Abstract
Diagnosing acute rejection after intestinal transplantation currently heavily relies on histopathological analysis of graft biopsies. However, the invasive risks associated with ileoscopic examination and the inaccessibility for biopsy after ileostomy closure hinder real-time detection of rejection responses. Molecules comprising the intestinal barrier have been identified as physiological and molecular biomarkers for various bowel conditions and systemic diseases. To investigate the potential of barrier function-related molecules in diagnosing rejection after intestinal transplantation, plasma samples were collected longitudinally from transplant recipients. The samples were categorized into “indeterminate for rejection (IND)” and “acute rejection (AR)” groups based on clinical diagnoses at each time point. The longitudinal association between plasma levels of these barrier function-related molecules and acute rejection was analyzed using the generalized estimating equations (GEE) method. Logistic GEE models revealed that plasma levels of claudin-3, occludin, sIgA, and zonulin were independent variables correlated with the clinical diagnosis of acute rejection. The subsequent prediction model demonstrated moderate ability in discriminating between IND and AR samples, with a sensitivity of 76.0%, specificity of 89.2%, and accuracy of 84.6%. In conclusion, monitoring plasma levels of claudin-3, occludin, sIgA, and zonulin shows great potential in aiding the diagnosis of acute rejection after intestinal transplantation.
Introduction
Intestinal transplantation (ITx) is considered the definitive treatment for patients with irreversible intestinal failure or life-threatening complications after long-term reliance on parenteral nutrition [1, 2]. The small intestine, with its abundant lymphoid tissue and diverse bacterial flora, has a higher incidence of acute rejection compared to other organ transplants [3, 4]. Approximately 50%–75% of small bowel transplantation patients experience acute rejection, ranging from mild forms with cryptic apoptosis to severe cases that result in ulcerative destruction of the epithelial mucosa, posing a challenge to graft and patient survival [3–6].
At present, the gold standard for diagnosing acute rejection following ITx depends on endoscopic observation and biopsy histology [7, 8]. However, the discontinuation of scheduled biopsies after ileostomy closure poses challenges in the early detection of acute rejection [9, 10]. Therefore, the identification of novel molecular biomarkers that can be non-invasively detected with high accuracy has been a crucial goal in aiding the clinical detection of rejection in intestinal transplantation [11–13].
The intestinal barrier plays a pivotal role in maintaining immune response homeostasis and immune tolerance by protecting the mucosal surface of the intestine [14–17]. The “microbiota-immune axis” concept has linked the intestinal barrier to various pathological conditions. Impairment of the intestinal barrier can lead to increased microbial translocation, inducing pro-inflammatory conditions in the intestine and subsequent systemic disorders [15–19]. Research has identified junctional molecules such as claudins, occludin, zonula occludens-1 (ZO-1), and regulatory proteins like secretory IgA (sIgA) and zonulin as potential biomarkers for several pathological conditions, including inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), food allergy, metabolic diseases, and leaky gut syndrome [20–23].
From 2007 to 2022, we conducted 31 ITx surgeries for 30 patients, with 5 year survival rates of 71.0% for patients and 51.6% for grafts, comparable to global figures [4, 5, 24]. To improve long-term outcomes by reducing graft loss related to acute rejection, we aimed to explore non-invasive biomarkers to enhance the accuracy and timeliness of acute rejection diagnosis. This study aimed to investigate the correlation between molecular levels of intestinal barrier components in plasma and the incidence of acute rejection, with the goal of developing a predictive model for diagnosing acute rejection.
Materials and Methods
Study Design and Sample Collection
To establish a time-series database for monitoring the plasma levels of intestinal barrier molecules in intestinal transplant recipients, plasma sample collection commenced on the day of transplantation, prior to the operation. Subsequent plasma collections followed the blood draw schedule outlined in the post-transplant monitoring protocol (see below). Blood samples were promptly transferred into heparin-containing tubes upon collection. After undergoing standardized centrifugation at 300×g, the plasma was divided into polypropylene tubes and stored at −80°C until analysis. The listing of plasma samples was documented based on the clinical manifestations and diagnosis recorded on each respective day.
Post-Transplant Monitoring Protocol and Diagnosis of Acute Rejection
At the time of ITx surgery, a Santulli’s proximal chimney ileostomy was created in each recipient for endoscopic examination and biopsy of the graft. The frequency of endoscopic examination was twice a week in the first month, once a week in the second month, once every other week in the third month, once a month in the fourth to sixth month, and whenever necessary.
The frequency of drawing blood was per day in the first week, twice a week in the second to the fourth week, once a week in the second month, once every other week in the third month, once a month in the fourth to sixth month, and whenever necessary.
The diagnosis of acute rejection was established through the pathological analysis of the biopsy, in conjunction with the identification of significant morphological changes in the graft mucosa during endoscopic examination [3, 25].
Quantification of Plasma Levels of the Intestinal Barrier Molecules
The plasma samples were thawed and vortexed before being subjected to ELISA assays. The procedure for detection and determination of their concentrations were performed according to the manufacturer’s protocols. The ELISA kits used in the study included: Citrulline (CEA505Ge, Cloud-Clone Corp., Katy, TX 77494, USA), Claudin-1 (CSB-EL005490HU, Cusabio Life Science, Houston, TX 77054, USA), Claudin-2 (CSB-EL005500HU, Cusabio Life Science), claudin-3 (CSB-EL005505HU, Cusabio Life Science), Claudin-4 (CSB-E17961h, Cusabio Life Science), L-FABP (HA404-1, Hycult Biotech Inc., Wayne, PA 19087, USA), Occludin (SEC228Hu, Cloud-Clone Corp.), sIgA (SEA641Hu, Cloud-Clone Corp.), zonular occludens-1 (CSB-E13916h, Cusabio Life Science) and zonulin (K5601, Immundiagnostik AG, 64625 Bensheim, Germany).
