whose expression led to the increased ATP production by affecting abundance of OXPHOS transcripts. In result, more energy for the abnormal proliferation of tapetal cells is produced (Zhong et al., 2021). Male sterility can be also generated by genome editing, as was shown for rice hexokinase hxk5 mutant and for Arabidopsis knockout lines in sarib and saric genes (Lee et al., 2019; Liang et al., 2020). Knockout of tfl1 gene in Brassica napus affected phase change and flowering timing (Sriboon et al., 2020).
In C. pepo female flowers are grown after initial male phase of development. Female flowers belong to organs with the highest number of specific genes; however, male flowers contain most differentially expressed genes. Female flower-specific genes coded proteins for cell wall biogenesis, including pectin catabolism and cell wall modification, pollen allergen Ole e 6-like (for pollen-stigma recognition) and Leu-rich extensins (pollen tube cell wall proteins), VIN3-like protein 2 - a novel TF involved in vernalization and for flowering promotion. In female flowers flowering promoting factor 1, some EARLY FLOWERING and Ultrapetala genes were also enriched. Male flowers specifically expressed EPIDERMAL PATTERNING FACTOR-like protein 6, a positive regulator of inflorescence development, Unusual floral organs gene for floral meristem determination and some TFs. They included male flower-specific ethylene-responsive TF 2-like from ERF family. Flowering-related genes also covered AP-2, EARLY FLOWERING, FCA, FLOWERING LOCUS T (for the florigen protein), PISTILLATA and Flowering time control FPA-like (Xanthopoulou et al., 2021). Among TFs regulating female flowering were NAC, ERF, bHLH, bZIP, MYB and C2H2 proteins. Interestingly, etr1b mutants (in the gene for one of ethylene receptors) displayed increased number of male flower nodes, indicating for the important role of etr genes in flowering (Segura et al., 2023). Participation of FLOWERING LOCUS T and FD TF (basic-leucine zipper TF) in flowering was investigated also in Lemna aequinoctialis (Yoshida et al., 2021).
Comparing with data above, distinct genes participated in the development of broccoli (Brassica oleracea var. italica) floral buds due to their morphological and anatomical specificity. The regulated DEGs between hybrid and parental cultivars coded proteins for the stress response, regulation of floral development and for the cellular signaling. In hybrid lines, DEGs for development and organ growth were affected. Overall, the upregulated DEGs coding proteins for growth and development, fatty acid and carbohydrate metabolism, protein synthesis and modifications prevailed in hybrid lines (Supplementary Table S2) (Li et al., 2018).
Seed transcriptomes
Seed endosperm and embryo develop after double fertilization. The seed cover, on the contrary, is formed from embryo stem cells. Having reproductive function, seeds protect the embryo from external conditions (Boesewinkel and Bouman, 1984; Martín-Gómez et al., 2020).
Transcriptomic analyzes designated multiple genes active under seed biogenesis and germination. Visium Gene Expression slides (10x Genomics) allowed for the spatial profiling of various cell transcriptomes during germination of barley (Hordeum vulgare) seeds (Peirats-Llobet et al., 2023). Obtained results confirmed 83%–90% transcripts known from previous studies (Supplementary Table S2) (Betts et al., 2017; Shulse et al., 2019; Zhu et al., 2022). In Arabidopsis seeds, aquaporin genes were expressed in mesocotyl, scutellum and coleorhiza, and lipid-transfer protein genes - in coleorhiza. Genes for cell wall modification proteins were active in radicle and scutellum. Genes for various endonucleases, thionins, taumatins and protein inhibitors were expressed in endosperm and aleurone layer. Specific proteases were active in endosperm (Peirats-Llobet et al., 2023). Some mitochondrial PPR proteins involved in RNA processing (e.g., FLOURY ENDOSPERM22 in rice) are also necessary for the proper endosperm development (Yang et al., 2023). Recently, spatially enhanced resolution omics sequencing (Stereo-seq) and scRNA-seq used to characterize transcriptomes of germinating rice (Oryza sativa) embryos, led to the discovery of novel scutellum type with own genetic markers. The activity of MTF2 gene for the mannitol transporter as well as TG3-1, SCL1-2 and CYS genes is notable in diverse tissues of rice grains (Yao et al., 2024). Using scRNA-seq, Liew et al. (2024) showed that Arabidopsis seed embryonic cells display similar transcriptomes under germination initiation when the transcriptional remodeling occurs and cell-specific TFs affect the germination rate. In addition to scRNA-seq, snRNA-seq was used to study spatial regulation of gene expression in isolated Arabidopsis embryo nuclei, parental-embryo relationships and tissue-specific gene imprinting (Kao et al., 2021; Picard et al., 2021).
The activity of multiple genes is dynamically altered under seed development (Figures 1, 4). Under seed germination, in various embryonic cell types, DEGs for nutrient metabolism, biosynthesis, and hormone biosynthesis are particularly notable (Yao et al., 2024). Genes for ribosomal proteins, RNA-binding proteins (including RNA splicing machinery) and elongation factors are active in embryo, particularly at later stages, when transcription and translation start rapidly. Interestingly, germination without transcriptional activity belongs to specific functions of barley embryos (Betts et al., 2017). In rice, early development of seeds is characterized by the presence of SCL1-2 cell type, in later steps environmental response-like pathways are activated. Specific marker genes, like MFT2 as well as SAG12-1, CER1 and PRP14 were activated during seed germination (Yao et al., 2024). Genes for secondary metabolite synthesis were active in the germinated seeds of Polygonatum cyrtonema (Liu R. et al., 2021), but not in barley grains, where it were upregulated at later stages of grain maturation (Peirats-Llobet et al., 2023). In general, highly expressed genes are enriched in Arabidopsis globular embryos and in the mature cotyledons. On the contrary, lowly expressed genes are active in the mature and post-mature stages of the green embryo, associated with dormancy transition (Figure 4) (Le et al., 2010).
During early seed imbibition, transporter genes (including mitochondrial proteins) are expressed in coleorhiza, embryo and scutellum of barley seeds. Genes for glycolytic enzymes, histone and protective protein (LEA) synthesis are also early induced in embryo; OXPHOS genes, however, are not highly expressed until the 24h-long imbibition when mitochondrial biogenesis rapidly increases. In later stages, activity of genes for aminoacid coenzyme, lipid and carbohydrate metabolism, mitochondrial transcription, nucleic acid processing and PPR proteins is notable. In some cases, genes for histone synthesis and RNA-binding proteins are active in dry seeds (Figure 4) (Narsai et al., 2017; Peirats-Llobet et al., 2023). The plastome expression is also necessary for seed development. For instance, some mTERFs (namely, mTERF2) can be targeted to chloroplasts; complete mTERF2 loss resulted in embryo lethality, but miRNA-directed knockdown of MTERF2 gene affected chlorophyll content and plant development (Lee et al., 2021).
Multiple TFs (including bZIP, bHLH and DREB proteins) display a complex spatiotemporal expression pattern under seed development. Among 48 diverse TFs, LEC, FUS, MEDEA and PEI were notable as Arabidopsis seed development regulators. Mutations that occurred in genes for these TFs resulted in embryo defects (Le et al., 2010). Seed germination is controlled by various NAC, bZIP, DOF, HB, AP/EREBP, WRKY proteins, however genes for bZIP and AP/EREBP TFs were downregulated after seed stratification (Narsai et al., 2017; Lee et al., 2023). For early seed development, MADS TFs are also essential; the single knockout mutant (generated by genome editing) of MADS78 or MADS79 in rice (Oryza sativa) showed aberrations in endosperm biogenesis and double mutants displayed delays in seed development (Paul et al., 2020). In the cotyledon stage, SAP TFs are distinctive. The globular stage is enriched with tissue-specific ARF and WRKY TFs. Mature and postmature greened stages have common multiple, including ARR, MYB and MADS proteins. bHLH TFs are abundant in radicle, BKNs are necessary for shoot apical meristem development and bZIPs are accumulated in scutellum and endosperm. Last, but not least, for the embryo root and hypophysis development, MP and TMO TFs respectively, are necessary (Figures 2, 4) (Peirats-Llobet et al., 2023).
Transcriptomic analyses of medicinal plant species for secondary metabolite synthesis
Transcriptomic analyses of medicinal plant species allow valuable comparisons of newly discovered genes with the existing data helping to understand the complexity of secondary metabolite biogenesis from various organs and developmental stages and to determine functions of encoded biomedical proteins (Wang et al., 2020; Guo et al., 2021). The potential of transcriptomes to code proteins necessary for the synthesis of valuable biomedical compounds (Figure 5) is discussed. Details on transcriptomes of medicinal plant species are presented in Supplementary Table S2.
