Abstract
Obesity is increasingly prevalent among candidates for kidney transplantation. Existing studies have shown conflicting post-transplant outcomes for obese patients which may relate to confounding bias from donor-related characteristics that were unaccounted for. We used ANZDATA Registry data to compare graft and patient survival between obese (BMI >27.5 kg/m2 Asians; >30 kg/m2 non-Asians) and non-obese kidney transplant recipients, while controlling for donor characteristics by comparing recipients of paired kidneys. We selected transplant pairs (2000–2020) where a deceased donor supplied one kidney to an obese candidate and the other to a non-obese candidate. We compared the incidence of delayed graft function (DGF), graft failure and death by multivariable models. We identified 1,522 pairs. Obesity was associated with an increased risk of DGF (aRR = 1.26, 95% CI 1.11–1.44, p < 0.001). Obese recipients were more likely to experience death-censored graft failure (aHR = 1.25, 95% CI 1.05–1.49, p = 0.012), and more likely to die with function (aHR = 1.32, 95% CI 1.15–1.56, p = 0.001), versus non-obese recipients. Long-term patient survival was significantly worse in obese patients with 10- and 15-year survival of 71% and 56% compared to 77% and 63% in non-obese patients. Addressing obesity is an unmet clinical need in kidney transplantation.
Introduction
Over the past four decades, the worldwide prevalence of obesity has tripled. In addition to the associations between obesity and hypertension, type 2 diabetes and coronary artery disease, obesity is clearly associated with premature mortality [1]. Obesity has therefore had a significant impact on community health as well as posing a major economic challenge to global healthcare systems. Obesity is increasingly prevalent in the end-stage kidney disease (ESKD) and kidney transplant populations [2,3]. In the US, the proportion of ESKD patients that were obese between 2008 and 2016 was nearly 40% [4].
Whilst kidney transplant recipients with high body mass index (BMI) are more like to develop post-transplant diabetes, congestive heart failure, atrial fibrillation, and cardiovascular death [5–7], transplantation offers a survival benefit for obese recipients compared to remaining on dialysis [8,9]. However, kidney transplant recipients with high BMI are at an increased risk of post-transplant complications, including prolonged wound healing, dehiscence, hernias, surgical site infections, deep vein thrombosis, and reintubation. These issues contribute to a longer hospital stay and higher hospital costs for transplantation in the obese [10–12].
The long-term graft and patient outcomes of obese recipients compared to non-obese recipients have remained controversial. When compared to non-obese recipients, some reports described an increased risk of graft failure and mortality for obese recipients whilst others have found no significant differences [12–15]. These disparate outcomes, may relate to the confounding bias of non-randomly distributed donor-related characteristics which were not accounted for [16–18]. Therefore, we sought to investigate the association between obesity and incidence of delayed graft function (DGF), graft survival and patient survival while controlling for donor characteristics by comparing obese and non-obese recipients of kidneys from a common donor, a matched-pair analysis. We hypothesized that obesity would increase the risk of DGF and lead to inferior graft and patient survival.
Materials and Methods
We extracted data from the Australia and New Zealand Dialysis and Transplant Registry (ANZDATA). The ANZDATA Registry is a clinical quality registry that collects comprehensive data from all patients with ESKD in Australia and New Zealand. Details of the structure and method of ANZDATA Registry data collection can be found on the Registry website (https://www.anzdata.org.au/anzdata/). We included all deceased donor kidney-only transplant pairs between 1 January 2000 and 31 December 2020, where a deceased donor supplied one kidney to an obese recipient and the other to a non-obese recipient. We excluded recipients under the age of 18, recipients of a deceased donor kidney retrieved outside Australia or New Zealand, and recipients of a second or subsequent transplant. We used the World Health Organization (WHO) classification of obesity as BMI greater than 30 kg/m2 for non-Asians, and greater than 27.5 kg/m2 for Asians due to differences in body habitus compared to the Western population [19–21]. Follow-up was until loss to follow-up, or 31 December 2020. The primary outcome was DGF which was defined as receipt of hemodialysis within 72 h after transplant prior to 2017, and receipt of hemodialysis within 7 days of transplantation after 2017 [22]. This modification to the definition of delayed graft function was due to a policy change made by ANZDATA in 2017. The secondary outcomes were death and death-censored graft failure.
We compared baseline characteristics of paired recipients using paired t-test or Wilcoxon’s signed rank test for continuous variables and McNemar’s test for dichotomous variables. We estimated the cumulative incidence of graft failure using Aalen-Johansen estimator to account for death as a competing event. We used Gray’s test to compare the cumulative incidence of graft failure in the presence of the competing risk of death. We used Kaplan-Meier curves to compare unadjusted patient survival. We used a logrank test to compare the probability of patient survival at different time points. We estimated the rate ratio of DGF for obese patients compared with non-obese patients, using conditional Poisson regression, adjusting for potential confounders [23–25]. As a sensitivity analysis, we repeated this analysis excluding patients who experienced graft failure within 90 days of transplantation. Time to graft failure and time to death were analyzed using Cox regression stratified by donor [24,25].
