Abstract
Main Problem: Preemptive kidney transplantation (PKT) is performed prior to dialysis initiation to avoid dialysis-related morbidity and mortality in children and adolescents. We undertook a systematic review to compare clinical outcomes in PKT versus kidney transplantation after dialysis initiation in paediatric patients.
Methods: The bibliographic search identified studies that compared paediatric recipients of a first or subsequent, living or deceased donor PKT versus non-preemptive kidney transplant. Methodological quality was assessed for all studies. Data were pooled using the random-effects model.
Results: Twenty-two studies (n = 22,622) were included. PKT reduced the risk of overall graft loss (relative risk (RR) .57, 95% CI: .49–.66) and acute rejection (RR: .81, 95% CI: .75–.88) compared to transplantation after dialysis. Although no significant difference was observed in overall patient mortality, the risk of patient death was found to be significantly lower in PKT patients with living donor transplants (RR: .53, 95% CI: .34–.83). No significant difference was observed in the incidence of delayed graft function.
Conclusion: Evidence from observational studies suggests that PKT is associated with a reduction in the risk of acute rejection and graft loss. Efforts should be made to promote and improve rates of PKT in this group of patients (PROSPERO).
Systematic Review Registration: https://clinicaltrials.gov/, CRD42014010565
Introduction
Kidney transplantation (KT) is the treatment of choice for children with end-stage kidney disease (ESKD) as it offers better survival and quality of life compared to treatment with dialysis (1, 2). Preemptive kidney transplantation (PKT) is performed before the initiation of dialysis to avoid the morbidity and mortality associated with dialysis (3, 4). Whether or not PKT also leads to improved clinical outcomes has been addressed by several studies but these report mixed findings. A USA registry analysis showed significantly better 5-year patient and graft survival rates in children transplanted preemptively vs. non-preemptively (nPKT) (5), whilst a multicentre retrospective cohort study from Japan found no difference in either patient survival or 5-year graft survival between these groups (6). Likewise, a number of single centre studies also show inconsistent results (7–10).
Historically, some centres believed that children with chronic kidney disease had to progress to ESKD requiring dialysis before being offered KT. The experience of dialysis would give children a sense of what life was like on dialysis leading to improved adherence post-transplant (11). This practice is no longer supported in most paediatric nephrology centres.
Paediatric ESKD patients differ from adult patients in terms of causes of ESKD, donor-recipient size mismatch, post-transplant complications, medication non-adherence, growth and development complications, and co-morbidities associated with the lower urinary tract (12). Therefore, it is important to evaluate the benefits of PKT specifically for the paediatric population. We undertook a systematic review to determine whether it is beneficial for paediatric patients to undergo KT before dialysis is initiated.
Materials and Methods
Registration of Protocol
This study was designed and reported according to the PRISMA guidelines (13). The protocol was prospectively registered with PROSPERO (CRD42014010565) (14).
Inclusion Criteria
Type of studies: Any study design, including registry analyses, cohort studies, case-control studies and case series comparing PKT with nPKT, were eligible for inclusion. Case reports, and narrative reviews, editorials without primary data and non-English studies were excluded. We included both full articles and congress abstracts, and also checked for overlap in case abstracts were later published as full texts.
Type of participants and intervention: Eligible studies included those that compared paediatric recipients of a first or subsequent, living donor (LD) or deceased donor (DD) PKT versus nPKT. We included studies that described their population as paediatric or reported an age range of up to 18 years. PKT was defined as transplantation prior to any initiation of peritoneal dialysis (PD) or haemodialysis (HD). nPKT refers to transplantation after any given period of PD or HD. No restrictions were imposed on pre-transplant dialysis duration (dialysis vintage). Studies reporting on recipients with either a history of a previous organ transplant other than kidney or recipients of multi-organ transplants were excluded.
Type of outcomes: The outcomes of interest were overall graft loss (non-censored for death), death-censored graft loss, patient death (from all causes), delayed graft function (DGF), incidence of acute rejection (any definition, including clinically suspected and biopsy-proven acute rejection), renal function [serum creatinine or estimated glomerular filtration rate (eGFR)], primary non-function, quality of life, return to school after transplantation, height/growth measures, and incidence of cardiovascular morbidity, infections and malignancy.
Search Strategy
As this review was part of a larger study that reviewed the available evidence for both paediatric and adult KT patients, a broad bibliographic search was carried out up to 31 July 2020 using a mixture of free text and controlled vocabulary terms (Supplementary Table S1), which retrieved references for both paediatric and adult studies. Five electronic databases including EMBASE, MEDLINE (OvidSP), Cochrane Central Register of Controlled Trials (CENTRAL), Web-of-science and Google Scholar were searched. No limits for date of publication or language were applied. The references of identified studies or review articles were scanned to find potentially eligible studies that may have been missed during the literature search. Attempts were made to contact the study authors in case of missing data or unclear study information.
Selection of Studies
The study selection was carried out in two stages by independent reviewers (RRM, LP, ST, and JL). Initially, titles and abstracts of the retrieved studies were screened against the inclusion/exclusion criteria, followed by full-text review of potentially eligible papers and final selection of the studies to be included in the review. Discrepancies between reviewers were resolved by consensus.
Data Extraction
Two reviewers (RRM and LP) independently extracted the data using a standardized data extraction sheet. Discrepancies between reviewers were solved by discussion. Where there was more than one publication of the same study, data were only extracted from the publication that had the most complete data or the largest sample size. We extracted data on general study information and demographics, and primary and secondary outcomes. Where possible, data for LD and DD were extracted separately.
Assessment of Methodological Quality
The methodological quality of the included studies, published as full text papers, was assessed by two independent authors (RRM and LP) using the Downs and Black checklist (15). Two out of the 27 items from the checklist were removed, i.e., the items relating to intervention compliance and the power of the study, as these were considered irrelevant or could not be calculated.
Statistical Analysis
Where at least three studies reported on an outcome, meta-analysis was performed using the statistical software R version 3.6.3. Data were pooled using the random-effects model to calculate the relative risk (RR) with a 95% confidence interval (CI). We planned to analyze data according to LD vs. DD, however, this was not always feasible as most studies combined LD and DD in their analyses. Hence, data were pooled regardless of whether they were LD and/or DD. Patient or graft survival rates were converted to the number of deaths and graft losses. Data on graft loss were categorized as either overall graft loss or death-censored graft loss. If a study neither defined graft loss nor specified whether the graft loss data was death-censored or non-censored for death, we categorized graft loss as being non-censored for death. We calculated a pooled estimate for the nPKT group if the study reported the results for nPKT according to different dialysis durations or separately for PD or HD. If a single study reported an outcome at more than one time point, the most recent follow-up data was used. Data were pooled for any duration of follow up. In order to account for the role of confounders in the analysis of the overall graft loss, we also calculated a pooled ratio consisting of adjusted ratios either calculated or directly extracted from the studies. Secondary analyses were conducted excluding smaller studies with overlapping countries and study periods to avoid duplicate use of data. If less than three studies reported on an outcome we summarized the results in a narrative review.
Heterogeneity was analyzed using the I2 statistic (16). Where heterogeneity was significant (I2 ≥ 50%), a mixed effect analysis was performed to explore its potential causes.
Results
Included Studies
The literature search retrieved 8,583 references. Following full-text analysis of 332 studies, 216 studies were excluded (Figure 1). Of the remaining 116 studies that met the inclusion criteria, 22 were identified as paediatric studies reporting on a total of 22,622 patients (Table 1). Cransberg (17) and Cransberg (18) were considered as one study due to insufficient data on the extent of overlap between the studies. Only the estimate for adjusted graft survival was extracted from Cransberg (18).
