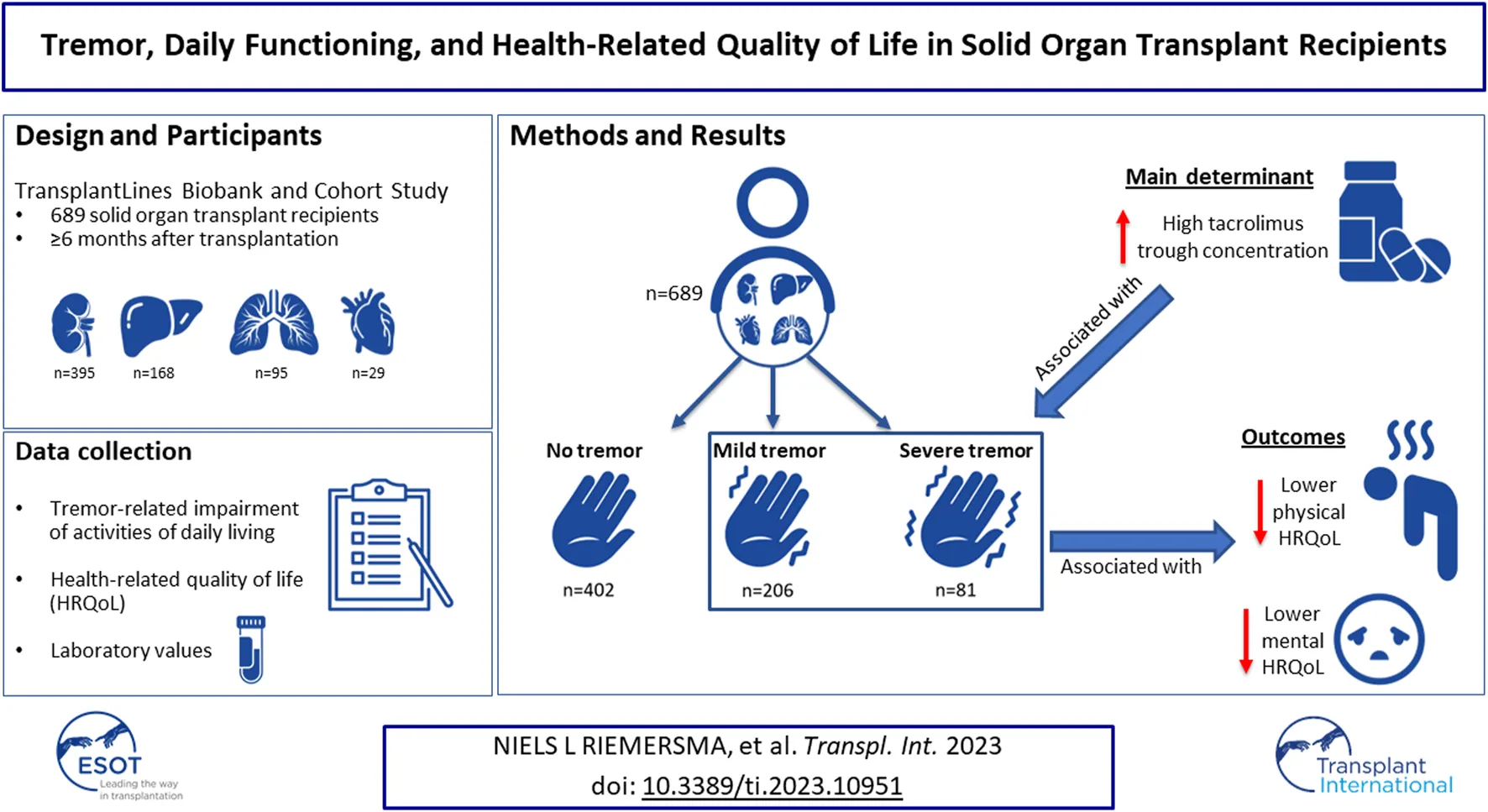
Abstract
Solid organ transplant recipients (SOTR) frequently report tremor. Data concerning tremor-related impairment and its potential impact on health-related quality of life (HRQoL) are lacking. This cross-sectional study assesses impact of tremor on activities of daily living and HRQoL using validated questionnaires among SOTR enrolled in the TransplantLines Biobank and Cohort Study. We included 689 SOTR (38.5% female, mean [±SD] age 58 [±14] years) at median [interquartile range] 3 [1–9] years after transplantation, of which 287 (41.7%) reported mild or severe tremor. In multinomial logistic regression analyses, whole blood tacrolimus trough concentration was an independent determinant of mild tremor (OR per µg/L increase: 1.11, 95% CI: 1.02 to 1.21, p = 0.019). Furthermore, in linear regression analyses, severe tremor was strongly and independently associated with lower physical and mental HRQoL (β = −16.10, 95% CI: −22.23 to −9.98, p < 0.001 and β = −12.68, 95% CI: −18.23 to −7.14, p < 0.001 resp.). SOTR frequently report tremor-related impairment of activities of daily living. Tacrolimus trough concentrations appeared as a main determinant of tremor among SOTR. The strong and independent association of tremor-related impairment with lower HRQoL warrants further studies into the effects of tacrolimus on tremor.
Clinical Trial Registration: ClinicalTrials.gov, Identifier NCT03272841.
Introduction
Solid organ transplantation has evolved from a scientific novelty to the preferred treatment for end-stage organ failure. For example, kidney, liver, lung, and heart transplantations strongly improve long-term survival in otherwise untreatable diseases (1–7). Unfortunately, even after successful transplantation, solid organ transplant recipients (SOTR) continue to have reduced health-related quality of life (HRQoL) compared to the general population (8–10). A suggested cause for this limited HRQoL is the need to adhere to a strict immunosuppressive maintenance regime, generally including calcineurin inhibitors (CNIs) (11). These CNIs, including cyclosporine and tacrolimus, are essential to prevent graft rejection, and are a cornerstone in current post-transplantation care (12). However, CNI-use is associated with multiple side effects, including nephrotoxicity and neurotoxicity (13, 14).
One of the most frequently reported side effects of CNIs is the development of tremor: rhythmic, sinusoidal oscillations of the limbs, head, or trunk (15). Medication-induced tremor may limit SOTR in performing activities of daily living (ADL), such as eating, writing, and personal hygiene, much like tremor interferes with ADL in other populations (16).
CNI-induced tremor generally occurs soon after initiation of CNI maintenance therapy (17), and occurs in up to half of SOTR using CNIs (18). The occurrence of CNI-induced tremor is reportedly dose-dependent, yet some patients experience tremor even at low dosages (18, 19). Currently, we lack the knowledge to what extent tremor leads to impairments in ADL, and how this may affect HRQoL among SOTR. In-depth investigation of the impact of tremor on ADL and HRQoL is therefore warranted and may add valuable information to previous studies that abstained from using tremor-related ADL impairment as primary outcome measurement (18).
We therefore aimed to assess the prevalence and severity of tremor-related ADL impairment among SOTR. Additionally, we aimed to identify clinical, biochemical, and pharmaceutical factors that may predispose SOTR for the development of tremor-related impairment. Finally, we assessed associations of tremor-related impairment with HRQoL.
Materials and Methods
Design and Study Population
For this cross-sectional study, data from the TransplantLines Biobank and Cohort Study (ClinicalTrials.gov Identifier: NCT03272841) were used (20). This ongoing cohort study includes SOTR and donors (aged ≥18 years) visiting the University Medical Center Groningen (UMCG, Groningen, The Netherlands). More detailed information on the study design, inclusion and exclusion criteria has been described previously (20). The study was conducted in accordance with the guidelines laid down in the Declarations of Helsinki and Istanbul and approved by the Institutional Review Board (METc 2014/077). The study protocol was reviewed and approved by the TransplantLines Scientific Committee (TxL 2022/004).