Statistical Analysis
This study employed generalized estimating equations (GEE) models to account for the effect of repeated measures, with Patient ID serving as the subject variable to define individual subjects within the dataset. Age and the concentrations of ten barrier function-related molecules were treated as continuous variables, while gender was considered as a categorical variable. The biopsy result was used as the binary outcome variable. Binary logistic GEE analysis was utilized to calculate the regression coefficients and odds ratios for the independent variables. The predictive probability of acute rejection and the clinical incidence of acute rejection were further analyzed using ROC (Receiver Operating Characteristic) curves.
Statistical analysis was conducted using SPSS software (version 22.0, IBM Corp., Chicago, IL, USA). The statistical data are presented as mean ± SE. The significance level was indicated by p-values, with a value of p < 0.05 considered statistically significant for all analyses.
Results
Patients and the Grouping of Plasma Samples
A total of 172 time-series plasma samples were collected from seven patients between September 2016 and June 2022, along with their corresponding medical records, including histopathological reports of graft biopsies during the same period. Plasma samples corresponding to non-rejection intestinal conditions (e.g., enteritis) and other systemic situations (e.g., sepsis) were excluded from the analysis. The basic information of the seven patients and the number of plasma samples collected are presented in Table 1. Next, based on clinical findings and/or biopsy reports on the day of blood collection, 143 plasma samples were categorized as IND (indeterminate for acute rejection, n = 93) and AR (acute rejection, n = 50). The mean plasma levels of ten intestinal barrier-related molecules are shown in Table 2.
TABLE 1
| Patient ID | Age | Gender | No. of plasma samples | Episode(s) of AR | Severity and timing of ARa |
|---|---|---|---|---|---|
| Pt-1 | 31 | Female | 42 | 4 | mild (D13) |
| severe (D30) | |||||
| severe (D175) | |||||
| severe (D234) | |||||
| Pt-2 | 58 | Male | 21 | 2 | mild (D21) |
| mild (D82) | |||||
| Pt-3 | 29 | Female | 22 | 0 | |
| Pt-4 | 37 | Male | 23 | 2 | severe (D16) |
| mild (D73) | |||||
| Pt-5 | 58 | Female | 14 | 1 | severe (D36) |
| Pt-6 | 28 | Female | 10 | 1 | mild (D20) |
| Pt-7 | 63 | Male | 11 | 0 |
Basic characteristics of the patients whose plasma samples were used in this research.
Timing of AR was represented as the day after transplant.
TABLE 2
| IND (Mean ± S.E.) | AR (Mean ± S.E.) | Unit | |
|---|---|---|---|
| N | 93 | 50 | |
| citrulline | 17.03 ± 0.50 | 16.21 ± 0.51 | ng/mL |
| claudin-1 | 309.76 ± 65.01 | 240.09 ± 15.05 | pg/mL |
| claudin-2 | 319.76 ± 38.20 | 276.58 ± 31.86 | pg/mL |
| claudin-3 | 76.20 ± 5.07 | 109.45 ± 8.04 | pg/mL |
| claudin-4 | 47.43 ± 5.29 | 50.62 ± 8.08 | pg/mL |
| L-FABP | 21.40 ± 2.30 | 16.52 ± 2.00 | ng/mL |
| occludin | 4.02 ± 0.43 | 2.34 ± 0.25 | ng/mL |
| sIgA | 120.17 ± 7.24 | 81.64 ± 5.26 | μg/mL |
| ZO-1 | 403.13 ± 25.04 | 437.91 ± 30.32 | pg/mL |
| zonulin | 5.99 ± 0.32 | 4.02 ± 0.24 | ng/mL |
Mean plasma levels of intestinal barrier molecules in the IND and AR groups.
Univariate Analysis
The association between plasma levels of intestinal barrier molecules and the diagnosis of AR was investigated by using univariate GEE analysis (Table 3). Among the examined variables, claudin-3 demonstrated a significant positive association with AR (coefficients = 0.013, p < 0.001). Conversely, citrulline demonstrated a significant negative association with acute rejection (coefficient = −0.121, p = 0.022). Notably, occludin and zonulin also exhibited significant negative association with acute rejection with the coefficients −0.339 (p = 0.010) and −0.367 (p < 0.001), respectively. The remaining variables, including claudin-1, claudin-2, claudin-4, L-FABP, sIgA, ZO-1, did not demonstrate statistically significant associations with AR in this univariate analysis (Table 3).
