Abstract
Utilizing assays that assess specific T-cell-mediated immunity against cytomegalovirus (CMV) holds the potential to enhance personalized strategies aimed at preventing and treating CMV in organ transplantation. This includes improved risk stratification during transplantation compared to relying solely on CMV serostatus, as well as determining the optimal duration of antiviral prophylaxis, deciding on antiviral therapy when asymptomatic replication occurs, and estimating the risk of recurrence. In this review, we initially provide an overlook of the current concepts into the immune control of CMV after transplantation. We then summarize the existent literature on the clinical experience of the use of immune monitoring in organ transplantation, with a particular interest on the outcomes of interventional trials. Current evidence indicates that cell-mediated immune assays are helpful in identifying patients at low risk for replication for whom preventive measures against CMV can be safely withheld. As more data accumulates from these and other clinical scenarios, it is foreseeable that these assays will likely become part of the routine clinical practice in organ transplantation.
Introduction
Despite the implementation of effective antiviral therapies and sensitive molecular diagnostic assays, cytomegalovirus (CMV) infection remains as a major complication after solid organ transplantation (SOT), threatening both graft function and survival [1].
While relevant advances have been made in the understanding of the immunobiology of CMV infection in the context of organ transplantation, little translation to clinical practice has been done so far. In this regard, the T-cell arm of adaptive immunity (hereafter cell-mediated immunity [CMI]), especially CMV-specific CD4+ and CD8+ T lymphocytes, has been well-recognized as a major immune mechanism driving antiviral control [2, 3]. Robust evidence has showed a close association between CMV-CMI and the risk of developing CMV infection in different transplant settings [4–6]. Yet, current immune-risk stratification of CMV infection relies on the serological mismatch between donors and recipients, based on the premise that seronegative recipients receiving a seropositive graft (D+/R−) are at the highest risk of developing primary CMV infection due to their naïve immune status, whereas seropositive patients (R+) receiving seropositive grafts are at an intermediate risk because of previous viral immunization which should provide sufficient protection against viral replication [7]. While such paradigm has helped to predict the advent of CMV infection, this approach encompasses important limitations as a proportion of R+ individuals may unpredictably develop CMV replication and also because of the widespread use of T-cell depleting therapies that convert previously immunized patients into naïve individuals against CMV [8]. To minimize the development of CMV infection, the use of universal antiviral prophylaxis or preemptive assessment of viral replication are the two main preventive strategies used [7]. However, either approach is far from being accurate as they do not personalize the type and duration of such preventive strategies, since the dynamic immune status specific to CMV is not being considered.
Recently, novel immune assays have been used in transplantation showing their capacity to accurately measure CMV-CMI [4, 7]. While interesting clinical associations have been reported between CMV-CMI and the risk of CMV infection after transplant, the different methodological nature of these assays -which provide diverse biological insight on functionality of immune responses-, the so far limited data coming from clinical trials, as well as the distinct clinical transplant settings evaluated, makes it difficult to establish robust conclusions on how to implement these new technologies into clinical practice with the aim of improving transplant outcomes.
In this review, we first summarize the main mechanisms involved in the immunobiology of CMV in transplantation, to then address the major advances made with the assessment of CMV-CMI using different immune-monitoring assays as well as the major drawbacks currently limiting the implementation of these assays.
Immunobiology of Cytomegalovirus Infection
CMV infection in SOT recipients results from primoinfection or reactivation. In these two situations, a complex multi layered cell response is required to inhibit CMV dissemination [9]. Five main cell types have been studied during CMV infection, three belonging to adaptive immunity (in particular CD8+ and CD4+ T cells, and to a lesser extent the B cells) (Figure 1). Importantly, some patients do not develop CMV disease despite the absence of any CMV-specific CD8+ and CD4+ T cells, suggesting that other actors belonging to innate immunity (such as NK and γδ cells) could also be necessary for CMV control.
FIGURE 1
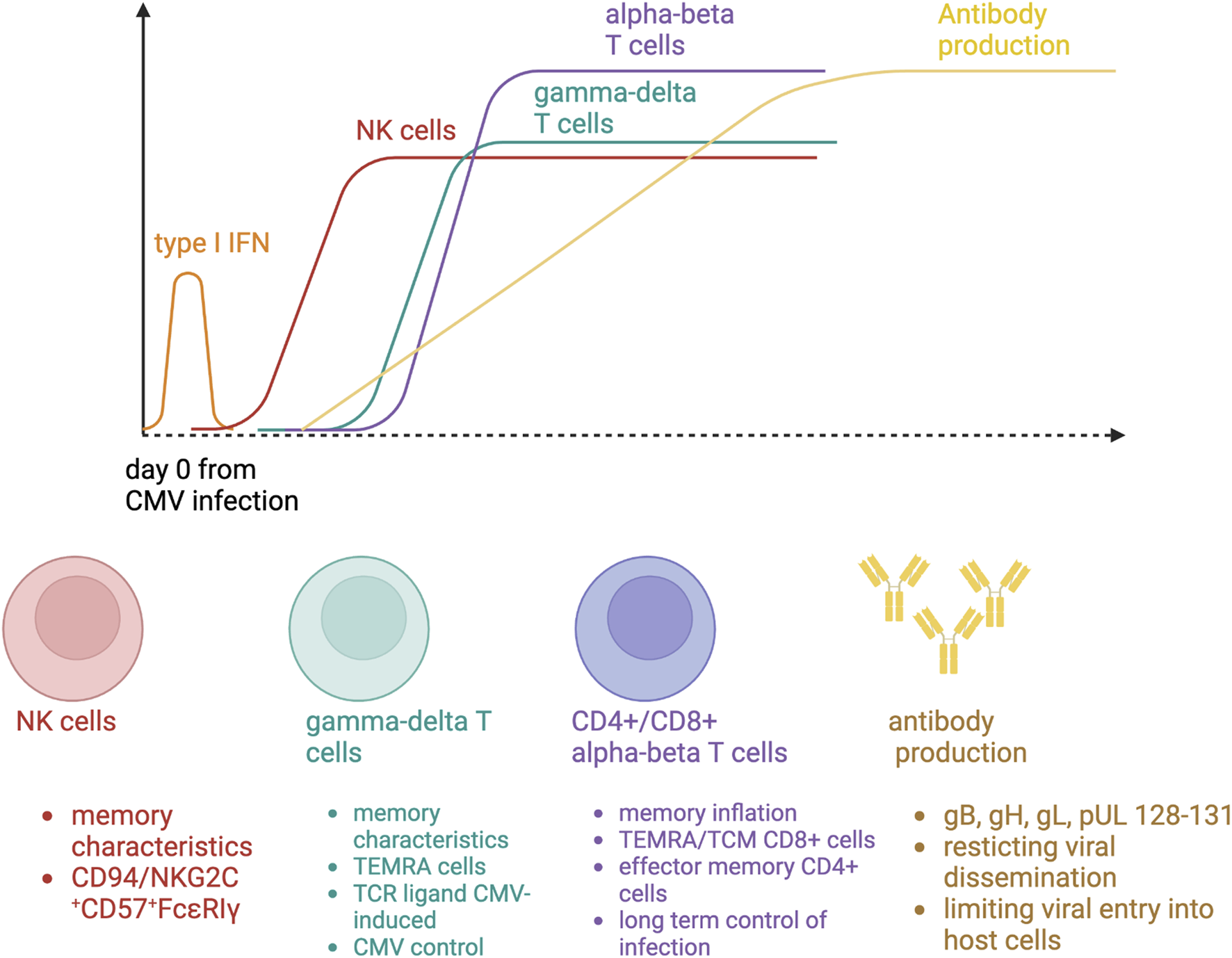
Immune responses to cytomegalovirus primary infection.
NK Cells
The monitoring of NK cells can be easily performed by flow cytometry with the following fluorochrome-coupled specific antibodies: CD3, CD16, CD56, NKG2C, CD57. In human, NK cell deficiency is associated with severe herpes viral infections, such as CMV [10]. Healthy human individuals with a history of CMV infection have an expanded population of NK cells expressing the activating CD94/NKG2C receptor [11]. In kidney transplant recipients, the number of circulating NK cell is correlated with NK cell-mediated cytotoxicity during CMV infection [12]. CMV R+ patients had preexisting memory-like NK cells (NKG2C+CD57+FcεRIγ−) at baseline and a subset of pre-memory-like NK cells (NKG2C+CD57+FcεRIγlow-dim) increases during CMV DNAemia. These cells expressed a higher cytotoxic profile than preexisting memory-like NK cells at the acute phase. At later phases of viremia, a subsequent accumulation of new memory-like NK cells has been reported [13]. NK cell clonal expansion is observed after CMV infection, leading to the development of immunological memory, two features belonging to an adaptive immune response. NK cell reactivity against CMV-infected cells results from a balance governed by the activation of receptors that sense alterations in the expression of ligands on the surface of CMV-infected cells. An increase in NK activating receptors could confer to the host a better protection against CMV infection.
γδ T Cells
In humans, γδ T cells are divided into two main subsets, based on their γ and δ T-cell receptor (TCR) chain expression: 1) the Vγ9Vδ2 γδT cells, expressing a δ2 chain, and 2) the non-Vγ9Vδ2 γδT cells. Initially, the involvement of non-Vγ9Vδ2 γδ T cells in the anti-CMV response was identified in the context of SOT or stem-cell transplantation. Five major observations suggest that non-Vγ9Vδ2 γδ T cells respond specifically to CMV:
- A longitudinal expansion of non-Vγ9Vδ2 γδ T cells is specifically observed in the peripheral blood of SOT recipients undergoing CMV infection [14, 15].
- CMV infection induces a restricted repertoire of non-Vγ9Vδ2 γδ T cells, suggesting an antigen-driven clonal selection [16].
- Non-Vγ9Vδ2 γδ T cells are poised for effector (particularly cytotoxic). During the course of CMV infection, non-Vγ9Vδ2 γδ T cells switch from a mainly naive phenotype (CD27+CD45RA+) towards a terminally differentiated effector memory (TEMRA) phenotype (CD27−CD45RA+), with the same kinetics than CMV-specific αβ T cells [17].
