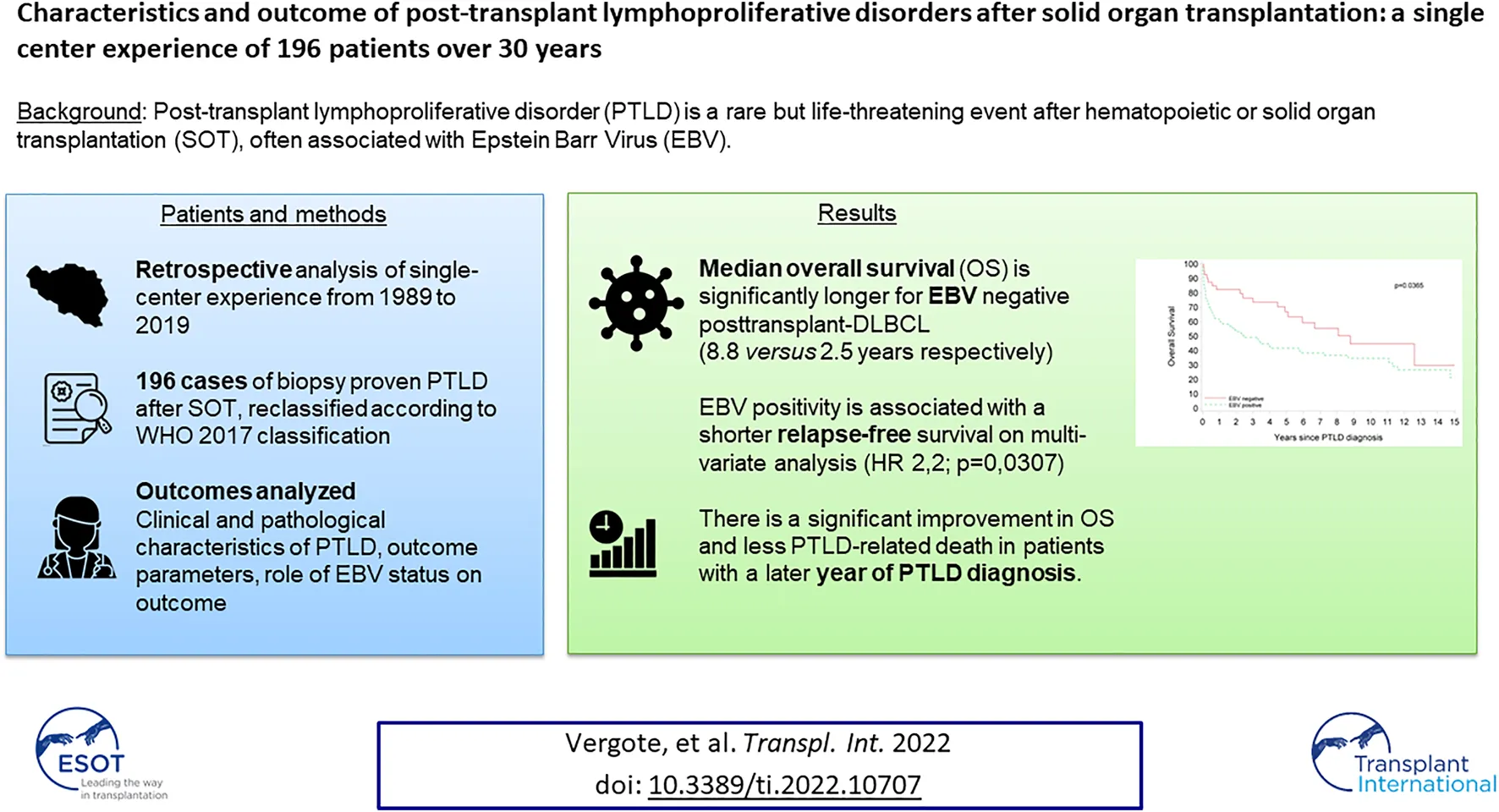
Abstract
Post-transplant lymphoproliferative disorder (PTLD) is a rare but life-threatening complication after transplantation. In this retrospective, monocentric study we aimed to collect real life data regarding PTLD and determine the role of Epstein Barr Virus (EBV) status and year of diagnosis on prognosis. We identified 196 biopsy-proven PTLD after solid organ transplantation (SOT) diagnosed at the University Hospitals Leuven (Belgium) from 1989 to 2019. EBV status was positive in 61% of PTLD. The median overall survival (OS) was 5.7 years (95% CI: 2.99–11.1). Although EBV positivity was not significantly correlated with OS in multivariate analyses (HR: 1.44 (95% CI: 0.93–2.24); p = 0.10), subgroup analysis showed a significantly better median OS for EBV negative post-transplant diffuse large B-cell lymphoma (DLBCL) compared to EBV positive post-transplant DLBCL (8.8 versus 2.5 years respectively; p = 0.0365). There was a significant relation between year of PTLD diagnosis and OS: the more recent the PTLD diagnosis, the lower the risk for death (adjusted HR: 0.962 (95% CI: 0.931–0.933); p = 0.017). In conclusion, the prognosis of PTLD after SOT has improved in the past decades. Our analysis shows a significant relation between EBV status and OS in post-transplant DLBCL.
Introduction
Post-transplant lymphoproliferative disorders (PTLD) are a heterogeneous group of lymphoid neoplasms following solid organ transplantation (SOT) and hematopoietic stem cell transplantation (HSCT)(1,2). The cumulative incidence of PTLD is estimated at 1% after 5 years and 2.1% after 10 years in adult kidney (-pancreas) transplant recipients (3). The risk of developing PTLD depends on the type of organ transplanted and incidence density (i.e. incidence adjusted for time under immunosuppression) ranges from 1.58 per 1000 person-years (kidney), up to 2.24 (heart), 2.44 (liver) and 5.72 (lung) (4-6). The pathogenesis is complex, but two major contributing factors are recognized. Firstly, most cases (60-70%) are associated with infection with the oncogenic Epstein-Barr Virus (EBV) (7-9). Secondly, there is a diminished T-cell immune surveillance due to the iatrogenic immunosuppression in transplant recipients (4,5). The pathogenesis of EBV negative (EBV(-)) PTLD remains the subject of debate. Several hypotheses have been suggested such as the “hit-and-run” hypothesis (where EBV initiates lymphomagenesis, but is then cleared), the role of other viruses (Cytomegalovirus, Human Herpes Virus 8...), chronic antigenic stimulation and long-term immunosuppression(4,10).
The World Health Organization (WHO) 2017 classification recognizes four types of PTLD (1): Non-destructive lesions (2); Polymorphic PTLD (3); Monomorphic PTLD (including B-, T- and natural killer (NK)-cell types) (4); classic Hodgkin lymphoma PTLD (2). Historically, PTLD represents a serious and potentially life-threatening complication of transplantation, with a reported survival rate of 60% after 5 years in kidney transplant recipients (3,5).
Several single- and multicenter reports have previously been published (11-14). However, they are often hampered by their heterogenous population and limited numbers of patients. Large reports from national registries often contain many more cases, but lack detailed information (3,15). Furthermore, significant progress has been made in the past 30 years including a new WHO 2017 classification and improvement of treatment by the introduction of monoclonal antibodies against CD20. Although genomic and transcriptional studies have recently demonstrated that EBV positive (EBV(+)) and EBV(−) PTLD carry different genomic signatures, the role of EBV status on prognosis remains unclear and patients are essentially treated the same (16,17).
Here, we describe one of the largest retrospective single-center series of PTLD after SOT, comprising 196 patients with histologically proven PTLD over a 30 year period. We previously reported our experience in PTLD, including 122 cases after SOT and 18 after HSCT (18). The goal of this report was to analyze a larger group of PTLD after SOT with longer follow-up. We aimed to investigate the role of EBV status on prognosis on a large real life cohort of PTLD and to find out whether prognosis has improved in the past decades.
Materials and Methods
Data Collection
This study was performed at the University Hospitals Leuven (Belgium), a tertiary hospital where all categories of SOT are performed. We reviewed all cases of histologically confirmed untreated PTLD after SOT, diagnosed in our hospital between January 1st, 1989 to December 31st, 2019 (Figure 1). Cases of indolent non-Hodgkin lymphoma (NHL) histology (n = 2), with the exception of EBV(+) marginal zone lymphoma, were excluded from analysis, since they are not included in the current WHO 2017 PTLD classification (2). All cases were reviewed by one expert hematopathologist (TT). Patient-related clinical characteristics included gender, age at PTLD diagnosis, Eastern Cooperative Oncology Group Performance status (ECOG PS) and pretransplant EBV serology. Transplant-related characteristics included type of organ transplanted, time from transplantation to PTLD diagnosis and type of immunosuppression. PTLD-related characteristics included: Ann Arbor Stage (19) at diagnosis, presence of B-symptoms, biochemical data (hemoglobin, creatinine clearance, albumin, lactate dehydrogenase (LDH)), number of extranodal sites involved, graft involvement and involvement of different organ systems, (sub)type of PTLD according to the WHO 2017 classification (2), presence of CD20 expression and EBV in the biopsy, year of PTLD diagnosis and data on treatment and outcome variables. If available, data on EBV polymerase chain reaction (PCR) in peripheral blood were collected. This study was approved by the Ethics Committee of University Hospitals/Catholic University Leuven (Ref: S62704 and S55498) and was conducted according to the ethical principles of the World Medical Association Declaration of Helsinki (20).
FIGURE 1
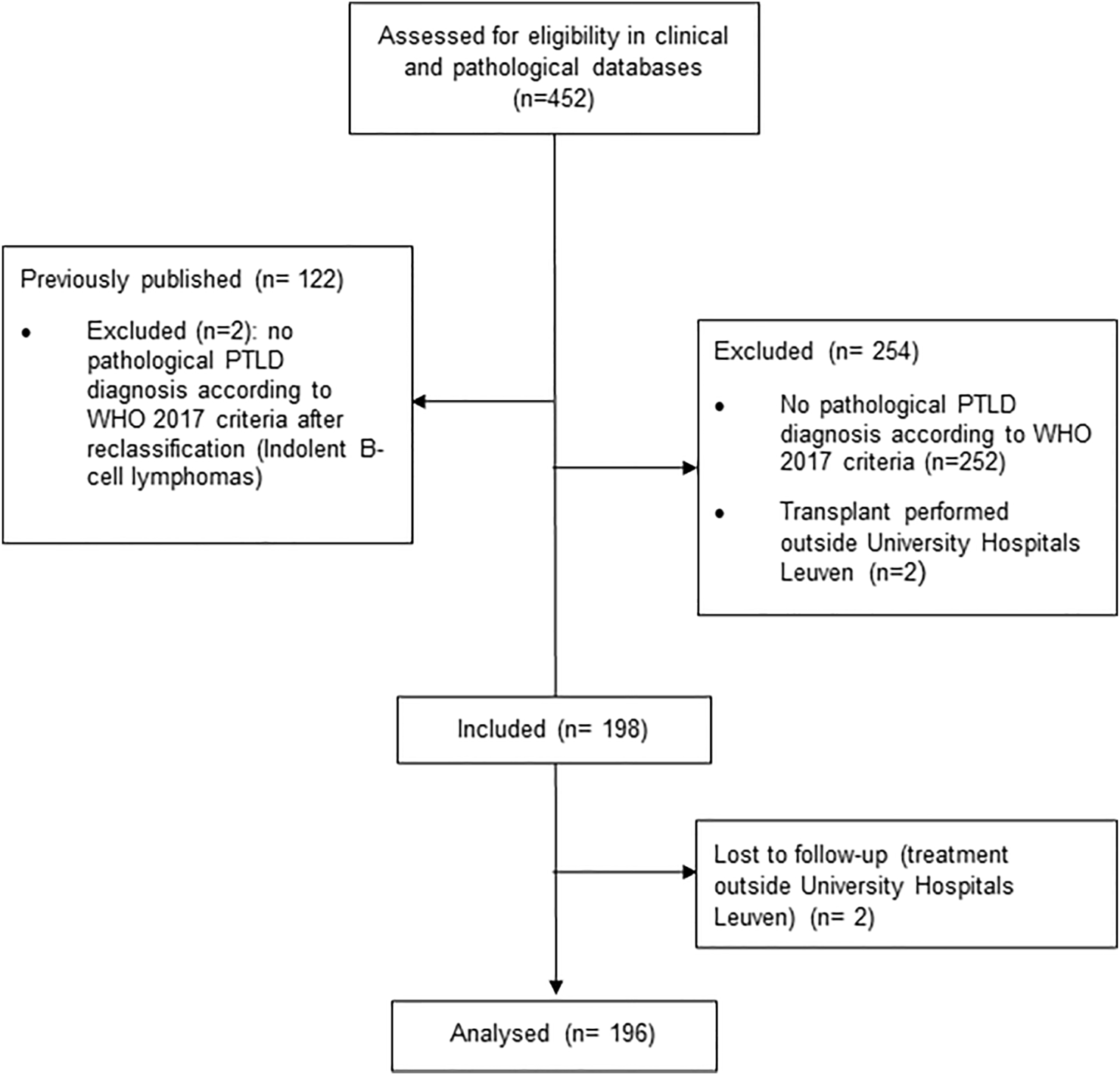
CONSORT flow diagram.
