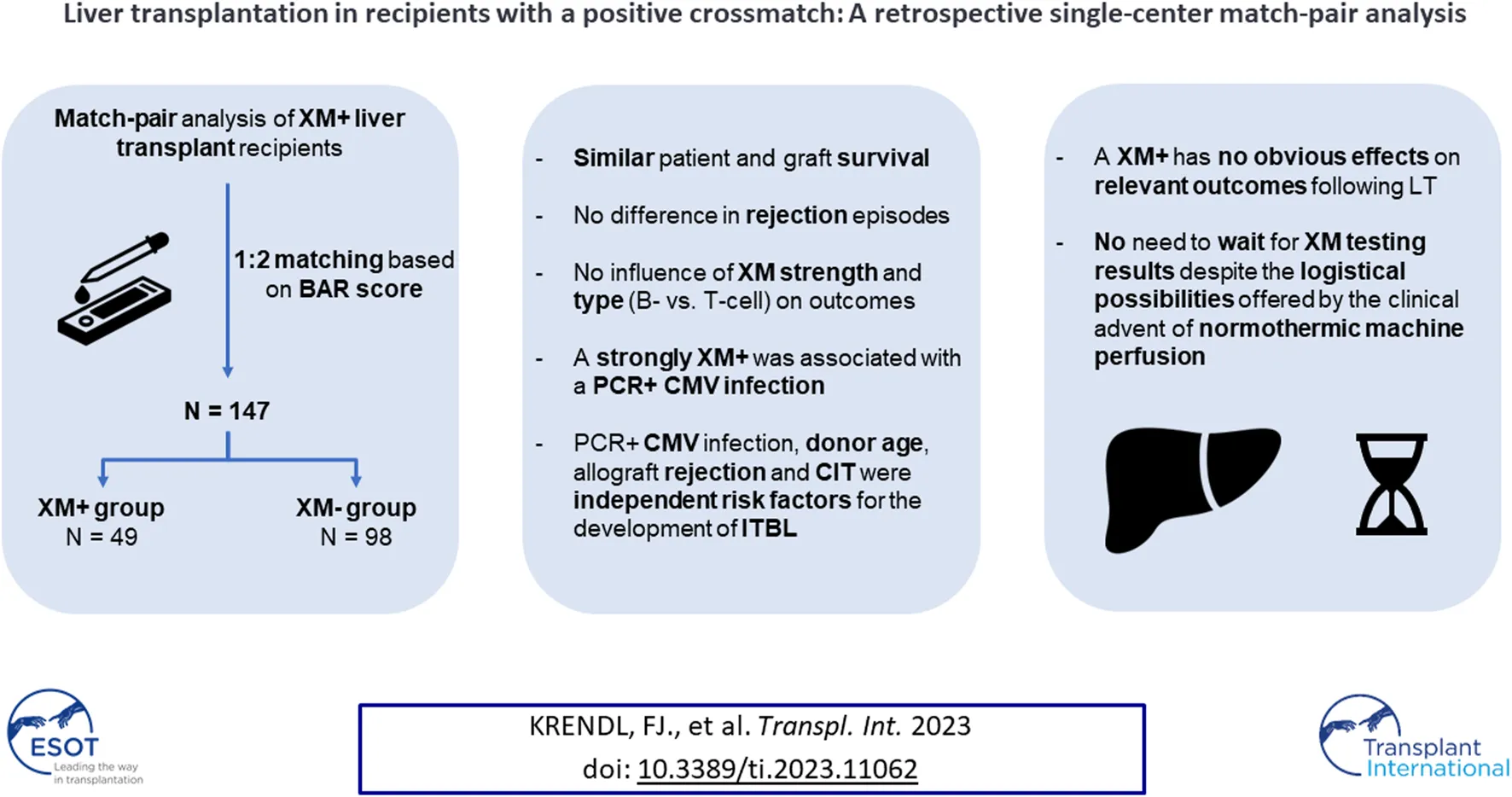
Abstract
A positive crossmatch (XM+) is considered a contraindication to solid abdominal organ transplantation except liver transplantation (LT). Conflicting reports exist regarding the effects of XM+ on post-transplant outcomes. The goal of this retrospective single-center analysis is to evaluate the influence of XM+ on relevant outcome parameters such as survival, graft rejection, biliary and arterial complications. Forty-nine adult patients undergoing LT with a XM+ between 2002 and 2017 were included. XM+ LT recipients were matched 1:2 with crossmatch negative (XM−) LT recipients based on the balance of risk (BAR) score. Patient and graft survival were compared using Kaplan-Meier survival analysis and the log-rank test. Comparative analysis of clinical outcomes in XM+ and XM− groups were conducted. Patient and graft survival were similar in XM+ and XM− patients. Rejection episodes did not differ either. Recipients with a strong XM+ were more likely to develop a PCR+ CMV infection. A XM+ was not associated with a higher incidence of biliary or arterial complications. Donor age, cold ischemia time, PCR+ CMV infection and a rejection episode were associated with the occurrence of ischemic type biliary lesions. A XM+ has no effects on patient and graft survival or other relevant outcome parameters following LT.
Introduction
A positive crossmatch (XM+) is usually considered a contraindication to all solid abdominal organ transplantations except liver transplantation (LT) (1, 2). Therefore, crossmatch testing is mandatory before pancreas, intestinal and kidney transplantation (3). However, in the context of LT the effect of a XM+ on post-transplant outcomes remains ill-defined and LT is commonly performed regardless of the crossmatch testing results, often even before these results become available (3–7).
Compared to other abdominal organs, the liver seems to be in a privileged immunological situation due to its dual afferent blood supply, its unique antigenic sinusoidal vasculature line by Kupffer cells and its ability to absorb preformed donor specific antibodies (DSAs) by secreting soluble antigens (8–10). Reports of combined liver and kidney transplantations in the presence of a XM+ in which the recipient became XM− within hours following transplantation underline the liver’s impressive immunologic capabilities (9, 11, 12).
Still, some authors suggest a link between inferior patient and graft survival and a higher rate of postoperative complications following LT in the presence of a XM+ (8, 13–18). Others, however, were not able to duplicate those findings (6, 10, 12, 19–25). Yet, focusing on a XM+ alone might not tell the full story as XM strength (26) and type (T cell vs. B cell) may play a significant role concerning post-transplant outcomes (3, 5, 17, 18). Fittingly, a T cell but not B cell dependent XM+ was reported to be associated with impaired graft survival (3). Historically, LT was essentially an emergency surgical procedure in order to keep cold ischemia time (CIT) short. While it seemed unthinkable to postpone a LT until crossmatch testing results become available only a few years ago, the advent of machine perfusion has changed clinical practice (27). Machine preservation offers the possibility to optimize transplant conditions including immunologic risk stratification pre-transplant. Considering these implications, it seems worthwhile to explore whether a XM+ influences post-transplant outcomes. Previous studies on this subject were hampered by a small number of patients and mostly lacked adequate controls and comparisons (15, 16, 26, 28, 29).
The aim of this match-pair analysis is to evaluate the influence of a XM+, including XM strength and type, on relevant clinical outcome parameters such as patient and graft survival, rejection episodes, biliary and arterial complications.
Patients and Methods
Study Population and Study Design
At the Medical University of Innsbruck, crossmatch testing is routinely performed for LT recipients. All adult patients who underwent XM+ deceased donor LT from donation after brain dead (DBD) donors between 2002 and 2017 were included. A 1:2 match-pair analysis was conducted, with patients who underwent LT with a negative crossmatch (XM−) serving as controls. Matching was performed based on the balance of risk (BAR) score (30, 31).
The study was conducted in accordance with the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board; protocol code 1034/2022. The results were reported according to the STROBE guidelines (32).
Immunosuppression and Postoperative Care
The standard immunosuppressive (IS) regimen for LT recipients at our center consisted of the following: Induction therapy with an intra-operative bolus of 500 mg methylprednisolone. As part of the PROTECT (33) and DIAMOND (34) trials, some patients received induction therapy with an interleukin 2 (IL2) antibody. Postoperatively patients received tacrolimus (Tac) (initial trough levels 6–8 ng/mL, gradually decreased to 6 ng/mL at 6 months, and 4–6 ng/mL at 12 months) and either mycophenolate mofetil (MMF) (1,000 mg twice daily) or mycophenolic acid (MPA) (720 mg twice daily). Steroids were gradually tapered to 5 mg prednisolone per day as part of the maintenance therapy. Complete steroid withdrawal was considered on an individual basis considering the side effect profile as well as the patient’s immunologic risk. Reasons to divert from our standard protocol were related to recipient factors. Conversion from Tac to cyclosporine A (CsA) was considered in case of long-QT syndrome, or tacrolimus associated neurotoxicity. MMF/MPA was switched to azathioprine (Aza) in case of gastrointestinal side effects or to avoid the teratogenic potential in female patients wishing to conceive.
