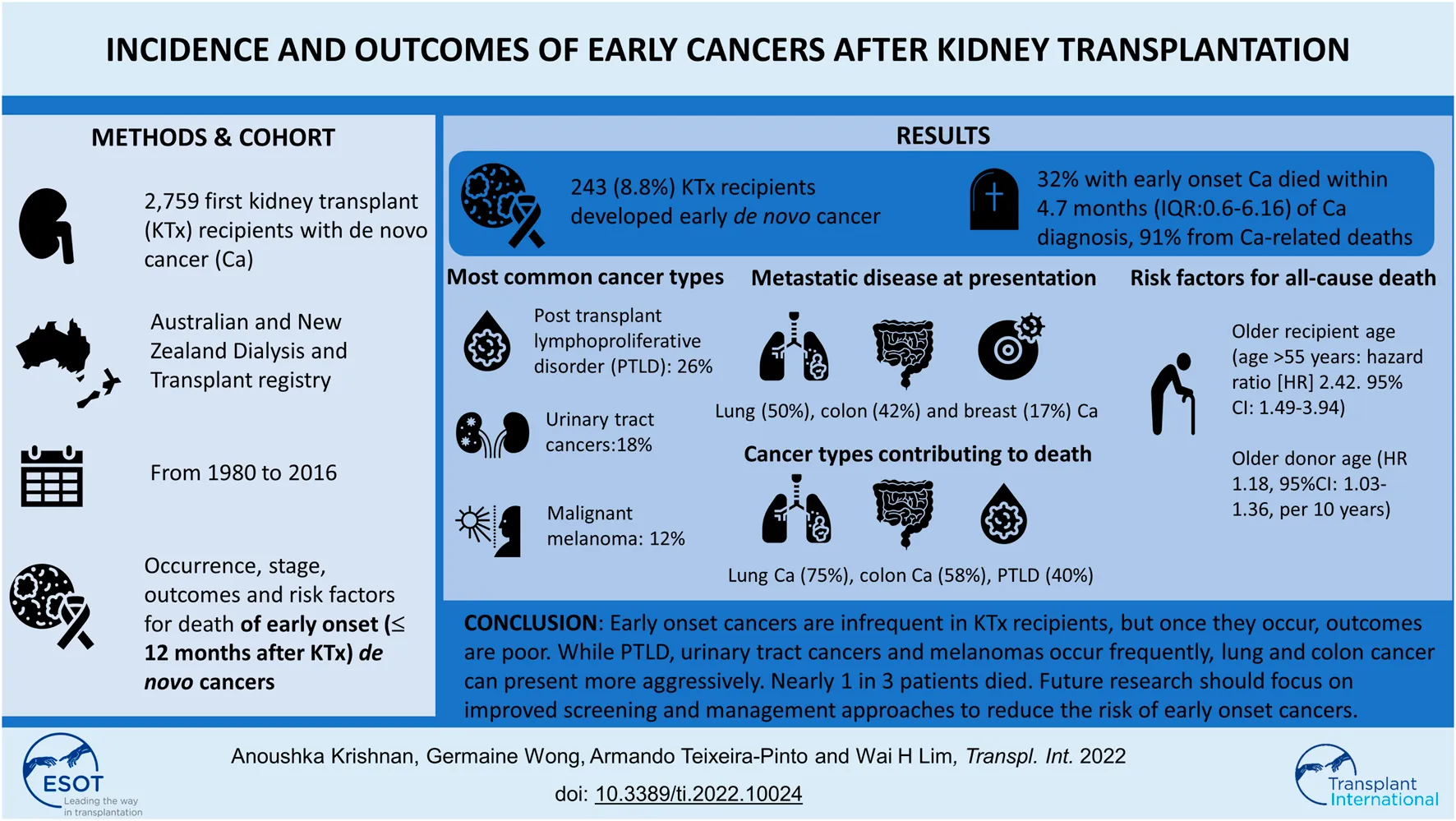
Abstract
Outcomes of early cancers after kidney transplantation are not well-understood. We included recipients of first live and deceased donor kidney transplants who developed de novo cancers in Australia and New Zealand between 1980–2016. We compared the frequency and stage of specific cancer types that developed early (≤12-months) and late (>12-months) post-transplantation. Risk factors for death were evaluated using multivariable Cox regression analyses. Of 2,759 recipients who developed de novo cancer, followed-up for 40,035 person-years, 243 (8.8%) patients were diagnosed with early cancer. Post-transplant lymphoproliferative disease, urinary cancers and melanoma were the most common cancer types (26%, 18%, and 12%) and the majority were either in-situ or locally invasive lesions (55%, 84%, and 86%). Tumors arising early from the gastrointestinal and respiratory systems were uncommon but aggressive, with 40% presenting with metastatic disease at time of diagnosis. Overall, 32% of patients with early cancers died within a median of 4.7 months (IQR:0.6–16) post-diagnosis and 91% were cancer-related deaths. Older recipient and donor age were associated with an increased risk of all-cause death. Early cancers, though infrequent in kidney transplant recipients, are associated with poor outcomes, as nearly 1 in 3 died from cancer-related death; with majority of deaths occurring within 12-months of cancer diagnosis.
Introduction
Cancer is a leading cause of death for many patients after kidney transplantation (1, 2). Compared to age and sex matched general population, cancer incidence and mortality rates are 2-3 times higher among transplant recipients (3). Epidemiological data have reported the mean time from transplantation to cancer diagnoses is approximately 6 years, suggesting that intensity of immunosuppression and cumulative drug exposure play key roles in cancer development (4, 5). However for some early cancers, such as post-transplant lymphoproliferative disease (PTLD) that commonly occur within a short timeframe after transplantation, the mechanistic pathways for cancer development may be different to those that occur later (5). Patients on dialysis are also at risk of certain cancers such as urinary tract cancer (6). Clinical practice guidelines recommend age-specific screening for potential transplant candidates and some guidelines suggest additional screening for kidney cancers in patients on dialysis (7). However, the sensitivity of these screening tests is imperfect (8) and may therefore, miss occult malignancies. Under the influence of immunosuppression, occult cancers may grow rapidly through deficiencies in tumor surveillance, and manifest early after transplantation.