Definitions
All PTLD cases required histopathological confirmation to be included. EBV in the biopsy was determined by Epstein-Barr-encoded RNA (EBER) in situ hybridization (ISH). Post-transplantation EBV surveillance was not performed systematically in our hospital. International Prognostic Index (IPI) was calculated as previously described (21).
For statistical reasons, patients with combined SOT were pooled according to the transplantation requiring the highest degree of immunosuppression. Patients with combined kidney-pancreas (n = 6) and kidney-liver (n = 3) were classified as kidney transplantation. Patients with combined heart-lung (n = 3) and liver-lung (n = 1) transplant were classified as lung transplantation. Lastly patients with combined heart-kidney (n = 1) and combined liver-pancreas (n = 1) were classified as heart and liver transplantation, respectively.
Response assessment after treatment was performed according to the Lugano criteria (22) and was based upon chart review of the available imaging protocols of computed tomography (CT) or positron emission tomography with 18F-fluorodeoxyglucose combined with CT ([18F]FDG-PET/CT), if possible including Deauville criteria (23). Timing of response assessment depended on the predefined initial treatment, e.g., after four cycles of rituximab for risk-stratified sequential treatment (24) and after four cycles of rituximab and four cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicine, vincristine and prednisolone) for sequential treatment(25). OS was calculated as time from biopsy-proven diagnosis till the date of death. Death was considered to be PTLD-related in any case where death was caused by either disease progression or a treatment-related complication. Relapse-free survival (RFS) was defined as time from biopsy-proven diagnosis till the date of relapse or death.
Statistical Methods
A description of the statistical methodology can be found in the Supplementary Material.
Results
Epidemiology
Between January 1st, 1989 and December 31st, 2019, 7497 patients received a SOT at our center. We identified 196 histologically confirmed cases of PTLD after SOT in the same period. Seventeen patients were pediatric (<18 years) and 179 were adults at time of PTLD diagnosis. There was a male predominance in the adult transplant recipients (58.3%), as well in the PTLD cohort (65.3%). We observed 19 (first decade: 1990–1999), 86 (second decade: 2000–2009) and 89 cases (third decade: 2010–2019), showing a significant increase from the first to the second decade (p < 0.0001) and stable number from the second to the third decade (p = 0.97) (Figure 2).
FIGURE 2
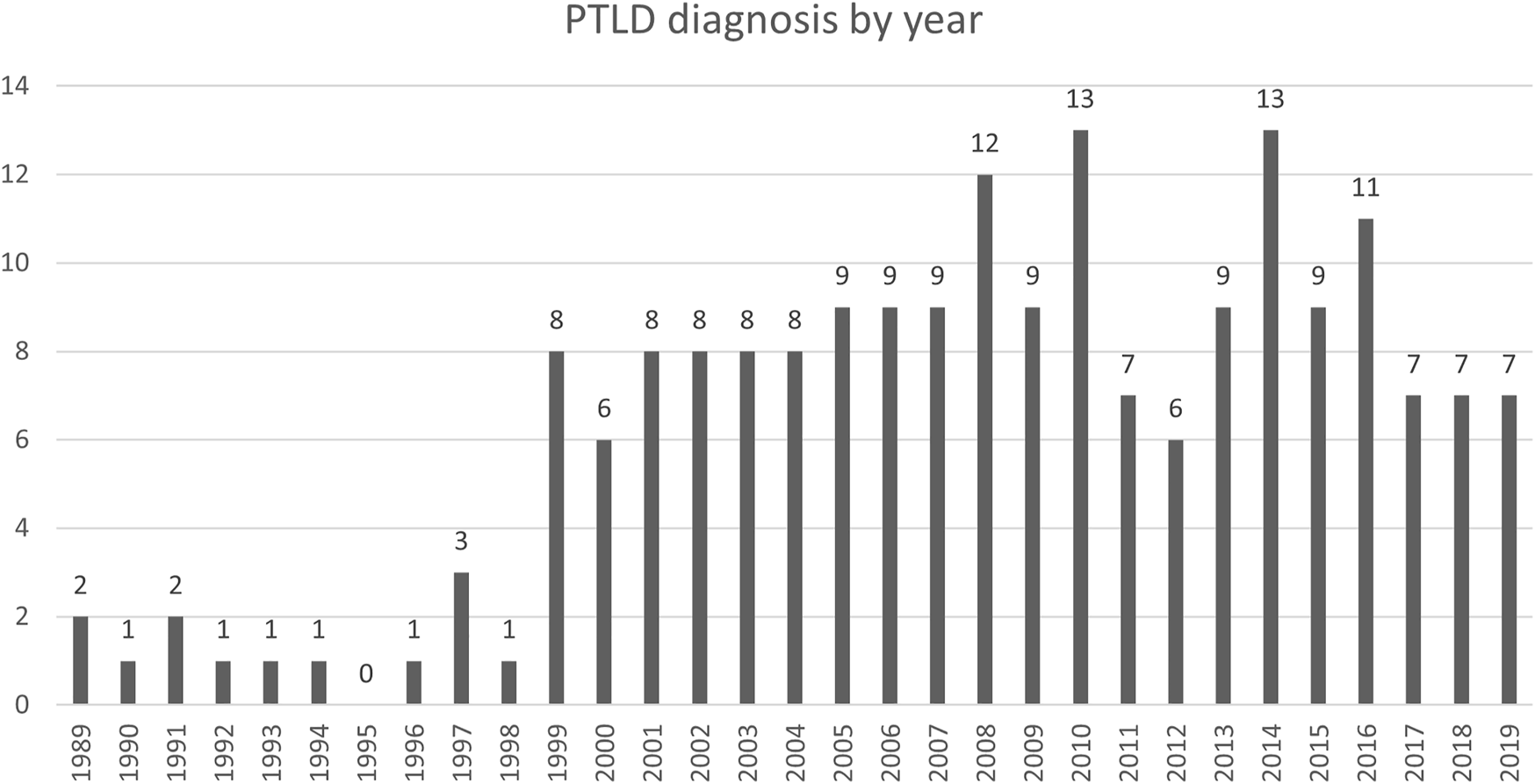
Absolute number of PTLD diagnosis by year.
Patient-, Transplant- and PTLD- Related Characteristics
Baseline patient characteristics are summarized in Table 1. The most common transplanted organs were kidney (n = 76; 38.8%), lung (n = 46; 23.5%), heart (n = 30; 15.3%) and liver (n = 29; 14.8%). EBV serology before transplantation was negative in 39/96 (40.6%) and positive in 57/96 patients (59.4%) with available data.
TABLE 1
| Years or number (%) | ||
|---|---|---|
| Age at diagnosis (years) | Median (IQR) | 54.1 (35.2-64.5) |
| Range | 3.5-83 | |
| Age at diagnosis | ≤60 years | 122 (62.2) |
| >60 years | 74 (37.8) | |
| Gender | Male | 128 (65.3) |
| Female | 68 (34.7) | |
| ECOG PS | 0-1 | 138 (70.8) |
| 2 | 42 (21.5) | |
| 3-4 | 15 (7.7) | |
| Unknown | 1 | |
| Transplanted organ | Heart | 30 (15.3) |
| Kidney | 76 (38.8) | |
| Lung | 46 (23.5) | |
| Liver | 29 (14.8) | |
| Heart-Kidney | 1 (0.5) | |
| Kidney-Pancreas | 6 (3.1) | |
| Kidney-Liver | 3 (1.5) | |
| Heart-Lung | 3 (1.5) | |
| Liver-Lung | 1 (0.5) | |
| Liver-Pancreas | 1 (0.5) | |
| IS at diagnosis | CNI | 189 (96.4) |
| AM | 152 (77.6) | |
| CS | 134 (68.4) | |
| Sirolimus | 1 (0.5) | |
| CNI + AM + CS | 99 (55.5) | |
| Induction | 94 (48%) | |
| Time between transplantation and PTLD (years) | Median (IQR) | 4.3 (1.0-10.6) |
| Range | 0.2-28 | |
| Pathology | Non-destructive | 16 (8.2) |
| Polymorphic | 11 (5.6) | |
| Monomorphic | 162 (82.7) | |
| Hodgkin | 6 (3.1) | |
| EBV(+) mucocutaneous ulcer | 1 (0.5) | |
| EBV ISH at diagnosis | Negative | 67 (26) |
| Positive | 119 (64) | |
| Unknown | 10 | |
| CD 20 expression at diagnosis | Negative | 31 (16.1) |
| Positive | 155 (80.3) | |
| Partially positive | 7 (3.6) | |
| Unknown | 3 | |
| Ann Arbor stage | I | 31 (17.4) |
| II | 20 (10.3) | |
| III | 23 (11.8) | |
| IV | 118 (60.5) | |
| Unknown | 1 | |
| B-symptoms | No | 133 (67.9) |
| Yes | 63 (32.1) | |
| Number of extranodal sites | None | 38 (19.5) |
| 1 | 67 (34.4) | |
| >1 | 90 (46.2) | |
| Unknown | 1 | |
| IPI | Low risk | 61 (31.6) |
| Low intermediate risk | 44 (22.8) | |
| High intermediate risk | 54 (27) | |
| High risk | 34 (17.6) | |
| Unknown | 3 | |
| Extranodal involvement | Graft involvement | 39 (19.9) |
| PCNSL | 12 (6.1) | |
| CNS involvement, not primary | 2 (1) | |
| Bone marrow involvement | 22 (14.6) | |
| GI involvement | 60 (30.8) | |
| Pulmonary involvement | 51 (28) | |
| Serum levels at diagnosis | Hemoglobin <10 g/dl | 70 (35.7) |
| LDH elevated | 87 (44.4) | |
| Albumin <35 g/L | 87 (29) | |
| Creatinine ≥1.5 mg/dl | 83 (42.3) |
Baseline patient characteristics of 196 patients with biopsy-proven PTLD after SOT.
AM, antimetabolites; CNI, calcineurin inhibitors; CNS, central nervous system; CS, corticosteroids; ECOG PS, eastern cooperative oncology group performance status; EBV(+), Epstein-Barr virus positive; EBV ISH, Epstein-Barr virus in situ hybridization; GI, gastro-intestinal; IPI, international prognostic index; IS, immunosuppressive therapy; LDH, lactate dehydrogenase; IQR, interquartile range; PCNSL, primary central nervous system lymphoma, PTLD, Post-transplant lymphoproliferative disorder.
The most frequent histological type was monomorphic PTLD (n = 162, 82.7%), with DLBCL being the most frequent subtype (n = 121; 74.7%). The cell of origin according to the Hans algorithm (28) was germinal center B-cell like (GCB) in 19/56 (33.9%) and non-germinal center B-cell like (non-GCB) in 37/56 (66.1%) in the posttransplant DLBCL-type (PT-DLBCL). These data were missing in 65 patients. The majority of GCB DLBCL were EBV(-) (94.7%), whereas the majority of non-GCB DLBCL were EBV(+) (78.4%).