FIGURE 1
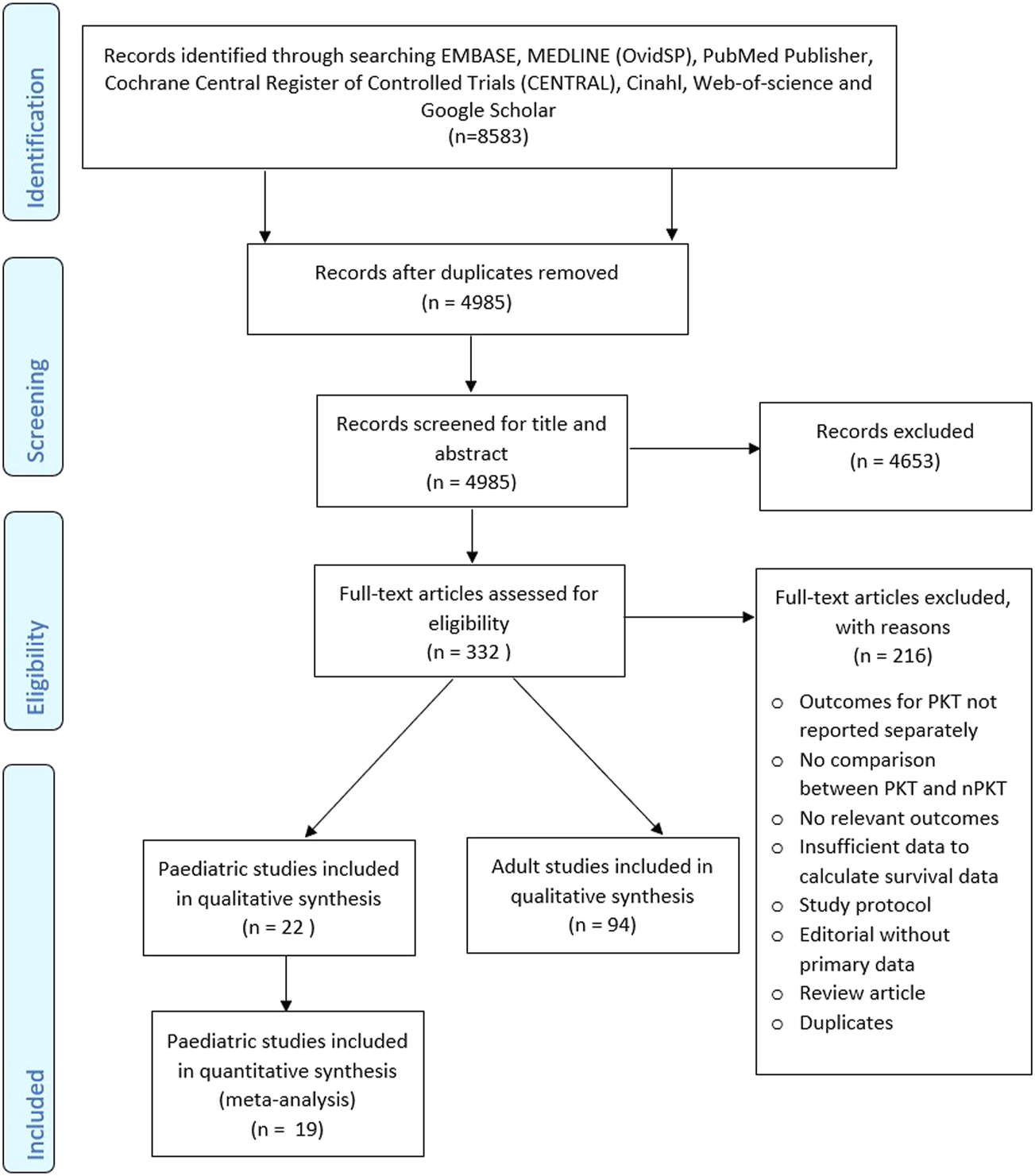
PRISMA flow diagram.
TABLE 1
| Author (year); country | Study design and setting | Paediatric definition | 1st Tx only | Number of patients | % Of HD in nPKT | HLA mismatch (Mean ± SD) | Duration of follow up | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Period when Tx was received | LD | DD | Total | ||||||||||
| PKT | nPKT | PKT | nPKT | PKT | nPKT | PKT | nPKT | ||||||
| Amaral (5) (2016); United States | Retrospective registry analysis; multicentre | <18 y | Yes | 1,104 | 2,266 | 564 | 3,593 | 7,527 | NR | 3.26 | 3.79 | NR | NR |
| January 2000–September 2012 | |||||||||||||
| Atkinson (24) (2020); United States | Prospective cohort study; multicentre | <17 y | Yes | 50 | 41 | 29 | 50 | 170 | 41.7 | — | — | Median: 3.8 yIQR: 1.8–5.8 y | NR |
| March 2006–January 2017 | |||||||||||||
| Butani (25) (2011); United States | Retrospective registry analysis; multicentre | <17 y | Yes | 730 | 1,354 | 273 | 1,249 | 3,606 | 47.6 | 2.8 ± 0* | — | 5 y | 5 y |
| January 1995–December 2000 | |||||||||||||
| Cransberg (17) (2006); Europe | Retrospective registry analysis; multicentre | <16 y | Yes | 86 | 132 | 70 | 825 | 1,113 | NR | 2.3 (LD); 2.6 (DD) | 2.1 (LD); 2.5 (DD) | Mean = Median = 5.3 y | Mean = Median = 5.3 y |
| Cransberg (18) (2000); Netherlands | January 1990–January 2000 | Range: 0–14.1 y | Range: 0–14.1 y | ||||||||||
| Cuervo (19) (2007); Mexico | Cohort study; single centre | NR | NR | 17 | 13 | 2 | 6 | 38 | NR | — | — | NR | NR |
| January 1995–December 2003 | |||||||||||||
| Duzova (32) (2009); Turkey | Retrospective cohort studies; single centre | NR | NR | 13 | 17 | 4 | 12 | 46 | NR | — | — | 5 y | 5 y |
| 2000–2008 | |||||||||||||
| Fitzwater (30) (1991); United States | Retrospective cohort studies; single centre | <18 y | Yes | 13 | 17 | 0 | 16 | 46 | 75.8 | — | — | Mean: 24 m | Mean ± SD: 19.5 ± 7 m |
| Until 1987 | |||||||||||||
| Flom (26) (1992); United States | Retrospective cohort studies; single centre | NR | No | 26 | 40 | 0 | 0 | 66 | 32.5 | — | — | Median: 3.5 yRange: 0.5–7.1 y | Median: 4.35 yRange: 0.6–7.3 y |
| January 1984–December 1990 | |||||||||||||
| Garcia (9) (2015); Brazil | Retrospective cohort study; single centre | NR | NR | 49 | 109 | 32 | 133 | 323 | 26.4 | — | — | Median: 36 mIQR: 13–68 m | Median: 42 mIQR: 17–69 m |
| January 2000–December 2010 | |||||||||||||
| Harada (6) (2001); Japan | Retrospective cohort studies; single centre | ≤18 y | NR | 9 | 20 | — | — | 29 | 45.0 | 2.2 ± 0.70 | 2.3 ± 0.87 | Mean ± SD: 42.4 ± 19.4 m | Mean ± SD: 68.3 ± 39.8 m |
| August 1987–December 1998 | |||||||||||||
| Kaya (20) (2018); Turkey | Retrospective cohort study; single centre2005–2017 | NR | NR | — | — | — | — | 230 | NR | — | — | Median: 7.23 yMean ± SD: 4.71 ± 2.61 y | Median: 7.23 yMean ± SD: 5.88 ± 9.38 y |
| Kim (27) (2019); Canada | Retrospective cohort study; single centre | <18 y | No | 54 | 98 | 21 | 151 | 324 | 51.0 | — | — | 1 y | 1 y |
| January 2000–December 2015 | |||||||||||||
| Kramer (21) (2012); Europe | Retrospective registry analysis; multicentre | >3 and <18 y | Yes | 321 | 435 | 123 | 950 | 1829 | NR | — | — | 8 y | 8 y |