For the current study, all enrolled SOTR with a functioning allograft for at least 6 months, with available data on tremor influence on ADL between September 2016 and November 2020, were included. A consort flow diagram is shown in Supplementary Figure S1.
Immunosuppressive Regimen
All included SOTR attended the outpatient clinic of the UMCG and were treated according to standard immunosuppressive therapies, with revision of therapy effectiveness at least once per year. Immunosuppressive maintenance therapies were generally tacrolimus-based, with addition of mycophenolate mofetil (MMF), and prednisolone. Other immunosuppressive drugs used less frequently were cyclosporine, everolimus, sirolimus, and azathioprine. Although immunosuppressive regimens may be adapted on an individual basis, standard protocol target trough concentrations of tacrolimus and cyclosporine in the UMCG are shown in Table 1.
TABLE 1
| Time after transplantation (months) | Tacrolimus trough concentration (µg/L) | Cyclosporine trough concentration (µg/L) | ||||
|---|---|---|---|---|---|---|
| 7–12 | 13–24 | >24 | 7–12 | 13–24 | >24 | |
| Kidney transplantation | 4–6 | 4–6 | 4–6 | 75–125 | 75–125 | 75–125 |
| Liver transplantation | ||||||
| aDual therapy | 7–10 | 7–10 | 7–10 | * | * | * |
| bTriple therapy | 5–7 | 5–7 | 5–7 | * | * | * |
| Lung transplantation | 7–10 | 7–10 | 7–10 | 150 | 100–150 | 100 |
| Heart transplantation | 7–9 | 7–9 | 7–9 | 150 | 125 | 125 |
| Small intestine transplantation | 10–15 | 10–15 | 10–15 | ∼ | ∼ | ∼ |
Immunosuppressive target trough concentration per transplant type in the UMCG.
aDual therapy consists of tacrolimus and prednisone, and btriple therapy additionally includes mycophenolate mofetil.
∼ target trough concentrations are not listed in UMCG protocols. *Cyclosporine is not used for this type of transplantation.
Laboratory Methods
Blood was drawn in the morning after an overnight fasting period of at least 8 h, including no medication use. All tacrolimus and cyclosporine whole blood trough concentrations were determined by validated liquid chromatography mass-spectrometry analyses at the UMCG (21). Other laboratory parameters were measured using routine laboratory methods (Roche Diagnostics, Basel, Switzerland).
Tremor Rating
Tremor severity was assessed using a Dutch translation of the Fahn-Tolosa-Marin (FTM) tremor rating scale part C (TRS-C) (22). This questionnaire is recommended for use in clinical practice (23). The TRS-C consists of eight questions to assess patient-perceived tremor occurrence during ADL, including writing, speaking, and bringing food or liquids to the mouth, and is provided in Supplementary Table S1. Furthermore, the questionnaire assesses the influence of tremor on personal hygiene, dressing, working, and social activities. As the TRS-C is designed to evaluate the impact of tremor on ADL, by nature, this questionnaire assesses severity over the previous couple of days of the patient’s life. The interviewers were trained to conduct the questionnaire during routine follow-up visits, and to interpret the answers of patients. To every question, patients graded the impairment tremor has on ADL with a score ranging from 0 (no influence of tremor on ADL) to 4 (severe influence of tremor on ADL). A total score was calculated by summing the individual scores, with a theoretical maximum score of 32 points. Patients with a TRS-C total score of 0, or with a TRS-C total score of 1 acquired because of mild disability of speech (i.e., shaky voice only when nervous), were classified as having no tremor. For data visualisation, and statistical analyses, tremor severity was rated according to severity: no tremor (defined as 0 points or 1 point on speech (“mild tremor only perceived when nervous” on the total TRS C score)), mild tremor (1–3 points), and severe tremor (≥4 points).
Health-Related Quality of Life
HRQoL was assessed using the 36-Item Short Form health survey, which is a validated questionnaire to assess several health domains, including physical functioning, role limitations due to physical health, role limitations due to emotional problems, vitality, emotional wellbeing, social functioning, pain, and general health perceptions (24). Subsequently, physical component summary (PCS) and mental component summary (MCS) scores were derived, with a higher score meaning a higher HRQoL. The PCS includes items from physical functioning, role limitations due to physical health, vitality, social functioning, pain, and general health perceptions, whereas the MCS includes items from role limitations due to emotional problems, vitality, emotional wellbeing, social functioning, and general health perceptions.
Additional Data Collection
Blood pressure and heart rate were measured with a semi-automatic device (Philips Suresign VS2+, Andover, Massachusetts, USA). Body weight and height were measured with participants wearing indoor clothing without shoes. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (kg/m2). Information on patients’ medical history was extracted from electronic patient records, and medication use was extracted from patient records and verified with the patient by a trained researcher. Diabetes was defined based on use of antidiabetic drugs, fasting glucose ≥7.0 mmol/L, and/or HbA1c ≥6.5%. Kidney glomerular filtration rate was estimated with the creatinine-based CKD-EPI equation (25). Anaemia was defined as a haemoglobin concentration <8.1 mmol/L for men and <7.5 mmol/L for women according to WHO criteria (26).
Statistical Analyses
Dispersion of TRS-C scores in total, and per transplant type, were visualized by means of pie charts and bar charts. Patient characteristics are presented and compared in patients without tremor, with mild tremor, and with severe tremor. Continuous variables are summarized as mean ± SD or median [interquartile range], depending on distribution, whereas categorical or dichotomous variables are presented as count (%). To assess differences between tremor severity groups, Analyses of Variance were used for normally distributed variables, Kruskal-Wallis tests for non-normally distributed variables and Chi-squared tests for categorical variables. To assess associations of tremor severity with clinical, biochemical and pharmacotherapeutic parameters, multinomial logistic regression analyses, adjusted for sex, age, and log2 time after transplantation were performed. Sensitivity analyses with additional adjustment for tacrolimus trough concentrations were performed. Also, exploratory subgroup analyses were performed to compare non-CNI users with no, mild, or severe tremor.
Differences in PCS and MCS between grades of tremor severity were visualised with boxplots. Analyses of variance were performed for testing significance of differences between the different grades. Furthermore, bivariable linear regression analyses with PCS and MCS as dependent variable were performed to assess associations of mild and severe tremor with HRQoL. In multivariable linear regression analyses we assessed the association of mild and severe tremor with HRQoL, while adjusting for potential confounders including age, sex, type of transplantation, log2 time after transplantation, polypharmacy, diabetes, anaemia, eGFR, use of tacrolimus, use of cyclosporine, employment status, educational level, and the presence of a partner. Potential presence of effect modification by age and sex was assessed by adding interaction terms to the linear regression models.
Scatterplots and QQ-plots were visually evaluated to assess data distribution. Non-normally distributed variables were transformed using a binary logarithm (log2) when necessary to meet the assumptions for regression. All statistical analyses were performed with IBM SPSS software (version 23.0, SPSS Inc., Chicago, IL, USA) and R (version 3.5.1, Vienna, Austria). In all analyses, a two-sided p-value <0.05 was considered statistically significant.
Results
Baseline Characteristics
In total, 689 SOTR were included in the current study, including kidney (n = 395, 57.3%), liver (n = 168, 24.4%), lung (n = 95, 13.8%), heart (n = 29, 4.2%), and small intestine (n = 2, 0.3%) transplant recipients with a mean ± SD age of 58.0 ± 13.7 years, of whom 38.5% were female. A detailed overview of baseline characteristics in patients with either no, mild, or severe tremor, is presented in Table 2. In brief, patients with more tremor-related impairment were more often lung transplant recipients, less often liver transplant recipients, more frequently used tacrolimus and tended to have higher tacrolimus trough concentrations. Furthermore, tremor-related impairment was more frequently reported in patients with polypharmacy, patients with lower eGFR, and patients with higher fasting glucose concentrations. Notably, patients with mild or severe tremor tended to have higher cyclosporin trough concentrations, although differences between the groups were not statistically significant.
