Abstract
Regenerative medicine has emerged as a novel alternative solution to organ failure which circumvents the issue of organ shortage. In preclinical research settings bio-artificial organs are being developed. It is anticipated that eventually it will be possible to launch first-in-human transplantation trials to test safety and efficacy in human recipients. In early-phase transplantation trials, however, research participants could be exposed to serious risks, such as toxicity, infections and tumorigenesis. So far, there is no ethical guidance for the safe and responsible design and conduct of early-phase clinical trials of bio-artificial organs. Therefore, research ethics review committees will need to look to related adjacent fields of research, including for example cell-based therapy, for guidance. In this systematic review, we examined the literature on early-phase clinical trials in these adjacent fields and undertook a thematic analysis of relevant ethical points to consider for early-phase clinical trials of transplantable bio-artificial organs. Six themes were identified: cell source, risk-benefit assessment, patient selection, trial design, informed consent, and oversight and accountability. Further empirical research is needed to provide insight in patient perspectives, as this may serve as valuable input in determining the conditions for ethically responsible and acceptable early clinical development of bio-artificial organs.
Introduction
For patients with end-stage organ failure, having an organ transplant is often the best and only cure. Advances in surgical techniques and immunosuppressive medication means that organ transplantation is now widely and successfully used. However, there are still important challenges to overcome, notably the shortage of donor organs and the short and long-term side effects of taking lifelong immunosuppressive medication.
In the last decade, the multi-disciplinary field of regenerative medicine has emerged. Regenerative medicine uses technologies such as tissue engineering and 3D bioprinting to (re)generate, repair or replace damaged tissues and organs. Regenerative medicine and tissue engineering are terms often used interchangeably in the scientific literature. In this article however we use the term regenerative medicine to refer to the aim of the intervention (to regenerate), and tissue engineering to refer to the method for creating regenerative products. Regenerative medicine could, by way of illustration, combine patient-derived cells (e.g., in the form of organoids made from induced pluripotent stem cells) with cutting-edge technologies such as tissue engineering, to develop transplantable personalized bio-artificial organs. For example, the European Commission-funded VANGUARD project aims to engineer a vascularized and immune-protected bio-artificial pancreas for transplantation into patients with Type I Diabetes. The ambition of the VANGUARD project1 is for the transplanted bio-artificial pancreas to produce insulin and treat the underlying diabetic disease without requiring the patient to take lifelong immunosuppressive medication. Similarly, in other disease areas, first steps are being taken towards the generation of transplantable bio-artificial organs, including livers (1), bladders (2), kidneys (3), hearts (4), small intestines (5) and lungs (6, 7). These bio-artificial organs are currently still at the preclinical stage and are being tested in laboratory settings or animal studies.
It is likely that researchers will reach a point at which sufficient preclinical evidence has been collected to suggest that bio-artificial organs might be beneficial and safe for humans. At that point, early-phase clinical trials will be initiated to test the safety and efficacy of these products in humans. In early-phase clinical trials, human research participants could be exposed to serious risks, such as toxicity, infections and tumorigenesis. This is especially so in regenerative medicine trials requiring invasive and non-reversible procedures, resulting in permanent alterations of participants’ bodies (8).
It is not clear to what extent existing ethics oversight and guidance for the conduct of clinical trials is applicable to or sufficient for the clinical translation of bio-artificial organs. First, drug authorities, including the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA), were originally set up to decide on marketing authorisation of pharmaceutical agents, not complex cell-based products. In Europe, bio-artificial organs are likely to be classified as Advanced Therapy Medicinal Products (ATMPs) (9), just like cell-based therapies. However, this classification may not completely cover the bio-artificial organ as, unlike most pharmaceutical agents, it is not a substance that can be injected or infused, but a complex product—more like a (cell-based) device—to be used in transplantation, which involves a (innovative) surgical intervention. Second, while there are internationally recognised guidelines for the ethical conduct of research involving human subjects, issued for instance by the Council for international Organization of Medical Science (CIOMS) (10) and the World Medical Association (WMA) (11), these guidelines should be expanded in order to make them applicable to the clinical translation of bio-artificial organs. The ethics guidelines of the International Society for Stem Cell Research (ISSCR) have been developed specifically for human stem cell research and clinical translation of cell-based interventions (12), but do not discuss applications of regenerative medicine in organ transplantation. Without the relevant guidance, it would be difficult for research ethics review committees (RECs) to evaluate the ethical acceptability of early-phase clinical trials of bio-artificial organs. Therefore, guidance on the safe and responsible design and conduct of early-phase clinical trials of transplantable bio-artificial organs should be developed.
In this systematic review we examined the published literature on early-phase clinical trials in the adjacent fields of regenerative medicine, including tissue-engineering, 3D bioprinting, cell-based therapy, organoid technology and synthetic biology. We undertook a thematic analysis of relevant ethical points to consider for early-phase clinical trials of transplantable bio-artificial organs. The results of our systematic review and thematic analysis will be valuable for researchers, research ethics review boards, policy makers and clinicians with an interest in regenerative medicine and involved in the translation of bio-artificial organs for clinical transplantation. However, above we hope our analysis will contribute to the preparation of robust guidelines and recommendations in this highly complex and evolving field.
Methods
We performed a systematic review of the literature, following the PRISMA statement, as far as applicable (see Supplementary Materials). The review protocol has not been published or registered. The authors (DJ, EB and EM) developed the search strategy in consultation with a university librarian. We conducted the literature search in September 2021, using seven scientific databases: PubMed, EMBASE, Medline, Web of Science Core Collection, Cochrane Central Register of Controlled Trials and PsycINFO. An additional systematic search of the grey literature (i.e., relevant literature published outside of commercial or academic publishing) was conducted in Google Scholar. Search strings were constructed by keywords and their truncation, and relevant database-specific subjects headings [MeSH terms] (see Supplementary Materials). Due to language barriers, only articles in English or Dutch were considered for full-text analysis. We screened all titles and abstracts until September 2021 with no restriction for date of publication. Only outdated research guidelines that have subsequently been updated were not included. Based on title and abstract, articles that fulfilled the inclusion criteria were selected. Two researches independently carried out the selection (DJ and EB). Articles were discussed in case of differences between DJ and EB in the selection to come to a consensus. Full-texts were screened by DJ. The articles that did not meet the inclusion criteria during full-text screening, were excluded. Finally, the reference lists of the articles selected for full-text screening were checked for scientific articles or other documents that may be relevant and included if inclusion criteria were fulfilled (by DJ) (see Figure 1).
FIGURE 1
Inclusion and Exclusion Criteria
The inclusion criteria of this systematic review were as follows: articles in the adjacent fields of regenerative medicine, tissue-engineering, 3D bioprinting, cell-based therapy, organoid technology, synthetic biology, and bio-artificial organs describing ethical points to consider (issues, questions or challenges) for early-phase clinical trials. Letters to the editor, editorials and opinion articles were included as non-research manuscripts. Articles that only discussed pre-clinical research were excluded from our sample. For reasons of feasibility, articles discussing transplantation of non-biological medical devices instead of biological materials (e.g., pacemakers, blood glucose monitors, insulin pumps, or cardioverter defibrators) and articles discussing engineering of specific tissues for purposes other than organ transplantation (e.g., engineering of brains and reproductive organs for research purposes) were excluded. Finally, conference abstracts and articles were excluded (Table 1).
TABLE 1
| Inclusion criteria | Exclusion criteria |
|---|---|
| Articles in the fields of regenerative medicine, tissue-engineering, 3D printing, cell-based therapy, organoid technology, synthetic biology and bio-artificial organs describing ethical points to consider (issues, questions, or challenges) for early-phase clinical trials | Letters to the editor |
| Editorials | |
| Opinion articles | |
| Non-biological medical devices | |
| Engineering a specific tissue only for research purpose | |
| Describing ethical issues associated with pre-clinical research only |
Inclusion and exclusion criteria.
Analyses and Syntheses
The method of qualitative content analysis was employed (13). Qualitative content analysis is an inductive (bottom-up) approach to categorize ethical considerations and to develop themes within a coding frame. One researcher (DJ) conducted the analyses. Firstly, codes were assigned to all the considerations mentioned in each publication. Secondly, themes (e.g., patient selection) were created out of these codes by DJ. Thirdly, DJ, EM and EB discussed whether the created words describing the themes were representative of the codes until agreement was reached. Finally, a coding framework was built out of the identified themes. The coding framework was used to systematically keep track of ethical considerations mentioned per article.
Qualitative Content Analysis
We did not conduct a quality appraisal procedure, as there are no suitable criteria for appraisal of the quality of the literature included. This is a well-documented limitation of systematic reviews of (bio) ethical literature (14, 15).
Results
The selection procedure is presented in a PRISMA Flow diagram (Figure 1). The search produced 2132 hits, of which 222 were deemed eligible on the basis of title and abstract, and 92 articles were included after reference checking and full-text screening. The publication dates ranged from January 2003 to March 2021 (Table 2).
