Abstract
The role of pre-transplant hypoalbuminemia and its impact on post-transplant outcomes in patients undergoing simultaneous pancreas-kidney (SPK) transplantation remains unclear. We analyzed all SPK recipients at our center, who had at least 2 weeks of pancreas and kidney graft survival and had serum albumin measured within 45 days pre-transplant. Recipients were categorized based on pretransplant albumin level as normal (≥4.0 g/dL, N = 222, 42%), mild hypoalbuminemia (≥3.5–<4.0 g/dL, N = 190, 36%), and moderate hypoalbuminemia (<3.5 g/dL, N = 120, 23%). Kidney delayed graft function (DGF), length of stay (LOS) after transplant, re-hospitalization within 30 days after discharge, and need for a return to the operating room (OR) related to transplant surgical complications, acute rejection, and uncensored and death-censored graft failure, within the first years post-transplant were outcomes of interest. A total of 532 SPK recipients were included. Mild or moderate hypoalbuminemia was not associated with DGF, LOS, re-hospitalization, or return to the OR in unadjusted or adjusted analyses. Similarly, mild or moderate hypoalbuminemia was not associated with a risk of graft rejection or graft failure. Among SPK recipients, pre-transplant hypoalbuminemia was not associated with worse outcomes and should not be the determining factor in selecting patients for SPK transplant.
Introduction
Albumin is the most abundant plasma protein in the human body and has many functions, including maintaining plasma colloid osmotic pressure, acting as a transport protein, buffering antioxidants, and having anticoagulant effects [1]. Hepatic synthesis of albumin depends on nutritional status and insulin stimulation; it can be decreased due to inflammatory cytokines, such as IL-6 and tumor necrosis factor-alpha [1, 2].
Hypoalbuminemia is multifactorial and results in increased capillary permeability and leakage. It may be related to the release of inflammatory cytokines, decreased nutritional intake, increased excretion, and altered rate of synthesis and catabolism of albumin [1–3]. Hypoalbuminemia is commonly seen in critically ill patients and is often associated with worse outcomes for a variety of medical and surgical conditions [4–9]. Hypoalbuminemia is associated with increased cardiovascular mortality as well as all-cause mortality in patients with end-stage renal disease (ESRD) undergoing hemodialysis [4]. Additionally, pre-operative hypoalbuminemia is associated with increased surgical morbidity, mortality, and increased risk of surgical complications [5–7]. In kidney-only transplantation, patients with pre-transplant hypoalbuminemia have an increased risk of BK and cytomegalovirus (CMV) infection [10]. However, pre-transplant hypoalbuminemia in kidney-only transplant recipients is not associated with an increased rate of re-admission, reoperation, or delayed graft function [11].
Simultaneous pancreas-kidney transplant (SPK) is a form of treatment for those with diabetes mellitus and ESRD as it restores euglycemia and slows the progression of diabetes complications [12, 13]. In these patients, hypoalbuminemia has been proposed to be caused by chronic systemic inflammation and reduced synthesis of albumin [14]. Additionally, post-operative hypoalbuminemia in SPK recipients is associated with an increased rate of CMV virus infection, graft loss, and a trend toward decreased survival [15]. However, the relationship between the severity of pre-transplant hypoalbuminemia and post-transplant outcomes among SPK recipients has not been thoroughly studied. We hypothesize that pre-transplant hypoalbuminemia is associated with an increased rate of postoperative complications in SPK recipients.
Materials and Methods
Study Population and Design
This single-center study from the University of Wisconsin-Madison included all adult SPK transplant recipients performed between 01/01/2001 and 12/31/2022. We excluded all recipients whose pancreas or kidney graft failed within 2 weeks of transplant, multiorgan transplant recipients (i.e., simultaneous liver - pancreas), or pancreas after kidney transplant recipients. In subgroup analysis, we analyzed SPK recipients even with graft failure within 2 weeks of transplant. This study was approved by the University of Wisconsin Institutional Review Board (IRB protocol number: 2014-1072). This study followed the Declaration of Helsinki. The clinical and research activities being reported were consistent with the Principles of the Declaration of Istanbul as outlined in “The Declaration of Istanbul on Organ Trafficking and Transplant Tourism.”
Only recipients with serum albumin levels measured within 45 days before transplant were included. In patients with multiple albumin levels measured within 45 days before transplant, we used the measurement closest to the transplant date. SPK recipients without albumin levels within the 45-day timeframe before transplant were excluded. Recipients were categorized based on pretransplant albumin levels as normal (≥4.0 g/dL), mild hypoalbuminemia (≥3.5–< 4.0 g/dL), and moderate hypoalbuminemia (<3.5 g/dL). Although, we initially planned to categorize the severe hypoalbuminemia group defined as <3.0 g/dL, this was not possible due to the extremely small sample size in this group with limited outcomes of interest, so for this reason they were included in the moderate hypoalbuminemia group. Pre or peri-transplant albumin transfusion to correct hypoalbuminemia has never been the current or past practice at our center, although few recipients may have received albumin infusion intraoperative or post-operative for various other indications. At no time during this study period or currently in our program was there a protocolized criterion for pretransplant serum albumin level to approve or disapprove SPK transplant eligibility.
Length of stay (LOS) after index transplant, kidney delayed graft function (DGF), and re-hospitalization within 30 days after discharge were perioperative outcomes of interest followed by the need for a return to the operating room (OR) related to transplant surgical complications, acute rejection of either grafts and uncensored and death-censored graft failure, within the 12 months post-transplant were short-term outcomes of interest. We limited outcomes to the first 12 months post-transplant to better correlate pre-transplant serum albumin levels and early post-transplant outcomes.
Variables and Definitions
Donor characteristics such as age, sex, race, body mass index (BMI), death due to cardiovascular causes, terminal serum creatinine, kidney donor profile index (KPDI), donation after circulatory death (DCD), pancreas cold ischemia time (CIT), and kidney CIT were reported. Information on recipients included age, sex, race, BMI, diabetes type, induction therapy, pre-emptive transplant, and early steroid withdrawal. Immunologic factors reported were panel reactive antibody (cPRA) > 20%, average Human Leukocyte Antigen (HLA) mismatch (of 6), and whether primary or prior transplant.
DGF was defined as a need for dialysis within one-week post-transplantation. Re-hospitalizations within 30 days post initial discharge and any unanticipated return to the operating room within 12 months post-SPK were included. Return to the operating/procedure room for an anticipated ureteric stent removal was not considered a return to the OR. All episodes of rejections were biopsy-proven within 12 months of transplant. Kidney uncensored graft failure was defined as all causes of graft failure including death, while death-censored graft failure (DCGF) was defined as a return to dialysis or re-transplantation, all within 2 weeks–12 months post-transplant. Similarly, pancreas uncensored graft failure was defined as all causes of pancreas graft failure including death. And pancreas DCGF was defined based on the current United Network for Organ Sharing criteria for pancreas graft failure, which include removal of the pancreas graft, re-registration for a pancreas transplant, registration for an islet transplant after receiving pancreas, or an insulin requirement that is ≥0.5 units/kg/day for 90 consecutive days [16, 17].
Serum Albumin Measurement
At our institution, serum albumin concentration measurements are conducted utilizing bromocresol assays. Before 2008, albumin was measured using bromocresol green assays. Since 2008, our institution has used bromocresol purple to assess serum albumin concentrations.
Reference ranges were adjusted to reflect the methodology of each test; prior institutional comparison of both assays on patient samples showed an average negative 0.56-g/dL bias in the newer bromocresol purple assay. This difference was considered to be within allowable limits for general clinical use.
Immunosuppressive Protocols
Our center-specific induction immunosuppression therapy was consistent throughout the study period, either a depleting agent (alemtuzumab or anti-thymocyte globulin) or a non-depleting agent (basiliximab) was utilized. Triple immunosuppression with tacrolimus, mycophenolic acid and prednisone taper was standard for all recipients. Few had early steroid withdrawal based on the immunological risk and patient request as previously described [18].
Biopsy and Rejection Protocols
The two most common indications for kidney biopsy were an unexplained rise in creatinine and proteinuria or the development of denovo DSA against HLA as described before [19]. Similarly, the common indications for pancreas biopsy were an unexplained rise in pancreatic enzymes, development of denovo DSA, and unexplained hyperglycemia [20]. If possible, we attempt to perform both graft biopsies in the setting of dual graft dysfunction [20].