TABLE 3
| Regression coefficient | Standard Error | Wald | p-value | OR | 95% C.I. for OR | ||
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| citrulline | −0.121 | 0.053 | 5.245 | 0.022 | 0.886 | 0.799 | 0.983 |
| claudin-1 | <0.001 | <0.001 | 0.056 | 0.813 | 1.000 | 0.999 | 1.001 |
| claudin-2 | <0.001 | 0.001 | 0.156 | 0.693 | 1.000 | 0.999 | 1.001 |
| claudin-3 | 0.013 | 0.003 | 15.078 | < 0.001 | 1.013 | 1.006 | 1.020 |
| claudin-4 | 0.003 | 0.003 | 0.814 | 0.367 | 1.003 | 0.997 | 1.009 |
| L-FABP | <0.001 | <0.001 | 0.840 | 0.360 | 1.000 | 1.000 | 1.000 |
| occludin | −0.339 | 0.132 | 6.601 | 0.010 | 0.712 | 0.550 | 0.923 |
| sIgA | −0.002 | 0.004 | 0.442 | 0.506 | 0.998 | 0.990 | 1.005 |
| ZO-1 | <0.001 | 0.001 | 0.361 | 0.548 | 1.000 | 0.999 | 1.002 |
| zonulin | −0.367 | 0.082 | 20.113 | < 0.001 | 0.693 | 0.590 | 0.813 |
Longitudinal association between the plasma levels of intestinal barrier molecules and acute rejection by univariate GEE analysis.
Multivariable Analysis
Further multivariate GEE analysis was conducted to better understand the collective impact of these molecules on the risk of acute rejection. In Table 4, three regression models revealed certain significant associations between plasma levels of intestinal barrier molecules and acute rejection. Claudin-3 demonstrated a consistent positive association with acute rejection across all models, with odds ratios (OR) of 1.026 (95% C.I. 1.012–1.040, p < 0.001) in model 1, 1.025 (95% C.I. 1.013–1.037, p < 0.001) in model 2, and 1.022 (95% C.I. 1.011–1.032, p < 0.001) in model 3. On the other hand, occludin showed consistent negative associations with acute rejection, with ORs of 0.566 (95% C.I. 0.390–0.820, p = 0.003) in model 1, 0.627 (95% C.I. 0.459–0.857, p = 0.003) in model 2, and 0.574 (95% C.I. 0.417–0.791, p = 0.001) in model 3. Zonulin also exhibited a significant negative association with acute rejection, with ORs of 0.743 (95% C.I. 0.582–0.947, p = 0.016) in model 1, 0.778 (95% C.I. 0.631–0.960, p = 0.019) in model 2, and 0.817 (95% C.I. 0.684–0.975, p = 0.025) in model 3. Additionally, sIgA demonstrated a significant negative association with acute rejection in model 1, with an OR of 0.986 (95% C.I. 0.973–0.999, p = 0.031). However, other variables, including citrulline, claudin-1, claudin-2, claudin-4, L-FABP, and ZO-1, did not exhibit statistically significant associations with acute rejection in the multivariable analysis models.
TABLE 4
| Model 1a | Model 2a | Model 3a | ||||
|---|---|---|---|---|---|---|
| ORb (95% C.I.) | p-value | OR (95% C.I.) | p-value | OR (95% C.I.) | p-value | |
| claudin-3 | 1.026 (1.012–1.040) | <0.001 | 1.025 (1.013–1.037) | <0.001 | 1.022 (1.011–1.032) | <0.001 |
| occludin | 0.566 (0.390–0.82) | 0.003 | 0.627 (0.459–0.857) | 0.003 | 0.574 (0.417–0.791) | 0.001 |
| zonulin | 0.743 (0.582–0.947) | 0.016 | 0.778 (0.631–0.960) | 0.019 | 0.817 (0.684–0.975) | 0.025 |
| sIgA | 0.986 (0.967–0.999) | 0.031 | 0.990 (0.978–1.001) | 0.082 | ||
| citrulline | 0.906 (0.791–1.039) | 0.157 | ||||
| claudin-1 | 1.000 (0.999–1.001) | 0.972 | ||||
| claudin-2 | 1.001 (0.999–1.004) | 0.315 | ||||
| claudin-4 | 1.007 (0.996–1.017) | 0.209 | ||||
| L-FABP | 1.000 (1.000–1.000) | 0.847 | ||||
| ZO-1 | 0.999 (0.996–1.001) | 0.258 | ||||
| QICCb | 150.572 | 139.552 | 145.405 | |||
| sensitivity | 72.0% | 76.0% | 68.0% | |||
| specificity | 94.6% | 89.2% | 79.6% | |||
| accuracy | 86.7% | 84.6% | 75.5% | |||
Multivariate GEE analyses of the association between the plasma levels of intestinal barrier molecules and acute rejection.
Analyses were adjusted for gender and age.
OR, odds ratio; QICC, corrected quasi likelihood under independence model criterion.
Evaluation of the AR Prediction Models
The model performance, as assessed by the QICC (corrected quasi-likelihood under the independence model criterion), showed that GEE model 2 had the lowest value (QICC = 139.552), suggesting a better fit compared to model 1 (QICC = 150.572) and model 3 (QIC = 145.405). The diagnostic sensitivity, specificity, and accuracy of model 2 were 76.0%, 89.2%, and 84.6%, respectively (Table 4).
The predictive probability of acute rejection was calculated for each sample using regression model 2, and the relationship between the predictive probability and the incidence of acute rejection was analyzed using the ROC curve. The AUC was calculated as 0.862 (95% C.I. 0.794 to 0.930, p < 0.001), with a model probability cut-off of 0.432 being identified as the optimal threshold (Figure 1).