Definitions
Crossmatch
All recipient sera were tested for cytotoxic antibodies against donor lymphocytes (CDC crossmatch). For the XM to be deemed positive more than 15% cytolysis had to be present. Additionally, a XM was defined as weakly positive when cytolysis ranged between 15% and 50% and strongly positive when cytolysis exceeded 50%. Cytotoxic cross-matching activity was tested before and after treatment with dithiothreitol (DTT) which inactivates IgM antibodies (35, 36). For XM strength analysis the post DTT treatment value was employed. In addition to XM strength, the XM type (T cell dependent vs. B cell dependent) was recorded.
Graft Loss and Graft Dysfunction
Graft loss was defined as patient death or the need for liver re-transplantation. Primary non-function was defined as peak AST ≥3000 IU/L plus at least one of the following criteria: INR ≥2.5, serum lactate ≥4 mmol/L and total bilirubin ≥10 mg/dL (values measured on postoperative day 3, biliary obstruction being excluded). Early allograft dysfunction (EAD) was defined according to the Olthoff criteria (37).
Rejections
Acute rejection was defined as biopsy proven rejection which required steroid bolus treatment (38). Steroid bolus treatment consisted of an intravenous steroid pulse of 500 mg methylprednisolone for three consecutive days. Chronic rejection was defined based on persistent laboratory abnormalities and histological confirmation (38).
Biliary Complications
Biliary complications were classified as bile duct leaks, biliary cast syndrome, anastomotic stenosis (AS) and non-anastomotic stenosis (NAS). Ischemic type biliary lesions (ITBL) were defined as NAS with or without biliary cast formation in the absence of hepatic artery stenosis or thrombosis (39–41).
Extended Criteria Donors
ECDs were defined according to the Eurotransplant Manual, Chapter 9: The Donor (42).
Outcomes
The primary outcome was patient and graft survival. Secondary outcomes included incidence and risk factors for rejection episodes as well as incidence, risk factors and type of biliary and arterial complications.
Statistical Analysis
A 1:2 optimal pair matching was performed with the goal of minimizing the absolute pairwise distances in the matched sample (median BAR score values XM+ 8.0 vs. XM− 7.5). For descriptive analysis, categorical variables were summarized with the help of absolute and relative (percentages) frequencies, continuous variables were summarized with means and standard deviation (SD) or medians and interquartile range (IQR) as appropriate. Comparative analysis of clinical outcomes in the XM+ and XM− group was conducted using the Chi-square or Fisher’s exact test (if one or more cells had an expected count of less than five) for categorical variables. The Mann–Whitney U test was used to compare continuous, not normally distributed variables. Any variable having a significant univariate test (p-value cut-off point of 0.25 based on the Wald test) was selected as a candidate for the multivariate analysis (43). Uni- and multivariate analyses were performed for the primary and secondary endpoints starting with a univariate analysis of each variable. Kaplan-Meier survival analysis was performed to compare patient and graft survival between XM+ patients and XM− patients using the log-rank test. Multivariate analysis for patient and graft survival endpoints was performed with Cox proportional hazards regression analysis. Logistic regression analysis was used to assess the effects of clinical parameters on secondary endpoints. Statistical analysis was conducted with SPSS (IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp).
Results
Recipient Characteristics
Forty-nine patients undergoing LT with a XM+ were matched 1:2 with XM− patients. Matching was performed based on the BAR score. The indications for LT and recipient demographics are presented in Table 1. The median recipient age was 58.0 years in the XM+ group compared to 59.0 years in the XM− group (p = 0.526). Patients in the XM+ group were more likely to be female [XM+ 36.7% (18 of 49) vs. XM− 19.4% (19 of 98), p = 0.022], have a lower BMI [XM+ 23.8 (21.6–27.1) vs. XM− 26.5 (23.9–29.1), p = 0.003] and a higher MELD score [XM+ 16.0 (12.0–21.0) vs. XM− 13.5 (8.8–17.0), p = 0.014] compared to patients in the XM− group. The groups were similar in terms of AB0 blood groups (p = 0.769), CMV mismatching (p = 0.228) and median follow-up (p = 0.304). Patients in the XM+ group had more commonly received a previous LT [XM+ 14.3% (7 of 49) vs. XM− 4.1% (4 of 98), p = 0.042]. The overall use of induction therapy was similar between the groups [XM+ 61.2% (30 of 49) vs. XM− 62.2% (61 of 98), p = 0.904]. However, XM+ patients more often received antibody induction with ATG [XM+ 4.1% (2 of 49) vs. XM− 0% (0 of 98), p = 0.110], although not statistically significant, and alemtuzumab [XM+ 8.2% (4 of 49) vs. XM− 1.0% (1 of 98), p = 0.042]. Yet, in a subgroup analysis, antibody induction had no significant influence on any of the explored outcome parameters including patient and graft survival.
TABLE 1
| All N = 147 | XM+ n = 49 | XM− n = 98 | p-value | |
|---|---|---|---|---|
| Age (years) | 59.0 (54.0–65.0) | 58.0 (53.5–64.0) | 59.0 (54.0–65.0) | 0.526 |
| Sex | 0.022 | |||
| Female | 37 (25.2) | 18 (36.7) | 19 (19.4) | |
| Male | 110 (74.8) | 31 (63.3) | 79 (80.6) | |
| BMI (kg/m2) | 25.9 (22.9–28.8) | 23.8 (21.6–27.1) | 26.5 (23.9–29.1) | 0.003 |
| MELD score | 16.0 (9.0–18.0) | 16.0 (12.0–21.0) | 13.5 (8.8–17.0) | 0.014 |
| Indication for LT | ||||
| AFLD | 58 (39.5) | 16 (32.7) | 42 (42.9) | 0.233 |
| NAFLD | 21 (14.3) | 8 (16.3) | 13 (13.3) | 0.617 |
| PBC | 7 (4.8) | 3 (6.1) | 4 (4.1) | 0.686 |
| PSC | 4 (2.7) | 0 (0.0) | 4 (4.1) | 0.302 |
| AIH | 4 (2.7) | 3 (6.1) | 1 (1.0) | 0.108 |
| Tumor | 62 (42.2) | 13 (26.5) | 49 (50.0) | 0.007 |
| Re - Tx | 11 (7.5) | 7 (14.3) | 4 (4.1) | 0.042 |
| Induction (yes/no) | 91 (61.9) | 30 (61.2) | 61 (62.2) | 0.904 |
| IL2 | 83 (57.2) | 24 (49.0) | 59 (61.5) | 0.151 |
| ATG | 2 (1.4) | 2 (4.1) | 0 (0.0) | 0.110 |
| Alemtuzumab | 5 (3.4) | 4 (8.2) | 1 (1.0) | 0.042 |
| Missing | 1 (0.7) | 0 (0.0) | 1 (1.0) | |
| ABO blood group | 0.769 | |||
| A | 58 (39.5) | 22 (44.9) | 36 (36.7) | |
| B | 14 (9.5) | 4 (8.2) | 10 (10.2) | |
| 0 | 61 (41.5) | 18 (36.7) | 43 (43.9) | |
| AB | 14 (9.5) | 5 (10.2) | 9 (9.2) | |
| CMV mismatch | 0.228 | |||
| D+/R- | 35 (23.8) | 9 (19.6) | 26 (26.8) | |
| D-/R+ | 38 (25.9) | 10 (21.7) | 28 (28.9) | |
| D+/R+ | 54 (36.7) | 23 (50.0) | 31 (32.0) | |
| D-/R- | 16 (10.9) | 4 (8.7) | 12 (12.4) | |
| Missing | 4 (2.7) | 3 (6.1) | 1 (1.0) | |
| Median follow-up (months) | 60.2 (25.0–98.6) | 70.7 (33.1–108.0) | 57.9 (23.8–96.8) | 0.304 |
Recipient characteristics in the matched cohort.