| January 1988–December 2007 | |||||||||||||
| Mahmoud (22) (1997); France | Retrospective cohort study; single centre | NR | NR | 8 | 8 | 32 | 55 | 103 | 82.5 | 3.3 | 3.3 | Mean: 3.3 y Range: 0.8–7.0 y | Mean: 3.2 yRange: 0.4–7.8 y |
| April 1987–December 1994 | |||||||||||||
| Marlais (28) (2018); United Kingdom | Retrospective registry analysis; multicentre | <18 y | NR | 607 | — | — | — | 2038 | 44.9 | — | — | NR | NR |
| January 2000–December 2015 | |||||||||||||
| Naderi (10), (2017); Iran | Retrospective cohort study; single centre | ≤18 y | No | — | — | — | — | 314 | 89.2 | — | — | Mean ± SD: 15.9 ± 4.0 y | Mean ± SD: 15.9 ± 4.0 y |
| 1989 to 2013 | Range: 0.5–20 y | Range: 0.5–20 y | |||||||||||
| Nevins (7) (1991); United States | Retrospective cohort study; single centre | <6 y | Yes | 31 | 24 | 2 | 13 | 70 | 56.8 | — | — | 5 y | 5 y |
| July 1979–October 1987 | |||||||||||||
| Offner (8) (1993); Germany | Retrospective cohort study; single centre | NR | Yes | 14 | 14 | 14 | 14 | 56 | NR | — | — | 5 y | 5 y |
| January 1970–September 1991 | |||||||||||||
| Reydit (29) (2017); France | Retrospective cohort study; multicentre | ≤18 y | Yes | - | - | - | - | 1920 | NR | — | — | Median: 7 y | Median: 7 y |
| 1995–2013 | |||||||||||||
| Sinha (31) (2010); United Kingdom | Cross-sectional study; single centre | NR | NR | 16 | 46 | 23 | 44 | 129 | 42.2 | 1.83 | 2.14 | Median: 4 yRange: 1–12 y | Median: 4 yRange:1–15 y |
| May 1993–November 2006 | |||||||||||||
| Splinter (33) (2018); Netherlands, Belgium and Germany | Cross-sectional study; multicentre | 8–18 y | NR | - | - | - | - | 150 | NR | — | — | N/A | N/A |
| October 2007–December 2014 | |||||||||||||
| Vats (23) (2000); United States | Retrospective registry analysis; multicentre | NR | Yes | 466 | 890 | 159 | 980 | 2,495 | 60.4 | — | — | Mean ± SD: 28.6 ± 19.5 m | Mean ± SD: 27.3 ± 19.0 m |
| 1992–1996 | |||||||||||||
Characteristics of the included studies.
PKT, preemptive kidney transplantation; nPKT, non-preemptive kidney transplantation; HD, haemodialysis; Tx, transplant; DD, deceased donor; LD, living donor; *, standard error; SD, standard deviation; IQR, interquartile range; y, years; m, months; NR, not reported; N/A, not applicable.
Methodological Quality
The methodological quality of the included studies varied with quality scores ranging from 10 to 19 out of a maximum possible score of 26 (Supplementary Table S2). Eleven studies adjusted for confounders in their analysis.
Patient Death
Ten studies (5–8, 17, 19–23) reported data on patient deaths. The pooled analysis showed no significant difference in the risk of patient death for PKT vs. nPKT (n = 13,490; RR: .77; CI: .53–1.11; p = .16; Figure 2). Heterogeneity was not significant (I2 = 35.13%). The difference in the risk remained nonsignificant after excluding four studies (8, 17, 20, 22) with overlapping countries and study periods (n = 11,988; RR: .86; CI: .53–1.39; p = .53; I2 = 57.94%; Supplementary Figure S1).
FIGURE 2
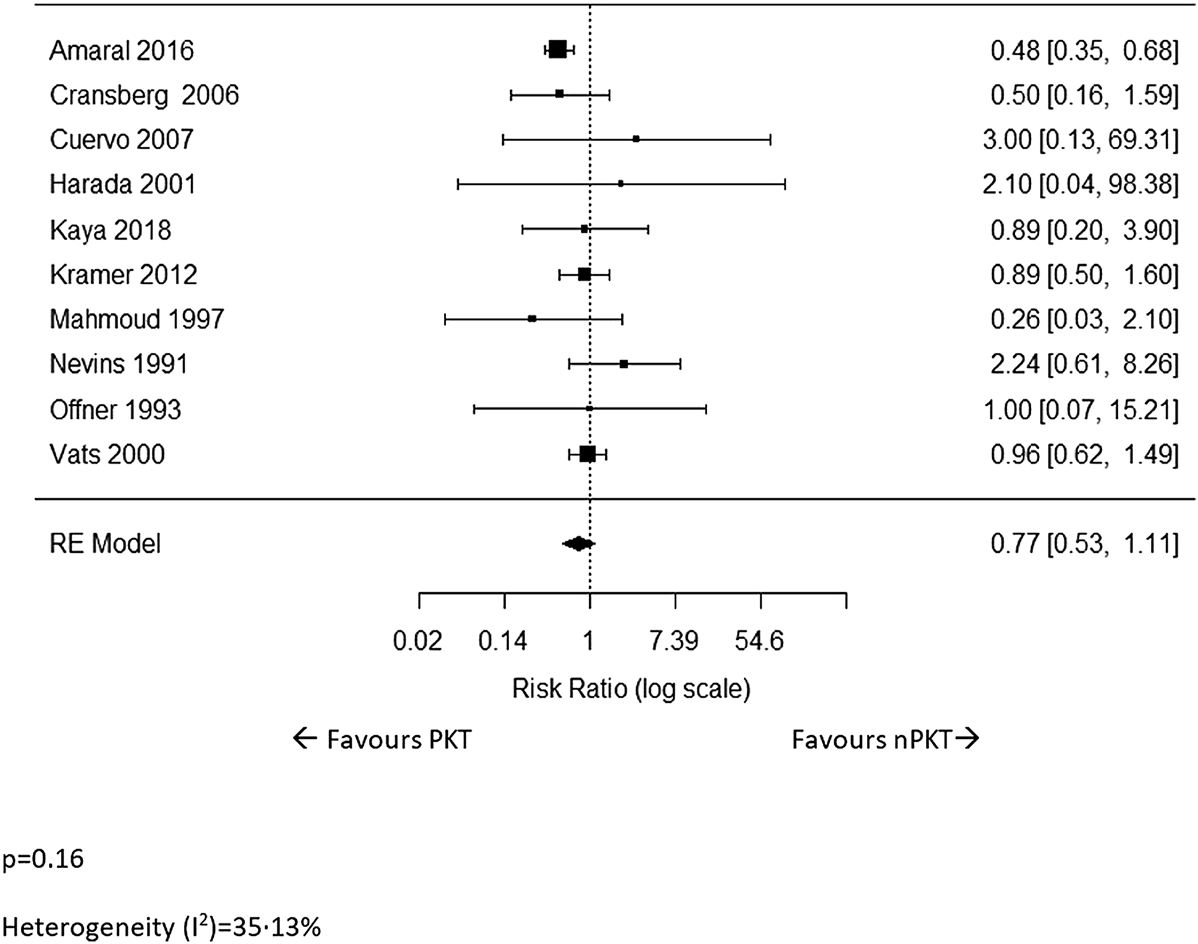
The relative risk of patient death for PKT vs. nPKT.