Similarly, the management of rejections was based on the severity and proximity from the transplant to the diagnosis of rejection as described before [19, 20]. Briefly, kidney T cell-mediated rejection (TCMR) was treated with steroid pulse plus/minus anti-thymocyte globulin. And antibody-mediated rejection (AMR) was treated with steroid pulse, intravenous immunoglobulin (IVIG), plus/minus rituximab, plus/minus plasmapheresis. Treatment of pancreas rejection was based on the type and severity of rejection and was graded by the Banff criteria. TCMR was treated with IV steroid pulse with or without anti-thymoglobulin 6–12 mg/kg in 4–10 divided doses, while mixed rejection was treated with steroids, anti-thymoglobulin, IVIG, and plasmapheresis. Early AMR was treated with steroids, IVIG, and plasmaphereis [20].
Statistical Analysis
Continuous data were compared using Student’s t-test or the Wilcoxon rank-sum test, where appropriate. Categorical data were analyzed using Fisher’s exact test or chi-square test. p-Values ≤ 0.05 were considered statistically significant. Multivariable logistic regression and Cox proportional hazard models were used to analyze associations of the pre-transplant serum albumin levels with various outcomes of interest. Variables considered to be associated with outcomes of interest from baseline characteristics were included in adjusted models. Outcomes of interest were also analyzed by Kaplan-Meier survival analysis. Analyses were performed in Stata SE 18.01.
Results
774 SPK recipients were transplanted during the study period, 23 were excluded due to early graft failure or early patient death all within 2 weeks of transplant, and 219 did not have serum albumin levels measured during the timeframe and were excluded from the study. With this, a total of 532 SPK recipients fulfilled our selection criteria. The details of recipient and donor baseline characteristics are summarized in Table 1. Recipient’s and donor’s characters differed across albumin categories in multiple variables, including the donor’s cause of death, the proportion of DCD donors, pancreas cold ischemia time, the proportion of previous transplant recipients, recipient’s sex, types of diabetes, induction immunosuppression and proportion of pre-emptive transplant recipients.
TABLE 1
| Variables | Overall (N = 532) | <3.5 (n = 120) | ≥3.5–<4.0 (n = 190) | ≥4.0 (n = 222) | P | |
|---|---|---|---|---|---|---|
| Donor Factors | Mean age (yrs) | 28.0 (12.2) | 27.7 (12.6) | 26.3 (11.0) | 29.7 (12.8) | 0.06 |
| Female (%) | 38.0 | 40.8 | 33.7 | 40.1 | 0.88 | |
| Non-white (%) | 13.2 | 12.5 | 13.7 | 13.1 | 0.92 | |
| Mean body mass index (kg/m2) | 23.7 (4.3) | 23.8 (4.0) | 23.8 (4.2) | 24.1 (4.5) | 0.35 | |
| Cause of death: Cardiovascular (%) | 19.0 | 17.5 | 13.2 | 24.8 | 0.01 | |
| Terminal serum Creatinine (mg/dL) | 1.03 (0.89) | 0.90 (0.50) | 1.08 (1.05) | 1.06 (0.91) | 0.15 | |
| Mean kidney donor profile index % | 20.2 (16.5) | 21.3 (17.8) | 17.3 (15.4) | 21.8 (16.2) | 0.79 | |
| Donation after circulatory death (%) | 14.3 | 19.2 | 15.3 | 10.8 | 0.03 | |
| Pancreas Cold ischemia time (hrs) | 14.5 (4.5) | 13.2 (4.2) | 14.5 (4.0) | 15.2 (4.8) | <0.001 | |
| Kidney Cold ischemia time (hrs) | 15.3 (4.4) | 14.8 (4.3) | 15.2 (3.9) | 15.6 (4.9) | 0.14 | |
| Immunologic Factors | cPRA >20% (%) | 14.2 | 11.5 | 17.9 | 12.2 | 0.95 |
| Mean HLA mismatch (of 6) | 4.3 (1.2) | 4.5 (1.2) | 4.3 (1.3) | 4.3 (1.2) | 0.23 | |
| Previous transplant (%) | 16.9 | 6.7 | 18.4 | 21.1 | 0.001 | |
| Recipients Factors | Means pre-transplant serum albumin level | 3.8 (0.6) | 3.0 (0.4) | 3.7 (0.1) | 4.3 (0.3) | <0.001 |
| Mean age (yrs) | 43.2 (9.3) | 43.3 (9.8) | 44.3 (9.7) | 42.2 (8.7) | 0.17 | |
| Female (%) | 39.7 | 47.5 | 41.6 | 33.8 | 0.01 | |
| Non-white (%) | 9.0 | 14.2 | 8.4 | 6.8 | 0.03 | |
| Mean body mass index (kg/m2) | 25.9 (3.9) | 25.2 (3.9) | 26.4 (4.0) | 25.7 (3.9) | 0.48 | |
| Diabetes type | ||||||
| Type I | 89.8 | 85.0 | 57.9 | 94.1 | 0.02 | |
| Type II/Other | 10.2 | 15.0 | 12.1 | 5.9 | ||
| Induction Immunosuppression (%) | ||||||
| Alemtuzumab | 35.5 | 24.2 | 32.1 | 44.6 | 0.003 | |
| Anti-thymocyte globulin | 24.3 | 30.0 | 26.3 | 19.4 | ||
| Basiliximab | 40.2 | 45.8 | 41.6 | 36.0 | ||
| Early steroid withdrawal (%) | 3.6 | 4.2 | 3.7 | 3.2 | 0.62 | |
| Pre-emptive transplant | 44.5 | 28.2 | 45.7 | 52.4 | <0.001 |
Baseline characteristics of participants.
Bold signifies statistical sigificant with p < 0.05.
Kidney Delayed Graft Function
A total of 7.3% of recipients had DGF (Table 2). 4.5% of recipients with normal albumin levels, 9% with mild, and 10% with moderate hypoalbuminemia group experienced DGF. Mild hypoalbuminemia (OR: 2.08; 95% CI: 0.93–4.67; p 0.07) and moderate hypoalbuminemia were not associated with risk of DGF (OR: 2.36; 95% CI: 0.99–5.63; p = 0.05) in an unadjusted model. This was further confirmed after adjustment of multiple variables in mild (OR: 1.69; 95% CI: 0.65–4.37; p = 0.28); moderate (OR: 1.52; 95% CI: 0.55–4.20; p = 0.42) hypoalbuminemia groups (Table 3).
TABLE 2
| Complications | DGF, % | LOS, mean (sd) | Rehospitalization, % | Re-operation, % | |
|---|---|---|---|---|---|
| Pre-Tx albumin (g/dL) | |||||
| ≥4.0 | 4.5 | 11.2 (6.5) | 32.4 | 25.7 | |
| ≥3.5–<4.0 | 9.0 | 10.4 (6.0) | 35.3 | 20.0 | |
| <3.5 | 10.0 | 12.1 (12.0) | 35.8 | 20.8 | |
| Overall | 7.3 | 11.1 (7.9) | 34.2 | 22.6 | |
Complications, DGF, LOS, rehospitalization, Re-operation.
TABLE 3
| Complications | Pre-Tx albumin | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | ||
| DGF | ≥4.0 | Ref | Ref | Ref | Ref | Ref | Ref |
| ≥3.5–<4.0 | 2.08 | 0.93, 4.67 | 0.07 | 1.69 | 0.65, 4.37 | 0.28 | |
| ≥3.0–<3.5 | 2.36 | 0.99, 5.63 | 0.051 | 1.52 | 0.55, 4.20 | 0.42 | |
Delayed graft function (n = 39).
Adjusted for age, sex, race, diabetes type, pre-emptive transplant, induction immunosuppression, pancreas cold time, donor age.
Length of Stay
The mean LOS among the entire cohort after index transplant was 11.1 ± 7.9 days (Table 2). The mean LOS in normal, mild, and moderate hypoalbuminemia recipients was 11.2 ± 6.5 days, 10.4 ± 6.0 days, and 12.1 ± 12.0 days, respectively. Mild hypoalbuminemia was not associated with increased or decreased LOS in unadjusted (OR: −0.78; 95% CI: −2.31, 0.76; p = 0. 32) or adjusted models (OR: −0.94; 95% CI: −2.49, 0.61; p = 0. 24). Moderate hypoalbuminemia was also not associated with LOS in unadjusted (OR: 0.92; 95% CI: −0.84, 2.61; p = 0. 31) or adjusted models (OR: 0.22; 95% CI: −1.61, 2.06; p = 0. 81) (Table 4).