TABLE 2
| Author | Title | Year | Journal | Research field |
|---|---|---|---|---|
| Aalto-Setälä et al. | Obtaining consent for future research with induced pluripotent cells: opportunities and challenges | 2009 | PLoS Biology | Cell-Based Therapy |
| Afshar et al. | Ethics of research on stem cells and regenerative medicine: ethical guidelines in the Islamic Republic of Iran | 2020 | Stem Cell Research & Therapy | Regenerative Medicine |
| No Author | European Medicines Agency, CAR Secretariat and US Food and Drug Administration | 2011 | Regenerative Medicine | Cell-Based Therapy |
| Apatoff et al. | Autologous stem cell therapy for inherited and acquired retinal disease | 2017 | Regenerative Medicine | Cell-Based Therapy |
| Attico et al. | Approaches for effective clinical application of stem cell transplantation | 2018 | Current Transplantation Reports | Cell-Based Therapy |
| Baker et al. | Ethical considerations in Tissue Engineering Research: case Studies in Translation | 2016 | Methods | Tissue Engineering |
| Bhangra et al. | Using Stem Cells to Grow Artificial Tissue for Peripheral Nerve Repair | 2016 | Stem Cells International | Cell-Based Therapy |
| Bliss et al. | Optimizing the Success of Cell Transplantation Therapy for stroke | 2010 | Neurobiology of Disease | Cell-Based Therapy |
| Bobba et al. | The current state of stem cell therapy for ocular disease | 2018 | Experimental Eye Research | Cell-Based Therapy |
| Bredenoord et al. | Human tissues in a dish: The research and ethical implication of organoid technology | 2017 | Science | Organoid Transplantation |
| Brignier et al. | Embryonic and adult stem cell therapy | 2010 | Journal of Allergy and Clinical Immunology | Cell-Based Therapy |
| Chan. | Current and emerging global themes in the bioethics of regenerative medicine: the tangled web of stem cell translation | 2017 | Regenerative Medicine | Cell-Based Therapy |
| Chan. | Research Translation and Emerging Health Technologies: Synthetic Biology and Beyond | 2018 | Health Care Anal | Synthetic Biology |
| Chung | Stem-cell-based Therapy in the field of urology: a review of stem cell basic science, clinical application and future directions in the treatment of various sexual and urinary conditions | 2015 | Expert Opinion in Biological Therapy | Cell-Based Therapy |
| Coombe et al. | Current approaches in regenerative medicine for the treatment of diabetes: introducing CRISPR/CAS9 technology and the case for non-embryonic stem cell therapy | 2018 | American Journal Stem Cells | Cell-Based Therapy |
| Court et al. | Bioartificial liver support devices: historical perspectives | 2003 | ANZ Journal of Surgery | Bioengineered Organs |
| Daley et al. | Setting Global Standards for Stem Cell Research and Clinical Translation: The 2016 ISSCR Guidelines | 2016 | Stem Cell Reports | Cell-Based Therapy |
| Davis et al. | The role of Stem Cells for Reconstructing the Lower Urinary Tracts | 2018 | Current Stem cell Research & Therapy | Cell-Based Therapy |
| Davidson. | Brave Pioneers or Clinical Cowboys? | 2010 | Cell Stem Cell | Cell-Based Therapy |
| De Vries et al. | Ethical Aspects of Tissue Engineering: A Review | 2008 | Tissue engineering | Tissue Engineering |
| De Windt et al. | Ethics in musculoskeletal regenerative medicine; guidance in choosing the appropriate comparator in clinical trials | 2019 | Osteoarthritis and Cartilage | Regenerative Medicine |
| Fears et al. | Inclusivity and diversity: Integrating international perspectives on stem cell challenges and potential | 2021 | Stem Cell Reports | Cell-Based Therapy |
| Fung et al. | Responsible Translation of Stem Cell Research: An Assessment of Clinical Trial Registration and Publications | 2017 | Stem Cell Reports | Cell-Based Therapy |
| Garg et al. | Stem Cell Therapies in Retinal Disorders | 2017 | Cells | Cell-Based Therapy |
| Genske et al. | Rethinking risk assessment for emerging technology first-in-human trials | 2016 | Medicine, Health Care and Philosophy | Synthetic Biology |
| Giancola et al. | Cell therapy: cGMP Facilities and manufacturing | 2012 | Muscles, Ligaments and Tendons Journal | Cell-Based Therapy |
| Gilbert et al. | Print Me an Organ? Ethical and Regulatory Issues Emerging from 3D Bioprinting in Medicine | 2018 | Science and Engineering Ethics | 3D Bioprinting |
| Goula et al. | Advanced Therapy Medicinal Products Challenges and Perspectives in Regenerative Medicine | 2020 | Journal of Clinical Medicine Research | Regenerative Medicine |
| Haake et al. | Concise Review: Towards the Clinical Translation of Induced Pluripotent Stem Cell-Derived Blood Cells- Ready for Take-Off | 2019 | Stem Cells Translational Medicine | Cell-Based Therapy |
| Habets et al. | The inherent ethical challenge of first-in-human pluripotent stem cell trials | 2014 | Regenerative Medicine | Cell-Based Therapy |
| Hara et al. | New Governmental Regulatory System for Stem Cell-Based Therapies in Japan | 2014 | Therapeutic Innovation & Regulatory Science | Cell-Based Therapy |
| Hayakawa et al. | A study on ensuring the quality and safety of pharmaceuticals and medical devices derived from the processing of allogeneic human somatic stem cells | 2015 | Regenerative Therapy | Cell-Based Therapy |
| Hildebrandt | Horses for courses: an approach to the qualification of clinical trial sites and investigators in ATMPs | 2020 | Drug Discovery Today | Cell-Based Therapy |
| Hug | Understanding voluntariness of consent in first-in-human cell therapy trials | 2020 | Regenerative Medicine | Cell-Based Therapy |
| Hyun | Allowing innovative Stem Cell-Based Therapies Outside of Clinical Trials: Ethical and Policy Challenges | 2010 | Journal of Law, Medicine and Ethics | Cell-Based Therapy |
| Hyun et al. | New ISSCR Guidelines Underscore Major Principles for Responsible Translational Stem Cell Research | 2008 | Cell Stem Cell | Cell-Based Therapy |
| Kim et al. | Report of the International Stem Cell Banking Initiative Workshop Activity: Current Hurdles and Progress in Seed-Stock Banking of Human Pluripotent Stem cells | 2017 | Stem Cells Translational Medicine | Cell-Based Therapy |
| King et al. | Ethical issues in stem cell research and therapy | 2014 | Stem Cell Research & Therapy | Cell-Based Therapy |
| Kleiderman et al. | Overcoming barriers to facilitate the regulation of multi-centre regenerative medicine clinical trials | 2018 | Stem Cell Research & Therapy | Regenerative Medicine |
| Knoepfler | From Bench to FDA to Bedside: US Regulatory Trends for New Stem Cell Therapies | 2015 | Advanced Drug Delivery Reviews | Cell-Based Therapy |
| Kusunose et al. | Informed consent in clinical trials using stem cells: Suggestions and points of attention from informed consent training workshops in Japan | 2015 | South African Journal of Bioethics and Law | Cell-Based Therapy |
| Lederer et al. | Neural stem cells: mechanisms of fate specification and nuclear reprogramming in regenerative medicine | 2008 | Biotechnology Journal | Cell-Based Therapy |
| Lee et al. | Conditional approvals for autologous stem cell-based interventions | 2018 | Perspectives in Biology and Medicine | Cell-Based Therapy |
| Levin et al. | Special Commentary: early Clinical Development of Cell Replacement Therapy: Considerations for the National Eye Institute Audacious Goals Initiative | 2017 | Ophthalmology | Cell-Based Therapy |
| Lim et al. | Whole Organ and Tissue Reconstruction in Thoracic Regenerative Surgery | 2013 | Mayo clinic Proceedings | Tissue Engineering |
| Liras | Future research and therapeutic applications of human stem cells: general, regulatory, and bioethical aspects | 2010 | Journal of translational Medicine | Cell-Based Therapy |
| Liu et al. | Advances in Pluripotent Stem Cells: History, Mechanisms, Technologies, And Applications§ | 2020 | Stem Cell Reviews and Reports | Cell-Based Therapy |
| Lomax et al. | Return of results in translational iPS cell research: considerations for donor informed consent | 2013 | Stem Cell Research & Therapy | Cell-Based Therapy |
| Lomax et al. | Regulated, reliable and reputable: Protect patients with uniform standards for stem cell treatments | 2020 | Stem Cells Translational Medicine | Cell-Based Therapy |
| Lowenthal et al. | Specimen Collection for Induced Pluripotent Stem Cell Research: Harmonizing the Approach to Informed Consent | 2012 | Stem Cells Translational Medicine | Cell-Based Therapy |
| Lowenthal et al. | Ethics and Policy Issues for Stem Cell Research and Pulmonary Medicine | 2014 | Chest | Cell-Based Therapy |
| Lu et al. | Tissue Engineered Constructs: Perspectives on Clinical Translation | 2015 | Annals of Biomedical Engineering | Tissue Engineering |
| Madariaga et al. | Bioengineering Kidneys for Transplantation | 2014 | Seminars in Nephrology | Bioengineered Organs |
| Maekawa et al. | Development of Novel Advanced Cell and Gene Therapy and GMP-Controlled Cell Processing | 2005 | Japan Medical Association journal | Cell-Based Therapy |
| Main et al. | Managing the potential and pitfalls during clinical translation of emerging stem cell therapies | 2014 | Clinical and Translational Medicine | Cell-Based Therapy |
| Masuda et al. | New Challenges for Intervertebral Disc Treatment Using Regenerative Medicine | 2010 | Tissue engineering | Regenerative Medicine |
| Moradi et al. | Research and therapy with induced pluripotent stem cells (iPSCs): Social, legal and ethical considerations | 2019 | Stem Cell Research & Therapy | Cell-Based Therapy |
| Nagamura | The Importance of Recruiting a Diverse Population for Stem Cell Clinical Trials | 2016 | Current Stem Cell Reports | Cell-Based Therapy |
| Naghieh et al. | Biofabrication Strategies for Musculoskeletal Disorders: Evolution towards Clinical Application | 2021 | Bioengineering | 3D Bioprinting |
| Nagpal et al. | PERSPECTIVES: Stroke survivors’ views on the design of an early‐phase cell therapy trial for patients with chronic ischaemic stroke | 2019 | Health Expectations | Cell-Based Therapy |
| Neri | Genetic Stability of Mesenchymal Stromal Cells for Regenerative Medicine Applications: A Fundamental Biosafety Aspect | 2019 | International Journal of Molecular Sciences | Cell-Based Therapy |
| Niemansburg et al. | Participant selection for preventive Regenerative Medicine trials: ethical challenges of selecting individuals at risk | 2015 | Journal of Medical ethics | Regenerative Medicine |
| Niemansburg et al. | Regenerative medicine interventions for orthopedic disorders: ethical issues in the translation into patient | 2013 | Regenerative Medicine | Regenerative Medicine |
| Niemansburg et al. | Ethical implications of regenerative medicine in orthopedics: an empirical study with surgeons and scientists in the field | 2014 | The spine Journal | Regenerative Medicine |
| O’Donnell et al. | Beyond the Present Constraints That Prevent a Wide Spread of Tissue Engineering and Regenerative Medicine Approaches | 2019 | Frontiers Bioengineering and Biotechnology | Regenerative Medicine |
| Oerlemans et al. | Regenerative Urology Clinical Trials: An Ethical Assessment of Road Blocks and Solution | 2013 | Tissue engineering | Tissue Engineering |
| Oerlemans et al. | Towards a Richer Debate on Tissue Engineering: A Consideration on the Basis of NEST-Ethics | 2012 | Science Engineering Ethics | Tissue Engineering |
| O’Keefe | American Society for Bone and Mineral Research- Orthopaedic Research Society Joint Task Force Report on Cell-Based Therapies | 2020 | Journal of Bone and Mineral Research | Cell-Based Therapy |
| Otto et al. | Ethical considerations in the translation of biofabrication technologies into clinic and society | 2016 | Biofabrication | 3D Bioprinting |
| Parent et al. | The ethics of testing and research of manufactured organs on brain-dead/recently deceased subjects | 2019 | Journal of Medical Ethics | Bioengineered Organs |
| Patuzzo et al. | 3D bioprinting Technology: Scientific Aspects and Ethical Issues | 2018 | Science and Engineering Ethics | 3D Bioprinting |
| Schneemann et al. | Ethical challenges for pediatric liver organoid transplantation | 2020 | Science Translational Medicine | Organoid Transplantation |
| Scopetti et al. | Mesenchymal stem cells in neurodegenerative diseases: Opinion review on ethical dilemmas | 2020 | World Journal of Stem Cells | Cell-Based Therapy |
| Sekar et al. | Current standards and ethical landscape of engineered issues—3D bioprinting perspective | 2021 | Journal of Tissue Engineering | 3D Bioprinting |
| Seok et al. | A Personalized 3D-Printed Model for Obtaining Informed | 2021 | Journal of Personalized Medicine | 3D Bioprinting |
| Consent Process for Thyroid Surgery: A Randomized Clinical Study Using a Deep Learning Approach with Mesh-Type 3D Modeling | ||||
| Shineha et al. | A Comparative Analysis of Attitudes on Communication Toward Stem Cell Research and Regenerative Medicine Between the Public and the Scientific Community | 2018 | Stem Cells Translational Medicine | Regenerative Medicine |
| Sievert et al. | Tissue Engineering for the Lower Urinary Tract: A Review of a State of the Art Approach | 2007 | European Urology | Tissue Engineering |
| Smith et al. | Challenging misinformation and engaging patients: characterizing a regenerative medicine consult service | 2020 | Regenerative Medicine | Regenerative Medicine |
| Sniecinski et al. | Emerging stem cell based strategies for treatment of childhood disease | 2018 | Transfusion and Apheresis Science | Cell-Based Therapy |
| Stegemann et al. | Cell therapy for bone repair: narrowing the gap between vision and practice | 2014 | European Cells and Materials | Cell-based therapy |
| Sugarman and Bredenoord | Real-time ethics engagement in biomedical research | 2020 | EMBO reports | Organoid transplantation |
| Sutherland and Mayer | Ethical and Regulatory Issues Concerning Engineered Tissues for Congenital Heart Repair | 2003 | Thoracic and Cardiovascular Surgery | Tissue Engineering |
| Takashima et al. | Lessons for reviewing clinical trials using induced pluripotent stem cells: examining the case of a first-in-human trial for age-related macular degeneration | 2018 | Regenerative Medicine | Cell-Based Therapy |
| Taylor et al. | Ethics of bioengineering organs and tissues | 2014 | Expert Opinion on Biological Therapy | Tissue Engineering |
| Trommelmans et al. | Ethical reflections on clinical trials with human tissue engineered products | 2008 | Journal of Medical Ethics | Tissue Engineering |
| Trommelmans et al. | Informing participants in clinical trials with ex vivo human tissue-engineered products: what to tell and how to tell it? | 2008 | Journal Tissue Engineering Regenerative Medicine | Tissue Engineering |
| Trommelmans et al. | An Exploratory Survey on the Views of European Tissue Engineers Concerning the Ethical Issues of Tissue Engineering Research | 2009 | Tissue Engineering | Tissue Engineering |
| Trommelmans et al. | Is tissue engineering a new paradigm in medicine? Consequences for the ethical evaluation of tissue engineering research | 2009 | Medical Health Care and Philosophy | Tissue Engineering |
| Tsang | Legal and ethical status of stem cells as medicinal products | 2005 | Advanced Drug Delivery | Cell-Based Therapy |
| Vijayavenkataraman et al. | 3D bioprinting - An Ethical, Legal and Social Aspects (ELSA) framework | 2016 | Bioprinting | 3D Bioprinting |
| Zamborsky et al. | Regenerative Medicine in Orthopaedics and Trauma: Challenges, Regulation and Ethical Issues | 2018 | Orthopaedics and Trauma | Cell-Based Therapy |
| Zocchi et al. | Regulatory, ethical, and technical considerations on regenerative technologies and adipose-derived mesenchymal stem cells | 2019 | European Journal of Plastic Surgery | Regenerative Medicine |
Included articles.