Patient death for LD transplants was reported in three studies (5, 6, 17). The pooled analysis revealed a significantly lower risk of patient death in PKT patients (n = 3,617; RR: .53; CI: .34–.83; p = .0054; I2 = 0%; Supplementary Figure S2).
Two studies (5, 17) reported data on patient survival for DD. Amaral et al (5) reported a significantly higher 5-year patient survival in the PKT versus nPKT group (97.5% vs. 95.0%; p = .004). However, in the Cransberg et al (17) study, patient survival at 6 years following transplantation was similar between these groups.
Graft Loss
Sixteen studies (5–10, 17, 20, 22–29) reported on overall graft loss. The meta-analysis revealed that the risk of graft loss following PKT was significantly lower than that of nPKT (n = 20,212; RR: .57; CI: .49–.66; p < .0001; I2 = 51.24%; Figure 3). Results were similar after excluding four (8, 24–26) studies with overlapping countries and study periods (n = 16,314; RR: .54; CI: .47–.62; p < .0001; I2 = 32.22%; Supplementary Figure S3). Eight of the 16 studies reported ratios adjusted for various confounders, using multivariate analyses or by matching the PKT and nPKT group (5, 6, 8, 9, 18, 22, 25, 29). Pooling of these adjusted ratios showed a similar result (n = 16,715; RR: .61; CI: .40–.92; p = .018; I2 = 60.7%; Supplementary Figure S4). The adjusted ratios and confounders are presented in Supplementary Table S3.
FIGURE 3
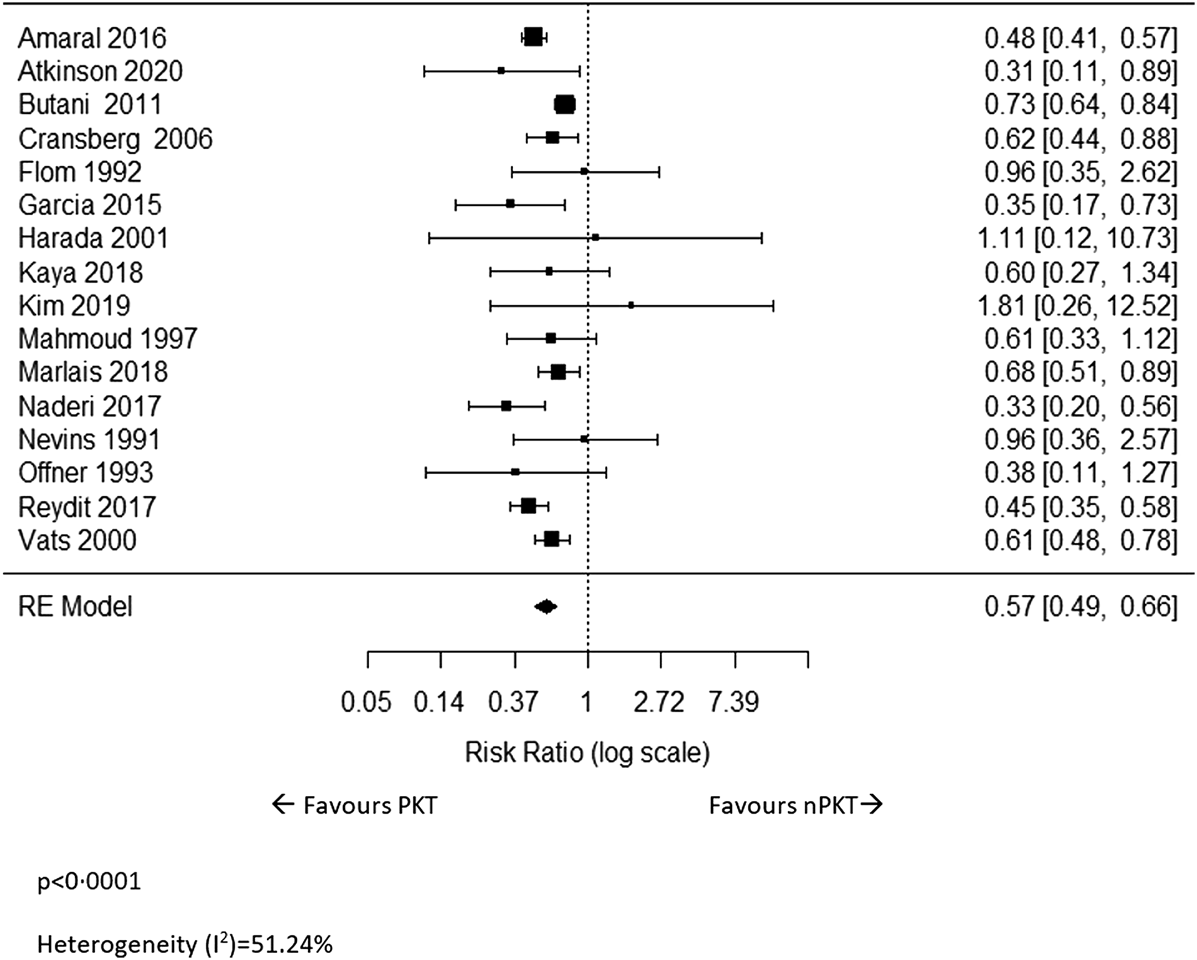
The relative risk of overall graft loss for PKT vs. nPKT.
In an attempt to explain the heterogeneity between studies for overall graft loss, a mixed-effect analysis was performed which looked at the role of four moderator variables: the percentage of HD patients in the nPKT group, length of follow-up, percentage of LD, and the year of publication (Supplementary Figures S5–S8). None of these variables were found to significantly influence the relative risk of graft loss. It may be worth noting that on visual inspection of the forest plot, the heterogeneity is in the size of effect rather than the direction of effect.
Five studies (5, 6, 23, 26, 27) reported on overall graft loss for LD, and the pooled analysis showed that PKT significantly reduced the risk of graft loss (n = 4,973; RR: .57; CI: .46–.69; p < .0001; I2 = 0%; Supplementary Figure S9).
Two studies (5, 23) reported on overall graft survival in DD recipients. Amaral et al (5) reported a significantly higher 5-year graft survival rate in PKT patients compared to nPKT patients (85.4% vs. 76.4%; p < .001). However, Vats et al (23) reported similar 3-year graft survival in PKT versus nPKT (PD and HD) patients.
Death-censored graft loss was reported in two studies (9, 30) for LD and DD data combined. Garcia et al (9) reported a higher 12-, 36-, 60- and 90-month death-censored graft survival rate, adjusted by donor type, in PKT patients compared with nPKT patients (97% vs. 87%; 92% vs. 79%; 86% vs. 72%; 76% vs. 65%, respectively). The difference was significant at 90 months (p < .05); however, the study did not clearly report if the differences were significant at the other time points. The study by Fitzwater et al (30), found no significant difference in the 2-year death-censored graft loss between PKT and nPKT.
Delayed Graft Function
DGF was reported in three studies (17, 25, 27). The RR for the incidence of DGF was .57 (n = 4,871; CI: .22–1.50; p = .26; Supplementary Figure S10). Heterogeneity was high (I2 = 81.51%). We could not explore heterogeneity as the number of studies was too small.
