Dear Editors,
Pre-exposition prophylaxis (PrEP) with monoclonal antibodies (mab) against severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) is used to prevent coronavirus disease 2019 (COVID-19) in high-risk individuals with insufficient response to vaccination (1, 2). Currently, cilgavimab/tixagevimab (Evusheld, AstraZeneca) remains the only mab combination approved for PrEP. A significant reduction of in vitro neutralization capacity against the Omicron BA.1 variant (B1.1.529) was observed for most mabs including cilgavimab/tixagevimab. A high rate of break-through infections including severe disease following cilgavimab/tixagevimab was observed for BA.1 (3). Sotrovimab (Xevudy, VIR Biotechnology GlaxoSmithKline) retained substantial in vitro neutralization capacity against BA.1, and a half-life of 48.8 days made it a candidate for an off-label use as PrEP in high-risk individuals. However, sotrovimab has shown a significantly reduced in vitro neutralization capacity against the Omicron BA.2 sub-lineage while cilgavimab/tixagevimab retained strong activity (4).
We used sotrovimab in an off-label indication as PrEP in kidney transplant recipients (KTR) without neutralizing antibodies after at least three COVID-19 vaccine doses at our institution (Medical University of Vienna, Austria) beginning in January 2022 (following the emergence of BA.1), and PrEP was changed to cilgavimab/tixagevimab in March 2022 (following the emergence of BA.2). PrEP was provided to KTR with antibody levels <264 BAU/mL following three vaccinations and no previous history of COVID-19.
In the present analysis, we longitudinally assessed the in vivo neutralization capacity of sotrovimab (n = 20) against BA.1 as well as BA.2 (proof of principle for sotrovimab as PrEP), and cilgavimab/tixagevimab (n = 30) against BA.2 (following the emergence of BA.2) for up to 8 weeks after PrEP (baseline characteristics are provided in Supplementary Table S1). Patients either received 1) 500 mg of sotrovimab intravenously (between January 12 and January 19, 2022) or 2) 300 mg of cilgavimab/tixagevimab by intramuscular injection (between March 4 and March 9, 2022). All patients were followed at the outpatient department of the Division of Nephrology and Dialysis at the Medical University in Vienna (IRB# 1362/2020; 1612/2021). Serum samples were collected at 4 and 8 weeks after antibody administration in all patients as well as 1 hour (only sotrovimab) and 2 weeks (both sotrovimab and cilgavimab/tixagevimab) in a subgroup of patients. Variant-specific live virus neutralization tests (NT) were performed with BA.1 and BA.2 variants. Detailed methods are provided in the supplementary material (5, 6). NT titers of serum samples ≥10 were considered positive. Neutralization titers are reported as median and Q1 and Q3.
All individuals receiving sotrovimab retained neutralization capacity against the BA.1 variant for 4 weeks follow up (FU), and all but one individual still exhibited neutralization capacity against BA.1 at 8 weeks FU. Median NT titers decreased from 30 (Q1, Q3: 30, 40) at 4 weeks to 20 (Q1, Q3: 15, 30) at 8 weeks FU (Figure 1A). In contrast, neutralizing capacity against the BA.2 variant in serum was only present in 60% at 4 weeks and further decreased to 15% at 8 weeks FU. In line, median NT titers against the BA.2 variant were also significantly lower (10 [Q1, Q3: <10, 10] and <10 [Q1, Q3: <10, <10] at 4 weeks and 8 weeks of FU, respectively; Figure 1A). However, analysis in the subgroup with measurements at 1 h and 2 weeks after sotrovimab infusion showed that all individuals had initially achieved neutralization capacity against BA.2 (40 [Q1, Q3: 30, 45] and 20 [Q1, Q3: 19, 30] at 1 h and 2 weeks, respectively).