Author name stated in bold: ethical considerations for early-phase regenerative trials are elaborately discussed in the paper.
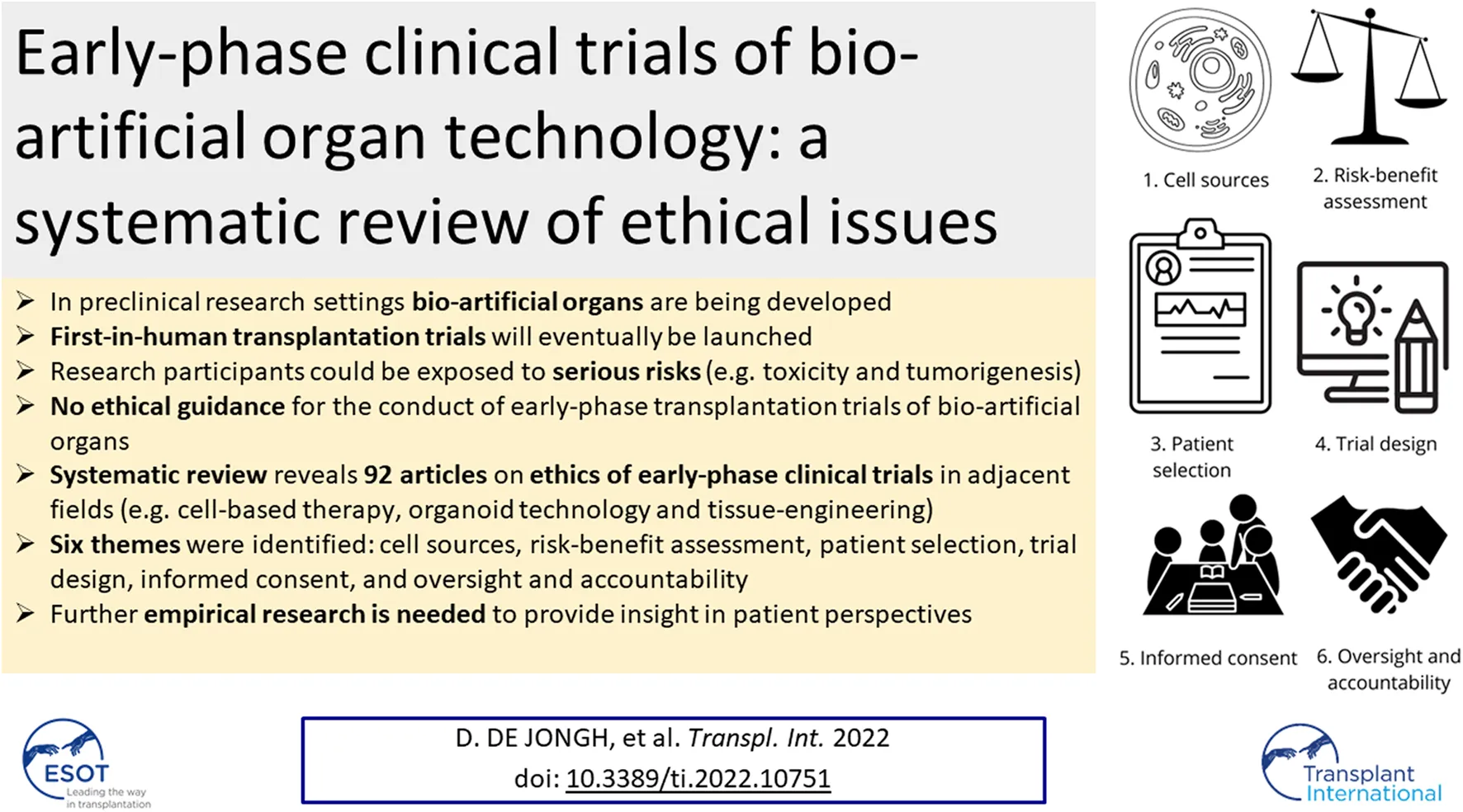
Themes
Six themes were identified: cell source, risk-benefit assessment, patient selection, trial design, informed consent, and oversight and accountability. The content of the article referring to the six identified ethical themes is summarized in Figure 2.
FIGURE 2
Research Fields
These six themes were found in seven different research fields (Table 2). The largest body of literature focusses on ethical considerations around early-phase trials in the field of cell-based therapy; 55 articles are published in this field, and the authoritative ISSCR guidelines are widely used (12, 16–26). There is less literature on ethical aspects of early-phase clinical trials in the field of 3D bioprinting, and organoid transplantation; seven articles were published on 3D bioprinting, three articles on bio-artificial organs, and two on organoid transplantation. Six empirical studies using questionnaires and interviews to investigate patients’ and professionals’ views on ethical considerations in early-phase clinical trials, were included. Seven papers were published in surgical journals.
Theme 1: Cell Sources
53 out of 92 articles mention ethical considerations related to the sources of cells used to generate complex tissue-engineered products such as bio-artificial or 3D bio-printed organs for transplantation into humans (9, 12, 16–24, 26–68). There are four types of cell sources: 1) xenogeneic cells, 2) autologous, 3) allogeneic donor, and 4) highly manipulated or/and genetically modified cells in humans, each with their own sets of ethical considerations (Table 3).
TABLE 3
| Cell source | Risks and benefits | Points to consider |
|---|---|---|
| Xenogeneic cells or tissue | Medical risks: Risk of zoonoses Individuals could object to use cells derived from animals on religious or socio-cultural grounds | - The use of animal cells should be minimized |
| - Components of animal origin should be replaced with human or chemically defined components whenever possible | ||
| - The use of viral transcription factor genes, retroviruses or pathogenic agents should be minimized | ||
| - Quality control systems, standard operating procedures (SOPs) and Good Manufacturing Practice (GMP) should be used | ||
| Autologous cells | Medical benefits: No immunological rejection | - It may not be possible to harvest sufficient numbers of patients’ cells |
| - The production cost could be high | ||
| - The timeframe for cell harvest could be insufficient for timely treatment | ||
| - Extra surgical interventions for participants could be necessary | ||
| - Quality control systems, SOPs and GMP should be used | ||
| Allogeneic donor cells | Medical risks: Immunological rejection and disease transmission | - Adequate donor consent should be obtained in a process that includes discussion of: aim of the research, return of research results, incidental findings, possibilities for withdrawal of consent, potential future research |
| - Additional safeguards should be adopted to protect personal data | ||
| Relational issues: Ownership and privacy issues Some donors may not want their cells to become an integral, growing part of another person. | - A policy should be developed on whether and how incidental findings of donor cell (genetic) screening should be returned to the cell donors and/or their relatives | |
| - Records on medical and family history of the donor of the cells should be obtained periodically | ||
| - Quality control systems, SOPs and GMP should be used | ||
| Highly manipulated and/or genetic modified cells | Medical risks: Unexpected behavior of cells or tissue (e.g., tumor formation, epigenetic or genetic instability) | - Strong pre-clinical data (of the safety and functions of the cells and or tissues) should be provided |
| - The use of manipulated cells should be minimized | ||
| - Participants should be monitored for a long time | ||
| - Researchers should adhere to cell processing and manufacturing protocols | ||
| - Quality control systems, SOPs and GMP should be used |
Points to consider in relation to cell sources.
Firstly, xenogeneic cells are associated with a risk of zoonosis (17, 20, 38, 47–49). For instance, issues related to the transmission of the infectious porcine retrovirus (PERV) from pig to human (69). Potential future patients could also reject the use of these cells to generate bio-artificial organs on religious grounds or for socio-cultural reasons (e.g., to protect animal rights/welfare) (33, 38, 48, 50, 52), even if their religious leaders take a more moderate stance (33). According to the literature, using these cells for transplantation into humans should be minimized as much as possible (12, 17, 38).
Secondly, the use of autologous cells (cells taken from the patient, who is both the donor and recipient) will make immunosuppressive therapy unnecessary (9, 16, 27–29, 33, 38–45, 68), and is perceived to carry fewer risks than the use of other cell types (33). However, challenges include the high production costs (29, 57, 70), extra surgical interventions for participants (50), the time required for their production (29, 40, 50, 57, 70), and the difficulty of standardizing manufacturing procedures (4057, 70).
Thirdly, besides the medical risks of transplanting allogeneic donor cell (cells taken from another human being), for example developing immunological problems, use of these cells also raises relational issues (20, 27, 30, 38, 41, 43, 63, 71, 72). Relational issues include questions such as: Who is the owner of the human cells once it is separated from the body (30,38,41,43)?; Can cells from the human body be subjected to laws regarding property rights (38,43)?, and; To what extent can the donor’s privacy and confidentiality be ensured by adopting additional measures (e.g., pseudonymisation) (20, 27, 30, 38, 41, 43, 63, 71, 72). Removing the donor’s personal information is often not desirable, because subsequent research may necessitate ongoing access to the information about the cell donor’s health status requiring personal data of the donor (e.g., their name and/or address) (20,52). Further, some donors may not want their cells to become an integral, growing part of another person (12, 20, 32, 52, 73). In addition, in the course of donor cell (genetic) screening, researchers should develop a policy on whether and how incidental findings (e.g., genetic risk) will be returned to the donors and/or their relatives (12, 20, 52, 63). Donors might consider their privacy violated if scientists know their future susceptibility to genetic disorders (52). Researchers should obtain an adequate informed consent from donors to respect their autonomy (12, 20, 22, 27, 28, 34, 38, 43, 45, 52, 57, 63, 67, 72–76), and give them some degree of insight and perhaps control over the use of donated materials by informing them about the types of incidental findings they wish to receive, future commercial applications, individualized research and therapeutic uses (12, 20, 27, 38, 43, 52, 72, 76), for instance by maintaining an ongoing dialogue with the donors (76). Moreover, to safeguard the health of the recipient over the years, it may be necessary to periodically obtain records on the medical and family history of the cell donor to monitor potential health risks, such as long-term immunological or tumorigenic reactions (12, 19, 20, 22, 27, 28, 32, 34, 35, 39, 41, 49, 51–53).