A dose-response analysis was performed to examine the association between the degree of obesity (i.e., class I, class II and class III) and clinical outcomes. Obesity was categorized as class I, class II and class III according to WHO guidelines (Table 3). We estimated the rate ratio of DGF using conditional Poisson regression and the hazard ratio of graft failure and death using Cox regression, adjusting for potential confounders.
The potential confounders considered were age at transplantation, sex, ethnicity, cause of kidney disease, duration of dialysis, dialysis modality prior to transplant, human leukocyte antigen (HLA) mismatch, ischemia time, maximum panel reactive antibodies, donor kidney side, pre-existing comorbidities including diabetes, chronic lung disease, cardiovascular disease (any of coronary artery, cerebrovascular or peripheral vascular), and non-skin cancer, acute rejection within 6 months of transplantation (for graft failure and death only), DGF (as a categorical variable, for graft failure and death only), and graft failure (as a time-varying covariate, for death only). We used stepwise selection methods where variables with a significance level of 0.20 were considered and included in the base multivariable model. We used backward selection method to remove variables that were not significant at the 0.05 level [26]. We used complete case analysis because the number of missing values was less than 5%. All analyses were performed using Stata Statistical Software: Release 14.2 (StataCorp., College Station, TX) This study was approved by the Ethics Review Committee of the Sydney Local Health District, Royal Prince Alfred Hospital Zone.
Results
Study Cohort
Between 1 January 2000, and 31 December 2020, 16,554 patients received their first kidney transplant in Australia and New Zealand. After inclusion and exclusion criteria were applied, 1,522 pairs were identified where a deceased donor supplied one kidney to an obese recipient and the other to a non-obese recipient (Figure 1). Follow-up time was 19,768 person-years in total, with a median follow-up time of 5.3 years (interquartile range 2.5–9.5 years). Nine of the obese recipients and seven of the non-obese recipients were lost to follow-up.
FIGURE 1
Donor and recipient baseline characteristics are summarized in Tables 1, 2. Baseline characteristics indicate that obese and non-obese recipients were comparable in terms of sex, time on dialysis, ischemia time, HLA mismatch and maximum panel reactive antibody percentage. The obese group included a higher proportion of recipients aged 50–65 (48% vs. 44%), p < 0.001), fewer people of Asian ancestry (12% vs. 15%, p < 0.001), more Indigenous people (17% vs. 11%, p < 0.001), more people with pre-existing diabetes (33% vs. 21%, p < 0.001) and comorbid cardiovascular disease (33% vs. 27%, p = 0.001) and more right-sided kidneys (55% vs. 45%, p < 0.001).
TABLE 1
| Factor | N = 1,522 n (%) |
|---|---|
| Age | |
| <18 | 79 (5) |
| 18–34 | 254 (17) |
| 35–49 | 426 (28) |
| 50–65 | 556 (37) |
| 65+ | 207 (14) |
| Male | 870 (57) |
| Body mass index (BMI) | |
| Underweight | 47 (3) |
| Normal | 556 (37) |
| Overweight | 534 (35) |
| Obese | 383 (25) |
| Terminal serum creatinine concentration, μmol/L | 96.4 ± 83.2 |
| Diabetes | 96 (6) |
| Hypertension | 388 (25) |
| Neurological determination of death (NDD) | 1,167 (77) |
| Cause of death | |
| Intracranial hemorrhage | 640 (44) |
| Traumatic brain injury | 285 (19) |
| Cerebral infarct | 94 (6) |
| Cerebral hypoxia/ischemia | 380 (26) |
| Other neurological condition | 12 (1) |
| Non-neurological condition | 59 (4) |
Donor characteristics.
TABLE 2
| Factor | Obese | Not obese | p-value |
|---|---|---|---|
| N = 1,522 | N = 1,522 | ||
| Age at transplant | n (%) | n (%) | <0.001 |
| 18–34 | 113 (7) | 193 (13) | |
| 35–49 | 430 (28) | 414 (27) | |
| 50–65 | 728 (48) | 667 (44) | |
| 65+ | 251 (16) | 248 (16) | |
| Male | 985 (65) | 991 (65) | 0.82 |
| Ethnicity | <0.001 | ||
| Caucasian | 1,001 (66) | 1,006 (66) | |
| Indigenous | 257 (17) | 162 (11) | |
| Asian | 185 (12) | 232 (15) | |
| Other | 79 (5) | 122 (8) | |
| Primary renal disease | <0.001 | ||
| GN | 575 (38) | 628 (41) | |
| Renovascular | 123 (8) | 112 (7) | |
| Diabetes | 351 (23) | 231 (15) | |
| Other | 473 (31) | 551 (36) | |
| Time since first RRT | 0.14 | ||
| 0–1 year | 173 (11) | 209 (14) | |
| 1–3 years | 594 (39) | 575 (38) | |
| Over 3 years | 755 (50) | 738 (48) | |
| Dialysis modality prior to transplant | 0.008 | ||
| Pre-emptive transplant | 11 (1) | 17 (1) | |
| HD | 1,106 (73) | 1,030 (68) | |
| PD | 405 (27) | 475 (31) | |
| Ischemia time [mean (sd)] | 12.1 (4.9) | 12.0 (5.0) | 0.52 |
| HLA mismatches | 0.58 | ||
| 0 | 46 (3) | 38 (2) | |
| 1–2 | 408 (27) | 427 (28) | |
| 3–4 | 483 (32) | 460 (30) | |
| 5–6 | 580 (38) | 596 (39) | |
| Maximum panel reactive antibodies | 0.50 | ||
| 0 | 918 (60) | 935 (61) | |
| 1–50 | 491 (32) | 465 (31) | |
| >50 | 110 (7) | 121 (8) | |
| Pre-existing comorbidities | |||
| Chronic lung disease | 130 (9) | 124 (8) | 0.69 |
| Cardiovascular disease | 501 (33) | 418 (27) | 0.001 |
| Diabetes | 504 (33) | 316 (21) | <0.001 |
| Right kidney | 832 (55) | 690 (45) | <0.001 |
Recipient and transplantation characteristics for obese and non-obese recipients.