FIGURE 5
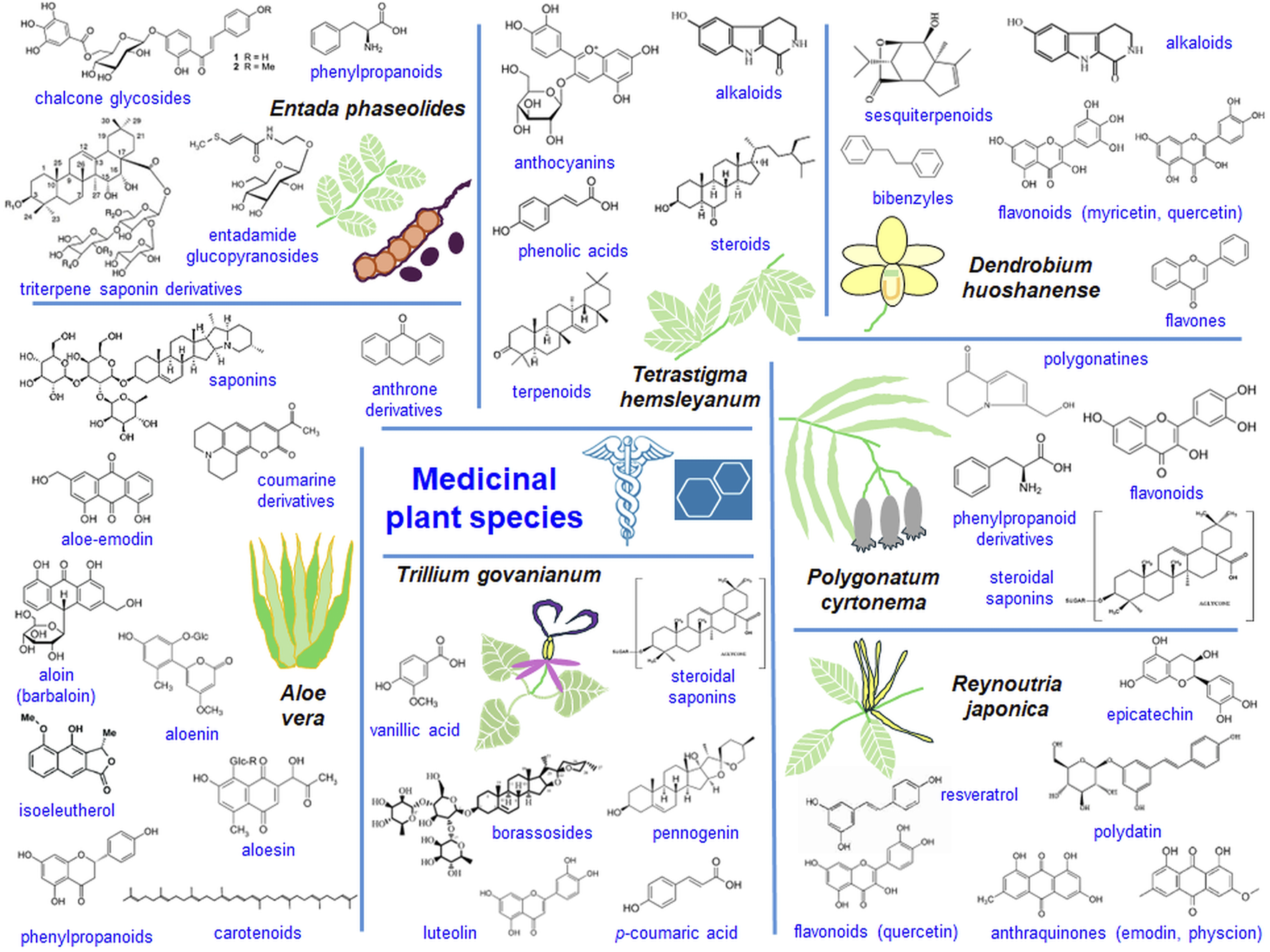
The pharmaceutical potential of selected medicinal plant species. Valuable compounds (formulas added) as secondary metabolism products of medicinal plant species were shown together with small species images.
Quinones, carotenoids and phytoestrogens
Aloe (Aloe vera) and Cape leadwort (Plumbago auriculata) produce of a wide range of quinone and carotenoid derivatives, including antraquinones, carotenoids and saponins (Figure 5). Numerous genes are involved in the biosynthesis of such compounds in these species (Choudhri et al., 2018; Li et al., 2024). The biosynthesis of the phenylpropanoid and terpenoid backbone is crucial for the biogenesis of plumbagin and saponin. In Cape leadwort, the biosynthesis of those compounds follows the mevalonic acid (MVA) pathway and specific “gateway enzymes” pathway. Li et al. (2024) used methyl jasmonate (MeJA) to increase the efficiency of plumbagin and saponin synthesis in P. auriculata. Genes for phenylpropanoid biosynthesis, cysteine and methionine metabolism, terpenoid, pantothenate, CoA and aminoacid biosynthesis were enriched among MeJa-responsive DEGs.
For the biosynthesis of environmental phytoestrogens, such as lignans with anticancer activity, flax (Linum usitatissimum) plants seem to be promising. To understand the relevance of lignan biogenesis for plant immunity responses, Danaeipour et al. (2023) characterized podophyllotoxin (PTOX) biogenesis pathway by flax transcriptome analysis (Supplementary Table S2).
Terpenoids and steroids
Few terpenoids (including monoterpenes) and steroids (including steroidal saponins) are synthesized by various medicinal plant species. They are accumulated efficiently in diverse organs (terpenoids in leaves and spikes, and steroids mainly in roots). Terpenoid biogenesis is particularly complex and involves two metabolic routes: MVA and methyl-erythritol-4-phosphate (MEP) pathways that may be present in a single plant species (Ju et al., 2024). In Trillium govanianum genes for terpenoid biogenesis were upregulated in leaves, while genes responsible for the synthesis of steroids appeared upregulated in roots (Figure 5) (Singh et al., 2017).
Sambong (Blumea balsamifera), an important species used in traditional Chinese medicine, is also a source of terpenoid compounds. Ju et al. (2024) investigated transcriptomes of B. balsamifera leaves at various developmental stages and found 116 DEGs coding proteins for MVA and MEP terpenoid biogenesis pathways.
Tissue- and developmentally specific terpenoid biogenesis in glandular trichomes of Nepeta tenuifolia was investigated by Liu L. et al. (2021). Five genes for the biogenesis of p-menthane monoterpene were identified within the (+)-menthone pathway. In addition, functional analyzes N. tenuifolia limonene 3-hydroxylase (L3OH) proved its relevance for further studies (Supplementary Table S2) (Liu L. et al., 2021).
Flavonoids, phenolic compounds and polysaccharides
Flavonoid compounds comprise flavonols (Han et al., 2023), flavone, flavonol, isoflavone and anthocyanins (Qiao et al., 2023), silymarin (Roy et al., 2018), phenylpropanoid derivatives (Liao et al., 2020), polyphenolic compounds, such as myristine and quercetin or C6C3C6-type flavonoids (Zhou et al., 2020; Yu et al., 2021) (Figure 5). Apricot fruits (Prunus persica) contain flavonols of antioxidant, antitumor and provascular activity. Numerous DEGs for enzymes of flavonol biogenesis were present in apricot fruit transcriptomes (Han et al., 2023). Characterization of transcriptomes from two cultivars of Artemisia allowed the identification of genes for the biosynthesis of flavonoids, phenylpropanoids and anthocyanins. Most genes coding enzymes for flavonoid synthesis were visibly upregulated in the ‘NYYY’ cultivar, which was particularly abundant in flavonoids (Qiao et al., 2023). Japanese thistle (Cirsium japonicum) belongs to Asiatic species with antitumor, proimmune, antidiabetic and hepatoprotective activity. It contains flavones, especially silymarin produced by the flavonoid and phenylpropanoid pathway. DEGs for flavonoid biogenesis were markedly upregulated in flowers (Roy et al., 2018). Transcriptomes of Madagascar periwinkle (Catharanthus roseus), important medicinal plant species, in etiolated, de-etiolated and greening cotyledons were investigated. Affected DEGs covered genes for phenylpropanoid metabolism enzymes (Supplementary Table S2) (Yu et al., 2021). Transcriptomes of leaves, roots and rhizomes of Solomon’s seal (Polygonatum cyrtonema), a species containing phenylpropanoids and flavonoids (Figure 5) were studied by Wang et al. (2019). Genes coding enzymes for phenylpropanoid and carbohydrate metabolism were distinctive (Wang et al., 2019).
Medicinal plant species are also a source of phenolic metabolites, including polyphenols, quinate and gallic acid derivatives, phenylpropanoids and lignins. Red alder (Alnus rubra) contains valuable bioactive phenolic metabolites. Hixson et al. (2024) analyzed the diverse genes coding for enzymes necessary for the synthesis of such compounds, including shikimate-chorismate-phenylalanine pathway, quinate and gallic acid derivatives, phenylpropanoid and lignin compounds, flavonoids as well as proanthocyanidins and related metabolites. Phenylpropanoid and lignin biosynthetic pathways were enriched among the studied DEGs.
Products of mevalonate, methylerythritol phosphate, shikimate and resveratrol biosynthesis pathways belongs to the most important polyphenolic compounds synthesized by medicinal plant species (Zheng et al., 2021). Japanese knotweed (Reynoutria japonica) contains resveratrol, flavonoids and bioactive anthraquinones, emodin and physcion, with antimicrobe and anticancer activities (Hong et al., 2016) (Figure 5). In R. japonica root, stem, leaf, flower and fruit transcriptomes (Supplementary Table S2) few genes coding key enzymes involved in the synthesis of resveratrol (including PAL, C4H, 4CL as well as STS/CHS synthases) were revealed (Zheng et al., 2021). Curcumin belongs to polyphenolic antioxidants used for the treatments of blood stasis and pain. Lu et al. (2020) analyzed transcriptomes of rhizomes of curcuma plants (Curcuma wenyujin) from two areas (Supplementary Table S2). Genes for terpene, curcumin and polysaccharide metabolism were upregulated in C. wenyujin from Wenzhou.
Dendrobium huoshanense stems are source of various alkaloids, sesquiterpenoids, flavonoids and flavones (Figure 5). In transcriptomes of leaves, stems and roots of D. huoshanense at least 103 genes involved in stem development and polysaccharide biosynthesis, 74 genes for glycosyltransferases and 15 genes involved in myricetin and quercetin synthesis were found (Zhou et al., 2020). Based on Meng et al. (2016) data, Shen et al. (2017) analyzed transcriptomes of flowers, leaves, roots and stems of another Dendrobium species, D. officinale (Supplementary Table S2). Between stems and roots, DEGs for proteins necessary for the metabolism of carbohydrates, pyruvate, dicarboxylates, purines and aminoacids were enriched. The potential for polysaccharide synthesis was also assessed in Dendrobium moniliforme (Yuan et al., 2019), where 1204 genes for carbohydrate metabolism were identified. Highly enriched genes were related to flavonoid metabolism among other categories. Another orchid species, Bletilla striata, is a source of various flavonoid and terpenoid compounds. The study of B. striata leaf, root and tuber transcriptomes allowed for finding multiple genes for metabolism of saccharides and flavonoids (Ma et al., 2022). The synthesis of methylated derivatives of flavonoids seems to be especially promising to increase their in-planta yield. In Eucalyptus nitida at least. 60 different DEGs for putative O-methyltransferases (OMTs) were selected and one of these enzymes converted flavanone pinocembrin into a methylated product in the functional test (Somaletha Chandran et al., 2022).