Other subtypes of monomorphic PTLD included plasmablastic lymphoma (n = 14; 8.6%), plasma cell malignancies (n = 3; 1.9%), T-cell NHL (T-NHL) (n = 8; 4.9%), Burkitt lymphoma (n = 8; 4.9%), Burkitt-like lymphoma with 11q aberration (n = 4; 2.5%), EBV(+) marginal zone lymphoma (n = 1; 0.6%) and B-NHL, undefined (n = 3; 1.9%).
Median time from transplant to PTLD diagnosis was 4.3 years (IQR: 1.0-10.6), with many cases occurring late (>1 year after transplantation) (n = 147; 75.0%) or very late (>10 years after transplantation) (30) (n = 46; 23.6%).
Treatment and Outcome
Treatment at first line consisted of reduction of immune suppression (RIS) (n = 178; 90.8%), rituximab (n = 120; 61.2%), chemotherapy (n = 41; 20.9%), surgery (n = 24; 12.2%), radiotherapy (n = 13; 6.6%), high-dose corticosteroids (n = 12; 6.1%) or antiviral treatment (n = 5; 2.6%). Ten patients (5.1%) received no treatment (7 supportive care, 3 spontaneous remissions of non-destructive PTLD). Eighty-three patients (42.3%) were treated with rituximab alone. Twenty-five patients were treated with RIS alone (12.8%) and 13 of these achieved a complete response (CR) (52%), of whom only 2 patients relapsed later on. Seventy-six patients (38.7%) in the cohort did not receive rituximab, mainly due to CD20 negativity (n = 26), treatment with RIS alone (n = 25), treatment in the pre-rituximab era (before 2000) (n = 19) and no treatment received (n = 10).
Response to first-line treatment was CR in 99 patients (50.5%), partial response in 25 (12.8%), stable disease in 9 (4.6%) and progressive disease in 40 patients (20.4%). Sixteen patients (8.2%) died during first line treatment and seven had received supportive care alone. Fifty-nine patients (30.1%) were refractory to first line treatment and 19 patients (9.7%) relapsed after achieving a CR. First line treatment according to histological subtypes is summarized in Figure 3.
FIGURE 3
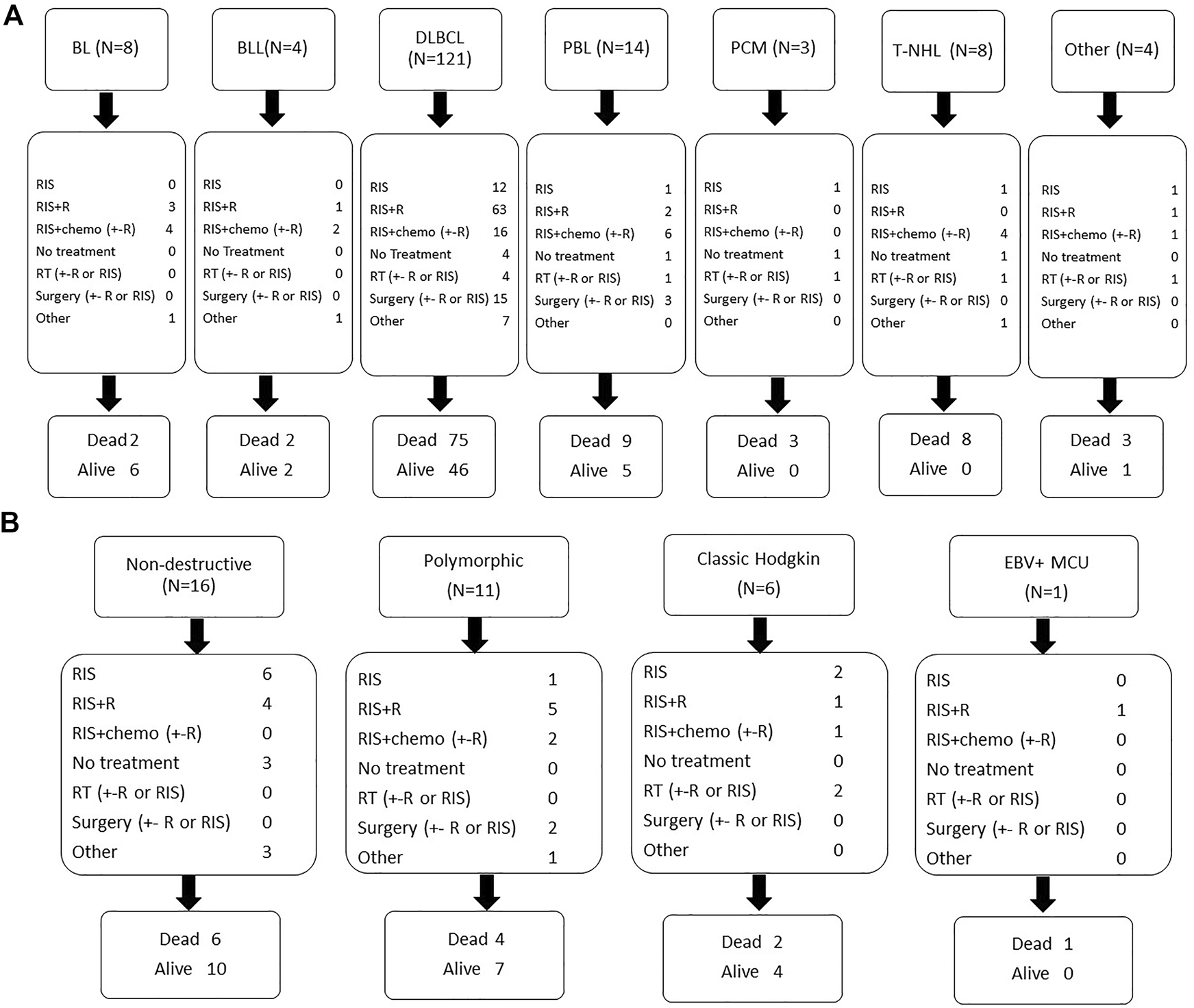
First line treatment and outcome according to histological subtype monomorphic PTLD (A) and other histological subtypes (B). Legend: BL: Burkitt lymphoma; BLL(11q): Burkitt-like lymphoma with 11q aberration; B-NHL,u: B-cell non-Hodgkin’s lymphoma, undefined; DLBCL: diffuse large B-cell lymphoma; MCU: mucocutaneous ulcus; MZL: marginal zone lymphoma; PBL: plasmablastic lymphoma; PCM: plasma cell malignancy; PTLD: post-transplant lymphoproliferative disorder; RIS: reduction of immunosuppression; R: rituximab; RT: radiotherapy; T-NHL: T-cell non-Hodgkin’s lymphoma.
After a median follow-up of 4.0 years (IQR: 0.5-8.8) after PTLD diagnosis, 115 patients (58.7%) died. Death was considered PTLD related in 46.1% (n = 53), non-PTLD related in 47% (n = 54) and unknown in 7% (n = 8). Other causes of death included mainly infections and other malignancies (Table 2). The cumulative incidence of PTLD-related death versus non-PTLD-related death is visualized in Figure 4.
TABLE 2
| Number (N = 115) | % | |
|---|---|---|
| PTLD progression | 47 | 40.9 |
| Infections | 21 | 18.3 |
| Other malignancies | 11 | 9.6 |
| CVA | 2 | 1.7 |
| Bleeding | 3 | 2.6 |
| Cardiac events | 7 | 6.1 |
| MOF | 5 | 4.3 |
| Other | 8 | 7 |
| Unknown | 11 | 9.6 |
Reasons of death.
CVA, cerebrovascular accident; MOF, multiple organ failure; PTLD, post-transplant lymphoproliferative disorder.
FIGURE 4
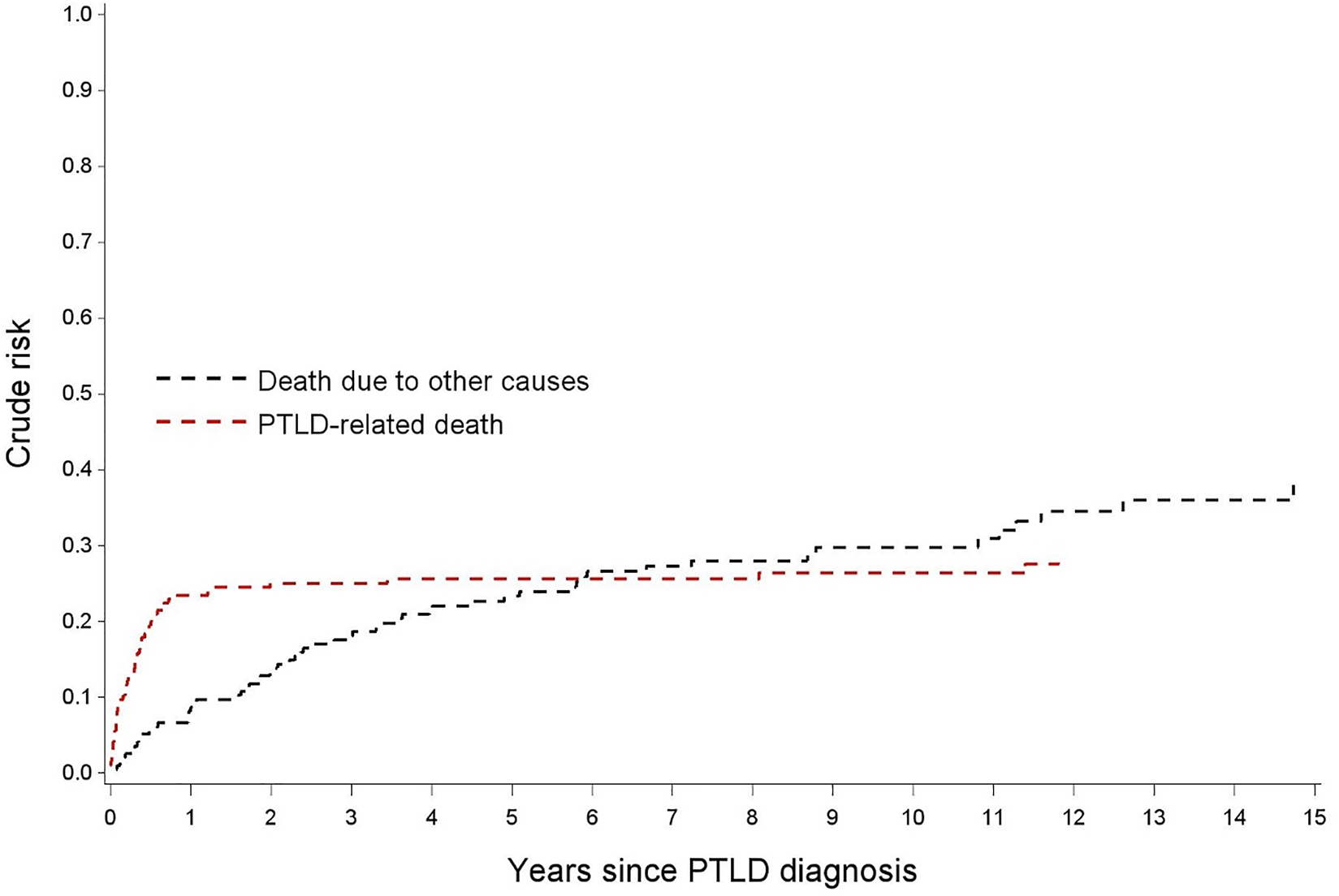
Nelson-Aalen estimates for the cumulative incidence of PTLD-related death and for death due to other causes.
OS rates after PTLD for the whole cohort were 67.8, 61.7 and 51.2% after 1, 2 and 5 years, respectively. The median OS was 5.7 years (95% CI 2.99–11.07). In the 99 patients achieving a CR after first line treatment, RFS was 87.9, 77.8 and 62.0% after 1, 2 and 5 years, respectively (Figure 5).