Prior research has not quantified the burden and outcomes of these early cancers after transplantation. Knowledge of the epidemiology of these cancers and their risk factors for adverse outcomes will help to identify complex and high-risk patients and facilitate appropriate interventions such as targeted cancer screening in this at-risk population. In this study we aimed to compare the frequency, types, sites and stage of cancers that occurred early compared to those that occurred later after transplantation. We also compared the risk of cancer-related and all-cause death between recipients with early and late cancers and defined the risk factors for deaths in patients with early cancer.
Materials and Methods
Study Population
Using data from the Australia and New Zealand Dialysis and Transplant (ANZDATA) registry, kidney failure patients who have received a first deceased and living donor kidney transplant in Australia and New Zealand between 1980 and 2016 and had developed “de novo” cancer after transplantation were included in the analyses. Recipients with a prior history of cancer (other than a history of non-melanoma skin cancer- NMSC) and known “donor-transmitted” and “donor-derived” cancers were excluded (Figure 1). De novo cancer was defined as a cancer that occurred in a kidney transplant recipient with no prior history of cancer before transplantation and included all cancer types except NMSC. Donor-transmitted cancers are those cancers which are present in the donated organ and tissue at transplantation, whereas donor-derived cancers are those that are of donor origin but developed de novo in the allograft after transplantation. Details of both donor-derived and donor-transmitted cancers are provided to the ANZDATA registry by the individual units. However, the ANZDATA registry does not verify whether the cancer cells were of donor origin.
FIGURE 1
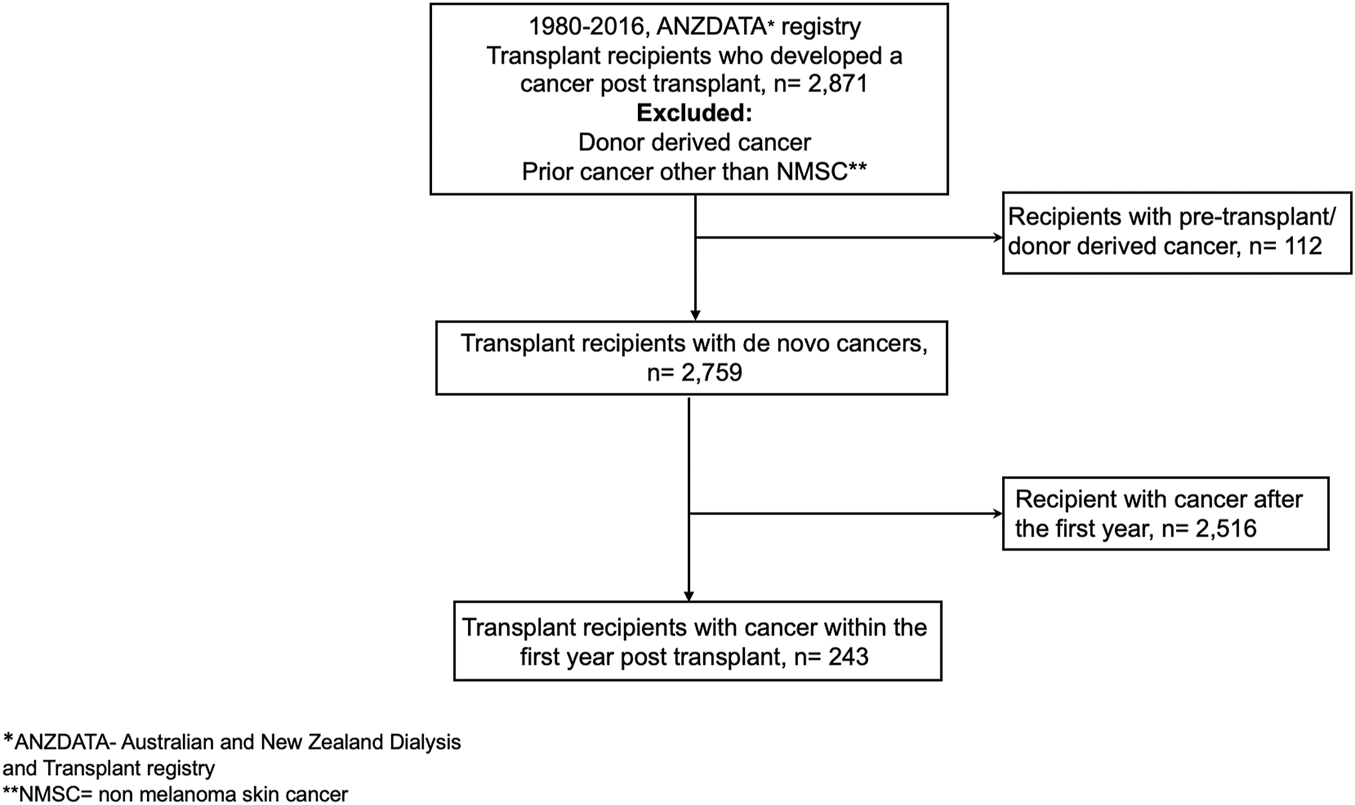
Participant flow.
The clinical and research activities being reported are consistent with the Principles of the Declaration of Istanbul as outlined in the “Declaration of Istanbul on Organ Trafficking and Transplant Tourism.” Ethics approval was obtained from the Human Research Ethics Committee of Western Australia, Australia. Written informed consents were sought from patients with kidney failure at time of entry into the registry, including the utilization of aggregate data for future research.
Exposure
Recipients were categorized according to whether they had developed early cancer or late cancer post kidney transplant. Early-onset cancers were defined as those cancers occurring within the first 12-months post transplantation, whereas late-onset cancers were defined as those occurring 12-months after transplantation.