TABLE 4
| Complications | Pre-Tx albumin | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|---|
| Coef | 95% CI | p-value | Coef | 95% CI | p-value | ||
| Length of stay | ≥4.0 | Ref | Ref | Ref | Ref | Ref | Ref |
| ≥3.5–<4.0 | −0.78 | −2.31, 0.76 | 0.32 | −0.94 | −2.49, 0.61 | 0.24 | |
| <3.5 | 0.92 | −0.84, 2.61 | 0.31 | 0.22 | −1.61, 2.06 | 0.81 | |
Length of stay, mean LOS.
Adjusted for age, sex, race, diabetes type, pre-emptive transplant, induction immunosuppression, pancreas cold time, donor age.
Rehospitalization
A total of 34.2% were re-hospitalizations within 30 days after the initial discharge from the index transplant (Table 2). The rate of rehospitalization in normal, mild, and moderate hypoalbuminemia recipients were 32.4%, 35.3%, and 35.8% respectively. Mild hypoalbuminemia was not associated with re-hospitalization rates in unadjusted (OR: 1.13; 95% CI: 0.75–1.71; p = 0.55) or adjusted models (OR: 1.31; 95% CI: 0.86–2.82; p = 0.21). Similarly, moderate hypoalbuminemia was not associated with re-hospitalization rates in unadjusted (OR: 1. 16; 95% CI: 0.73–1.86; p = 0.53) or adjusted models (OR: 1. 34; 95% CI: 0.81–2.23; p = 0. 25) (Table 5). The most common indications for re-hospitalization were gastrointestinal symptoms including, nausea, vomiting, diarrhea, and abdominal pain followed by hypo or hypertension.
TABLE 5
| Complications | Pre-Tx albumin | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | ||
| Re-hospitalization | ≥4.0 | Ref | Ref | Ref | Ref | Ref | Ref |
| ≥3.5–<4.0 | 1.13 | 0.75, 1.71 | 0.55 | 1.31 | 0.86, 2.82 | 0.21 | |
| <3.5 | 1.16 | 0.73, 1.86 | 0.53 | 1.34 | 0.81, 2.23 | 0.25 | |
Re-hospitalization within 30 days after initial discharge (n = 182).
Adjusted for age, sex, race, diabetes type, pre-emptive transplant, induction immunosuppression, pancreas cold time, donor age.
Reoperations Within 2 Weeks–12 Months
A total of 22.6% of recipients returned to the operating room within 2 weeks–12 months post-transplant (Table 2). 25.7% of patients with normal albumin levels, 20% with mild, and 20.8% with moderate hypoalbuminemia were reoperated within the stated timeframe. Mild hypoalbuminemia was not associated with increased reoperation rates in unadjusted (OR: 0.59; 95% CI: 0.29–1.18; p = 0.14) or adjusted models (OR: 0.54; 95% CI: 0.26, 1.12; p = 0.10). Similarly, moderate hypoalbuminemia was not associated with increased reoperation rates in unadjusted (OR: 1.04; 95% CI: 0.52–2.04; p = 0.92) or adjusted models (OR: 0.80; 95% CI: 0.38–1.66; p = 0. 54) (Table 6). This was further confirmed by the Kaplan-Meier survival analysis curve (Figure 1). The most common indications for return to the operating room were intraabdominal fluid collection followed by intrabdominal bleeding.
TABLE 6
| Complications | Pre-Tx albumin | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | ||
| Re-operation | ≥4.0 | Ref | Ref | Ref | Ref | Ref | Ref |
| ≥3.5–<4.0 | 0.59 | 0.29, 1.18 | 0.14 | 0.54 | 0.26, 1.12 | 0.10 | |
| <3.5 | 1.04 | 0.52, 2.04 | 0.92 | 0.80 | 0.38, 1.66 | 0.54 | |
Need to go to OR related to transplant within 12 months (n = 48).
Adjusted for age, sex, race, diabetes type, pre-emptive transplant, induction immunosuppression, pancreas cold time, donor age.
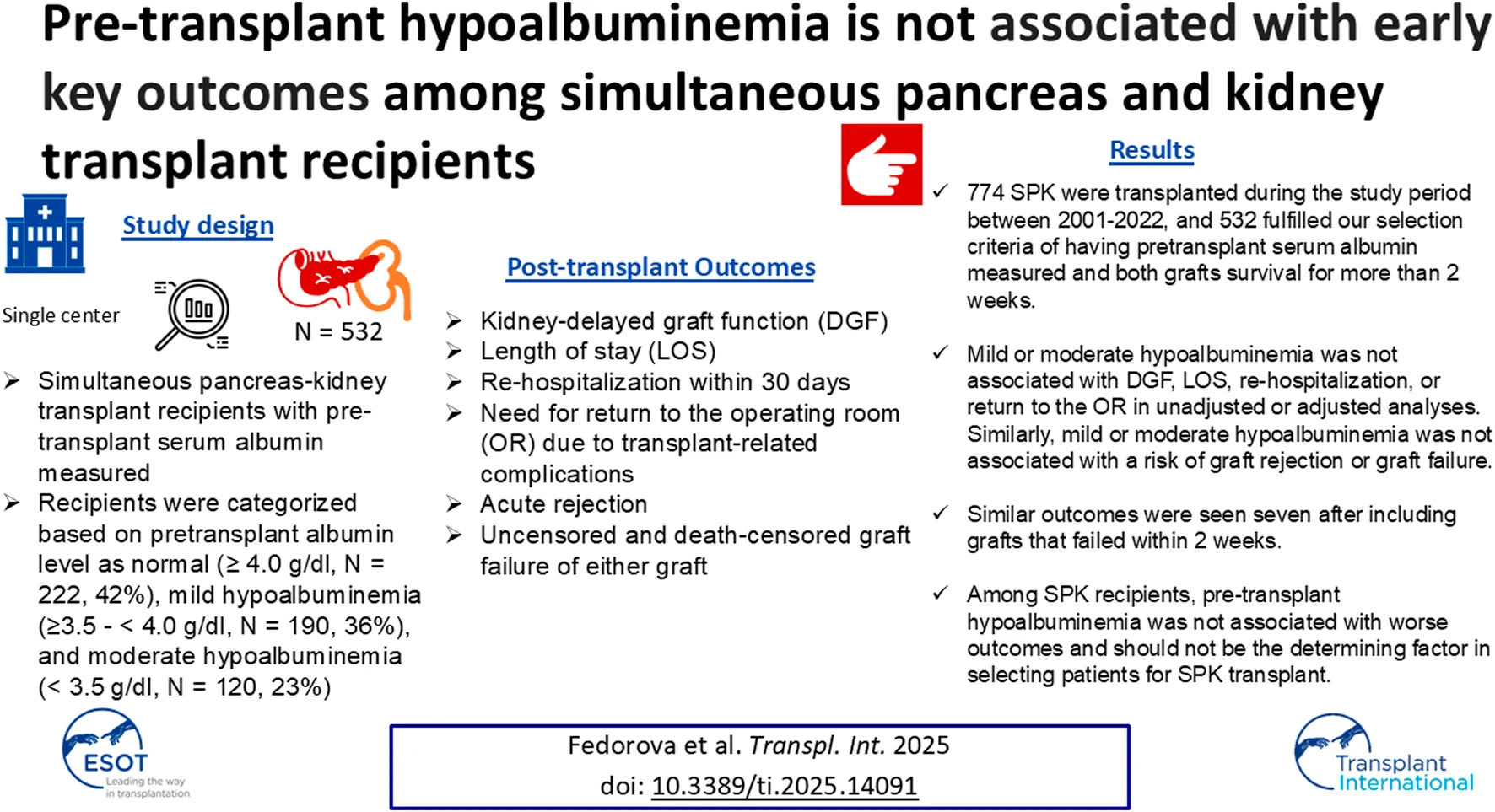
FIGURE 1
No significant differences in the incidence rate ratio of re-operation within 2 weeks–12 months post-transplant by serum albumin levels. The thin solid line represents the normal pre-transplant serum albumin levels group, the thick dashed line represents the mild hypoalbuminemia group and the thick solid line represents the moderate hypoalbuminemia group.