- The non-Vγ9Vδ2 T cell clones or cell lines can inhibit CMV dissemination and kill CMV-infected cells, in vitro [18]. Moreover, non-Vγ9Vδ2 γδ T cell expansion is associated with recovery from CMV infection without recurrence [15].
- Non-Vγ9Vδ2 T cells recognize native antigens, which are expressed at the cell surface during stress conditions (for instance CMV infection) such as reactive oxygen species (ROS) production, or AMP-activated protein kinase (AMPK)-dependent metabolic reprogramming. One example of CMV-induced γδ TCRs ligands is Annexin A2 [19].
Gamma-delta T cells can be easily monitored in clinical routine thanks to flow cytometry using a commercially available kit gathering fluorochrome coupled specific antibodies for CD45, CD3, Vδ2 and PAN-δ.
B Cells
While the advent of long-lasting humoral immunity toward a primary viral infection is universally accepted, the contribution of antibodies for protection against and control of CMV replication in transplant recipients is still a matter of debate. Data coming from experimental models suggest a key role of B cells through CMV-specific antibody release, particularly in restricting viral dissemination and in limiting disease severity [20, 21]. CMV-specific neutralizing antibodies appear during the first 4 weeks after primary infection and are mainly directed against CMV glycoprotein B, but also H, L, and pUL128-131, all of them involved in cell attachment, penetration, and fusion of the viral envelope to the cell membrane of the host [22]. The association shown between the former use of CMV-specific immunoglobulins as prophylaxis and better outcomes among liver transplant recipients also suggests to some extend a protective role of humoral immunity against viral replication [23].
Notably, in clinical transplantation, some R+ transplant individuals remain at high risk of CMV infection despite detectable humoral immunity, suggesting either a low avidity or poor neutralizing activity of the antibody response. Post-transplant IgM and IgG antibody seroconversion has been shown not to be a reliable predictor of CMV disease [24]. Furthermore, some of D+/R− patients (20%–30%) do not develop CMV infection after transplantation, suggesting either an optimal antibody seroconversion early after transplantation or the presence of preformed CMV-specific memory B cells prior to transplantation even though undetectable circulating CMV-specific IgG antibodies [25].
CMV-Specific CD8+ T Cells
During primary infection, CMV-specific CD8+ T cells exhibit an antigen-driven early-differentiating phenotype (CD27+CD28+ CD45RO+CD45RA−) armed for cytotoxicity [26, 27]. After viral clearance in healthy CMV R+ individuals, CMV-specific CD8+ T cells can represent up to 10% of the memory CD8+ lymphocyte pool, a process described as memory inflation [28]. There are two main subsets of CMV-specific CD8+ T cells: a) a central memory cell population (CD27+ CD28− CD45RO+) with low cytotoxic potential but high proliferation ability, and b) a TEMRA cell population, representing up to 75% of CMV-specific CD8+ T cells (CD27− CD28− CD45RA+), with a low proliferation ability but a major cytotoxic potential. TEMRA cells are resupplied from central memory cells and naive precursors.
During primary infection, the CMV-specific CD8+ T cell population is polyclonal. On the opposite, few epitope‐specific clones are predominant at the chronic phase. More than half of individuals have CD8+ T cell recognizing CMV peptides from the three following open reading frames (UL48, UL83, UL123). UL123 (immediate-early [IE]-1)-specific CD8+ T cells are associated with less CMV reactivation in SOT recipients, likely because UL123 is the first CMV protein to be expressed in infected cells. In vitro, CMV-specific CD8+ T cells can kill autologous CMV-infected cells and inhibit CMV dissemination. In mouse models, late effector CD8+ T cells maintain long-term control of viral replication [29].
CMV-Specific CD4+ T Cells
After a primary infection in SOT recipients, CMV-specific CD4+ T cells can be detected 1 week after the occurrence of CMV DNAemia [30], more specifically those CD4+ CD28-granzyme B+ cells [30, 31]. At the chronic phase of infection after viral clearance, CMV-specific CD4+ T cells represent up to 9% of the memory T lymphocyte pool. They exhibit an effector memory phenotype (CD27− CD28− CD45RA−). More than half of individuals have CD4+ T cells recognizing CMV peptides transcribed from the five following open reading frames (UL55, UL83, UL86, UL99, UL122). CD4+ T cells play a central role in anti-CMV immunity by clearing cells loaded with CMV peptides, helping B cells to mount a specific humoral response against viral antigens and CD8+ T cells to perform their effector functions [32].
Immunosuppressive Therapy and CMV Immune Response
CMV-CMI is abrogated for one to 3 months after anti thymocyte globulin induction [8] and reduced in patients having received high-dose steroids [33]. Rejection is usually treated by these two drugs and is therefore a risk factor for CMV disease [34, 35]. In vitro, tacrolimus is a potent inhibitor of CMV-specific cytokines release [36], and completely inhibits activation and proliferation of CMV-specific T cells [37]. On the opposite, belatacept demonstrated minimal inhibitory effects on CMV-specific T cells likely because of an absence of effect on cells lacking CD28 [36, 37]. While the antiviral immune response against CMV measured in vitro appears preserved under belatacept [38], high‐risk belatacept‐treated recipients show defects in sustaining CMV control [39], and exhibit high incidence of atypical life-threatening CMV diseases [40]. Further research is needed to elucidate this gap. Finally, a dysfunctional T-cell profile (with high PD1, low CD85j expression) has been observed in CMV-infected patients receiving mycophenolic acid. On the contrary, everolimus can improve T-cell fitness and transform dysfunctional into functional cells, along with better control of CMV [41]. In summary, the analysis of these five cells types could be useful for transplant physicians to understand the impact of the immunosuppressive regimen on CMV-specific T response.
Observational Data on the Clinical Application of CMV Immune-Monitoring
A growing number of observational studies have assessed in recent years the clinical usefulness of CMV-CMI monitoring to guide patient management in different SOT populations [42]. This research mainly includes single-center studies—with some multicenter experiences [8, 33, 43–47]— and has been performed in a wide range of clinical risk scenarios (Table 1). The most common methodologies used for the measurement of CMV-CMI is the reference technique of intracellular cytokine staining (ICS) by flow cytometry [42, 44, 45, 59, 61, 62, 67–71] and the different platforms for interferon (IFN)-γ release assay (IGRA) [4, 43, 46–56, 60, 63, 65, 66, 72–75]. Out of these immune assays, only three are currently commercially available: the quantiFERON®-CMV (QTF-CMV) (Qiagen, Hilden; Germany), the T-SPOT®.CMV (Oxford Immunotec, Abingdon, United Kingdom) and the T-Track®CMV (Mikrogen, Neuried, Germany). Available experience with the major histocompatibility complex (MHC)-tetramer staining method is more limited [64], whereas a few studies have compared the diagnostic accuracy of different approaches [54, 57, 76]. In most cases the primary study outcome is any CMV viremia, regardless of the presence or absence of symptoms or the level of DNAemia, or less often clinically significant viremia requiring antiviral therapy [46]. Since R+ patients typically have a low incidence of CMV disease [77, 78], the few studies that have primarily investigated the role of CMV-CMI monitoring to predict the occurrence of symptomatic infection (viral syndrome or end-organ disease) are focused on the high-risk group D+/R− patients [43, 48, 58]. Notably each platform has different readouts that are directly related to the nature of each immune assay. In general, all assays measure T-cell mediated effector immune responses of IFN-Ɣ production in response to two main immunogenic CMV antigens, phosphoprotein 65 (pp65) and IE-1 [79]. Importantly, while ELISA-based assays do not provide the individual response to each CMV antigen, flow-cytometry and ELISpot-based assays do deliver such the specific immunes, thus better illustrating the global burden of immune responses against CMV.
TABLE 1
| Clinical scenario | Predicted event | Supporting studies | Monitoring method | Proposed intervention |
|---|---|---|---|---|
| High-risk patients (D+/R−, T-cell-depleting antibodies, lung transplantation) during antiviral prophylaxis or at the time of discontinuation | Late-onset diseasea | Yes [43, 46, 48–58] | QTF-CMV, ELISpot | Prolong antiviral prophylaxis or close monitoring for viremia if inadequate response |
| Pre-transplant assessment in intermediate-risk patients (R+ with no other factors) | Post-transplant viremia and/or disease | Yes [4, 44, 47, 51, 59, 60] | QTF-CMV, ELISpot, ICS | Initiate antiviral prophylaxis or close monitoring for viremia in patients with inadequate response (D+/RNR) |
| Intermediate-risk patients (R+) on preemptive therapy with no concurrent viremia | Subsequent viremia and/or disease | Yes [42, 44, 49, 51, 52, 61–64] | ICS, QTF-CMV, ELISpot, MHC-tetramer staining | Reduce the frequency and/or discontinue monitoring of viremia if adequate response |
| Intermediate-risk patients (R+) on preemptive therapy with asymptomatic viremia | Spontaneous clearance | Yes [65, 66] | QTF-CMV | Withhold antiviral therapy if adequate response |
| Active CMV infection or disease during antiviral treatment | Response to antiviral treatment | No | Decrease immunosuppression and/or modify antivirals if inadequate response | |
| Active CMV infection or disease after discontinuation of antiviral treatment | Post-treatment relapse | Yes [67] | ICS | Initiate secondary prophylaxis if inadequate response |
| Acute graft rejection treated with steroid boluses and/or T-cell-depleting antibodies | Disease following anti-rejection therapy | No | (Re)initiate prophylaxis if inadequate response |
Summary of observational studies assessing the potential application of CMV-CMI monitoring in different clinical scenarios.
CMV, cytomegalovirus; D, donor; ELISpot, enzyme-linked immunosorbent spot assay; ICS, intracellular cytokine staining; QTF-CMV, QuantiFERON-CMV assay; MHC, major histocompatibility complex; R, recipient.