FIGURE 1
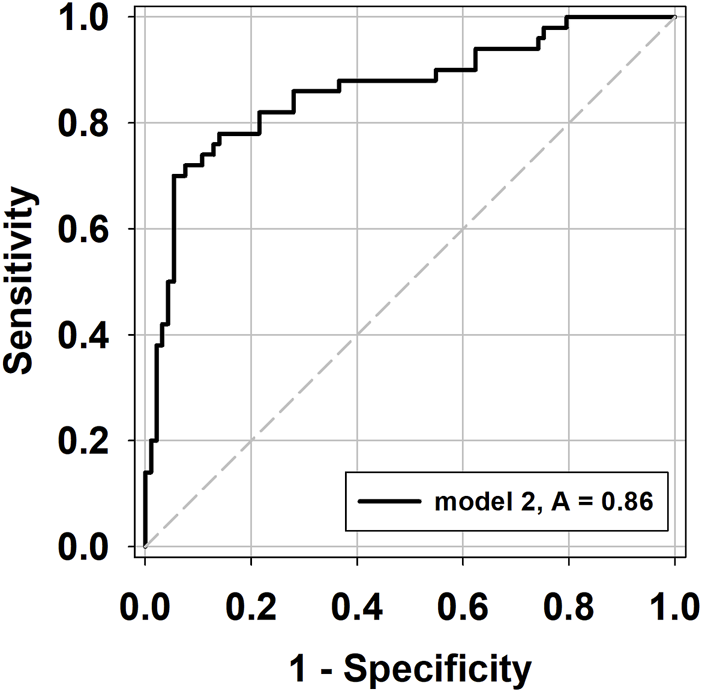
ROC curves of the predictive probability (PP) values from GEE model 2 in the prediction of acute rejection.
Discussion
In the present study, our results demonstrated that there were significant changes of claudin-3, occludin, sIgA, and zonulin during the onset of acute rejection after intestinal transplantation. These four molecules were independent factors most related to the clinical diagnoses of acute rejection, with that the increase in claudin-3 was associated with higher probability of acute rejection while increased occludin, sIgA and zonulin were negatively associated with acute rejection.
Endoscopic examination and tissue biopsy, as the most conventional method for graft monitoring, is still holds as the most definite way of confirming the diagnoses of rejection after intestinal transplantation [8, 9]. The search for non-invasive biomarkers for diagnosing acute rejection had been on in the recent decade. For example, blood citrulline and stool calprotectin had been considered as potential biomarkers for this purpose. Decreased citrulline was reported to reflect reduced enterocyte mass and intestinal insufficiency during acute rejection [11, 26, 27]; increased fecal calprotectin implicated ongoing immune responses in the intestine [28]. However, the lack of diagnostic specificity had limited their application in diagnosing acute rejection [29–31].
The molecules regulating intestinal barrier function had been identified as biomarkers to evaluate intestinal permeability thus being applied in the diagnosis of inflammatory bowel diseases [20–23]. We therefore investigated the applicability of these biomarkers in the detection of acute rejection after intestinal transplantation. As the results shown, we have tracked down to four molecules with different roles in barrier functions.
Secretory IgA serves as a crucial defense effector in the intestinal barrier, playing a key role in microbial neutralization and immune exclusion It is produced by plasma cells in the epithelial lamina propria, transported across epithelial cells, and then secreted into the lumen [32, 33]. Quantifying sIgA in serum or saliva has been applied for diagnosing Crohn’s disease (CD), ulcerative colitis (UC), and mucositis, with elevated levels observed in active CD and reduced levels in UC [34–36]. In our study, we found a negative association between sIgA levels and the onset of acute rejection (Table 4), suggesting altered sIgA production or depletion during rejection. Intestinal microbial stimulation and Th1-inhibiting/Th2-stimulating cytokines play a role in balancing sIgA levels [37]. Given that Th1-inhibiting cytokines (e.g., IL-6, IFN-γ and TNF-α) are involved in acute rejection, the downregulation of sIgA could serve as an early indicator of the acute rejection-associated Th1 immune response.
Altered expression of claudins in intestinal tissue has been extensively studied in patients with various intestinal disorders. Reduced expression of claudin-1 was observed in patients with inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS) [38, 39], while an increase in claudin-2 was found in the inflamed epithelium of patients with ulcerative colitis (UC) [40, 41]. The variation in claudin-3 and claudin-4 expression in IBD remains controversial, with studies reporting both reduced and increased expression [42–44]. In our study, we found a significant association between claudin-3 and acute rejection (Table 4), suggesting increased levels of claudin-3 in circulation due to intestinal tissue destruction during rejection.
The expression of occludin has shown variability in intestinal biopsies of patients with Crohn’s disease (CD) and ulcerative colitis (UC), suggesting inconsistent patterns in occludin expression within these studies [45, 46]. However, limited research has explored the use of plasma occludin as a marker for intestinal diseases. Interestingly, plasma occludin has gained attention in the context of blood-brain barrier damage, demonstrating fluctuating levels of occludin in different types of stroke [47].