Values are presented as medians or absolute numbers with IQRs and percentages in parentheses. Italic values show significant p-values. AFLD, alcoholic fatty liver disease; AIH, autoimmune hepatitis; ATG, anti-thymocyte globulin; BAR, balance of risk; BMI, body mass index; CMV, cytomegalovirus; COD, cause of death; CVA, cerebrovascular accident; ET-DRI, Eurotransplant donor risk index; MELD, model for end-stage liver disease; NAFLD, non-alcoholic fatty liver disease. IL2, interleukin 2. IQR, interquartile range; PBC, primary biliary cirrhosis; PSC, primary sclerosing cholangitis; PCR, polymerase chain reaction. Re-Tx, re-transplantation. SAB, subarachnoid hemorrhage; XM, crossmatch.
Donor Characteristics
Donor age [XM+ 55.0 (41.5–65.5) vs. XM− 52.0 (43.0–62.0), p = 0.639], and ET-DRI [XM+ 1.67 (1.40–1.91) vs. XM− 1.57 (1.39–1.86), p = 0.659] were similar between groups. The overall ET-DRI was 1.64, suggesting that very good quality grafts were used in this cohort. XM+ recipients more commonly received a graft from a female donor [XM+ female 61.2% (30 of 49) vs. XM− 42.9% (42 of 98), p = 0.036] and donor BMI was significantly lower in the XM+ group compared to the XM− group [XM+ 24.2 (22.6–26.2) vs. XM− 26.8 (23.9–29.8), p = 0.001]. Donor BMI and liver steatosis correlated directly with each other (p = 0.001). Anhepatic time [XM+ 51.0 (43.3–57.8) vs. XM− 57.0 (47.8–65.3), p = 0.007] and warm ischemic time (WIT) [XM+ 41.5 (36.0–51.0) vs. XM− 47.5 (41.0–56.0), p = 0.008] were significantly shorter in the XM+ group. University of Wisconsin (UW) solution was more commonly used as a preservation solution in the XM+ compared to the XM− group [XM+ 37.5% (18 of 49) vs. XM− 19.4% (19 of 98), p = 0.018] (Table 2).
TABLE 2
| All N = 147 | XM+ n = 49 | XM− n = 98 | p-value | |
|---|---|---|---|---|
| Age (years) | 53.0 (42.0–62.3) | 55.0 (41.5–65.5) | 52.0 (43.0–62.0) | 0.639 |
| Sex | 0.036 | |||
| Female | 72 (49.0) | 30 (61.2) | 42 (42.9) | |
| Male | 75 (51.0) | 19 (38.8) | 56 (57.1) | |
| BMI (kg/m2) | 25.7 (22.9–29.0) | 24.2 (22.6–26.2) | 26.8 (23.9–29.8) | 0.001 |
| COD | 0.132 | |||
| Trauma | 37 (25.3) | 16 (32.6) | 21 (21.4) | |
| Anoxia | 11 (7.5) | 1 (2.0) | 10 (10.2) | |
| CVA | 96 (65.3) | 31 (63.2) | 65 (66.3) | |
| Other | 2 (1.3) | 0 (0.0) | 2 (2.0) | |
| Missing | 1 (0.7) | 1 (2.0) | 0 (0.0) | |
| ECD | 109 (74.7) | 33 (68.8) | 76 (77.6) | 0.251 |
| Preservation | 0.018 | |||
| UW | 37 (25.3) | 18 (37.5) | 19 (19.4) | |
| HTK | 109 (74.7) | 30 (62.5) | 79 (80.6) | |
| Missing | 1 (0.7) | 1 (2.0) | 0 (0.0) | |
| Anhepatic time (min) | 54.0 (46.0–63.0) | 51.0 (43.3–57.8) | 57.0 (47.8–65.3) | 0.007 |
| WIT (min) | 46.0 (39.0–55.0) | 41.5 (36.0–51.0) | 47.5 (41.0–56.0) | 0.008 |
| CIT (h) | 8.6 (7.5–10.0) | 8.8 (7.5–10.7) | 8.4 (7.5–9.8) | 0.316 |
| ET-DRI | 1.64 (1.40–1.88) | 1.67 (1.40–1.91) | 1.57 (1.39–1.86) | 0.659 |
Donor characteristics and operative data in the matched cohort.
Values are presented as medians or absolute numbers with IQRs and percentages in parentheses; Italic values show significant p-values. BMI, body mass index; COD, cause of death; CVA, cerebrovascular accident; ECD, extended criteria donor; ET-DRI, Eurotransplant donor risk index; HTK, histidine-tryptophan-ketoglutarate. IQR, interquartile range; SAB, subarachnoid hemorrhage; UW, University of Wisconsin; WIT, warm ischemia time; XM, crossmatch.
Early Allograft Dysfunction
The EAD rate was similar in XM+ and XM− patients [XM+ 24.5% (12 of 49) vs. XM− 37.8% (37 of 98), p = 0.138] (Table 3). XM strength or type had no influence on EAD rates. EAD, however, was associated with a positive CMV PCR. Univariate analysis showed recipient BMI, graft steatosis, donor gGT and XM type to be risk factors for the development of EAD. Considering these factors for multivariate analysis, only donor gGT remained as a statistically significant factor for the development of EAD (p = 0.045).
TABLE 3
| All N = 147 | XM+ n = 49 | XM− n = 98 | p-value | |
|---|---|---|---|---|
| EAD | 49 (33.3) | 12 (24.5) | 37 (37.8) | 0.138 |
| Rejection | 17 (11.6) | 7 (14.3) | 10 (10.2) | 0.466 |
| Acute | 12 (8.2) | 5 (10.2) | 7 (7.1) | 0.535 |
| Chronic | 5 (3.4) | 2 (4.1) | 3 (3.1) | 1.000 |
| Biliary complications | 61 (41.5) | 21 (42.9) | 40 (40.8) | 0.813 |
| Bile duct leaks | 22 (15.0) | 5 (10.2) | 17 (17.3) | 0.252 |
| AS | 37 (25.2) | 14 (28.6) | 23 (23.5) | 0.502 |
| NAS | 15 (10.2) | 6 (12.2) | 9 (9.2) | 0.563 |
| ITBL | 15 (10.2) | 6 (12.2) | 9 (9.2) | 0.563 |
| Casts | 20 (13.6) | 6 (12.2) | 14 (14.3) | 0.734 |
| Arterial complications | 13 (8.8) | 2 (4.1) | 11 (11.2) | 0.220 |
| Stenosis | 2 (1.4) | 0 (0.0) | 2 (2.0) | 0.553 |
| Thrombosis | 6 (4.1) | 1 (2.0) | 5 (5.1) | 0.664 |
| Dissection | 6 (4.1) | 1 (2.0) | 5 (5.1) | 0.664 |
| CMV PCR + | 31 (20.7) | 14 (28.6) | 17 (17.3) | 0.116 |
Clinical outcomes and complications.
Values are presented as absolute numbers with percentages in parentheses. AS, anastomotic stricture. XM+, positive crossmatch. XM−, negative crossmatch. CMV, cytomegalovirus; EAD, early allograft dysfunction; ITBL, ischemic type biliary lesion; NAS, non-anastomotic stricture; PCR, polymerase chain reaction; PNF, primary non-function.
Rejection Episodes
Rejection episodes did not differ significantly between XM+ and XM− recipients [XM+ 14.3% (7 of 49) vs. XM− 10.2% (10 of 98), p = 0.466]. XM strength (p = 0.400) and type (p = 0.282) had no influence on the incidence of rejection episodes. Acute and chronic rejection rates were similar between groups [acute: XM+ 10.2% (5 of 49) vs. XM− 7.1% (7 of 98), p = 0.535; chronic: XM+ 4.1% (2 of 49) vs. XM− 3.1% (3 of 98), p = 1.000] (Table 3). Patients with a documented episode of allograft rejection tended to have more biliary complications than those without a rejection episode but that difference proved not to be statistically significant [58.8% (10 of 17) vs. 39.2% (51 of 130), p = 0.123]. Neither a CMV mismatch at LT (p = 0.546) nor a positive CMV PCR (p = 0.758) following LT was associated with the occurrence of rejection episodes.