Refers to the occurrence of CMV, disease after discontinuing antiviral prophylaxis with ganciclovir or valganciclovir (usually administered for 100–200 days).
As shown in Table 1, the available literature is not equally distributed across the different clinical scenarios involved. One of the most immediate applications of CMV-CMI monitoring is the individualization of the length of prophylaxis. Rather than the fixed-duration regimen of 3–6 months of valganciclovir—up to 12 months for lung transplant recipients—recommended by the current guidelines for high-risk patients [7, 80], the knowledge of the CMV-CMI functionality would allow for prematurely discontinuing prophylaxis in patients that have mounted a protective response, or prolonging it beyond the standard schedule in the presence of a negative (non-reactive) assay result. Manuel et al. provided early data on the usefulness of the QTF-CMV assay in a multicenter cohort of 127 D+/R− patients. The presence of a positive (reactive) assay at the end of valganciclovir prophylaxis was associated with a lower 12 months incidence of CMV disease as compared to negative or indeterminate results (6.4% versus 22.2%, respectively; p-value < 0.001), yielding a positive predictive value (PPV) for immune protection of 90% (95% confidence interval [95% CI]: 74–98). Interestingly, those patients with an indeterminate QTF-CMV result—suggestive of a profoundly abrogated immunity or absence of CMV peptide recognition—had the highest incidence of late disease [43]. These findings have been subsequently confirmed in different SOT populations [53, 57, 75]. On the other hand, a recent study has suggested that the predictive accuracy in this clinical scenario of commercially available ELISpot assays is superior of that of the QTF-CMV assay [57]. A similar conclusion may be drawn from a meta-analysis in kidney transplant recipients [81]. The next natural step is to apply this evidence to the clinical decision-making process. In addition to the interventional studies reviewed in the next section, a retrospective study in lung transplant recipients reported a lower incidence of high-level CMV replication by using a QTF-CMV-guided strategy of extended valganciclovir prophylaxis (5–11 months) as compared to a fixed 5 months regimen (43.1% versus 60.3%, respectively; p-value < 0.001) [55]. These results were replicated using the T-SPOT®.CMV in a distinct cohort of R+ lung transplant recipients [82].
Although the ability of the QTF-CMV assay to stratify the risk of late CMV disease following the discontinuation of prophylaxis has been demonstrated for the D+/R− constellation, some studies restricted to R+ kidney transplant recipients receiving T-cell-depleting induction therapy (ATG) [54] or R+ lung transplant recipients [56] failed to find significant differences in the occurrence of viral reactivation between patients with reactive or non-reactive results. It has been proposed that the diagnostic accuracy of the QTF-CMV assay to predict protection from low-level infection among R+ patients might be improved by increasing the threshold for IFN-γ production used to define a positive result [54]. In addition, more sensitive techniques not restricted to CD8+ T-cell responses, such as ICS by flow cytometry and ELISpot-based assays, would perform better in this scenario, at the expense of being more time-consuming and costly [83].
The predominant population of R+ seropositive SOT recipients without ATG has been traditionally considered as an intermediate risk for CMV events, and either preemptive therapy or antiviral prophylaxis are recommended as prevention methods [7, 80]. A major contribution of the strategies for measuring the CMV-CMI has been the identification of a subgroup of R+ patients that lacks or displays very weak effective T-cell-mediated responses against CMV at the pre-transplant evaluation (non-reactive recipients [RNR]) despite their positive anti-CMV IgG serological status. The proportion of R+ patients with no detectable baseline CMV-CMI has been estimated at about 20%–30% [44, 59, 60, 84, 85]. From a functional perspective, these patients should be considered closer to the seronegative recipients (R−) than to the so-called intermediate-risk (R+) group, which would result in a higher susceptibility to post-transplant infection if they receive an organ from a seropositive donor [25]. In a study in kidney and lung transplant recipients, Cantisán et al. found that D+/RNR patients faced a markedly increased risk of CMV replication as compared to R+ patients with a positive (reactive) pre-transplant QTF-CMV assay (adjusted odds ratio [OR]: 10.49; 95% CI: 1.88–58.46) [60]. Comparable results have been obtained with the ICS technique [44, 59] or an ELISpot assay [51, 85]. An early assessment at post-transplant day 15 provides a predictive capacity significantly higher than at the pre-transplant evaluation since some transplant recipients with robust preformed CMV-CMI may significantly decrease their functional CMV-CMI after induction immunosuppression therapy, even in absence of ATG [44]. In this regard and unlike the QTF-CMV assay, the knowledge of the specific CMV-CMI against each individual CMV antigen that is provided by ELISpot-based assays, may further help to better stratify patients according to three distinct immunological risks, this is, at low, high, and at intermediate risk if one response against one of the two antigen is absent or very low [33]. Some factors have been reported to be associated with the absence of QTF-CMV reactivity among R+ SOT candidates such as profound lymphopenia, younger age, the type of organ to be transplanted, presence of certain recipient HLA genotypes and of non-HLA-A1/non-HLA-A2 alleles [84]. The latter finding is not unexpected as the presentation to the CD8+ T-cells of the viral epitopes contained in the “antigen tube” of the assay is restricted through some HLA class I alleles [86, 87].
Finally, some studies have been conducted to investigate the usefulness of post-transplant CMV-CMI monitoring among intermediate-risk recipients preemptively managed to predict protection against the development of CMV infection or, once established, the capacity of spontaneous clearance of viremia [42, 44, 49, 51, 52, 61–66]. These results pointed to the predominance of CD8+ T-cells in the early response to primary infection—or re-infection in the D+/R+ constellation—and CD4+ T-cells in the long-term control of latent infection [42, 44, 61]. The assessment of CMV-CMI at the onset of asymptomatic CMV viremia may be also useful to discern the patients that will spontaneously clear the infection from those who would eventually benefit from preemptive therapy. By applying the cut-off value for QTF-CMV positivity of ≥0.2 IU/mL of IFN-γ, Lisboa et al. reported a sensitivity and specificity in this clinical scenario of 82.8% and 75.0%, respectively, yielding a negative predictive value to predict virologic and/or clinical progression of asymptomatic viremia of 54.5% and a PPV of 90.9% to predict spontaneous clearance [65].
Few observational studies have also explored the role innate cells (NK and Non-Vγ9Vδ2 γδT cells) in different scenarios. For instance, pretransplant peripheral blood NKG2C+ NKG2A- NK cells could protect from CMV infection in kidney transplant recipients independently of the presence of CMV-specific T cells [88]. The NKG2C+ NK cell proportion in the bronchoalveolar lavage could also be a relevant biomarker for assessing risk of subsequent CMV viremia in lung transplant recipients [89]. During acute CMV infection, the NKG2C+ NK cells proliferate, become NKG2C(hi), and finally acquire CD57, a marker of “memory” NK cells that have been expanded in response to infection [90]. During CMV disease, non-Vγ9Vδ2 γδT cells expansion was correlated to the resolution of CMV infection and the emergence of CMV resistance in kidney transplant recipients, but more importantly was able to predict the absence of recurrence [15, 91]. A prospective clinical trial is ongoing to confirm this last finding (SPARCKLING study: NCT03339661).
Finally, as a complement to the assessment of the functionality of the CMV-specific T-cell response, other immunological biomarkers have been proposed to improve the process of risk stratification in the SOT population. This includes the assessment of antibodies targeting the pentameric complex (gH/gL/pUL128/pUL130/pUL131A), post-transplant hypogammaglobulinemia, absolute counts of total lymphocytes or peripheral blood subpopulations, as well as genetic markers. A detailed account of the advantage and limitations of these assays is summarized in Table 2.
TABLE 2
| Immunological biomarker | Rationale | Diagnostic performance, advantages and limitations | Selected studies |
|---|---|---|---|
| Serum immunoglobulin levels | Severe IgG HGG (usually defined by the threshold of <400–500 mg/dL) as a quantitative surrogate of the humoral immune response | Easily available and economical (nephelometry). Potentially reversible by IVIg/SCIg replacement therapy. Lack of specificity for CMV infection risk | [92, 93] |
| Total lymphocyte count | Lymphopenia (usually defined by the threshold of <0.5–0.75 × 103 cells/μL) as a quantitative surrogate of the T-cell-mediated immune response | Easily available and economical. Lack of specificity for CMV infection risk | [94–97] |
| Peripheral blood lymphocyte subpopulations | Enumeration of peripheral blood CD4+ and CD8+ T-cell counts at different post-transplant time points by automated flow cytometry | Less time- and labor-consuming than CMV-CMI monitoring. Lack of specificity for CMV infection risk. Simultaneous risk assessment for other opportunistic infections. Of particular usefulness in patients receiving T-cell-depleting agents | [98, 99] |
| SNP in genes orchestrating innate and adaptive responses (pattern recognition receptors and interferons) | Protective effect associated to SNPs within TLR9 and IFNL3 genes. Risk-conferring effect associated to SNPs within TLR2, MBL2, DC-SIGN, IL10 and IFNG genes | Attempts of polygenic risk scores (lacking external validation). Modest risk modification effect attributable to a given SNP. Lack of dedicated GWAS studies | [100–104] |
| Intracellular ATP production in CD4+ T-cells | Quantification of intracellular ATP release in CD4+ T-cells stimulated with a potent non-specific mitogen (phytohemagglutinin A), which would provide an overall functional evaluation of T-cell-mediated immunity | FDA-approved commercial assay (ImmuKnow®, Cylex). Lack of validated cut-off values to predict CMV infection. Time- and labor-consuming. Potentially affected by sample storage time | [56, 105] |
Other immunological approaches proposed within the risk assessment for post-transplant CMV infection.
ATP, adenosine triphosphate; CMV, cytomegalovirus; CMVCMI, cytomegalovirus-specific cell-mediated immunity; FDA, food and drug administration; GWAS, genomed-wide association study; HGG, hypogammaglobulinemia; IVIg, intravenous immunoglobulin; SCIg, subcutaneous immunoglobulin; SNP, single-nucleotide polymorphism.