Data Collection
Baseline characteristics recorded by the ANZDATA registry included donor factors of age, type and sex; recipient characteristics of age, sex, ethnicity, body mass index, waiting time prior to transplantation, comorbid conditions at time of transplantation (presence or absence of diabetes, coronary artery disease, cerebrovascular disease and peripheral vascular disease), primary causes of kidney failure; and transplant-related factors including the number of human leukocyte antigen (HLA) mismatches, total ischemic time (in hours), induction (none, interleukin-2 receptor therapy and T-cell depleting therapy) and initial immunosuppressive therapies (prednisolone, calcineurin-inhibitor and anti-metabolite therapies) and transplant era (categorized into 1980–1989, 1990–1999 and 2000–2016 transplant periods).
Ascertainment of De Novo Cancers
De novo cancers occurring post-kidney transplantation were reported to the ANZDATA registry. The registry does not verify the histology of the de novo cancers, but the cancer records within the ANZDATA registry are accurate with a high concordance rate compared to those reported to the New South Wales Cancer Registry (9), a mandatory requirement for cancer reporting in New South Wales. De novo cancers are recorded according to cancer sites and cell types according to the International Classification of Disease for Oncology, edition 3, first revision (ICD-O-3.1) (10).
Clinical Outcomes
The primary outcomes included the frequency, types, sites, stage (including presence of lymph node involvement and distant metastatic disease) and occurrence of cancer recurrence. Other outcomes included treatment of the de novo cancers in recipients with early cancers and comparison of the risk of cancer-related and all-cause death between recipients with early cancer and those with late cancer. We also defined the risk factors for all-cause deaths in recipients with early cancers.
Statistical Analyses
Data were expressed as number (proportion), mean and standard deviation (SD) and median and interquartile range (IQR) where appropriate, with comparisons between groups by chi-square test, analysis of variance (ANOVA) and Kruskal–Wallis test, respectively. We compared the frequency, cancer types, stage and outcomes of patients who developed early cancers with those who developed cancers 12 months after transplantation. The treatment patterns, responses to treatment and outcome of early-onset cancers were also described. Kaplan Meier survival curves were constructed for all-cause and cancer-specific mortality in recipients with early cancers and stratified by site-specific cancer types. The log-rank test was used to test the trend of all-cause and cancer-specific survival functions across the cancer types. Survival time was censored at the date of the clinical outcome or on 31 December 2017. The cumulative survivals (and 95%CI) from the time of cancer diagnosis till the time of death were calculated for patients with early and late-onset cancers. Adjusted multivariable cox regression models were used to evaluate the risk factors for all-cause mortality in patients with early-onset cancers. Covariates with p-values of <0.25 in the unadjusted association for all-cause mortality were included in the multivariable analyses. Proportional hazard assumptions were checked, and two-way interactions were tested. The final model retained the covariates that remained significant after adjustment using a backward stepwise strategy. Variables included in the final multi-variable model included donor age, recipient age (stratified as <35, 35–55 and over 55 years), sex, race (Indigenous Australians, Maori and other), smoking status (stratified as current smoker, ex-smoker or non-smoker), induction immunosuppression, initial anti-metabolite therapy (none, azathioprine and mycophenolic acid) and transplant era.
Analyses were undertaken using SPSS V10 statistical software program (SPSS Inc., North Sydney, Australia), R (version 3.6) and STATA (version 11 StataCorp LP, College Station, TX). P-values of <0.05 were considered statistically significant.
Results
Study Population
Between 1980 and 2016, a total of 21,844 patients received a first kidney transplant. Of these, 2,871 kidney transplant recipients developed cancer(s) post-transplantation, with 2,759 (96.1%) recipients developing de novo cancers post-transplant and 112 (3.9%) with either pre-transplant or donor derived cancers, respectively. Of the 2,759 recipients with de novo cancers, 243 (8.8%) developed de novo cancers within the first 12 months post-transplantation (Figure 1). The median (IQR) patient-follow up time for all recipients was 13.4 years (7.84–20.44) resulting in 40,006 patient-years of follow up with shorter median (IQR) follow-up periods for those who developed early cancer (4.8 [1.6–10.9]) years with 1,699 patient-years of follow-up.
Baseline characteristics of the study population with early and late onset de novo cancers are shown in Table 1. Recipients who developed early cancer were older (mean [SD] age: 50.8 [15.4] vs. 45.3 [14.2] years, p < 0.001), more likely to have pre-transplant diabetes (16.5% vs. 10.5%, p = 0.002) and coronary artery disease (12.3% vs. 6%, p < 0.001) and received kidneys from older donors (mean [SD] age: 42.3 [17.1] vs. 35.7 [18.6] years, p < 0.001) compared to those who developed late cancers. Additionally, a higher proportion of early de novo cancers developed in later transplant era (after the year 2000) [63.8% vs. 33.8%, p < 0.001]. The incidence rate of early onset cancer was 0.01 (95%CI: 0.007, 0.013) per 1000-person-days between 1980–1989, 0.02 (95%CI: 0.013, 0.022) per 1000-person-days between 1990–1999 and 0.09 (95%CI: 0.08, 0.10) per 1000-person-days after the year 2000.