TABLE 2
| Variables | No tremor (n = 402) | Mild tremor (n = 206) | Severe tremor (n = 81) | p-value |
|---|---|---|---|---|
| Tremor rating scale-C score | 0 [0-0] | 2.0 [1.0–2.0] | 5.0 [4.0–7.0] | - |
| Recipient | ||||
| Female sex | 158 (39.3%) | 75 (36.4%) | 32 (39.5%) | 0.7 |
| Age at visit (years) | 54.4 ± 13.6 | 54.9 ± 14.2 | 58.1 ± 13.0 | 0.1 |
| Transplant type* | ||||
| Kidney (n = 395) | 238 (59.2%) | 112 (54.4%) | 45 (55.6%) | 0.5 |
| Liver (n = 168) | 111 (27.6%) | 44 (21.4%) | 13 (16.0%) | 0.042 |
| Lung (n = 95) | 40 (10.0%) | 40 (19.4%) | 15 (18.5%) | 0.002 |
| Heart (n = 29) | 13 (3.2%) | 9 (4.4%) | 7 (8.6%) | 0.1 |
| Small intestine (n = 2) | 0 (100.0%) | 1 (0.5%) | 1 (1.2%) | 0.1 |
| Polypharmacy (>4 drugs) | 340 (84.6%) | 183 (88.8%) | 77 (95.1%) | 0.025 |
| Diabetes | 101 (25.1%) | 54 (26.2%) | 21 (25.9%) | 1.0 |
| Anaemia | 81 (24.7%) | 52 (30.4%) | 25 (35.7%) | 0.1 |
| Body mass index (kg/m2) | 27.0 ± 4.8 | 26.6 ± 4.7 | 27.0 ± 5.3 | 0.5 |
| Kidney transplant characteristics | ||||
| Donor age (years) | 51.0 [39.0–59.0] | 52.0 [40.0–62.0] | 51.0 [34.8–61.0] | 0.2 |
| Living donor | 134 (33.3%) | 71 (34.5%) | 24 (29.6%) | 0.7 |
| Time after transplantation (years) | 3.0 [1.0–10.0] | 2.0 [0.0–9.0] | 2.0 [1.0–11.0] | 0.1 |
| Laboratory measurements | ||||
| eGFR creatinine (mL/min/1.73m2) | 59.6 ± 22.4 | 55.9 ± 20.3 | 53.8 ± 24.2 | 0.041 |
| Creatinine (µmol/L) | 112.0 [89.3–139.8] | 118.0 [98.0–140.0] | 116.0 [88.0–168.0] | 0.1 |
| Haemoglobin (mmol/L) | 8.3 ± 1.1 | 8.1 ± 1.2 | 8.1 ± 1.2 | 0.1 |
| HbA1c (mmol/mol) | 38.0 [34.0–43.0] | 39.0 [35.0–44.8] | 39.0 [35.0–44.3] | 0.2 |
| Glucose (mmol/L) | 5.6 [5.1–6.5] | 5.8 [5.2–6.8] | 5.8 [5.3–7.0] | 0.030 |
| Vitamin B12 (pmol/L) | 305.0 [245.0–418.5] | 307.5 [239.5–422.0] | 313.0 [223.0–467.5] | 0.9 |
| Folic acid (nmol/L) | 13.8 [9.9–18.1] | 12.8 [10.0–17.9] | 12.3 [9.6–20.7] | 0.4 |
| aTacrolimus (µg/L) | 5.7 ± 2.4 | 6.3 ± 2.5 | 6.3 ± 2.1 | 0.022 |
| bCyclosporine (µg/L) | 65.0 [49.0–98.0] | 104.0 [83.5–111.0] | 88.0 [36.5–120.3] | 0.1 |
| Medication | ||||
| Calcineurin inhibitor use | 341 (84.8%) | 183 (88.8%) | 70 (86.4%) | 0.4 |
| No use | 61 (15.2%) | 23 (11.2%) | 11 (13.6%) | 0.4 |
| Tacrolimus | 290 (72.1%) | 170 (82.5%) | 64 (79.0%) | 0.014 |
| Cyclosporine | 48 (11.9%) | 13 (6.3%) | 7 (8.6%) | 0.1 |
| mTOR inhibitor | 21 (5.2%) | 13 (6.3%) | 5 (6.2%) | 0.8 |
| Proliferation inhibitor | 309 (76.9%) | 165 (80.1%) | 67 (82.7%) | 0.4 |
| Prednisolone or prednisone | 322 (80.1%) | 172 (83.5%) | 72 (88.9%) | 0.1 |
| Beta blockers | 161 (40.0%) | 84 (41.0%) | 35 (43.2%) | 0.9 |
| Short or long acting bronchodilators | 20 (5.0%) | 10 (4.9%) | 6 (7.4%) | 0.6 |
Characteristics of 689 SOTR without tremor, with mild tremor, or with severe tremor, based on the TRS-C total score.
*Percentages were calculated by dividing the number of patients in each transplant type by the total number of all solid organ transplant patients with no/mild/severe tremor. Bold type indicates significance of results.
eGFR, estimated glomerular filtration rate as calculated using CKD-EPI formula. Normally distributed data are presented as mean ± standard deviation, skewed data as median [interquartile range], and categorical data as number (valid percentage). p-values represent significance of differences between tremor severity groups as assessed with Analyses of Variance, Kruskal-Wallis or Chi-squared tests, depending on distribution. Data were available in a514 and b62 patients.
Occurrence of Tremor and Impact on Activities of Daily Life
206 (29.9%) SOTR reported mild tremor and 81 (11.8%) reported severe tremor. Mild or severe tremor was reported in 39.7% among kidney transplant recipients, 33.9% among liver transplant recipients, 57.9% among lung transplant recipients, and 55.1% among heart transplant recipients. TRS-C scores of all SOTR and per organ type are shown in Figures 1A, B and 2 respectively. Additional bar charts of TRS-C total score per organ type are shown in Supplementary Figures S2A–D. Among patients that reported impairment because of tremor, median TRS-C score was 2.0 [1.0–4.0] [range 0–19]. Notably, patients reported that tremor-related impairment was most pronounced during writing and bringing food to the mouth. Furthermore, 18 (2.6%) patients reported changes in social activities because of tremor. Scores per TRS-C question are visualized by means of bar charts in Supplementary Figures S3A–H.