Interventional Studies Evaluating CMV Immune Monitoring Strategies
The evidence generated by clinical trials on the use of CMV-CMI in transplant recipients is more limited. Most randomized controlled trials have focused on using the CMV-CMI assays for determining the duration of antiviral prophylaxis in intermediate or high-risk patients, particularly in kidney transplant recipients. In these studies, analysis of CMV-CMI has been performed using either the QTF-CMV or an ELISpot-CMV assay (Table 3).
TABLE 3
| Study author | Number of patients | Type of organ transplant | CMV serostatus | Cell-mediated immune assay | Intervention | Main results |
|---|---|---|---|---|---|---|
| [106] | 118 | Lung | R+ and D+/R− | QTF-CMV | Test at 5, 8 and 11 months, stop prophylaxis if test positive | Lower CMV replication in the allograft and longer duration of antiviral prophylaxis in the intervention group |
| [107] | 150 | Kidney | R+ on ATG | QTF-CMV | Test at 30, 45, 60, 90 days, stop prophylaxis if test positive | Similar incidence of CMV replication/disease, shorter duration of antiviral, lower incidence of neutropenia in the intervention group |
| [108] | 185 | Kidney (164) and liver (21) | R+ on ATG and D+/R− | T-Track-CMV | Test at 30, 60, 90 days (R+ and D+/R−), 120, 150, 180 (D+/R−), stop prophylaxis if test positive | Similar incidence of CMV replication/disease, shorter duration of antiviral in the intervention group |
| [109] | 27 | All SOT | R+ and D+/R− | QTF-CMV | Test at the end of therapy for CMV replication, add secondary prophylaxis in case of negative result | Lower incidence of CMV relapse in patients with a positive test |
| [110] | 160 | Kidney | R+ | T-SPOT.CMV | Stratify patients at transplant in low vs. high-risk according to test result. Then randomize to preemptive vs. prophylaxis | Higher incidence of CMV replication in high-risk group. Better performance of antiviral prophylaxis strategy in both groups |
Summary of the intervention studies on the application of CMV-CMI assays in SOT recipients.
ATG, anti-thymocyte globulin; CMV, cytomegalovirus; D, donor; QTF-CMV, QuantiFERON-CMV assay; R, recipient.
In the study by [106] 118 lung transplant recipients were randomized to receive a fixed duration of antiviral prophylaxis (5 months), or a duration based on the results of the QTF-CMV assay, performed at 5, 8 and 11 months after transplantation. Antiviral prophylaxis was continued in case of a negative result of the assay in the intervention group. CMV replication measured by PCR in the bronchoalveolar lavage was observed in 58% in the control group as compared to 37% in the intervention group (p = 0.03), and this effect was probably due to the longer duration of prophylaxis in patients in the intervention group. A significant number of patients (39%), mostly D+/R−, remained with undetectable CMV-CMI at the end of prophylaxis period.
In the TIMOVAL trial [107], R+ kidney transplant recipients receiving induction therapy with ATG were randomized to receive a fixed duration of 3 months of prophylaxis (control group) or a duration based of immune-monitoring every 2–4 weeks using the QTF-CMV assay. Despite receiving ATG, up to 45% of patients had a QTF-CMV result as soon as 30 days after transplantation. Incidence of CMV infection (17% immune-monitoring vs. 13% control) was similar between groups while duration of antiviral prophylaxis was shorter in the intervention group. Incidence of neutropenia was lower in the immune-monitoring arm.
In the CMV-CMI study from Switzerland [108] 185 kidney or liver transplant R+ recipients receiving ATG or D+/R− were randomized to receive 3 or 6 months of prophylaxis (depending on the risk group) or immune monitoring once monthly with the T-Track-CMV®. Overall, the incidence of clinically significant CMV infections was similar between groups (30.9% immune-monitoring vs. 31.1% group) although non-inferiority was not proven (p = 0.06). The duration of antiviral prophylaxis was significantly shorter in the intervention group (−26 days, p < 0.001). The impact of the intervention was more pronounced in R+ patients.
Kumar et al. [109] performed a single-arm interventional study using a QTF-CMV assay at the end of antiviral therapy for clinically significant CMV infection (both CMV disease and asymptomatic replication). Patients with a positive QTF-CMV result did not receive additional antiviral therapy while patients with a negative result received valganciclovir for 8 additional weeks. Of the 27 SOT recipients included, 14 patients had detectable IFN-γ levels and 13 had undetectable levels. Only 1/14 (7%) patient with a positive assay result had a relapse of CMV replication in contrast with 9/13 (69%) in the group with a negative assay result.
Finally, in the RESPECT trial [26], Jarque et al. used the T-SPOT. CMV at the time of transplant to stratify patients as being low-risk (positive assay) or at high-risk (negative assay) based on IE-1 CMV-CMI for predicting post transplant CMV replication. Patients were then randomized to receive antiviral prophylaxis or a preemptive approach. Patients with a positive CMV-CMI test had significantly lower rates of CMV replication/disease irrespective of the preventive strategy used. However, the best performance of the assay was when performed at 15 days post transplant (81% of CMV infection if test negative vs. 9% if test positive).
Although more interventional studies would be desirable to better delineate the clinical scenarios for the use of CMV-CMI monitoring in SOT recipients, a summary of the main data available is provided below.
• A CMV-CMI assay can be used in the pre transplant period (if no T-cell depletion will be used) to identify those patients with a negative or low pre-transplant CMV-CMI and thus being at higher risk of CMV infection and therefore to choose the most appropriate preventive strategy against CMV. However, a positive CMV-CMI test prior to transplantation may lead to misleading predictive interpretations since a proportion of these patients may become high risk after transplantation due to induction immunosuppressive therapy.
• In patients receiving universal prophylaxis, the most appropriate population for using these assays seems to be the CMV-seropositive patients receiving ATG (as proposed in the TIMOVAL and CMV-CMI trials). According to these studies, as soon as 1 month post transplant, the majority of patients (45%–62%) mount a measurable CMI response against CMV, associated with a low risk for developing CMV disease. A potential strategy for these patients can be to perform a single-point assay at 4–6 weeks after transplant and to stop antivirals if the test is positive. In case of a negative result, an extension of prophylaxis or a preemptive approach could be applied. Figure 2 illustrate a potential management of R+ patients according to the use of ATG.
• In patients managed with a preemptive approach, a CMV-CMI assay could be used in CMV-seropositive patients without receiving ATG (based on the RESPECT trial [110]). Here the risk of significant CMV replication is much lower and the probability to reach a detectable immune response much higher than in patients receiving ATG. A potential strategy for these patients can be to perform a single-point assay at 2 weeks after transplant and to stop PCR monitoring if the test is positive (Figure 2).
• There is limited data for high-risk D+/R− patients. In the CMV CMI study [108], the impact of the use of CMI assays was less visible in the high-risk group, mainly because the mounting of immunity was achieved later after transplant, and in only a minority of patients. A potential strategy in this population would consist in assessing CMV-CMI between 4 and 6 months post transplant and stop prophylaxis in case of a positive assay. Given the suboptimal sensitivity of CMV-CMI assays in this population, a negative result should not foster the extension of prophylaxis, but rather a closer follow-up after discontinuation of antivirals.
FIGURE 2
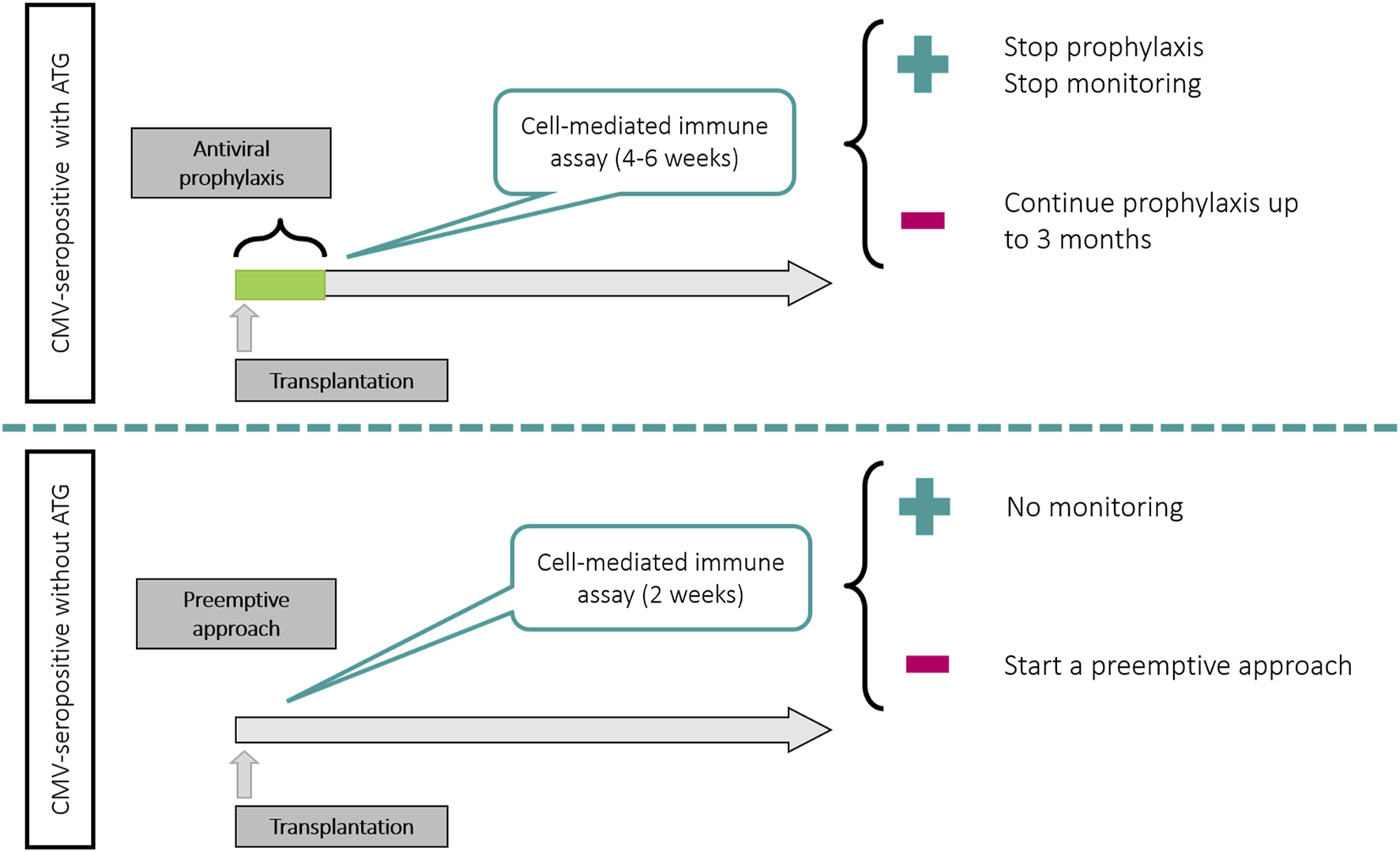
Potential uses of CMV-CMI assays in CMV-seropositive patients according to preventive strategy and use of T-cell depleting antibodies.