Among TFs regulating flavonoid biogenesis pathways were AP2-ERF, bHLH, bZIP, C2C2, TIFY, MADS, MYB, TCP, bHLH, C3H, B3, HB, E2F, GRAS, WRKY, SBP and TRAF proteins (Yu et al., 2021; Han et al., 2023; Qiao et al., 2023). TFs regulating the expression pattern of genes for polysaccharide biosynthesis included MYB, AP2-EREBP, WRKY, bHLH, zinc finger C3H and C2H2 and NAC proteins (Wang et al., 2019).
Glycosides, anthocyanins and macrocyclic peptides
Neopicrorhiza scrophulariiflora accumulates picroside I and picroside II, anti-inflammatory, anti-cancer and anti-bacterial iridoid glycosides. Rao et al. (2025) identified 200 different flavonoid and >60 terpenoid compounds in this species. Among others, DEGs between investigated tissues coded proteins for the metabolism of terpenoids, polyketides and phenylpropanoids. At least 74 and 43 annotated genes coded proteins for iridoid and picroside biogenesis, respectively. Matchbox bean (Entada phaseolides) is a source of glycosides and glucopyranosides (Figure 5). DEGs for phenylpropanoid biosynthesis and cyanoamino acid metabolism were enriched between leaves and roots of E. phaseolides (Liao et al., 2020).
Medicinal plant species accumulating anthocyanins could also synthesize alkaloids, flavonoids, steroids and terpenoids (Yan et al., 2020; Ni et al., 2021). Analysis of the purple and green leaf transcriptomes of Tetrastigma hemsleyanum, a species with antibiotic properties, allowed identification of DEGs mainly involved in anthocyanin and carotenoid biosynthesis (Figure 5) (Yan et al., 2020). Ni et al. (2021) studied candidate genes involved in anthocyanin biosynthesis in sugarcane (Saccharum officinarum). The transcriptomes from rinds and piths of three cultivars of sugarcane were compared. The enriched pathways for DEGs between investigated tissues included the ones for steroid, phenylpropanoid and flavonoid biosynthesis, tryptophan metabolism, indole alkaloid biosynthesis as well as sesquiterpenoid and triterpenoid biosynthesis. At least 50 DEGs for enzymes of anthocyanin biogenesis were identified (Supplementary Table S2).
Cyclotides, which belong to stable disulfide-rich macrocyclic peptides, belong to promising drugs. Asian pigeonwings (Clitoria ternatea), a perennial medicinal plant species, is the only known fabaceous plant producing large amounts of cyclotides. In pods and stems of C. ternatea multiple cyclotide genes are highly expressed. At least 71 cyclotide precursor sequences were found, including 26 entirely novel ones. A pathway for cyclotide biogenesis, with precursor processing was also proposed (Kalmankar et al., 2020).
Discussion
Studies employing RNA-seq to study organ-specific and medicinal plant species transcriptomes
Current data on plant transcriptomes can be obtained from various studies on transcriptomes of multiple tissues, single cells or single organelles (e.g., scRNA-seq or snRNA-seq). From 2010 the number of reports on plant organ-specific transcriptomes increased notably. Studies on transcriptomes of roots and leaves dominate since 2020. Papers on generative organ and seed transcriptomes were less in quantity in this period. Recently, the number of reports on transcriptomes of medicinal plant species and single-cell transcriptomes has doubled in the last 5 years. Between 2010 and 2025 ca. 9,200 studies on root, leaf, flower and seed specific transcriptomes were published. The data for transcriptomes of medicinal plant species have been deposited in >1,600 publications between 2010 and 2025. Those numbers depicts the continuous growth of interest of plant organ transcriptomes in current plant research (Figure 6).
FIGURE 6
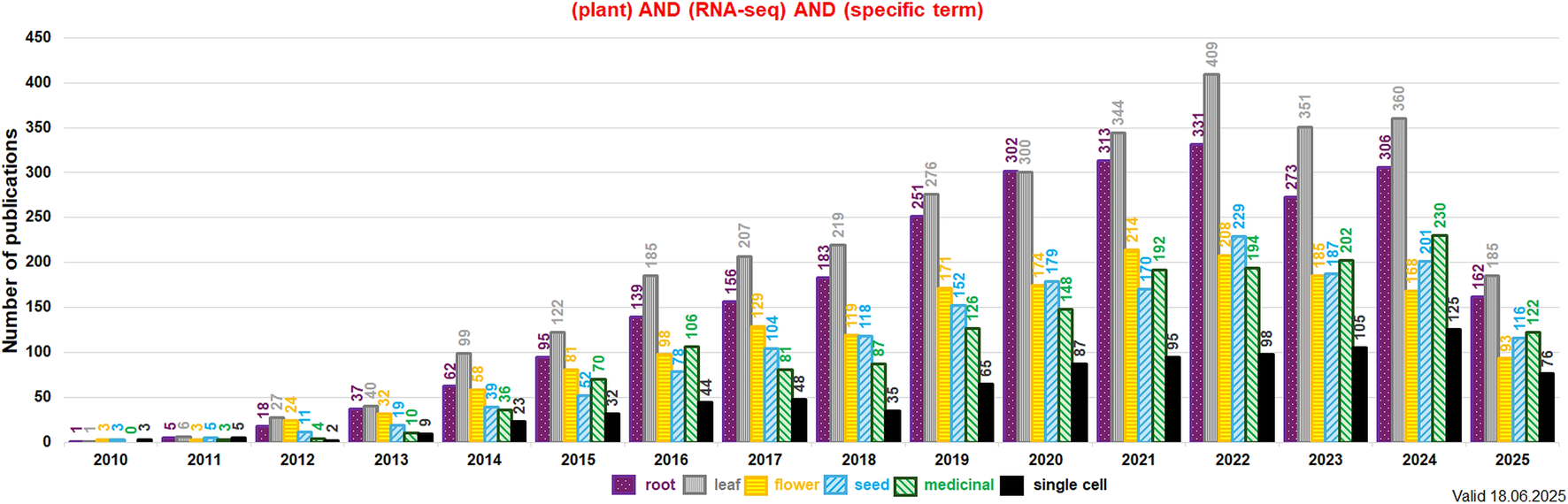
The detailed number of publications per year related to RNA-seq and investigated plant organs, medicinal plant species and single cell studies (values indicated above each bar; data for subterms indicated by the various colors and checking patterns). Keywords used in the NCBI PubMed search (https://pubmed.ncbi.nlm.nih.gov/) included: “plant,” “RNA-seq,” and the given subterm (indicated in the legend below the histogram). Data for roots, leaves, flowers, seeds, medicinal plant species and scRNA-seq were presented from 2010 onward. The analysis was performed in June 2025.
Transcriptomic patterns across diverse organs
Functional terms related to the organ-specific DEGs are summarized in Figure 1. This comparison indicates the high specificity of organ transcriptomes. Genes jointly affected in diverse plant organs encode proteins participating in important activities, including hormonal signaling, aminoacid metabolism, cell organization, DNA synthesis, protein fate and intracellular transport. TFs common for all plant organs comprise mostly bHLH, MYB and NAC proteins (Figures 1, 2).
Vascularization is enriched by genes mainly involved in carbohydrate metabolism, cell divisions and cell wall reorganization. TFs for diverse aspects of vascularization processes are particularly abundant and notably vary from TFs regulating development of other plant tissues and organs (Figures 1–3).
In roots, genes coding proteins for chromatin assembly, cell wall biogenesis, cytokinesis and cytoskeleton remodeling, cell cycle, detoxication, hormonal response, DNA replication, high-energy compound catabolism, histone biogenesis, polysaccharide and phenylpropanoid synthesis, and vesicle transport are notable. Those functions likely reflect root growth, organogenesis, plasticity and responsiveness. In particular, distinct genes are active in primary and lateral roots as well as in root endodermis. Root organogenesis is regulated by multiple TFs, including the specific proteins governing development of root hairs, meristem, endodermis, primary and lateral roots (Figures 1–3).
Leaf transcriptomes particularly depends on organ polarity and organization. They are especially abundant in photosynthetic protein transcripts. Energy conversions, synthesis of secondary metabolites (including wax), various morphogenic functions, protein assembly, redox homeostasis belong to main functions of leaf-affected genes. Various TFs are involved in the organogenesis of leaf tissues or morphological zones, however AHL, bHLH, MYB and WRKY TFs overlaps between them. In senescing leaves genes for autophagy and diverse compound degradation are accompanied by transcriptomic alterations leading to the rearrangement of organellar biogenesis; distinct TFs are also active (Figure 3).
Flower transcriptomes are enriched with the activity of genes for the vegetative to generative phase shift as well as with genes for stimulus detection, fatty acid and carbohydrate metabolism, floral meristem identity, hormonal signaling, pigment synthesis, photoperiod regulation, pollen development and production of secondary metabolites. The activity of various TFs is necessary for flower development, including specific TFs for the pigment biogenesis (Figures 1, 2).
Compared to vegetative organs, seeds have quite distinct transcriptomes. They code proteins for water homeostasis, transcription and translation machinery, protective and transporter activities and for starch metabolism. Seed transcriptome is in a very complex manner remodeled in various tissues during seed development, imbibition and germination (with notable mobilization of organellar biogenesis in those processes). Seeds contain also a vast number of specific TFs, which are differentially switched on during seed maturation (Figures 1, 2, 4).
Future directions and concluding remarks
In this review, organ-specific alterations from a number of studies on plant model and crop species were analyzed with a focus on mostly affected protein genes including genes for organ-specific TFs. The involvement of multiple marker genes and genes coding for organellar proteins in plant development was also highlighted. In general, under plant organogenesis, organ-specific genes are switched on and off in a highly complex, spatiotemporal manner.
From the variety of studied species, it is obvious that basic transcriptomic studies should be expanded to less-studied model, crop and medicinal plant species, which produce a particularly wide range of valuable secondary metabolites (Yan et al., 2020; Ju et al., 2024; Li et al., 2024). All future research should be also validated in field conditions, because transcriptomic patterns vary from controlled to field grown conditions and the production of medicinal compounds in natural conditions can be more effective.