FIGURE 1
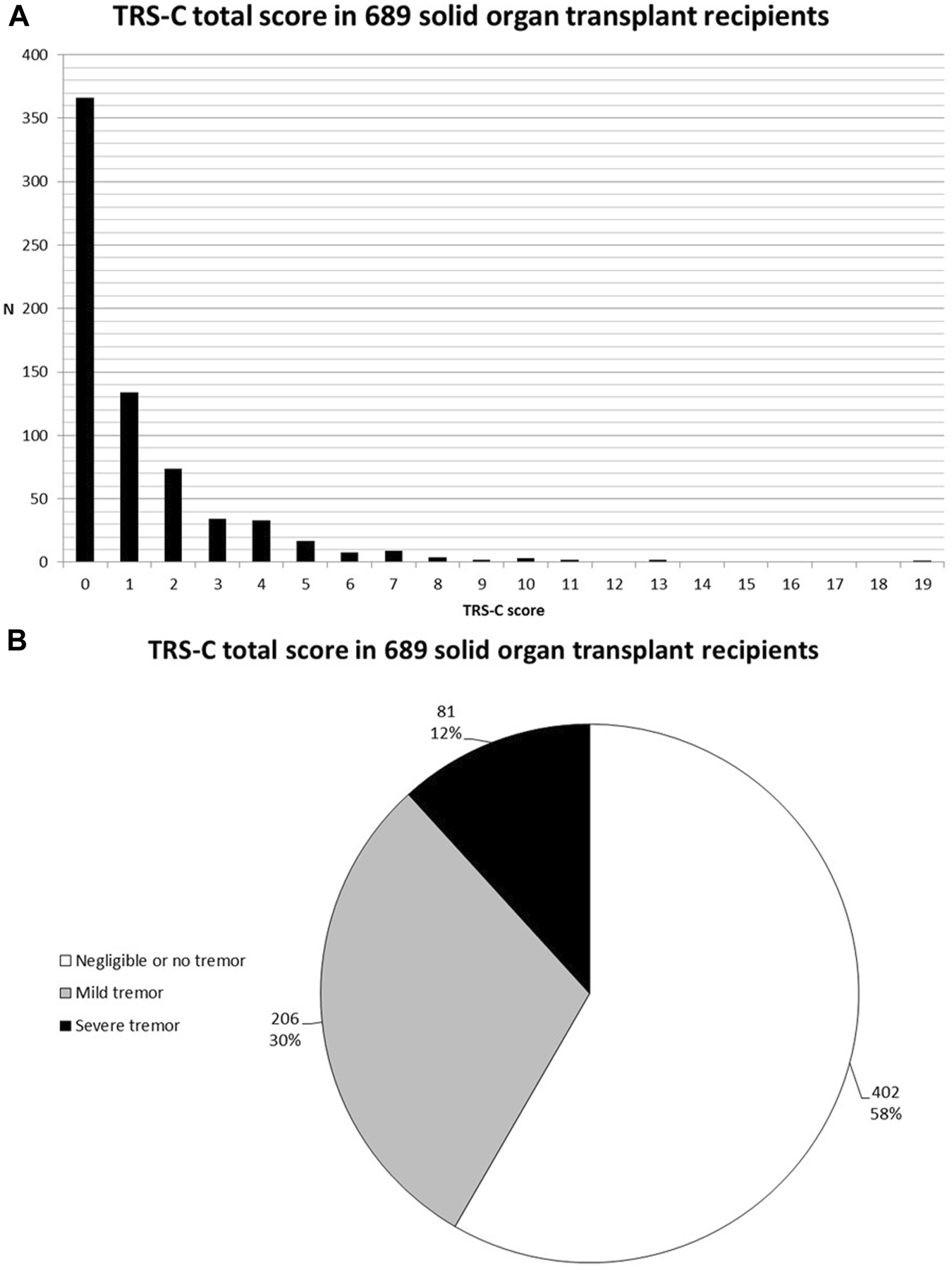
(A) Bar chart of TRS-C total score in 689 SOTR. (B) Pie-chart of tremor severity in 689 SOTR.
FIGURE 2
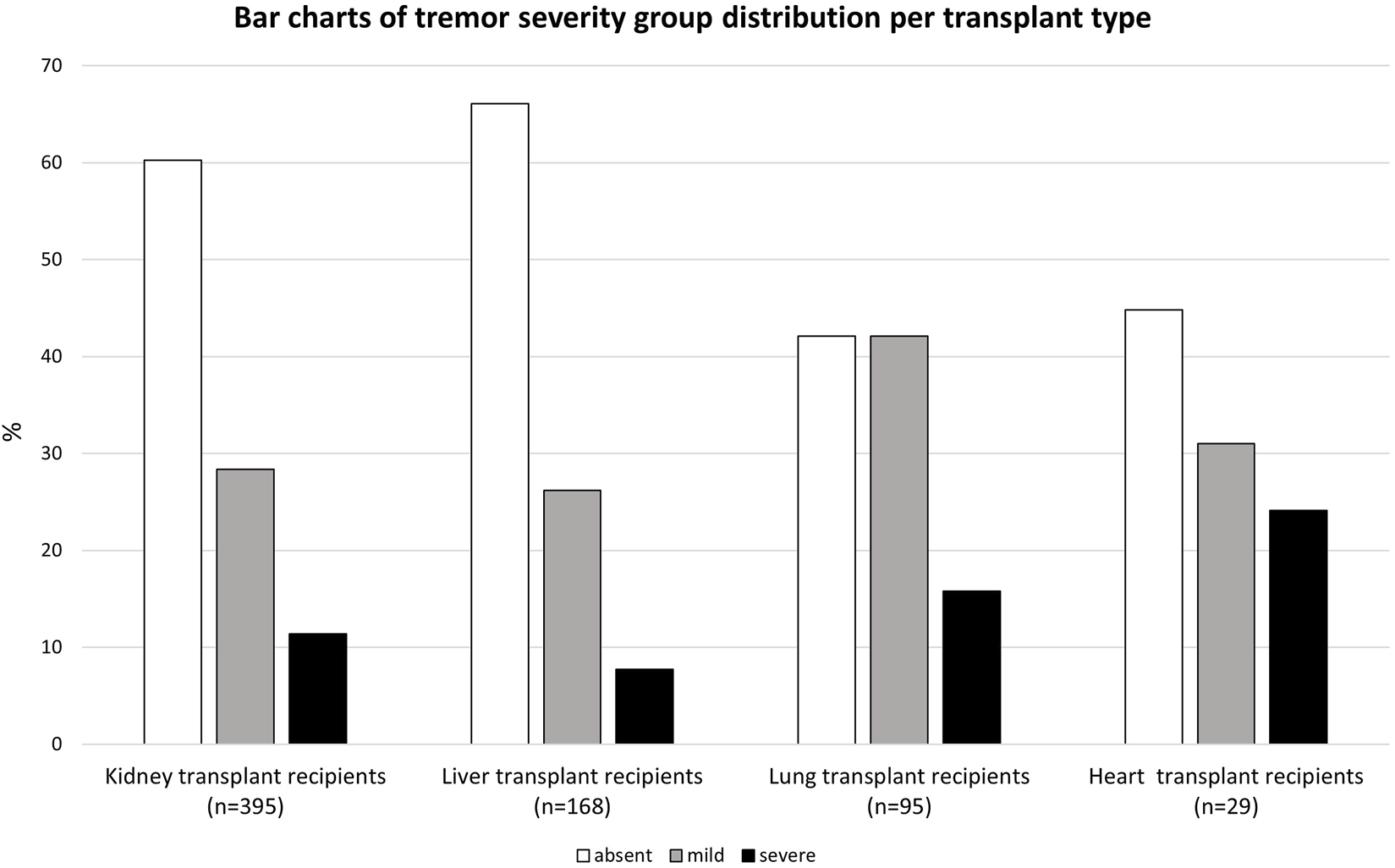
Bar charts of tremor severity group distribution per transplant type.
Determinants of Tremor-Related Impairment
Results of multinomial logistic regression analyses adjusted for age, sex, and log2 time after transplantation with presence of mild or severe tremor as dependent variable are shown in Table 3. Whole blood tacrolimus trough concentration was a determinant of mild tremor, independent of age, sex, and log2 time after transplantation (OR per μg/L increase: 1.11, 95% CI: 1.02 to 1.21, p = 0.019). Notably, higher whole blood tacrolimus trough concentration also tended to be associated with more severe tremor, although this observation was not statistically significant (OR per μg/L increase: 1.11, 95% CI: 0.98 to 1.26, p = 0.1). Furthermore, lung transplantation was associated with mild tremor; heart transplantation, polypharmacy and higher creatinine were associated with severe tremor. These associations remained similar after adjustment for tacrolimus trough concentrations (Supplementary Table S2). Notably, of the 95 patients who did not use calcineurin inhibitors, 34 (36%) reported mild or severe tremor. Exploratory subgroup analyses showed that non-CNI users with tremor more often had diabetes (Supplementary Table S3). Use of concomitant medication that may affect tremor, such as beta blockers and bronchodilators, was not associated with mild of severe tremor.