Conclusion
In this review we show the advances made in the field of CMV immune-risk stratification with the development of new sensitive assays measuring CMV-CMI. While most of the studies strongly suggest an added value of measuring CMV-CMI to better stratify the risk of CMV, in particular among R+ SOT recipients, yet some concerns arise when translating these immune tools into clinical practice; the precise predictive values illustrating the risk at the patient-individual level should be noted with caution to ultimately establish safe, guided preventive strategies. Specific cut-offs, the biological insight provided by each type of assay, and the precise clinical settings where to be implemented need to be further investigated through the implementation of clinical trials.
With the implementation of artificial intelligence, including highly powerful machine-learning algorithms, the combination of distinct clinical as well as immunological variables at distinct biological level could further refine the individual risk of transplant patients to develop CMV infection. Notably, this is the ultimate goal of the large multicenter European project (HORUS1) by developing a dynamic multidimensional biomarker algorithm to robustly assess the risk of developing CMV infection.
Therefore, an effort should be made among the transplant community to confirm the added value of cell-mediated immune assays over current clinical management, as though if confirmed, they could revolutionize the management of CMV infection by personalizing the type and duration of preventive therapy against CMV infection after SOT.
Statements
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
OB’s research is supported by a research grant network from the RICORS (Redes de Investigación Cooperativa Orientadas a Resultados en Salud) consortia by the Instituto de Salud Carlos III and co-funded by European Union (ERDF/ESF)- A way to build Europe. MF-R holds a research contract “Miguel Servet” (CP18/00073) from the Instituto de Salud Carlos III, Spanish Ministry of Science and Innovation, also co-funded by the European Union. OB, HK, LC, and OM participate in the HORUS project, which has received funding from HORIZON Europe under the grant agreement No.101057651.
Footnotes
References
1.
Raval AD Kistler KD Tang Y Murata Y Snydman DR . Epidemiology, Risk Factors, and Outcomes Associated With Cytomegalovirus in Adult Kidney Transplant Recipients: A Systematic Literature Review of Real-World Evidence. Transpl Infect Dis (2021) 23(2):e13483. 10.1111/tid.13483
2.
Griffiths P Reeves M . Pathogenesis of Human Cytomegalovirus in the Immunocompromised Host. Nat Rev Microbiol (2021) 19(12):759–73. 10.1038/s41579-021-00582-z
3.
Zangger N Oxenius A . T Cell Immunity to Cytomegalovirus Infection. Curr Opin Immunol (2022) 77:102185. 10.1016/j.coi.2022.102185
4.
Bhugra A Khodare A Agarwal R Pamecha V Gupta E . Role of Cytomegalovirus Specific Cell-Mediated Immunity in the Monitoring of Cytomegalovirus Infection Among Living Donor Liver Transplantation Adult Recipients: A Single-Center Experience. Transpl Infect Dis (2023) 25(1):e14011. 10.1111/tid.14011
5.
Kotton CN Torre-Cisneros J International CMVSF Aguado JM Alain S Baldanti F et al Cytomegalovirus in the Transplant Setting: Where Are We Now and What Happens Next? A Report From the International CMV Symposium 2021. Transpl Infect Dis (2022) 24(6):e13977. 10.1111/tid.13977
6.
Lucia M Crespo E Cruzado JM Grinyo JM Bestard O . Human CMV-Specific T-Cell Responses in Kidney Transplantation; Toward Changing Current Risk-Stratification Paradigm. Transpl Int (2014) 27(7):643–56. 10.1111/tri.12318
7.
Kotton CN Kumar D Caliendo AM Huprikar S Chou S Danziger-Isakov L et al The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-Organ Transplantation. Transplantation (2018) 102(6):900–31. 10.1097/TP.0000000000002191
8.
Kaminski H Jarque M Halfon M Taton B Di Ascia L Pfirmann P et al Different Impact of rATG Induction on CMV Infection Risk in D+R- and R+ KTRs. J Infect Dis (2019) 220(5):761–71. 10.1093/infdis/jiz194
9.
Kaminski H Marseres G Cosentino A Guerville F Pitard V Fournie JJ et al Understanding Human γδ T Cell Biology Toward a Better Management of Cytomegalovirus Infection. Immunol Rev (2020) 298(1):264–88. 10.1111/imr.12922
10.
Mace EM Orange JS . Emerging Insights Into Human Health and NK Cell Biology From the Study of NK Cell Deficiencies. Immunol Rev (2019) 287(1):202–25. 10.1111/imr.12725
11.
Guma M Angulo A Vilches C Gomez-Lozano N Malats N Lopez-Botet M . Imprint of Human Cytomegalovirus Infection on the NK Cell Receptor Repertoire. Blood (2004) 104(12):3664–71. 10.1182/blood-2004-05-2058
12.
Venema H van den Berg AP van Zanten C van Son WJ van der Giessen M The TH . Natural Killer Cell Responses in Renal Transplant Patients With Cytomegalovirus Infection. J Med Virol (1994) 42(2):188–92. 10.1002/jmv.1890420216
13.
Ishiyama K Arakawa-Hoyt J Aguilar OA Damm I Towfighi P Sigdel T et al Mass Cytometry Reveals Single-Cell Kinetics of Cytotoxic Lymphocyte Evolution in CMV-Infected Renal Transplant Patients. Proc Natl Acad Sci U S A (2022) 119(8):e2116588119. 10.1073/pnas.2116588119
14.
Dechanet J Merville P Berge F Bone-Mane G Taupin JL Michel P et al Major Expansion of Gammadelta T Lymphocytes Following Cytomegalovirus Infection in Kidney Allograft Recipients. J Infect Dis (1999) 179(1):1–8. 10.1086/314568
15.
Kaminski H Garrigue I Couzi L Taton B Bachelet T Moreau JF et al Surveillance of γδ T Cells Predicts Cytomegalovirus Infection Resolution in Kidney Transplants. J Am Soc Nephrol (2016) 27(2):637–45. 10.1681/ASN.2014100985
16.
Dechanet J Merville P Lim A Retiere C Pitard V Lafarge X et al Implication of Gammadelta T Cells in the Human Immune Response to Cytomegalovirus. J Clin Invest (1999) 103(10):1437–49. 10.1172/JCI5409
17.
Couzi L Pitard V Netzer S Garrigue I Lafon ME Moreau JF et al Common Features of Gammadelta T Cells and CD8(+) Alphabeta T Cells Responding to Human Cytomegalovirus Infection in Kidney Transplant Recipients. J Infect Dis (2009) 200(9):1415–24. 10.1086/644509
18.
Halary F Pitard V Dlubek D Krzysiek R de la Salle H Merville P et al Shared Reactivity of V{delta}2(neg) {gamma}{delta} T Cells Against Cytomegalovirus-Infected Cells and Tumor Intestinal Epithelial Cells. J Exp Med (2005) 201(10):1567–78. 10.1084/jem.20041851
19.
Marlin R Pappalardo A Kaminski H Willcox CR Pitard V Netzer S et al Sensing of Cell Stress by Human γδ TCR-Dependent Recognition of Annexin A2. Proc Natl Acad Sci U S A (2017) 114(12):3163–8. 10.1073/pnas.1621052114
20.
Boppana SB Britt WJ . Antiviral Antibody Responses and Intrauterine Transmission After Primary Maternal Cytomegalovirus Infection. J Infect Dis (1995) 171(5):1115–21. 10.1093/infdis/171.5.1115
21.
Jonjic S Pavic I Polic B Crnkovic I Lucin P Koszinowski UH . Antibodies Are Not Essential for the Resolution of Primary Cytomegalovirus Infection But Limit Dissemination of Recurrent Virus. J Exp Med (1994) 179(5):1713–7. 10.1084/jem.179.5.1713
22.
Genini E Percivalle E Sarasini A Revello MG Baldanti F Gerna G . Serum Antibody Response to the gH/gL/pUL128-131 Five-Protein Complex of Human Cytomegalovirus (HCMV) in Primary and Reactivated HCMV Infections. J Clin Virol (2011) 52(2):113–8. 10.1016/j.jcv.2011.06.018
23.
Falagas ME Snydman DR Ruthazer R Griffith J Werner BG Freeman R et al Cytomegalovirus Immune Globulin (CMVIG) Prophylaxis Is Associated With Increased Survival After Orthotopic Liver Transplantation. The Boston Center for Liver Transplantation CMVIG Study Group. Clin Transpl (1997) 11(5):432–7.
24.
Humar A Mazzulli T Moussa G Razonable RR Paya CV Pescovitz MD et al Clinical Utility of Cytomegalovirus (CMV) Serology Testing in High-Risk CMV D+/R-Transplant Recipients. Am J Transpl (2005) 5(5):1065–70. 10.1111/j.1600-6143.2005.00797.x
25.