Acute Rejection Within 2 Weeks–12 Months
A total of 19.4% of SPK recipients developed pancreas rejection and 12.2% developed kidney rejection. Pre-transplant hypoalbuminemia, either mild or moderate was not associated with acute rejection in either adjusted or unadjusted models (Table 7). This was further confirmed by the Kaplan-Meier Survival analysis curve (Figures 2, 3).
TABLE 7
| Complications | Pre-Tx albumin | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | ||
| Pancreas acute rejection (n = 103) | ≥4.0 | Ref | Ref | Ref | Ref | Ref | Ref |
| ≥3.5–<4.0 | 1.13 | 0.74, 1.74 | 0.57 | 1.16 | 0.75, 1.71 | 0.51 | |
| <3.5 | 0.86 | 0.51, 1.47 | 0.58 | 0.81 | 0.46, 1.41 | 0.45 | |
| Kidney acute rejection (n = 65) | ≥4.0 | Ref | Ref | Ref | Ref | Ref | Ref |
| ≥3.5–<4.0 | 0.97 | 0.56, 1.70 | 0.92 | 0.98 | 0.55, 1.73 | 0.94 | |
| <3.5 | 1.02 | 0.55, 1.93 | 0.94 | 0.81 | 0.41, 1.59 | 0.54 | |
Acute rejection within 12 months.
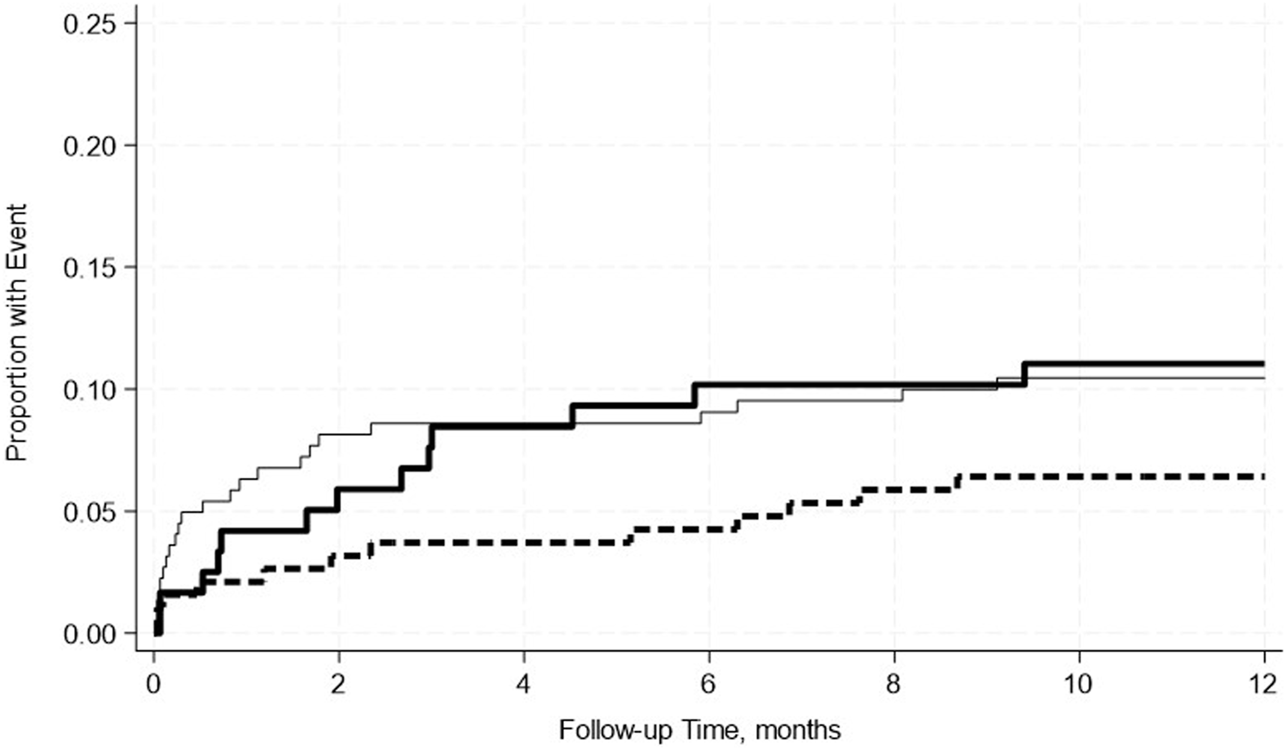
FIGURE 2
No significant differences in the incidence rate ratio of pancreas rejection within 2 weeks–12 months post-transplant by serum albumin levels. The thin solid line represents the normal pre-transplant serum albumin levels group, the thick dashed line represents the mild hypoalbuminemia group and the thick solid line represents the moderate hypoalbuminemia group.
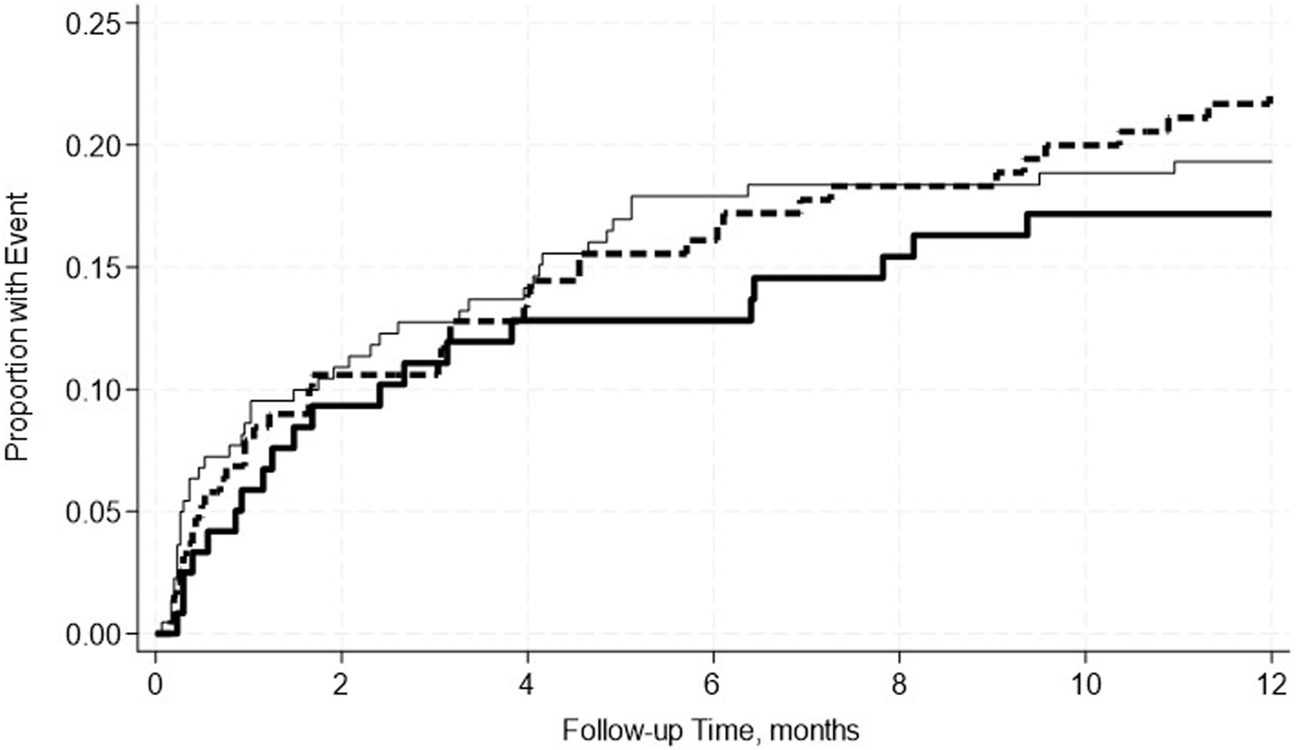
FIGURE 3
No significant differences in the incidence rate ratio of kidney rejection within 2 weeks–12 months post-transplant by serum albumin levels. The thin solid line represents the normal pre-transplant serum albumin levels group, the thick dashed line represents the mild hypoalbuminemia group and the thick solid line represents the moderate hypoalbuminemia group.