TABLE 3
| Baseline variables | No tremor (n = 402) | Mild tremor (n = 206) | Severe tremor (n = 81) | N | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |||
| Recipient | ||||||
| *Female sex | Ref. | 0.91 (0.64–1.29) | 0.6 | 1.05 (0.64–1.71) | 0.9 | 689 |
| *Age at visit (per 10 years) | Ref. | 1.03 (0.91–1.16) | 0.7 | 1.23 (1.02–1.48) | 0.029 | 689 |
| Transplant type | ||||||
| Kidney (n = 269) | Ref. | Ref. | Ref. | Ref. | Ref. | - |
| Liver (n = 154) | Ref. | 0.98 (0.63–1.53) | 0.9 | 0.64 (0.32–1.27) | 0.2 | 689 |
| Lung (n = 75) | Ref. | 2.21 (1.34–3.63) | 0.002 | 1.96 (1.00–3.87) | 0.1 | 689 |
| Heart (n = 29) | Ref. | 1.63 (0.67–3.96) | 0.3 | 2.74 (1.02–7.38) | 0.047 | 689 |
| Polypharmacy (>4 drugs) | Ref. | 1.26 (0.73–2.15) | 0.4 | 3.13 (1.08–9.08) | 0.036 | 689 |
| Diabetes | Ref. | 1.08 (0.73–1.59) | 0.7 | 0.94 (0.54–1.65) | 0.8 | 689 |
| Anaemia | Ref. | 1.30 (0.86–1.96) | 0.2 | 1.70 (0.98–2.97) | 0.1 | 569 |
| Body mass index (kg/m2) | Ref. | 0.98 (0.94–1.01) | 0.2 | 0.99 (0.94–1.04) | 0.8 | 686 |
| Kidney transplant characteristics | ||||||
| log2 donor age (years) | Ref. | 1.23 (0.90–1.68) | 0.2 | 0.87 (0.59–1.27) | 0.5 | 642 |
| Living donor | Ref. | 0.95 (0.66–1.38) | 0.8 | 0.85 (0.50–1.46) | 0.6 | 689 |
| *log2 time after transplantation (years) | Ref. | 0.90 (0.81–0.99) | 0.036 | 0.96 (0.83–1.11) | 0.6 | 689 |
| Laboratory measurements | ||||||
| eGFR creatinine (mL/min/1.73m2) | Ref. | 0.99 (0.99–1.00) | 0.1 | 0.99 (0.98–1.00) | 0.2 | 650 |
| log2 creatinine (µmol/L) | Ref. | 1.24 (0.88–1.76) | 0.2 | 1.71 (1.08–2.70) | 0.022 | 650 |
| Haemoglobin (mmol/L) | Ref. | 0.87 (0.74–1.02) | 0.1 | 0.82 (0.65–1.03) | 0.1 | 649 |
| log2 HbA1c (mmol/mol) | Ref. | 1.70 (0.97–2.97) | 0.1 | 1.39 (0.62–3.12) | 0.4 | 645 |
| log2 glucose (mmol/L) | Ref. | 1.54 (0.92–2.56) | 0.1 | 1.64 (0.82–3.29) | 0.2 | 624 |
| log2 vitamin B12 (pmol/L) | Ref. | 1.02 (0.78–1.32) | 0.9 | 1.12 (0.78–1.60) | 0.6 | 636 |
| log2 folic acid (nmol/L) | Ref. | 0.97 (0.75–1.25) | 0.8 | 1.08 (0.75–1.55) | 0.7 | 577 |
| Tacrolimus (µg/L) | Ref. | 1.11 (1.02–1.21) | 0.019 | 1.11 (0.98–1.26) | 0.1 | 514 |
| log2 cyclosporine (µg/L) | Ref. | 2.12 (0.94–4.81) | 0.1 | 1.32 (0.46–3.75) | 0.6 | 62 |
| Medication | ||||||
| Calcineurin inhibitor use | Ref. | 1.17 (0.66–2.06) | 0.6 | 1.11 (0.51–2.40) | 0.8 | 689 |
| No use | Ref. | Ref. | Ref. | Ref. | Ref. | - |
| Tacrolimus | Ref. | 1.49 (0.83–2.68) | 0.2 | 1.38 (0.62–3.08) | 0.4 | 689 |
| Cyclosporine | Ref. | 0.74 (0.34–1.62) | 0.5 | 0.82 (0.29–2.28) | 0.7 | 689 |
| mTOR inhibitor | Ref. | 1.17 (0.57–2.40) | 0.7 | 1.17 (0.42–3.23) | 0.8 | 689 |
| Proliferation inhibitor | Ref. | 1.10 (0.72–1.68) | 0.7 | 1.42 (0.75–2.68) | 0.3 | 689 |
| Prednisolone or prednisone | Ref. | 1.16 (0.74–1.81) | 0.5 | 1.92 (0.91–4.05) | 0.1 | 689 |
| Beta blockers | Ref. | 0.97 (0.68–1.38) | 0.9 | 1.04 (0.64–1.71) | 09 | 689 |
| Short or long acting bronchodilators | Ref. | 0.95 (0.43–2.08) | 0.9 | 1.39 (0.54–3.62) | 0.50 | 689 |
Multinomial logistic regression analyses of tremor severity with adjustment for age, sex, and log2 time after transplantation in 689 solid organ transplant recipients.
Bold type indicates significance of results. log2, the binary logarithm; eGFR, estimated glomerular filtration rate as calculated using CKD-EPI formula. Intestinal transplant recipients are excluded in analyses due to low number of participants (n = 2). *Presented numbers represent regression coefficients of the concerned variable in a model including age, sex, and log2 time after transplantation.
Health-Related Quality of Life
SOTR that reported mild or severe tremor had significantly lower physical and mental HRQoL, as visualized in Figure 3 (p < 0.001 for both). In bivariable linear regression analyses, both mild and severe tremor were associated with a lower physical HRQoL, with the strongest association for severe tremor (PCS: β = −5.64, 95% CI: −9.34 to 1.94, p = 0.003 and β = −15.87, 95% CI: −21.26 to −10.48, p < 0.001 resp.). A similar pattern was found for mental HRQoL (MCS: β = −3.50, 95% CI: −6.68 to −0.31, p = 0.032 and β = −11.22, 95% CI: −15.86 to −6.57, p < 0.001 resp.). These associations remained similar after adjustment for age, sex, type of transplantation, log2 time after transplantation, polypharmacy, diabetes, anaemia, eGFR, use of tacrolimus, and use of cyclosporine (Table 4). When we additionally adjusted for employment status, educational level, and the presence of a partner, results remained generally similar, although the association between mild tremor and physical HRQoL was no longer statistically significant. No interactions of age and sex were present for the association of tremor severity with HRQoL.