Lucia M Crespo E Melilli E Cruzado JM Luque S Llaudo I et al Preformed Frequencies of Cytomegalovirus (CMV)-Specific Memory T and B Cells Identify Protected CMV-Sensitized Individuals Among Seronegative Kidney Transplant Recipients. Clin Infect Dis (2014) 59(11):1537–45. 10.1093/cid/ciu589
26.
Appay V Dunbar PR Callan M Klenerman P Gillespie GM Papagno L et al Memory CD8+ T Cells Vary in Differentiation Phenotype in Different Persistent Virus Infections. Nat Med (2002) 8(4):379–85. 10.1038/nm0402-379
27.
Gamadia LE Remmerswaal EB Weel JF Bemelman F van Lier RA Ten Berge IJ . Primary Immune Responses to Human CMV: A Critical Role for IFN-Gamma-Producing CD4+ T Cells in Protection Against CMV Disease. Blood (2003) 101(7):2686–92. 10.1182/blood-2002-08-2502
28.
Sylwester AW Mitchell BL Edgar JB Taormina C Pelte C Ruchti F et al Broadly Targeted Human Cytomegalovirus-Specific CD4+ and CD8+ T Cells Dominate the Memory Compartments of Exposed Subjects. J Exp Med (2005) 202(5):673–85. 10.1084/jem.20050882
29.
Klenerman P Oxenius A . T Cell Responses to Cytomegalovirus. Nat Rev Immunol (2016) 16(6):367–77. 10.1038/nri.2016.38
30.
Rentenaar RJ Gamadia LE van DerHoek N van Diepen FN Boom R Weel JF et al Development of Virus-Specific CD4(+) T Cells During Primary Cytomegalovirus Infection. J Clin Invest (2000) 105(4):541–8. 10.1172/JCI8229
31.
van Leeuwen EM Remmerswaal EB Vossen MT Rowshani AT Wertheim-van Dillen PM van Lier RA et al Emergence of a CD4+CD28-Granzyme B+, Cytomegalovirus-Specific T Cell Subset After Recovery of Primary Cytomegalovirus Infection. J Immunol (2004) 173(3):1834–41. 10.4049/jimmunol.173.3.1834
32.
Casazza JP Betts MR Price DA Precopio ML Ruff LE Brenchley JM et al Acquisition of Direct Antiviral Effector Functions by CMV-Specific CD4+ T Lymphocytes With Cellular Maturation. J Exp Med (2006) 203(13):2865–77. 10.1084/jem.20052246
33.
Banas B Steubl D Renders L Chittka D Banas MC Wekerle T et al Clinical Validation of a Novel Enzyme-Linked Immunosorbent Spot Assay-Based In Vitro Diagnostic Assay to Monitor Cytomegalovirus-Specific Cell-Mediated Immunity in Kidney Transplant Recipients: A Multicenter, Longitudinal, Prospective, Observational Study. Transpl Int (2018) 31(4):436–50. 10.1111/tri.13110
34.
Jorgenson MR Descourouez JL Lyu B Astor BC Garg N Smith JA et al The Risk of Cytomegalovirus Infection After Treatment of Acute Rejection in Renal Transplant Recipients. Clin Transpl (2019) 33(8):e13636. 10.1111/ctr.13636
35.
Santos CA Brennan DC Fraser VJ Olsen MA . Delayed-Onset Cytomegalovirus Disease Coded During Hospital Readmission After Kidney Transplantation. Transplantation (2014) 98(2):187–94. 10.1097/TP.0000000000000030
36.
Egli A Kumar D Broscheit C O'Shea D Humar A . Comparison of the Effect of Standard and Novel Immunosuppressive Drugs on CMV-Specific T-Cell Cytokine Profiling. Transplantation (2013) 95(3):448–55. 10.1097/TP.0b013e318276a19f
37.
Xu H Perez SD Cheeseman J Mehta AK Kirk AD . The Allo- and Viral-Specific Immunosuppressive Effect of Belatacept, But Not Tacrolimus, Attenuates With Progressive T Cell Maturation. Am J Transpl (2014) 14(2):319–32. 10.1111/ajt.12574
38.
Schaenman J Rossetti M Pickering H Sunga G Wilhalme H Elashoff D et al Preservation of Antiviral Immunologic Efficacy Without Alloimmunity After Switch to Belatacept in Calcineurin Inhibitor-Intolerant Patients. Kidney Int Rep (2023) 8(1):126–40. 10.1016/j.ekir.2022.10.015
39.
Magua W Johnson AC Karadkhele GM Badell IR Vasanth P Mehta AK et al Impact of Belatacept and Tacrolimus on Cytomegalovirus Viral Load Control and Relapse in Moderate and High-Risk Cytomegalovirus Serostatus Kidney Transplant Recipients. Transpl Infect Dis (2022) 24(6):e13983. 10.1111/tid.13983
40.
Chavarot N Divard G Scemla A Amrouche L Aubert O Leruez-Ville M et al Increased Incidence and Unusual Presentations of CMV Disease in Kidney Transplant Recipients After Conversion to Belatacept. Am J Transpl (2021) 21(7):2448–58. 10.1111/ajt.16430
41.
Kaminski H Marseres G Yared N Nokin MJ Pitard V Zouine A et al mTOR Inhibitors Prevent CMV Infection Through the Restoration of Functional αβ and γδ T Cells in Kidney Transplantation. J Am Soc Nephrol (2022) 33(1):121–37. 10.1681/ASN.2020121753
42.
Egli A Binet I Binggeli S Jager C Dumoulin A Schaub S et al Cytomegalovirus-Specific T-Cell Responses and Viral Replication in Kidney Transplant Recipients. J Transl Med (2008) 6:29. 10.1186/1479-5876-6-29
43.
Manuel O Husain S Kumar D Zayas C Mawhorter S Levi ME et al Assessment of Cytomegalovirus-Specific Cell-Mediated Immunity for the Prediction of Cytomegalovirus Disease in High-Risk Solid-Organ Transplant Recipients: A Multicenter Cohort Study. Clin Infect Dis (2013) 56(6):817–24. 10.1093/cid/cis993
44.
Fernandez-Ruiz M Gimenez E Vinuesa V Ruiz-Merlo T Parra P Amat P et al Regular Monitoring of Cytomegalovirus-Specific Cell-Mediated Immunity in Intermediate-Risk Kidney Transplant Recipients: Predictive Value of the Immediate Post-Transplant Assessment. Clin Microbiol Infect (2019) 25(3):381 e1–381381.e10. 10.1016/j.cmi.2018.05.010
45.
San-Juan R Navarro D Garcia-Reyne A Montejo M Munoz P Carratala J et al Effect of Long-Term Prophylaxis in the Development of Cytomegalovirus-Specific T-Cell Immunity in D+/R-Solid Organ Transplant Recipients. Transpl Infect Dis (2015) 17(5):637–46. 10.1111/tid.12417
46.
Kumar D Chin-Hong P Kayler L Wojciechowski D Limaye AP Osama Gaber A et al A Prospective Multicenter Observational Study of Cell-Mediated Immunity as a Predictor for Cytomegalovirus Infection in Kidney Transplant Recipients. Am J Transpl (2019) 19(9):2505–16. 10.1111/ajt.15315
47.
Paez-Vega A Poyato A Rodriguez-Benot A Guirado L Fortun J Len O et al Analysis of Spontaneous Resolution of Cytomegalovirus Replication After Transplantation in CMV-Seropositive Patients With Pretransplant CD8+IFNG+ Response. Antivir Res (2018) 155:97–105. 10.1016/j.antiviral.2018.05.006
48.
Kumar D Chernenko S Moussa G Cobos I Manuel O Preiksaitis J et al Cell-Mediated Immunity to Predict Cytomegalovirus Disease in High-Risk Solid Organ Transplant Recipients. Am J Transpl (2009) 9(5):1214–22. 10.1111/j.1600-6143.2009.02618.x
49.
Abate D Saldan A Fiscon M Cofano S Paciolla A Furian L et al Evaluation of Cytomegalovirus (CMV)-Specific T Cell Immune Reconstitution Revealed that Baseline Antiviral Immunity, Prophylaxis, or Preemptive Therapy But Not Antithymocyte Globulin Treatment Contribute to CMV-Specific T Cell Reconstitution in Kidney Transplant Recipients. J Infect Dis (2010) 202(4):585–94. 10.1086/654931
50.
Weseslindtner L Kerschner H Steinacher D Nachbagauer R Kundi M Jaksch P et al Prospective Analysis of Human Cytomegalovirus DNAemia and Specific CD8+ T Cell Responses in Lung Transplant Recipients. Am J Transpl (2012) 12(8):2172–80. 10.1111/j.1600-6143.2012.04076.x
51.
Bestard O Lucia M Crespo E Van Liempt B Palacio D Melilli E et al Pretransplant Immediately Early-1-Specific T Cell Responses Provide Protection for CMV Infection After Kidney Transplantation. Am J Transpl (2013) 13(7):1793–805. 10.1111/ajt.12256
52.
Abate D Saldan A Mengoli C Fiscon M Silvestre C Fallico L et al Comparison of Cytomegalovirus (CMV) Enzyme-Linked Immunosorbent Spot and CMV Quantiferon Gamma Interferon-Releasing Assays in Assessing Risk of CMV Infection in Kidney Transplant Recipients. J Clin Microbiol (2013) 51(8):2501–7. 10.1128/JCM.00563-13
53.
Deborska-Materkowska D Perkowska-Ptasinska A Sadowska A Gozdowska J Ciszek M Serwanska-Swietek M et al Diagnostic Utility of Monitoring Cytomegalovirus-Specific Immunity by QuantiFERON-Cytomegalovirus Assay in Kidney Transplant Recipients. BMC Infect Dis (2018) 18(1):179. 10.1186/s12879-018-3075-z
54.
Fernandez-Ruiz M Rodriguez-Goncer I Parra P Ruiz-Merlo T Corbella L Lopez-Medrano F et al Monitoring of CMV-Specific Cell-Mediated Immunity With a Commercial ELISA-Based Interferon-Gamma Release Assay in Kidney Transplant Recipients Treated With Antithymocyte Globulin. Am J Transpl (2020) 20(8):2070–80. 10.1111/ajt.15793
55.