FIGURE 5
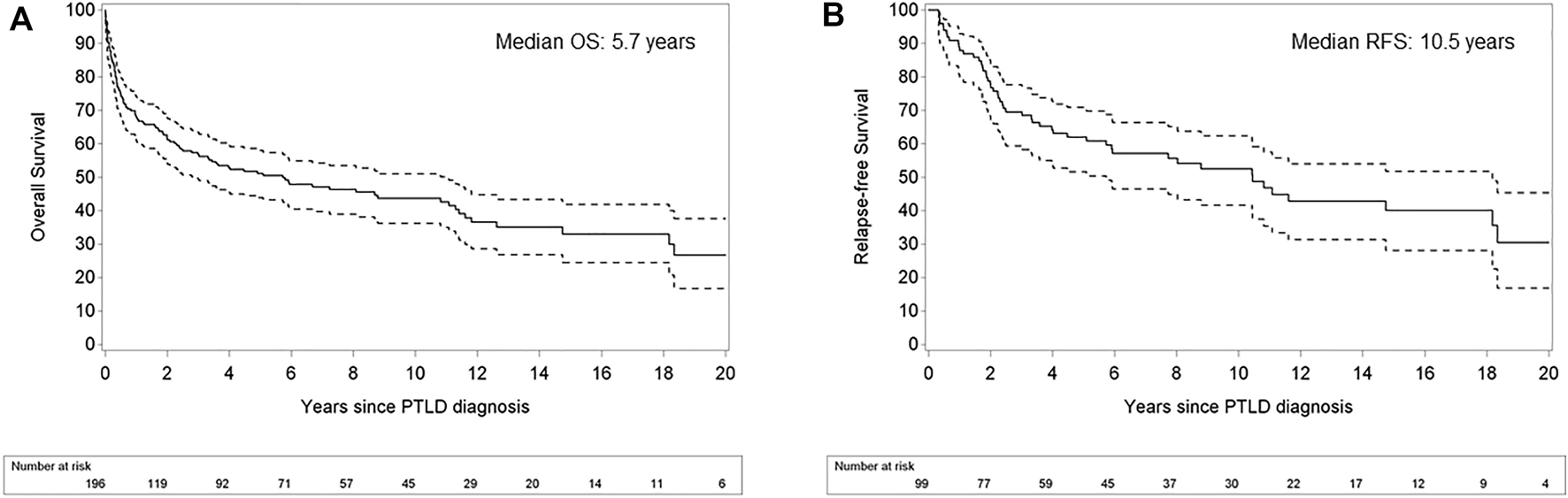
Kaplan Meier plots for overall survival in patients with post-transplant lymphoproliferative disorder (A) and relapse-free survival after achievement of complete response (B). Legend: Dashed lines refer to the pointwise 95% confidence interval. OS: overall survival; RFS: relapse-free survival.
Uni- and Multivariate Analysis of Factors Influencing Outcome
Factors influencing CR rate in first line, PTLD-related death, OS, and RFS are summarized in Tables 3–6, respectively.
TABLE 3
| Univariate | Multivariatea | p-value | ||
|---|---|---|---|---|
| Variable | Odds Ratio (95% CI) | p-value | Odds Ratio (95% CI) | |
| Age at transplantation (years) | 0.984 (0.970;1.000) | 0.0430 | ||
| Age at PTLD diagnosis (years) | 0.980 (0.966;0.995) | 0.0096 | 0.989 (0.973;1.006) | 0.2045 |
| Age at PTLD diagnosis >60 years | 0.437 (0.242;0.790) | 0.0061 | ||
| EBV ISH positivity | 1.351 (0.741;2.463) | 0.3257 | 1.454 (0.758;2.788) | 0.2596 |
| Female gender | 0.809 (0.449;1.459) | 0.4818 | ||
| Transplanted organ | 0.5318 | |||
| Kidneyb | 0.647 (0.280;1.496) | 0.3082 | ||
| Liverb | 0.483 (0.174;1.342) | 0.1627 | ||
| Lungb | 0.583 (0.234;1.450) | 0.2458 | ||
| Graft organ involved | 1.267 (0.622;2.581) | 0.5145 | ||
| Monomorphic histology | 0.423 (0.193;0.924) | 0.0309 | ||
| CNS involvement | 0.9992 | |||
| PCNSL | 0.978 (0.304;3.147) | 0.9706 | ||
| Secondary | 0.978 (0.060;15.879) | 0.9877 | ||
| Extranodal disease | 0.534 (0.257;1.107) | 0.0916 | ||
| Elevated LDH | 0.305 (0.169;0.550) | <0.0001 | ||
| CD20 positivity | 0.3238 | |||
| Positive | 1.779 (0.808;3.913) | 0.1524 | ||
| Partially positive | 1.187 (0.225;6.260) | 0.8394 | ||
| Hypoalbuminemia | 0.672 (0.378;1.194) | 0.1752 | ||
| IPI score | 0.657 (0.528;0.817) | 0.0002 | 0.659 (0.522;0.833) | 0.0005 |
| ECOG PS | 0.0017 | |||
| ECOG 2c | 0.560 (0.279;1.126) | 0.1036 | ||
| ECOG 3/4c | 0.115 (0.025;0.529) | 0.0055 | ||
| Ann Arbor stage III-IV | 0.451 (0.236;0.864) | 0.0163 | ||
| Year of PTLD diagnosis | 0.961 (0.922;1.003) | 0.0661 | 0.955 (0.913;0.999) | 0.0436 |
Univariate and multivariate analysis (Logistic regressions) of factors influencing complete response rate.
EBV status was added into the multivariate model obtained after backward selection
Compared to heart transplant.
Compared to ECOG PS 0-1.
95% CI, 95% confidence interval; PTLD, post-transplant lymphoproliferative disorder; ECOG PS, eastern cooperative oncology group performance status; EBV ISH, Epstein-Barr Virus in situ hybridization; LDH, lactate dehydrogenase; IPI, international prognostic index; PCNSL, primary central nervous system lymphoma.
TABLE 4
| Univariate | Multivariatea | |||
|---|---|---|---|---|
| Variable | Hazard Ratio (95% CI) | p-value | Hazard Ratio (95% CI) | p-value |
| Age at transplantation (years) | 1.029 (1.012;1.045) | 0.0007 | ||
| Age at PTLD diagnosis (years) | 1.030 (1.013;1.047) | 0.0006 | ||
| Age at PTLD diagnosis >60 years | 2.798 (1.617;4.842) | 0.0002 | ||
| EBV ISH positivity | 1.670 (0.884;3.157) | 0.1143 | 1.155 (0.591;2.255) | 0.6730 |
| Female gender | 0.860 (0.478;1.549) | 0.6161 | ||
| Transplanted organ | 0.9320 | 0.0162 | ||
| Kidneyb | 0.855 (0.392;1.867) | 0.6945 | 1.124 (0.498;2.534) | 0.7787 |
| Liverb | 1.068 (0.424;2.694) | 0.8883 | 2.477 (0.924;6.639) | 0.0714 |
| Lungb | 1.013 (0.438;2.342) | 0.9762 | 4.074 (1.456;11.399) | 0.0075 |
| Graft organ involved | 0.834 (0.407;1.710) | 0.6207 | 0.322 (0.135;0.772) | 0.0111 |
| Monomorphic histology | 3.365 (1.211;9.352) | 0.0200 | ||
| CNS involvement | 0.2785 | |||
| PCNSL | 2.021 (0.802;5.094) | 0.1359 | ||
| Secondary | 2.820 (0.388;20.494) | 0.3055 | ||
| Extranodal disease | 2.782 (1.105;7.003) | 0.0298 | ||
| Elevated LDH | 5.274 (2.799;9.937) | <0.0001 | ||
| CD20 positivity | 0.3068 | |||
| Positive | 0.587 (0.307;1.122) | 0.1073 | ||
| Partially positive | 0.708 (0.158;3.166) | 0.6510 | ||
| Hypoalbuminemia | 3.566 (1.939;6.561) | <0.0001 | 2.398 (1.256;4.577) | 0.0080 |
| IPI score | 1.935 (1.562;2.399) | <0.0001 | 1.978 (1.554;2.519) | <0.0001 |
| ECOG PS | <0.0001 | |||
| ECOG 2c | 2.196 (1.163;4.148) | 0.0153 | ||
| ECOG 3/4c | 9.207 (4.581;18.504) | <0.0001 | ||
| Ann Arbor stage III-IV | 4.306 (1.711;10.836) | 0.0019 | ||
| Year of PTLD diagnosis | 0.951 (0.916;0.988) | 0.0100 | 0.937 (0.897;0.979) | 0.0038 |
Univariate and multivariate analysis (Cox regressions) of patients characteristics related to PTLD related death.
EBV status was added into the multivariate model obtained after backward selection.
Compared to heart transplant.
Compared to ECOG PS 0-1.
Abbreviations: 95% CI: 95% confidence interval; PTLD: post-transplant lymphoproliferative disorder; ECOG PS: eastern cooperative oncology group performance status; EBV ISH: Epstein-Barr Virus in situ hybridization; LDH: lactate dehydrogenase; IPI: international prognostic index; PCNSL: primary central nervous system lymphoma.
TABLE 5
| Univariate | Multivariatea | |||
|---|---|---|---|---|
| Variable | Hazard Ratio (95% CI) | p-value | Hazard Ratio (95% CI) | p-value |
| Age at transplantation (years) | 1.040 (1.028;1.052) | <0.0001 | ||
| Age at PTLD diagnosis (years) | 1.041 (1.028;1.053) | <0.0001 | 1.035 (1.022;1.049) | <0.0001 |
| Age at PTLD diagnosis >60 years | 3.389 (2.321;4.948) | <0.0001 | ||
| EBV ISH positivity | 1.475 (0.975;2.232) | 0.0659 | 1.441 (0.928;2.239) | 0.1037 |
| Female gender | 0.998 (0.670;1.484) | 0.9903 | 1.290 (0.837;1.986) | 0.2483 |
| Transplanted organ | 0.6780 | 0.0161 | ||
| Kidneyb | 0.854 (0.506;1.442) | 0.5553 | 1.197 (0.685;2.093) | 0.5282 |
| Liverb | 1.186 (0.637;2.209) | 0.5912 | 2.291 (1.181;4.445) | 0.0142 |
| Lungb | 0.972 (0.546;1.729) | 0.9226 | 2.091 (1.084;4.033) | 0.0278 |
| Graft organ involved | 1.091 (0.690;1.725) | 0.7088 | ||
| Monomorphic histology | 2.468 (1.381;4.409) | 0.0023 | ||
| CNS involvement | 0.6513 | |||
| PCNSL | 1.422 (0.691;2.925) | 0.3393 | ||
| Secondary | 1.224 (0.171;8.792) | 0.8405 | ||
| Extranodal disease | 1.879 (1.121;3.151) | 0.0167 | ||
| Elevated LDH | 2.922 (1.997;4.275) | <0.0001 | ||
| CD20 positivity | 0.2877 | |||
| Positive | 0.751 (0.466;1.210) | 0.2393 | ||
| Partially positive | 0.394 (0.092;1.683) | 0.2085 | ||
| Hypoalbuminemia | 2.758 (1.873;4.062) | <0.0001 | 1.956 (1.289;2.967) | 0.0016 |
| IPI score | 1.612 (1.399;1.856) | <0.0001 | 1.346 (1.154;1.570) | 0.0002 |
| ECOG PS | <0.0001 | |||
| ECOG 2c | 1.715 (1.127;2.608) | 0.0117 | ||
| ECOG 3/4c | 4.815 (2.636;8.795) | <0.0001 | ||
| Ann Arbor stage III-IV | 1.902 (1.211;2.989) | 0.0053 | ||
| Year of PTLD diagnosis | 0.968 (0.942;0.995) | 0.0196 | 0.962 (0.931;0.993) | 0.0172 |
Univariate and multivariate analysis (Cox regressions) of patient characteristics related to overall survival.
Year of PTLD diagnosis was added into the multivariate model obtained after backward selection
Compared to heart transplant.