Graft Failures Within 2 Weeks–12 Months
A total of 7.3% of SPK recipients experienced uncensored graft failure and 4.7% experienced pancreas DCGF. Similarly, 3.3% experienced kidney uncensored graft failure, and 1.1% experienced kidney DCGF (Table 8). Pre-transplant hypoalbuminemia either mild or moderate was not associated with either uncensored or death-censored graft failure in either adjusted or unadjusted models. This was further confirmed by the Kaplan-Meier Survival analysis curve (Figures 4–7).
TABLE 8
| Complications | Pre-Tx albumin | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | HR | 95% CI | p-value | ||
| Uncensored pancreas graft failure (n = 39) | ≥4.0 | Ref | Ref | Ref | Ref | Ref | Ref |
| ≥3.5–<4.0 | 0.96 | 0.47, 1.94 | 0.90 | 1.10 | 0.53, 2.31 | 0.80 | |
| <3.5 | 0.87 | 0.38, 2.02 | 0.75 | 0.86 | 0.35, 2.11 | 0.75 | |
| Death censored pancreas graft failure (n = 25) | ≥4.0 | Ref | Ref | Ref | Ref | Ref | Ref |
| ≥3.5–<4.0 | 0.77 | 0.32, 1.90 | 0.58 | 0.97 | 0.38, 2.49 | 0.96 | |
| <3.5 | 0.77 | 0.27, 2.19 | 0.63 | 0.79 | 0.26, 2.40 | 0.68 | |
| Uncensored kidney graft failure (n = 18) | ≥4.0 | Ref | Ref | Ref | Ref | Ref | Ref |
| ≥3.5–<4.0 | 1.56 | 0.54, 4.50 | 0.41 | 1.52 | 0.51, 4.57 | 0.45 | |
| <3.5 | 1.24 | 0.35, 4.40 | 0.74 | 1.12 | 0.29, 4.27 | 0.87 | |
| Death censored kidney graft failure (n = 6) | ≥4.0 | Ref | Ref | Ref | Ref | Ref | Ref |
| ≥3.5–<4.0 | 1.17 | 0.24, 5.79 | 0.85 | X | x | X | |
| <3.5 | x | X | x | x | x | X | |
| Death with functioning graft (n = 16) | ≥4.0 | Ref | Ref | Ref | Ref | Ref | Ref |
| ≥3.5–<4.0 | 1.16 | 0.37, 3.59 | 0.80 | 1.20 | 0.37, 3.91 | 0.77 | |
| <3.5 | 1.24 | 0.34, 4.39 | 0.74 | 1.14 | 0.30, 4.35 | 0.84 | |
Graft failure within 12 months.
FIGURE 4
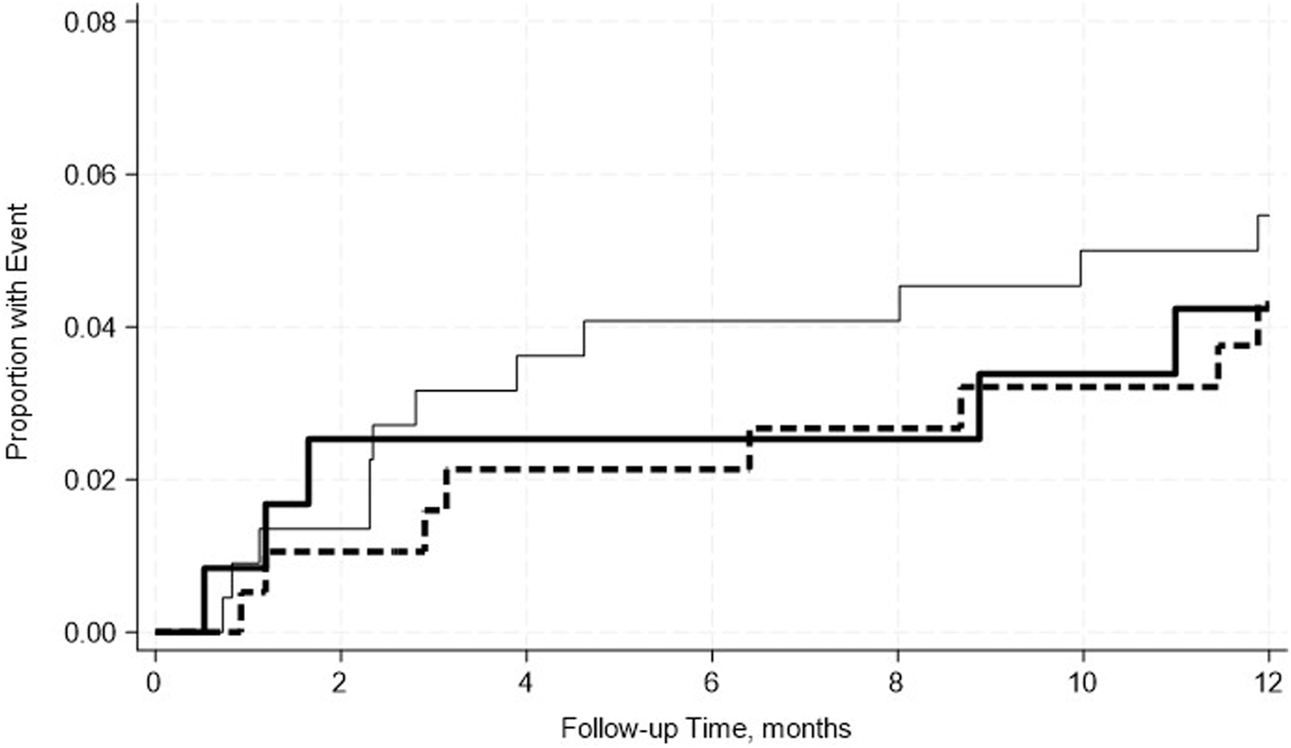
No significant differences in the incidence rate ratio of the pancreas death censored graft failure within 2 weeks–12 months post-transplant by serum albumin levels. The thin solid line represents the normal pre-transplant serum albumin levels group, the thick dashed line represents the mild hypoalbuminemia group, and the thick solid line represents the moderate hypoalbuminemia group.
FIGURE 5
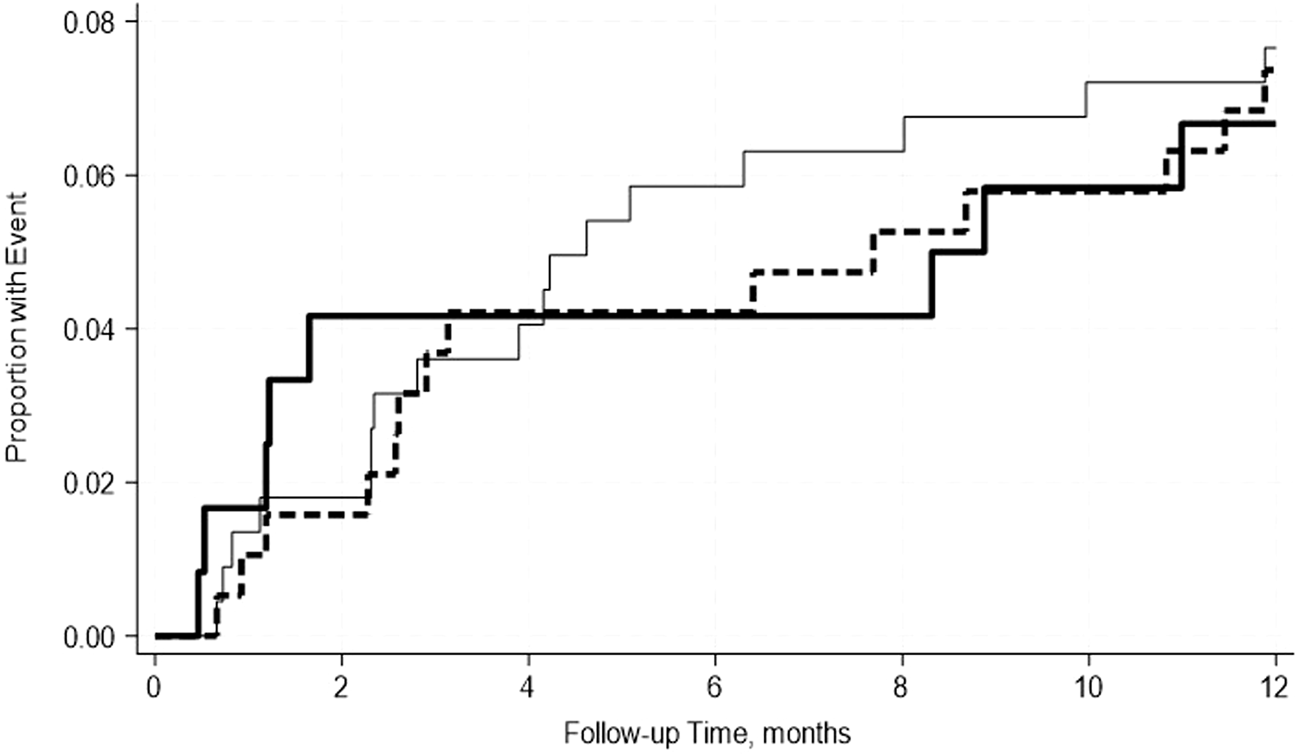
No significant differences in the incidence rate ratio of the pancreas uncensored graft failure within 2 weeks–12 months post-transplant by serum albumin levels. The thin solid line represents the normal pre-transplant serum albumin levels group, the thick dashed line represents the mild hypoalbuminemia group, and the thick solid line represents the moderate hypoalbuminemia group.