FIGURE 3
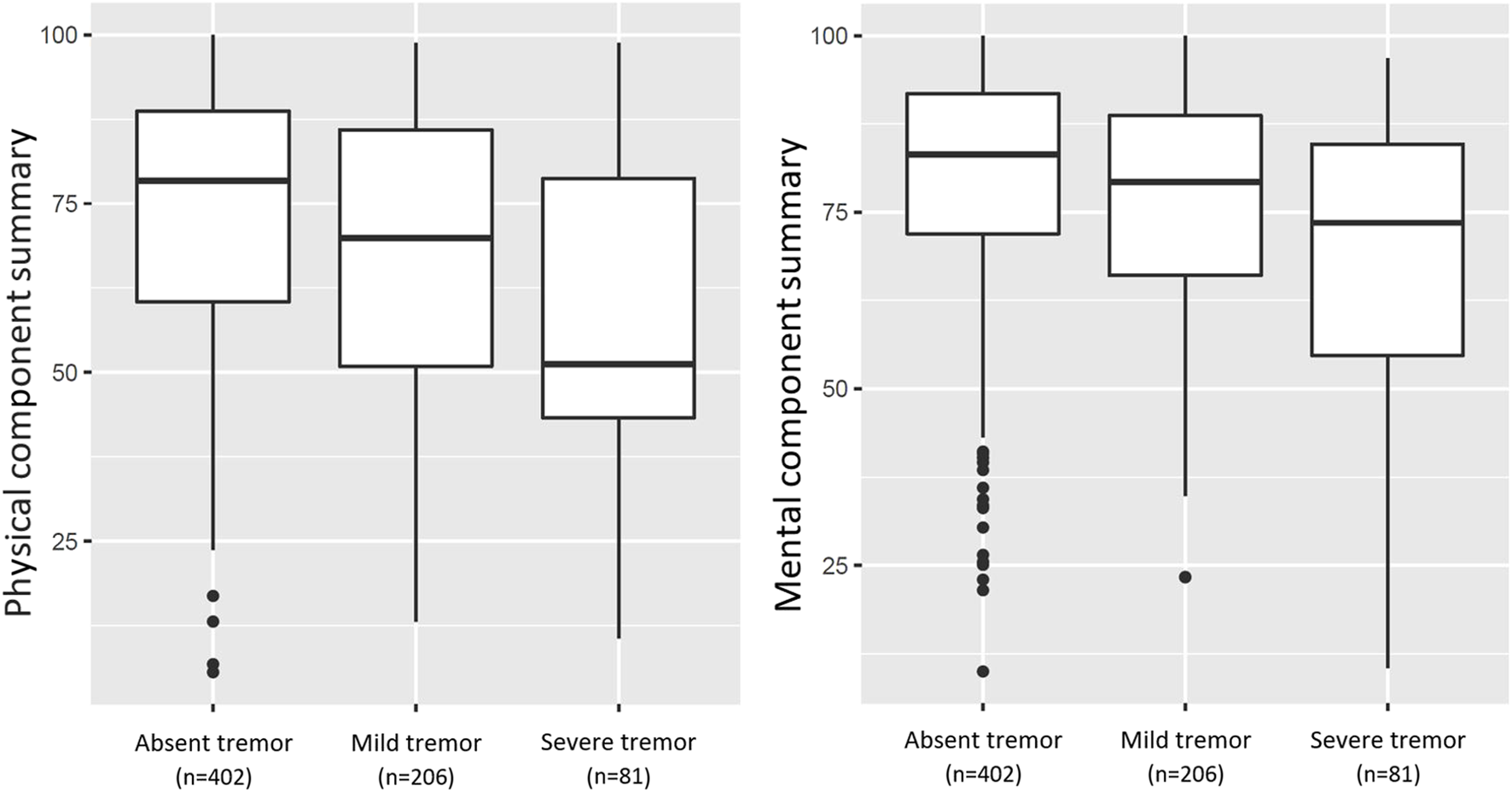
Boxplots of PCS, and MCS for HRQoL in 689 SOTR (mild and severe tremor present in 206 (29.9%) and 81 (11.8%) SOTR, respectively). The PCS, and MCS differed significantly between groups as calculated using Analyses of Variance (p < 0.001; p < 0.001, resp.).
TABLE 4
| Independent variable | Physical component summary | Mental component summary | ||
|---|---|---|---|---|
| β (95% CI) | p-value | β (95% CI) | p-value | |
| Crude | ||||
| No tremor | Ref. | n/a | Ref. | n/a |
| Mild tremor | −5.64 (−9.34 to −1.94) | 0.003 | −3.50 (−6.68 to −0.31) | 0.032 |
| Severe tremor | −15.87 (−21.26 to −10.48) | <0.001 | −11.22 (−15.86 to −6.57) | <0.001 |
| Model 1 | ||||
| No tremor | Ref. | n/a | Ref. | n/a |
| Mild tremor | −5.76 (−9.42 to −2.10) | 0.002 | −3.70 (−6.86 to −0.54) | 0.022 |
| Severe tremor | −15.14 (−20.50 to −9.79) | <0.001 | −11.54 (−16.16 to −6.93) | <0.001 |
| Model 2 | ||||
| No tremor | Ref. | n/a | Ref. | n/a |
| Mild tremor | −5.18 (−8.88 to −1.49) | 0.006 | −3.58 (−6.79 to −0.38) | 0.028 |
| Severe tremor | −14.36 (−19.76 to −8.97) | <0.001 | −11.41 (−16.09 to −6.73) | <0.001 |
| Model 3 | ||||
| No tremor | Ref. | n/a | Ref. | n/a |
| Mild tremor | −4.97 (−8.62 to −1.31) | 0.008 | −3.52 (−6.72 to −0.31) | 0.032 |
| Severe tremor | −13.83 (−19.17 to −8.48) | <0.001 | −11.24 (−15.93 to −6.56) | <0.001 |
| Model 4 | ||||
| No tremor | Ref. | n/a | Ref. | n/a |
| Mild tremor | −4.89 (−8.78 to −1.01) | 0.014 | −3.70 (−7.21 to −0.20) | 0.038 |
| Severe tremor | −15.08 (−20.61 to −9.56) | <0.001 | −11.89 (−16.87 to −6.90) | <0.001 |
| Model 5 | ||||
| No tremor | Ref. | n/a | Ref. | n/a |
| Mild tremor | −4.86 (−8.75 to −0.96) | 0.015 | −3.74 (−7.25 to −0.23) | 0.037 |
| Severe tremor | −15.05 (−20.59 to −9.52) | <0.001 | −11.92 (−16.91 to −6.92) | <0.001 |
| Model 6 | ||||
| No tremor | Ref. | n/a | Ref. | n/a |
| Mild tremor | −5.03 (−8.92 to −1.13) | 0.012 | −3.80 (−7.32 to −0.28) | 0.034 |
| Severe tremor | −15.35 (−20.88 to −9.81) | <0.001 | −12.06 (−17.06 to −7.06) | <0.001 |
| Model 7 | ||||
| No tremor | Ref. | n/a | Ref. | n/a |
| Mild tremor | −4.09 (−8.42 to 0.24) | 0.06 | −4.05 (−7.97 to −0.13) | 0.043 |
| Severe tremor | −16.10 (−22.23 to −9.98) | <0.001 | −12.68 (−18.23 to −7.14) | <0.001 |
Linear regression analyses of tremor severity with physical and mental HRQoL.
95% CI, 95% confidence interval; PCS, physical component summary; MCS, mental component summary; eGFR, estimated glomerular filtration rate; HRQoL, health-related quality of life. Model 1: adjusted for age and sex; model 2: model 1 + type of transplantation and log2 time after transplantation; model 3: model 2 + polypharmacy; model 4: model 3 + diabetes and anaemia; model 5: model 4 + eGFR; model 6: model 5 + use of tacrolimus and use of cyclosporine; model 7: model 6 + employment status, education level, and presence of a partner.
Discussion
This study shows that mild or severe tremor frequently impairs daily life activities of SOTR. Tacrolimus trough concentration was a main determinant of tremor. Importantly, mild and severe tremor-related impairments were strongly associated with lower HRQoL, independent of other known determinants of tremor.