FIGURE 1
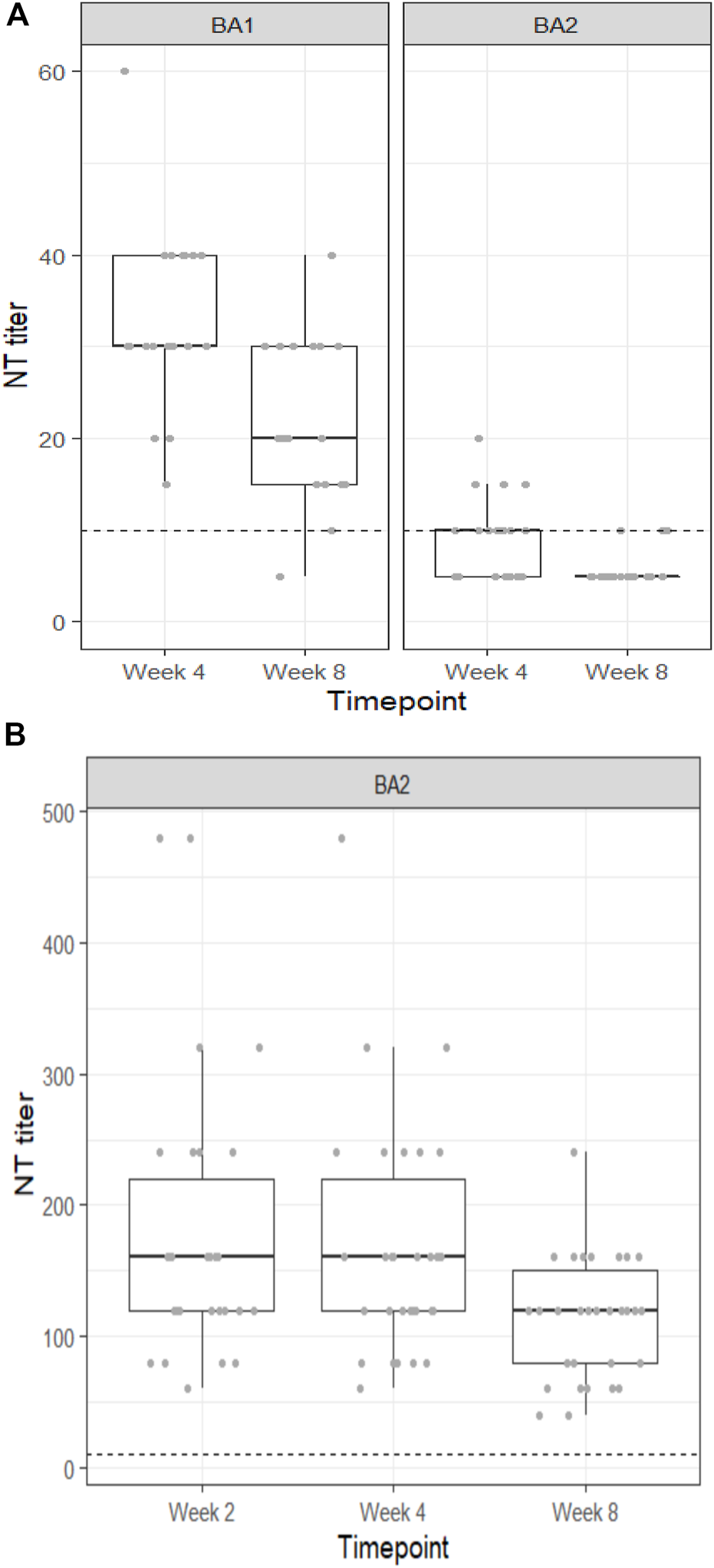
(A) Neutralization titers of patient sera against the SARS-CoV-2 Omicron BA.1 and BA.2 variants at four and 8 weeks after prophylactic infusion of sotrovimab. (B) Neutralization titers of patient sera against the SARS-CoV-2 Omicron BA.2 variant at 2, 4, and 8 weeks after prophylactic infusion of cilgavimab/tixagevimab.
Patients receiving cilgavimab/tixagevimab had significantly higher neutralization titers against BA.2 than those receiving sotrovimab at all time points: 160 [Q1, Q3: 120, 220], 160 [Q1, Q3: 120, 220] and 120 [Q1, Q3: 80, 150] at 2, 4, and 8 weeks, respectively (Figure 1B).
We could show that sotrovimab retains neutralization capacity against BA.1 for at least 8 weeks while only having a limited neutralization activity against the BA.2 variant. Cilgavimab/tixagevimab on the contrary shows strong in vivo neutralization of BA.2 for at least 8 weeks. Our data support that PrEP has to be adapted based on immune-evasion characteristics of pre-dominant SARS-CoV-2 variants.
Statements
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics committee of the Medical University of Vienna. The patients/participants provided their written informed consent to participate in this study.
Author contributions
KS, LW, AH, RO, and RR-S conceptualized the study. KS, AH, and JC performed the analyses. AH and RR-S wrote the draft of the manuscript. All authors approved the mansucript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2022.10906/full#supplementary-material
References
1.
Reindl-Schwaighofer R Heinzel A Jabbour R Mayrdorfer M . Comparison of SARS-CoV-2 Antibody Response 4 Weeks after Homologous vs Heterologous Third Vaccine Dose in Kidney Transplant Recipients: A Randomized Clinical Trial. JAMA Intern Med (2021) 182:165–71. 10.1001/jamainternmed.2021.7372
2.
Ducloux D Courivaud C . REGEN-cov Antibody Combination to Prevent COVID-19 Infection in Kidney Transplant Recipient without Detectable Antibody Response to Optimal Vaccine Scheme. Kidney Int (2022) 101(3):645–6. 10.1016/j.kint.2021.12.015
3.
Benotmane I Velay A Gautier-Vargas G Olagne J Obrecht A Cognard N et al Breakthrough COVID-19 Cases Despite Prophylaxis with 150 Mg of Tixagevimab and 150 Mg of Cilgavimab in Kidney Transplant Recipients. Am J Transpl (2022) 22:2675–81. 10.1111/ajt.17121
4.
Iketani S Liu L Guo Y Chan JFW Huang Y . Antibody Evasion Properties of SARS-CoV-2 Omicron Sublineages. Nature (2022) 604:553–6. 10.1038/s41586-022-04594-4
5.
Sieber J Mayer M Schmidthaler K Kopanja S Camp JV Popovitsch A et al Long-Lived Immunity in SARS-CoV-2-Recovered Children and its Neutralizing Capacity against Omicron. Front Immunol (2022) 13:882456. 10.3389/fimmu.2022.882456
6.
Medits I Springer DN Graninger M Camp JV Holtl E Aberle SW et al Different Neutralization Profiles after Primary SARS-CoV-2 Omicron BA.1 and BA.2 Infections. Front Immunol (2022) 13:946318. 10.3389/fimmu.2022.946318
Summary
Keywords
COVID-19, kidney transplantation, antiviral, prophylaxis, SARS-CoV2
Citation
Stiasny K, Weseslindtner L, Heinzel A, Camp JV, Oberbauer R and Reindl-Schwaighofer R (2022) SARS-CoV-2 Omicron BA.1/BA.2 Neutralization up to 8 Weeks After PrEP With Sotrovimab or Cilgavimab/Tixagevimab. Transpl Int 35:10906. doi: 10.3389/ti.2022.10906
Received
15 September 2022
Accepted
29 November 2022
Published
12 December 2022
Volume
35 - 2022
Updates
Copyright
© 2022 Stiasny, Weseslindtner, Heinzel, Camp, Oberbauer and Reindl-Schwaighofer.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andreas Heinzel, andreas.heinzel@meduniwien.ac.at
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.