Dear Editors,
Pancreatic islet isolation relies on the complete perfusion of digestive enzymes throughout the pancreas for dissociation of the extracellular matrix to digest pancreatic tissue and maximize the islet yield [1–3]. Enzymes are perfused in the pancreatic duct by retrograde cannulation (RC) or through combined ante- and retrograde cannulation (ARC) by dissecting the pancreas at the neck [4, 5]. Incomplete enzyme perfusion is often observed in pancreases of patients with chronic pancreatitis undergoing total pancreatectomy with islet autotransplantation (TPIAT) and at the dissection surface during ARC procedures. Here we describe intraparenchymal injections (IPI) of digestive enzymes as a potential solution to overcome incomplete perfusion.
All data were collected on consecutive human pancreatic islet isolations for clinical use between December 2014 and February 2024 in the Leiden University Medical Center. Pancreases for allogeneic islet transplantation were allocated by Eurotransplant. Pancreases for autologous islet transplantation were obtained after total pancreatectomy. Islet isolations were performed using an adapted version of the semi-automated method [4, 6]. RC was the standard method of cannulation, ARC was used if RC proved challenging. Experienced members of the islet isolation team examined the pancreas for hypoperfused tissue areas and performed intraparenchymal injections of digestive enzymes using 25–30 gauge needles until those areas were distended. IPI is demonstrated in Supplementary Video S1. Further details are provided in the Supplementary Methods.
Data from 253 consecutive islet isolations from donor pancreases intended for allogeneic islet transplantation, and 26 islet isolations from pancreases intended for autologous islet transplantation were included. Allogeneic organ donors had a mean age of 47.9 ± 12.7 years, 45.5% were female, and the body mass index was 27.3 ± 5.1 kg/m2. In procedures involving donor pancreases, RC was performed in 218 (86.2%) and ARC in 35 (13.8%) of the isolations (Supplementary Table S1). Patients with an indication for total pancreatectomy and islet autotransplantation had a mean age of 45.5 ± 14.9 years, 65.4% were female, the body mass index was 24.0 ± 4.1 kg/m2, and 84.6% had a history of chronic pancreatitis (Supplementary Table S2)
In islet isolations for autologous transplantation, digestion with IPI was higher (IPI 81.4% ± 15.5% vs without IPI 55.0% ± 27.4%, 95% CI of change: 7.82–45.02, p = 0.01, Figure 1D). Median islet yield was 5,540 (IQR 3,100–7,330) IEQ/g with IPI and 2,570 (IQR 1,870–3,230) IEQ/g without IPI (p = 0.05, Figure 1E) (Supplementary Table S3). We found that ductal cannulation with enzyme perfusion was not possible in 6 of these islet isolations. In these 6 isolations, we performed intraparenchymal enzyme injections only and isolated between 190.000 and 705.000 IEQ (range 2972–9503 IEQ/kg, Figures 1F, G; Supplementary Table S4). Five out of 6 islet preparations were transplanted. One of these islet products could not be transplanted because of a high endotoxin concentration.
FIGURE 1