TABLE 1
| Early cancers (n = 243, n, %) | Late cancers (n = 2,516, n, %) | p-values | |
|---|---|---|---|
| Donor characteristics | |||
| Age (years, mean [SD]) | 42.3 (17.1) | 35.7 (18.6) | <0.001 |
| Female gender (n, %) | 118 (48.6) | 1004 (39.9) | 0.001 |
| Type | 0.03 | ||
| Deceased | 179 (73.7) | 1927 (76.6) | |
| Live | 64 (26.3) | 589 (23.4) | |
| Recipient characteristics | |||
| Age (years, mean [SD]) | 50.8 (15.4) | 45.3 (14.2) | <0.001 |
| Female gender (n, %) | 98 (40.3) | 1051 (41.8) | 0.66 |
| Race (n, %) | 0.61 | ||
| Caucasian | 208 (85.5) | 2224 (88.4) | |
| Aboriginals/Maori | 11 (4.6) | 87 (3.4) | |
| Others | 24 (9.9) | 205 (8.2) | |
| Diabetes (n, %) | 40 (16.5) | 265 (10.5) | 0.002 |
| Coronary artery disease (n, %) | 30 (12.3) | 152 (6.0) | <0.001 |
| Peripheral vascular disease (n, %) | 10 (4.1) | 80 (3.2) | 0.09 |
| Cerebrovascular disease (n, %) | 9 (3.7) | 41 (1.6) | 0.04 |
| Smoker (n, %) | 0.19 | ||
| Non- smokers | 116 (57.4) | 1008 (52.4) | |
| Former smokers | 67 (33.2) | 655 (34.0) | |
| Current smokers | 19 (9.4) | 262 (13.6) | |
| Cause of ESKD (n, %) | 0.03 | ||
| Glomerulonephritis | 104 (42.8) | 1186 (47.1) | |
| Cystic | 32 (13.2) | 374 (14.9) | |
| Diabetes | 30 (12.3) | 201 (8.0) | |
| Vascular | 16 (6.6) | 96 (3.8) | |
| Analgesic nephropathy | 11 (4.5) | 107 (4.3) | |
| Others | 50 (20.6) | 552 (21.9) | |
| Viral serology | |||
| CMV | <0.001 | ||
| Negative | 65 (26.7) | 565 (22.5) | |
| Positive | 138 (56.8) | 1143 (45.4) | |
| Unknown | 40 (16.5) | 808 (32.2) | |
| EBV | <0.001 | ||
| Negative | 43 (17.7) | 237 (9.4) | |
| Positive | 134 (55.1) | 1061 (42.1) | |
| Unknown | 66 (27.2) | 1218 (48.5) | |
| Immunology/transplant | |||
| Waiting time (days, mean [SD]) | 868 (777) | 774 (768) | 0.07 |
| Ischemic time (hours, mean [SD]) | 11.1 (7.4) | 12.5 (7.9) | 0.01 |
| Transplant era (n, %) | <0.001 | ||
| 1980-1989 | 30 (12.3) | 667 (26.5) | |
| 1990-1999 | 58 (23.9) | 998 (39.7) | |
| After 2000 | 155 (63.8) | 851 (33.8) | |
| Induction immunosuppression | <0.001 | ||
| None | 131 (54) | 1917 (76) | |
| Interleukin-2 receptor therapy | 102 (42) | 446 (18) | |
| T-cell depleting therapy | 10 (4) | 153 (6) | |
| Maintenance immunosuppression | |||
| Steroids (Prednisolone) | 226 (93) | 2245 (89) | 0.06 |
| Calcineurin inhibitors | <0.001 | ||
| None | 16 (7) | 297 (12) | |
| Cyclosporine | 145 (60) | 1833 (73)) | |
| Tacrolimus | 82 (33) | 386 (15) | |
| Anti-metabolites | <0.001 | ||
| None | 27 (11) | 321 (13) | |
| Azathioprine | 60 (25) | 1246 (49) | |
| Mycophenolate mofetil/sodium | 156 (64) | 949 (38) | |
Baseline characteristics of patients who developed early (within 12 months) and late (after 12 months) de novo cancer post-transplant (n = 2,759).
Recipients who developed early cancer in the latter era (after 2000) were older compared to those who developed cancer in the earlier eras (p ≤ 0.01, Supplementary Table S1). The proportion of incident kidney transplant recipients with early-onset cancers was similar across the three eras of 1980-1989 (0.8%), 1990–1999 (1.2%) and 2000–2016 (1.2%) (p = 0.36, Supplementary Figure S1).
Cancer Types of Early-Onset and Late-Onset De Novo Cancers
The median (IQR) time to cancer onset was 205 days (107–298) in those with early-onset cancer compared to 2,083 days (1,675–4,914) in those with late-onset cancer. The three most common types of cancers in those who developed early de novo cancers were PTLD (25%), urinary tract cancers (18%) and malignant melanoma (12%). For late-onset cancers, the three most common types of de novo cancers were PTLD (14%), urinary tract cancers (13.6%) and melanomas (10.6%) (Table 2). Supplementary Table S2 demonstrates differences between recipients of living and deceased donor kidneys who developed early cancers. The distribution of the three most frequently occurring cancers (PTLD, urinary cancers and melanomas) were similar between the two groups.
TABLE 2
| Cancer type (n, %) | Cancer within 12 months (n = 243) | Cancer after 12 months (n = 2,516) | p-value |
|---|---|---|---|
| Post-transplant lymphoproliferative disease | 62 (25.5) | 351 (14) | <0.001 |
| Urinary tract cancera | 44 (18.1) | 342 (13.6) | 0.05 |
| Melanoma | 29 (11.9) | 266 (10.6) | 0.51 |
| Other GI tractb | 17 (7.0) | 152 (6.0) | 0.35 |
| Genital3 | 15 (6.2) | 262 (10.4) | 0.04 |
| Colorectal | 12 (4.9) | 197 (7.8) | 0.10 |
| Breast | 12 (4.9) | 143 (5.7) | 0.23 |
| Prostate | 9 (3.7) | 172 (6.8) | 0.06 |
| Lung | 8 (3.3) | 182 (7.2) | 0.02 |
| Thyroid | 7 (2.9) | 54 (2.1) | 0.45 |
| Brain | 3 (1.2) | 24 (1.0) | 0.18 |
| Lip | 1 (0.4) | 30 (1.2) | 0.27 |
| Unknown origin | 2 (0.8) | 70 (2.8) | 0.006 |
| Others | 22 (9.1) | 271 (10.8) | 0.68 |
Distribution of cancer types amongst patients who developed early cancer compared to those who developed late cancer.
Cancers of the kidney, ureters and bladder.
Other gastrointestinal tract cancers (including gall bladder, small intestine, bile duct, pancreas, liver, stomach, esophagus).
Cervix, ovaries, uterus, penile.