Lastly, the use of highly manipulated cells (i.e., cells of which the biological nature or structural function has been altered during the manufacturing process) and/or genetically modified cells raises safety concerns, and requires more quality controls to avoid undesired events (9, 12, 18, 20–23, 27, 28, 33, 35, 40, 50, 61, 63). For instance, these cells could have an increased risk of being tumorigenic, genetically unstable or toxic (12, 18, 35). Therefore, some authors recommend avoiding the use of manipulated cells whenever possible (e.g., tumor formation, epigenetic or genetic instability) (9, 12, 18, 20, 22). However, cell manipulation and/or genetic modification might be useful and even necessary for the generation of a bio-artificial organ (e.g., to repair disease-causing mutations) (20). Cells used in tissue-engineered products are often differentiated in vitro prior to being combined with a scaffolding material, for example collagen, to form artificial tissue, therefore tissue-engineered products are mostly classified as more than minimally manipulated (18).
Theme 2: Risk-Benefit Assessment
One of the conditions for ethically responsible clinical research is a favorable risk-benefit ratio (Table 4). This means that the risks and burdens of trial participation must be outweighed by the expected scientific or social value and the (potential) benefits for individual participants (12, 16, 21, 23, 24, 28, 29, 32, 34, 37, 45, 50, 53, 57, 64, 66–69, 77, 78) (Figure 3). The requirement of a favorable risk-benefit is difficult to meet in early-phase research, because the potential direct benefits to individual research participants in these trials are limited and uncertain (69). In the absence of direct medical benefit, justification of exposing individual research participants to potential harms in early-phase clinical trials is sought in expected scientific and/or social value (24, 30, 50, 66, 79). These include the benefits gained for science and society: generalizable knowledge and health gains for future patients (50). Knowledge of the working mechanism and the interaction of a regenerative medicine technology with the body, gathered in early-phase clinical trials, is necessary to move these technologies to the next clinical phase of clinical development (24, 30, 50, 66, 69). The anticipated social value of bio-artificial organs is potentially high, as they are intended as cures for patients with end-stage organ failure and might be more cost-effective than existing organ replacement therapies (66). At this stage, however, the social value is highly uncertain.
FIGURE 3
TABLE 4
| Points to consider in relation to risk-benefit assessment |
|---|
| - Researchers should provide robust pre-clinical data (i.e. safety and efficacy of the product should be rigorously demonstrated in laboratory tests and animal models) |
| - Personalization of the bio-artificial organ makes the product variable; therefore, the quality control and safety requirements of mass manufacturing do not apply |
| - Researchers should monitor and follow up participants for a long time after the study |
| - Efforts should be made not only to minimize the risks, but also to maximize the scientific and social value of a trial, in order to improve the risk-benefit ratio |
| - Clinical teams who conduct clinical trials of bioartificial organs should have experience with regenerative medicine technologies and with post-trial follow-up care |
Points to consider in relation to risk-benefit assessment.
Transplanting regenerative medicine into human recipients requires an irreversible (innovative) surgical procedure, which is associated with risks of harms and complications. Once the regenerative product is implanted in the body, it may not be possible to completely remove it (50). For instance, surgical removal of the product will be impractical or associated with greater risks [i.e., infections or complications of anesthesia (33)], and there will be some irreversible changes, such as scarring (50, 70). In addition, unlike non-biological medical devices, the regenerative product will most likely interact and integrate with the rest of the body, which may have uncertain, possibly unforeseeable long-term adverse health events for the recipient (16, 18, 21, 23, 24, 27, 28, 31–34, 37–40, 48, 50, 58, 62, 66–70, 72, 73, 77, 79–86).
When researchers are dealing with uncertain but potentially high risks, they are advised, before undertaking an early-phase clinical trial, to provide preclinical evidence of high internal validity (e.g., through replication) and external validity (e.g. through careful study design) (12, 16, 23, 27–29, 31, 34–37, 43, 46, 49–51, 53, 57, 59, 61, 62, 64–69, 77, 79–81, 84, 85, 87–90). Some argue that large animals should be used, because these animals can better imitate the human anatomy and/or pathology than small animals (1281). Others recommend to involve unbiased third parties to repeat some of the research (69). Even if robust preclinical evidence is available using these strategies, some unexpected risk will inevitably remain, such as unforeseeable long-term adverse health events for the recipient. Researchers should be aware that preclinical evidence from animal models may not correctly predict the duration, function and interaction that occur in a human body (16, 24, 27, 31, 34, 37, 39, 50, 65, 68, 79–82). In addition, the personalization of regenerative medicine makes the product variable, therefore, the quality control and safety requirements of mass manufacturing for external validity do not apply (32, 34, 35, 48). A major benefit of personalization, however, is that it may take away or reduce the need for the use of life-long immunosuppressive therapy for recipients, and avoid well-known side effects such as infections and nephropathy (45, 69).
To detect health risks associated with potential long-term adverse events, such as genetic instability, undirected or uncontrolled cell growth, research participants must be carefully monitored (16, 19, 21, 23, 24, 28, 29, 32, 34, 42, 46, 50, 58, 64, 67–70, 81–83, 85), with long-term follow-up (12, 19, 21, 23, 27–29, 32, 34, 35, 37, 38, 40, 46, 50, 51, 53, 62, 66–70, 73, 79, 81, 85, 87, 91, 92). On the one hand, intensive monitoring may be perceived as reassuring or beneficial by research participants (50, 83, 93). On the other hand, possible life-long follow-up could also be burdensome for participants (50). Given the complexity of tissue-engineered products, clinical teams conducting these studies should have experience with other regenerative medicine therapies (e.g., cell-based therapy) and with post-trial follow-up care (81).
Theme 3: Patient Selection
In the patient selection procedure, a new kind of trade-off has to be made: against enormous benefits stand potentially large risks (e.g., tumour formation). Selection of patients in early-phase clinical trials is a major ethical theme in the literature (12, 27, 31–34, 37, 42, 43, 45, 48, 50, 66, 67, 69, 70, 77, 81, 82, 94). Potential target groups can be divided into 5 categories: healthy individuals, individuals at risk, children, patient with early-stage disease and patients with end-stage disease (Table 5). First, it is considered unacceptable to ask 1) healthy individuals for clinical studies of regenerative medicine applications, especially of tissue-engineered products which are designed to function in the body of the recipient, given the high risks (34) and lack of benefit (32,34). Also, when regenerative applications are personalized (i.e., composed, in part, of patient-derived material), the only eligible recipient will likely be the patient themselves (48). Second, the scholarly literature contains arguments in favour of the selection of 2) individuals at risks, with 3) early-stage disease (31, 37, 48, 50, 69, 77, 81, 94), and 4) children (37, 38, 48, 78). These individuals are relatively healthy, if a regenerative medicine application is used into one of these groups, it may help 1) to achieve more health benefit, and 2) to prevent (long-term) severe complications (31, 37, 48, 50, 69, 77, 81, 94). On the other hand, it is uncertain whether these individuals, who may not have developed or will develop symptoms at all, will indeed come to suffer from end-stage organ failure at all and be in need for a transplant. At the same time, as the procedure is novel, risky and invasive, their current physical condition could worsen significantly (50). Lastly, based on the literature, the most eligible patients for early-phase clinical trials are patients who have reached the 5) end-stage of their disease (12, 27, 31, 33, 34, 42, 43, 45, 48, 66, 69, 70, 81, 82, 94). These patients no (or no longer) have effective or suitable treatment options at the time of enrolment and may be facing limited life expectancy (12, 27, 31, 33, 34, 42, 43, 45, 48, 66, 69, 70, 81, 82, 94). When serious complications occur, they may have less to lose than healthy individuals or patients with stable disease (, 12, 32–34, 48, 50, 66, 67, 77, 94). Also, for patients who have reached the end-stage of their disease, a bio-artificial organ could potentially be associated with greater medical benefits.
TABLE 5
| Suggested research participants for early-phase clinical trials | Reasons for and against selection |
|---|---|
| Healthy individuals | For |
| - Healthy individuals are most resilient to physical harms (thus, harms are minimized) | |
| Against | |
| - No clinical value for the participant | |
| - Risks are too high | |
| Individuals at risk | For |
| - No symptoms | - Less damage to the body from disease or disease-related complications, which could lead to better health outcomes compared to more advanced disease stages |
| - Risk factors for disease | - Disease can be prevented |
| Against | |
| - Risks could be too high | |
| - Unnecessary treatment (participants may not develop the disease) | |
| Early-stage patients | For |
| - Mild to moderate disease | - Less damage to the body from disease or disease-related complications, which could lead to better health outcomes compared to more advanced disease stages |
| - Medically controlled disease | Against |
| - Risks are too high | |
| - Alternative treatment options may be available | |
| - Treatment could worsen the disease | |
| Children | For |
| Diagnosed with the disease | - Less damage to the body |
| - Serious complications can be prevented | |
| - Benefit can be enjoyed the longest | |
| Against | |
| - Risks may be too high | |
| - Alternative treatment options may be available | |
| - The disease may not proceed to advanced stages | |
| - Long-term follow-up may be burdensome for the participants | |
| - Children are unable to provide informed consent | |
| Advanced-stage/end-stage patients | For |
| - Severe disease | - There is an unmet medical need, as effective treatment options are not or no longer available |
| - Unstable disease | - Potential for medical benefit from participation in the trial |
| - No or no longer a suitable treatment option available | - Less to lose when serious complications occur |
| Against | |
| - The body is already damaged; this damage might be irreparable | |
| - Treatment could worsen the disease |
Points to consider in relation to patient selection.
Theme 4: Trial Design
Intervention
Six articles in our sample argued that the traditional model for clinical translation—phases I to phases II, III and IV, in which toxicity and/or efficacy of new drugs are tested—may not be suitable for clinical trials of transplantable applications of regenerative medicine in humans (17, 24, 37, 38, 62, 81). Schneemann et al. proposed that early-phase transplantation trials should combine safety and efficacy outcomes in their trial design to maximise participants’ chances at obtaining medical benefit (37). Schneemann et al. suggested participants should be given a “dose” (in the context of bio-artificial organs: a certain quantity of engineered tissue) that is expected to be therapeutic, and efficacy should be added as an outcome measure (37). Combined safety and efficacy trials are associated with lower risks and costs than traditional studies, which could have positive effects on the likelihood of successful clinical development and help prevent promising interventions from failing (17, 81).
Outcomes
In the literature, relevant outcome measures for regenerative medicine clinical trials are discussed in 18 papers (12, 16, 19, 21, 24, 32, 34, 37, 43, 50, 61, 64, 69, 77, 80, 81, 87, 94). Both clinical outcome measures (e.g., survival rate or functional status) and patient-reported outcome measures (PROMs) (e.g., quality of life or experienced symptoms) are considered important (12, 21, 34, 43, 69, 77, 81, 87, 94). In later stages of clinical development and implementation, registries should be set up so that real-world outcome data can be collected to facilitate fair evaluation of the benefits of this technology. In addition, in later stages researchers should not only measure clinical outcome measures, but also PROMs, in order to ensure that new technologies not only affect biological parameters favourably, but also improve patients’ lives (37, 69, 94). By giving potential participants the opportunity to define outcome measures, they become active stakeholders in the trial design (37, 69, 78, 94). Further, asking patients to define outcomes could help increase the enrolment of participants in the trial (21, 37, 69, 94).
Skills and Materials
Authors also suggest to involve surgeons early on in the trial design, since they know what surgical skills and materials are needed to perform surgical trials safely (43, 37, 35, 12, 87). Clinical translation of bio-artificial organs in transplantation may require surgeons to learn new techniques and develop new instruments, therefore minimizing the number of surgeons involved is suggested (Table 6). Additionally, different surgeons may learn and refine surgical techniques in different ways, which may (temporarily) affect the outcomes of trials (34, 68, 95). Therefore, it is advised to account for a learning curve and for variability in experience between surgeons (32, 68, 66, 77, 96).