DGF for LD was reported in two studies (17,27). Cransberg et al (17) showed a slightly higher incidence of DGF in PKT patients (3.5% vs. 2.4%), but did not report if this difference was significant. No significant difference was observed between PKT vs. nPKT in terms of DGF in the study by Kim et al (27).
The only study that reported on DGF in DD patients was Cransberg et al (17), which observed no difference in the DGF rate between PKT and nPKT.
Acute Rejection
Incidence of acute rejection was reported in seven studies (6, 17, 22, 25–27, 30). The pooled analysis revealed that the risk of acute rejection in PKT patients was significantly lower than that of nPKT patients (n = 4,897; RR: .81; CI: .75–.88; p < .0001; Figure 4). Heterogeneity was low (I2 = 0%). Similar results were observed after excluding Fitzwater et al (30) from the analysis due to overlapping country and study period (n = 4,851; RR: .81; CI: .74–.87; p < .0001; I2 = 0%; Supplementary Figure S11). Of the seven studies, only two (6, 22) adjusted for confounders; hence, a pooled estimate of the adjusted acute rejection rate could not be calculated.
FIGURE 4
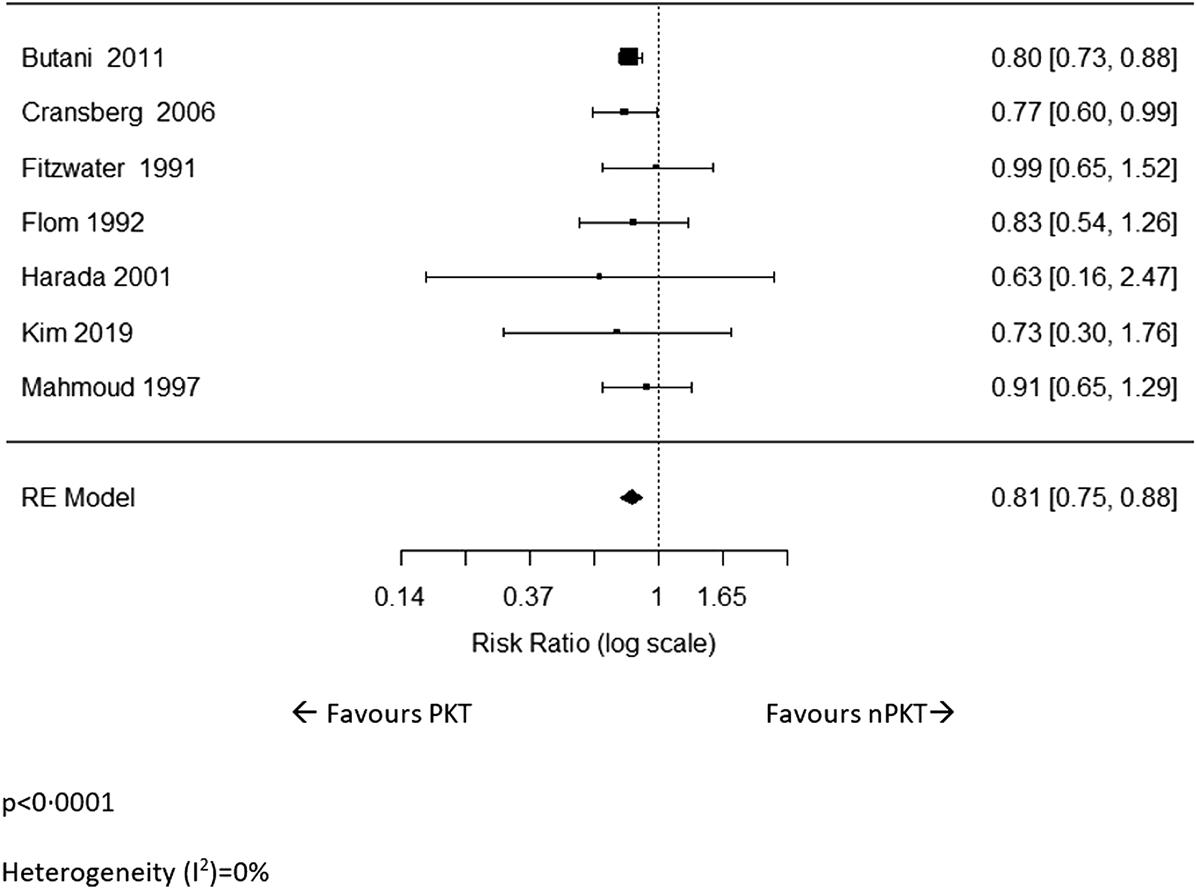
The relative risk of acute rejection for PKT vs. nPKT.
Three studies (6, 26, 27) reported on the rate of acute rejection for LD. Although the effect size was similar to the overall analysis, it did not reach statistical significance (n = 247; RR: .79; CI: .55–1.15; p = .22; I2 = 0%; Supplementary Figure S12).
Cransberg et al (17) was the only study that included data on acute rejection for DD. In the study, a significantly higher percentage of patients remained acute rejection-free following PKT than after nPKT (52% vs. 37%; p = .039) at 3 years.
Cardiovascular Morbidity, Infections and Malignancy
Two studies reported cardiovascular morbidity outcomes (17, 31). Cransberg et al (17) measured the incidence of severe hypertension between PKT vs. nPKT at one, three and 5 years post-transplant, and found significantly lower incidence of severe hypertension in the PKT group in the third year (40% vs. 64%; p = .016), among patients with DD transplants. The study by Sinha and Marks (31) also showed a significantly lower incidence of hypertension in the PKT versus nPKT group (31% vs. 53%; p = .02) for combined LD and DD data. No studies reported on infections and malignancy.
Renal Function
Renal function was reported in six studies as either eGFR or serum creatinine, with four studies (20, 22, 30, 32) reporting on LD and DD data combined. Mahmoud et al (22) evaluated the mean GFR at one and 4 years post-transplant, and found no statistical differences in the GFR values between the PKT and nPKT group at both follow-ups. The study by Kaya et al (20) also showed no significant difference in the mean GFR between these groups within a median follow-up of 7.23 years. Duzova et al (32) measured the mean GFR values at one, two, three and 5 years after transplantation, and reported a significantly lower mean GFR in the PKT group only in the third year (mean ± standard deviation (SD): 86 ± 31 ml/min/m2 vs. 101 ± 31 ml/min/m2; p < .05). Likewise, Fitzwater et al (30) reported no statistical differences in the serum creatinine levels between PKT and nPKT at 1 month, 3 months, 6 months, 1 year and 2 years post-transplant.
Two studies (26, 27) reported renal function for LD only. Kim et al (27) reported no differences between PKT and nPKT in the median GFR at 1 month and 1 year. Flom et al (26) reported a higher mean (±SD) GFR for PKT (68 ± 28 ml/min/1.73 m2) versus nPKT (HD and PD) (both 60 ± 26 ml/min/1.73 m2), calculated over a median follow-up of 3.5, 3.6 and 5.1 years for PKT, PD and HD respectively. However, the study did not report whether this difference was significant.
Primary Non-Function
No studies reported on primary-non function.