Our results confirm that SOTR frequently experience tremor-related impairment of ADL. A previous study reported similar tremor prevalence compared to our study (18). However, the current study provides additional insights, because we assessed prevalence based on impact of tremor on ADL. The pathophysiology of CNI-induced tremor remains unknown. However, the transplant population tends to show lower TRS-C scores compared to patients with essential tremor (27). Our results show that tacrolimus trough concentrations are associated with tremor-related impairment among SOTR. This is further corroborated by the finding that tremor-related impairment is higher among SOTR with higher target trough concentrations (e.g., lung and heart transplant recipients). Also, patients with severe tremor had higher creatinine blood concentrations, which is consistent with the notion that tacrolimus is both nephrotoxic and neurotoxic (18, 19). However, alternatively, renal impairment may also predispose patients to tremor, so tacrolimus use does not necessarily relate to the occurrence of both nephro- and neurotoxicity in the same patient and claims regarding causality cannot be drawn from the current study. Nevertheless, our findings support the previously suggested notion that the occurrence of CNI side effects is dose-dependent (18, 19). Generally, factors including vitamin B12, HbA1c and glucose are regarded as key determinants of tremor in different populations (28), and concomitant medication such as bronchodilators and betablockers are known to affect tremor. Our results highlight that these factors appear to be less important among SOTR, and that CNI use appears to be the main cause of tremor in this population.
The notion that tacrolimus trough concentrations are independently associated with tremor-related impairment among SOTR is important for treating physicians to consider during treatment regimens. Furthermore, this notion highlights the need for alternative immunosuppressive treatment regimens that are less likely to cause tremor, while maintaining low risks of rejection. Since dose-dependency seems to be key in the pathophysiology of medication-induced tremor, extended-release preparations may be able to reduce tremor prevalence by reducing peak-to-trough variability (19). Langone et al. have reported promising results in patients using extended-release tacrolimus, showing a significantly lower peak-to-trough variability, lower tremor prevalence, and a higher quality of life (19). However, this study included a small number of only kidney transplant recipients. Therefore, future interventional studies are required for strengthening these results.
The potential impact of tremor on the lives of SOTR is further highlighted by the associations of severe tremor with both lower mental and physical HRQoL. These results are generally in line with findings in previous smaller studies. For example, Langone et al. reported an improved quality of life after a reduction in tremor severity, in a small cohort of 38 kidney transplant recipients (19).
A major strength of this study is the large number of included SOTR with available data regarding subjective tremor and HRQoL. Additionally, we included kidney, liver, lung, heart, and small intestine transplant recipients, allowing for evaluation of tremor prevalence among these patient groups. In addition, the cohort was well-characterized, allowing us to adjust for many potential confounders, including extensive clinical data, laboratory measurements, and treatment regimens. A limitation of the current study is that the participants mainly used tacrolimus-based treatment regimens, and associations of cyclosporine and other immunosuppressive drugs with tremor could therefore be incompletely studied. In addition, since patients were not evaluated before transplantation, the presence and exacerbation of preconditions which may cause tremor could not be assessed. Non-CNI users with tremor more frequently had diabetes compared to those without diabetes, which may partly explain the high prevalence of tremor in this subgroup. Nevertheless, this observation cannot fully explain the tremor occurrence in this subgroup, and future studies are needed to gain insights into the tremor susceptibility of some SOTR without CNI-use. Lastly, due to the observational design of the study, we cannot draw conclusions regarding causality of our findings. Moreover, the current cross-sectional study cannot identify trajectories of tremor before and after transplantation, and therefore longitudinal studies assessing the determinants of tremor are warranted. Such studies may also help to account for potential tacrolimus dosage adaptations that clinicians may conduct in patients with severe tremor.
SOTR frequently report tremor-related impairment of ADL. Tacrolimus trough concentrations appeared a main determinant of tremor among SOTR. The strong and independent association of tremor-related impairment with lower HRQoL warrants further studies into the effects of tacrolimus on tremor.
Members of Transplantlines Investigators
Coby Annema, Stephan J. L. Bakker, Stefan P. Berger, Hans Blokzijl, Frank A. J. A. Bodewes, Marieke T. de Boer, Kevin Damman, Martin H. de Borst, Arjan Diepstra, Gerard Dijkstra, Caecilia S. E. Doorenbos, Rianne M. Douwes, Michele F. Eisenga, Michele E. Erasmus, C. Tji Gan, Antonio W. Gomes Neto, Eelko Hak, Bouke G. Hepkema, Frank Klont, Tim J. Knobbe, Daan Kremer, Henri G. D. Leuvenink, Willem S. Lexmond, Vincent E. de Meijer, Hubert G. M. Niesters, Gertrude J. Nieuwenhuijs - Moeke, L. Joost van Pelt, Robert A. Pol, Adelita V. Ranchor, Jan Stephan F. Sanders, Marion J. Siebelink, Riemer J. H. J. A. Slart, J. Casper Swarte, Daan J. Touw, Marius C. van den Heuvel, Coretta van Leer-Buter, Marco van Londen, Erik A. M. Verschuuren, Michel J. Vos, Rinse K. Weersma.
Statements
Data availability statement
The datasets presented in this article are not readily available because public participant data sharing is not included in the TransplantLines informed consent forms. Data requests are only legally allowed after approval by the TransplantLines Scientific Committee upon reasonable request, in accordance with the medical ethical committee allowance and the UMCG Biobank Regulations. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by METc University Medical Centre Groningen 2014/077. The patients/participants provided their written informed consent to participate in this study.
Author contributions
NR and DK wrote the draft manuscript. NR and DK performed data analyses. DK, AG-N, and ME performed the research and contributed to data collection. TK contributed to data collection, interpretation, and manuscript revisions. CG, SN, HB, VM, KD, GD, JE, SB, and DT were responsible for the research design and manuscript revisions. SB and AS were responsible for the research design, contributed to data interpretation and manuscript revisions. All authors read and approved the final manuscript.
Funding
The authors declare that the TransplantLines Biobank and Cohort study received funding from Astellas BV (TransplantLines Biobank and Cohort study), Chiesi Pharmaceuticals BV (PA-SP/PRJ-2020-9136), and co-financed by the Dutch Ministry of Economic Affairs and Climate Policy by means of the PPP-allowance made available by the Top Sector Life Sciences & Health to stimulate public-private partnerships. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication. Some authors are funded by unrestricted grants: VM was supported by a VENI research grant by the Dutch Research Council (NWO; grant #09150161810030), a research grant from the Dutch Ministry of Economic Affairs (Health∼Holland Public Private Partnership grant #PPP-2019-024), and a research grant from the Dutch Society for Gastroenterology (NVGE #01-2021); AS was supported by a Mandema Stipend received from the Junior Scientific Masterclass Groningen (MS-2018). ME was supported by a Mandema Stipend received from the Junior Scientific Masterclass Groningen (MS-2021).
Conflict of interest
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2023.10951/full#supplementary-material
Abbreviations
ADL, Activities of daily living; CNI, Calcineurin inhibitor; FTM TRS-C, Fahn-Tolosa-Marin tremor rating scale C; HRQoL, Health-related quality of life; MCS, Mental component summary; PCS, Physical component summary; SOTR, Solid organ transplant recipients.
References
1.
Yazigi NA . Long Term Outcomes after Pediatric Liver Transplantation. Pediatr Gastroenterol Hepatol Nutr (2013) 16(4):207–18. 10.5223/pghn.2013.16.4.207
2.
Yang LS Shan LL Saxena A Morris DL . Liver Transplantation: A Systematic Review of Long-Termquality of Life. Liver Int (2014) 34(9):1298–313. 10.1111/liv.12553
3.
Yusen RD Edwards LB Kucheryavaya AY Benden C Dipchand AI Dobbels F et al The Registry of the International Society for Heart and Lung Transplantation: Thirty-First Adult Lung and Heart-Lung Transplant Report - 2014; Focus Theme: Retransplantation. J Heart Lung Transplant (2014) 33(10):1009–24. 10.1016/j.healun.2014.08.004
4.