In addition, modern methodologies including gene editing and advanced gene engineering should be broadened to allow (1) to study the relevance of additional marker genes or mainly affected organ-specific DEGs under plant development and (2) to increase the potential of secondary metabolite synthesis. Gene editing could particularly be used for precise plant breeding by (1) knock-out of genes negatively affecting yield or useful agronomical traits, (2) knock-in and gene replacement to introduce new alleles or to decrease trait multiplicity and to modify promoters or coding sequence in developmentally important genes. Those attempts may be used to improve the crop yield or to modify plant phenotype (Chen et al., 2019). For instance, tomato (Solanum lycopersicum) lines with the edited SlCHRC gene (coding for carotenoid binding protein regulating chromoplast development in fruits) displayed greener phenotype with lowered carotenoid and plastoglobuli content, contrary to lines overexpressing this protein (Wang et al., 2024a). By virus-induced gene silencing (VIGS), Huang et al. (2022) knocked-down cotton (Gossypium hirsutum) genes for Vir-like m6A methyltransferase associated proteins which affected plastid-dependent and independent leaf development by altering expression of multiple target genes. In this review some other examples of editing of developmentally important genes were also presented (Wang et al., 2017; Lee et al., 2019; Liang et al., 2020; Paul et al., 2020; Sriboon et al., 2020; Liu et al., 2024). In addition, Cao et al. (2024) proposed a simple transformation and gene editing protocol which should be prospective for numerous medicinal plant species. It employs Agrobacterium- mediated root transformation for the delivery of editing reagent.
Key metabolic pathways of pharmaceutical importance should be more effectively investigated in medicinal plant species. Obviously, further studies are awaited to understand better how transcriptomic analyses can improve synthesis of natural products in plant-based bioreactors. Those attempts are needed (1) to scale up metabolite synthesis, (2) to synthesize more modified or novel compounds and (3) to optimize parallel synthesis of multiple compounds for obtaining more efficient yield. The ability of the synthesis of selected compounds can be, however, hampered by the insufficient transcriptomic data (Kalmankar et al., 2020; Danaeipour et al., 2023; Rao et al., 2025) which should be improved in terms of complexity.
Statements
Author contributions
MR designed the paper, compiled the literature, supervised submission, wrote, edited and reviewed the first manuscript draft, and prepared all figures and supplementary tables. The author revised manuscript, figures and supplementary files, read, and approved the submitted version.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Supported by the Excellent Initiative – Research University (ID-UB) program at the Adam Mickiewicz University, Poznań and KNOW RNA Research Center at Adam Mickiewicz University, Poznań, grant no. 01/KNOW2/2014.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/abp.2025.14609/full#supplementary-material
Glossary
- ABC
ATP-Binding cassette
- ABI/VP
ABA-INSENSITIVE/VIVIPAROUS
- ACL
ATP-Citrate lyase
- AGL
AGAMOUS LIKE
- AHL
AT-HOOK MOTIF NUCLEAR LOCALIZED PROTEIN 23
- AIL
AINTEGUMENTA-LIKE
- ANAC
NAC Domain containing protein
- ANT
AINTEGUMENTA
- AP
APETALA
- APL
ALTERED PHLOEM DEVELOPMENT
- ARF
auxin responsive factor
- ARG
arginase
- ARR
authentic response regulator
- ATAF
Arabidopsis thaliana activating factor
- ATHB
small homeodomain-leucine zipper family
- ATL
ARABIDOPSIS TOXICOS EN LEVADURA
- AUX/IAA
auxin/indole-3-acetic acid
- B3
B3 DNA-binding domain
- BAM
receptor kinase
- BELL
BEL1-like
- BFN
bifunctional nuclease
- bHLH
basic helix–loop–helix
- BIM
BES1-INTERACTING MYC-LIKE, a bHLH transcription factor
- BKN
barley knox
- BSK
BRASSINOSTEROID-SIGNALING KINASE
- bZIP
basic (region) leucine zipper
- CAMTA
calmodulin binding transcription activator
- CBF
C-repeat binding factor
- C2C2
TF with zinc finger
- CCOAOMT
caffeoyl-CoA O-methyltransferase
- CCR
cinnamoyl-CoA:NADP reductase
- CDK
cyclin-dependent kinase
- CER
ECERIFERUM
- C2H2
cysteine 2/histidine 2
- C3H
cysteine 3/histidine
- C4H
cinnamate 4-hydroxylase
- CHRC
chromoplast-specific carotenoid-binding protein
- CHS
chalcone synthase
- 4CL
4-coumarate: CoA ligase
- CLE
CLAVATA3/ESR-RELATED
- CMS
cytoplasmic male sterility
- CNA
CORONA
- CO
CONSTANS
- COBL
COBRA-LIKE
- COX
cytochrome c oxidase
- CRY
cryptochrome
- CUC
cup-shaped cotyledon
- CYS
cystatin
- cyt.
cytochrome
- DEG
differentially expressed gene
- DFR
dihydroflavonol 4-reductase
- DMAPP
dimethylallyl pyrophosphate
- DOF/Dof
DNA-binding with one finger
- DREB
dehydration-responsive element-binding
- EF
early flowering
- E2F
transcription factor binding DNA through the E2 recognition site
- ELP
EXTENSIN-LIKE PROTEIN
- EREBP
ethylene-responsive element binding protein
- ERF
ethylene response factor
- ETR
ethylene receptor
- FAMA
a regulator of stomatal development, TF
- FAR
FAR-RED IMPAIRED RESPONSE
- FCA
FLOWERING LOCUS CA
- FD
FLOWERING LOCUS D
- FE
ALTERED PHLOEM DEVELOPMENT, a phloem-specific Myb-related protein
- F3′5′H
flavonoid 3′,5′-hydroxylase
- FIE
fertilization-independent endospermia
- FKF
FLAVIN-BINDING KELCH REPEAT F-BOX
- FMA
FAMA, A BHLH TRANSCRIPTION FACTOR
- FPA
FLOWERING PATHWAY
- FUL
FRUITFUL
- FUS
fused in sarcoma
- G2
Golden 2
- GATA
(T/A)GATA(A/G)-binding transcription factors
- GER
GERMIN
- GH
glycosyl hydrolase
- GI
GIGANTEA
- GID
GIBBERELLIN-INSENSITIVE DWARF
- GL
GLABRA2
- GO
gene ontology
- GPAT
glycerol-3-phosphate acyltransferase
- GPI
glycosylphosphatidylinositol
- GRAS
GIBBERELLIN-ACID INSENSITIVE/REPRESSOR of GA1/SCARECROW
- GRF
growth regulating factor
- HAN
HANABA TANARU
- HAP
transcription factor complex
- HAT
histone acetylases
- HB
homeobox
- HCT
hydroxycinnamoyl transferase
- HD
homeodomain
- HDT
histone deacetylase
- HLH
helix-loop-helix
- HLZ
Homeobox-Leucine Zipper
- HPLC
high pressure liquid chromatography
- HSF
heat shock factor
- hxk
hexokinase
- IFL
INTERFASCICULAR FIBERLESS
- IPP
isopentenyl pyrophosphate
- IRX
IRREGULAR XYLEM
- JA
jasmonic acid
- KAN
KANADI
- KCS
3-ketoacyl-CoA synthase
- KNAT
KNOTTED-like homeobox of ARABIDOPSIS THALIANA
- KNOX
KNOTTED1-like homeobox
- LAC
laccase
- LACS
long-chain acyl-CoA synthetase
- LBD
LATERAL ORGAN BOUNDARIES DOMAIN
- LEA
late-embryogenesis abundant
- LEC
little elongation complex
- Lhc
light-harvesting complex
- LHY
Late Elongated Hypocotyl
- L3OH
limonene 3-hydroxylase
- LRL
ROOTHAIRLESS LIKE
- LRS
lateral roots
- MADS
MINICHROMOSOME MAINTENANCE 1/AGAMOUS/DEFICIENS/SERUM RESPONSE FACTOR
- MEDEA
motif enrichment in differential elements of accessibility
- MEE
MATERNAL EFFECT EMBRYO ARREST
- MeJA
methyl jasmonate
- MEP
methyl-erythritol-4-phosphate
- MIF
mini zinc-finger
- miRNA
micro-RNA
- MP
MONOPTEROS
- mTERF
mitochondrial transcription termination factor
- MTF
metal regulatory transcription factor
- MTF2
mannitol transporter protein
- MYB
myeloblastosis
- MVA
mevalonic acid
- NAC
no apical meristem/ATAF1/cup-shaped cotyledon
- Nam
no apical meristem
- NCED
9-cis-epoxycarotenoid dioxygenase
- ncRNA
non-coding RNA
- NDH
NADH dehydrogenase, chlororespirating
- NF-Y
nuclear transcription factor Y
- NYE
NON-YELLOWING
- NPY
NAKED PINS IN YUC MUTANTS
- Ole e
allergen from the pollen of olive tree (Olea europaea)
- OMT
O-methyltransferase
- ORF/orf
open reading frame
- OXPHOS
oxidative phosphorylation
- PA
phloem-associated
- PAL
phenylalanine ammonia lyase
- PASPA
PUTATIVE ASPARTIC PROTEINASE A3
- PcG
Polycomb group
- PEAR
PHLOEM EARLY DNA-BINDING-WITH-ONE-FINGER
- PEI
Cys3His zinc finger domain-containing protein
- PER
peroxidase
- PHB
PHABULOSA
- PHD
plant homeodomain
- PHV
PHAVOLUTA
- PHY
phytochrome
- PIN
PINFORMED
- PLT
PLETHORA
- PRR
PSEUDO RESPONSE REGULATOR
- PSB/psb
photosystem II subunits
- PXE/PXY
PHLOEM INTERCALATED WITH XYLEM
- PTOX
podophyllotoxin
- RBCS
Rubisco small subunit
- RCP
reduced carotenoid pigmentation/ROOT CAP
- REV
REVOLUTA
- RNA-seq
RNA sequencing
- RPL
ribosomal large subunit
- RPS
ribosomal small subunit
- RSI
ROSINA
- SAP
STERILE APETALA
- SAG
senescence-associated gene
- SBP
SQUAMOSA Promoter Binding Protein
- SCL
scutellum epidermis/layer
- SCR
SCARECROW
- scRNA-seq
single cell RNA sequencing
- SEO
sieve element occlusion
- SHR
SHORT ROOT
- siRNA
small interfering RNA
- SMB
SOMBRERO
- SMXL
SUPPRESOR OF MAX-LIKE
- SNF
sucrose non-fermentable
- snRNA-seq
single nucleus RNA sequencing
- SOC
suppressor of overexpression of constans
- STH
saitohin
- STS
stachyose synthase
- SUC
SUCROSE TRANSPORTER
- Tat
twin-arginine translocation
- TCA
tricarboxylic acid cycle
- TCP
TEOSINTE BRANCHED1/CYCLOIDEA/PROLIFERATING CELL FACTOR
- TG3-1
HYBRID PROLINE- OR GLYCINE-RICH PROTEIN 5
- TF
transcription factor
- tfl
terminal flowering
- TIFY
conserved core motif TIF[F/Y]XG
- TLP
Tubby-like protein
- TMO
TARGET OF MONOPTEROS
- TRAF
Tumor necrosis factor receptor-associated factors
- VIGS
virus-induced gene silencing
- VIN
VERNALIZATION INSENSITIVE
- VND
VASCULAR-RELATED NAC-DOMAIN
- WER
WEREWOLF
- WGD
whole-genome duplication
- WOL
WOODEN LEG
- WOX
WUSCHEL-Related Homeobox
- WRKY
transcription factor family
- XCP
xylem peptidase
- ZIP
leucine zipper
References
1
Asaf S. Khan A. L. Aaqil Khan M. Muhammad Imran Q. Kang S.-M. Al-Hosni K. et al (2017). Comparative analysis of complete plastid genomes from wild soybean (Glycine soja) and nine other Glycine species. PLoS ONE12, e0182281. 10.1371/journal.pone.0182281
2
Barkan A. Goldschmidt-Clermont M. (2000). Participation of nuclear genes in chloroplast gene expression. Biochimie82, 559–572. 10.1016/S0300-9084(00)00602-7
3
Berkowitz O. Xu Y. Liew L. C. Wang Y. Zhu Y. Hurgobin B. et al (2021). RNA‐seq analysis of laser microdissected Arabidopsis thaliana leaf epidermis, mesophyll and vasculature defines tissue‐specific transcriptional responses to multiple stress treatments. Plant J.107, 938–955. 10.1111/tpj.15314
4
Best C. Mizrahi R. Ostersetzer-Biran O. (2020a). Why so complex? The intricacy of genome structure and gene expression, associated with angiosperm mitochondria, may relate to the regulation of embryo quiescence or dormancy-intrinsic blocks to early plant life. Plants9, 598. 10.3390/plants9050598
5
Best C. Sultan L. Murik O. Ostersetzer-Biran O. (2020b). Insights into the mitochondrial transcriptome landscapes of two Brassicales plant species, Arabidopsis thaliana (var. Col-0) and Brassica oleracea (var. botrytis). Endocyt. Cell Res.30, 16–38.