GN, Glomerulonephritis; HD, hemodialysis; PD, peritoneal dialysis; HLA, Human Leukocyte Antigen; RRT, renal replacement therapy.
Outcomes
Delayed Graft Function
A greater proportion of obese recipients experienced DGF compared to non-obese recipients (39% vs. 30%, p < 0.001). Conditional Poisson regression demonstrated an increased risk of DGF for obese recipients versus their non-obese pair (aRR = 1.27, 95% CI 1.12–1.44, p < 0.001), after adjusting for dialysis modality prior to transplant, ischemia time and pre-existing cardiovascular disease and accounting for donor-related factors (Supplementary Table S1).
Sensitivity analysis, excluding those patients who experienced graft failure within 90 days of transplantation, showed a similar effect of obesity on DGF to the primary analysis (aRR = 1.29, 95% CI 1.12–1.48, p < 0.001).
Graft Failure
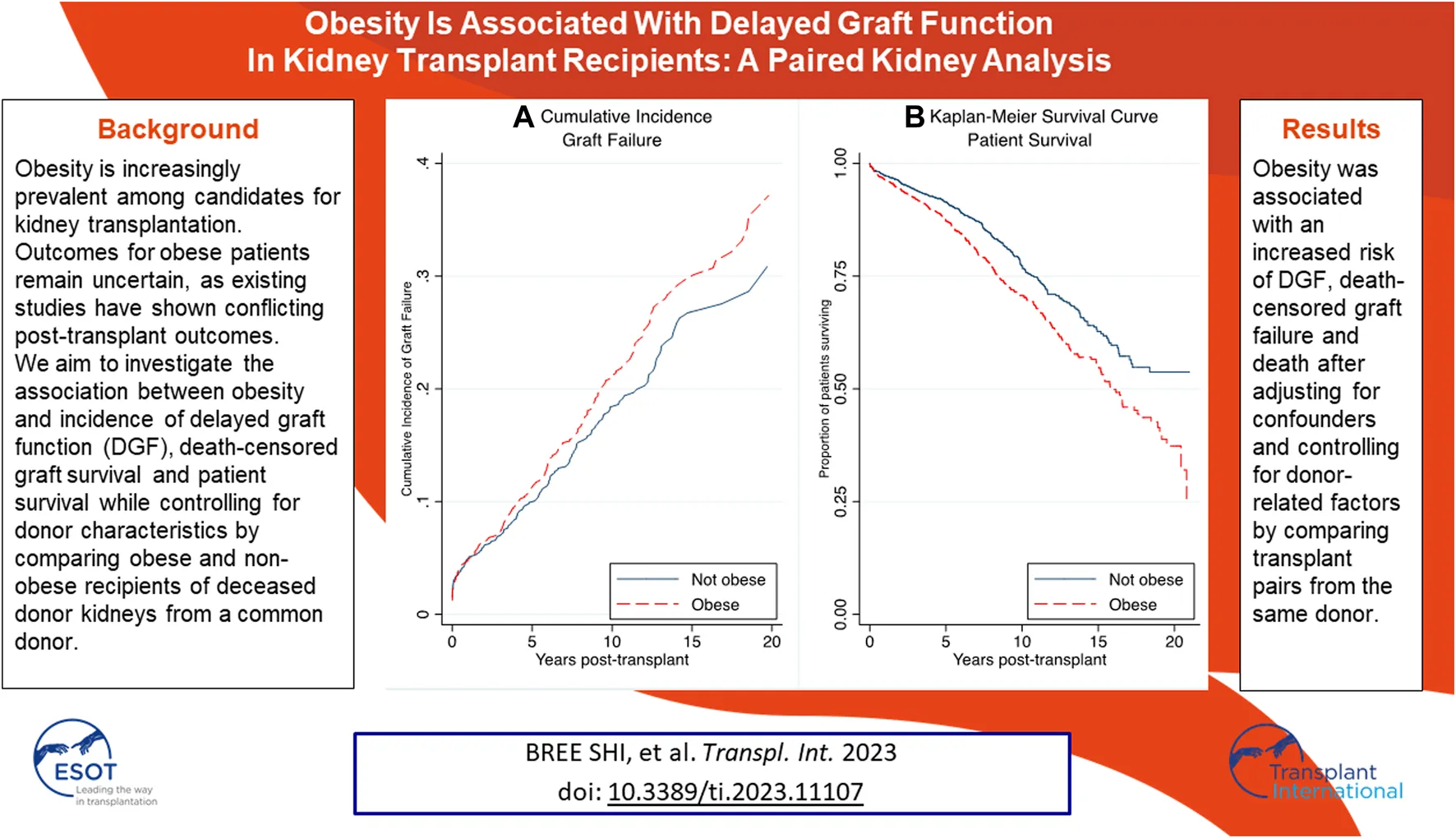
Unadjusted graft failure was more common amongst obese recipients (Figure 2). Cumulative incidence of graft failure at 5 years was not affected by obesity status (11% obese vs. 10% non-obese), however, obese recipients were found to have a higher incidence of long-term graft failure with 10- and 15-year cumulative incidence of 21% and 30% compared to 18% and 27% in non-obese patients. The Gray’s test confirmed a significant difference on the overall incidence of graft failure between obese and non-obese recipients (p = 0.044). On multivariable analysis, obesity was confirmed as an independent risk factor for death-censored graft failure. Obesity was associated with a higher risk of death-censored graft failure after adjusting for DGF, donor kidney side, age, ethnicity and HLA mismatch (aHR = 1.25, 95% CI 1.05–1.49, p = 0.012) (Supplementary Table S2). Recipients who experienced delayed graft function were more likely to experience death-censored graft failure (aHR = 1.84, 95% CI 1.39–2.44, p < 0.001).
FIGURE 2
Patient Survival
There were 342 (22%) deaths in the obese group compared to 260 (17%) (p < 0.001). Death from cardiovascular disease was the most prominent cause of death amongst the obese recipients, with 105 cardiovascular deaths (31%) compared to 65 (25%) among the non-obese recipients. Obesity was strongly associated with inferior survival in both the short and long-term (p < 0.001) (Figure 3). Short and long-term patient survival was significantly worse in obese recipients with 5-, 10- and 15-year survival of 87%, 71% and 56% compared to 91%, 77% and 63% in non-obese patients (p = 0.017, p < 0.001, p < 0.001). In