Biliary Complications
Of 147 patients, 61 (41.5%) developed biliary complications (Table 3). There was no significant difference in overall biliary complications between the XM+ and XM− group [XM+ 42.9% (21 of 49) vs. XM− 40.8% (40 of 98), p = 0.813]. Bile duct leaks occurred in 10.2% (XM+ 5 of 49) vs. 17.3% (XM− 17 of 98), (p = 0.252), anastomotic strictures in 28.6% (XM+ 14 of 49) vs. 23.5% (XM− 23 of 98), (p = 0.502), non-anastomotic strictures in 12.2% (XM+ 6 of 49) vs. 9.2% (XM− 9 of 98), (p = 0.563) and biliary casts in 12.2% (XM+ 6 of 49) vs. 14.3% (XM− 14 of 98), (p = 0.734). In all NAS cases the hepatic artery was patent without stenosis or thrombosis and therefore, according to the pre-specified definition, these cases were all recorded as ITBL. Recipients with ITBL received organs from older donors [donor age median 64.0 years (48.0–76.0) vs. 52.0 years (42.0–61.0), p = 0.027] and the duration of the CIT was longer [CIT median 9.8 h (8.3–11.4) vs. 8.5 h (7.5–9.8), p = 0.038]. ET-DRI, a score incorporating donor age and CIT, was also significantly higher for recipients with ITBL [ET-DRI median 2.00 (1.74–2.30) vs. 1.57 (1.38–1.84), p = 0.002]. An episode of active CMV replication was associated with the occurrence of ITBL (p = 0.018). Univariate analysis revealed donor age, CIT, ET-DRI, allograft rejection and active CMV replication as risk factors for the development of ITBL. Considering these parameters for multivariate analysis (except for ET-DRI, as this a composite parameter) the most independent significant factor was allograft rejection [OR 7.773 (95% CI 1.878–31.169), p = 0.005] followed by donor age [OR 1.076 (95% CI 1.021–1.135), p = 0.006], active CMV replication [OR 4.096 (95% CI 1.180–14.219), p = 0.026] and duration of CIT [OR 1.315 (95% CI 1.032–1.676), p = 0.027] (Table 4). Out of 61 patients with a biliary complication, 50 patients (82.0%) required an endoscopic retrograde cholangiopancreatography, 15 patients (24.6%) underwent a re-operation while 13 patients (21.3%) required a re-transplantation. Patients with an ITBL were more likely to require a re-transplantation [33.3% (5 of 15) vs. 12.1% (16 of 132), p = 0.042]. Overall, patients with biliary complications had a significantly higher graft loss rate compared to patients without biliary complications [47.5% (29 of 61) vs. 27.9% (24 of 86), p = 0.015]. Neither XM strength nor XM type were associated with the development of biliary complications or ITBL.
TABLE 4
| OR | 95% CI | p-value | |
|---|---|---|---|
| Rejection | 7.773 | 1.878–32.169 | 0.005 |
| Donor Age | 1.076 | 1.021–1.135 | 0.006 |
| CMV PCR + | 4.096 | 1.180–14.219 | 0.026 |
| CIT | 1.315 | 1.032–1.676 | 0.027 |
Factors influencing ITBL - Multivariate analysis.
CI, confidence interval; CIT, cold ischemia time; CMV, cytomegalovirus; ITBL, ischemic type biliary lesions; OR, odds ratio; PCR, polymerase chain reaction.
Arterial Complications
In total, 13 patients (8.8%) developed arterial complications. The incidence of arterial complications did not differ between patients with and those without a positive crossmatch [XM+ 4.1% (2 of 49) vs. XM− 11.2% (11 of 98), p = 0.220]. No difference regarding the incidence of hepatic artery thrombosis (HAT) was noted between groups [XM+ 2.0% (1 of 49) vs. XM− 5.1% (5 of 98), p = 0.664].
CMV Infection
Overall, 20.7% of recipients developed a CMV infection (CMV PCR+). XM status was not associated with CMV PCR+ [XM+ 28.6% (14 of 49) vs. XM− 17.3% (17 of 98), p = 0.116]. Neither was XM type (p = 0.312). However, XM strength was associated with a CMV PCR+ [XM strong 50% (9 of 18) vs. XM weak 16.7% (5 of 30), p = 0.022]. CMV mismatch status at LT was associated with a subsequent CMV infection (D-/R- 0, D+/R- 4, D-/R+ 9, D+/R+ 17, p = 0.019).
Patient and Graft Survival
Mean patient survival was similar in patients with (XM+) and those without (XM−) a positive crossmatch [XM+ 134.7 months (95% CI 107.5–161.9) vs. XM− 117.2 months (95% CI 105.5–128.9), p = 0.398]. One- and five-year patient survival rates are shown in Figure 1. Mean graft survival was comparable between groups [XM+ 114.4 months (95% CI 90.4–138.5) vs. XM− 97.8 months (95% CI 84.5–111.2), p = 0.834]. One- and five-year graft survival rates are shown in Figure 2. No single parameter, including XM strength or type, was found to affect patient or graft survival in univariate Cox regression analysis. Re-transplantation rates [XM+ 8.2% (4 of 49) vs. XM− 17.3 (17 of 98), p = 0.234] did not differ significantly between groups. One primary non-function (PNF) was recorded in the XM+ group, whereas no PNF occurred in the XM− group.
FIGURE 1
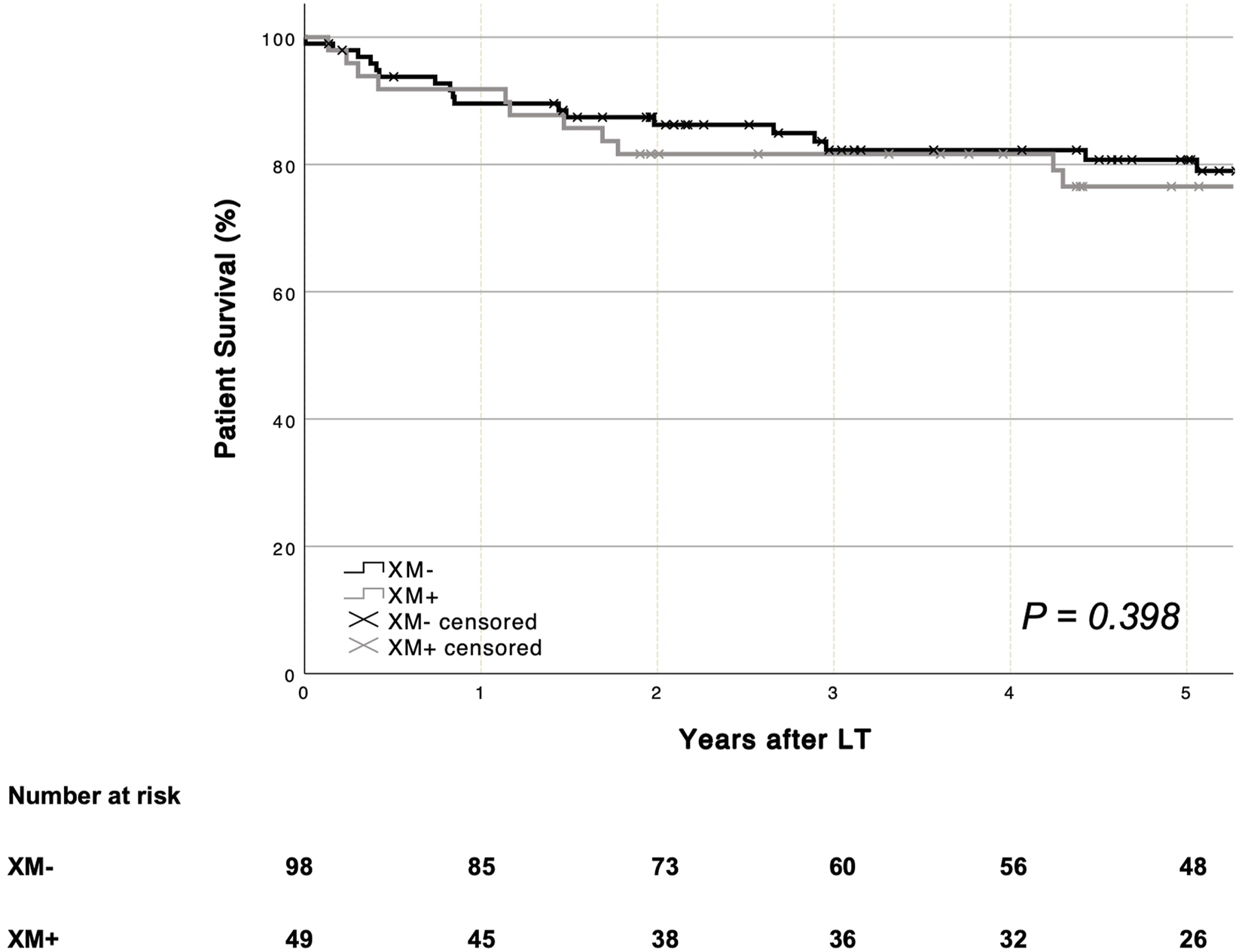
Overall patient survival was similar for recipients with and without a positive XM (log-rank p = 0.398). LT, liver transplantation. XM, crossmatch.