Quality of Life
Quality of life was reported in only two studies (6, 33). Splinter et al (33) assessed the health-related quality of life (HRQoL) of patients who spent at least 6 months on their treatment modality, using the PedsQL™ questionnaire. The PedsQL™ consisted of five major domains, including physical health, emotional functioning, social functioning, school functioning, and psychosocial health. The mean ± SD HRQoL scores for physical health was significantly higher in the PKT vs. nPKT group (78.6 ± 18.0 vs. 70.4 ± 20.5; p < .05), but showed no differences between the groups for the other domains. Harada et al (6) asked patients about the benefits and disadvantages of renal transplantation. The percentage of patients that reported feeling satisfied with the improvement in their physical condition was significantly higher in the PKT vs. the nPKT group (p < .01). On the other hand, a significantly higher percentage of patients in the nPKT group reported satisfaction related to the freedom from restrictions of liquid intake, daily diet and time spent on dialysis, following renal transplantation (p < .01). No significant differences were observed between the two groups regarding disadvantages felt due to renal transplantation, which included anxiety about the fate of the renal graft and annoyance resulting from frequent hospital visits and daily medications.
Return to School
No studies reported data on return to school.
Height/Growth
Three studies (6, 8, 31) reported findings on the height/growth of patients. Harada et al (6) assessed the mean ± SD heights of the patients at transplantation and at one and 3 years post-transplant, using the national cross-sectional standard growth chart for boys and girls. The study showed significantly better mean ± SD height in the PKT vs. nPKT group at transplantation (−.84 ± 0.73 vs. −2.86 ± 1.93; p < .05) and at 3 years post-transplant (−.53 ± 1.65 vs. −3.22 ± 1.94; p < 0.05), only for patients less than 15 years old. Sinha and Marks (31), who measured the height of the patients at the last clinical visit (range 1–15 years) using the median standard deviation score (SDS), found no significant differences in the scores between the two groups. Similar results were reported by Offner et al (8), who also used the median SDS to measure the height of the patients at 1 year post-transplantation.
Primary Kidney Transplant
Secondary analyses comparing PKT versus nPKT patients with primary KT are presented in Supplementary Figures S13–S15.
Discussion
The available evidence from observational studies suggests that PKT significantly lowers the risk of graft loss and acute rejection compared to nPKT. PKT patients with LD transplants are seen to benefit from a reduced risk of patient death as well as overall graft loss. Most studies in our review showed nonsignificant differences in post-transplant renal function between PKT and nPKT patients. Regarding other outcomes, such as cardiovascular morbidity, quality of life and height/growth, it was not possible to draw firm conclusions due to the limited evidence available. However, with regard to quality of life, patients reported improvement in physical condition better in the PKT than the nPKT group. There were not enough data to draw firm conclusions regarding different outcomes for DD and LD kidney transplantation.
Our results agree with the findings of the systematic review by Abramowicz et al (34), which looked at a combination of paediatric and adult KT recipients and suggested PKT offers better allograft survival. The same benefit has been observed in studies performed on adult PKT patients (35, 36). Research explaining the reasons for this benefit, especially specific to paediatric patients, is scarce. It is possible that several confounding factors have accounted for some or all of this observed survival advantage. Studies have shown that rates of PKT are significantly higher in children who are white versus other races, and males versus females (37–39). This may result in selection bias, which in turn may affect graft survival.
We attempt to explain the association between PKT and higher graft survival by analysing data in adult studies because of the lack of data on paediatric patients. It should, however, be noted that it remains unclear to what extent these adult data can be applied to the paediatric patients. Firstly, some authors have speculated that the association of between PKT and a reduced risk of graft loss may have been influenced by higher residual renal function of native kidney observed in PKT patients at transplantation, compared to nPKT patients. However, three studies have found that PKT with higher pre-transplant eGFR is not linked to better graft survival (40–42), suggesting that pre-transplant residual renal function may not be one of the major factors affecting graft survival. Secondly, the survival benefit of PKT may be due to the avoidance of comorbidities, such as cardiovascular disease, that are associated with dialysis (43). A study by Prezelin-Reydit et al (44), however, found that the adjusting for cardiovascular comorbidities and diabetes mellitus did not alter the link between PKT and the reduction in the hazard of graft failure. This agrees with our subgroup analysis of adjusted risks, which still showed a graft survival advantage for PKT. Lastly, as PKT take place earlier in a patient’s natural history of disease compared to nPKT, there are concerns that this “lead time” may bias observational studies to favour PKT as the optimal treatment modality (11, 45). However, Gill et al (36) demonstrated that PKT and nPKT patients with at least 2 years of allograft survival established similar baseline GFR levels at 6 months post-transplant, disapproving the hypothesis that the graft survival benefit linked to PKT may be a consequence of lead time bias due to earlier transplantation of PKT patients with preserved native kidney function.
Another significant finding in our meta-analysis is a lower incidence of acute rejection in PKT patients which may be explained by the biological differences observed in the immune reactivity of PKT versus nPKT patients (11). These differences are not yet well understood and are somewhat counterintuitive; therefore, further in-depth immunological studies into T cell senescence and allo-immunity in both groups are warranted.
This study had several weaknesses. It only included observational studies, which by nature are frequently subject to confounding and bias, which may lead to false-positive findings (46). Additionally, although current paediatric kidney transplantation guidance advises PKT whenever possible, in reality, some non-adherent children may be initiated on dialysis before receiving a transplant. This practice introduces a bias and it may be an additional unaccounted confounder in our results. The small number of studies in some of the pooled analyses preclude finding convincing evidence for the outcomes, for example for delayed graft function. Heterogeneity was high for some of the outcomes, and could not always be explored due to the small number of studies. Definitions of reported outcomes were not clearly stated for some studies, e.g., overall graft survival or death-censored graft survival. We were unable to perform separate analyses for LD versus DD patients for most outcomes due to limited number of studies that presented these data separately. It was also unclear from some of the included studies whether there were any pre-emptive second transplants included in the study populations. Although we attempted to address the possible role of confounding variables, such as socio-economic status, health literacy, psychosocial support, lead time bias and recurrence of primary ESKD, on overall graft survival by pooling adjusted ratios, this is limited to the adjustments used in the original analyses and additional confounders may still be present. Another limitation is the inconsistent reporting of dialysis vintage, making it difficult to assess the impact of different durations of dialysis on transplant outcomes.
Our systematic review also highlights the inconsistent and poor reporting of certain outcomes that are relevant to paediatric ESKD patients, such as cardiovascular disease and quality of life. Studies have shown that absence from school, social engagement, symptoms (feeling ill or pain), hospitalisation, poor sleep and fatigue are important to children with ESKD (47–49), however, these outcomes were poorly reported or not reported at all by the studies included in the review. Future studies should report the core outcomes established by the SONG-Kids initiative (50) to ensure that outcomes relevant to children are included in research proposals.
In conclusion, systematic review of observational studies showed that paediatric PKT patients have a lower risk of overall graft loss and acute rejection than nPKT patients. While no difference was seen in overall patient mortality, PKT appeared to significantly lower the risk of patient death in LD patients. Therefore, it is important to develop pathways that ensure PKT options for as many paediatric ESKD patients as possible, especially emphasising on living donation. With education of paediatric patients and carers early in the disease process about LD PKT, a timely transplant or timely waitlisting for DD KT (in absence of LD options) can be achieved for many patients. This also calls for a redesign of the default renal replacement therapy pathway, which unfortunately is still set to dialysis before transplantation.
Statements
Author’s note
The abstract of the paper received the 2021 Marius Renard Award for Best Abstract on Paediatric Transplantation (a joint ESOT and IPTA (International Pediatric Transplant Association) award) during the 2021 ESOT congress.