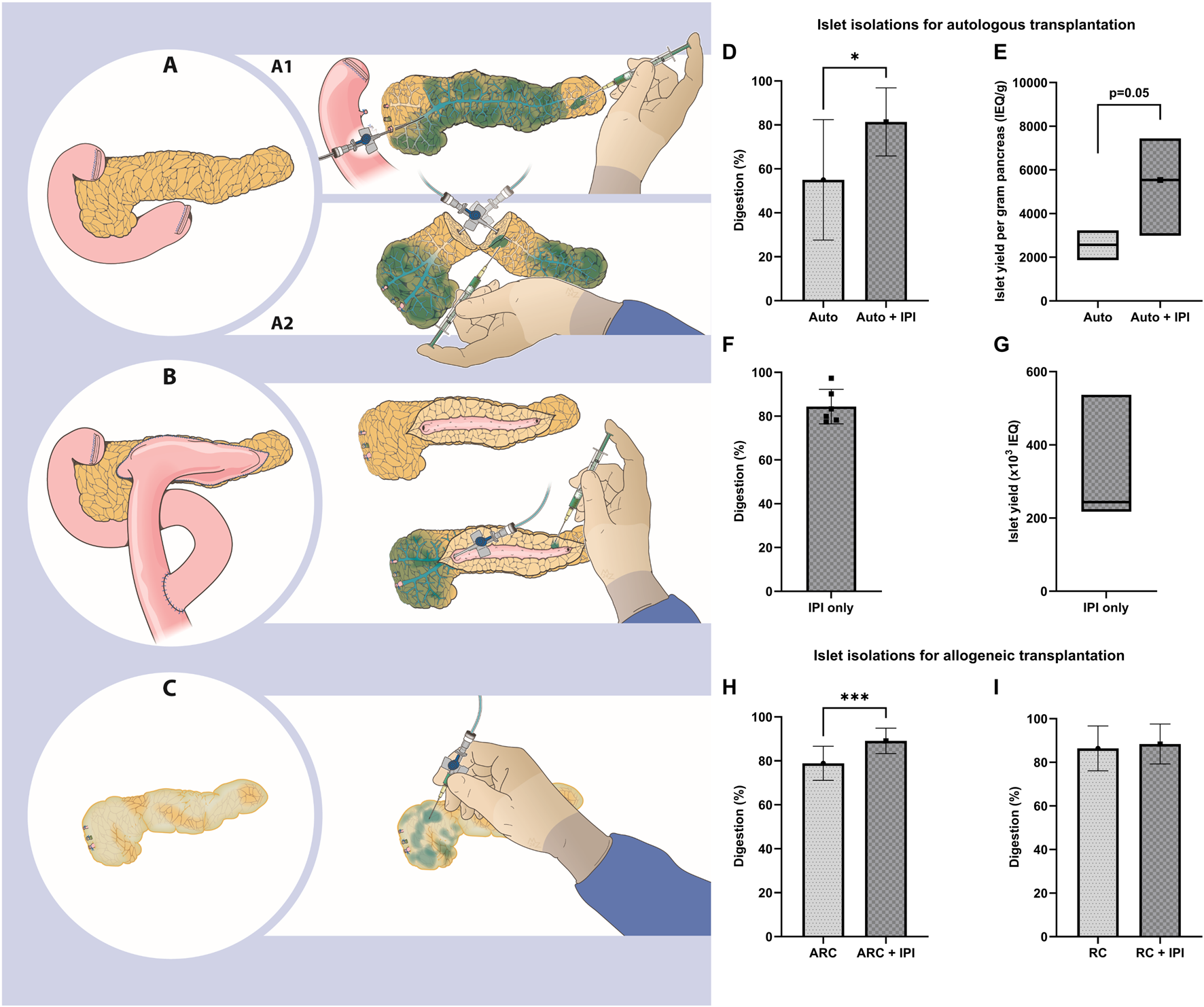
(A) The starting point for islet isolation with the pancreas connected to the duodenum. A1) The pancreas is separated from the duodenum and perfused by retrograde cannulation (RC) of the pancreatic duct. Certain tissue areas are poorly perfused and intraparenchymal injections (IPI) are performed to deliver enzymes (depicted in blue). A2) The pancreas is cut at the neck and enzymes are perfused by ante- and retrograde cannulation (ARC) of the pancreatic duct. Parenchyma around the cut surface is poorly perfused and IPI is performed. (B) A pancreas after pancreaticojejunostomy. The jejunum is removed and the pancreatic duct exposed. Enzymes are perfused by ARC and IPI. (C) A smaller, fibrotic pancreas. Ductal cannulation is not possible and IPI is performed. (D-G) Islet isolations for autologous transplantation. (D) Percentage digestion (mean ± standard deviation). (E) islet yield per gram pancreas (median and interquartile range). (F) Percentage digestion (mean ± standard deviation) and (G) islet yield (median and interquartile range) using only IPI. (H, I) Islet isolations for allogeneic transplantation showing percentage digestion (mean ± standard deviation) for ARC (H) and RC (I). IEQ, islet equivalent.
In islet isolations for allogeneic transplantation, we found a higher digestion in ARC isolations with IPI of 10.0%pt. (95% CI: 5.99–14.08, p < 0.001, Figure 1H), without a difference in islet yield per gram pancreas. For RC islet isolations, digestion and islet yield per gram pancreas were similar between the isolations with and without IPI (Figure 1I).
Generation of a maximal number of viable and functional islets is the most important goal for islet isolation. In this observational study we show that intraparenchymal injection is unlikely to have a negative effect on islet yield. The potential contribution of intraparenchymal enzyme injections was demonstrated in 6 isolations for autologous islet transplantation with a sufficient islet yield for autotransplantation. Digestion rate and islet yield of isolations using ARC in donor pancreas and of isolations for TPIAT were higher when IPI was performed based on the presence of hypoperfused pancreas parenchyma. In RC isolations, similar digestion and islet yield were present.
IPI could be considered in pancreases with an altered anatomy, such as after dissection of the neck for ARC (Figure 1A) and after previous pancreatic surgery. Damage to the pancreas due to dissection, which is inherent to ARC, leads to hypoperfusion and subsequent incomplete digestion. Fibrosis, calcification (Supplementary Figures S1A, C) and previous surgery (e.g., Frey, Beger, Puestow procedures; Figures 1B, C) are often present when pancreases are presented for isolation in the context of autologous islet transplantation [7]. These surgical procedures may render classical perfusion methods inadequate and negatively affect islet yield [8]. In these instances, intraparenchymal injections may facilitate more complete perfusion of the parenchyma with digestive enzymes, potentially supporting digestion and islet yield.