TABLE 6
| Trial design | Points to consider |
|---|---|
| Intervention | - Researchers should set up combined efficacy and safety trials |
| Outcomes | - Patients should be actively involved in research design as stakeholders |
| 1. Patient-reported (e.g., quality of life, treatment satisfaction and experienced symptoms) | - PROMs should be developed for later-phase clinical trials and adopted in trial design |
| 2. Professional defined (survival rate, functional status and biological parameters) | |
| Skills and materials | - Learning curves of surgeons should be corrected for |
| - The effects of the risks associated with surgical procedures on the outcomes of trials should be corrected |
points to consider in relation to trial design.
Theme 5: Informed Consent
The ethical requirements of clear informed consent is mentioned frequently in the literature (12, 16, 17, 20–25, 27, 29, 31–34, 37, 38, 43, 45, 50–52, 59, 60, 64–69, 75–77, 79, 81, 83, 85, 89, 90, 92, 93, 97, 98). Valid informed consent requires that participants must be adequately informed about relevant aspects of research participation, including the aim of the procedure, duration of the study, their right to withdraw, and the risks and benefits implications of the trial (Table 7). Less often mentioned as an essential component in informed consent is information on the specific composition of the regenerative medicine application, although some authors find it important (33, 81, 83). One survey showed that participants want to be especially informed about issues that could directly affect their health status, such as foreseeable risks, impact on quality of life and safety measures (83). Participants are worried about the risks associated with genetic manipulation of transplantable tissue and about commercialization of cells (33, 83).
TABLE 7
| Procedural | Substantial |
|---|---|
| - Informed consent from participants with decisional capacity or their legally authorized | - Potential risks, benefits and uncertainties |
| representative should be obtained | |
| - Relevant information about the trial, should also be presented visually | - Composition of the product |
| - Patients should be encouraged to ask questions | - The irreversible nature of the intervention |
| - Scientific jargon should be avoided by using only simple words or easily understood terminology | - How adverse events will be dealt with - The right and practical difficulty |
| - The teach-back method, exams or questionnaires could be used to ensure that participants | to withdraw |
| understand the relevant information | - How life-long follow up will be organized |
| - Participants should be encouraged to ask independent experts/patient advocates for advice or assistance in the decision-making process | - The possibility to consent for partial or complete autopsy in the event of death |
| - Participants need to be informed that the intervention is not likely to provide direct medical benefits |
points to consider in relation to informed consent.
Given the lack of evidence on the risks, however, it could be difficult for researchers to provide full disclosure. Rather, participants should be made aware of the uncertainties surrounding the risks and benefits of investigational regenerative medicine technologies (20, 21, 23, 24, 32–34, 65, 72, 81, 98). Participants should be given the opportunity to consult an independent expert (33, 98), and can be offered psychological support (81), or consult a patient advocates (81), to assist them in the decision-making process (33, 60, 81, 83, 84, 98). To minimize “the therapeutic misconception,” the (sometimes) mistaken belief among research participants that they will benefit from trial participation, measures should be taken to ensure that research participants are aware of the fact that research is conducted not with the goal of providing them medical treatment, but of obtaining generalizable information (12, 16, 17, 21, 24, 25, 29, 31, 33, 37, 50, 57, 60, 64, 67, 69, 81, 93, 97, 98). Researchers should avoid presenting the potential of the product in an overly optimistic light, overestimating the possible benefits, or giving unrealistic timelines for it to reach the clinic (30). Also, to strengthen comprehension, researchers are advised to present information about the trial not only in writing but also visually (33, 60, 68, 79), encourage patients to ask questions, and avoid scientific jargon by using only simple words or easily understood terminology during the informed consent process (20–22, 29, 31, 57, 69, 93, 98). Researchers may use the teach-back method (98) or even an “exam” or questionnaire (33) to ensure that participants understand the information and make an informed choice (33, 34, 81, 98, 99). Participants must also be aware that participating in a trial might diminish their chances of getting access to future treatment opportunities (21,48,50).
A widely endorsed norm in research ethics is that participants should always have the right to withdraw their consent without negative consequences for the health care they receive. However, for participants in early-phase clinical trials of regenerative medicine technologies, withdrawal may be complicated (34). While it may be possible to withdraw from follow-up, removal of bio-artificial organs (in their entirety) may not be possible. For this reason, the opportunities for withdrawal or lack thereof, and the implications of trial participation for the future health and safety of participants must be discussed beforehand, as part of the informed consent process (34). In particular, research participants should be aware of the need for a long-term follow-up and the possibility of (long-term) adverse events (32, 34, 81). Lastly, some authors suggest informing and asking participants to provide consent for a partial or complete autopsy after their death. Obtaining this information will improve the scientific value of the study and contribute to the safety of future research participants (12).
Theme 6: Oversight and Accountability
The literature suggests that researchers should be especially careful when communicating with patients, physicians, other stakeholders, and the general public about regenerative medicine applications, as overly optimistic expectations might easily arise (17, 21, 22, 25, 29, 46, 52, 57, 62, 64, 67, 69, 78, 80, 81, 86, 90, 93, 94, 100) (Table 8). The ways in which research is represented in the media affects societal perspectives and frames policy debates (17, 67, 86, 100). In frontier science, of which research on bio-artificial organ transplantation is an example, researchers might wish or feel compelled to attract media attention to obtain financial support (17). However, they should refrain from inaccurate or incomplete representation of research, as this could ultimately have negative consequences for the advancement of the field and the integrity. For instance, researchers should avoid sharing findings with the press before peer review (17, 62) or could follow the ISSCR guidelines with regard to the conduct, public engagement and accountability of clinical trials (12, 16). In addition, researchers should be open to (international) collaboration between scientists, ethicists and clinicians (18, 22, 23, 25, 28, 35, 36, 38, 39, 41, 45, 50, 54, 57, 63–65, 73, 77, 81, 84–86, 89, 96, 100–102) and the conduct of interdisciplinary dialogues, involving scientists, such as engineers and biologists, but also patients, clinicians, policy makers, industry partners, ethicists, and the general public (17, 24, 29, 35, 37, 38, 46, 55, 64, 73, 80, 81, 84, 86, 90, 93) to encourage responsible innovation, and build and maintain long-term trust in research and the development of regenerative medicine applications. Adopting a similar strategy around bio-artificial organ technologies is highly desirable.
TABLE 8
| Oversight and accountability | Points to consider |
|---|---|
| Public awareness and patient engagement | - The information should be publicly available |
| - Interdisciplinary dialogues between scientists, ethicists, patients, policy-makers, clinicians, industry partners, and the general public should be stimulated | |
| - Dissemination of non-peer-reviewed research results should be avoided | |
| - Participants should be referred to patient advocacy groups | |
| - Participants should have an active role in research (e.g., as active stakeholders) | |
| Strengthening of RECs | - RECs should be expanded with experts in regenerative medicine/organ transplantation or set up advisory boards or specialized working groups to support RECs |
| - Patient representatives should be invited to participate in RECs | |
| - Educational activities should be organized for RECs | |
| Stimulate (data) transparency, minimize publication bias and diminish selective reporting to create long-term trust in research | - Preclinical researchers should publish negative, positive and inconclusive results |
| - Researchers should pre-register clinical trials | |
| - Data monitoring plans should be put in place | |
| - Researchers, clinicians and regulators should be stimulated to collaborate | |
| - Guidance should be periodically revised |
points to consider in relation to oversight and accountability.
All research involving clinical applications of regenerative medicine must be subjected to independent RECs for approval. The main task of these oversight bodies is to ensure ethical conduct of clinical research and to protect human research participants. However, it is uncertain whether existing RECs have sufficient specific technical and clinical expertise in the fields of both organ transplantation and regenerative medicine to be able to evaluate the risks associated with bio-artificial organ transplantation trials. Multiple authors have proposed to set up specialized RECs or advisory boards with experts from various backgrounds for the evaluation of clinical trials of regenerative medicine technologies (9, 16, 19, 20, 22, 24, 28, 29, 32, 45, 46, 62–65, 67, 69, 77, 78, 80, 85, 92). These experts could assist RECs in assessing the scientific underpinnings of the clinical trial protocols and the risks of abnormal product function and proliferation (16). According to some, such specialized RECs should ideally also include lay people (21, 80). Moreover, authors recommend providing education opportunities for surgeons, researchers, nurses and ethicist in training, on the ethical aspects related to ATMPs (9, 20–22, 29, 36, 40, 45, 64, 65, 69, 70, 73, 77, 87, 92, 93).
Researchers should pre-register clinical trials and publish understandable and complete data on each step along the research pathway regardless of whether the data is positive, negative or inconclusive (12, 16, 24, 28, 29, 69, 80, 81). Being transparent about data could also inspire other researchers to go into new research directions (69).
Discussion
In the rapidly evolving field of regenerative medicine, it is important that early-phase clinical trials are performed in a responsible and ethically acceptable way. Such trials can lead to unforeseeable serious harm for research participants, as, for instance, has occurred during early-phase clinical trials of gene therapies in the 1990s, in which research participants have died (103). Yet clinical translation of bio-artificial organ technologies has the potential to make available life-saving therapeutic products to patients suffering from end-stage organ failure and to remove the need of (life-long) immunosuppressive therapy, which has hitherto been a serious disadvantage of organ transplantation.
To our knowledge, this is the first systematic review of the literature on early-phase clinical trials in regenerative medicine, tissue engineering, cell-based therapy, bio-engineered organs, organoid transplantation, synthetic biology, and 3D bioprinting, which summarizes relevant ethical points to consider in early-phase research on transplantable bio-artificial organs. Our review reveals that a significant body of literature exists on ethical considerations around early-phase trials in the field of cell-based therapy. However, there is strikingly little literature on ethical aspects of early-phase clinical trials in the field of 3D bioprinting, and organoid transplantation. There is also little attention for ethical aspects of early-phase regenerative medicine trials in surgery; only seven papers were published in surgical journals. A further noticeable finding in this review was the paucity of empirical ethics research in the scientific fields that were included in the review: only six empirical studies were found (21, 77, 83, 93, 94, 98), three of which focussed on the perceived ethical challenges of regenerative medicine among professionals in the field (21, 77, 83), and three of which focussed on patients’ perspectives (93, 94, 98) on ethical considerations for early-phase clinical regenerative trials. Yet insight in patients’ perspectives is essential to assessing the social value of new technologies and to determining the conditions under which it should be offered to patients.
In total, six themes were identified in the literature: cell source, risk-benefit assessment, patient selection, trial design, informed consent, and oversight and accountability. We found that ethical considerations around cell sources were mentioned most often, which is consistent with an earlier review of the ethical aspects of tissue engineering by de Vries et al (38). For each of the six themes, we have distilled and discussed ethical points to consider, which can be valuable for research groups and RECs who will be setting up or evaluating early-phase clinical transplantation trials of bio-artificial organs in the future, and for health care professionals working in the field of organ transplantation with an interest in innovative technologies. Below, we would like to reflect on important points made on two themes: trial design and informed consent. These themes are underrepresented in the literature, and need specific attention before early-phase bio-artificial organ transplantation trials can be initiated, and evaluated by RECs.