the multivariable model, obesity was found to be strongly associated with worse patient survival. Obese recipients had an increased risk of death compared to non-obese recipients (aHR = 1.32, 95% CI 1.15–1.56, p = 0.001) (Supplementary Table S3). Significant determinants of death that were included in the final model were graft failure, older age, Indigenous ethnicity, diabetes as primary renal disease, length of time on dialysis and pre-existing cardiovascular disease. Graft failure was adjusted as a time-varying covariate in the model. Recipients with graft failure had a much higher risk of death (aHR = 2.84, 95% CI 2.00–4.03, p < 0.001).
FIGURE 3
Degree of Obesity and Clinical Outcomes
We performed a dose-response analysis to examine the association between the degree of obesity and clinical outcomes. The 1,522 obese recipients were classified as 1,173 (77%) class I; 304 (20%) class II and 45 (3%) class III (Table 3). We combined obesity classes II and III due to insufficient patient numbers in obesity class III.
TABLE 3
| Classification | BMI, kg/m2, non-Asians | BMI, kg/m2, Asians | n (%) |
|---|---|---|---|
| Obese class I | 30–34.9 | 27.5–32.4 | 1,173 (77) |
| Obese class II | 35–39.9 | 32.5–37.4 | 304 (20) |
| Obese class III | 40+ | 37.5+ | 45 (3) |
Degree of obesity was categorized into obese class I, obese class II, and obese class III according to World Health Organization guidelines.
When comparing with non-obese recipients, class II/III obese recipients had a 1.44 higher rate of DGF whilst class I obese recipients had a 1.20 higher rate. This trend was not statistically significant when comparing class I obese recipients to class II/III obese recipients (Figure 4. aRR 1.20, 95% CI 0.88–1.62, p = 0.25). A similar non-significant trend was found for death-censored graft failure and death. Class II/III obese recipients had a 1.67 higher rate of death-censored graft failure compared to a 1.16 higher rate for class I obese recipients (aHR 1.45, 95% CI 0.95–2.21, p = 0.085). Class II/III obese recipients had a 1.42 higher rate of death compared to a 1.26 higher rate for class I obese recipients (aHR 1.10, 95% CI 0.71–1.71, p = 0.66).
FIGURE 4
Discussion
In this paired analysis, we controlled for unmeasured donor-related characteristics by comparing outcomes of kidneys from the same donor and demonstrated that obese recipients were more likely to experience DGF, death-censored graft failure and death after deceased donor kidney transplantation when comparing with non-obese recipients.