FIGURE 2
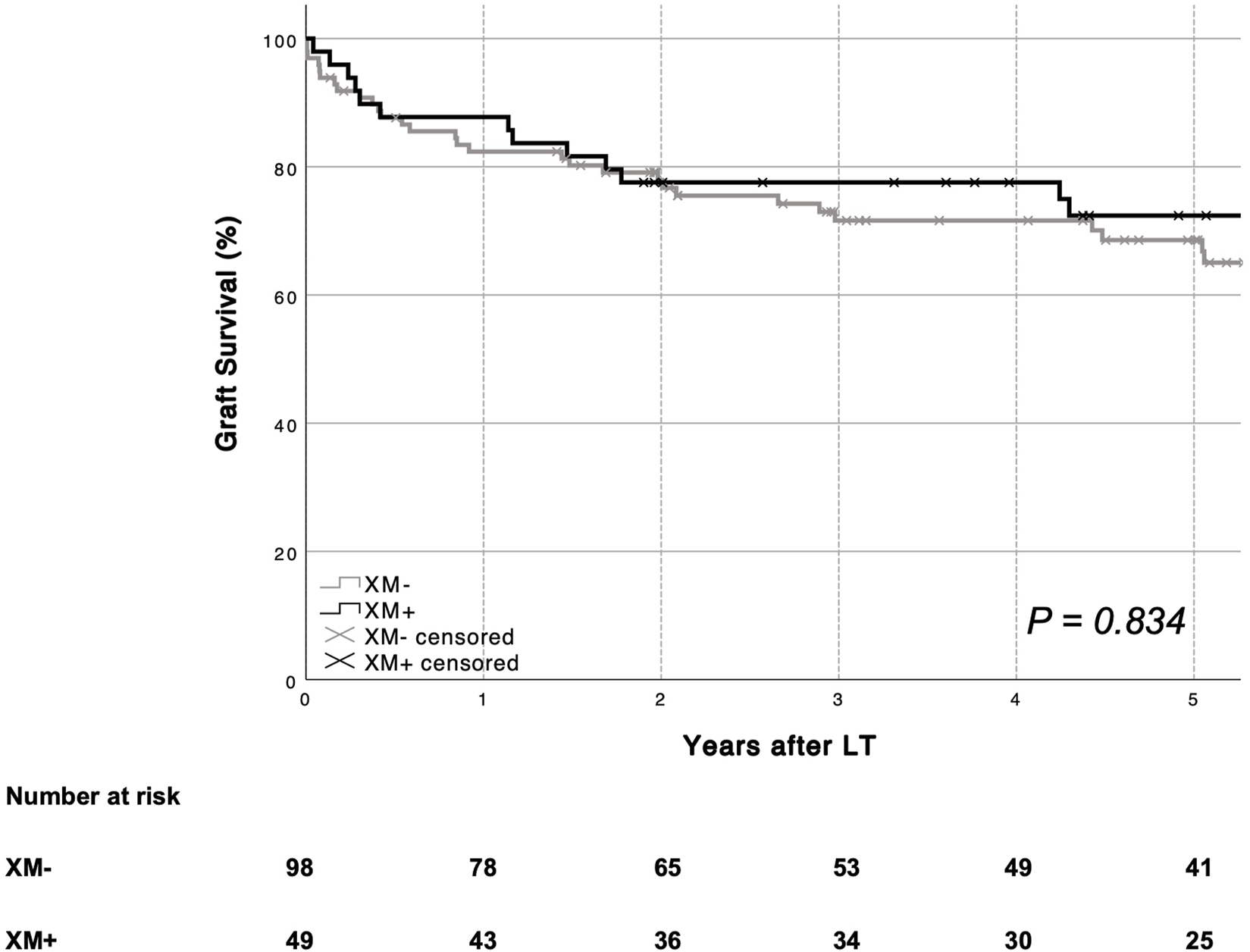
Overall graft survival according to XM status is shown. No difference in graft survival was seen based on recipient XM status (log-rank p = 0.834). LT, liver transplantation. XM, crossmatch.
Cause of Death
Overall, 37 out of 147 patients (25.2%) died during the observation period. Of those 37 patients, 13 (35%) died due to post-transplant malignancies, eight (22%) due to septic complications, six (16%) had recurrence of disease, six (16%) died of unknown causes, two (5%) died due to graft vs. host disease, one (3%) due to cardiovascular events and one (3%) due to other, non-specified reasons. Overall, 28 patients (76%) died with a functioning graft [XM+ 63% (10 of 16) vs. XM− 86% (18 of 21), p = 0.136].
Discussion
This analysis comparing XM+ and XM− LT recipients over the course of a 16-year period demonstrated that a XM+ has no obvious effects on patient and graft survival and does not appear to influence any of the relevant clinical outcome parameters following LT such as rejection episodes, biliary or arterial complications. Furthermore, neither XM type nor strength had any influence on post-transplant outcomes.
Known risk factors for XM+ are female recipient sex, previous LT as well as immunologic indications for LT such as autoimmune hepatitis (AIH) (6, 14, 24). In contrast to an analysis by Ruiz et al. (6), patients with AIH were not at risk for a XM+ in our study. Considering that only four patients in our cohort underwent LT for AIH this finding needs to be viewed cautiously. However, similar to results reported by Ruiz et al. and others (8, 13, 24, 44, 45), we found a higher number of female recipients and re-transplantations in the XM+ group; attributable to previous pregnancies, blood transfusions during or in the aftermath of the primary transplant operation and sensitization caused by the initial graft itself. We also found the recipient BMI to be lower in XM+ recipients, which is in accordance with the finding that the XM+ group encompassed more female recipients.
A high rate of antibody induction (61.9%) was observed in the study cohort. This can be explained by the fact that our center took part in two IL2 antibody induction studies (PROTECT (33) and DIAMOND (34)) during the study period. While the overall antibody induction rate did not differ between XM+ and XM− negative patients, XM+ patients were more likely to receive alemtuzumab (although the absolute number was small). Interestingly, XM strength did not correlate with the use of antibody induction. However, XM strength did correlate with subsequent PCR+ CMV infections.
Overall, the number of rejection episodes was similar between our XM+ and XM− recipients. Previous studies have reported higher rejection rates in XM+ recipients (13, 14, 17, 46, 47). However, almost all of these studies used different definitions of what constitutes a positive XM. Charco et al. (13) and Bathgate et al. (14) defined a XM+ as cytolysis greater than 20%, while Takaya et al. (44) defined a XM+ as cytolysis of 50% or more. Furthermore, IS regimens differed between study centers (14, 17, 46, 47), and most of these studies were conducted decades ago when IS regimens were less intensive with lower CsA and Tac target levels. While originally reporting a higher complication rate in recipients with a XM+ Takaya et al. showed, in a follow-up study, that comparable outcomes can be achieved with a more intense IS regimen (48). The more intense IS regimen used in the follow-up study constitutes the standard IS regimen today at most transplant centers including ours (45). This might explain why, in more recent studies with more intense IS regimens, a XM+ had no influence on the occurrence of rejection episodes, patient and graft survival as well as overall complications (24, 25, 45, 49), which is in accordance with our observations. To the contrary: in a recent study by Ünlü et al. (50) LT recipients perceived to be at an increased immunologic risk received more intense IS leading to higher infectious complications without providing any graft or patient survival benefit. Considering the liver’s privileged immunologic status, a more intense IS for XM+ recipients might be unnecessary and even harmful. Accordingly, when analyzing their 20-year experience with XM+ LT recipients Ruiz et al. (6) found no association between a XM+ and graft complications as well as patient and graft survival.
Compared to previous studies (44, 51, 52), we were unable to find any association between a XM+, including XM strength and type, and the occurrence of biliary complications. Unsurprisingly, patients with biliary complications had a higher graft loss rate and patients with ITBL required re-transplantation more often. ITBL remain one of the most worrisome complications following LT. Immunologic factors have been implicated in the pathogenesis of ITBL in addition to ischemia reperfusion injury and bile salt toxicity (39). While a XM+ had no influence on ITBL development in our study, allograft rejection as well as a positive CMV PCR were associated with an increased risk for the development of ITBL in uni- and multivariate analysis; as were older donor age and prolonged CIT, both well known risk factors for the development of ITBL. Furthermore, XM strength was positively associated with subsequent PCR+ CMV infections. Previous clinical studies have shown acute rejection and active CMV replication to be immunologic risk factor for the development of biliary complications in the context of LT (53-56). Interestingly, a PCR+ CMV infection in immunocompromised HIV positive patients has been known to cause destruction in the biliary tree for a long time, a condition termed AIDS cholangiopathy (57). In a study examining the effects of a CMV infection on rat liver grafts Martelius et al. provided experimental data supporting the role of CMV in the pathogenesis of bile duct injury (58). CMV infection leads to upregulation of MHC antigens and expression of vascular adhesion molecules such as VCAM-1 and ICAM-1 through secretion of pro-inflammatory cytokines (58, 59). Similarly, allograft rejection is thought to induce an inflammatory state at the local level leading to endothelial injury (60, 61). Since viability of the biliary tree depends on the oxygen rich arterial blood supply, an immune-mediated micro-vasculopathy may result in ischemic type injury to the bile ducts, providing a possible pathophysiological explanation for our findings (52, 62, 63).