Author contributions
RRM: Literature search, data collection, methodological quality assessment, data analysis, data interpretation, writing, and project administration. SK: data analysis, data interpretation, revising, and writing. JS: data interpretation, revising, and writing. SM: data interpretation, revising, and writing. JL: Conceptualization, literature search, data collection, data interpretation, revising, and writing. ST: Conceptualization, literature search, data collection, data interpretation, revising, and writing. FD: Conceptualization, data interpretation, revising, writing, and supervision. LP: Conceptualization, literature search, data collection, methodological quality assessment, data analysis, data interpretation, revising, writing, project administration, funding acquisition, and supervision.
Funding
The study was supported by a grant from the Philip Allison Foundation. The funder had no role in the study design, data collection, analysis, interpretation of the data, decision to publish and/or the preparation of the manuscript.
Acknowledgments
We would like to acknowledge Wichor Bramer, Biomedical Information Specialist, Erasmus Medical Centre, Rotterdam, The Netherlands, for designing and running the searches.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2022.10315/full#supplementary-material
Abbreviations
CI, confidence interval; DD, deceased donor; DGF, delayed graft function; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; HD, haemodialysis; HRQoL, health-related quality of life; KT, kidney transplantation; LD, living donor; nPKT, non-preemptive kidney transplantation; PD, peritoneal dialysis; PKT, preemptive kidney transplantation; RR, relative risk; SD, standard deviation; SDS, standard deviation score.
References
1.
McDonald SP Craig JC . Long-term Survival of Children with End-Stage Renal Disease. N Engl J Med (2004) 350:2654–62. 10.1056/nejmoa031643
2.
Goldstein SL Graham N Burwinkle T Warady B Farrah R Varni JW . Health-related Quality of Life in Paediatric Patients with ESRD. Pediatr Nephrol (2006) 21:846–50. 10.1007/s00467-006-0081-y
3.
Ku E McCulloch CE Ahearn P Grimes BA Mitsnefes MM . Trends in Cardiovascular Mortality Among a Cohort of Children and Young Adults Starting Dialysis in 1995 to 2015. JAMA Netw Open (2020) 3:e2016197. 10.1001/jamanetworkopen.2020.16197
4.
Galiyeva DB Jackson CA Wild SH Burns S Hughes D Traynor JP et al Long-term All-Cause Mortality and Cardiovascular Outcomes in Scottish Children after Initiation of Renal Replacement Therapy: A National Cohort Study. Pediatr Nephrol (2020) 35:677–85. 10.1007/s00467-019-04430-4
5.
Amaral S Sayed BA Kutner N Patzer RE . Preemptive Kidney Transplantation Is Associated with Survival Benefits Among Paediatric Patients with End-Stage Renal Disease. Kidney Int (2016) 90:1100–8. 10.1016/j.kint.2016.07.028
6.
Harada H Seki T Nonomura K Chikaraishi T Takeuchi I Morita K et al Pre-emptive Renal Transplantation in Children. Int J Urol (2001) 8:205–11. 10.1046/j.1442-2042.2001.00285.x
7.
Nevins TE Danielson G . Prior Dialysis Does Not Affect the Outcome of Paediatric Renal Transplantation. Pediatr Nephrol (1991) 5:211–4. 10.1007/BF01095954
8.
Offner G Hoyer PF Meyer B Pichlmayr R Brodehl J . Pre-emptive Renal Transplantation in Children and Adolescents. Transpl Int (1993) 6:125–8. 10.1007/BF00336353
9.
Garcia CD Bittencourt VB Rohde RW Dickel S Pires I Tumba K et al Pre-emptive Paediatric Kidney Transplantation or Not? Transplant Proc (2015) 47:954–7. 10.1016/j.transproceed.2015.03.019
10.
Naderi G Latif A Karimi S Tabassomi F Esfahani ST . The Long-Term Outcome of Paediatric Kidney Transplantation in Iran: Results of a 25-year Single-center Cohort Study. Int J Organ Transpl Med (2017) 8:85–96.
11.
Mange KC Weir MR . Preemptive Renal Transplantation: Why Not?Am J Transpl (2003) 3:1336–40. 10.1046/j.1600-6143.2003.00232.X
12.
Cho MH . Paediatric Kidney Transplantation Is Different from Adult Kidney Transplantation. Korean J Pediatr (2018) 61:205–9. 10.3345/kjp.2018.61.7.205
13.
Liberati A Altman DG Tetzlaff J Mulrow C Gotzsche PC Ioannidis JPA et al The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies that Evaluate Healthcare Interventions: Explanation and Elaboration. Bmj (2009) 339:b2700. 10.1136/bmj.b2700
14.
Lafranca J Pengel L Bramer W Dor F . Outcome of Preemptive versus Non-preemptive Kidney Transplantation: A Systematic Review. PROSPERO 2014 CRD42014010565 (2014). Available from: https://www.crd.york.ac.uk/PROSPERO/export_record_pdf.php (accessed May 4, 2020).
15.
Downs SH Black N . The Feasibility of Creating a Checklist for the Assessment of the Methodological Quality Both of Randomised and Non-randomised Studies of Health Care Interventions. J Epidemiol Community Health (1998) 52:377–84. 10.1136/jech.52.6.377
16.
Higgins JPT Thompson SG . Quantifying Heterogeneity in a Meta-Analysis. Statist Med (2002) 21:1539–58. 10.1002/sim.1186
17.
Cransberg K Smits JMA Offner G Nauta J Persijn GG . Kidney Transplantation without Prior Dialysis in Children: The Eurotransplant Experience. Am J Transpl (2006) 6:1858–64. 10.1111/j.1600-6143.2006.01405.x
18.
Cransberg K van Gool JD Davin JC de Jong MCJW Darby M Boendermaker ME et al Paediatric Renal Transplantations in the Netherlands. Pediatr Transpl (2000) 4:72–81. 10.1034/j.1399-3046.2000.00079.x
19.
Cuervo E de la Torre A Romero B et al Preemptive Renal Transplantation, One Single center Experience [Abstract# 237]. Pediatr Transpl (2007) 11:89.
20.
Kaya G Koyun M Comak E et al Long-term Results of Preemptive Kidney Transplantation [Abstract P-367]. Pediatr Nephrol (2018) 33:1951. 10.1007/s00467-017-3825-y
21.
Kramer A Stel VS Geskus RB Tizard EJ Verrina E Schaefer F et al The Effect of Timing of the First Kidney Transplantation on Survival in Children Initiating Renal Replacement Therapy. Nephrol Dial Transpl (2012) 27:1256–64. 10.1093/ndt/gfr493
22.
Mahmoud A Saïd M-H Dawahra M Hadj-Aïssa A Schell M Faraj G et al Outcome of Preemptive Renal Transplantation and Pretransplantation Dialysis in Children. Pediatr Nephrol (1997) 11:537–41. 10.1007/s004670050333
23.
Vats AN Donaldson L Fine RN Chavers BM . Pretransplant Dialysis Status and Outcome of Renal Transplantation in North American Children: a Naprtcs Study12. Transplantation (2000) 69:1414–9. 10.1097/00007890-200004150-00035
24.
Atkinson MA Roem JL Gajjar A Warady BA Furth SL Muñoz A . Mode of Initial Renal Replacement Therapy and Transplant Outcomes in the Chronic Kidney Disease in Children (CKiD) Study. Pediatr Nephrol (2020) 35:1015–21. 10.1007/s00467-019-04416-2
25.
Butani L Perez RV . Effect of Pretransplant Dialysis Modality and Duration on Long-Term Outcomes of Children Receiving Renal Transplants. Transplantation (2011) 91:447–51. 10.1097/TP.0b013e318204860b
26.
Flom LS Reisman EM Donovan JM Zaontz MR Stein J Firlit CF et al Favorable Experience with Pre-emptive Renal Transplantation in Children. Pediatr Nephrol (1992) 6:258–61. 10.1007/bf00878362
27.