Taylor DO Edwards LB Boucek MM Trulock EP Aurora P Christie J et al Registry of the International Society for Heart and Lung Transplantation: Twenty-Fourth Official Adult Heart Transplant Report-2007. J Heart Lung Transplant (2007) 26(8):769–81. 10.1016/j.healun.2007.06.004
5.
Parmar A Vandriel SM Ng VL . Health-related Quality of Life after Pediatric Liver Transplantation: A Systematic Review. Liver Transplant (2017) 23(3):361–74. 10.1002/lt.24696
6.
Seiler A Klaghofer R Ture M Komossa K Martin-Soelch C Jenewein J . A Systematic Review of Health-Related Quality of Life and Psychological Outcomes after Lung Transplantation. J Heart Lung Transplant (2016) 35(2):195–202. 10.1016/j.healun.2015.07.003
7.
Hariharan S Israni AK Danovitch G . Long-Term Survival after Kidney Transplantation. New Engl J Med (2021) 385(8):729–43. 10.1056/nejmra2014530
8.
Kugler C Gottlieb J Warnecke G Schwarz A Weissenborn K Barg-Hock H et al Health-related Quality of Life after Solid Organ Transplantation: A Prospective, Multiorgan Cohort Study. Transplantation (2013) 96(3):316–23. 10.1097/TP.0b013e31829853eb
9.
van Sandwijk MS Al Arashi D van de Hare FM van der Torren JMR Kersten MJ Bijlsma JA et al Fatigue, Anxiety, Depression and Quality of Life in Kidney Transplant Recipients, Haemodialysis Patients, Patients with a Haematological Malignancy and Healthy Controls. Nephrol Dial Transpl (2019) 34(5):833–8. 10.1093/ndt/gfy103
10.
Tackmann E Dettmer S . Health-related Quality of Life in Adult Heart-Transplant Recipients—A Systematic Review. Herz (2020) 45(5):475–82. 10.1007/s00059-018-4745-8
11.
Kugler C Fischer S Gottlieb J Tegtbur U Welte T Goerler H et al Symptom Experience after Lung Transplantation: Impact on Quality of Life and Adherence. Clin Transpl (2007) 21(5):590–6. 10.1111/j.1399-0012.2007.00693.x
12.
Neuwirt H Rudnicki M Schratzberger P Pirklbauer M Kronbichler A Mayer G . Immunosuppression after Renal Transplantation. Memo - Mag Eur Med Oncol (2019) 12(3):216–21. 10.1007/s12254-019-0507-4
13.
Krensky AM Azzi JR Hafler DA . Immunosuppressants and Tolerogens. In: BruntonLLHilal-DandanRKnollmannBC, editors. Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 13e. McGraw-Hill Education (2017).
14.
Naesens M Kuypers DRJ Sarwal M . Calcineurin Inhibitor Nephrotoxicity. Clin J Am Soc Nephrol (2009) 4(2):481–508. 10.2215/CJN.04800908
15.
Bhatia KP Bain P Bajaj N Elble RJ Hallett M Louis ED et al Consensus Statement on the Classification of Tremors. From the Task Force on Tremor of the International Parkinson and Movement Disorder Society. Move Disord (2018) 33(1):75–87. 10.1002/mds.27121
16.
Thangavelu K Talk AC Clark GI Dissanayaka NNW . Psychosocial Factors and Perceived Tremor Disability in Essential Tremor. Neurosci Biobehav Rev (2020) 108(2019):246–53. 10.1016/j.neubiorev.2019.10.021
17.
Morgan JC Kurek JA Davis JL Sethi KD . Insights into Pathophysiology from Medication-Induced Tremor. Tremor and Other Hyperkinetic Movements (2017) 7:442–10. 10.7916/D8FJ2V9Q
18.
Erro R Bacchin R Magrinelli F Tomei P Geroin C Squintani G et al Tremor Induced by Calcineurin Inhibitor Immunosuppression: a Single-centre Observational Study in Kidney Transplanted Patients. J Neurol (2018) 265(7):1676–83. 10.1007/s00415-018-8904-x
19.
Langone A Steinberg SM Gedaly R Chan LK Shah T Sethi KD et al Switching STudy of Kidney TRansplant PAtients with Tremor to LCP-TacrO (STRATO): An Open-Label, Multicenter, Prospective Phase 3b Study. Clin Transpl (2015) 29(9):796–805. 10.1111/ctr.12581
20.
Eisenga MF Gomes-Neto AW Van Londen M Ziengs AL Douwes RM Stam SP et al Rationale and Design of TransplantLines: A Prospective Cohort Study and Biobank of Solid Organ Transplant Recipients. BMJ Open (2018) 8(12):e024502–13. 10.1136/bmjopen-2018-024502
21.
Koster RA Dijkers ECF Uges DRA . Robust, High-Throughput LC-MS/MS Method for Therapeutic Drug Monitoring of Cyclosporine, Tacrolimus, Everolimus, and Sirolimus in Whole Blood. Ther Drug Monit (2009) 31(1):116–25. 10.1097/FTD.0b013e318192304c
22.
Fahn S Tolosa E Marin C . Clinical Rating Scale for Tremor. In: Parkinson’s Disease and Movement Disorders (1993). p. 225–34.
23.
Elble R Bain P João Forjaz M Haubenberger D Testa C Goetz CG et al Task Force Report: Scales for Screening and Evaluating Tremor: Critique and Recommendations. Move Disord (2013) 28(13):1793–800. 10.1002/mds.25648
24.
Hays RD Morales LS . The RAND-36 Measure of Health-Related Quality of Life. Ann Med (2001) 33(5):350–7. 10.3109/07853890109002089
25.
Levey AS Stevens LA Schmid CH Zhang YL Castro AF 3rd Feldman HI et al A New Equation to Estimate Glomerular Filtration Rate. Ann Intern Med (2009) 150(9):604–12. 10.7326/0003-4819-150-9-200905050-00006
26.
Goddard WP Murray I Long RG Scott B Barton R Salman M et al Iron Deficiency Anaemia. Bmj (1997) 314(7096):1759. 10.1136/bmj.314.7096.1759
27.
van der Stouwe AMM Broersma M Buijink AWG van Rootselaar AF Maurits NM . Limited Correlations between Clinician-Based and Patient-Based Measures of Essential Tremor Severity. Parkinsonism Relat Disord (2015) 21(6):654–7. 10.1016/j.parkreldis.2015.03.004
28.
Crawford P Zimmerman EE . Tremor: Sorting through the Differential Diagnosis. Am Fam Physician (2018) 97(3):180–6.
Summary
Keywords
tremor, solid organ transplant recipient, calcineurin inhibitors, health-related quality of life, ADL impairment
Citation
Riemersma NL, Kremer D, Knobbe TJ, Gan CT, Nolte S, Gomes-Neto AW, Blokzijl H, de Meijer VE, Damman K, Eisenga MF, Drost G, Elting JWJ, Touw DJ, Berger SP, Bakker SJL, van der Stouwe AMM and Transplantlines Investigators (2023) Tremor, Daily Functioning, and Health-Related Quality of Life in Solid Organ Transplant Recipients. Transpl Int 36:10951. doi: 10.3389/ti.2023.10951
Received
04 October 2022
Accepted
23 February 2023
Published
16 March 2023
Volume
36 - 2023
Updates
Copyright
© 2023 Riemersma, Kremer, Knobbe, Gan, Nolte, Gomes-Neto, Blokzijl, de Meijer, Damman, Eisenga, Drost, Elting, Touw, Berger, Bakker, van der Stouwe and Transplantlines Investigators.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Niels L. Riemersma, N.L.Riemersma@umcg.nl
Members of Transplantlines Investigators are listed at the end of article
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.