Strengths and Limitations
The study compared XM+ with XM− LT using a 1:2 match-pair design. Matching was performed based on the BAR score which has shown to correlate best with post-transplant outcomes compared to other published risk scores (30, 31). Strengths of our study include the prospectively maintained LT database at our center, the match-pair analysis and the relatively long follow-up. Limitations of the present study include the retrospective design and a possible bias concerning the selection of participants beyond the data displayed in the demographics. Despite performing a match-pair analysis in order to guarantee a homogenous comparison group, differences in donor and recipient characteristics did exist between the XM+ and XM− group. The donor BMI was significantly lower, and anhepatic as well as WIT were significantly shorter in the XM+ group compared to the XM− group. This may introduce a bias as a lower donor BMI and shorter ischemia times could imply favorable outcomes. Furthermore, the recipients’ MELD score was found to be higher in the XM+ group. However, the BAR score which, among other factors, includes the MELD score and correlates with relevant outcome parameters following LT was used for match-pair analysis to mitigate potential biases. None of these factors had any significant influence on patient or graft survival in our cohort when performing univariate Cox proportional hazards regression analysis (Supplementary Tables S1, S2) as well as when adjusting for these differences in baseline characteristics in a multivariate Cox regression model (Supplementary Tables S3, S4). Also, University of Wisconsin (UW) solution was more commonly used than Histidine-Tryptophan-Ketoglutarate (HTK) solution as a preservation solution in the XM+ group. UW used to be the gold standard for static cold storage perfusion of liver grafts but preservation with HTK is reported to be clinically equivalent (64, 65). Concerns regarding the higher viscosity of UW leading to an incomplete flush of the peribiliary glands and an increase in ITBL have been voiced. However, these concerns have not materialized (66). Moreover, the type of preservation solution had no significant influence on the development of ITBL in our recipients in univariate binary logistic regression analysis (Supplementary Table S5).
Conclusion
In the present era of LT, a XM+ has no effects on graft and patient survival as well as postoperative complications. Therefore, our center policy will not change, and we will continue to transplant patients without waiting for XM testing results despite the logistical possibilities offered by the advent of normothermic machine perfusion. A PCR+ CMV infection was more likely to occur in recipients with a strongly positive XM. Together with allograft rejection, donor age and CIT, a PCR+ CMV infection was among the strongest independent predictor for the development of ITBL. Patients with ITBL had higher re-transplantation rates than patients without ITBL.
Statements
Data availability statement
Data is available upon reasonable request form the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Ethikkommission der Medizinischen Universität Innsbruck. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
FJK: Study design, data acquisition, data analysis, interpretation of the data, drafting and revising the manuscript; MF: Data acquisition, interpretation of the data, drafting and revising the manuscript; FM: Interpretation of the data, drafting and revising the manuscript; AB: Data acquisition, interpretation of the data, drafting and revising the manuscript; AV: Data acquisition, interpretation of the data, drafting and revising the manuscript; BC: Interpretation of the data, drafting and revising the manuscript; TR: Interpretation of the data, drafting and revising the manuscript; MM: Interpretation of the data, drafting and revising the manuscript; CM: Interpretation of the data, drafting and revising the manuscript; MR: Study design, data analysis, interpretation of the data, drafting and revising the manuscript; HU: Study design, data analysis, interpretation of the data, drafting and revising the manuscript; DÖ: Interpretation of the data, drafting and revising the manuscript; RO: Interpretation of the data, drafting and revising the manuscript; SS: interpretation of the data, drafting and revising the manuscript; AW: Study design, data acquisition, data analysis, interpretation of the data, drafting and revising the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2023.11062/full#supplementary-material
Glossary
- AIH
autoimmune hepatitis
- AS
anastomotic stenosis
- AST
aspartate transferase
- ATG
anti-thymocyte globulin
- BAR score
balance of risk score
- BMI
body mass index
- CDC
cytotoxic dependent cytotoxicity
- CIT
cold ischemia time
- CMV
cytomegalovirus
- COD
cause of death
- CsA
cyclosporine A
- CVA
cerebrovascular accident
- DCD
donation after cardiocirculatory death
- DSA
donor specific antibody
- DTT
dithiothreitol
- EAD
early allograft dysfunction
- ECD
extended criteria donor
- ET-DRI
Eurotransplant donor risk index
- gGT
gamma-glutamyltransferase
- HAT
hepatic artery thrombosis
- HTK
histidine-tryptophan-ketoglutarate
- IL2
interleukin 2
- IS
immunosuppressive
- ITBL
ischemic type biliary lesion
- INR
international normalized ratio
- IQR
interquartile range
- LT
liver transplantation
- MELD
model of end-stage liver disease
- MMF
mycophenolate mofetil
- MPA
mycophenolic acid
- NAFLD
non-alcoholic fatty liver disease
- NAS
non-anastomotic stenosis
- PBC
primary biliary cirrhosis
- PCR
polymerase chain reaction
- PSC
primary sclerosing cholangitis
- Re-Tx
re-transplantation
- SAB
subarachnoid hemorrhage
- SD
standard deviation
- Tac
tacrolimus
- UW
University of Wisconsin
- WIT
warm ischemia time
- XM
crossmatch
- XM+
positive crossmatch
- XM−
negative crossmatch
References
1.
Shin M Moon HH Kim JM Park JB Kwon CHD Kim SJ et al Significance of True-Positive and False-Positive Pretransplantation Lymphocytotoxic Crossmatch in Primary Liver Allograft Outcomes. Transplantation (2013) 15(11):1410–7. 10.1097/TP.0b013e31828d155a
2.
Tait BD Süsal C Gebel HM Nickerson PW Zachary AA Claas FHJ et al Consensus Guidelines on the Testing and Clinical Management Issues Associated with HLA and Non-HLA Antibodies in transplantation. Transplantation (2013) 95(1):19–47. 10.1097/TP.0b013e31827a19cc
3.
Opelz G Döhler B Süsal C . Analysis of Positive Kidney, Heart, and Liver Transplant Crossmatches Reported to the Collaborative Transplant Study. Hum Immunol (2009) 70(8):627–30. 10.1016/j.humimm.2009.04.009
4.
Sugawara Y Tamura S Kaneko J Togashi J Makuuchi M Kokudo N . Positive Lymphocytotoxic Crossmatch Does Not Adversely Affect Survival in Living Donor Liver Transplantation. Dig Surg (2009) 26(6):482–6. 10.1159/000253873
5.
Na GH Kim EY Hong TH You YK Kim DG . Effects of Preoperative Positive Cross-Match and HLA Mismatching on Early Acute Cellular Rejection and Graft Survival in Living Donor Liver Transplantation. Ann Transpl17 (2015) 553–60. 10.12659/AOT.894466
6.
Ruiz R Tomiyama K Campsen J Goldstein RM Levy MF McKenna GJ et al Implications of a Positive Crossmatch in Liver Transplantation: a 20-year Review. Liver Transplant (2012) 18(4):455–60. 10.1002/lt.22474
7.
Al-Sibae MR Koffron AJ Raofi V . Does a Positive Pretransplant Crossmatch Affect Long-Term Outcome in Liver Transplantation?Transplantation (2011) 91(3):261–2. 10.1097/TP.0b013e318204758c
8.
Demetris AJ Nakamura K Yagihashi A Takaya S Hartman GG et al A Clinicopathological Study of Human Liver Allograft Recipients Harboring Preformed IgG Lymphocytotoxic Antibodies. Hepatology (1992) 16(3):671–81. 10.1002/hep.1840160310
9.
Fung J Griffin M Duquesnoy R Shaw B Starzl T . Successful Sequential Liver-Kidney Transplantation in a Patient with Performed Lymphocytotoxic Antibodies. Transpl Proc. (1987) 19(1):767–8.