FIGURE 6
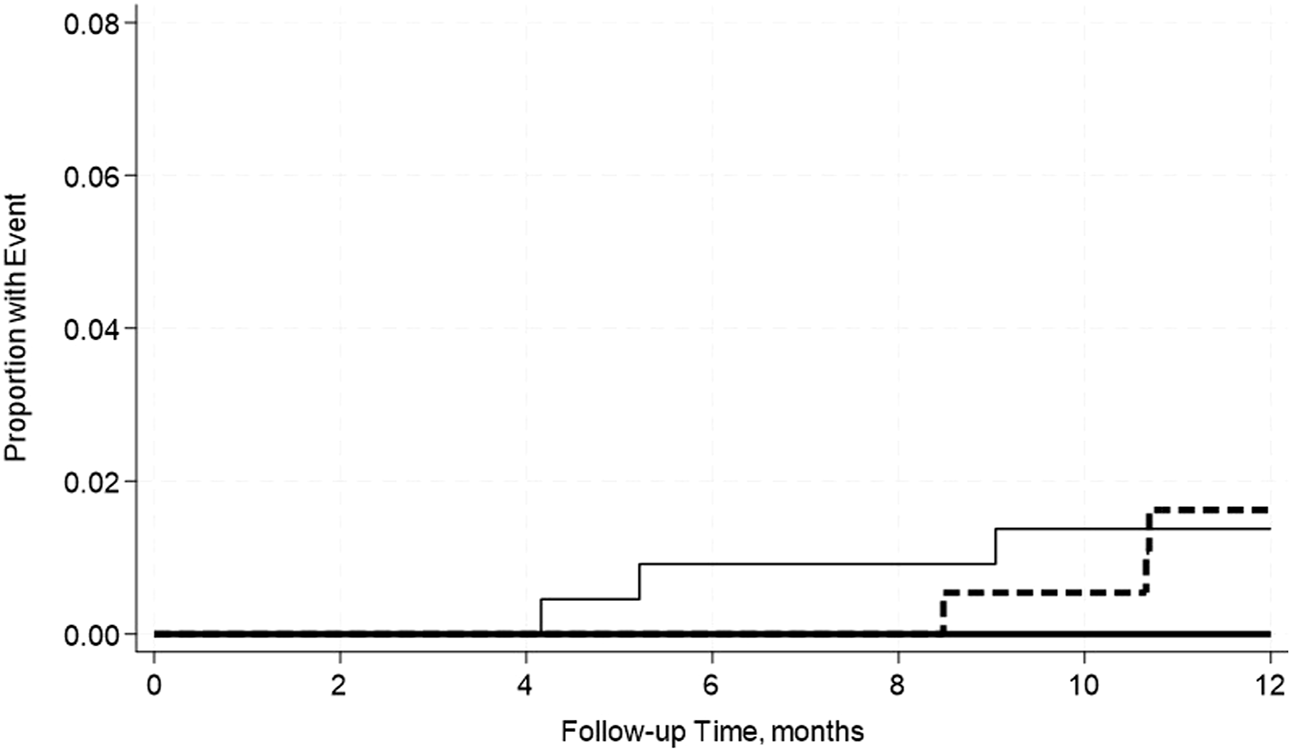
No significant differences in the incidence rate ratio of kidney death censored graft failure within 2 weeks–12 months post-transplant by serum albumin levels. The thin solid line represents the normal pre-transplant serum albumin levels group, the thick dashed line represents the mild hypoalbuminemia group and the thick solid line represents the moderate hypoalbuminemia group.
FIGURE 7
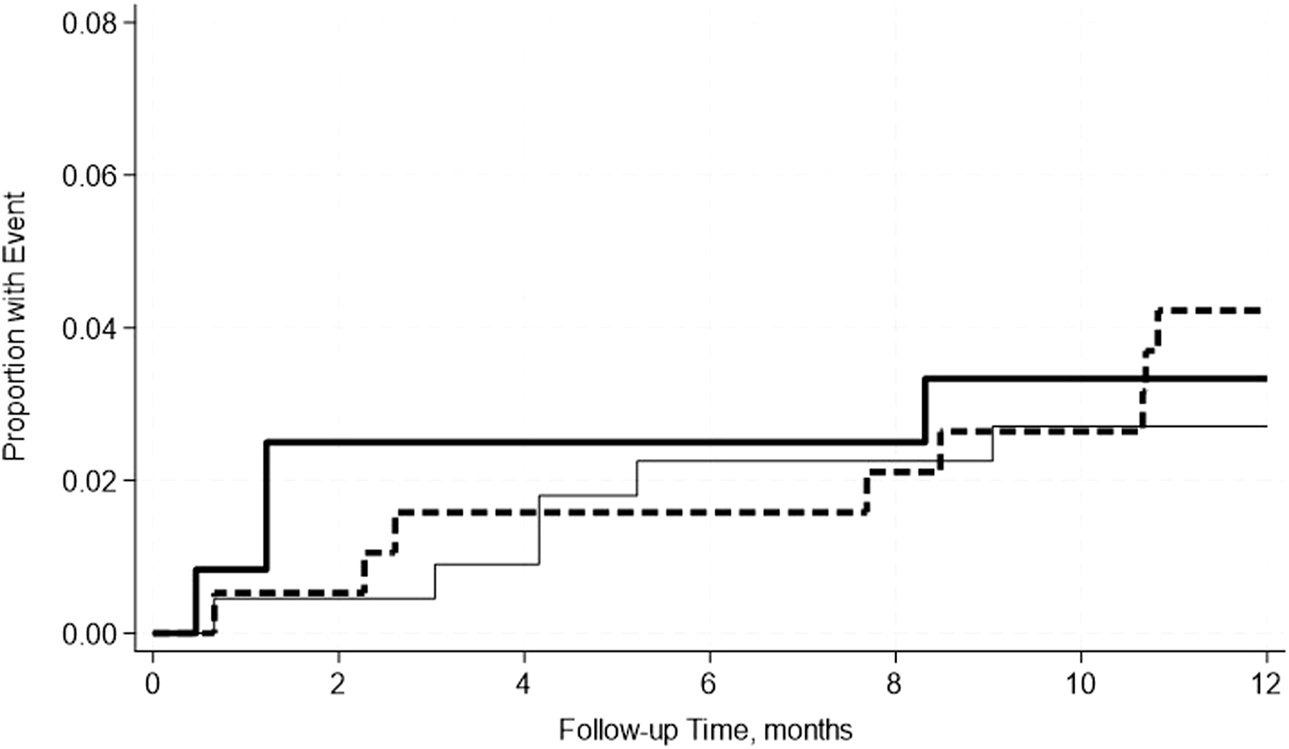
No significant differences in the incidence rate ratio of kidney uncensored graft failure within 2 weeks–12 months post-transplant by serum albumin levels. The thin solid line represents the normal pre-transplant serum albumin levels group, the thick dashed line represents the mild hypoalbuminemia group and the thick solid line represents the moderate hypoalbuminemia group.
Also, 3.0% of SPK recipients experienced death with both functional grafts, and similar to the previous findings, neither mild nor moderate pre-transplant hypoalbuminemia was associated with death with both functional grafts (Table 8).