6
Betts N. S. Berkowitz O. Liu R. Collins H. M. Skadhauge B. Dockter C. et al (2017). Isolation of tissues and preservation of RNA from intact, germinated barley grain. Plant J.91, 754–765. 10.1111/tpj.13600
7
Boesewinkel F. D. Bouman F. (1984). “The seed: structure,” in Embryology of angiosperms. Editor JohriB. M. (Berlin, Heidelberg: Springer), 567–610. 10.1007/978-3-642-69302-1_12
8
Bonke M. Thitamadee S. Mähönen A. P. Hauser M.-T. Helariutta Y. (2003). APL regulates vascular tissue identity in Arabidopsis. Nature426, 181–186. 10.1038/nature02100
9
Booth M. W. Breed M. F. Kendrick G. A. Bayer P. E. Severn-Ellis A. A. Sinclair E. A. (2022). Tissue-specific transcriptome profiles identify functional differences key to understanding whole plant response to life in variable salinity. Biol. Open11, bio059147. 10.1242/bio.059147
10
Breeze E. Harrison E. McHattie S. Hughes L. Hickman R. Hill C. et al (2011). High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. Plant Cell23, 873–894. 10.1105/tpc.111.083345
11
Cahoon A. Nauss J. Stanley C. Qureshi A. (2017). Deep transcriptome sequencing of two green algae, Chara vulgaris and Chlamydomonas reinhardtii, provides no evidence of organellar RNA editing. Genes8, 80. 10.3390/genes8020080
12
Cao X. Xie H. Song M. Zhao L. Liu H. Li G. et al (2024). Simple method for transformation and gene editing in medicinal plants. J. Integr. Plant Biol.66, 17–19. 10.1111/jipb.13593
13
Castañón-Suárez C. A. Arrizubieta M. Castelán-Muñoz N. Sánchez-Rodríguez D. B. Caballero-Cordero C. Zluhan-Martínez E. et al (2024). The MADS-box genes SOC1 and AGL24 antagonize XAL2 functions in Arabidopsis thaliana root development. Front. Plant Sci.15, 1331269. 10.3389/fpls.2024.1331269
14
Chen J. Liu F. Tang Y. Yuan Y. Guo Q. (2014). Transcriptome sequencing and profiling of expressed genes in phloem and xylem of ramie (Boehmeria nivea L. Gaud). PLoS ONE9, e110623. 10.1371/journal.pone.0110623
15
Chen K. Wang Y. Zhang R. Zhang H. Gao C. (2019). CRISPR/Cas genome editing and precision plant breeding in agriculture. Annu. Rev. Plant Biol.70, 667–697. 10.1146/annurev-arplant-050718-100049
16
Chen Y. Tong S. Jiang Y. Ai F. Feng Y. Zhang J. et al (2021). Transcriptional landscape of highly lignified poplar stems at single-cell resolution. Genome Biol.22, 319. 10.1186/s13059-021-02537-2
17
Cho K.-S. Cho J.-H. Im J.-S. Choi J.-G. Park Y.-E. Hong S.-Y. et al (2017). The complete mitochondrial genome sequences of potato (Solanum tuberosum L., Solanaceae). Mitochondrial DNA Part B2, 781–782. 10.1080/23802359.2017.1398607
18
Choudhri P. Rani M. Sangwan R. S. Kumar R. Kumar A. Chhokar V. (2018). De novo sequencing, assembly and characterisation of Aloe vera transcriptome and analysis of expression profiles of genes related to saponin and anthraquinone metabolism. BMC Genomics19, 427. 10.1186/s12864-018-4819-2
19
Chrobok D. Law S. R. Brouwer B. Lindén P. Ziolkowska A. Liebsch D. et al (2016). Dissecting the metabolic role of mitochondria during developmental leaf senescence. Plant Physiol.172, 2132–2153. 10.1104/pp.16.01463
20
Danaeipour Z. Garoosi G. Tohidfar M. Bakhtiarizadeh M. R. Mirjalili M. H. (2023). Comprehensive RNA-Seq-based study and metabolite profiling to identify genes involved in podophyllotoxin biosynthesis in Linum album Kotschy ex Boiss. (Linaceae). Sci. Rep.13, 9219. 10.1038/s41598-023-36102-7
21
Daniell H. Lin C.-S. Yu M. Chang W.-J. (2016). Chloroplast genomes: Diversity, evolution, and applications in genetic engineering. Genome Biol.17, 134. 10.1186/s13059-016-1004-2
22
Denyer T. Ma X. Klesen S. Scacchi E. Nieselt K. Timmermans M. C. P. (2019). Spatiotemporal developmental trajectories in the Arabidopsis root revealed using high-throughput single-cell RNA sequencing. Dev. Cell48, 840–852.e5. 10.1016/j.devcel.2019.02.022
23
Dinneny J. R. Yanofsky M. F. (2004). Vascular patterning: Xylem or phloem?Curr. Biol.14, R112–R114. 10.1016/j.cub.2004.01.017
24
Dobrogojski J. Adamiec M. Luciński R. (2020). The chloroplast genome: A review. Acta Physiol. Plant.42, 98. 10.1007/s11738-020-03089-x
25
Du J. Wang Y. Chen W. Xu M. Zhou R. Shou H. et al (2023). High-resolution anatomical and spatial transcriptome analyses reveal two types of meristematic cell pools within the secondary vascular tissue of poplar stem. Mol. Plant16, 809–828. 10.1016/j.molp.2023.03.005
26
Du K. Xu Y. Wang N. Qin L. Tao J. (2024). Transcriptomic remodeling occurs during cambium activation and xylem cell development in Taxodium ascendens. Curr. Issues Mol. Biol.46, 11927–11941. 10.3390/cimb46110708
27
Efroni I. Mello A. Nawy T. Ip P.-L. Rahni R. DelRose N. et al (2016). Root regeneration triggers an embryo-like sequence guided by hormonal interactions. Cell165, 1721–1733. 10.1016/j.cell.2016.04.046
28
Eshed Y. Baum S. F. Perea J. V. Bowman J. L. (2001). Establishment of polarity in lateral organs of plants. Curr. Biol.11, 1251–1260. 10.1016/S0960-9822(01)00392-X
29
Eshed Y. Izhaki A. Baum S. F. Floyd S. K. Bowman J. L. (2004). Asymmetric leaf development and blade expansion in Arabidopsis are mediated by KANADI and YABBY activities. Development131, 2997–3006. 10.1242/dev.01186
30
Forster B. P. Heberle-Bors E. Kasha K. J. Touraev A. (2007). The resurgence of haploids in higher plants. Trend Plant Sci.12, 368–375. 10.1016/j.tplants.2007.06.007
31
Galbraith D. W. (2012). Flow cytometry and fluorescence-activated cell sorting in plants: The past, present, and future. Biomedica30, 65. 10.7705/biomedica.v30i0.824
32
Gautam V. Sarkar A. K. (2015). Laser assisted microdissection, an efficient technique to understand tissue specific gene expression patterns and functional genomics in plants. Mol. Biotechnol.57, 299–308. 10.1007/s12033-014-9824-3
33
Gonzalez-Jorge S. Ha S.-H. Magallanes-Lundback M. Gilliland L. U. Zhou A. Lipka A. E. et al (2013). CAROTENOID CLEAVAGE DIOXYGENASE4 is a negative regulator of β-carotene content in Arabidopsis seeds. Plant Cell25, 4812–4826. 10.1105/tpc.113.119677
34
Guo J. Huang Z. Sun J. Cui X. Liu Y. (2021). Research progress and future development trends in medicinal plant transcriptomics. Front. Plant Sci.12, 691838. 10.3389/fpls.2021.691838
35
Guo X. Wang Y. Zhao C. Tan C. Yan W. Xiang S. et al (2025). An Arabidopsis single-nucleus atlas decodes leaf senescence and nutrient allocation. Cell188, 2856–2871.e16. 10.1016/j.cell.2025.03.024
36
Han X. Wang J. Wang G. Dong F. Nie P. Xue X. (2023). Transcriptome and metabolome analysis of flavonol synthesis in apricot fruits. Front. Plant Sci.14, 1187551. 10.3389/fpls.2023.1187551
37
Hirsch C. N. Robin Buell C. (2013). Tapping the promise of genomics in species with complex, nonmodel genomes. Annu. Rev. Plant Biol.64, 89–110. 10.1146/annurev-arplant-050312-120237
38
Hixson K. K. Meng Q. Moinuddin S. G. A. Kwon M. Costa M. A. Cort J. R. et al (2024). RNA-seq and metabolomic analyses of beneficial plant phenol biochemical pathways in red alder. Front. Plant Sci.15, 1349635. 10.3389/fpls.2024.1349635
39
Hong M. Tan H. Li S. Cheung F. Wang N. Nagamatsu T. et al (2016). Cancer stem cells: The potential targets of Chinese medicines and their active compounds. Int. J. Mol. Sci.17, 893. 10.3390/ijms17060893
40
Huang Q. Huang X. Deng J. Liu H. Liu Y. Yu K. et al (2016). Differential gene expression between leaf and rhizome in Atractylodes lancea: A comparative transcriptome analysis. Front. Plant Sci.7, 348. 10.3389/fpls.2016.00348
41
Huang X. Abuduwaili N. Wang X. Tao M. Wang X. Huang G. (2022). Cotton (Gossypium hirsutum) VIRMA as an N6-methyladenosine RNA methylation regulator participates in controlling chloroplast-dependent and independent leaf development. Int. J. Mol. Sci.23, 9887. 10.3390/ijms23179887
42
Huang Y.-J. Liu L.-L. Huang J.-Q. Wang Z.-J. Chen F.-F. Zhang Q.-X. et al (2013). Use of transcriptome sequencing to understand the pistillate flowering in hickory (Carya cathayensis Sarg.). BMC Genomics14, 691. 10.1186/1471-2164-14-691
43