10.
Donaldson PT Thomson LJ Heads A Underhill JA Vaughan RW Rolando N et al IgG Donor-specific Crossmatches Are Not Associated with Graft Rejection or Poor Graft Survival after Liver Transplantation. An Assessment by Cytotoxicity and Flow Cytometry. Transplantation (1995) 60(9):1016–23.
11.
Neumann UP Lang M Moldenhauer A Langrehr JM Glanemann M Kahl A et al Significance of a T-Lymphocytotoxic Crossmatch in Liver and Combined Liver-Kidney Transplantation. Transplantation (2001) 71(8):1163–8. 10.1097/00007890-200104270-00025
12.
Gordon RD Fung JJ Markus B Fox I IwatSuki S Esquivel CO et al The Antibody Crossmatch in Liver Transplantation. Surgery (1986) 100(4):705–15.
13.
Charco R Vargas V Balsells J Lazaro JL Murio E JaurriEta E et al Influence of Anti-HLA Antibodies and Positive T-Lymphocytotoxic Crossmatch on Survival and Graft Rejection in Human Liver Transplantation. J Hepatol (1996) 24(4):452–9. 10.1016/s0168-8278(96)80166-8
14.
Bathgate AJ McColl M Garden OJ Forsythe JL Madhavan KK Hayes PC . The Effect of a Positive T-Lymphocytotoxic Crossmatch on Hepatic Allograft Survival and Rejection. Liver Transpl Surg (1998) 4(4):280–4. 10.1002/lt.500040411
15.
Katz SM Kimball PM Ozaki C Monsour H Clark J Cavazos D et al Positive Pretransplant Crossmatches Predict Early Graft Loss in Liver Allograft Recipients. Transplantation (1994) 57(4):616–20.
16.
Muro M Marin L Miras M Moya-Quiles R Minguela A Sanchez-Bueno F et al Liver Recipients Harbouring Anti-donor Preformed Lymphocytotoxic Antibodies Exhibit a Poor Allograft Survival at the First Year after Transplantation: Experience of One centre. Transpl Immunol (2005) 14(2):91–7. 10.1016/j.trim.2005.03.013
17.
Nikaein A Backman L Jennings L Levy MF Goldstein R Gonwa T et al HLA Compatibility and Liver Transplant Outcome. Improved Patient Survival by HLA and Cross-Matching. Transplantation (1994) 58(7):786–92.
18.
Talbot D Bell A Shenton BK Hussein KA Manas D Gibbs P et al The Flow Cytometric Crossmatch in Liver Transplantation. Transplantation (1995) 59(5):737–40. 10.1097/00007890-199503150-00017
19.
Iwatsuki S Rabin BS Shaw BW Jr. Starzl TE . Liver Transplantation against T Cell-Positive Warm Crossmatches. Transpl Proc. (1984) 16(6):1427–9.
20.
Doyle HR Marino IR Morelli F Doria C Aldrighetti L McMichael J et al Assessing Risk in Liver Transplantation. Special Reference to the Significance of a Positive Cytotoxic Crossmatch. Ann Surg (1996) 224(2):168–77. 10.1097/00000658-199608000-00009
21.
Goggins WC Fisher RA Kimball PM Wolfe L Hill BE Pietruszka TD et al The Impact of a Positive Crossmatch upon Outcome after Liver Transplantation. Transplantation (1996) 62(12):1794–8. 10.1097/00007890-199612270-00019
22.
Lang M Neumann U Knoop M Bechstein WO Neuhaus P . Impact of Immunosuppression in Liver Transplantation across a Positive Crossmatch. Transpl Proc. (1998) 30(4):1466–7. 10.1016/s0041-1345(98)00318-2
23.
Dagher L Chavez R Taylor C Smith ST Alexander G Jamieson N . Influence of Positive Crossmatch in the Outcome of Liver Transplantation. Transpl Proc. (1999) 31(6):2363. 10.1016/s0041-1345(99)00379-6
24.
Matinlauri IH Höckerstedt KA Isoniemi HM . Equal Overall Rejection Rate in Pre-transplant Flow-Cytometric Cross-Match Negative and Positive Adult Recipients in Liver Transplantation. Clin Transpl (2005) 19(5):626–31. 10.1111/j.1399-0012.2005.00364.x
25.
Joo DJ Ju MK Huh KH Kim MS Choi GH Choi JS et al Does Lymphocyte Cross-Matching Predict Acute Rejection and Graft Survival in Liver Transplantation? Transpl Proc. (2012) 44(2):418–20. 10.1016/j.transproceed.2012.01.071
26.
Leonard GR Shike H Uemura T Gaspari JL Ruggiero FM Shah RA et al Liver Transplantation with a Strongly Positive Crossmatch: Case Study and Literature Review. Liver Transplant (2013) 19(9):1001–10. 10.1002/lt.23694
27.
Cardini B Oberhuber R Fodor M Hautz T Margreiter C Resch T et al Clinical Implementation of Prolonged Liver Preservation and Monitoring through Normothermic Machine Perfusion in Liver Transplantation. Transplantation (2020) 104(9):1917–28. 10.1097/TP.0000000000003296
28.
Karuppan S Ericzon BG Möller E . Relevance of a Positive Crossmatch in Liver Transplantation. Transpl Int (1991) 4(1):18–25. 10.1007/BF00335511
29.
Doyle HR Marino IR . Effect of HLA Match in Liver Transplantation. Transplantation (1995) 60(1):112–3. 10.1097/00007890-199507150-00024
30.
Boecker J Czigany Z Bednarsch J Amygdalos I Meister F Santana DAM et al Potential Value and Limitations of Different Clinical Scoring Systems in the Assessment of Short- and Long-Term Outcome Following Orthotopic Liver Transplantation. PLoS One (2019) 14:e0214221. 10.1371/journal.pone.0214221
31.
Dutkowski P Oberkofler CE Slankamenac K Puhan MA Schadde E Mullhaupt B et al Are There Better Guidelines for Allocation in Liver Transplantation? A Novel Score Targeting justice and Utility in the Model for End-Stage Liver Disease Era. Ann Surg (2011) 254(5):745–53. 10.1097/SLA.0b013e3182365081
32.
von Elm E Altman DG Egger M Pocock SJ Gøtzsche PC Vandenbroucke JP et al The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. Int J Surg (2014) 12(12):1495–9. 10.1016/j.ijsu.2014.07.013
33.
Fischer L Klempnauer J Beckebaum S Metselaar HJ Neuhaus P Schemmer P et al A Randomized, Controlled Study to Assess the Conversion from Calcineurin-Inhibitors to Everolimus after Liver Transplantation–PROTECT. Am J Transpl (2012) 12(7):1855–65. 10.1111/j.1600-6143.2012.04049.x
34.
TruneČka P Klempnauer J Bechstein WO Pirenne J Friman S Zhao A et al Renal Function in De Novo Liver Transplant Recipients Receiving Different Prolonged-Release Tacrolimus Regimens-The DIAMOND Study. Am J Transpl (2015) 15(7):1843–54. 10.1111/ajt.13182
35.
Pirofsky B Rosner ER . DTT Test: a New Method to Differentiate IgM and IgG Erythrocyte Antibodies. Vox Sang (1974) 27(5):480–8. 10.1111/j.1423-0410.1974.tb02446.x
36.
Okuno T Kondelis N . Evaluation of Dithiothreitol (DTT) for Inactivation of IgM Antibodies. J Clin Pathol (1978) 31(12):1152–5. 10.1136/jcp.31.12.1152
37.
Olthoff KM Kulik L Samstein B Kaminski M Abecassis M Emond J et al Validation of a Current Definition of Early Allograft Dysfunction in Liver Transplant Recipients and Analysis of Risk Factors. Liver Transplant (2010) 16(8):943–9. 10.1002/lt.22091
38.
van Leeuwen OB Bodewes SB Lantinga VA Haring MPD Thorne AM Bruggenwirth IMA et al Sequential Hypothermic and Normothermic Machine Perfusion Enables Safe Transplantation of High-Risk Donor Livers. Am J Transpl (2022) 22:1658–70. 10.1111/ajt.17022
39.
Buis CI Hoekstra H Verdonk RC Porte RJ . Causes and Consequences of Ischemic-type Biliary Lesions after Liver Transplantation. J Hepatobiliary Pancreat Surg (2006) 13(6):517–24. 10.1007/s00534-005-1080-2
40.