There are no previous studies on how to deal with hypoperfused pancreatic parenchyma during isolation. A strength of this study is the inclusion of consecutive islet isolations of pancreases for both allogeneic and autologous islet transplantation. Study limitations include its retrospective, observational nature and judgement of hypoperfusion by experienced members of the islet isolation team. In order to obtain more robust information of the contribution of IPI on islet isolation outcome, randomized studies with or without IPI, and more objective assessment of hypoperfusion should be performed.
In conclusion, intraparenchymal injections may improve digestion and islet yield, representing a potential addition to current islet isolation practice.
Statements
Data availability statement
The datasets presented in this article are not readily available because instutition-guided restrictions may be applicable. Requests to access the datasets should be directed to ME, m.a.engelse@lumc.nl.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements. Oral informed consent was obtained from the individuals for the publication of any potentially identifiable images or data included in this article as per institution guidelines.
Author contributions
MT, DJC, EK, and ME participated in the design of the research. MT, DJC, YC, EW, JS, JD, CV, MH, ER, SM, BB, VH, and ME participated in performing the research. MT participated in data analysis. MT, DJC, EK, and ME participated in the preparation of the article. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The LUMC islet isolation program has been supported by the Dutch Diabetes Research Foundation, Stichting DON and the Bontius Foundation.
Acknowledgments
We would like to acknowledge Manon Zuurmond for the illustrations and the patients for their consent to use the images. Declaration of generative AI and AI-assisted technologies in the writing process. During the preparation of this work the authors used ChatGPT-4o (OpenAI) in order to check spelling and harmonize text. After using this tool, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2025.13507/full#supplementary-material
References
1.
Szot GL Lee MR Tavakol MM Lang J Dekovic F Kerlan RK et al Successful Clinical Islet Isolation Using a GMP-Manufactured Collagenase and Neutral Protease. Transplantation (2009) 88(6):753–6. 10.1097/TP.0b013e3181b443ae
2.
Balamurugan AN Loganathan G Lockridge A Soltani S Wilhelm J Beilman G et al Islet Isolation from Pancreatitis Pancreas for Islet Autotransplantation. Islets of Langerhans (2015) 1199–227. 10.1007/978-94-007-6686-0_48
3.
Wang Y Danielson KK Ropski A Harvat T Barbaro B Paushter D et al Systematic Analysis of Donor and Isolation Factor's Impact on Human Islet Yield and Size Distribution. Cell Transpl (2013) 22(12):2323–33. 10.3727/096368912X662417
4.
Ricordi C Lacy PE Finke EH Olack BJ Scharp DW . Automated Method for Isolation of Human Pancreatic Islets. Diabetes (1988) 37(4):413–20. 10.2337/diab.37.4.413
5.
Lakey JR Warnock GL Shapiro AM Korbutt GS Ao Z Kneteman NM et al Intraductal Collagenase Delivery into the Human Pancreas Using Syringe Loading or Controlled Perfusion. Cell Transpl (1999) 8(3):285–92. 10.1177/096368979900800309
6.
Doppenberg JB Nijhoff MF Engelse MA de Koning EJP . Clinical Use of Donation after Circulatory Death Pancreas for Islet Transplantation. Am J Transpl (2021) 21(9):3077–87. 10.1111/ajt.16533
7.
Mou Y Song Y Chen HY Wang X Huang W Liu XB et al Which Surgeries Are the Best Choice for Chronic Pancreatitis: A Network Meta-Analysis of Randomized Controlled Trials. Front Surg (2021) 8:798867. 10.3389/fsurg.2021.798867
8.
Wang H Desai KD Dong H Owzarski S Romagnuolo J Morgan KA et al Prior Surgery Determines Islet Yield and Insulin Requirement in Patients with Chronic Pancreatitis. Transplantation (2013) 95(8):1051–7. 10.1097/TP.0b013e3182845fbb
Summary
Keywords
intraparenchymal injection, pancreatic islet, islet isolation, digestion, collagenase
Citation
Tol MC, Cornelissen D-J, Chae YS, van der Wel EJ, Sijtsma JC, Doppenberg JB, Vermeulen CJ, Hanegraaf MAJ, van Rossenberg EH, Mieog JSD, Bonsing BA, Huurman VAL, de Koning EJP and Engelse MA (2025) Intraparenchymal Enzyme Injections in Islet Isolations With Incomplete Ductal Perfusion of Enzymes. Transpl. Int. 38:13507. doi: 10.3389/ti.2025.13507
Received
09 July 2024
Accepted
03 April 2025
Published
30 April 2025
Volume
38 - 2025
Updates
Copyright
© 2025 Tol, Cornelissen, Chae, van der Wel, Sijtsma, Doppenberg, Vermeulen, Hanegraaf, van Rossenberg, Mieog, Bonsing, Huurman, de Koning and Engelse.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marten A. Engelse, m.a.engelse@lumc.nl
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.