Studies examining the impact of obesity on kidney transplant outcomes have shown conflicting results, but may be confounded by unmeasured donor-related characteristics. These may include donor kidney function and proteinuria, pre-renal insults to the donor kidney during terminal illness, use of inotropic medications and nephrotoxin exposure, many of which are not adequately captured nor accounted for in existing studies, The majority of published studies have reported an increased risk of delayed graft function for obese recipients [12,13,15]. However, the impact of DGF on long-term transplant outcomes including graft and patient survival remains contentious. Our results are consistent with two systematic review and meta-analyses which showed an increased risk of graft failure for obese recipients compared to non-obese recipients [14,15]. In terms of overall mortality, two meta-analyses reported an increased risk of death for obese recipients, in line with our results [12,14]. One systematic review and meta-analysis reported no association between obesity and overall mortality [27], however, this analysis included only six studies that reported hard transplant outcomes. Another systematic review and meta-analysis reported that there was an increased risk of graft failure and death only for studies that included obese patients who were transplanted before 2000, but no association for those transplanted after 2000 [13]. This contradicts our study result which included patients transplanted after 2000 only.
We found a trend towards increasing risks of DGF, graft failure and death with increasing degrees of obesity, however, this increase was not statistically significant. The number of recipients with class II/III obesity in our study was small and likely inadequately powered to provide certainty. A recent US registry study reported 27% lower odds of DGF (p < 0.001) for recipients with BMI >30–35 versus BMI >35 kg/m2, though no difference in graft or patient survival at a median follow-up of 3.9 years [28].
Our study provides detailed insights from a large, bi-national kidney transplant registry over a 20-year period. We examined a different BMI cut-off for the Asian population that has significant structural variations compared to the Western population. Donor-related factors, which could potentially impact outcomes such as DGF, were carefully accounted and unmeasured confounders were evenly matched by the use of a matched-pair analysis. As randomized controlled trials to compare outcomes for obese versus non-obese recipients are not feasible, we believe the paired analysis we have performed provides the most rigorous assessment of the impact of obesity on hard outcomes following kidney transplantation.
Obesity has more than doubled worldwide in the past 20 years. Although our study has demonstrated that obesity was strongly associated with an increased risk of DGF and inferior long-term outcomes, previous work has clearly indicated that transplantation yields superior outcomes compared to remaining on dialysis for the majority of obese candidates for transplantation [8,9,29]. Our findings should be used to inform patients and providers of the increased risks associated with transplantation for obese recipients. Rather than avoiding transplantation for the obese, these data should encourage the pursuit of strategies to improve outcomes, such as weight-loss management prior to transplantation and improvements in peri-operative management to reduce the incidence of DGF and other complications associated with obesity. This poses two key questions: (1) can transplant management be optimized for obese recipients; and (2) can weight loss before or post-transplant improve transplant outcomes for obese candidates. Some studies have reported an “obesity paradox” where a decrease in BMI for dialysis patients was associated with worse graft and patient survival [30–33]. However, in these studies there was no clear indication of whether the weight loss was intentional, or unintentional due to disease progression or comorbidities. The reason behind the paradox remains unknown. Hypotheses include that obese patients may be less prone to protein energy wasting [34], have a better appetite and well-preserved energy stores, have better hemodynamic tolerance, stem cell mobilization, hemodynamic tolerance, and more efficient disposal of lipophilic uremic toxins [35,36]. A healthy lifestyle that is beneficial to the general public has been shown to improve mortality in chronic kidney disease (CKD) patients [37]. Intentional weight loss in the pre-transplant population may reduce the risk of wound infection, DGF, death-censored failure and reduce the length of hospitalization and alleviates the financial burden on transplant programs [38]. Weight-management programs for CKD patients that include a renal-specific diet, regular exercise combined with anti-obesity medication have been reported to be effective in weight reduction, with improved functional ability, graft function and significantly longer adverse event-free period for the combined outcome of all-cause mortality, myocardial infarction, stroke, and hospitalization for congestive heart failure [39–41]. Another possible intervention is bariatric surgery. A recent study reported a lowering of 7 kg/m2 in BMI in the long-term and a median of 2.4 years longer life expectancy in the bariatric surgery cohort compared to usual obesity care [42]. However, there is very limited data on the outcomes of bariatric surgery on dialysis and kidney transplant patients. In a retrospective cohort study, researchers demonstrated lower all-cause mortality at 5 years for obese ESKD patients who had undergone bariatric surgery [43]. In another retrospective study, bariatric surgery before or after kidney transplantation was reported to be associated with reduced risk of graft failure and mortality compared to control with no bariatric surgery [44]. More data are required to determine if bariatric surgery does improve long-term outcomes from kidney transplantation.