Compared to ECOG PS 0-1.
95% CI, 95% confidence interval; PTLD, post-transplant lymphoproliferative disorder; ECOG PS, eastern cooperative oncology group performance status; EBV ISH, Epstein-Barr Virus in situ hybridization; LDH, lactate dehydrogenase; IPI, international prognostic index; PCNSL, primary central nervous system lymphoma.
TABLE 6
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variable | Hazard Ratio (95% CI) | p-value | Hazard Ratio (95% CI) | p-value |
| Age at transplantation (years) | 1.039 (1.022;1.057) | <0.0001 | ||
| Age at PTLD diagnosis (years) | 1.047 (1.029;1.066) | <0.0001 | 1.054 (1.034;1.074) | <0.0001 |
| Age at PTLD diagnosis >60 years | 3.576 (2.047;6.247) | <0.0001 | ||
| EBV ISH positivity | 1.261 (0.678;2.346) | 0.4647 | 2.183 (1.075;4.432) | 0.0307 |
| Female gender | 0.989 (0.541;1.810) | 0.9726 | ||
| Transplanted organ | 0.2155 | 0.0103 | ||
| Kidneya | 1.000 (0.486;2.056) | 0.9993 | 1.585 (0.734;3.424) | 0.2414 |
| Livera | 1.782 (0.736;4.313) | 0.2003 | 5.244 (1.904;14.446) | 0.0013 |
| Lunga | 0.645 (0.266;1.561) | 0.3306 | 1.398 (0.510;3.831) | 0.5153 |
| Graft organ involved | 0.903 (0.453;1.801) | 0.7726 | ||
| Monomorphic histology | 1.519 (0.759;3.041) | 0.2376 | ||
| CNS involvement | 0.5534 | |||
| PCNSL | 0.862 (0.267;2.782) | 0.8044 | ||
| Secondary | ND | 0.9884 | ||
| Extranodal disease | 1.352 (0.694;2.631) | 0.3751 | ||
| Elevated LDH | 2.200 (1.248;3.879) | 0.0064 | ||
| CD20 positivity | 0.1585 | |||
| Positive | 1.317 (0.522;3.319) | 0.5598 | ||
| Partially positive | ND | 0.9873 | ||
| Hypoalbuminemia | 2.371 (1.354;4.152) | 0.0025 | ||
| IPI score | 1.417 (1.146;1.751) | 0.0013 | ||
| ECOG PS | 0.0417 | |||
| ECOG 2b | 1.924 (1.054;3.515) | 0.0332 | ||
| ECOG 3/4b | ND | 0.9897 | ||
| Ann Arbor stage III-IV | 1.143 (0.644;2.027) | 0.6479 | ||
| Year of PTLD diagnosis | 0.969 (0.931;1.009) | 0.1280 | 0.975 (0.929;1.024) | 0.3078 |
Univariate and multivariate analysis (Cox regressions) of patient characteristics related to relapse free survival.
Compared to heart transplant.
Compared to ECOG PS 0-1.
95% CI, 95% confidence interval; PTLD, post-transplant lymphoproliferative disorder; ECOG PS, eastern cooperative oncology group performance status; EBV ISH, Epstein-Barr Virus in situ hybridization; LDH, lactate dehydrogenase; IPI, international prognostic index; PCNSL, primary central nervous system lymphoma; ND, not determined.
Higher age at transplantation, higher age at PTLD diagnosis, monomorphic histology, elevated LDH, higher IPI, poor ECOG PS (3,4) and advanced Ann Arbor stage were statistically significant adverse factors for CR rate in univariate analysis. In multivariate analysis a higher IPI score and a higher year of PTLD diagnosis were related to a lower CR rate.
Higher age at transplantation, higher age at PTLD diagnosis, monomorphic histology, extranodal disease, elevated LDH, hypoalbuminemia, higher IPI, poor ECOG PS (>1), advanced Ann Arbor stage are significantly related to PTLD-related death in univariate analysis using Cox regression models. Similar results were obtained using Fine and Gray models (results not shown). In the multivariate model hypoalbuminemia, higher IPI-score, graft organ involvement and type of transplanted organ (lung versus heart) were retained as factors associated with worse outcome. A higher year of PTLD diagnosis was associated with less PTLD-related death in uni- and multivariate analysis.
Higher age at transplantation, higher age at PTLD diagnosis, monomorphic histology, extranodal disease, elevated LDH, hypoalbuminemia, a higher IPI-score, ECOG >1, advanced Ann Arbor stage were significantly adverse factors for OS in univariate analysis. In the multivariate model the IPI-score, higher age at diagnosis, hypoalbuminemia, type of transplanted organ (liver and lung transplantation compared to heart) were retained as poor prognostic factors. Higher year of PTLD diagnosis was associated with a longer OS in uni- and multivariate analysis.
Higher age at transplantation, higher age at PTLD diagnosis, elevated LDH, hypoalbuminemia, higher IPI, poor ECOG PS were significant adverse factors for RFS in univariate analysis. In the multivariate model higher age at diagnosis, EBV positivity and liver transplantation were considered prognostic factors worse RFS.
In summary, IPI was an important prognostic factor, significantly related to all four outcomes in univariate analysis and to CR rate, PTLD-related death and OS in multivariate analysis. Furthermore, hypoalbuminemia was a poor prognostic factor for PTLD-related death, OS and RFS in univariate analysis and for PTLD-related death and OS in multivariate analysis. Type of transplanted organ was significantly related to RFS, PTLD-related death and OS in multivariate analysis.
EBV
EBV status, as determined by EBV ISH at the time of diagnosis, was positive in 119 of the 186 evaluable cases (64%). The number of positive EBV was higher in early (<1 year after transplantation) PTLD cases (n = 43; 89.6%) compared to late PTLD (n = 76; 55.1%). EBV positivity was associated with type of grafted organ (highest in lung, lowest in liver transplantation) and organ-involvement in the whole PTLD cohort. There was no association between EBV status and other clinical factors (Table 7).
TABLE 7
| EBV negative (N = 67) | EBV positive (N = 119) | p | |
|---|---|---|---|
| Male Gender | 45 (67.2%) | 76 (63.2%) | 0.75 |
| Transplanted organ | |||
| Heart | 8 (12%) | 21 (17.7%) | 0.02 |
| Liver | 14 (20.1%) | 13 (10.9%) | |
| Lung | 11 (16.4%) | 39 (32.8%) | |
| Kidney | 34 (50.8%) | 46 (38.7%) | |
| Graft organ involvement | 7 (10.5%) | 29 (24.4%) | 0.021 |
| Monomorphic PTLD | 54 (80.6%) | 98 (82.4%) | 0.84 |
| CNS involvement | 2 (3%) | 12 (10.1%) | 0.27 |
| CD20 positive | 52 (78.8%) | 96 (82.1%) | 0.27 |
| Decreased albumin | 26 (38.5%) | 57 (50%) | 0.16 |
| Median age at PTLD (years) | 56 | 52.6 | 0.18 |
| Median IPI | 2 | 2 | 0.37 |
| Initial therapy | 0.090 | ||
| RIS alone | 5 (7.5%) | 17 (14.3%) | |
| RIS + other (excluding R/chemo) | 5 (7.5%) | 13 (10.9%) | |
| RIS + R | 40 (59.7%) | 54 (45.4%) | |
| RIS + chemo | 10 (14.9%) | 9 (7.6%) | |
| RIS + R + chemo | 3 (4.5%) | 15 (12.6%) | |
| Other | 4 (6.0%) | 11 (9.2%) |
Comparison of baseline characteristics in relation to EBV status.
chemo, chemotherapy; EBV, Epstein-Barr Virus; IPI, international prognostic index; CNS, central nervous system; PTLD, Post-transplant lymphoproliferative disorder, R, rituximab; RIS, reduction of immunosuppression.
EBV status at diagnosis was not significantly related to OS in univariate (hazard ratio (HR): 1.48 (95% CI: 0.975–2.232); p = 0.066) and multivariate analysis (HR: 1.44 (95% CI: 0.928–2.239); p = 0.10). However, there was a trend towards worse OS for the EBV(+) PTLD. There was also no significant relation between EBV status and CR (odds ratio (OR): 1.35 (95% CI: 0.741–2.463); p = 0.33) and PTLD-related death (HR: 1.67 (95% CI: 0.884–3.157); p = 0.11) in univariate, nor in multivariate analysis ((OR: 1.45 (95% CI: 0.758–2.788); p = 0.26) and (HR: 1.15 (0.591–2.255); p = 0.67), respectively). However, there was a relation between EBV status and RFS in the multivariate model, where EBV positivity was a risk factor (HR: 2.29 (95% CI: 1.146–4.595); p = 0.02) (Figure 6).
FIGURE 6
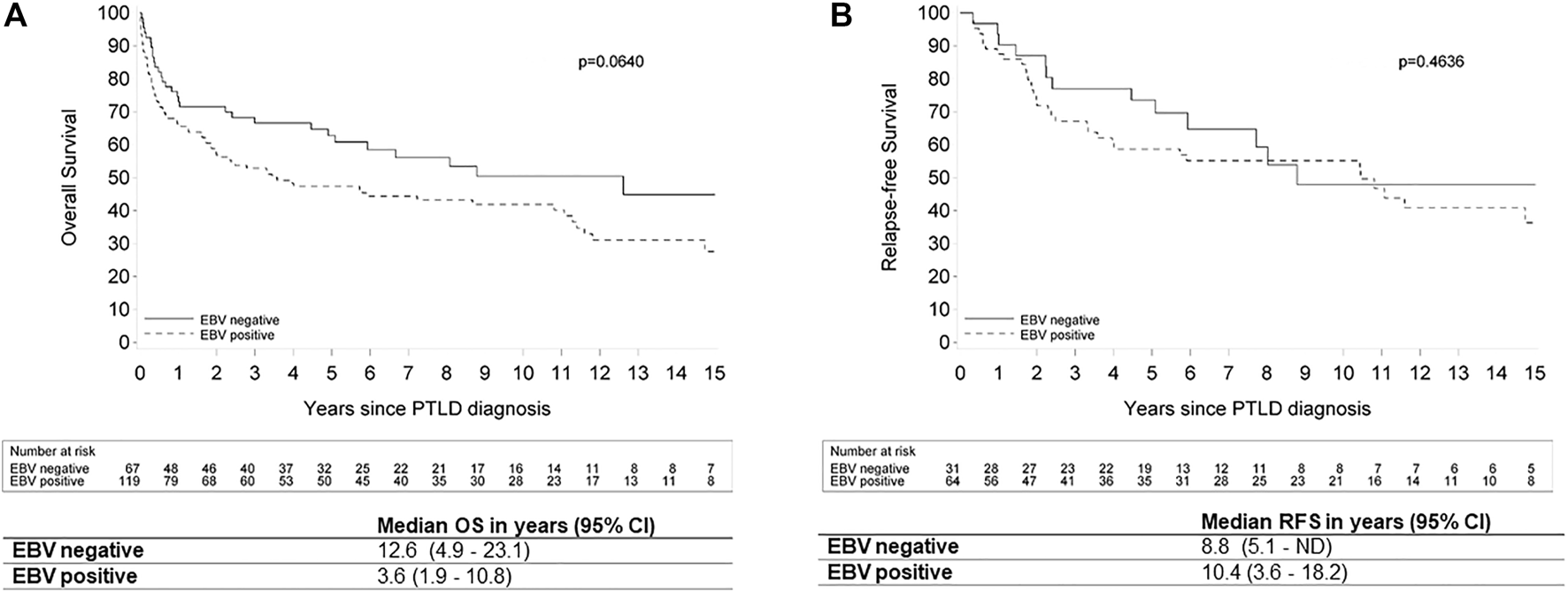
Kaplan Meier plots for (A) overall survival and (B) relapse-free survival by EBV status in patients with post-transplant lymphoproliferative disorder. Legend: EBV: Epstein-Barr Virus; OS: overall survival; ND: not determined; PTLD: post-transplant lymphoproliferative disorder; RFS: relapse-free survival; 95% CI: 95% confidence interval.