Cancer Stage and Outcomes of Early-Onset and Late-Onset De Novo Cancers
Of recipients who had early-onset cancers, 25% (n = 61) developed or presented with advanced stage disease (lymph node involvement or distant metastases). At the time of presentation, 50% of lung, 42% of colorectal and 17% of breast cancers had evidence of advanced disease (Figure 2A). For late-onset cancers, 45% of lung, 41% of colorectal and 26% of breast cancers had evidence of advanced disease at the time of diagnosis. In contrast, 9% and 14% of early kidney cancers and melanomas and 16% and 6% of these late cancers respectively, presented with evidence of advanced disease. Kaplan-Meier survival curves for all-cause mortality and cancer mortality according to the most common site-specific cancer types are shown in Figure 2B.
FIGURE 2
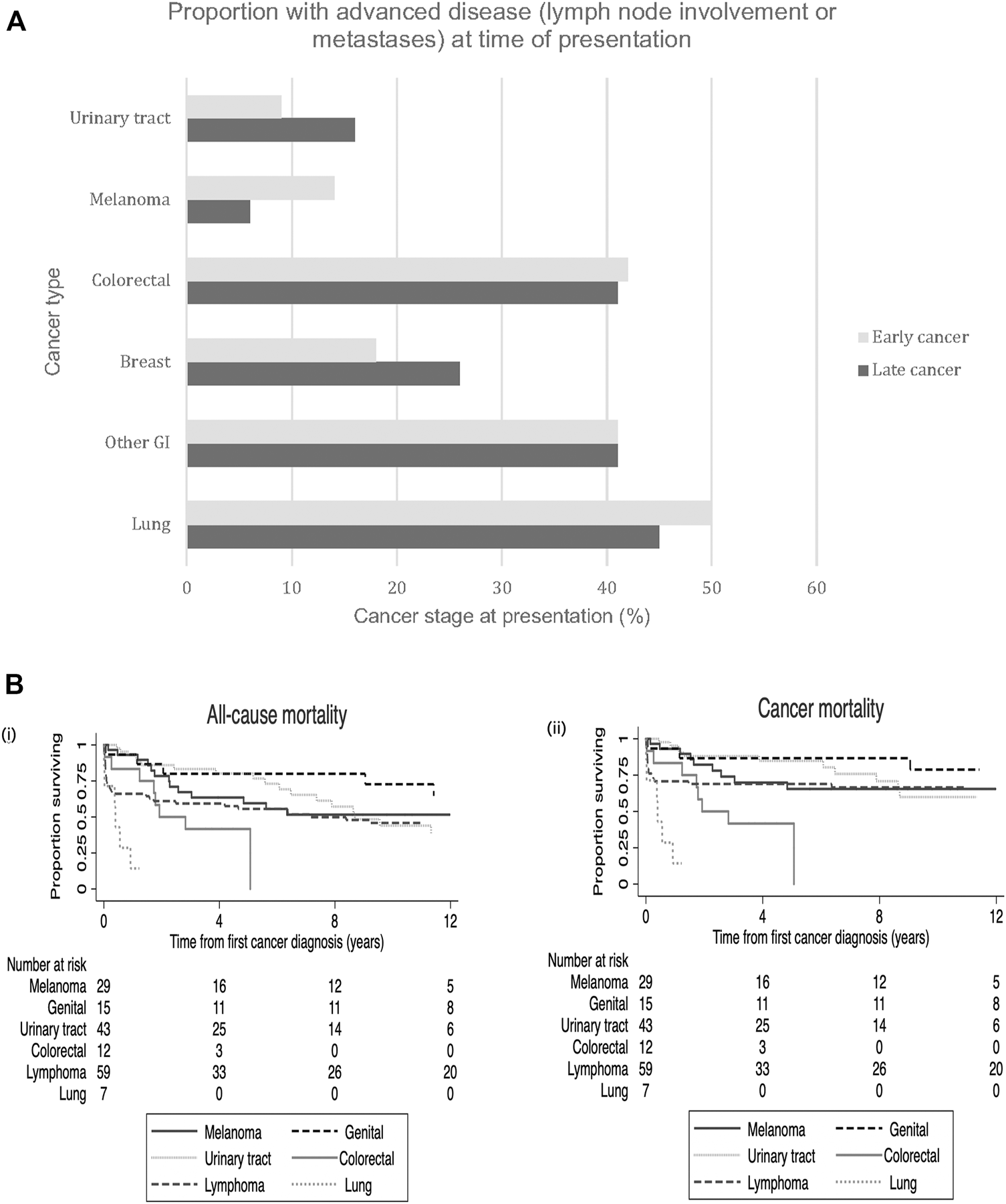
(A) Site-specific cancer types. Proportion of early site-specific cancers presenting with advanced stage disease (lymph node involvement or metastases) at time of cancer presentation in those with early vs. late de novo cancers. Legend: GI- gastrointestinal. (B) Kaplan Meier survival curves with number at risk tables for all-cause mortality (i) and cancer mortality (ii) according to the six common site-specific early de novo cancers.
Among recipients who developed de novo early-onset cancers, 77 (32%) died with 70 (91%) deaths attributed to cancer related deaths (Table 3). The median (IQR) time from cancer diagnosis to cancer-specific and all-cause deaths was 145 days (IQR: 20–464) and 144 days (20–505), respectively. For recipients with late-onset cancers, 1,473 (59%) recipients died with 977 (39%) attributed to cancer-related deaths. The median time from cancer diagnosis to cancer-specific and all-cause deaths were 229 days (53–781) and 427 days (80–1,623), respectively. Figures 3, 4 shows the Kaplan Meier curves of cancer-related deaths and all-cause deaths for recipients with early and late-onset de novo cancer; both from time of transplant and from time of cancer diagnosis, with majority of deaths being related to cancer in those who developed early de novo cancer.