Several limitations should be noted in considering our analysis. First, it is a retrospective registry study that depends on the quality of data captured. Second, the analysis used BMI as the only indicator for categorizing obesity, which does not differentiate between fat and muscle mass, nor between visceral and subcutaneous fat. Other methods such as waist circumference, waist-to-hip ratio, in vivo neutron activation analysis (IVNAA), densitometry, deuterium oxide dilution, and dual energy X-ray absorptiometry (DXA) are also available and may enhance specificity. However, such measures are not routinely used in candidate assessment and are not reported to ANZDATA. Third, there may be other potential confounders that are unaccounted for, such as social status, genetic factors, immunosuppression and drug dosing. Fourth, even though significant confounders were adjusted for in the model, residual confounding is still possible. Five, indication of whether dialysis is required after transplantation may vary between centers resulting in potential center effect for DGF which was not accounted for. Six, there may be a loss of statistical power due to pairing. However, we believe that it is important to utilize a matched pair analysis to minimize bias due to donor-related characteristics, such as donor kidney function, hemodynamic instability during organ procurement, use of vasoactive medications and exposure to nephrotoxins, all of which are captured crudely or not at all in registry data. Finally, the study cohort was predominantly Caucasian. The remaining non-Caucasian patient group was heterogeneous, with 40% and 23% of the Indigenous group being Australian Aboriginal and New Zealand Mauri, and 25% and 23% of the Asian group being Indian and Chinese, respectively. Therefore, the comparison between Caucasian and non-Caucasian patients in our study is different from the same comparison in the US where around 70% of non-Caucasian patients were Black/African American [45].
In conclusion, our study demonstrates a relationship between obesity and post-transplant outcomes after carefully controlling for donor-related factors in a paired kidney analysis. Addressing obesity is an unmet clinical need in kidney transplantation. Transplantation is recommended for many obese candidates as it is acknowledged to yield superior outcomes to dialysis. However, design and evaluation of strategies to: (1) optimize transplant management for obese recipients; and (2) reduce the prevalence of obesity among transplant candidates are required.
Statements
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: The data are not available due to privacy and ethical considerations. Requests to access these datasets should be directed to https://anzdata.org.au/.
Author contributions
BS—prepared the study protocol, acquired the data, designed and conducted the statistical analysis, interpreted the results, drafted the manuscript, edited the manuscript and approved the final version of the manuscript. TY—conceived the study, designed the statistical analysis, interpreted the results, edited the manuscript and approved the final version of the manuscript. JX—prepared the study protocol, acquired the data and approved the final version of the manuscript. KW—interpreted the results, edited the manuscript and approved the final version of the manuscript. JL—conceived the study, designed the statistical analysis, interpreted the results, edited the manuscript and approved the final version of the manuscript. SC—conceived the study, designed the statistical analysis, interpreted the results, edited the manuscript and approved the final version of the manuscript.
Acknowledgments
The data reported here have been supplied by Australia and New Zealand Dialysis and Transplant (ANZDATA) Registry.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2023.11107/full#supplementary-material
Abbreviations
aHR, adjusted hazard ratio; ANZDATA, the Australia and New Zealand Dialysis and Transplant registry; aRR, adjusted rate ratio; BMI, body mass index; CI, confidence interval; CKD, chronic kidney disease; DGF, delayed graft function; ESKD, end-stage kidney disease; GN, Glomerulonephritis; HD, hemodialysis; HLA, human leukocyte antigen; PD, peritoneal dialysis; RRT, renal replacement therapy; WHO, World Health Organization.
References
1.
World Health Organization. Obesity and Overweight (2021). Available at: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (Accessed March 23, 2022).
2.
GoreJLPhamPTManovichGMWilkinsonAHRosenthalJTLipshutzGSet alObesity and Outcome Following Renal Transplantation. Am J Transpl (2006) 6(2):357–63. 10.1111/j.1600-6143.2005.01198.x
3.
FriedmanANMiskulinDCRosenbergIHLeveyAS. Demographics and Trends in Overweight and Obesity in Patients at Time of Kidney Transplantation. Am J Kidney Dis (2003) 41(2):480–7. 10.1053/ajkd.2003.50059
4.
WakamGKSheetzKHGerhardingerLMontgomeryJRWaitsSA. Population-based Trends in Obesity and Kidney Transplantation Among Patients with End-Stage Kidney Disease. Transpl Direct (2021) 7(12):e787. 10.1097/txd.0000000000001163
5.
ParikhCRKlemPWongCYalavarthyRChanL. Obesity as an Independent Predictor of Posttransplant Diabetes Mellitus. Transpl P (2003) 35(8):2922–6. 10.1016/j.transproceed.2003.10.074
6.
LentineKLSchnitzlerMAAbbottKCLiLBurroughsTEIrishWet alDe Novo Congestive Heart Failure after Kidney Transplantation: A Common Condition with Poor Prognostic Implications. Am J Kidney Dis (2005) 46(4):720–33. 10.1053/j.ajkd.2005.06.019
7.
YoungJBNeumayerHHGordonRD. Pretransplant Cardiovascular Evaluation and Posttransplant Cardiovascular Risk. Kidney Int (2010) 78(118):S1–S7. 10.1038/ki.2010.209
8.
GillJSLanJDongJRoseCHendrenEJohnstonOet alThe Survival Benefit of Kidney Transplantation in Obese Patients. Am J Transpl (2013) 13(8):2083–90. 10.1111/ajt.12331
9.
KrishnanNHigginsRShortAZehnderDPitcherDHudsonAet alKidney Transplantation Significantly Improves Patient and Graft Survival Irrespective of BMI: A Cohort Study. Am J Transpl (2015) 15(9):2378–86. 10.1111/ajt.13363
10.