A total of 17 SPK recipients had early pancreas DCGF, 3 had early kidney DCGF, and 3 died with both functional grafts, all within 2 weeks post-transplant, and were not included in the main analysis of the study. When analyzing the data in this subgroup of SPK recipients, pretransplant hypoalbuminemia was not associated with those outcomes of interest either in the mild or moderate hypoalbuminemia group in the unadjusted model (Supplementary Table S1).
Also when including these recipients with early pancreas graft failure, pretransplant hypoalbuminemia was not associated with either grafts uncensored or DCGF or death either in the mild or moderate hypoalbuminemia group in the unadjusted model or adjusted model (Supplementary Table S2).
Discussion
In this large cohort of 532 SPK recipients, we found that pre-transplant hypoalbuminemia was not significantly associated with any detrimental early post-transplant outcomes. We looked for various common early post-transplant outcomes and events including kidney DGF, length of stay, readmission, re-operation, and graft failure, and none of these feared outcomes were associated with pre-transplant hypoalbuminemia.
Hypoalbuminemia is a known risk factor for various detrimental outcomes in patients who suffer from diabetes mellitus and ESRD. Inflammation has been identified to play a key role in the pathogenesis of hypoalbuminemia in this group of patients. Chronic systemic inflammation that suppresses both innate and acquired immunity ultimately places patients with concomitate diabetes and ESRD at higher risk of life-threatening infections, morbidity, and death [4, 21, 22]. Therefore, we expected to observe a negative effect of hypoalbuminemia on both patient and graft survival. Breyer et al., also did not observe worse outcomes among kidney transplant recipients, although they report a lower risk of acute rejection based on the degree of hypoalbuminemia [11].
Patients with diabetes and ESRD are at higher risk for malnutrition [23]. Among solid organ transplant recipients, malnutrition has been associated with various poor clinical outcomes among liver, kidney, heart, and lung transplant recipients [23, 24].
In the past, serum albumin was thought to be an acceptable sole marker of nutritional status but has since been widely researched and refuted [23]. A retrospective cohort study from 2006 found no association between serum albumin and mortality risk among lung transplant recipients but found a higher risk of death in recipients with a low prealbumin level (<18 g/dL) [25]. Still, pre-albumin and albumin levels should not be considered valid tools for malnutrition diagnosis, as they are influenced by multiple factors including inflammation and fluid status. According to Evans et al, albumin and prealbumin as acute phase proteins do not consistently or predictably change with weight loss, calorie restriction, or nitrogen balance and more accurately indicate the severity of the inflammatory response rather than poor nutrition status [26]. In October 2020, the American Society for Parenteral and Enteral Nutrition (ASPEN) published a position paper stating that albumin and prealbumin are not components of any accepted definitions of malnutrition [26]. Extrapolating from previous data among various solid organ transplants, even among SPK recipients, our results support that serum albumin levels should not be used to assess nutritional status in this unique population. Although, out of the scope of this study, in the future, if an association between malnutrition and outcomes among SPK recipients is to be sought, should include various other nutritional tools and should not solely rely on the serum albumin levels.
To the best of our knowledge, there is no other study assessing the risk of pre-transplant hypoalbuminemia in SPK recipients with various post-transplant outcomes. In one study, Becker et al reported that persistent post-SPK hypoalbuminemia was associated with an increased risk of CMV infection, both kidney and pancreas graft failure, and a trend toward increased risk of overall patient mortality [15]. In another study, among kidney-only transplant recipients, Anderson et al. reported that hypoalbuminemia was an independent risk factor for overall graft failure after kidney transplantation [27]. Furthermore, several other studies showed that kidney transplant recipients with hypoalbuminemia were at increased risk for all-cause mortality, cardiovascular mortality, graft failure, DGF, acute rejection, BK, and CMV infections [10, 28–31].
To the best of our knowledge, this is the first study that investigated mild and moderate hypoalbuminemia and its associations with numerous post-transplant outcomes among patients undergoing SPK. Data that originated from a single-center hospital study allowed us to provide nuanced, granular data points, that reflected a homogeneous approach to transplant practices involving both medical and surgical patient management. These unique characteristics are unavailable with large multicenter registry datasets. Nonetheless, our study had several limitations. We were unable to identify the exact causes of hypoalbuminemia. Additionally, it was out of the scope of this study to assess the risk of infections and malignancies based on the pre-transplant serum albumin levels. Also, we did not assess the outcomes based on the changes in serum albumin levels post transplant. Lastly, due to having stringent selection criteria for SPK, most of the recipients were likely to be in relatively good health, and despite having mild/moderate hypoalbuminemia they were able to recover from SPK transplantation no differently than those without hypoalbuminemia. Also, we did not assess the various pre-transplant risk factors that usually coexist with hypoalbuminemia including pre-transplant peritoneal dialysis modality, frailty, nutritional status, muscle mass, fluid overload etc.
To summarize, the outcomes of this study have significant clinical importance showing that various degrees of hypoalbuminemia, were not associated with inferior outcomes particularly when it comes to death-censored and death uncensored graft failure. We conclude that patients with mild or moderate hypoalbuminemia, as defined in this study, who are otherwise acceptable candidates for SPK, should be considered for transplantation. Undoubtedly, future research on this topic is necessary to address the limitations reported above. Also, research to identify some of the easily available biomarkers predicting various post-transplant outcomes in these populations will be beneficial.
Summary
Simultaneous pancreas-kidney transplant (SPK) has become a growing form of treatment for those with diabetes mellitus and ESRD as it restores euglycemia and slows the progression of diabetes complications. In these patients, hypoalbuminemia has been proposed to be caused by chronic systemic inflammation and reduced synthesis of albumin. However, the role of pre-transplant hypoalbuminemia and its impact on post-transplant outcomes in patients undergoing SPK transplantation remains unclear. In this study, we studied 532 SPK recipients at our center with mild and moderate hypoalbuminemia. Outcomes of interest included kidney delayed graft function (DGF), length of stay (LOS) after transplant, re-hospitalization within 30 days after discharge, and need for a return to the operating room related to transplant surgical complications, acute rejection, and uncensored and death-censored graft failure, within the first years post-transplant. Mild or moderate hypoalbuminemia was not associated with DGF, LOS, re-hospitalization, return to the operating room, graft rejection, or graft failure. The outcomes of this study have significant clinical importance showing that mild or moderate hypoalbuminemia, was not associated with inferior outcomes, concluding that these patients are acceptable candidates for SPK.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by University of Wisconsin- Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because Retrospective study. Written consent particularly to this study was not obtained.
Author contributions
EF: concept, design, and manuscript preparation, editing. SN: manuscript preparation. DK: editing. JO: editing. DA: editing. CT: editing. DA-A: editing. DM: editing. BA: analysis, editing. SP: original concept, design, manuscript preparation, editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by an unrestricted research grant from the Virginia Lee Cook Foundation to DM.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2025.14091/full#supplementary-material
Abbreviations
ABMR, Antibody-Mediated Rejection; aHR, Adjusted Hazard Ratio; BMI, Body Mass Index; CIT, Cold Ischemia Time; CMV, Cytomegalovirus; cPRA, Calculated Panel Reactive Antibody; DCGF, Death Censored Graft Failure; DCD, Donation After Circulatory Death; ESRD, End Stage Renal Disease; HLA, Human Leukocyte Antigen; KDPI, Kidney Donor Profile Index; KDGF, Kidney Delayed Graft Function; LOS, Length of Stay; SPK, Simultaneous Pancreas and Kidney; TCMR, T-cell Mediated Rejection.
Footnotes
References
1.
GattaAVerardoABolognesiM. Hypoalbuminemia. Intern Emerg Med (2012) 7(S3):193–9. 10.1007/s11739-012-0802-0
2.
NicholsonJPWolmaransMRParkGR. The Role of Albumin in Critical Illness. Br J Anaesth (2000) 85(4):599–610. 10.1093/bja/85.4.599
3.
SoetersPBWolfeRRShenkinA. Hypoalbuminemia: Pathogenesis and Clinical Significance. J Parenter Enter Nutr (2019) 43(2):181–93. 10.1002/jpen.1451
4.
HerselmanMEsauNKrugerJMLabadariosDMoosaMR. Relationship Between Serum Protein and Mortality in Adults on Long-Term Hemodialysis: Exhaustive Review and Meta-Analysis. Nutrition (2010) 26(1):10–32. 10.1016/j.nut.2009.07.009
5.