A subgroup analysis of all cases of PT-DLBCL showed that EBV ISH was positive in 77 of the 117 evaluable cases (65.8%). Furthermore, we saw a significantly better median OS for EBV(−) PT-DLBCL compared to EBV(+) PT-DLBCL (8.8 versus 2.5 years respectively; p = 0.0365).There was no significant relation between EBV status and RFS in this group (p = 0.8852) (Figure 7).
FIGURE 7
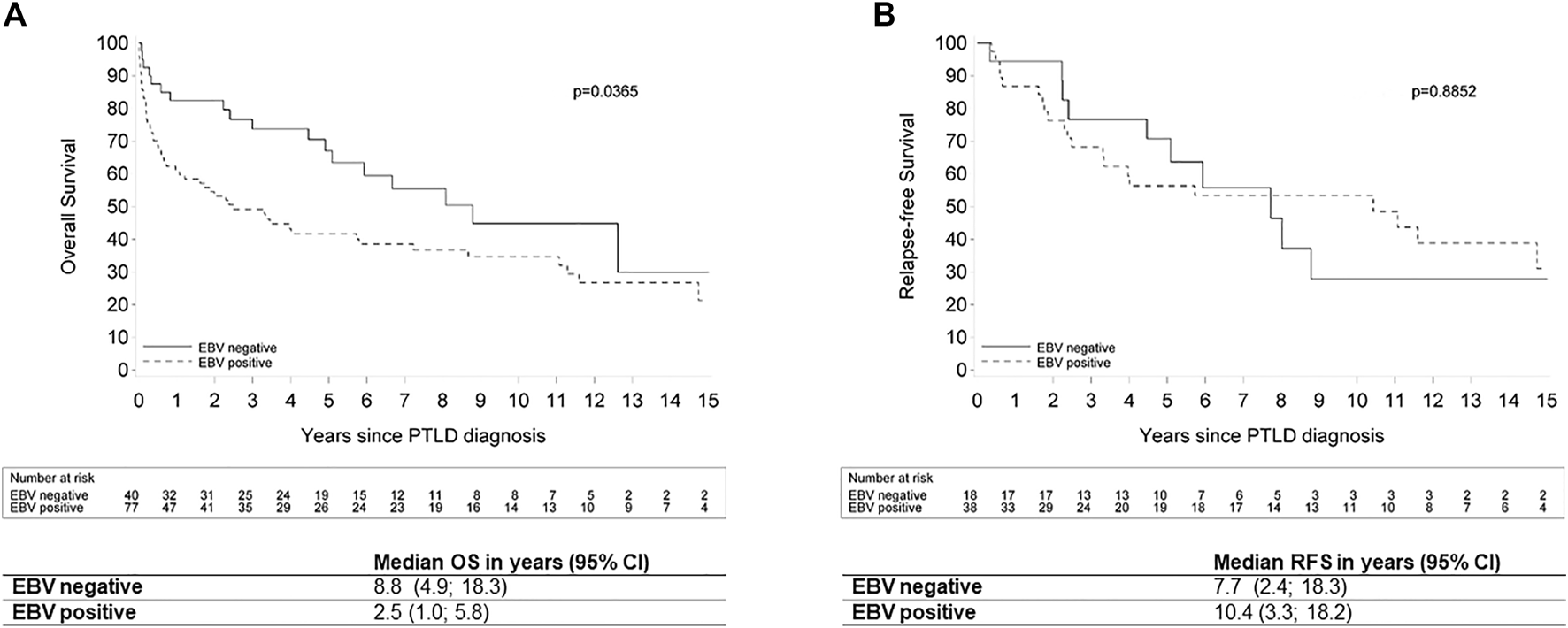
Kaplan Meier plots for (A) overall survival and (B) relapse-free survival by EBV status in patients with post-transplant diffuse large B-cell lymphoma. Legend: EBV: Epstein-Barr Virus; OS: overall survival; ND: not determined; PTLD: post-transplant lymphoproliferative disorder; RFS: relapse-free survival; 95% CI: 95% confidence interval.
EBV PCR in blood was positive in 107 of 142 evaluable cases (75.4%). However, EBV PCR was more often positive in EBV ISH positive cases (91% of 89 evaluable cases), than in EBV ISH negative cases (52% of 50 evaluable cases). This resulted in a sensitivity of 91% and specificity of 48% for EBV PCR in predicting EBV ISH positivity.
Era of PTLD Diagnosis
There was a significant relation between year of PTLD diagnosis and OS, that persisted after correction for differences in patient mix in the multivariate model: the more recent the PTLD diagnosis, the lower the risk for death (HR: 0.97 (95% CI: 0.942–0.995; p = 0.0196) and adjusted HR: 0.962 (95%CI: 0.931–0.933; p = 0.017) in the Cox multivariate model.
A similar result was obtained for PTLD-related death: HR: 0.951 (95% CI: 0.916–0.988; p = 0.01) and adjusted HR: 0.935 (95% CI: 0.896–0.977; p = 0.0024) for the year of PTLD diagnosis in the multivariate Cox model. A similar conclusion was obtained in the Fine and Gray model (results not shown). However, there was no evidence of a significant relation between year of PTLD diagnosis and CR or RFS.
Discussion
We investigated the baseline characteristics, outcome, role of EBV and era of PTLD diagnosis on outcome in a large cohort of biopsy-proven PTLD after SOT. We noticed a high proportion of late (>1 year after transplantation: n = 147; 75%) and very late PTLD (>10 years after transplantation; n = 46; 23.6%) in our analysis. Several reports have recently suggested that the incidence of early EBV(+) PTLD is decreasing (3, 11, 31). In our cohort the proportion of early PTLD was stable over the first, second and third decade (21.1%, 20.9% and 28.1% respectively). Other groups have suggested that a decrease in early PTLD might be a result of pre-emptive EBV viral load monitoring. However, this has not been confirmed in a recent report (11) and this strategy has not been implemented in our series. Other factors influencing the incidence of early PTLD include the changes in immunosuppressive regimens and decreased use of T-cell depleting induction therapy (32-35).
The median age at diagnosis in the current study was 54.1 years, which is comparable to previous reports (26,36-38). PTLD is typically diagnosed at an advanced stage (72.3%) with extra-nodal involvement (80.5%). Gastro-intestinal involvement (30.8%) was the most frequent extra-nodal site involved. We observed 12 cases of PCNSL (6.1%) in our cohort, less than the previously reported 10% of all PTLDs (39-41). However, it is difficult to draw definite conclusions regarding the incidence of PCNSL in PTLD due to the small group size. By far the most commonly observed histologic type of PTLD in our study was monomorphic PTLD (82.7%), with DLBCL as the most frequent subtype. Non-destructive and classic Hodgkin lymphoma PTLD were rare, as previously reported in the literature. Furthermore, we noted only 11 cases (5.6%) of polymorphic PTLD, which is less than previously reported (3, 26, 37, 42). A more recent report noted a similar rate, with 5.7% polymorphic PTLD in a single center analysis of 227 adult PTLD after SOT (14). Tsai et al. also reported that PTLD morphology has changed over the past 3 decades, with a gradual increase in the number of monomorphic PTLD and a steady number of polymorphic PTLD (38). This seems to be corroborated by our results.
Burkitt lymphoma type PTLD is a rare entity, with only 8 cases over 30 years in our study. However, their prognosis is relatively good as 6 patients are currently alive and still in remission after treatment with intensified immuno/chemotherapy. We encountered 4 cases of Burkitt-like lymphoma with 11q aberration, a rare entity known to be more prevalent in immunocompromised patients (43). Furthermore, we encountered 8 T-NHLs, of which 2 were classified as hepatosplenic T-cell lymphoma and 3 cases were primary cutaneous T-NHL. Prognosis was very poor in these patients with 6 of them dying within 1 year after the diagnosis. The poor prognosis of T-cell PTLD has previously been reported (29, 44-48). A more recent report by Barba et al showed that the outcome in 58 T/NK-cell PTLD after kidney transplantation was worse than in 148 T/NK-cell lymphomas in non-transplanted (49). They noted that transplant recipients received less anthracycline-based therapy, probably out of fear of complications in this fragile population. EBV(+) mucocutaneous ulcer has recently been described as an indolent entity occurring in patients with age-related or iatrogenic immunosuppression (2). It is currently classified as a separate entity (outside PTLD) in the WHO 2017 classification (2). However, it can occur in the post-transplant setting and needs to be considered in the differential diagnosis. We reclassified only one case of EBV(+) mucocuteanous ulcer in our cohort, which was originally classified as monomorphic PTLD, DLBCL type.
Most cases of PTLD are related to EBV. However, more recent reports suggest that up to 50% of PTLDs are EBV(−) (50). In our cohort EBV ISH was positive in 64% of all evaluable cases. Analysis of EBV DNA viremia showed a high sensitivity (91%), but low specificity (48%) in predicting EBV ISH status. Previous studies have shown that transplant recipients with PTLD have a higher viral load then recipients without PTLD. Furthermore, a higher or rapidly increasing viral load is associated with a higher risk of PTLD (4, 51-54). The low specificity of the EBV PCR in our series could possibly be attributed to the low cut-off value used (>2.7 log copies/ml or >2.18 log EBV IU/ml).
Genomic and transcriptional studies have recently demonstrated that EBV(+) and EBV(−) PTLD carry different genomic signatures(16,17). The genomic aberrations in EBV(−) PTLD are less complex and indistinguishable from those in immunocompetent DLBCL. This has led to the hypothesis that EBV(+) PT-DLBCL represent true PTLD and that EBV(−) PT-DLBCL could be considered as de novo lymphomas in transplant recipients (16,17). EBV(+) and EBV(-) PT-DLBCL have some different clinical characteristics. In particular, EBV(+) PT-DLBCL typically occurs early and is most often non-GCB type, whereas EBV(−) PT-DLBCL occurs later and is typically of GCB type. Furthermore, polymorphic or non-destructive lesions are usually EBV(+)(4, 16, 55). Despite these differences both groups are essentially treated with the same therapy (except EBV-specific adoptive immunotherapy). The impact of EBV status on treatment response or prognosis remains unclear (50, 56). In our cohort we found no significant relation between EBV status and CR, PTLD-related death or OS. However, we observed a significant relation between EBV status and OS in PT-DLBCL, with clinically meaningful improved survival in EBV(-) PT-DLBCL compared to EBV(+) PT-DLBCL (8.8 years versus 2.5 years, respectively). Previous reports have shown conflicting results on the relation between EBV status and OS (13, 14, 18, 25, 50, 57, 58).
As only 21 patients were treated before 2000 (when rituximab became available in Belgium), no comparison could be made regarding outcomes in the pre- and post-rituximab era. However, we investigated the impact of date of PTLD diagnosis on outco-me parameters. We observed a significant improvement in OS and a diminished PTLD-related death rate with later year of PTLD diagnosis. This relation was not found with CR and RFS. It seems that the prognosis of PTLD has improved over the past decades, although the responses to first line treatment have not. Possible explanations for this finding could be achievement of deeper responses, better supportive care and risk-stratified sequential therapy (patients not achieving CR to rituximab monotherapy can still be rescued with R-CHOP chemotherapy).
RIS remains the cornerstone of PTLD treatment. Twenty-five patients were treated with RIS alone and 13 of these achieved a CR (52%). Reported response rates to RIS have been very variable, however the largest earlier reported single-center retrospective analysis of 67 PTLDs after SOT treated with RIS alone, reported an overall response rate of 45% (37% CR) (59). Responses have been known to be higher in non-destructive lesions and in EBV(+) PTLD (4). The higher rate of responses in our cohort might reflect the higher ratio of non-destructive and polymorphic lesions. Of note, RIS may be related to subsequent onset of (chronic) rejection, for instance in lung transplant recipients, which requires increased clinical surveillance (60).