PhamPTDanovitchGMPhamPT. Kidney Transplantation in the Obese Transplant Candidates: To Transplant or Not to Transplant?Semin Dial (2013) 26(5):568–77. 10.1111/sdi.12109
11.
ZrimSFurlongTGraceBMeadeA. Body Mass index and Postoperative Complications in Kidney Transplant Recipients. Nephrology (2012) 17(6):582–7. 10.1111/j.1440-1797.2012.01621.x
12.
LafrancaJAIjermansJNBetjesMGDorFJ. Body Mass index and Outcome in Renal Transplant Recipients: a Systematic Review and Meta-Analysis. Bmc Med (2015) 13(1):111. 10.1186/s12916-015-0340-5
13.
NicolettoBBFonsecaNKOManfroRCGonçalvesLFSLeitãoCBSouzaGC. Effects of Obesity on Kidney Transplantation Outcomes: a Systematic Review and Meta-Analysis. Transplantation (2014) 98(2):167–76. 10.1097/tp.0000000000000028
14.
AhmadiSFZahmatkeshGStrejaEMolnarMZRheeCMKovesdyCPet alBody Mass Index and Mortality in Kidney Transplant Recipients: A Systematic Review and Meta-Analysis. Am J Nephrol (2014) 40(4):315–24. 10.1159/000367812
15.
HillCJCourtneyAECardwellCRMaxwellAPLucarelliGVerouxMet alRecipient Obesity and Outcomes after Kidney Transplantation: a Systematic Review and Meta-Analysis. Nephrol Dial Transpl (2015) 30(8):1403–11. 10.1093/ndt/gfv214
16.
MerionRMAshbyVBWolfeRADistantDAHulbert-ShearonTEMetzgerRAet alDeceased-Donor Characteristics and the Survival Benefit of Kidney Transplantation. Jama (2005) 294(21):2726–33. 10.1001/jama.294.21.2726
17.
MassieABLuoXChowEKHAlejoJLDesaiNMSegevDL. Survival Benefit of Primary Deceased Donor Transplantation with High‐KDPI Kidneys. Am J Transpl (2014) 14(10):2310–6. 10.1111/ajt.12830
18.
SummersDMJohnsonRJAllenJFuggleSVCollettDWatsonCJet alAnalysis of Factors that Affect Outcome after Transplantation of Kidneys Donated after Cardiac Death in the UK: a Cohort Study. Lancet (2010) 376(9749):1303–11. 10.1016/s0140-6736(10)60827-6
19.
WHO Expert Consultation. Appropriate Body-Mass index for Asian Populations and its Implications for Policy and Intervention Strategies. Lancet (2004) 363(9403):157–63. 10.1016/s0140-6736(03)15268-3
20.
World Health Organization, International Diabetes Institute, International Association for the Study of Obesity, International Obesity Task Force. The Asia-Pacific Perspective: Redefining Obesity and its Treatment. WHO (2000).
21.
LowSChinMCMaSHengDDeurenberg-YapM. Rationale for Redefining Obesity in Asians. Ann Acad Med Singap (2009) 38(1):66–74. 10.47102/annals-acadmedsg.v38n1p66
22.
Australia and New Zealand Dialysis and Transplant Registry. ANZDATA Data Dictionary (2023). Available at: https://www.anzdata.org.au/anzdata/services/data-management/data-dictionary/ (Retrieved March 1, 2023).
23.
HardinJWHilbeJM. Generalized Linear Models and Extensions Fourth Edition. College Station: Stata Press (2018).
24.
CummingsPMcKnightB. Analysis of Matched Cohort Data. Stata J (2004) 4(3):274–81. 10.1177/1536867x0400400305
25.
CummingsPMcKnightBGreenlandS. Matched Cohort Methods for Injury Research. Epidemiol Rev (2003) 25(1):43–50. 10.1093/epirev/mxg002
26.
ChowdhuryMZITurinTC. Variable Selection Strategies and its Importance in Clinical Prediction Modelling. Fam Med Community Heal (2020) 8(1):e000262. 10.1136/fmch-2019-000262
27.
LadhaniMCraigJCIrvingMClaytonPAWongG. Obesity and the Risk of Cardiovascular and All-Cause Mortality in Chronic Kidney Disease: a Systematic Review and Meta-Analysis. Nephrol Dial Transpl (2017) 32(3):439–49. 10.1093/ndt/gfw075
28.
SureshkumarKKChopraBJosephsonMAShahPBMcGillRL. Recipient Obesity and Kidney Transplant Outcomes: A Mate-Kidney Analysis. Am J Kidney Dis (2021) 78(4):501–10.e1. 10.1053/j.ajkd.2021.02.332
29.
BennettWMMcEvoyKMHenellKRPidikitiSDouzdjianVBatiukT. Kidney Transplantation in the Morbidly Obese: Complicated but Still Better Than Dialysis. Clin Transpl (2011) 25(3):401–5. 10.1111/j.1399-0012.2010.01328.x
30.
ScholdJDSrinivasTRGuerraGReedAIJohnsonRJWeinerIDet alA ‘Weight‐Listing’ Paradox for Candidates of Renal Transplantation?Am J Transpl (2007) 7(3):550–9. 10.1111/j.1600-6143.2006.01629.x
31.