GibbsJCullWHendersonWDaleyJHurKKhuriSF. Preoperative Serum Albumin Level as a Predictor of Operative Mortality and Morbidity: Results From the National VA Surgical Risk Study. Arch Surg (1999) 134(1):36–42. 10.1001/archsurg.134.1.36
6.
XuRHaoMZhouWLiuMWeiYXuJet alPreoperative Hypoalbuminemia in Patients Undergoing Cardiac Surgery: A Meta-Analysis. Surg Today (2023) 53(8):861–72. 10.1007/s00595-022-02566-9
7.
CurranSApruzzesePKendallMCDe OliveiraG. The Impact of Hypoalbuminemia on Postoperative Outcomes After Outpatient Surgery: A National Analysis of the NSQIP Database. Can J Anesth Can Anesth (2022) 69(9):1099–106. 10.1007/s12630-022-02280-7
8.
JellingeMEHenriksenDPHallasPBrabrandM. Hypoalbuminemia Is a Strong Predictor of 30-Day All-Cause Mortality in Acutely Admitted Medical Patients: A Prospective, Observational, Cohort Study. PLoS One(2014) 22(8):e105983. 10.1371/journal.pone.0105983
9.
MeyerCPRios-DiazAJDalelaDRaviPSoodAHanskeJet alThe Association of Hypoalbuminemia With Early Perioperative Outcomes – A Comprehensive Assessment Across 16 Major Procedures. Am J Surg (2017) 214(5):871–83. 10.1016/j.amjsurg.2016.11.023
10.
SrivastavaABodnarJOsmanFJorgensonMRAstorBCMandelbrotDAet alSerum Albumin Level Before Kidney Transplant Predicts Post-Transplant BK and Possibly Cytomegalovirus Infection. Kidney Int Rep (2020) 5(12):2228–37. 10.1016/j.ekir.2020.09.012
11.
BreyerIAstorBCSrivastavaAAzizFGargNMohamedMAet alPre‐Transplant Hypoalbuminemia Is Not Associated With Worse Short‐Term Outcomes Among Kidney Transplant Recipients. Clin Transpl (2023) 37(2):e14862. 10.1111/ctr.14862
12.
GruessnerRWGGruessnerAC. The Current State of Pancreas Transplantation. Nat Rev Endocrinol (2013) 9(9):555–62. 10.1038/nrendo.2013.138
13.
WhiteSAShawJASutherlandDE. Pancreas Transplantation. Pancreas Transplant (2009) 373:1808–17. 10.1016/S0140-6736(09)60609-7
14.
KaysenGARathoreVShearerGCDepnerTA. Mechanisms of Hypoalbuminemia in Hemodialysis Patients. Kidney Int (1995) 48(2):510–6. 10.1038/ki.1995.321
15.
BeckerBNBeckerYTHeiseyDMLeversonGECollinsBHOdoricoJSet alThe Impact of Hypoalbuminemia in Kidney-Pancreas Transplant Recipients1. Transplantation (1999) 68(1):72–5. 10.1097/00007890-199907150-00014
16.
StrattaRJFarneyACFridellJA. Analyzing Outcomes Following Pancreas Transplantation: Definition of a Failure or Failure of a Definition. Am J Transpl (2022) 22(6):1523–6. 10.1111/ajt.17003
17.
OPTN/UNOS Pancreas Transplantation Committee Definition of Pancreas Graft Failure (2015). Available from: https://optn.transplant.hrsa.gov/media/1572/policynotice_20150701_pancreas.pdf (Accessed: September 2, 2024).
18.
ParajuliSArunachalamASwansonKJAzizFGargNRedfieldRRet alOutcomes AFTer Simultaneous Kidney‐Pancreas Versus Pancreas AFTEr Kidney Transplantation in the Current Era. Clin Transpl (2019) 33(12):e13732. 10.1111/ctr.13732
19.
ParajuliSJoachimEAlagusundaramoorthySBlazelJAzizFGargNet alSubclinical Antibody-Mediated Rejection After Kidney Transplantation: Treatment Outcomes. Transplantation (2019) 103(8):1722–9. 10.1097/TP.0000000000002566
20.
ParajuliSArpaliEAstorBCDjamaliAAzizFRedfieldRRet alConcurrent Biopsies of Both Grafts in Recipients of Simultaneous Pancreas and Kidney Demonstrate High Rates of Discordance for Rejection as Well as Discordance in Type of Rejection - a Retrospective Study. Transpl Int (2018) 31(1):32–7. 10.1111/tri.13007
21.
PiferTBMcculloughKPPortFKGoodkinDAMaroniBJHeldPJet alMortality Risk in Hemodialysis Patients and Changes in Nutritional Indicators: DOPPS. Kidney Int (2002) 62(6):2238–45. 10.1046/j.1523-1755.2002.00658.x
22.
SridharNRJosyulaS. Hypoalbuminemia in Hemodialyzed End Stage Renal Disease Patients: Risk Factors and Relationships - a 2 Year Single Center Study. BMC Nephrol (2013) 14(1):242. 10.1186/1471-2369-14-242
23.
LordenHEngelkenJSprangKRolfsonMMandelbrotDParajuliS. Malnutrition in Solid Organ Transplant Patients: A Review of the Literature. Clin Transpl (2023) 37(11):e15138. 10.1111/ctr.15138
24.
LordenHEngelkenJSprangKRolfsonMMandelbrotDParajuliS. Pretransplant Malnutrition, Particularly With Muscle Depletion Is Associated With Adverse Outcomes After Kidney Transplantation. Transpl Direct (2024) 10(5):e1619. 10.1097/TXD.0000000000001619
25.
González-CastroALlorcaJSuberviolaBDíaz-RegañónGOrdóñezJMiñambresE. Influence of Nutritional Status in Lung Transplant Recipients. Transpl Proc (2006) 38(8):2539–40. 10.1016/j.transproceed.2006.08.084
26.
EvansDCCorkinsMRMaloneAMillerSMogensenKMGuenterPet alThe Use of Visceral Proteins as Nutrition Markers: An ASPEN Position Paper. Nutr Clin Pract (2021) 36(1):22–8. 10.1002/ncp.10588
27.
AndersonBKhalilKEvisonFNathJSharifA. Hypoalbuminaemia at Time of Surgery Is Associated With an Increased Risk for Overall Graft Loss After Kidney Transplantation. Nephrology (2019) 24(8):841–8. 10.1111/nep.13481
28.
ThongprayoonCCheungpasitpornWChewcharatAMaoMAThirunavukkarasuSKashaniKB. Impacts of Admission Serum Albumin Levels on Short-Term and Long-Term Mortality in Hospitalized Patients. QJM Int J Med (2020) 113(6):393–8. 10.1093/qjmed/hcz305
29.
MolnarMZKovesdyCPBunnapradistSStrejaEMehrotraRKrishnanMet alAssociations of Pretransplant Serum Albumin With Post-Transplant Outcomes in Kidney Transplant Recipients. Am J Transpl (2011) 11(5):1006–15. 10.1111/j.1600-6143.2011.03480.x
30.
YangSWChoiJYKwonOJ. The Impact of Pretransplantation Serum Albumin Levels on Long-Term Renal Graft Outcomes. Transpl Proc (2013) 45(4):1379–82. 10.1016/j.transproceed.2012.10.063
31.
DahlbergRMuthBSamaniegoMHofmannRMPirschJDjamaliA. One-Year Serum Albumin Is an Independent Predictor of Outcomes in Kidney Transplant Recipients. J Ren Nutr (2010) 20(6):392–7. 10.1053/j.jrn.2010.03.008
Summary
Keywords
hypoalbuminemia, SPK, DGF, patient survival, graft survival
Citation
Fedorova E, Nehring Firmino S, Kaufman DB, Odorico JS, Aufhauser D, Thiessen C, Al-Adra DP, Mandelbrot D, Astor BC and Parajuli S (2025) Pre-Transplant Hypoalbuminemia Is Not Associated With Early Key Outcomes Among Simultaneous Pancreas and Kidney Transplant Recipients. Transpl Int 38:14091. doi: 10.3389/ti.2025.14091
Received
20 November 2024
Accepted
06 January 2025
Published
20 January 2025
Volume
38 - 2025
Updates
Copyright
© 2025 Fedorova, Nehring Firmino, Kaufman, Odorico, Aufhauser, Thiessen, Al-Adra, Mandelbrot, Astor and Parajuli.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sandesh Parajuli, sparajuli@medicine.wisc.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.