Zonulin is an important regulator of barrier function that can disrupt the tight junctions between cells [48]. Previous research has highlighted the association between increased zonulin expression and various conditions, including inflammatory bowel disease (IBD), food allergy, diabetes, arthritis, liver disease, and aging [49–52]. In our study, we initially hypothesized that higher zonulin levels would contribute to the compromised intestinal integrity observed during acute rejection. However, contrary to our expectations, we found lower levels of zonulin in the acute rejection group. It is worth noting that the intestinal epithelial cells are a significant source of zonulin [53]. The reduction in zonulin levels in the acute rejection group could potentially be attributed to impaired or dysfunctional intestinal cells during the onset of acute rejection.
Our investigation into predictive factors for acute rejection, including claudin-3, occludin, sIgA, and zonulin, has illuminated distinct roles in maintaining intestinal barrier integrity. While claudin-3, occludin, and zonulin consistently emerged as significant factors associated with acute rejection in both univariate and multivariable analyses, sIgA demonstrated significance when other variables were considered. Excluding sIgA from model 2 led to reduced prediction sensitivity and accuracy in model 3, underscoring its crucial contribution.
ROC curves were generated to determine the optimal cutoff values for claudin-3, occludin, sIgA, and zonulin in predicting the occurrence of acute rejection. The analysis revealed that claudin-3 levels above 90.32 pg/mL (p < 0.001), occludin levels below 2.55 pg/mL (p = 0.185), sIgA levels below 63.37 μg/mL (p < 0.001), and zonulin levels below 2.95 ng/mL (p < 0.001) were indicative of the diagnosis of acute rejection, as depicted in Supplementary Figure S1. It is important to note, however, that the ROC analyses did not take into consideration the potential impact of repeated measurements within individual samples. Therefore, these cutoff values should not be used for clinical purposes at this time.
The quest for acute rejection-specific biomarkers is a challenging endeavor. Low specificity in differentiating acute rejection from enteritis complicates conclusive outcomes [31]. Due to the dispersed distribution of samples representing enteritis and sepsis outcomes within our patient cohort, we opted not to include these groups in our GEE analysis. However, we conducted a detailed comparison of relative changes in claudin-3, occludin, sIgA, and zonulin levels across the IND, AR, enteritis, and sepsis samples, as outlined in Supplementary Table S1. Noteworthy differences emerged among these sample groups, with both enteritis and sepsis-related samples displaying elevated concentrations of barrier markers compared to the IND group. In sepsis cases, we observed an exceptionally high mean level of claudin-3, spanning a wide range. This suggests the possibility that a simultaneous increase in these markers might indicate pathological conditions such as enteritis and sepsis. Importantly, this finding highlights that elevations in claudin-3 alone may not reliably indicate acute rejection, emphasizing the need for a more comprehensive diagnostic framework or a combination of markers.
Furthermore, our prediction model revealed a significant insight: the variation trends in sIgA and zonulin for patients with acute rejection were opposite to those observed in patients with other inflammatory or ulcerative intestinal diseases, both in existing literature and our own data. The mean values of sIgA and zonulin were relatively correlated with the severity of acute rejection with the AR-severe group displaying greater significance (p = 0.011 for sIgA; p = 0.002 for zonulin) than the AR-mild group (p = 0.023 for sIgA; p = 0.006 for zonulin) (Supplementary Table S2; Supplementary Figure S2). This finding holds significant potential when differential diagnoses must be made, providing a valuable advantage.
Our study, although illuminating, faces certain limitations, primarily due to a small number of patients and sample size. Enhancing the model’s sensitivity, specificity, and accuracy would benefit from additional laboratory data, including white blood cell counts, immunosuppressant concentrations, liver function parameters, and renal function indicators. Another limitation stems from the restricted quantity of plasma samples, limiting the exploration of potential molecules associated with barrier function. However, the innovative aspect of our study lies in our statistical approach, acknowledging the importance of individual variations. Accounting for repeated measures within each patient enables the capture of dynamic trends in diagnostic markers, creating a more comprehensive and reliable basis for detecting rejection. This approach differentiates our study from prior research, emphasizing the need to consider nuanced variations for a more accurate diagnosis.
Conclusion
In conclusion, our study has identified claudin-3, sIgA, and zonulin as promising non-invasive biomarkers for diagnosing acute rejection in recipients of intestinal transplants. Notably, this is the pioneering investigation to employ GEE analysis for comparing plasma levels of intestinal barrier molecules in the rejection and non-rejection phases of intestinal transplant recipients. We anticipate that our model holds significant potential to enhance post-transplant monitoring of intestinal grafts, ultimately advancing patient care in this critical domain.
Statements
Data availability statement
The datasets presented in this article are not readily available to preserve individuals’ privacy under local IRB regulation. Requests to access the datasets should be directed to yahuitsi@saturn.yzu.edu.tw.
Ethics statement
The studies involving humans were approved by Research Ethics Review Committee of Far Eastern Memorial Hospital (New Taipei City, Taiwan) (No. 105025-F). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YC and C-YC designed the study; YC, Y-HT, and S-HT participated in the acquisition, analysis, and interpretation of the data, and drafted the initial manuscript; YC, Y-HT, S-HT, and C-YC revised the manuscript and approved the manuscript to be published. All authors contributed to the article and approved the submitted version.
Funding
Supported by Ministry of Science and Technology, Taiwan (grant No. MOST 108-2314-B-418-012-MY2 and 110-2314-B-418-001-MY3 awarded to YC) and Far Eastern Memorial Hospital–National Yang Ming University Joint Research Program (grant No. 110DN23) awarded to YC and C-YC.