Sanchez-Urdazpal L Gores GJ Ward EM Maus TP Wahlstrom HE Moore SB et al Ischemic-type Biliary Complications after Orthotopic Liver Transplantation. Hepatology (1992) 16(1):49–53. 10.1002/hep.1840160110
41.
Fodor M Cardini B Peter W Weissenbacher A Oberhuber R Hautz T et al Static Cold Storage Compared with Normothermic Machine Perfusion of the Liver and Effect on Ischaemic-type Biliary Lesions after Transplantation: a Propensity Score-Matched Study (2021) 108(9):1082–9. 10.1093/bjs/znab118
42.
Foundation E . Chapter 9: The Donor (2017). Available from: https://www.eurotransplant.org/professionals/eurotransplant-manual/(Accessed 12 08, 2021).
43.
Bursac Z Gauss CH Williams DK Hosmer DW . Purposeful Selection of Variables in Logistic Regression. Source code Biol Med (2008) 3:17. 10.1186/1751-0473-3-17
44.
Takaya S Jain A Yagihashi A TaKeuchi K et al Increased Bile Duct Complications And/or Chronic Rejection in Crossmatch Positive Human Liver Allografts. Transpl Proc. (1999) 31(5):2028–31. 10.1016/s0041-1345(99)00256-0
45.
Taner T Gandhi MJ Sanderson SO Poterucha CR De Goey SR Stegall MD et al Prevalence, Course and Impact of HLA Donor-specific Antibodies in Liver Transplantation in the First Year. Am J Transpl (2012) 12(6):1504–10. 10.1111/j.1600-6143.2012.03995.x
46.
Takaya S Bronsther O Iwaki Y NaKamura K Abu-Elmagd K YagihAshi A et al The Adverse Impact on Liver Transplantation of Using Positive Cytotoxic Crossmatch Donors. Transplantation (1992) 53(2):400–6. 10.1097/00007890-199202010-00026
47.
Charco R Balsells J Murio E Lázaro JL Margarit C Martorell J . Adverse Impact of High Panel-Reactive Antibody (PRA) and Positive Cytotoxic Crossmatch in Liver Transplantation. Transpl Int (1994) 7(1):S94–6. 10.1111/j.1432-2277.1994.tb01319.x
48.
Takaya S Iwaki Y Starzl TE . Liver Transplantation in Positive Cytotoxic Crossmatch Cases Using FK506, High-Dose Steroids, and Prostaglandin E1. Transplantation (1992) 54(5):927–9. 10.1097/00007890-199211000-00031
49.
Lunz J Ruppert KM Cajaiba MM Isse K Bentlejewski CA Minervini M et al Re-examination of the Lymphocytotoxic Crossmatch in Liver Transplantation: Can C4d Stains Help in Monitoring? Am J Transpl (2012) 12(1):171–82. 10.1111/j.1600-6143.2011.03786.x
50.
Ünlü S Lachmann N Jara M Ritschl PV Wiering L Eurich D et al Treatment of Anti-HLA Donor-specific Antibodies Results in Increased Infectious Complications and Impairs Survival after Liver Transplantation. J Clin Med (2020) 9:3986. 10.3390/jcm9123986
51.
Batts KP Moore SB Perkins JD Wiesner RH Grambsch PM Krom RA . Influence of Positive Lymphocyte Crossmatch and HLA Mismatching on Vanishing Bile Duct Syndrome in Human Liver Allografts. Transplantation (1988) 45(2):376–9. 10.1097/00007890-198802000-00026
52.
Musat AI Agni RM Wai PY Pirsch JD Lorentzen DF Powell A et al The Significance of Donor-specific HLA Antibodies in Rejection and Ductopenia Development in ABO Compatible Liver Transplantation. Am J Transpl (2011) 11(3):500–10. 10.1111/j.1600-6143.2010.03414.x
53.
Halme L Hockerstedt K Lautenschlager I . Cytomegalovirus Infection and Development of Biliary Complications after Liver Transplantation. Transplantation. Jun15(2003):75. 10.1097/01.tp.0000064620.08328.e5
54.
Magro B Tacelli M Mazzola A Conti F Celsa C . Biliary Complications after Liver Transplantation: Current Perspectives and Future Strategies. Hepatobiliary Surg Nutr (2021) 10(1):76–92. 10.21037/hbsn.2019.09.01
55.
Pirenne J Monbaliu D Aerts R Desschans B Liu Q Cassiman D et al Biliary Strictures after Liver Transplantation: Risk Factors and Prevention by Donor Treatment with Epoprostenol. Transpl Proc. (2009) 41(8):3399–402. 10.1016/j.transproceed.2009.09.026
56.
Nemes B Gámán G Doros A . Biliary Complications after Liver transplantation. Expert Rev Gastroenterol Hepatol (2015) 9(4):447–66. Expert Rev Gastroenterol Hepatol. 10.1586/17474124.2015.967761
57.
Margulis SJ Honig CL Soave R Govoni AF Mouradian JA Jacobson IM . Biliary Tract Obstruction in the Acquired Immunodeficiency Syndrome. Ann Intern Med (1986) 105(2):207–10. 10.7326/0003-4819-105-2-207
58.
Martelius T Krogerus L Höckerstedt K Bruggeman C Lautenschlager I . Cytomegalovirus Infection Is Associated with Increased Inflammation and Severe Bile Duct Damage in Rat Liver Allografts. Hepatology (1998) 27(4):996–1002. 10.1002/hep.510270415
59.
Lautenschlager I Halme L Höckerstedt K Krogerus L Taskinen E . Cytomegalovirus Infection of the Liver Transplant: Virological, Histological, Immunological, and Clinical Observations. Transpl Infect Dis (2006) 8(1):21–30. 10.1111/j.1399-3062.2006.00122.x
60.
Ronca V Wootton G Milani C Cain O . The Immunological Basis of Liver Allograft Rejection. Front Immunol (2020) 11:2155. 10.3389/fimmu.2020.02155
61.
Senter-Zapata M Khan AS Subramanian T Vachharajani N Dageforde LA Wellen JR et al Patient and Graft Survival: Biliary Complications after Liver Transplantation. J Am Coll Surgeons (2018) 226(4):484–94. 10.1016/j.jamcollsurg.2017.12.039
62.
Lautenschlager I Höckerstedt K Jalanko H Loginov R Salmela K TaskinEn E et al Persistent Cytomegalovirus in Liver Allografts with Chronic Rejection. Hepatology (1997) 25(1):190–4. 10.1053/jhep.1997.v25.pm0008985289
63.
Campbell P . Clinical Relevance of Human Leukocyte Antigen Antibodies in Liver, Heart, Lung and Intestine Transplantation. Curr Opin Organ Transpl (2013) 18(4):463–9. 10.1097/MOT.0b013e3283636c71
64.
Feng L Zhao N Yao X Sun X Du L Diao X et al Histidine-tryptophan-ketoglutarate Solution vs. University of Wisconsin Solution for Liver Transplantation: a Systematic Review. Liver Transplant (2007) 13(8):1125–36. 10.1002/lt.21208
65.
Rosado J Guarrera JV . UW versus HTK for Static Preservation in Liver Transplantation: Is There a "Solution Effect" on Outcomes?Transplantation (2018) 102(11):1791–2. 10.1097/TP.0000000000002407
66.
Kaltenborn A Gwiasda J Amelung V et al Comparable Outcome of Liver Transplantation with Histidine-Tryptophan-Ketoglutarate vs. University of Wisconsin Preservation Solution: a Retrospective Observational Double-center Trial. BMC Gastroenterol (2014) 14:169. 10.1186/1471-230x-14-169
Summary
Keywords
liver transplantation, biliary complications, graft survival, crossmatch, CMV
Citation
Krendl FJ, Fodor M, Messner F, Balog A, Vales A, Cardini B, Resch T, Maglione M, Margreiter C, Riedmann M, Ulmer H, Öfner D, Oberhuber R, Schneeberger S and Weissenbacher A (2023) Liver Transplantation in Recipients With a Positive Crossmatch: A Retrospective Single-Center Match-Pair Analysis. Transpl Int 36:11062. doi: 10.3389/ti.2023.11062
Received
17 November 2022
Accepted
20 February 2023
Published
02 March 2023
Volume
36 - 2023
Updates
Copyright
© 2023 Krendl, Fodor, Messner, Balog, Vales, Cardini, Resch, Maglione, Margreiter, Riedmann, Ulmer, Öfner, Oberhuber, Schneeberger and Weissenbacher.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Annemarie Weissenbacher, annemarie.weissenbacher@i-med.ac.at
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.