TABLE 3
| Multiple incident cancers (n, %) | Number of cancers (n) | First cancer causing allograft failure (n, %) | First cancer causing Death (n, %) | |
|---|---|---|---|---|
| Cancer first 12 months | 39 (16.0) | 243 | 3 (1.2) | 77 (31.7) |
| Melanoma | 29 | 0 | 7 (24.1) | |
| Urinary tracta | 44 | 1 (2.3) | 7 (15.9) | |
| Lymphoproliferative disease | 62 | 1 (1.6) | 25 (40.3) | |
| Colorectal | 12 | 0 | 7 (58.3) | |
| Other GIb | 17 | 0 | 14 (82.4) | |
| Lung | 8 | 0 | 6 (75.0) | |
| Brain | 3 | 0 | 1 (33.3) | |
| Genitalc | 15 | 0 | 1 (6.7) | |
| Prostate | 9 | 0 | 1 (11.1) | |
| Breast | 12 | 0 | 0 | |
| Thyroid | 7 | 0 | 0 | |
| Lip | 1 | 0 | 0 | |
| Unknown origin | 2 | 0 | 2 (100.0) | |
| Others | 22 | 1 (4.5) | 6 (27.3) |
Outcomes of early cancer.
Cancer occurring >12 months post-transplant: 13.4% multiple cancers, 1.2% first cancer causing allograft failure and 31.7% first cancer causing death.
Cancers of the kidney, ureters and bladder.
Other gastrointestinal tract cancers (including gall bladder, small intestine, bile duct, pancreas, liver, stomach, esophagus).
Cervix, ovaries, uterus, penile.
FIGURE 3
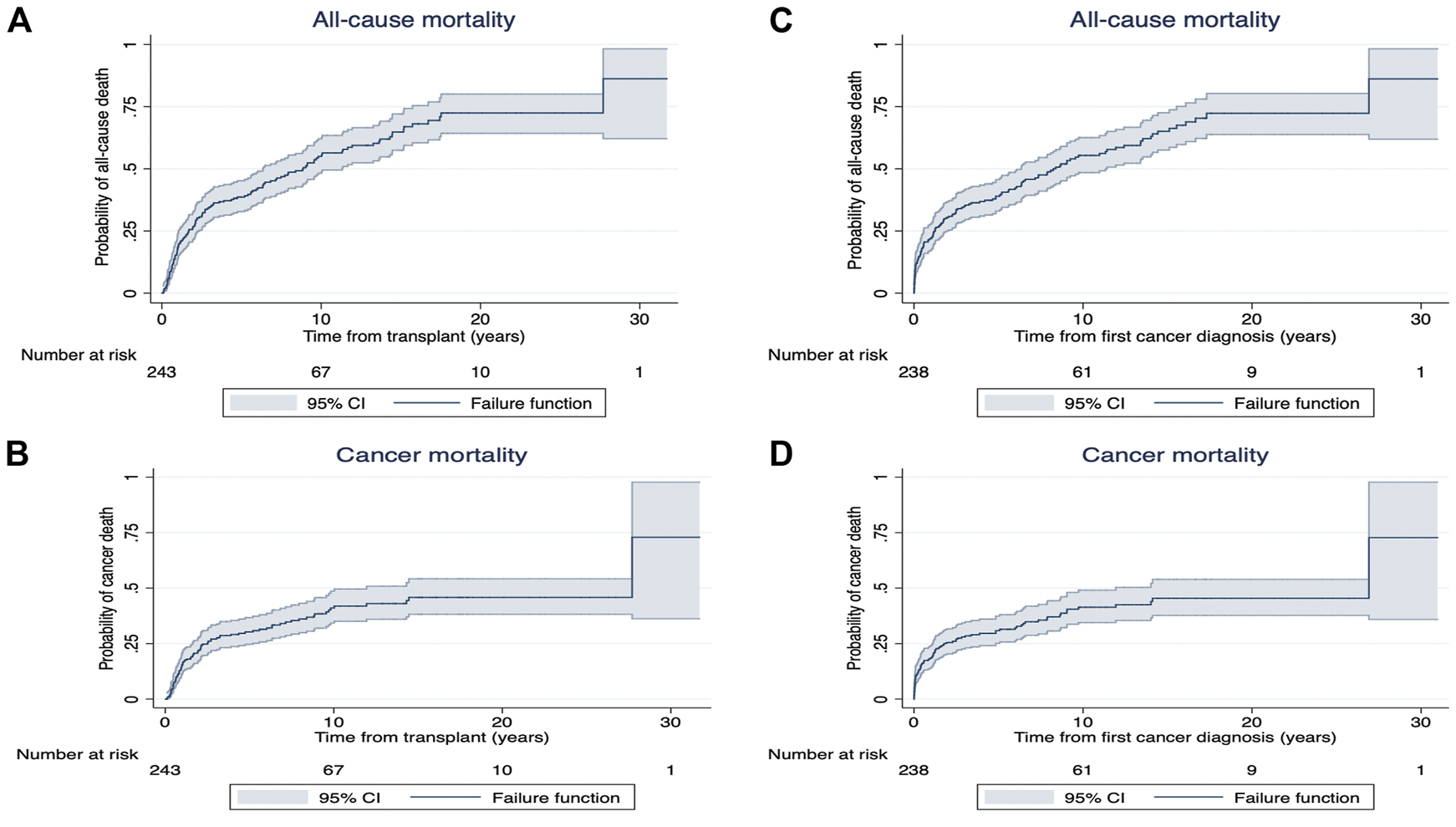
Kaplan Meier survival curves for all-cause mortality (A) and cancer mortality (B) from time of exposure (from transplant [years]) and all-cause mortality (C) and cancer mortality (D) from time from first cancer diagnosis (years) in recipients with early de novo cancer.