Imadi S. R. Kazi A. G. Ahanger M. A. Gucel S. Ahmad P. (2015). Plant transcriptomics and responses to environmental stress: An overview. J. Genet.94, 525–537. 10.1007/s12041-015-0545-6
44
Jia Y. Chen C. Gong F. Jin W. Zhang H. Qu S. et al (2022). An Aux/IAA family member, RhIAA14, involved in ethylene-inhibited petal expansion in rose (Rosa hybrida). Genes13, 1041. 10.3390/genes13061041
45
Jiao Y. Hu Q. Zhu Y. Zhu L. Ma T. Zeng H. et al (2019). Comparative transcriptomic analysis of the flower induction and development of the Lei bamboo (Phyllostachys violascens). BMC Bioinforma.20, 687. 10.1186/s12859-019-3261-z
46
Ju Z. Liang L. Zheng Y. Shi H. Zhao W. Sun W. et al (2024). Full-length transcriptome sequencing and RNA-seq analysis offer insights into terpenoid biosynthesis in Blumea balsamifera (L.) DC. Genes15, 285. 10.3390/genes15030285
47
Kalmankar N. V. Venkatesan R. Balaram P. Sowdhamini R. (2020). Transcriptomic profiling of the medicinal plant Clitoria ternatea: Identification of potential genes in cyclotide biosynthesis. Sci. Rep.10, 12658. 10.1038/s41598-020-69452-7
48
Kao P. Schon M. A. Mosiolek M. Enugutti B. Nodine M. D. (2021). Gene expression variation in Arabidopsis embryos at single-nucleus resolution. Development148, dev199589. 10.1242/dev.199589
49
Kenrick P. Crane P. R. (1997). The origin and early diversification of land plants: a cladistic study. Washington, DC: Smithsonian Institution Press.
50
Khalilzadeh M. Weber K. C. Dutt M. El-Mohtar C. A. Levy A. (2022). Comparative transcriptome analysis of Citrus macrophylla tree infected with Citrus tristeza virus stem pitting mutants provides new insight into the role of phloem regeneration in stem pitting disease. Front. Plant Sci.13, 987831. 10.3389/fpls.2022.987831
51
Kim D.-H. Doyle M. R. Sung S. Amasino R. M. (2009). Vernalization: Winter and the timing of flowering in plants. Annu. Rev. Cell Dev. Biol.25, 277–299. 10.1146/annurev.cellbio.042308.113411
52
Kortz A. Hochholdinger F. Yu P. (2019). Cell type-specific transcriptomics of lateral root formation and plasticity. Front. Plant Sci.10, 21. 10.3389/fpls.2019.00021
53
Kress W. J. Soltis D. E. Kersey P. J. Wegrzyn J. L. Leebens-Mack J. H. Gostel M. R. et al (2022). Green plant genomes: what we know in an era of rapidly expanding opportunities. Proc. Natl. Acad. Sci. U.S.A.119, e2115640118. 10.1073/pnas.2115640118
54
Le B. H. Cheng C. Bui A. Q. Wagmaister J. A. Henry K. F. Pelletier J. et al (2010). Global analysis of gene activity during Arabidopsis seed development and identification of seed-specific transcription factors. Proc. Natl. Acad. Sci. U.S.A.107, 8063–8070. 10.1073/pnas.1003530107
55
Lee K. Leister D. Kleine T. (2021). Arabidopsis mitochondrial transcription termination factor mTERF2 promotes splicing of group IIB introns. Cells10, 315. 10.3390/cells10020315
56
Lee S.-K. Kim H. Cho J.-I. Nguyen C. D. Moon S. Park J. E. et al (2020). Deficiency of rice hexokinase HXK5 impairs synthesis and utilization of starch in pollen grains and causes male sterility. J. Exp. Bot.71, 116–125. 10.1093/jxb/erz436
57
Lee T. A. Nobori T. Illouz-Eliaz N. Xu J. Jow B. Nery J. R. et al (2023). A single-nucleus atlas of seed-to-seed development in Arabidopsis. bioRxiv. 10.1101/2023.03.23.533992
58
Li C. Lin H. Chen A. Lau M. Jernstedt J. Dubcovsky J. (2019). Wheat VRN1, FUL2 and FUL3 play critical and redundant roles in spikelet development and spike determinacy. Development146, dev175398. 10.1242/dev.175398
59
Li H. Yuan J. Wu M. Han Z. Li L. Jiang H. et al (2018). Transcriptome and DNA methylome reveal insights into yield heterosis in the curds of broccoli (Brassica oleracea L var. italic). BMC Plant Biol.18, 168. 10.1186/s12870-018-1384-4
60
Li P. Ponnala L. Gandotra N. Wang L. Si Y. Tausta S. L. et al (2010). The developmental dynamics of the maize leaf transcriptome. Nat. Genet.42, 1060–1067. 10.1038/ng.703
61
Li R. Wang Z. Wang J.-W. Li L. (2023). Combining single-cell RNA sequencing with spatial transcriptome analysis reveals dynamic molecular maps of cambium differentiation in the primary and secondary growth of trees. Plant Commun.4, 100665. 10.1016/j.xplc.2023.100665
62
Li Y. Zhao Z. Hu J. Lei T. Chen Q. Li J. et al (2024). MeJA-induced hairy roots in Plumbago auriculata L. by RNA-seq profiling and key synthase provided new insights into the sustainable production of plumbagin and saponins. Front. Plant Sci.15, 1411963. 10.3389/fpls.2024.1411963
63
Liang X. Li S.-W. Gong L.-M. Li S. Zhang Y. (2020). COPII components Sar1b and Sar1c play distinct yet interchangeable roles in pollen development. Plant Physiol.183, 974–985. 10.1104/pp.20.00159
64
Liao W. Mei Z. Miao L. Liu P. Gao R. (2020). Comparative transcriptome analysis of root, stem, and leaf tissues of Entada phaseoloides reveals potential genes involved in triterpenoid saponin biosynthesis. BMC Genomics21, 639. 10.1186/s12864-020-07056-1
65
Liew L. C. You Y. Auroux L. Oliva M. Peirats-Llobet M. Ng S. et al (2024). Establishment of single-cell transcriptional states during seed germination. Nat. Plants10, 1418–1434. 10.1038/s41477-024-01771-3
66
Lin T. Lashbrook C. C. Cho S. K. Butler N. M. Sharma P. Muppirala U. et al (2015). Transcriptional analysis of phloem-associated cells of potato. BMC Genomics16, 665. 10.1186/s12864-015-1844-2
67
Liu D. Ellison E. E. Myers E. A. Donahue L. I. Xuan S. Swanson R. et al (2024). Heritable gene editing in tomato through viral delivery of isopentenyl transferase and single-guide RNAs to latent axillary meristematic cells. Proc. Natl. Acad. Sci. U.S.A.121, e2406486121. 10.1073/pnas.2406486121
68
Liu G. Wu Z. Luo J. Wang C. Shang X. Zhang G. (2023). Genes expression profiles in vascular cambium of Eucalyptus urophylla × Eucalyptus grandis at different ages. BMC Plant Biol.23, 500. 10.1186/s12870-023-04500-8
69
Liu H. Hu D. Du P. Wang L. Liang X. Li H. et al (2021). Single‐cell RNA‐seq describes the transcriptome landscape and identifies critical transcription factors in the leaf blade of the allotetraploid peanut (Arachis hypogaea L.). Plant Biotechnol. J.19, 2261–2276. 10.1111/pbi.13656
70
Liu K. Feng S. Pan Y. Zhong J. Chen Y. Yuan C. et al (2016). Transcriptome analysis and identification of genes associated with floral transition and flower development in sugar apple (Annona squamosa L.). Front. Plant Sci.7, 1695. 10.3389/fpls.2016.01695
71
Liu L. Yin M. Lin G. Wang Q. Zhou P. Dai S. et al (2021). Integrating RNA-seq with functional expression to analyze the regulation and characterization of genes involved in monoterpenoid biosynthesis in Nepeta tenuifolia Briq. Plant Physiol. Biochem.167, 31–41. 10.1016/j.plaphy.2021.07.026
72
Liu R. Lu J. Xing J. Du M. Wang M. Zhang L. et al (2021). Transcriptome and metabolome analyses revealing the potential mechanism of seed germination in Polygonatum cyrtonema. Sci. Rep.11, 12161. 10.1038/s41598-021-91598-1
73
Logan D. C. (2006). Plant mitochondrial dynamics. Biochim. Biophys. Acta (BBA) - Mol. Cell Res.1763, 430–441. 10.1016/j.bbamcr.2006.01.003
74
Lu L. Liu P. Yang Y. Zhang Y. Wang C. Feng J. et al (2020). Transcriptome analysis of Curcuma wenyujin from Haikou and Wenzhou, and a comparison of the main constituents and related genes of Rhizoma Curcumae. PLoS ONE15, e0242776. 10.1371/journal.pone.0242776
75
Ma K. Luo X. Han L. Zhao Y. Mamat A. Li N. et al (2021). Transcriptome profiling based on Illumina- and SMRT-based RNA-seq reveals circadian regulation of key pathways in flower bud development in walnut. PLoS ONE16, e0260017. 10.1371/journal.pone.0260017
76
Ma X. Tang K. Tang Z. Dong A. Xiao H. Meng Y. et al (2022). An organ‐specific transcriptomic atlas of the medicinal plant Bletilla striata: protein‐coding genes, microRNAs, and regulatory networks. Plant Genome15, e20210. 10.1002/tpg2.20210
77
Margulis L. (1970). Origin of eukaryotic cells: evidence and research implications for a theory of the origin and evolution of microbial, plant, and animal cells on the Precambrian earth. New Haven: Yale University Press.