MolnarMZStrejaEKovesdyCPBunnapradistSSampaioMSJingJet alAssociations of Body Mass Index and Weight Loss with Mortality in Transplant‐Waitlisted Maintenance Hemodialysis Patients. Am J Transpl (2011) 11(4):725–36. 10.1111/j.1600-6143.2011.03468.x
32.
Kalantar-ZadehKKoppleJDKilpatrickRDMcAllisterCJShinabergerCSGjertsonDWet alAssociation of Morbid Obesity and Weight Change over Time with Cardiovascular Survival in Hemodialysis Population. Am J Kidney Dis (2005) 46(3):489–500. 10.1053/j.ajkd.2005.05.020
33.
Kalantar-ZadehKKuwaeNWuDYShantoufRSFouqueDAnkerSDet alAssociations of Body Fat and its Changes over Time with Quality of Life and Prospective Mortality in Hemodialysis Patients. Am J Clin Nutr (2006) 83(2):202–10. 10.1093/ajcn/83.2.202
34.
GlicklichDMustafaMR. Obesity in Kidney Transplantation: Impact on Transplant Candidates, Recipients, and Donors. Cardiol Rev (2019) 27(2):63–72. 10.1097/CRD.0000000000000216
35.
StenvinkelPLindholmB. Resolved: being fat is good for dialysis patients: the Godzilla effect: con. J Am Soc Nephrol (2008) 19(6):1059–64. 10.1681/01.asn.0000926896.67402.33
36.
CarreroJJQureshiARAxelssonJAvesaniCMSulimanMEKatoSet alComparison of Nutritional and Inflammatory Markers in Dialysis Patients with Reduced Appetite. Am J Clin Nutr (2007) 85(3):695–701. 10.1093/ajcn/85.3.695
37.
RicardoACMaderoMYangWAndersonCMenezesMFischerMJet alAdherence to a Healthy Lifestyle and All-Cause Mortality in CKD. Clin J Am Soc Nephro (2013) 8(4):602–9. 10.2215/cjn.00600112
38.
SeverMSZoccaliC. Moderator’s View: Pretransplant Weight Loss in Dialysis Patients: Cum Grano Salis. Nephrol Dial Transpl (2015) 30(11):1810–3. 10.1093/ndt/gfv333
39.
CookSAMacLaughlinHMacdougallIC. A Structured Weight Management Programme Can Achieve Improved Functional Ability and Significant Weight Loss in Obese Patients with Chronic Kidney Disease. Nephrol Dial Transpl (2008) 23(1):263–8. 10.1093/ndt/gfm511
40.
MacLaughlinHLCookSAKariyawasamDRosekeMNiekerkMVMacdougallIC. Nonrandomized Trial of Weight Loss with Orlistat, Nutrition Education, Diet, and Exercise in Obese Patients with CKD: 2-Year Follow-Up. Am J Kidney Dis (2010) 55(1):69–76. 10.1053/j.ajkd.2009.09.011
41.
MacLaughlinHLHallWLCondryJSandersTMacdougallIC. Participation in a Structured Weight Loss Program and All-Cause Mortality and Cardiovascular Morbidity in Obese Patients with Chronic Kidney Disease. J Ren Nutr (2015) 25(6):472–9. 10.1053/j.jrn.2015.05.001
42.
CarlssonLSjöholmKJacobsonPAndersson-AssarssonJCSvenssonPATaubeMet alLife Expectancy after Bariatric Surgery in the Swedish Obese Subjects Study. New Engl J Med (2020) 383(16):1535–43. 10.1056/nejmoa2002449
43.
SheetzKHGerhardingerLDimickJBWaitsSA. Bariatric Surgery and Long-Term Survival in Patients with Obesity and End-Stage Kidney Disease. Jama Surg (2020) 155(7):581–8. 10.1001/jamasurg.2020.0829
44.
CohenJBLimMATewksburyCMTorres-LandaSTrofe-ClarkJAbtPLet alBariatric Surgery before and after Kidney Transplantation: Long-Term Weight Loss and Allograft Outcomes. Surg Obes Relat Dis (2019) 15(6):935–41. 10.1016/j.soard.2019.04.002
45.
United States Renal Data System. USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States (2021). Available at: https://adr.usrds.org/2021/end-stage-renal-disease/7-transplantation (Accessed March 23, 2022).
Summary
Keywords
kidney transplantation, patient survival, graft survival, obesity, DGF
Citation
Shi B, Ying T, Xu J, Wyburn K, Laurence J and Chadban SJ (2023) Obesity is Associated With Delayed Graft Function in Kidney Transplant Recipients: A Paired Kidney Analysis. Transpl Int 36:11107. doi: 10.3389/ti.2023.11107
Received
05 December 2022
Accepted
11 May 2023
Published
30 May 2023
Volume
36 - 2023
Updates
Copyright
© 2023 Shi, Ying, Xu, Wyburn, Laurence and Chadban.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Steven J Chadban, steve.chadban@health.nsw.gov.au
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.