FIGURE 4
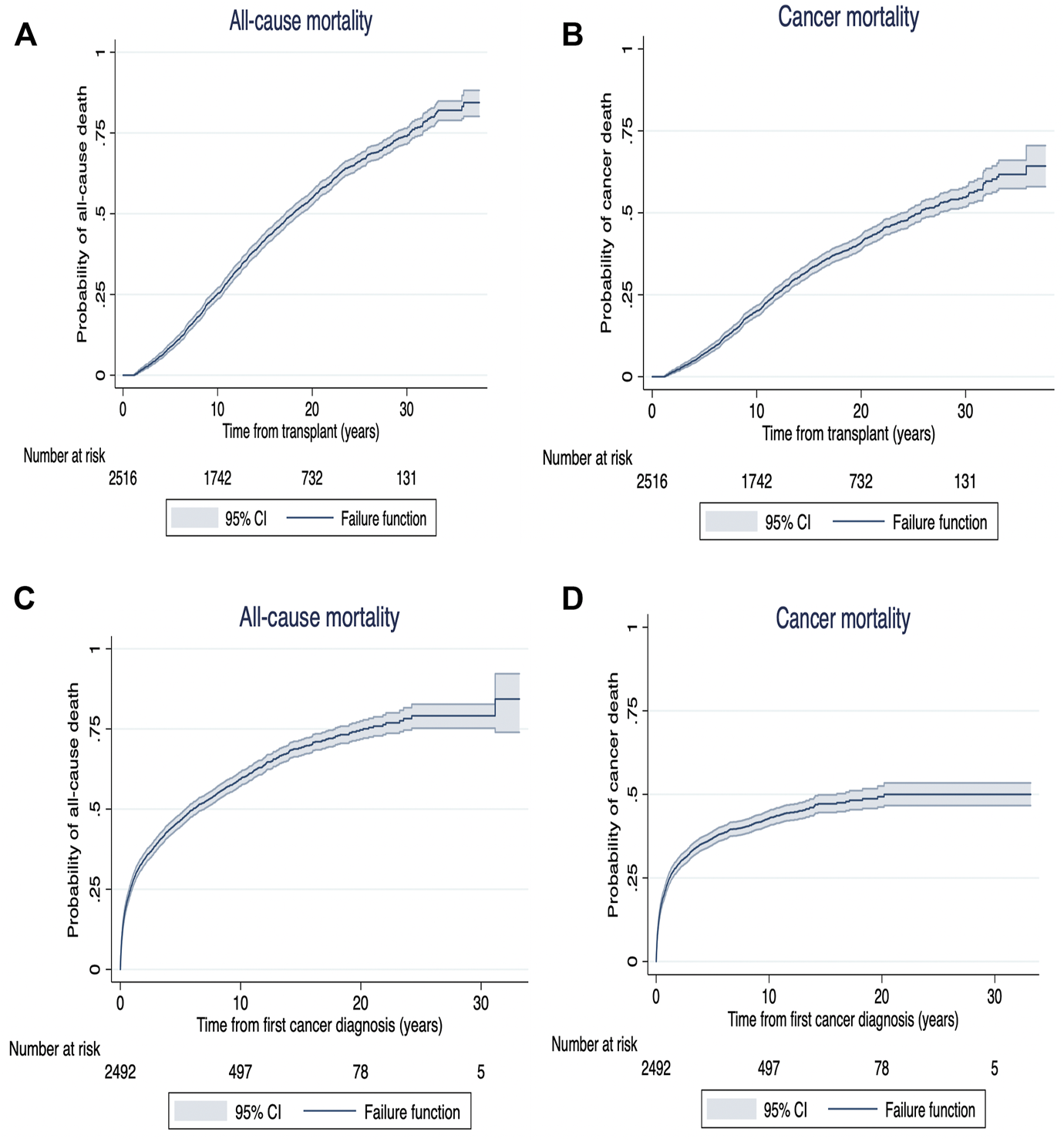
Kaplan Meier survival curves for all-cause mortality (A) and cancer mortality (B) from time of exposure (from transplant [years]) and all-cause mortality (C) and cancer mortality (D) from time from cancer diagnosis (years) in recipients with late (>12 months) de novo cancer.
Following cancer diagnosis, the overall patient survivals at 1, 5 and 10 years for recipients who developed early de novo cancer were 77% (95%CI: 70.7, 81.4), 46% (95%CI: 39.7, 52.2) and 25% (95%CI: 19.8, 30.7). In recipients who developed late de novo cancers, the overall patient survivals at 1, 5 and 10 years were 73% (95%CI: 70.7, 74.2), 41% (95%CI: 38.6, 42.3) and 20% (95%CI: 18.2, 21.3) (Supplementary Figure S1).
Early-Onset Cancers Outcomes-Deaths, Recurrent and Second Cancers
Most of the early cancer related deaths were associated with lung (75%) and colorectal (58.3%) cancers (Table 3).
Of those who developed lung cancer, 75% (n = 6) died, with the median (IQR) time to death of 142 days (6–236). The median (IQR) age at diagnosis was 56 years (50–62) with 50% being males. 50% (n = 3) of the patients presented with advanced stage disease at the time of presentation.
Of those who developed early colorectal cancer, 58% (n = 7) died, with the median (IQR) time to death of 651 days (96–924). The median (IQR) age at diagnosis was 59 years (49–69) with 57% being males. 57% (n = 4) of the recipients presented with advanced stage disease at the time of presentation.
Of the more common cancer types, 25 (40%) recipients died from PTLD (median [IQR] age at diagnosis was 48 [28–56] years) while 7 (16%) died from urinary tract cancers (median [IQR] age at diagnosis was 56 [49–60] years), with nearly 50% of those dying from the latter presenting with advanced stage disease. A detailed description of all the early onset de novo cancers that had contributed to premature mortality is shown in Table 3.
Of all recipients with early-onset cancers (n = 243), onset of a second (new) cancer or recurrence of de novo cancer occurred in 39 recipients (16%), with a median (IQR) time to cancer occurrence of 1,165 (71–2,309) days. The most common cancers were those that involved the urinary tract, lung and the gastrointestinal tracts (15% each). Seven (18%) of these second cancers occurred within 1 year of the primary malignancy.
In this cohort, 77% (n = 33) recipients developed a second new malignancy at a different site within a median (IQR) of 1,414 (435–2,423) days, of which, the most common cancer sites were lung cancer (n = 6, 18%) closely followed by cancer of the urinary tract (n = 5, 15%). Additionally, 15% (n = 6) recipients had recurrence of the de novo primary malignancy within a median (IQR) of 5 (0–159) days, of which 33% (n = 2) had recurrence of melanomas and 33% (n = 2) had recurrence of transitional cell cancer of the urinary tract.
Treatments of cancers that resulted in death were diverse and included various combinations of surgical resection, chemotherapy, radiotherapy and reduction of immunosuppressive medications (Table 4).