Kim JK Lorenzo AJ Farhat WA Chua ME Ming JM Dos Santos J et al A Comparison of post‐transplant Renal Function in Pre‐emptive and post‐dialysis Paediatric Kidney Transplant Recipients. Pediatr Transpl (2019) 23:e13377. 10.1111/petr.13377
28.
Marlais M Martin K Marks S . 120 Improved Renal Allograft Survival for Pre-emptive Paediatric Renal Transplant Recipients in the united kingdom. Arch Dis Child (2018) 103:A48. 10.1136/goshabs.120
29.
Reydit M Salomon R Macher MA et al Pre-emptive Kidney Transplantation Is Associated with Improved Graft Survival in Children: Data from the French Renal Registry [Abstract O-33]. Pediatr Nephrol (2017) 32:1657. 10.1007/s00467-017-3753-x
30.
Fitzwater DS Brouhard BH Garred D Cunningham RJ Novick AC Steinmuller D . The Outcome of Renal Transplantation in Children without Prolonged Pre-transplant Dialysis. Clin Pediatr (Phila) (1991) 30:148–52. 10.1177/000992289103000302
31.
Sinha R Marks SD . Comparison of Parameters of Chronic Kidney Disease Following Paediatric Preemptive versus Non-preemptive Renal Transplantation. Pediatr Transpl (2010) 14:583–8. 10.1111/j.1399-3046.2010.01334.x
32.
Duzova A Bilginer Y Aki FT et al Preemptive Renal Transplantation in a Mediterranean Country [Abstract# 155]. Pediatr Transpl (2009) 13:82.
33.
Splinter A Tjaden LA Haverman L Adams B Collard L Cransberg K et al Children on Dialysis as Well as Renal Transplanted Children Report Severely Impaired Health-Related Quality of Life. Qual Life Res (2018) 27:1445–54. 10.1007/s11136-018-1789-4
34.
Abramowicz D Hazzan M Maggiore U Peruzzi L Cochat P Oberbauer R et al Does Pre-emptive Transplantation versus post Start of Dialysis Transplantation with a Kidney from a Living Donor Improve Outcomes after Transplantation? A Systematic Literature Review and Position Statement by the Descartes Working Group and ERBP. Nephrol Dial Transpl (2016) 31:691–7. 10.1093/ndt/gfv378
35.
Abou Ayache R Bridoux F Pessione F Thierry A Belmouaz M Leroy F et al Preemptive Renal Transplantation in Adults. Transplant Proc (2005) 37:2817–8. 10.1016/j.transproceed.2005.05.039
36.
Gill JS Tonelli M Johnson N Pereira BJG . Why Do Preemptive Kidney Transplant Recipients Have an Allograft Survival Advantage?Transplantation (2004) 78:873–9. 10.1097/01.tp.0000130204.80781.68
37.
Patzer RE Sayed BA Kutner N McClellan WM Amaral S . Racial and Ethnic Differences in Paediatric Access to Preemptive Kidney Transplantation in the United States. Am J Transpl (2013) 13:1769–81. 10.1111/ajt.12299
38.
Pruthi R O'Brien C Casula A Braddon F Lewis M Maxwell H et al UK Renal Registry 15th Annual Report: Chapter 4 Demography of the UK Paediatric Renal Replacement Therapy Population in 2011. Nephron Clin Pract (2013) 123:81–92. 10.1159/000353323
39.
Freeman MA Myaskovsky L . An Overview of Disparities and Interventions in Paediatric Kidney Transplantation Worldwide. Pediatr Nephrol (2015) 30:1077–86. 10.1007/s00467-014-2879-3
40.
Ishani A Ibrahim HN Gilbertson D Collins AJ . The Impact of Residual Renal Function on Graft and Patient Survival Rates in Recipients of Preemptive Renal Transplants. Am J Kidney Dis (2003) 42:1275–82. 10.1053/j.ajkd.2003.08.030
41.
Akkina SK Connaire JJ Snyder JJ Matas AJ Kasiske BL . Earlier Is Not Necessarily Better in Preemptive Kidney Transplantation. Am J Transpl (2008) 8:2071–6. 10.1111/j.1600-6143.2008.02381.x
42.
Grams ME Massie AB Coresh J Segev DL . Trends in the Timing of Pre-emptive Kidney Transplantation. Jasn (2011) 22:1615–20. 10.1681/asn.2011010023
43.
Kasiske BL Snyder JJ Matas AJ Ellison MD Gill JS Kausz AT . Preemptive Kidney Transplantation: The Advantage and the Advantaged. Jasn (2002) 13:1358–64. 10.1097/01.asn.0000013295.11876.c9
44.
Prezelin-Reydit M Combe C Harambat J Jacquelinet C Merville P Couzi L et al Prolonged Dialysis Duration Is Associated with Graft Failure and Mortality after Kidney Transplantation: Results from the French Transplant Database. Nephrol Dial Transpl (2019) 34:538–45. 10.1093/ndt/gfy039
45.
Clayton P McDonald S Chadban S . Lead Time Bias in Pre-emptive Living Kidney Donor Transplantation. Transplantation (2014) 98:143–4. 10.1097/00007890-201407151-00443
46.
Dekkers OM Vandenbroucke JP Cevallos M Renehan AG Altman DG Egger M . COSMOS-E: Guidance on Conducting Systematic Reviews and Meta-Analyses of Observational Studies of Etiology. Plos Med (2019) 16:e1002742. 10.1371/journal.pmed.1002742
47.
Roumelioti M-E Wentz A Schneider MF Gerson AC Hooper S Benfield M et al Sleep and Fatigue Symptoms in Children and Adolescents with CKD: A Cross-Sectional Analysis from the Chronic Kidney Disease in Children (CKiD) Study. Am J Kidney Dis (2010) 55:269–80. 10.1053/j.ajkd.2009.09.021
48.
Nicholas DB Picone G Selkirk EK . The Lived Experiences of Children and Adolescents with End-Stage Renal Disease. Qual Health Res (2011) 21:162–73. 10.1177/1049732310382789
49.
Tjaden L Tong A Henning P Groothoff J Craig JC . Children's Experiences of Dialysis: a Systematic Review of Qualitative Studies. Arch Dis Child (2012) 97:395–402. 10.1136/archdischild-2011-300639
50.
Tong A Samuel S Samuel S Zappitelli M Dart A Furth S et al Standardised Outcomes in Nephrology-Children and Adolescents (SONG-Kids): A Protocol for Establishing a Core Outcome Set for Children with Chronic Kidney Disease. Trials (2016) 17:401. 10.1186/s13063-016-1528-5
Summary
Keywords
outcomes, meta-analysis, systematic review, paediatric, preemptive kidney transplantation
Citation
Rana Magar R, Knight S, Stojanovic J, Marks SD, Lafranca JA, Turner S, Dor FJMF and Pengel LHM (2022) Is Preemptive Kidney Transplantation Associated With Improved Outcomes when Compared to Non-preemptive Kidney Transplantation in Children? A Systematic Review and Meta-Analysis. Transpl Int 35:10315. doi: 10.3389/ti.2022.10315
Received
20 December 2021
Accepted
12 January 2022
Published
17 March 2022
Volume
35 - 2022
Updates
Copyright
© 2022 Rana Magar, Knight, Stojanovic, Marks, Lafranca, Turner, Dor and Pengel.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liset H. M. Pengel, liset.pengel@nds.ox.ac.uk, orcid.org/0000-0001-9620-8639
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.