First, when designing clinical trials, researchers should not focus exclusively on gathering data on clinical outcomes, but also on understanding research participants’ perspectives. Qualitative studies of patients’ perspectives can help elucidate their needs and preferences with regard to the set-up and conduct of clinical trials, the use of outcome measures, the design and performance characteristics of the product that is being developed, the type of follow-up care that will be offered, etc., so that the process of clinical development and the resulting bio-artificial organ technologies are optimally aligned with patients’ perspectives, to improve their quality of life. Also, trials should be designed such that data on long-term clinical outcomes of transplantable bioartificial organ technologies can be gathered. An exploratory survey among European tissue-engineers by Trommelmans et al. found that the majority of respondents insisted on long-term follow-up (83). Given the irreversibility of transplantation of bio-artificial organs and its potential for adverse events emerging only after a long time, long-term follow-up procedures may be essential in trials of bio-artificial organs. This requires long-term—possibly even lifelong—commitment of participants (34), and long-term trust relationships between researchers and patients. Barriers to long-term follow-up studies frequently reported include outdated contact information, lack of financial reimbursement for follow-up services, and direct and indirect costs charged to participants (104,105). Researchers in regenerative medicine could learn from prior experiences in overcoming these barriers. One such strategy is to discuss the long-term follow-up planning with participants during the informed consent procedure (106). Additional research is needed to identify barriers specific to long-term follow-up of bio-artificial organ transplantation trials, and to develop strategies for overcoming them.
Second, during the informed procedure, researchers should communicate reasonably foreseeable risks and benefits associated with participation in clinical trials. However, little guidance exists on how researchers should communicate such risk and benefits in cutting edge early-phase research (107, 108), in which there is a high degree of uncertainty surrounding these risks and benefits due to limited knowledge. There are concerns that researchers might overestimate and exaggerate the benefits in early-phase clinical trials, which is a potential source of “therapeutic misconception” (109, 110). For instance, Kimmelman et al. (110) analysed patient information and informed consent documents on risky, novel, experimental early-phase gene-transfer trials for seriously ill patients, and concluded that these were often inappropriately optimistic about the direct benefits for individual participants. The results of this study are relevant, because early-phase bio-artificial organs will also be risky and experimental. To prevent therapeutic misconception, researchers should provide realistic information to participants about the individual medical benefits and uncertainties of participation in early-phase clinical trials.
We consider it remarkable that it is often recommended, in various research fields, to use questionnaires, or extraordinarily written or oral exams, to check whether research participants have understood relevant information about clinical trial participation (16, 21, 33, 108, 110–112). It is believed that the exam approach will leave more time for the researcher, during a subsequent informed consent discussion, to focus on the aspects about which the participant’s knowledge is not yet sufficient, and tailor the process to the participant’s individual informational needs (113). However, it is unclear whether this focus on formally “testing” participants’ knowledge of (the science underlying) the trial will lead to better informed, more autonomous decisions about research participation. It may also place more responsibility or liability on research participants when—deciding about—participating in novel, possibly risky trials. Further research will be needed to understand and improve communication about risks and benefits of participation in early-phase clinical trials of bio-artificial organs.
We did not limit this review to one specific bio-artificial organ type. Instead, we developed a general list of ethical points to consider for all bio-artificial organ technologies. However, these points to consider may play out differently in specific bio-artificial organ technologies, and may vary with organ type; for instance, to a greater extent than for hearts, lungs, and livers, there are alternative (organ replacement) therapies available for pancreases or kidneys. This difference may affect risk-benefit assessment and patient selection of a clinical trial, which needs to be taken into account.
In conclusion, there is no specific ethical guidance for the safe and responsible design and conduct of early-phase clinical trials of transplantable bio-artificial organs. However, we have shown that ethical considerations from adjacent research fields may be useful for early-phase transplantable bio-artificial organs trials. In particular, the irreversibility, uncertainty of outcomes, the ethical considerations around the cell sources used to generate the product (e.g., donor cells), and the need for life-long follow-up studies makes clinical translation of bio-artificial organ technologies ethically contentious. Ethical themes that researchers and RECs should consider when designing or evaluating studies include cell source, risk-benefit assessment, patient selection, trial design, informed consent, and oversight and accountability. Patient engagement and empirical studies of patients’ perspectives on (organ-) specific bio-artificial organ technologies will be essential to realizing the social value of research and clinical translation of bio-artificial organs, and to ensuring adequate informed consent for research participation.
VANGUARD Consortium Partners
Members of the VANGUARD consortium are as follows: Ekaterine Berishvili, Laura Mar Fonseca, Fanny Lebreton, Kevin Bellofatto, Juliette Bignard (Department of Surgery, University of Geneva, Geneva, Switzerland); Jochen Seissler, Leila Wolf-van Buerck, Mohsen Honarpisheh, Yichen Zhang, Yutian Lei, Monika Pehl (Diabetes Centre—Campus Innenstadt, Medizinische Klinik und Poliklinik IV, Klinikum der Ludwig-Maximilians-Universität München, Germany); Antonia Follenzi, Christina Olgasi, Alessia Cucci, Chiara Borsotti, Simone Assanelli (Department of Health Sciences, University of Piemonte Orientale, Novara, Italy); Lorenzo Piemonti, Antonio Citro, Silvia Pellegrini, Cataldo Pignatelli, Francesco Campo (IRCCS Ospedale San Raffaele, Diabetes Research Institute, Milano, Italy); Olivier Thaunat (Dept. Transplantation, Nephrology and Clinical Immunology, Lyon Claude Bernard University, Lyon, France); Devi Mey, Chiara Parisotto, Giovanna Rossi (European Society for Organ Transplantation, Padova, Italy); Patrick Kugelmeier, Petra Wolint, Markus Mühlemann, Karolina Pal-Kutas (Kugelmeiers AG, Erlenbach, Switzerland); Marco Cavallaro, Julia Götz, Jeanette Müller (Accelopment Switzerland Ltd.).
Statements
Author contributions
DdJ, EM, AC, MS, and EB contributed to conception and design of the manuscript. DdJ wrote the first draft of the manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
Funding
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement no. 874700.
Acknowledgments
The authors would like to thank S.T.G. Meertens-Gunput from the Erasmus MC Medical Library for her assistance in developing and updating the systematic literature search.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Materials for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2022.10751/full#supplementary-material
Supplementary Data Sheet S1PRISMA checklist.
Supplementary Data Sheet S2Capsule sentence summary.
Supplementary Data Sheet S3Search term.
Footnotes
1.^VANGUARD. New generation cell therapy: bioartificial pancreas to cure type 1 diabetes. https://vanguard-project.eu/ (Accessed 1 July 2022).
References
1.
MazzaGRomboutsKRennie HallAUrbaniLVinh LuongTAl-AkkadWet alDecellularized Human Liver as a Natural 3D-Scaffold for Liver Bioengineering and Transplantation. Sci Rep (2015) 5:13079–15. 10.1038/srep13079
2.
AtalaABauerSBSokerSYooJJRetikAB. Tissue-engineered Autologous Bladders for Patients Needing Cystoplasty. Lancet (2006) 367:1241–6. 10.1016/S0140-6736(06)68438-9
3.
JeremyJSJacquesGSarahGGabrielGJosephPVHaraldCO. Regeneration and Experimental Orthotopic Transplantation of a Bioengineered Kidney. Nat Med (2013) 19:646–51. 10.1038/nm.3154
4.
GuyetteJPCharestJMMillsRWJankBJMoserPTGilpinSEet alBioengineering Human Myocardium on Native Extracellular Matrix. Circ Res (2016) 118:56–72. 10.1161/CIRCRESAHA.115.306874
5.
KitanoKSchwartzDMZhouHGilpinSEWojtkiewiczGRRenXet alBioengineering of Functional Human Induced Pluripotent Stem Cell-Derived Intestinal Grafts. Nat Commun (2017) 8:765. 10.1038/s41467-017-00779-y
6.
WilkinsonDCAlva-OrnelasJASucreJMSVijayarajPDurraARichardsonWet alDevelopment of a Three-Dimensional Bioengineering Technology to Generate Lung Tissue for Personalized Disease Modeling. Stem Cell Transl Med (2017) 6:622–33. 10.5966/sctm.2016-0192
7.
OhataKOttHC. Human-scale Lung Regeneration Based on Decellularized Matrix Scaffolds as a Biologic Platform. Surg Today (2020) 50:633–43. 10.1007/s00595-020-02000-y
8.
KimmelmanJ. Ethics, Ambiguity Aversion, and the Review of Complex Translational Clinical Trials. Bioethics (2012) 26:242–50. 10.1111/j.1467-8519.2010.01856.x
9.
KnoepflerPS. From Bench to FDA to Bedside: US Regulatory Trends for New Stem Cell Therapies. Adv Drug Deliv Rev (2015) 82:192–6. 10.1016/j.addr.2014.12.001
10.
BallantyneAErikssonS. Research Ethics Revised: The New CIOMS Guidelines and the World Medical Association Declaration of Helsinki in Context. Bioethics (2019) 33:310–1. 10.1111/bioe.12581
11.
World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA - J Am Med Assoc (2013) 310:2191–4. 10.1001/jama.2013.281053
12.
DaleyGQQHyunIApperleyJFFBarkerRAABenvenistyNBredenoordALLet alSetting Global Standards for Stem Cell Research and Clinical Translation: The 2016 ISSCR Guidelines. Stem Cel Rep (2016) 6:787–97. 10.1016/J.STEMCR.2016.05.001
13.
SchreierM. Qualitative Content Analysis. In: The SAGE Handbook of Qualitative Data Analysis. London: SAGE Publications (2014). p. 170–83. 10.4135/9781446282243.n12
14.
MertzM. How to Tackle the Conundrum of Quality Appraisal in Systematic Reviews of Normative Literature/information? Analysing the Problems of Three Possible Strategies (Translation of a German Paper). BMC Med Ethics (2019) 20:81–12. 10.1186/s12910-019-0423-5
15.
MertzMStrechDKahrassH. What Methods Do Reviews of Normative Ethics Literature Use for Search, Selection, Analysis, and Synthesis? In-Depth Results from a Systematic Review of Reviews. Syst Rev (2017) 261. 10.1186/s13643-017-0661-x
16.
HyunILindvallOÄhrlund-RichterLCattaneoECavazzana-CalvoMCossuGet alNew ISSCR Guidelines Underscore Major Principles for Responsible Translational Stem Cell Research. Cell Stem Cell (2008) 3:607–9. 10.1016/j.stem.2008.11.009
17.
BredenoordALCleversHKnoblichJA. Human Tissues in a Dish: The Research and Ethical Implications of Organoid Technology. Science (2017) 80:eaaf9414–7. 10.1126/science.aaf9414
18.
BhangraKSBusuttilFPhillipsJBRahimAA. Using Stem Cells to Grow Artificial Tissue for Peripheral Nerve Repair. Stem Cell Int (2016) 2016:7502178. 10.1155/2016/7502178
19.
NeriS. Genetic Stability of Mesenchymal Stromal Cells for Regenerative Medicine Applications: A Fundamental Biosafety Aspect. Int J Mol Sci (2019) E2406. 10.3390/ijms20102406
20.
MoradiSMahdizadehHŠarićTKimJHaratiJShahsavaraniHet alResearch and Therapy with Induced Pluripotent Stem Cells (iPSCs): Social, Legal, and Ethical Considerations. Stem Cel Res Ther (2019) 10:341–13. 10.1186/S13287-019-1455-Y
21.
KusunoseMNagamuraFMutoK. Informed Consent in Clinical Trials Using Stem Cells: Suggestions and Points of Attention from Informed Consent Training Workshops in Japan. S Afr J Bioeth L (2015) 8:49. 10.7196/SAJBL.8016
22.
KleidermanEBoilyAHasiloCKnoppersBM. Overcoming Barriers to Facilitate the Regulation of Multi-centre Regenerative Medicine Clinical Trials. Stem Cel Res Ther (2018) 9:307. 10.1186/s13287-018-1055-2
23.