Acknowledgments
The authors would like to thank the members of Liver Disease Prevention & Treatment Research Foundation (Taipei City, Taiwan) for their technical support, and the Clinical Research Core Laboratory of Taipei Veterans General Hospital for providing experimental space and facilities.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2023.11595/full#supplementary-material
Abbreviations
AR, acute rejection; CD, Crohn’s disease; IND, indeterminate for rejection; IBS, irritable bowel syndrome; IBD, inflammatory bowel disease; ITx, intestinal transplantation; GEE, generalized estimating equations; L-FABP, liver-type fatty acid-binding protein; OR, odds ratio; ROC, Receiver Operating Characteristic; sIgA, secretory immunoglobulin A; UC, ulcerative colitis; ZO-1, zonula occludens-1.
References
1.
Kaufman SS Avitzur Y Beath SV Ceulemans LJ Gondolesi GE Mazariegos GV et al New Insights into the Indications for Intestinal Transplantation: Consensus in the Year 2019. Transplantation (2020) 104:937–46. 10.1097/TP.0000000000003065
2.
Woodward JM Massey D Sharkey L . The Long and Short of IT: Intestinal Failure-Associated Liver Disease (IFALD) in Adults-Recommendations for Early Diagnosis and Intestinal Transplantation. Frontline Gastroenterol (2020) 11:34–9. 10.1136/flgastro-2018-101069
3.
Huard G Schiano TD Moon J Iyer K . Severe Acute Cellular Rejection after Intestinal Transplantation Is Associated with Poor Patient and Graft Survival. Clin Transpl (2017) 31:e12956. 10.1111/ctr.12956
4.
Grant D Abu-Elmagd K Mazariegos G Vianna R Langnas A Mangus R et al Intestinal Transplant Registry Report: Global Activity and Trends. Am J Transpl (2015) 15:210–9. 10.1111/ajt.12979
5.
Amin A Farmer DG . Current Outcomes after Pediatric and Adult Intestinal Transplantation. Curr Opin Organ Transpl (2019) 24:193–8. 10.1097/MOT.0000000000000608
6.
Loo L Vrakas G Reddy S Allan P . Intestinal Transplantation: A Review. Curr Opin Gastroenterol (2017) 33:203–11. 10.1097/MOG.0000000000000358
7.
Chung CS Lee TH Chiu CT Chen Y . Snowmelt Sign" and "Corkscrew Microvessels" Predicting Epithelium Regeneration after Acute Rejection of Small-Bowel Transplantation: A Case Report. Transpl Proc (2017) 49:2419–21. 10.1016/j.transproceed.2017.11.006
8.
Carroll RE . Endoscopic Follow-Up of Intestinal Transplant Recipients. Gastroenterol Clin North Am (2018) 47:381–91. 10.1016/j.gtc.2018.01.012
9.
Crismale JF Mahmoud D Moon J Fiel MI Iyer K Schiano TD . The Role of Endoscopy in the Small Intestinal Transplant Recipient: A Review. Am J Transpl (2020) 21(5):1705–12. 10.1111/ajt.16354
10.
Venick RS . Grant Monitoring after Intestinal Transplantation. Curr Opin Organ Transpl (2021) 26:234–9. 10.1097/MOT.0000000000000847
11.
David AI Selvaggi G Ruiz P Gaynor JJ Tryphonopoulos P Kleiner GI et al Blood Citrulline Level Is an Exclusionary Marker for Significant Acute Rejection after Intestinal Transplantation. Transplantation (2007) 84:1077–81. 10.1097/01.tp.0000287186.04342.82
12.
Varkey J . Graft Assessment for Acute Rejection after Intestinal Transplantation: Current Status and Future Perspective. Scand J Gastroenterol (2021) 56:13–9. 10.1080/00365521.2020.1847318
13.
Lauro A Marino IR Matsumoto CS . Advances in Allograft Monitoring after Intestinal Transplantation. Curr Opin Organ Transpl (2016) 21:165–70. 10.1097/MOT.0000000000000279
14.
Thoo L Noti M Krebs P . Keep Calm: The Intestinal Barrier at the Interface of Peace and War. Cell Death Dis (2019) 10:849. 10.1038/s41419-019-2086-z
15.
Camilleri M Madsen K Spiller R Greenwood-Van Meerveld B Verne GN . Intestinal Barrier Function in Health and Gastrointestinal Disease. Neurogastroenterol Motil (2012) 24:503–12. 10.1111/j.1365-2982.2012.01921.x
16.
Vancamelbeke M Vermeire S . The Intestinal Barrier: A Fundamental Role in Health and Disease. Expert Rev Gastroenterol Hepatol (2017) 11:821–34. 10.1080/17474124.2017.1343143
17.
Horowitz A Chanez-Paredes SD Haest X Turner JR . Paracellular Permeability and Tight junction Regulation in Gut Health and Disease. Nat Rev Gastroenterol Hepatol (2023) 20(7):417–32. 10.1038/s41575-023-00766-3
18.
Konig J Wells J Cani PD Garcia-Rodenas CL MacDonald T Mercenier A et al Human Intestinal Barrier Function in Health and Disease. Clin Transl Gastroenterol (2016) 7:e196. 10.1038/ctg.2016.54
19.
Bischoff SC Barbara G Buurman W Ockhuizen T Schulzke JD Serino M et al Intestinal Permeability-Aa New Target for Disease Prevention and Therapy. BMC Gastroenterol (2014) 14:189. 10.1186/s12876-014-0189-7
20.