Gardiner BJ Lee SJ Robertson AN Cristiano Y Snell GI Morrissey CO et al Real-World Experience of Quantiferon-CMV Directed Prophylaxis in Lung Transplant Recipients. J Heart Lung Transpl (2022) 41(9):1258–67. 10.1016/j.healun.2022.05.004
56.
Monforte V Sintes H Ussetti P Castejon R Perez VL Laporta R et al Assessment of Quantiferon®-CMV and Immuknow® Assays in CMV-Seropositive Lung Transplant Recipients to Stratify Risk of CMV Infection. Arch Bronconeumol (2022) 58(8):614–7. 10.1016/j.arbres.2021.10.002
57.
Gliga S Fiedler M Dornieden T Achterfeld A Paul A Horn PA et al Comparison of Three Cellular Assays to Predict the Course of CMV Infection in Liver Transplant Recipients. Vaccines (Basel). (2021) 9(2):88. 10.3390/vaccines9020088
58.
San-Juan R Navarro D Garcia-Reyne A Montejo M Munoz P Carratala J et al Effect of Delaying Prophylaxis Against CMV in D+/R-Solid Organ Transplant Recipients in the Development of CMV-Specific Cellular Immunity and Occurrence of Late CMV Disease. J Infect (2015) 71(5):561–70. 10.1016/j.jinf.2015.06.013
59.
Lopez-Oliva MO Martinez V Buitrago A Jimenez C Rivas B Escuin F et al Pretransplant CD8 T-Cell Response to IE-1 Discriminates Seropositive Kidney Recipients at Risk of Developing CMV Infection Posttransplant. Transplantation (2014) 97(8):839–45. 10.1097/01.TP.0000438025.96334.eb
60.
Cantisan S Lara R Montejo M Redel J Rodriguez-Benot A Gutierrez-Aroca J et al Pretransplant Interferon-Gamma Secretion by CMV-Specific CD8+ T Cells Informs the Risk of CMV Replication After Transplantation. Am J Transpl (2013) 13(3):738–45. 10.1111/ajt.12049
61.
Sester M Sester U Gartner B Heine G Girndt M Mueller-Lantzsch N et al Levels of Virus-Specific CD4 T Cells Correlate With Cytomegalovirus Control and Predict Virus-Induced Disease After Renal Transplantation. Transplantation (2001) 71(9):1287–94. 10.1097/00007890-200105150-00018
62.
Gerna G Lilleri D Chiesa A Zelini P Furione M Comolli G et al Virologic and Immunologic Monitoring of Cytomegalovirus to Guide Preemptive Therapy in Solid-Organ Transplantation. Am J Transpl (2011) 11(11):2463–71. 10.1111/j.1600-6143.2011.03636.x
63.
Pongsakornkullachart K Chayakulkeeree M Vongwiwatana A Kantakamalakul W Skulratanasak P Phoompoung P . QuantiFERON-Cytomegalovirus Assay for Prediction of Cytomegalovirus Viremia in Kidney Transplant Recipients: Study From High Cytomegalovirus Seroprevalence Country. Front Cel Infect Microbiol (2022) 12:893232. 10.3389/fcimb.2022.893232
64.
Sund F Lidehall AK Claesson K Foss A Totterman TH Korsgren O et al CMV-Specific T-Cell Immunity, Viral Load, and Clinical Outcome in Seropositive Renal Transplant Recipients: A Pilot Study. Clin Transpl (2010) 24(3):401–9. 10.1111/j.1399-0012.2009.00976.x
65.
Lisboa LF Kumar D Wilson LE Humar A . Clinical Utility of Cytomegalovirus Cell-Mediated Immunity in Transplant Recipients With Cytomegalovirus Viremia. Transplantation (2012) 93(2):195–200. 10.1097/TP.0b013e31823c1cd4
66.
Andreani M Albano L Benzaken S Cassuto E Jeribi A Caramella A et al Monitoring of CMV-Specific Cell-Mediated Immunity in Kidney Transplant Recipients With a High Risk of CMV Disease (D+/R-): A Case Series. Transpl Proc. (2020) 52(1):204–11. 10.1016/j.transproceed.2019.11.002
67.
Pipeling MR John ER Orens JB Lechtzin N McDyer JF . Primary Cytomegalovirus Phosphoprotein 65-Specific CD8+ T-Cell Responses and T-Bet Levels Predict Immune Control During Early Chronic Infection in Lung Transplant Recipients. J Infect Dis (2011) 204(11):1663–71. 10.1093/infdis/jir624
68.
Radha R Jordan S Puliyanda D Bunnapradist S Petrosyan A Amet N et al Cellular Immune Responses to Cytomegalovirus in Renal Transplant Recipients. Am J Transpl (2005) 5(1):110–7. 10.1111/j.1600-6143.2003.00647.x
69.
Benmarzouk-Hidalgo OJ Cisneros JM Cordero E Martin-Pena A Sanchez B Martin-Gandul C et al Therapeutic Effect of the Acquisition of Cytomegalovirus-Specific Immune Response During Preemptive Treatment. Transplantation (2011) 91(8):927–33. 10.1097/TP.0b013e3182115ba2
70.
Mena-Romo JD Perez Romero P Martin-Gandul C Gentil MA Suarez-Artacho G Lage E et al CMV-Specific T-Cell Immunity in Solid Organ Transplant Recipients at Low Risk of CMV Infection. Chronology and Applicability in Preemptive Therapy. J Infect (2017) 75(4):336–45. 10.1016/j.jinf.2017.05.020
71.
Lopez-Oliva MO Martinez V Rodriguez-Sanz A Alvarez L Santana MJ Selgas R et al Pre-Transplant Assessment of Pp65-Specific CD4 T Cell Responses Identifies CMV-Seropositive Patients Treated With rATG at Risk of Late Onset Infection. Clin Immunol (2020) 211:108329. 10.1016/j.clim.2019.108329
72.
Westall GP Mifsud NA Kotsimbos T . Linking CMV Serostatus to Episodes of CMV Reactivation Following Lung Transplantation by Measuring CMV-Specific CD8+ T-Cell Immunity. Am J Transpl (2008) 8(8):1749–54. 10.1111/j.1600-6143.2008.02294.x
73.
Lochmanova A Lochman I Tomaskova H Marsalkova P Raszka J Mrazek J et al Quantiferon-CMV Test in Prediction of Cytomegalovirus Infection After Kidney Transplantation. Transpl Proc (2010) 42(9):3574–7. 10.1016/j.transproceed.2010.07.101
74.
Jarque M Melilli E Crespo E Manonelles A Montero N Torras J et al CMV-Specific Cell-Mediated Immunity at 3-Month Prophylaxis Withdrawal Discriminates D+/R+ Kidney Transplants at Risk of Late-Onset CMV Infection Regardless the Type of Induction Therapy. Transplantation (2018) 102(11):e472–e80. 10.1097/TP.0000000000002421
75.
Chiereghin A Potena L Borgese L Gibertoni D Squarzoni D Turello G et al Monitoring of Cytomegalovirus (CMV)-Specific Cell-Mediated Immunity in Heart Transplant Recipients: Clinical Utility of the QuantiFERON-CMV Assay for Management of Posttransplant CMV Infection. J Clin Microbiol (2018) 56(4):e01040-17. 10.1128/JCM.01040-17
76.
Gabanti E Lilleri D Scaramuzzi L Zelini P Rampino T Gerna G . Comparison of the T-Cell Response to Human Cytomegalovirus (HCMV) as Detected by Cytokine Flow Cytometry and QuantiFERON-CMV Assay in HCMV-Seropositive Kidney Transplant Recipients. New Microbiol (2018) 41(3):195–202.
77.
Manuel O Kralidis G Mueller NJ Hirsch HH Garzoni C van Delden C et al Impact of Antiviral Preventive Strategies on the Incidence and Outcomes of Cytomegalovirus Disease in Solid Organ Transplant Recipients. Am J Transpl (2013) 13(9):2402–10. 10.1111/ajt.12388
78.
Fernandez-Ruiz M Arias M Campistol JM Navarro D Gomez-Huertas E Gomez-Marquez G et al Cytomegalovirus Prevention Strategies in Seropositive Kidney Transplant Recipients: An Insight Into Current Clinical Practice. Transpl Int (2015) 28(9):1042–54. 10.1111/tri.12586
79.
Wills MR Carmichael AJ Mynard K Jin X Weekes MP Plachter B et al The Human Cytotoxic T-Lymphocyte (CTL) Response to Cytomegalovirus Is Dominated by Structural Protein Pp65: Frequency, Specificity, and T-Cell Receptor Usage of Pp65-Specific CTL. J Virol (1996) 70(11):7569–79. 10.1128/JVI.70.11.7569-7579.1996
80.
Torre-Cisneros J Aguado JM Caston JJ Almenar L Alonso A Cantisan S et al Management of Cytomegalovirus Infection in Solid Organ Transplant Recipients: SET/GESITRA-SEIMC/REIPI Recommendations. Transpl Rev (Orlando) (2016) 30(3):119–43. 10.1016/j.trre.2016.04.001
81.
Ruan Y Guo W Liang S Xu Z Niu T . Diagnostic Performance of Cytomegalovirus (CMV) Immune Monitoring With ELISPOT and QuantiFERON-CMV Assay in Kidney Transplantation: A PRISMA-Compliant Article. Medicine (Baltimore) (2019) 98(16):e15228. 10.1097/MD.0000000000015228
82.
Donadeu L Revilla-Lopez E Jarque M Crespo E Torija A Bravo C et al CMV-Specific Cell-Mediated Immunity Predicts a High Level of CMV Replication After Prophylaxis Withdrawal in Lung Transplant Recipients. J Infect Dis (2021) 224(3):526–31. 10.1093/infdis/jiaa727
83.