The median OS in our cohort was 5.7 years. This is less than reported in the prospective phase II PTLD-1 and PTLD-2 trials, with a median OS of 6.6 years (24,25). However, only CD20-positive PTLD were included in these PTLD-1 and 2 trials. More recent real-world data showed a 3 years OS of 65.9% in CD20-positive PTLD treated with rituximab-based therapy (61). The IPI-score remained the most important poor prognostic factor in multivariate analysis for OS, CR and PTLD-related death in the current study, in concordance with earlier reports. Hypoalbuminemia and type of organ transplanted (liver and lung) were also retained in our multivariate model as poor prognostic factors for OS.
This study is limited by its retrospective design. Treatment of PTLD has obviously changed over the past decades with the incorporation of rituximab into first line treatment of CD20-positive PTLD since the early 2000s. Furthermore, some data regarding EBV serology and EBV PCR in blood were missing, since this only came into practice in the last 2 decades. Some patients reported in the current study were also reported in a previous publication (18). However, the latter study also included PLTD after HSCT and the follow-up was shorter than in the current study. In addition, we reclassified all PTLD according to the WHO 2017 classificiation (2) and added more detailed histopathological data (such as cell of origin).
In conclusion, this retrospective analysis provides real world data on 196 biopsy-proven PTLD cases, to the best of our knowledge the second largest single-institution cohort published in the literature. The OS of our patients increased in the past decade, resulting in a median OS of 5.7 years for the whole cohort. We observed a significantly improved OS for EBV(−) PT-DLBCL compared to EBV(+) PT-DLBCL.
Statements
Data availability statement
Data concerns health-related information of the patients and therefore cannot be given away freely. If needed, the first author can be contacted to obtain the data.
Ethics statement
This study was approved by the Ethics Committee of University Hospitals/Catholic University Leuven (Ref: S62704 and S55498). Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
VV, TT, and DD participated in concept and design and drafting of the article. VV, TT, DD, and SF participated in data extraction. VV, CMD, SF, WL, BS, AU, JC, GV, RV, TT, and DD participated in critical revision of the article for intellectually important content.
Conflict of interest
VV reports consultancy fees from Beigene, BMS/Cellgene, Gilead/Kite, speaker fees from from Janssen, travel support from Amgen, Abbvie; all paid to her institution. CMD reports consultancy fees from Sirtex, PSI CRO, Terumo and Ipsen and speaker fees from Ipsen; all paid to his institution. WL reports consultancy fees from Boston-Scientific, Cook Medical, CLS Behring, Echosens, Evive Biotech, Genfit, Norgine, Abbvie, Gore and Intercept.; all paid to institution. TT reports consultancy and speaker fees from EUSApharma; all paid to his institution. TT holds a Mandate for Fundamental and Translational Research from the ‘Stichting tegen Kanker’ (2014-083 and 2019-091). DD reports grants/research support from Roche; personal fees/honoraria from Takeda, Novartis, Amgen, Atara Biotherapeutics, Incyte; all paid to his institution. DD holds a mandate for Clinical and Translational Research from “Kom op tegen Kanker” (2017/10908/2816). RV is a senior clinical research fellow of the Research Foundation Flanders (FWO). BS is a senior clinical investigator of the Research Foundation Flanders (1842919N) and received funding from the Foundation Against Cancer (Stichting tegen Kanker; C/2020/1380).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2022.10707/full#supplementary-material
Glossary
- [18F]FDG-PET/CT,
Positron emission tomography with 18F-fluorodeoxyglucose combined with computed tomography
- ATG,
anti-thymocyte globulin
- B-NHL,
B-cell non-Hodgkin lymphoma
- CI,
Confidence Interval
- CNS,
Central nervous system
- CR,
complete response
- CT,
computed tomography
- DLBCL,
diffuse large B-cell lymphoma
- EBER,
Epstein Barr-encoded RNA
- EBV,
Epstein Barr Virus
- EBV(+),
Epstein Barr Virus positive
- EBV(-),
Epstein Barr Virus negative
- EBV ISH,
Epstein Barr Virus in situ hybridization
- ECOG PS,
Eastern Cooperative Oncology Group Performance status
- GCB,
germinal center B-cell like
- GI,
gastro-intestinal
- HR,
hazard ratio
- HSCT,
hematopoietic stem cell transplantation
- IPI,
International Prognostic Index
- IQR,
interquartile range
- LDH,
lactate dehydrogenase
- NHL,
non-Hodgkin lymphoma
- OR,
Odds Ratio
- OS,
overall survival
- PCNSL,
primary central nervous system lymphoma
- PCR,
polymerase chain reaction
- PTLD,
Post-transplant lymphoproliferative disorder
- PT-DLBCL,
Post-transplant diffuse large B-cell lymphoma
- R-CHOP,
rituximab, cyclophosphamide, doxorubicine, vincristine and prednisolone
- RFS,
relapse-free survival
- RIS,
reduction of immune suppression
- SOT,
solid organ transplantation
- T-NHL,
T-cell non-Hodgkin lymphoma
- WHO,
World Health Organization
References
1.
Penn I . Cancers Complicating Organ Transplantation. N Engl J Med (1990) 323:1767–9. 10.1056/NEJM199012203232510
2.
Swerdlow SH Campo E Harris NL . WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon: IARC (2017). p. 453–62.
3.
Caillard S Lamy FX Quelen C Dantal J Lebranchu Y Lang P et al Epidemiology of Posttransplant Lymphoproliferative Disorders in Adult Kidney and Kidney Pancreas Recipients: Report of the French Registry and Analysis of Subgroups of Lymphomas. Am J Transpl (2012) 12(3):682–93. 10.1111/j.1600-6143.2011.03896.x
4.
Dierickx D Habermann TM . Post-Transplantation Lymphoproliferative Disorders in Adults. N Engl J Med (2018) 378(6):549–62. 10.1056/NEJMra1702693
5.
Dharnidharka VR . Comprehensive Review of post–organ Transplant Hematologic Cancers. Am J Transpl (2018) 18(3):537–49. 10.1111/ajt.14603
6.
Sampaio MS Cho YW Qazi Y Bunnapradist S Hutchinson IV Shah T . Posttransplant Malignancies in Solid Organ Adult Recipients: an Analysis of the U.S. National Transplant Database. Transplantation (2012) 94:990–8. 10.1097/TP.0b013e318270bc7b
7.
Allen UD Preiksaitis JK . Post-Transplant Lymphoproliferative Disorders, Epstein-Barr Virus Infection, and Disease in Solid Organ Transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin Transpl (2019) 33(9):e13652–22. 10.1111/ctr.13652
8.
Shannon-Lowe C Rickinson AB Bell AI . Epstein-barr Virus-Associated Lymphomas. Philos Trans R Soc Lond B Biol Sci (2017) 372(1732):20160271. 10.1098/rstb.2016.0271
9.
Green M Michaels MG . Epstein-barr Virus Infection and Posttransplant Lymphoproliferative Disorder. Am J Transpl (2013) 13(3):41–54. 10.1111/ajt.12004
10.
Morscio J Tousseyn T . Recent Insights in the Pathogenesis of post-transplantation Lymphoproliferative Disorders. World J Transpl (2016) 6(3):505–16. 10.5500/wjt.v6.i3.505
11.
Peters AC Akinwumi MS Cervera C Mabilangan C Ghosh S Lai R et al The Changing Epidemiology of Posttransplant Lymphoproliferative Disorder in Adult Solid Organ Transplant Recipients over 30 Years: A Single-center Experience. Transplantation (2018) 102(9):1553–62. 10.1097/TP.0000000000002146
12.
Maksten EF Vase MØ Kampmann J D’Amore F Møller MB Strandhave C et al Post-Transplant Lymphoproliferative Disorder Following Kidney Transplantation: A Population-Based Cohort Study. Transpl Int (2016) 29(4):483–93. 10.1111/tri.12744
13.
Evens AM David KA Helenowski I Nelson B Kaufman D Kircher SM et al Multicenter Analysis of 80 Solid Organ Transplantation Recipients with Post-Transplantation Lymphoproliferative Disease: Outcomes and Prognostic Factors in the Modern Era. J Clin Oncol (2010) 28:1038–46. 10.1200/JCO.2009.25.4961
14.
King RL Khurana A Mwangi R Fama A Ristow KM Maurer MJ et al Clinicopathologic Characteristics, Treatment, and Outcomes of Post-transplant Lymphoproliferative Disorders: A Single-Institution Experience Using 2017 WHO Diagnostic Criteria. Hemasphere (2021) 5(10):e640. 10.1097/HS9.0000000000000640
15.
Zaffiri L Long A Neely ML Cherikh WS Chambers DC Snyder LD . Incidence and Outcome of post-transplant Lymphoproliferative Disorders in Lung Transplant Patients: Analysis of ISHLT Registry. J Hear Lung Transpl (2020). 10.1016/j.healun.2020.06.010
16.
Morscio J Dierickx D Ferreiro JF Herreman A Van Loo P Bittoun E et al Gene Expression Profiling Reveals clear Differences between EBV-Positive and EBV-Negative Posttransplant Lymphoproliferative Disorders. Am J Transpl (2013) 13(5):1305–16. 10.1111/ajt.12196
17.
Finalet Ferreiro J Morscio J Dierickx D Vandenberghe P Gheysens O Verhoef G et al EBV-Positive and EBV-Negative Posttransplant Diffuse Large B Cell Lymphomas Have Distinct Genomic and Transcriptomic Features. Am J Transpl (2016) 16(2):414–25. Available from: https://pubmed.ncbi.nlm.nih.gov/26780579/.
18.
Dierickx D Tousseyn T Sagaert X Fieuws S Wlodarska I Morscio J et al Single-center Analysis of Biopsy-Confirmed Posttransplant Lymphoproliferative Disorder: Incidence, Clinicopathological Characteristics and Prognostic Factors. Leuk Lymphoma (2013) 54(11):2433–40. 10.3109/10428194.2013.780655
19.
Rosenberg SA . Validity of the Ann Arbor Staging Classification for the Non-hodgkin’s Lymphomas. Cancer Treat Rep (1977) 61(6):1023–7.
20.
Association WM . World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA (2013) 310(20):2191–4. 10.1001/jama.2013.281053
21.
A predictive model for aggressive non-Hodgkin’s lymphoma. A Predictive Model for Aggressive Non-hodgkin's Lymphoma. N Engl J Med (1993) 329(14):987–94. 10.1056/NEJM199309303291402
22.
Barrington SF Mikhaeel NG Kostakoglu L Meignan M Hutchings M Müeller SP et al Role of Imaging in the Staging and Response Assessment of Lymphoma: Consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol (2014) 32(27):3048–58. 10.1200/JCO.2013.53.5229
23.
Meignan M Gallamini A Haioun C Haioun C . Report on the First International Workshop on Interim-PET Scan in Lymphoma. Leuk Lymphoma (2009) 50(8):1257–60. 10.1080/10428190903040048
24.
Trappe RU Dierickx D Zimmermann H Morschhauser F Mollee P Zaucha JM et al Response to Rituximab Induction Is a Predictive Marker in B-Cell post-transplant Lymphoproliferative Disorder and Allows Successful Stratification into Rituximab or R-Chop Consolidation in an International, Prospective, Multicenter Phase II Trial. J Clin Oncol (2017) 35(5):536–43. 10.1200/JCO.2016.69.3564
25.
Trappe R Oertel S Leblond V Mollee P Sender M Reinke P et al Sequential Treatment with Rituximab Followed by CHOP Chemotherapy in Adult B-Cell post-transplant Lymphoproliferative Disorder (PTLD): The Prospective International Multicentre Phase 2 PTLD-1 Trial. Lancet Oncol (2012) 13(2):196–206. 10.1016/S1470-2045(11)70300-X
26.
Caillard S Porcher R Provot F Dantal J Choquet S Durrbach A et al Post-Transplantation Lymphoproliferative Disorder after Kidney Transplantation: Report of a Nationwide French Registry and the Development of a New Prognostic Score. J Clin Oncol (2013) 31(10):1302–9. 10.1200/JCO.2012.43.2344
27.