HyunI. Allowing Innovative Stem Cell-Based Therapies outside of Clinical Trials: Ethical and Policy Challenges. J L Med Ethics (2010) 38:277–85. 10.1111/J.1748-720X.2010.00488.X
24.
HabetsMGJLVan DeldenJJMBredenoordAL. The Inherent Ethical challenge of First-In-Human Pluripotent Stem Cell Trials. Regen Med (2014) 9:1–3. 10.2217/rme.13.83
25.
FungMYuanYAtkinsHShiQBubelaT. Responsible Translation of Stem Cell Research: An Assessment of Clinical Trial Registration and Publications. Stem Cel Rep (2017) 8:1190–201. 10.1016/j.stemcr.2017.03.013
26.
BrignierACGewirtzAM. Embryonic and Adult Stem Cell Therapy. J Allergy Clin Immunol (2010) 125:S336–44. 10.1016/j.jaci.2009.09.032
27.
HayakawaTAoiTUmezawaAOzawaKSatoYSawaYet alA Study on Ensuring the Quality and Safety of Pharmaceuticals and Medical Devices Derived from the Processing of Allogeneic Human Somatic Stem Cells. Regen Ther (2015) 2:70–80. 10.1016/J.RETH.2015.05.001
28.
LirasA. Future Research and Therapeutic Applications of Human Stem Cells: General, Regulatory, and Bioethical Aspects. J Transl Med (2010) 8:131. 10.1186/1479-5876-8-131
29.
MainHMunsieMO’ConnorMD. Managing the Potential and Pitfalls during Clinical Translation of Emerging Stem Cell Therapies. Clin Transl Med (2014) 3:10. 10.1186/2001-1326-3-10
30.
OttoIABreugemCCMaldaJBredenoordAL. Ethical Considerations in the Translation of Regenerative Biofabrication Technologies into Clinic and Society. Biofabrication (2016) 8. 10.1088/1758-5090/8/4/042001
31.
VijayavenkataramanSLuWFFuhJYH. 3D Bioprinting – an Ethical, Legal and Social Aspects (ELSA) Framework. Bioprinting (2016) 1:11–21. 10.1016/j.bprint.2016.08.001
32.
TrommelmansLSellingJDierickxK. Is Tissue Engineering a New Paradigm in Medicine? Consequences for the Ethical Evaluation of Tissue Engineering Research. Med Health Care Philos (2009) 12:459–67. 10.1007/s11019-009-9192-0
33.
TrommelmansLSellingJDierickxK. Informing Participants in Clinical Trials with Ex Vivo Human Tissue-Engineered Products: what to Tell and How to Tell it?J Tissue Eng Regen Med (2008) 2:236–41. 10.1002/TERM.82
34.
TrommelmansLSellingJDierickxK. Ethical Reflections on Clinical Trials with Human Tissue Engineered Products. J Med Ethics (2008) e1. 10.1136/jme.2007.022913
35.
SutherlandFWHMayerJE. Ethical and Regulatory Issues Concerning Engineered Tissues for Congenital Heart Repair. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu (2003) 6:152–63. 10.1053/PCSU.2003.50012
36.
StegemannJPVerrierSGebhardFLaschkeMWMartinISimpsonHet alCell Therapy for Bone Repair: Narrowing the gap between Vision and Practice. Eur Cel Mater (2014) 27:1–4. 10.22203/ECM.V027SA01
37.
SchneemannSABoersSNvan Delden JjmNieuwenhuisEESFuchsSABredenoordAL. Ethical Challenges for Pediatric Liver Organoid Transplantation. Sci Transl Med (2020) 12:8471. 10.1126/scitranslmed.aau8471
38.
De VriesRBMOerlemansATrommelmansLDierickxKGordijnB. Ethical Aspects of Tissue Engineering: A Review. Tissue Eng B Rev (2008) 14:367–75. 10.1089/ten.teb.2008.0199
39.
GiancolaRBonfiniTIaconeA. Cell Therapy: cGMP Facilities and Manufacturing. Muscles Ligaments Tendons J (2012) 2:243–7.
40.
MadariagaMLLOttHC. Bioengineering Kidneys for Transplantation. Semin Nephrol (2014) 34:384–93. 10.1016/J.SEMNEPHROL.2014.06.005
41.
NaghiehSLindbergGTamaddonMLiuC. Biofabrication Strategies for Musculoskeletal Disorders: Evolution towards Clinical Applications. Bioengineering (2021) 8. 10.3390/bioengineering8090123
42.
LevinLAMillerJWZackDJFriedlanderMSmithLEH. Special Commentary: Early Clinical Development of Cell Replacement Therapy: Considerations for the National Eye Institute Audacious Goals Initiative. Ophthalmology (2017) 124:926–34. 10.1016/J.OPHTHA.2017.02.017
43.
O’DonnellBTIvesCJMohiuddinOABunnellBA. Beyond the Present Constraints that Prevent a Wide Spread of Tissue Engineering and Regenerative Medicine Approaches. Front Bioeng Biotechnol (2019) 7:95. 10.3389/FBIOE.2019.00095
44.
O’KeefeRJTuanRSLaneNEAwadHABarryFBunnellBAet alAmerican Society for Bone and Mineral Research-Orthopaedic Research Society Joint Task Force Report on Cell-Based Therapies. J Bone Miner Res (2020) 35:3–17. 10.1002/JBMR.3839
45.
SekarMPBudharajuHZenniferASethuramanSVermeulenNSundaramurthiDet alCurrent Standards and Ethical Landscape of Engineered tissues—3D Bioprinting Perspective. J Tissue Eng (2021) 6:20417314211027677–33. 10.1177/20417314211027677
46.
ScopettiMSanturroAGattoVRussaRLAManettiFErricoSDet alMesenchymal Stem Cells in Neurodegenerative Diseases: Opinion Review on Ethical Dilemmas. World J Stem Cell (2020) 12:168–77. 10.4252/WJSC.V12.I3.168
47.
CourtFGWemyss-HoldenSADennisonARMaddernGJ. Bioartificial Liver Support Devices: Historical Perspectives. ANZ J Surg (2003) 73:739–48. 10.1046/j.1445-2197.2003.02741.x
48.
GilbertFO’ConnellCDMladenovskaTDoddsS. Print Me an Organ? Ethical and Regulatory Issues Emerging from 3D Bioprinting in Medicine. Sci Eng Ethics (2018) 24:73–91. 10.1007/S11948-017-9874-6
49.
LiuGDavidBTTrawczynskiMFesslerRG. Advances in Pluripotent Stem Cells: History, Mechanisms, Technologies, and Applications. Stem Cel Rev Rep (2020) 16:3–32. 10.1007/S12015-019-09935-X
50.
NiemansburgSLVan DeldenJJMDhertWJABredenoordAL. Regenerative Medicine Interventions for Orthopedic Disorders: Ethical Issues in the Translation into Patients. Regen Med (2013) 8:65–73. 10.2217/RME.12.112
51.
BobbaSDi GirolamoNMunsieMChenFPébayAHarkinDet alThe Current State of Stem Cell Therapy for Ocular Disease. Exp Eye Res (2018) 177:65–75. 10.1016/j.exer.2018.07.019
52.
Aalto-SetäläKConklinBRLoB. Obtaining Consent for Future Research with Induced Pluripotent cells:Opportunities and Challenges. Plos Biol (2009) 7:e1000042. 10.1371/journal.pbio.1000042
53.
TsangL. Legal and Ethical Status of Stem Cells as Medicinal Products. Adv Drug Deliv Rev (2005) 57:1970–80. 10.1016/j.addr.2005.08.005
54.
ChungE. Stem-cell-based Therapy in the Field of Urology: A Review of Stem Cell Basic Science, Clinical Applications and Future Directions in the Treatment of Various Sexual and Urinary Conditions. Expert Opin Biol Ther (2015) 15:1623–32. 10.1517/14712598.2015.1075504
55.
CoombeLKadriAMartinezJFTatacharVGallicanoGI. Current Approaches in Regenerative Medicine for the Treatment of Diabetes: Introducing CRISPR/CAS9 Technology and the Case for Non-embryonic Stem Cell Therapy. Am J Stem Cell (2018) 7:104–13.
56.
GargAYangJLeeWTsangS. Stem Cell Therapies in Retinal Disorders. Cells (2017) 6:4. 10.3390/CELLS6010004
57.
KingNMPPerrinJ. Ethical Issues in Stem Cell Research and Therapy. Stem Cel Res Ther (2014) 5:85. 10.1186/SCRT474
58.
LedererCWSantamaN. Neural Stem Cells: Mechanisms of Fate Specification and Nuclear Reprogramming in Regenerative Medicine. Biotechnol J (2008) 3:1521–38. 10.1002/BIOT.200800193
59.
ZamborskyRKilianMCsobonyeiovaMDanisovicL. Regenerative Medicine in Orthopaedics and Trauma: Challenges, Regulation and Ethical Issues. Ortop Traumatol Rehabil (2018) 20:173–80. 10.5604/01.3001.0012.0764
60.
TakashimaKInoueYTashiroSMutoK. Lessons for Reviewing Clinical Trials Using Induced Pluripotent Stem Cells: Examining the Case of a First-In-Human Trial for Age-Related Macular Degeneration. Regen Med (2018) 13:123–8. 10.2217/rme-2017-0130
61.
SievertKDAmendBStenzlA. Tissue Engineering for the Lower Urinary Tract: A Review of a State of the Art Approach. Eur Urol (2007) 52:1580–9. 10.1016/J.EURURO.2007.08.051
62.
HaraASatoDSaharaY. New Governmental Regulatory System for Stem Cell-Based Therapies in Japan. Ther Innov Regul Sci (2014) 48:681–8. 10.1177/2168479014526877
63.
KimJHKurtzAYuanBZZengFLomaxGLoringJFet alReport of the International Stem Cell Banking Initiative Workshop Activity: Current Hurdles and Progress in Seed-Stock Banking of Human Pluripotent Stem Cells. Stem Cell Transl Med (2017) 6:1956–62. 10.1002/SCTM.17-0144
64.
FearsRAkutsuHAlentajan-AletaLTCaicedoACampos de CarvalhoACČolićMet alInclusivity and Diversity: Integrating International Perspectives on Stem Cell Challenges and Potential. Stem Cel Rep (2021) 16:1847–52. 10.1016/J.STEMCR.2021.07.003
65.
HildebrandtM. Horses for Courses: an Approach to the Qualification of Clinical Trial Sites and Investigators in ATMPs. Drug Discov Today (2020) 25:265–8. 10.1016/J.DRUDIS.2019.10.003
66.
de WindtTSNiemansburgSLVonkLAvan DeldenJMRoesKCBDhertWJAet alEthics in Musculoskeletal Regenerative Medicine; Guidance in Choosing the Appropriate Comparator in Clinical Trials. Osteoarthritis Cartilage (2019) 27:34–40. 10.1016/j.joca.2018.08.022
67.
LowenthalJSugarmanJ. Ethics and Policy Issues for Stem Cell Research and Pulmonary Medicine. Chest (2015) 147:824–34. 10.1378/CHEST.14-1696
68.
OerlemansAJMFeitzWFJVan LeeuwenEDekkersWJM. Regenerative Urology Clinical Trials: An Ethical Assessment of Road Blocks and Solutions. Tissue Eng Part B Rev (2013) 19:41–7. 10.1089/TEN.TEB.2012.0136
69.
BakerHBMcQuillingJPKingNMP. Ethical Considerations in Tissue Engineering Research: Case Studies in Translation. Methods (2017) 99:135–44. 10.1016/J.YMETH.2015.08.010
70.
LeeTLLysaghtT. Conditional Approvals for Autologous Stem Cell-Based Interventions: Conflicting Norms and Institutional Legitimacy. Perspect Biol Med (2018) 61:59–75. 10.1353/PBM.2018.0027
71.