78
Martín-Gómez J. J. Gutiérrez del Pozo D. Ucchesu M. Bacchetta G. Cabello Sáenz de Santamaría F. Tocino Á. et al (2020). Seed morphology in the Vitaceae based on geometric models. Agronomy10, 739. 10.3390/agronomy10050739
79
Mason P. J. Furtado A. Marquardt A. Hodgson-Kratky K. Hoang N. V. Botha F. C. et al (2020). Variation in sugarcane biomass composition and enzymatic saccharification of leaves, internodes and roots. Biotechnol. Biofuels13, 201. 10.1186/s13068-020-01837-2
80
Mason P. J. Hoang N. V. Botha F. C. Furtado A. Marquardt A. Henry R. J. (2022). Comparison of the root, leaf and internode transcriptomes in sugarcane (Saccharum spp. hybrids). Curr. Res. Biotechnol.4, 167–178. 10.1016/j.crbiot.2022.02.005
81
McFadden G. I. (2001). Primary and secondary endosymbiosis and the origin of plastids. J. Phycol.37, 951–959. 10.1046/j.1529-8817.2001.01126.x
82
Melzer S. Lens F. Gennen J. Vanneste S. Rohde A. Beeckman T. (2008). Flowering-time genes modulate meristem determinacy and growth form in Arabidopsis thaliana. Nat. Genet.40, 1489–1492. 10.1038/ng.253
83
Meng Y. Yu D. Xue J. Lu J. Feng S. Shen C. et al (2016). A transcriptome-wide, organ-specific regulatory map of Dendrobium officinale, an important traditional Chinese orchid herb. Sci. Rep.6, 18864. 10.1038/srep18864
84
Mishima K. Fujiwara T. Iki T. Kuroda K. Yamashita K. Tamura M. et al (2014). Transcriptome sequencing and profiling of expressed genes in cambial zone and differentiating xylem of Japanese cedar (Cryptomeria japonica). BMC Genomics15, 5920. 10.1186/1471-2164-15-219
85
Morris J. L. Puttick M. N. Clark J. W. Edwards D. Kenrick P. Pressel S. et al (2018). The timescale of early land plant evolution. Proc. Natl. Acad. Sci. U.S.A.115, E2274-E2283. 10.1073/pnas.1719588115
86
Mutasa-Gottgens E. Hedden P. (2009). Gibberellin as a factor in floral regulatory networks. J. Exp. Bot.60, 1979–1989. 10.1093/jxb/erp040
87
Narsai R. Gouil Q. Secco D. Srivastava A. Karpievitch Y. V. Liew L. C. et al (2017). Extensive transcriptomic and epigenomic remodelling occurs during Arabidopsis thaliana germination. Genome Biol.18, 172. 10.1186/s13059-017-1302-3
88
Nelson T. Tausta S. L. Gandotra N. Liu T. (2006). Laser microdissection of plant tissue: What you see is what you get. Annu. Rev. Plant Biol.57, 181–201. 10.1146/annurev.arplant.56.032604.144138
89
Ni Y. Chen H. Liu D. Zeng L. Chen P. Liu C. (2021). Discovery of genes involved in anthocyanin biosynthesis from the rind and pith of three sugarcane varieties using integrated metabolic profiling and RNA-seq analysis. BMC Plant Biol.21, 214. 10.1186/s12870-021-02986-8
90
Paul P. Dhatt B. K. Miller M. Folsom J. J. Wang Z. Krassovskaya I. et al (2020). MADS78 and MADS79 are essential regulators of early seed development in rice. Plant Physiol.182, 933–948. 10.1104/pp.19.00917
91
Peirats-Llobet M. Yi C. Liew L. C. Berkowitz O. Narsai R. Lewsey M. G. et al (2023). Spatially resolved transcriptomic analysis of the germinating barley grain. Nucleic Acids Res.51, 7798–7819. 10.1093/nar/gkad521
92
Pellicer J. Hidalgo O. Dodsworth S. Leitch I. (2018). Genome size diversity and its impact on the evolution of land plants. Genes9, 88. 10.3390/genes9020088
93
Picard C. L. Povilus R. A. Williams B. P. Gehring M. (2021). Transcriptional and imprinting complexity in Arabidopsis seeds at single-nucleus resolution. Nat. Plants7, 730–738. 10.1038/s41477-021-00922-0
94
Provan J. Powell W. Hollingsworth P. M. (2001). Chloroplast microsatellites: New tools for studies in plant ecology and evolution. Trends Ecol. and Evol.16, 142–147. 10.1016/S0169-5347(00)02097-8
95
Prudencio Á. S. Hoeberichts F. A. Dicenta F. Martínez-Gómez P. Sánchez-Pérez R. (2021). Identification of early and late flowering time candidate genes in endodormant and ecodormant almond flower buds. Tree Physiol.41, 589–605. 10.1093/treephys/tpaa151
96
Qiao Y. Wu L. Yang S. Wang Q. Gu H. Wei L. et al (2023). Metabolomic and transcriptomic analyses provide insights into variations in flavonoids contents between two Artemisia cultivars. BMC Plant Biol.23, 288. 10.1186/s12870-023-04295-8
97
Rao K. Liu S. Tang X. Jia G. Yang S. Ren C. et al (2025). Integrative analysis of metabolite and transcriptome reveals the biosynthetic pathway and candidate genes for iridoid glycoside biosynthesis in Neopicrorhiza scrophulariiflora (Pennell) D.Y.Hong. Front. Plant Sci.16, 1527477. 10.3389/fpls.2025.1527477
98
Roberts W. R. Roalson E. H. (2017). Comparative transcriptome analyses of flower development in four species of Achimenes (Gesneriaceae). BMC Genomics18, 240. 10.1186/s12864-017-3623-8
99
Rockwell N. C. Lagarias J. C. Bhattacharya D. (2014). Primary endosymbiosis and the evolution of light and oxygen sensing in photosynthetic eukaryotes. Front. Ecol. Evol.2. 10.3389/fevo.2014.00066
100
Roszak P. Heo J. Blob B. Toyokura K. Sugiyama Y. De Luis Balaguer M. A. et al (2021). Cell-by-cell dissection of phloem development links a maturation gradient to cell specialization. Science374, eaba5531. 10.1126/science.aba5531
101
Roy N. S. Kim J.-A. Choi A.-Y. Ban Y.-W. Park N.-I. Park K.-C. et al (2018). RNA-seq de novo assembly and differential transcriptome analysis of Korean medicinal herb Cirsium japonicum var. spinossimum. Genomics Inf.16, e34. 10.5808/GI.2018.16.4.e34
102
Rurek M. (2016). Participation of non-coding RNAs in plant organelle biogenesis. Acta Biochim. Pol.63, 653–663. 10.18388/abp.2016_1346
103
Rurek M. Smolibowski M. (2024). Variability of plant transcriptomic responses under stress acclimation: A review from high throughput studies. Acta Biochim. Pol.71, 13585. 10.3389/abp.2024.13585
104
Růžička K. Ursache R. Hejátko J. Helariutta Y. (2015). Xylem development-from the cradle to the grave. New Phytol.207, 519–535. 10.1111/nph.13383
105
Sánchez-Cabrera M. Jiménez-López F. J. Narbona E. Arista M. Ortiz P. L. Romero-Campero F. J. et al (2021). Changes at a critical branchpoint in the anthocyanin biosynthetic pathway underlie the blue to orange flower color transition in Lysimachia arvensis. Front. Plant Sci.12, 633979. 10.3389/fpls.2021.633979
106
Schmidt-Rohr K. (2021). O2 and other high-energy molecules in photosynthesis: Why plants need two photosystems. Life11, 1191. 10.3390/life11111191
107
Schneider G. F. Dekker C. (2012). DNA sequencing with nanopores. Nat. Biotechnol.30, 326–328. 10.1038/nbt.2181
108
Segura M. García A. Benítez Á. Martínez C. Jamilena M. (2023). Comparative RNA-seq analysis between monoecious and androecious plants reveals regulatory mechanisms controlling female flowering in Cucurbita pepo. Int. J. Mol. Sci.24, 17195. 10.3390/ijms242417195
109
Shen C. Guo H. Chen H. Shi Y. Meng Y. Lu J. et al (2017). Identification and analysis of genes associated with the synthesis of bioactive constituents in Dendrobium officinale using RNA-Seq. Sci. Rep.7, 187. 10.1038/s41598-017-00292-8
110
Sheng Y. Gao H. Yu C. Huang G. Wang K. Wang K. et al (2025). The developmental transcriptome dynamics of current-year shoot utilized as scion in Camellia chekiangoleosa. BMC Plant Biol.25, 712. 10.1186/s12870-025-06715-3
111
Shi Z. Han X. Wang G. Qiu J. Zhou L. Chen S. et al (2022). Transcriptome analysis reveals chrysanthemum flower discoloration under high-temperature stress. Front. Plant Sci.13, 1003635. 10.3389/fpls.2022.1003635
112
Shulse C. N. Cole B. J. Ciobanu D. Lin J. Yoshinaga Y. Gouran M. et al (2019). High-throughput single-cell transcriptome profiling of plant cell types. Cell Rep.27, 2241–2247.e4. 10.1016/j.celrep.2019.04.054
113
Singh P. Singh G. Bhandawat A. Singh G. Parmar R. Seth R. et al (2017). Spatial transcriptome analysis provides insights of key gene(s) involved in steroidal saponin biosynthesis in medicinally important herb Trillium govanianum. Sci. Rep.7, 45295. 10.1038/srep45295