Genser L Aguanno D Soula HA Dong L Trystram L Assmann K et al Increased Jejunal Permeability in Human Obesity Is Revealed by a Lipid challenge and Is Linked to Inflammation and Type 2 Diabetes. J Pathol (2018) 246:217–30. 10.1002/path.5134
21.
Nascimento JC Matheus VA Oliveira RB Tada SFS Collares-Buzato CB . High-Fat Diet Induces Disruption of the Tight Junction-Mediated Paracellular Barrier in the Proximal Small Intestine before the Onset of Type 2 Diabetes and Endotoxemia. Dig Dis Sci (2020) 66(10):3359–74. 10.1007/s10620-020-06664-x
22.
Atreya R Neurath MF . IBD Pathogenesis in 2014: Molecular Pathways Controlling Barrier Function in IBD. Nat Rev Gastroenterol Hepatol (2015) 12:67–8. 10.1038/nrgastro.2014.201
23.
Lissner D Schumann M Batra A Kredel LI Kuhl AA Erben U et al Monocyte and M1 Macrophage-Induced Barrier Defect Contributes to Chronic Intestinal Inflammation in IBD. Inflamm Bowel Dis (2015) 21:1297–305. 10.1097/MIB.0000000000000384
24.
Chen Y Tseng S Koh C Chung C Weng C Tsai Y . Zinc Deficiency and Long-Term Outcome in Cases after Isolated Intestinal Transplantation in Taiwan. Transpl Proc (2018) 50:2771–4. 10.1016/j.transproceed.2018.03.094
25.
Wu T Abu-Elmagd K Bond G Nalesnik MA Randhawa P Demetris AJ . A Schema for Histologic Grading of Small Intestine Allograft Acute Rejection. Transplantation (2003) 75:1241–8. 10.1097/01.TP.0000062840.49159.2F
26.
Pappas PA Tzakis AG Gaynor JJ Carreno MR Ruiz P Huijing F et al An Analysis of the Association between Serum Citrulline and Acute Rejection Among 26 Recipients of Intestinal Transplant. Am J Transpl (2004) 4:1124–32. 10.1111/j.1600-6143.2004.00469.x
27.
Gondolesi G Ghirardo S Raymond K Hoppenhauer L Surillo D Rumbo C et al The Value of Plasma Citrulline to Predict Mucosal Injury in Intestinal Allografts. Am J Transpl (2006) 6:2786–90. 10.1111/j.1600-6143.2006.01513.x
28.
Cagnola H Scaravonati R Cabanne A Bianchi C Gruz F Errea A et al Evaluation of Calprotectin Level in Intestinal Content as an Early Marker for Graft Rejection. Transpl Proc (2010) 42:57–61. 10.1016/j.transproceed.2009.12.013
29.
Fragkos KC Forbes A . Citrulline as a Marker of Intestinal Function and Absorption in Clinical Settings: A Systematic Review and Meta-Analysis. United Eur Gastroenterol J (2018) 6:181–91. 10.1177/2050640617737632
30.
Lansing M Turner JM Wizzard P Lavallee CM Lim DW Muto M et al Plasma Citrulline Is Not a Biomarker for Intestinal Adaptation in Short Bowel Syndrome, Studied in Piglets: A Model for Human Neonates. Pediatr Surg Int (2019) 35:657–63. 10.1007/s00383-019-04475-4
31.
Rumbo M Oltean M . Intestinal Transplant Immunology and Intestinal Graft Rejection: From Basic Mechanisms to Potential Biomarkers. Int J Mol Sci (2023) 24:4541. 10.3390/ijms24054541
32.
Fagarasan S Honjo T . Intestinal IgA Synthesis: Regulation of Front-Line Body Defences. Nat Rev Immunol (2003) 3:63–72. 10.1038/nri982
33.
Pabst O Slack E . IgA and the Intestinal Microbiota: The Importance of Being Specific. Mucosal Immunol (2020) 13:12–21. 10.1038/s41385-019-0227-4
34.
Pietrzak B Tomela K Olejnik-Schmidt A Mackiewicz A Schmidt M . Secretory IgA in Intestinal Mucosal Secretions as an Adaptive Barrier against Microbial Cells. Int J Mol Sci (2020) 21:9254. 10.3390/ijms21239254
35.
Gong Y Niu W Tang Y Zhang Q Liu S Liu X et al Aggravated Mucosal and Immune Damage in a Mouse Model of Ulcerative Colitis with Stress. Exp Ther Med (2019) 17:2341–8. 10.3892/etm.2019.7162
36.
Arsenescu R Bruno ME Rogier EW Stefka AT McMahan AE Wright TB et al Signature Biomarkers in Crohn's Disease: Toward a Molecular Classification. Mucosal Immunol (2008) 1:399–411. 10.1038/mi.2008.32
37.
Wu Y Kudsk KA DeWitt RC Tolley EA Li J . Route and Type of Nutrition Influence IgA-Mediating Intestinal Cytokines. Ann Surg (1999) 229:662–7. discussion 7-8. 10.1097/00000658-199905000-00008
38.
Ivanov AI Nusrat A Parkos CA . The Epithelium in Inflammatory Bowel Disease: Potential Role of Endocytosis of Junctional Proteins in Barrier Disruption. Novartis Found Symp (2004) 263:115–24. discussion 24-32, 211-8. 10.1002/0470090480.ch9
39.