Engels EA Pfeiffer RM Fraumeni JF Kasiske BL Israni AK Snyder JJ et al Spectrum of Cancer Risk Among US Solid Organ Transplant Recipients. JAMA - J Am Med Assoc (2011) 30617:1891–901. 10.1001/jama.2011.1592
28.
Hans CP Weisenburger DD Greiner TC Gascoyne RD Delabie J Ott G et al Confirmation of the Molecular Classification of Diffuse Large B-Cell Lymphoma by Immunohistochemistry Using a Tissue Microarray. Blood (2004) 103(1):275–82. 10.1182/blood-2003-05-1545
29.
Rajakariar R Bhattacharyya M Norton A Sheaff M Cavenagh J Raftery MJ et al Post Transplant T-Cell Lymphoma: A Case Series of Four Patients from a Single Unit and Review of the Literature. Am J Transpl (2004) 4:1534–8. 10.1111/j.1600-6143.2004.00521.x
30.
Opelz G Döhler B . Lymphomas after Solid Organ Transplantation: A Collaborative Transplant Study Report. Am J Transpl (2004) 4(2):222–30. 10.1046/j.1600-6143.2003.00325.x
31.
Sampaio MS Cho YW Shah T Bunnapradist S Hutchinson IV . Impact of EpsteinBarr Virus Donor and Recipient Serostatus on the Incidence of post-transplant Lymphoproliferative Disorder in Kidney Transplant Recipients. Nephrol Dial Transpl (2012) 27:2971–9. 10.1093/ndt/gfr769
32.
Fernberg P Edgren G Adami J Ingvar A Bellocco R Tufveson G et al Time Trends in Risk and Risk Determinants of Non-hodgkin Lymphoma in Solid Organ Transplant Recipients. Am J Transpl (2011) 11:2472–82. 10.1111/j.1600-6143.2011.03704.x
33.
Van Leeuwen MT Grulich AE Webster AC McCredie MRE Stewart JH McDonald SP et al Immunosuppression and Other Risk Factors for Early and Late Non-hodgkin Lymphoma after Kidney Transplantation. Blood (2009) 114:630–7. 10.1182/blood-2009-02-202507
34.
Na R Laaksonen MA Grulich AE Meagher NS McCaughan GW Keogh AM et al Iatrogenic Immunosuppression and Risk of Non-hodgkin Lymphoma in Solid Organ Transplantation: A Population-Based Cohort Study in Australia. Br J Haematol (2016) 174:550–62. 10.1111/bjh.14083
35.
Sampaio MS Cho YW Shah T Bunnapradist S Hutchinson IV . Association of Immunosuppressive Maintenance Regimens with Posttransplant Lymphoproliferative Disorder in Kidney Transplant Recipients. Transplantation (2012) 93:73–81. 10.1097/TP.0b013e31823ae7db
36.
Romero S Montoro J Guinot M Almenar L Andreu R Balaguer A et al Post-Transplant Lymphoproliferative Disorders after Solid Organ and Hematopoietic Stem Cell Transplantation. Leuk Lymphoma () 60(1):142–50. 10.1080/10428194.2018.1474462
37.
Ghobrial IM Habermann TM Maurer MJ Geyer SM Ristow KM Larson TS et al Prognostic Analysis for Survival in Adult Solid Organ Transplant Recipients with post-transplantation Lymphoproliferative Disorders. J Clin Oncol (2005) 23(30):7574–82. 10.1200/JCO.2005.01.0934
38.
Tsai DE Bagley S Reshef R Shaked A Bloom RD Ahya V et al The Changing Face of Adult Posttransplant Lymphoproliferative Disorder: Changes in Histology between 1999 and 2013. Am J Hematol (2018) 93(7):874–81. 10.1002/ajh.25116
39.
Evens AM Choquet S Kroll-Desrosiers AR Jagadeesh D Smith SM Morschhauser F et al Primary CNS Posttransplant Lymphoproliferative Disease (PTLD): An International Report of 84 Cases in the Modern Era. Am J Transpl (2013) 13:1512–22. 10.1111/ajt.12211
40.
Mahale P Shiels MS Lynch CF Engels EA . Incidence and Outcomes of Primary central Nervous System Lymphoma in Solid Organ Transplant Recipients. Am J Transpl (2018) 18:453–61. 10.1111/ajt.14465
41.
Cavaliere R Petroni G Lopes MB Schiff D O’Neill BP Plotkin SR et al Primary central Nervous System post-transplantation Lymphoproliferative Disorder: An International Primary central Nervous System Lymphoma Collaborative Group Report. Cancer (2010) 116:863–70. 10.1002/cncr.24834
42.
Montanari F Radeski D Seshan V Alobeid B Bhagat G O’Connor OA . Recursive Partitioning Analysis of Prognostic Factors in post-transplant Lymphoproliferative Disorders (PTLD): A 120 Case Single Institution Series. Br J Haematol (2015) 171(4):491–500. 10.1111/bjh.13621
43.
Ferreiro JF Morscio J Dierickx D Marcelis L Verhoef G Vandenberghe P et al Post-Transplant Molecularly Defined Burkitt Lymphomas Are Frequently MYC-Negative and Characterized by the 11q-Gain/loss Pattern. Haematologica (2015) 100:e275–9. 10.3324/haematol.2015.124305
44.
Swerdlow SH . T-Cell and NK-Cell Posttransplantation Lymphoproliferative Disorders. Am J Clin Pathol (2007) 127:887–95. 10.1309/LYXN3RGF7D7KPYG0
45.
Tiede C Maecker-Kolhoff B Klein C Kreipe H Hussein K . Risk Factors and Prognosis in T-Cell Posttransplantation Lymphoproliferative Diseases. Transplantation (2013) 95:479–88. 10.1097/tp.0b013e3182762e07
46.
Hanson MN Morrison VA Peterson BA Stieglbauer KT Kubic VL McCormick SR et al Posttransplant T-Cell Lymphoproliferative Disorders - an Aggressive, Late Complication of Solid-Organ Transplantation. Blood (1996) 88:3626–33. 10.1182/blood.v88.9.3626
47.
Herreman A Dierickx D Morscio J Camps J Bittoun E Verhoef G et al Clinicopathological Characteristics of Posttransplant Lymphoproliferative Disorders of T-Cell Origin: Single-center Series of Nine Cases and Meta-Analysis of 147 Reported Cases. Leuk Lymphoma (2013) 54:2190–9. 10.3109/10428194.2013.775436
48.
Koff JL Li JX Zhang X Switchenko JM Flowers CR Waller EK . Impact of the Posttransplant Lymphoproliferative Disorder Subtype on Survival. Cancer (2018) 124(11):2327–36. 10.1002/cncr.31339
49.
Barba T Bachy E Maarek A Fossard G Genestier L Anglicheau D et al Characteristics of T and NK-Cell Lymphomas after Renal Transplantation: a French National Multicentric Cohort Study. Transplantation (2021) 105:1858–68. 10.1097/TP.0000000000003568
50.
Luskin MR Heil DS Tan KS Choi S Stadtmauer EA Schuster SJ et al The Impact of EBV Status on Characteristics and Outcomes of Posttransplantation Lymphoproliferative Disorder. Am J Transpl (2015) 15:2665–73. 10.1111/ajt.13324
51.
Cho YU Chi HS Jang S Park SH Park CJ . Pattern Analysis of Epstein-Barr Virus Viremia and its Significance in the Evaluation of Organ Transplant Patients Suspected of Having Posttransplant Lymphoproliferative Disorders. Am J Clin Pathol (2014) 141:268–74. 10.1309/AJCP9WYEXKOL9YUV
52.
Stevens SJC Verschuuren EAM Pronk I Van Der Bij W Harmsen MC The TH et al Frequent Monitoring of Epstein-Barr Virus DNA Load in Unfractionated Whole Blood Is Essential for Early Detection of Posttransplant Lymphoproliferative Disease in High-Risk Patients. Blood (2001) 97:1165–71. 10.1182/blood.v97.5.1165
53.
Tsai DE Douglas L Andreadis C Vogl DT Arnoldi S Kotloff R et al EBV PCR in the Diagnosis and Monitoring of Posttransplant Lymphoproliferative Disorder: Results of a Two-Arm Prospective Trial. Am J Transpl (2008) 8:1016–24. 10.1111/j.1600-6143.2008.02183.x
54.
Wagner HJ Wessel M Jabs W Smets F Fischer L Offner G et al Patients at Risk for Development of Posttransplant Lymphoproliferative Disorder: Plasma versus Peripheral Blood Mononuclear Cells as Material for Quantification of Epstein-Barr Viral Load by Using Real-Time Quantitative Polymerase Chain Reaction. Transplantation (2001) 72(6):1012–9. 10.1097/00007890-200109270-00006
55.
Ferla V Rossi FG Goldaniga MC Baldini L . Biological Difference between Epstein–Barr Virus Positive and Negative Post-transplant Lymphoproliferative Disorders and Their Clinical Impact. Front Oncol (2020) 10:506. 10.3389/fonc.2020.00506
56.
Kinch A Baecklund E Backlin C Ekman T Molin D Tufveson G et al A Population-Based Study of 135 Lymphomas after Solid Organ Transplantation: The Role of Epstein-Barr Virus, Hepatitis C and Diffuse Large B-Cell Lymphoma Subtype in Clinical Presentation and Survival. Acta Oncol (2014) 53(5):669–79. 10.3109/0284186X.2013.844853
57.
Santarsieri A Rudge JF Amin I Gelson W Parmar J Pettit S et al Incidence and Outcomes of post-transplant Lymphoproliferative Disease after 5365 Solid-Organ Transplants over a 20-year Period at Two UK Transplant Centres. Br J Haematol (2022) 197:310–9.
58.
Jagadeesh D Tsai DE Wei W Bustamante JA Wagner-Johnston ND Berg S et al Post-Transplant Lymphoproliferative Disorder (PTLD) after Solid Organ Transplant (SOT): A Multicenter Real World Analysis (RWA) of 877 Patients (Pts) Treated in the Modern Era. J Clin Oncol (2020) 38(15):e20026. 10.1200/jco.2020.38.15_suppl.e20026
59.
Reshef R Vardhanabhuti S Luskin MR Heitjan DF Hadjiliadis D Goral S et al Reduction of Immunosuppression as Initial Therapy for Posttransplantation Lymphoproliferative Disorder. Am J Transpl (2011) 11:336–47. 10.1111/j.1600-6143.2010.03387.x
60.
Leyssens A Dierickx D Verbeken EK Tousseyn T Verleden SE Vanaudenaerde BM et al Post-Transplant Lymphoproliferative Disease in Lung Transplantation: A Nested Case-Control Study. Clin Transpl (2017) 31(7):e12983. 10.1111/ctr.12983
61.
Burns DM Clesham K Hodgson YA Fredrick L Haughton J Lannon M et al Real-world Outcomes with Rituximab-Based Therapy for Posttransplant Lymphoproliferative Disease Arising after Solid Organ Transplant. Transplantation (2020) 104(12):2582–90. 10.1097/TP.0000000000003183
Summary
Keywords
epidemiology, transplantation, outcome, prognosis, post-transplant lymphoproliferative disorder, Epstein Barr Virus
Citation
Vergote VKJ, Deroose CM, Fieuws S, Laleman W, Sprangers B, Uyttebroeck A, Van Cleemput J, Verhoef G, Vos R, Tousseyn T and Dierickx D (2022) Characteristics and Outcome of Post-Transplant Lymphoproliferative Disorders After Solid Organ Transplantation: A Single Center Experience of 196 Patients Over 30 Years. Transpl Int 35:10707. doi: 10.3389/ti.2022.10707
Received
15 June 2022
Accepted
29 November 2022
Published
14 December 2022
Volume
35 - 2022
Updates
Copyright
© 2022 Vergote, Deroose, Fieuws, Laleman, Sprangers, Uyttebroeck, Van Cleemput, Verhoef, Vos, Tousseyn and Dierickx.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vibeke K. J. Vergote, Vibeke.Vergote@uzleuven.be, orcid.org/0000-0003-1100-5600
†These authors share senior authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.