Fernandez-Ruiz M Redondo N Parra P Ruiz-Merlo T Rodriguez-Goncer I Polanco N et al Comparison of Intracellular Cytokine Staining Versus an ELISA-Based Assay to Assess CMV-Specific Cell-Mediated Immunity in High-Risk Kidney Transplant Recipients. J Med Virol (2023) 95(4):e28733. 10.1002/jmv.28733
84.
Cantisan S Rodelo-Haad C Paez-Vega A Nieto A Vaquero JM Poyato A et al Factors Related to the Development of CMV-Specific CD8+ T Cell Response in CMV-Seropositive Solid Organ Transplant Candidates. Am J Transpl (2015) 15(3):715–22. 10.1111/ajt.13012
85.
Schachtner T Stein M Reinke P . CMV-Specific T Cell Monitoring Offers Superior Risk Stratification of CMV-Seronegative Kidney Transplant Recipients of a CMV-Seropositive Donor. Transplantation (2017) 101(10):e315–e25. 10.1097/TP.0000000000001825
86.
Fernandez-Ruiz M Corrales I Amat P Gonzalez E Andres A Navarro D et al Influence of Age and HLA Alleles on the CMV-Specific Cell-Mediated Immunity Among CMV-Seropositive Kidney Transplant Candidates. Am J Transpl (2015) 15(9):2525–6. 10.1111/ajt.13370
87.
Walker S Fazou C Crough T Holdsworth R Kiely P Veale M et al Ex Vivo Monitoring of Human Cytomegalovirus-Specific CD8+ T-Cell Responses Using QuantiFERON-CMV. Transpl Infect Dis (2007) 9(2):165–70. 10.1111/j.1399-3062.2006.00199.x
88.
Ataya M Redondo-Pachon D Llinas-Mallol L Yelamos J Heredia G Perez-Saez MJ et al Pretransplant Adaptive NKG2C+ NK Cells Protect Against Cytomegalovirus Infection in Kidney Transplant Recipients. Am J Transpl (2020) 20(3):663–76. 10.1111/ajt.15658
89.
Calabrese DR Chong T Wang A Singer JP Gottschall M Hays SR et al NKG2C Natural Killer Cells in Bronchoalveolar Lavage Are Associated With Cytomegalovirus Viremia and Poor Outcomes in Lung Allograft Recipients. Transplantation (2019) 103(3):493–501. 10.1097/TP.0000000000002450
90.
Lopez-Verges S Milush JM Schwartz BS Pando MJ Jarjoura J York VA et al Expansion of a Unique CD57⁺NKG2Chi Natural Killer Cell Subset During Acute Human Cytomegalovirus Infection. Proc Natl Acad Sci U S A (2011) 108(36):14725–32. 10.1073/pnas.1110900108
91.
Lafarge X Merville P Cazin MC Berge F Potaux L Moreau JF et al Cytomegalovirus Infection in Transplant Recipients Resolves When Circulating Gammadelta T Lymphocytes Expand, Suggesting a Protective Antiviral Role. J Infect Dis (2001) 184(5):533–41. 10.1086/322843
92.
Florescu DF Kalil AC Qiu F Schmidt CM Sandkovsky U . What Is the Impact of Hypogammaglobulinemia on the Rate of Infections and Survival in Solid Organ Transplantation? A Meta-Analysis. Am J Transpl (2013) 13(10):2601–10. 10.1111/ajt.12401
93.
Greenberger PA Walker CL Fitzsimons TE Roberts M . Hypogammaglobulinemia Associated With Cytomegalovirus Pneumonia. J Infect Dis (1991) 163(3):631–3. 10.1093/infdis/163.3.631
94.
Gardiner BJ Nierenberg NE Chow JK Ruthazer R Kent DM Snydman DR . Absolute Lymphocyte Count: A Predictor of Recurrent Cytomegalovirus Disease in Solid Organ Transplant Recipients. Clin Infect Dis (2018) 67(9):1395–402. 10.1093/cid/ciy295
95.
Yoon M Oh J Chun KH Lee CJ Kang SM . Post-Transplant Absolute Lymphocyte Count Predicts Early Cytomegalovirus Infection After Heart Transplantation. Sci Rep (2021) 11(1):1426. 10.1038/s41598-020-80790-4
96.
Perry WA Paulus JK Price LL Snydman DR Chow JK . Association Between Lymphopenia at 1 Month Posttransplant and Infectious Outcomes or Death in Heart Transplant Recipients. Clin Infect Dis (2021) 73(11):e3797–e3803. 10.1093/cid/ciaa1800
97.
Schoeberl AK Zuckermann A Kaider A Aliabadi-Zuckermann A Uyanik-Uenal K Laufer G et al Absolute Lymphocyte Count as a Marker for Cytomegalovirus Infection After Heart Transplantation. Transplantation (2023) 107(3):748–52. 10.1097/TP.0000000000004360
98.
Calarota SA Chiesa A De Silvestri A Morosini M Oggionni T Marone P et al T-Lymphocyte Subsets in Lung Transplant Recipients: Association Between Nadir CD4 T-Cell Count and Viral Infections After Transplantation. J Clin Virol (2015) 69:110–6. 10.1016/j.jcv.2015.06.078
99.
Fernandez-Ruiz M Lopez-Medrano F Allende LM Andres A Garcia-Reyne A Lumbreras C et al Kinetics of Peripheral Blood Lymphocyte Subpopulations Predicts the Occurrence of Opportunistic Infection After Kidney Transplantation. Transpl Int (2014) 27(7):674–85. 10.1111/tri.12321
100.
Manuel O Wojtowicz A Bibert S Mueller NJ van Delden C Hirsch HH et al Influence of IFNL3/4 Polymorphisms on the Incidence of Cytomegalovirus Infection After Solid-Organ Transplantation. J Infect Dis (2015) 211(6):906–14. 10.1093/infdis/jiu557
101.
Fernandez-Ruiz M Corrales I Arias M Campistol JM Gimenez E Crespo J et al Association Between Individual and Combined SNPs in Genes Related to Innate Immunity and Incidence of CMV Infection in Seropositive Kidney Transplant Recipients. Am J Transpl (2015) 15(5):1323–35. 10.1111/ajt.13107
102.
Redondo N Navarro D Aguado JM Fernandez-Ruiz M . Human Genetic Polymorphisms and Risk of Viral Infection After Solid Organ Transplantation. Transpl Rev (Orlando) (2022) 36(1):100669. 10.1016/j.trre.2021.100669
103.
Redondo N Rodriguez-Goncer I Parra P Ruiz-Merlo T Lopez-Medrano F Gonzalez E et al Influence of Single-Nucleotide Polymorphisms in TLR3 (Rs3775291) and TLR9 (Rs352139) on the Risk of CMV Infection in Kidney Transplant Recipients. Front Immunol (2022) 13:929995. 10.3389/fimmu.2022.929995
104.
Bodro M Cervera C Linares L Suarez B Llopis J Sanclemente G et al Polygenic Innate Immunity Score to Predict the Risk of Cytomegalovirus Infection in CMV D+/R- Transplant Recipients. A Prospective Multicenter Cohort Study. Front Immunol (2022) 13:897912. 10.3389/fimmu.2022.897912
105.
Perez-Jacoiste Asin MA Fernandez-Ruiz M Lopez-Medrano F Aquilino C Gonzalez E Ruiz-Merlo T et al Monitoring of Intracellular Adenosine Triphosphate in CD4(+) T Cells to Predict the Occurrence of Cytomegalovirus Disease in Kidney Transplant Recipients. Transpl Int (2016) 29(10):1094–105. 10.1111/tri.12816
106.
Westall GP Cristiano Y Levvey BJ Whitford H Paraskeva MA Paul E et al A Randomized Study of Quantiferon CMV-Directed Versus Fixed-Duration Valganciclovir Prophylaxis to Reduce Late CMV After Lung Transplantation. Transplantation (2019) 103(5):1005–13. 10.1097/TP.0000000000002454
107.
Paez-Vega A Gutierrez-Gutierrez B Aguera ML Facundo C Redondo-Pachon D Suner M et al Immunoguided Discontinuation of Prophylaxis for Cytomegalovirus Disease in Kidney Transplant Recipients Treated With Antithymocyte Globulin: A Randomized Clinical Trial. Clin Infect Dis (2022) 74(5):757–65. 10.1093/cid/ciab574
108.
Manuel O Laager M Hirzel C Neofytos D Walti LN Hoenger G et al Immune Monitoring-Guided vs Fixed Duration of Antiviral Prophylaxis Against Cytomegalovirus in Solid-Organ Transplant Recipients. A Multicenter, Randomized Clinical Trial. Clin Infect Dis (2023):ciad575. 10.1093/cid/ciad575
109.
Kumar D Mian M Singer L Humar A . An Interventional Study Using Cell-Mediated Immunity to Personalize Therapy for Cytomegalovirus Infection After Transplantation. Am J Transpl (2017) 17(9):2468–73. 10.1111/ajt.14347
110.
Jarque M Crespo E Melilli E Gutierrez A Moreso F Guirado L et al Cellular Immunity to Predict the Risk of Cytomegalovirus Infection in Kidney Transplantation: A Prospective, Interventional, Multicenter Clinical Trial. Clin Infect Dis (2020) 71(9):2375–85. 10.1093/cid/ciz1209
Summary
Keywords
immune monitoring, cytomegalovirus management, innate immunity, preventive strategies, antiviral prophylaxis
Citation
Bestard O, Kaminski H, Couzi L, Fernández-Ruiz M and Manuel O (2023) Cytomegalovirus Cell-Mediated Immunity: Ready for Routine Use?. Transpl Int 36:11963. doi: 10.3389/ti.2023.11963
Received
25 August 2023
Accepted
26 October 2023
Published
07 November 2023
Volume
36 - 2023
Updates
Copyright
© 2023 Bestard, Kaminski, Couzi, Fernández-Ruiz and Manuel.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Oriol Manuel, oriol.manuel@chuv.ch
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.