Tang Y Clayburgh DR Mittal N Goretsky T Dirisina R Zhang Z et al Epithelial NF-kappaB Enhances Transmucosal Fluid Movement by Altering Tight junction Protein Composition after T Cell Activation. Am J Pathol (2010) 176:158–67. 10.2353/ajpath.2010.090548
40.
Heller F Florian P Bojarski C Richter J Christ M Hillenbrand B et al Interleukin-13 Is the Key Effector Th2 Cytokine in Ulcerative Colitis that Affects Epithelial Tight Junctions, Apoptosis, and Cell Restitution. Gastroenterology (2005) 129:550–64. 10.1016/j.gastro.2005.05.002
41.
Luettig J Rosenthal R Barmeyer C Schulzke JD . Claudin-2 as a Mediator of Leaky Gut Barrier during Intestinal Inflammation. Tissue Barriers (2015) 3:e977176. 10.4161/21688370.2014.977176
42.
Prasad S Mingrino R Kaukinen K Hayes KL Powell RM MacDonald TT et al Inflammatory Processes Have Differential Effects on Claudins 2, 3 and 4 in Colonic Epithelial Cells. Lab Invest (2005) 85:1139–62. 10.1038/labinvest.3700316
43.
Amasheh S Dullat S Fromm M Schulzke JD Buhr HJ Kroesen AJ . Inflamed Pouch Mucosa Possesses Altered Tight Junctions Indicating Recurrence of Inflammatory Bowel Disease. Int J Colorectal Dis (2009) 24:1149–56. 10.1007/s00384-009-0737-8
44.
Lu Z Ding L Lu Q Chen YH . Claudins in Intestines: Distribution and Functional Significance in Health and Diseases. Tissue Barriers (2013) 1:e24978. 10.4161/tisb.24978
45.
Kuo WT Shen L Zuo L Shashikanth N Ong M Wu L et al Inflammation-induced Occludin Downregulation Limits Epithelial Apoptosis by Suppressing Caspase-3 Expression. Gastroenterology (2019) 157:1323–37. 10.1053/j.gastro.2019.07.058
46.
Yamamoto-Furusho JK Mendivil EJ Fonseca-Camarillo G . Differential Expression of Occludin in Patients with Ulcerative Colitis and Healthy Controls. Inflamm Bowel Dis (2012) 18:E1999. 10.1002/ibd.22835
47.
Lasek-Bal A Kokot A Gendosz de Carrillo D Student S Pawletko K Krzan A et al Plasma Levels of Occludin and Claudin-5 in Acute Stroke Are Correlated with the Type and Location of Stroke but Not with the Neurological State of Patients-Preliminary Data. Brain Sci (2020) 10:831. 10.3390/brainsci10110831
48.
Tripathi A Lammers KM Goldblum S Shea-Donohue T Netzel-Arnett S Buzza MS et al Identification of Human Zonulin, a Physiological Modulator of Tight Junctions, as Prehaptoglobin-2. Proc Natl Acad Sci U S A (2009) 106:16799–804. 10.1073/pnas.0906773106
49.
Kim JH Heo JS Baek KS Kim SY Kim JH Baek KH et al Zonulin Level, a Marker of Intestinal Permeability, Is Increased in Association with Liver Enzymes in Young Adolescents. Clin Chim Acta (2018) 481:218–24. 10.1016/j.cca.2018.03.005
50.
Vanuytsel T Vermeire S Cleynen I . The Role of Haptoglobin and its Related Protein, Zonulin, in Inflammatory Bowel Disease. Tissue Barriers (2013) 1:e27321. 10.4161/tisb.27321
51.
Moreno-Navarrete JM Sabater M Ortega F Ricart W Fernandez-Real JM . Circulating Zonulin, a Marker of Intestinal Permeability, Is Increased in Association with Obesity-Associated Insulin Resistance. PLoS One (2012) 7:e37160. 10.1371/journal.pone.0037160
52.
Qi Y Goel R Kim S Richards EM Carter CS Pepine CJ et al Intestinal Permeability Biomarker Zonulin Is Elevated in Healthy Aging. J Am Med Dir Assoc (2017) 18:810 e1–810. 10.1016/j.jamda.2017.05.018
53.
Fasano A Not T Wang W Uzzau S Berti I Tommasini A et al Zonulin, a Newly Discovered Modulator of Intestinal Permeability, and its Expression in Coeliac Disease. Lancet (2000) 355:1518–9. 10.1016/S0140-6736(00)02169-3
Summary
Keywords
biomarkers, acute rejection, intestinal transplant, intestinal barrier, noninvasive
Citation
Chen Y, Tseng S-H, Chen C-Y and Tsai Y-H (2023) Application of Intestinal Barrier Molecules in the Diagnosis of Acute Cellular Rejection After Intestinal Transplantation. Transpl Int 36:11595. doi: 10.3389/ti.2023.11595
Received
19 May 2023
Accepted
22 August 2023
Published
08 September 2023
Volume
36 - 2023
Updates
Copyright
© 2023 Chen, Tseng, Chen and Tsai.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chih-Yen Chen, chency@vghtpe.gov.tw; Ya-Hui Tsai, yahuitsi@saturn.yzu.edu.tw
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.