114
Sloan D. B. Alverson A. J. Chuckalovcak J. P. Wu M. McCauley D. E. Palmer J. D. et al (2012a). Rapid evolution of enormous, multichromosomal genomes in flowering plant mitochondria with exceptionally high mutation rates. PLoS Biol.10, e1001241. 10.1371/journal.pbio.1001241
115
Sloan D. B. Keller S. R. Berardi A. E. Sanderson B. J. Karpovich J. F. Taylor D. R. (2012b). De novo transcriptome assembly and polymorphism detection in the flowering plant Silene vulgaris (Caryophyllaceae). Mol. Ecol. Resour.12, 33–343. 10.1111/j.1755-0998.2011.03079.x
116
Somaletha Chandran K. Humphries J. Goodger J. Q. D. Woodrow I. E. (2022). Molecular characterisation of flavanone O-methylation in Eucalyptus. Int. J. Mol. Sci.23, 3190. 10.3390/ijms23063190
117
Sriboon S. Li H. Guo C. Senkhamwong T. Dai C. Liu K. (2020). Knock-out of TERMINAL FLOWER 1 genes altered flowering time and plant architecture in Brassica napus. BMC Genet.21, 52. 10.1186/s12863-020-00857-z
118
Srikanth A. Schmid M. (2011). Regulation of flowering time: All roads lead to Rome. Cell. Mol. Life Sci.68, 2013–2037. 10.1007/s00018-011-0673-y
119
Tasaki K. Watanabe A. Nemoto K. Takahashi S. Goto F. Sasaki N. et al (2022). Identification of candidate genes responsible for flower colour intensity in Gentiana triflora. Front. Plant Sci.13, 906879. 10.3389/fpls.2022.906879
120
Tenorio Berrío R. Verstaen K. Vandamme N. Pevernagie J. Achon I. Van Duyse J. et al (2022). Single-cell transcriptomics sheds light on the identity and metabolism of developing leaf cells. Plant Physiol.188, 898–918. 10.1093/plphys/kiab489
121
Tyagi P. Singh D. Mathur S. Singh A. Ranjan R. (2022). Upcoming progress of transcriptomics studies on plants: an overview. Front. Plant Sci.13, 1030890. 10.3389/fpls.2022.1030890
122
Wang C. Peng D. Zhu J. Zhao D. Shi Y. Zhang S. et al (2019). Transcriptome analysis of Polygonatum cyrtonema hua: identification of genes involved in polysaccharide biosynthesis. Plant Methods15, 65. 10.1186/s13007-019-0441-9
123
Wang H.-Z. Dixon R. A. (2012). On-off switches for secondary cell wall biosynthesis. Mol. Plant5, 297–303. 10.1093/mp/ssr098
124
Wang X. Li N. Li W. Gao X. Cha M. Qin L. et al (2020). Advances in transcriptomics in the response to stress in plants. Glob. Med. Genet.07, 030–034. 10.1055/s-0040-1714414
125
Wang Y. Huan Q. Li K. Qian W. (2021). Single-cell transcriptome atlas of the leaf and root of rice seedlings. J. Genet. Genom.48, 881–898. 10.1016/j.jgg.2021.06.001
126
Wang Y. Luo Y. Guo X. Li Y. Yan J. Shao W. et al (2024b). A spatial transcriptome map of the developing maize ear. Nat. Plants10, 815–827. 10.1038/s41477-024-01683-2
127
Wang Y. Meng Z. Liang C. Meng Z. Wang Y. Sun G. et al (2017). Increased lateral root formation by CRISPR/Cas9-mediated editing of arginase genes in cotton. Sci. China Life Sci.60, 524–527. 10.1007/s11427-017-9031-y
128
Wang Y. Tian C. Na Q. Zhu C. Cao H. Zhang M. et al (2024a). The role of SlCHRC in carotenoid biosynthesis and plastid development in tomato fruit. Int. J. Biol. Macromol.281, 136354. 10.1016/j.ijbiomac.2024.136354
129
Weber H. (2002). Fatty acid-derived signals in plants. Trends Plant Sci.7, 217–224. 10.1016/S1360-1385(02)02250-1
130
Xanthopoulou A. Montero-Pau J. Picó B. Boumpas P. Tsaliki E. Paris H. S. et al (2021). A comprehensive RNA-Seq-based gene expression atlas of the summer squash (Cucurbita pepo) provides insights into fruit morphology and ripening mechanisms. BMC Genomics22, 341. 10.1186/s12864-021-07683-2
131
Yan J. Qian L. Zhu W. Qiu J. Lu Q. Wang X. et al (2020). Integrated analysis of the transcriptome and metabolome of purple and green leaves of Tetrastigma hemsleyanum reveals gene expression patterns involved in anthocyanin biosynthesis. PLoS ONE15, e0230154. 10.1371/journal.pone.0230154
132
Yang H. Wang Y. Tian Y. Teng X. Lv Z. Lei J. et al (2023). Rice FLOURY ENDOSPERM22, encoding a pentatricopeptide repeat protein, is involved in both mitochondrial RNA splicing and editing and is crucial for endosperm development. J. Integr. Plant Biol.65, 755–771. 10.1111/jipb.13402
133
Yao J. Chu Q. Guo X. Shao W. Shang N. Luo K. et al (2024). Spatiotemporal transcriptomic landscape of rice embryonic cells during seed germination. Dev. Cell59, 2320–2332.e5. 10.1016/j.devcel.2024.05.016
134
Yoshida A. Taoka K. Hosaka A. Tanaka K. Kobayashi H. Muranaka T. et al (2021). Characterization of frond and flower development and identification of FT and FD genes from duckweed Lemna aequinoctialis Nd. Front. Plant Sci.12, 697206. 10.3389/fpls.2021.697206
135
Yu B. Pan Y. Liu Y. Chen Q. Guo X. Tang Z. (2021). A comprehensive analysis of transcriptome and phenolic compound profiles suggests the role of flavonoids in cotyledon greening in Catharanthus roseus seedling. Plant Physiol. Biochem.167, 185–197. 10.1016/j.plaphy.2021.07.028
136
Yuan Y. Zhang J. Kallman J. Liu X. Meng M. Lin J. (2019). Polysaccharide biosynthetic pathway profiling and putative gene mining of Dendrobium moniliforme using RNA-Seq in different tissues. BMC Plant Biol.19, 521. 10.1186/s12870-019-2138-7
137
Zhang H. (2019). The review of transcriptome sequencing: Principles, history and advances. IOP Conf. Ser. Earth Environ. Sci.332, 042003. 10.1088/1755-1315/332/4/042003
138
Zhang J. Tang Y. Pu X. Qiu X. Wang J. Li T. et al (2022). Genetic and transcriptomic dissection of an artificially induced paired spikelets mutant of wheat (Triticum aestivum L.). Theor. Appl. Genet.135, 2543–2554. 10.1007/s00122-022-04137-5
139
Zhao Y. Xu W. Zhang Y. Sun S. Wang L. Zhong S. et al (2021). PPR647 protein is required for chloroplast RNA editing, splicing and chloroplast development in maize. Int. J. Mol. Sci.22, 11162. 10.3390/ijms222011162
140
Zheng L. Zhou C. Li T. Yuan Z. Zhou H. Tamada Y. et al (2021). Global transcriptome analysis reveals dynamic gene expression profiling and provides insights into biosynthesis of resveratrol and anthraquinones in a medicinal plant Polygonum cuspidatum. Ind. Crops Prod.171, 113919. 10.1016/j.indcrop.2021.113919
141
Zhong X. Chen D. Cui J. Li H. Huang Y. Kang J. (2021). Comparative analysis of the complete mitochondrial genome sequences and anther development cytology between maintainer and Ogura-type cytoplasm male-sterile cabbage (B. oleracea Var. capitata). BMC Genomics22, 646. 10.1186/s12864-021-07963-x
142
Zhou H. Song X. Lu M.-Z. (2024). Growth-regulating factor 15-mediated vascular cambium differentiation positively regulates wood formation in hybrid poplar (Populus alba × P. glandulosa). Front. Plant Sci.15, 1343312. 10.3389/fpls.2024.1343312
143
Zhou P. Pu T. Gui C. Zhang X. Gong L. (2020). Transcriptome analysis reveals biosynthesis of important bioactive constituents and mechanism of stem formation of Dendrobium huoshanense. Sci. Rep.10, 2857. 10.1038/s41598-020-59737-2
144
Zhu M. Taylor I. W. Benfey P. N. (2022). Single-cell genomics revolutionizes plant development studies across scales. Development149, dev200179. 10.1242/dev.200179
Summary
Keywords
medicinal plant species, plant organs, plant transcriptome, RNA-seq, secondary metabolites
Citation
Rurek M (2025) Diversity of organ-specific plant transcriptomes. Acta Biochim. Pol. 72:14609. doi: 10.3389/abp.2025.14609
Received
11 March 2025
Accepted
26 June 2025
Published
16 July 2025
Volume
72 - 2025
Edited by
Grzegorz Wegrzyn, University of Gdansk, Poland
Reviewed by
Grzegorz Wegrzyn, University of Gdansk, Poland
Shiv Rattan, Agricultural Research Organization, Volcani Center, Israel
Updates
Copyright
© 2025 Rurek.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michał Rurek, rurek@amu.edu.pl
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.