OerlemansAJMVan Den BergPPVan LeeuwenEDekkersWJM. Ethical Issues Regarding the Donation and Source of Cells for Tissue Engineering: a European Focus Group Study. Tissue Eng Part B Rev (2011) 17:229–34. 10.1089/TEN.TEB.2010.0683
72.
OerlemansAJMvan HoekMECvan LeeuwenEvan der BurgSDekkersWJM. Towards a Richer Debate on Tissue Engineering: a Consideration on the Basis of NEST-Ethics. Sci Eng Ethics (2013) 19:963–81. 10.1007/S11948-012-9419-Y
73.
GoulaAGkiokaVMichalopoulosEKatsimpoulasMNoutsiasMSarriEFet alAdvanced Therapy Medicinal Products Challenges and Perspectives in Regenerative Medicine. J Clin Med Res (2020) 12:780–6. 10.14740/JOCMR3964
74.
HaakeKAckermannMLachmannN. Concise Review: Towards the Clinical Translation of Induced Pluripotent Stem Cell-Derived Blood Cells—Ready for Take-Off. Stem Cell Transl Med (2019) 8:332–9. 10.1002/SCTM.18-0134
75.
LomaxGPShepardKA. Return of Results in Translational iPS Cell Research: Considerations for Donor Informed Consent. Stem Cel Res Ther (2013) 4:6. 10.1186/SCRT154
76.
LowenthalJLipnickSRaoMHullSC. Specimen Collection for Induced Pluripotent Stem Cell Research: Harmonizing the Approach to Informed Consent. Stem Cell Transl Med (2012) 1:409–21. 10.5966/sctm.2012-0029
77.
NiemansburgSLVan DeldenJJMÖnerFCDhertWJABredenoordAL. Ethical Implications of Regenerative Medicine in Orthopedics: An Empirical Study with Surgeons and Scientists in the Field. Spine J (2014) 14:1029–35. 10.1016/j.spinee.2013.10.016
78.
ChanS. Research Translation and Emerging Health Technologies: Synthetic Biology and beyond. Health Care Anal (2018) 26:310–25. 10.1007/s10728-016-0334-2
79.
NiemansburgSLHabetsMGJLDhertWJADelden Jjm vanBredenoordAL. Participant Selection for Preventive Regenerative Medicine Trials: Ethical Challenges of Selecting Individuals at Risk. J Med Ethics (2015) 41:914–6. 10.1136/MEDETHICS-2014-102625
80.
GenskeAEngel-GlatterS. Rethinking Risk Assessment for Emerging Technology First-In-Human Trials. Med Health Care Philos (2016) 19:125–39. 10.1007/s11019-015-9660-7
81.
TaylorDACaplanALMacchiariniP. Ethics of Bioengineering Organs and Tissues. Expert Opin Biol Ther (2014) 14:879–82. 10.1517/14712598.2014.915308
82.
AfsharLAghayanHRSadighiJArjmandBHashemiSMBasiriMet alEthics of Research on Stem Cells and Regenerative Medicine: Ethical Guidelines in the Islamic Republic of Iran. Stem Cel Res Ther (2020) 11:396. 10.1186/s13287-020-01916-z
83.
TrommelmansLSellingJDierickxK. An Exploratory Survey on the Views of European Tissue Engineers Concerning the Ethical Issues of Tissue Engineering Research. Tissue Eng Part B Rev (2009) 15:241–7. 10.1089/TEN.TEB.2008.0647
84.
SugarmanJBredenoordAL. Real-time Ethics Engagement in Biomedical Research: Ethics from Bench to Bedside. EMBO Rep (2020) 21:e49919–4. 10.15252/embr.201949919
85.
SeokJYoonSRyuCHKimSKRyuJJungYS. A Personalized 3D-Printed Model for Obtaining Informed Consent Process for Thyroid Surgery: A Randomized Clinical Study Using a Deep Learning Approach with Mesh-type 3D Modeling. J Pers Med (2021) 574. 10.3390/JPM11060574
86.
DavisonAC. Brave Pioneers or Clinical Cowboys?Cell Stem Cell (2010) 6:504–5. 10.1016/j.stem.2010.05.011
87.
AtticoESceberrasVPellegriniG. Approaches for Effective Clinical Application of Stem Cell Transplantation. Curr Transpl Rep (2018) 5:244–50. 10.1007/s40472-018-0202-0
88.
ApatoffMBLSengilloJDWhiteECBakhoumMFBassukAGMahajanVBet alAutologous Stem Cell Therapy for Inherited and Acquired Retinal Disease. Regen Med (2018) 13:89–96. 10.2217/rme-2017-0089
89.
LuLArbitHMHerrickJLSegovisSGMaranAYaszemskiMJ. Tissue Engineered Constructs: Perspectives on Clinical Translation. Ann Biomed Eng (2015) 43:796–804. 10.1007/S10439-015-1280-0
90.
PatuzzoSGoracciGGasperiniLCilibertiR. 3D Bioprinting Technology: Scientific Aspects and Ethical Issues. Sci Eng Ethics (2018) 24:335–48. 10.1007/S11948-017-9918-Y
91.
NagamuraF. The Importance of Recruiting a Diverse Population for Stem Cell Clinical Trials. Curr Stem Cel Rep (2016) 2:321–7. 10.1007/s40778-016-0062-4
92.
LomaxGPTorresAMillanMT. Regulated, Reliable, and Reputable: Protect Patients with Uniform Standards for Stem Cell Treatments. Stem Cell Transl Med (2020) 9:547–53. 10.1002/SCTM.19-0377
93.
ShinehaRInoueYIkkaTKishimotoAYashiroY. A Comparative Analysis of Attitudes on Communication toward Stem Cell Research and Regenerative Medicine between the Public and the Scientific Community. Stem Cell Transl Med (2018) 7:251. 10.1002/SCTM.17-0184
94.
NagpalAHillierSMiltonAGHamilton-BruceMAKoblarSA. PERSPECTIVES: Stroke Survivors’ Views on the Design of an Early-phase Cell Therapy Trial for Patients with Chronic Ischaemic Stroke. Health Expect (2019) 22:1069–77. 10.1111/hex.12932
95.
ZocchiMLVindigniVPaganiAPirroOContiGSbarbatiAet alRegulatory, Ethical, and Technical Considerations on Regenerative Technologies and Adipose-Derived Mesenchymal Stem Cells. Eur J Plast Surg (2019) 426(42):531–48. 10.1007/S00238-019-01571-5
96.
MasudaKLotzJC. New Challenges for Intervertebral Disc Treatment Using Regenerative Medicine. Tissue Eng Part B Rev (2010) 16:147–58. 10.1089/TEN.TEB.2009.0451
97.
LimMLJungebluthPAjalloueianFFriedrichLHGilevichIGrinnemoKHet alWhole Organ and Tissue Reconstruction in Thoracic Regenerative Surgery. Mayo Clin Proc (2013) 88:1151–66. 10.1016/J.MAYOCP.2013.03.011
98.
SmithCMartin-LillieCHiganoJDTurnerLPhuSArthursJet alChallenging Misinformation and Engaging Patients: Characterizing a Regenerative Medicine Consult Service. Regen Med (2020) 15:1427–40. 10.2217/rme-2020-0018
99.
HugK. Understanding Voluntariness of Consent in First-In-Human Cell Therapy Trials. Regen Med (2020) 15:1647–60. 10.2217/RME-2019-0126
100.
DavisNFCunnaneEMMulvihillJJQuinlanMRBoltonDMWalshMTet alThe Role of Stem Cells for Reconstructing the Lower Urinary Tracts. Curr Stem Cel Res Ther (2018) 13:458–65. 10.2174/1574888x13666180426113907
101.
MaekawaTKimuraSKasaiY. Development of Novel Advanced Cell and Gene Therapy and GMP-Controlled Cell Processing. Jpn Med Assoc J (2005) 48:81–4.
102.
SniecinskiISeghatchianJ. Emerging Stem Cell Based Strategies for Treatment of Childhood Diseases. Transfus Apher Sci (2018) 57:311–5. 10.1016/J.TRANSCI.2018.05.011
103.
LehrmanS. Virus Treatment Questioned after Gene Therapy Death. Nature (1999) 401:517–8. 10.1038/43977
104.
WatermanADDewMADavisCLMcCabeMWainrightJLForlandCLet alLiving-donor Follow-Up Attitudes and Practices in U.S. Kidney and Liver Donor Programs. Transplantation (2013) 95:883–8. 10.1097/TP.0B013E31828279FD
105.
HendersonMLThomasAGShafferAMassieABLuoXHolscherCMet alThe National Landscape of Living Kidney Donor Follow-Up in the United States. Am J Transpl (2017) 17:3131–40. 10.1111/AJT.14356
106.
KeshvaniNFeurerIDRumbaughEDreherAZavalaEStanleyMet alEvaluating the Impact of Performance Improvement Initiatives on Transplant Center Reporting Compliance and Patient Follow-Up after Living Kidney Donation. Am J Transpl (2015) 15:2126–35. 10.1111/ajt.13265
107.
KahrassHBossertSSchürmannCStrechD. Details of Risk–Benefit Communication in Informed Consent Documents for Phase I/II Trials. Clin Trials (2021) 18:71–80. 10.1177/1740774520971770
108.
BenjaminySKowalSPMacDonaldIMBubelaT. Communicating the Promise for Ocular Gene Therapies: Challenges and Recommendations. Am J Ophthalmol (2015) 160:408–15. 10.1016/j.ajo.2015.05.026
109.
HendersonGEDavisAMKingNMPEasterMMZimmerCRRothschildBBet alUncertain Benefit: Investigators’ Views and Communications in Early Phase Gene Transfer Trials. Mol Ther (2004) 10:225–31. 10.1016/J.YMTHE.2004.05.013
110.
KimmelmanJPalmour N. Therapeutic Optimism in the Consent Forms of Phase 1 Gene Transfer Trials: an Empirical Analysis. J Med Ethics (2005) 31:209–14. 10.1136/jme.2003.006247
111.
BeckSBernerAMBignellGBondMCallananMJChervovaOet alPersonal Genome Project UK (PGP-UK): A Research and Citizen Science Hybrid Project in Support of Personalized Medicine. BMC Med Genomics (2018) 108. 10.1186/s12920-018-0423-1
112.
GordonEJDaudACaicedoJCCameronKAJayCFryerJet alInformed Consent and Decision-Making about Adult-To-Adult Living Donor Liver Transplantation: a Systematic Review of Empirical Research. Transplantation (2011) 92:1285–96. 10.1097/TP.0B013E31823817D5
113.
SpoonEQWKortramKIsmailSYNieboerDD’anconaFCHChristiaansMHLet alLiving Kidney Donor Knowledge of provided Information and Informed Consent: The PRINCE Study. J Clin Med (2022) 11:698. 10.3390/jcm11030698
Summary
Keywords
ethics, regenerative medicine, bioengineering, research ethics, first-in-human clinical trials, bio-artificial organs, clinical trials, early-phase clinical trials
Citation
de Jongh D, Massey EK, Cronin AJ, Schermer MHN, Bunnik EM and the VANGUARD Consortium (2022) Early-Phase Clinical Trials of Bio-Artificial Organ Technology: A Systematic Review of Ethical Issues. Transpl Int 35:10751. doi: 10.3389/ti.2022.10751
Received
08 July 2022
Accepted
07 October 2022
Published
31 October 2022
Volume
35 - 2022
Updates
Copyright
© 2022 de Jongh, Massey, Cronin, Schermer, Bunnik and the VANGUARD Consortium.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dide de Jongh, d.dejongh.1@erasmusmc.nl
A full list of the members of the “VANGUARD consortium” can be found in “VANGUARD Consortium Partners”
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.