TABLE 4
| Cancer type, Sex distribution and median age (IQR) | Initial cancer location, type and number | Stage at presentation/number/time to advanced disease | Treatment |
|---|---|---|---|
| Melanoma (n = 7), M3, F4, Median age 61 (54–62) | Skin-7 | Metastatic lesion- 6 (2 metastasized at initial presentation | Local excision- 6 |
| - 4 metastasized later; Median 15 months [12–27]) | Chemotherapy and reduction IS- 1 | ||
| No treatment- 1 | |||
| Urinary tract (n = 7)a, M6, F1, Median age 56 (49–60) | Urinary bladder- 5 (3x TCC, 2x adenocarcinoma)—4 invasive presentation) | Metastatic lesions- 3 | Bladder excision-2 Chemotherapy- 1 |
| Native kidney-2 (adenocarcinoma) | - Kidney: 1- at presentation | Kidney mass excision- 1 | |
| - Bladder:2- at 7 and 10 months | |||
| Lymphoproliferative disease (n = 25), M16, F9, Median age 48 (28–56) | Lymph nodes/blood/bone marrow- 10 | In situ lesion- 4 | Reduced IS- 10 |
| Liver- 2 | Invasive lesion- 7 | Chemotherapy- 5 | |
| Small intestine- 2 | Metastatic lesion- 12 (all at presentation) | Radiotherapy- 5 | |
| Brain- 3 | Local lymph nodes- 1 | Local excision (small intestine- 1) | |
| Colon- 2 | Unknown- 1 | No treatment- 8 | |
| Kidney- 2 | Unknown- 1 | ||
| Lung- 2 | |||
| Unknown primary- 2 | |||
| Colorectal (n = 7), M4, F3, Median age: 59 (49–69) | Colon- 5 | Invasive- 3 | Local excision- 3 |
| Recto-sigmoid- 2 (adenocarcinoma- 1, squamous cell carcinoma- 1) | Metastatic lesion- 4 at presentation | Radiotherapy-1 | |
| All 3 invasive metastasized at 8, 13 and 55m post-diagnosis | Chemotherapy- 3 | ||
| No treatment- 1 | |||
| Other GI (n = 14)b, M 11, F3, Median age: 59 (35–66) | Pancreas- 5 (adenocarcinoma) | Metastatic lesions- 8 (6 at presentation: pancreas- 3, oesophagus- 2, small bowel- 1, stomach- 1) | Pancreas: none- 2, reduction in IS- 2, chemotherapy- 1 |
| Stomach- 2 (adenocarcinoma) | Invasive- 4 | Stomach: none- 1, chemotherapy- 1 | |
| Oesophageal- 2, (adenocarcinoma) | Oesophageal: Chemo-radiotherapy- 2 | ||
| Ampulla of Vater- 2 | Ampulla of Vater: None- 2 | ||
| Hepatocellular carcinoma- 1 | Small intestine: Excision- 1 | ||
| Small intestine- 1 (adenocarcinoma) | Oropharynx: Excision, reduction IS and radiotherapy- 1 | ||
| Oropharynx- 1 | HCC: excision- 1 | ||
| Lung (n = 6), M3, F3, Median age: 56 (50–62) | Adenocarcinoma- 2 | Metastatic lesion- 4 (3 at presentation, 1 at 4 months) | Radiotherapy- 2 |
| Small cell cancer- 2 Large cell cancer- 1 | Invasive- 2 | Chemotherapy- 1 | |
| Unknown- 1 | Multiple- 2 (chemo-radiotherapy and reduction IS- 1) | ||
| None- 1 |
Characteristics of early cancers contributing to death.
M, males, F, females.
Cancers of the kidney, ureters and bladder
Other gastrointestinal tract cancers (including gall bladder, small intestine, bile duct, pancreas, liver, stomach, esophagus).
Factors Associated With All-Cause Mortality in Early-Onset Cancers
Risk factors associated with all-cause death among those with early cancers were older recipient age [>55 years: 2.42 (1.49–3.94), ref: 35-55 years] and older donor age [1.18 (1.03–1.36), per 10-years].
Discussion
In this large contemporaneous cohort of kidney transplant recipients with de novo cancers spanning over 3 decades, we have shown that almost 1 in 10 of these cancers occurred within the first 12 months post-transplantation. The most common cancer types were PTLD, malignant melanoma and cancers of the urinary tract, and typically, most of these cancers were of early stage at the time of presentation. On the contrary, recipients with other cancer types such as cancers of the digestive and respiratory systems tend to present with advanced stage disease. Overall, 32% of patients with early cancers died within a median of 4.7 months (IQR: 0.6–16) post-diagnosis and 91% were cancer-related deaths. Characteristics associated with an increased risk of death in recipients with early-onset cancer included increasing donor and recipient age.
Early cancers after transplantation are devastating events with a high burden of morbidity and mortality. Additionally, treatment strategies lack robust trial-based evidence and usually consist of surgical resection, radiotherapy and judicious reduction in immunosuppression with regular monitoring for cancer progression and allograft function. Certain strategies such as cancer screening may reduce the incidence of late-stage cancer through early detection, allowing interventions to be instigated early and before transplantation when the disease is still at a precancerous stage. Most clinical practice guidelines recommended routine age and sex-specific population-based cancer screening prior to listing (7). These include biennial bowel screening using either fecal immunochemical testing, or 5-years flexible sigmoidoscopy, biennial mammography for breast cancer, low-dose computer-tomography for lung cancer screening, and routine cervical screening using human papillomavirus test (HPV) for oncogenic cervical genotypes and pre-cancerous cervical lesions prior to transplantation (7). Despite these recommendations, uptake for screening in general among our candidates with chronic kidney disease is likely to be low and may potentially explain the late presentation of certain cancer types such as lung and gastrointestinal cancers within the early months after transplantation. While we do not routinely collect screening data in our transplant candidates, our prior work has indicated that the uptake of certain cancer screenings such as breast and cervical cancer are quite low amongst patients with kidney disease (11). Patients with kidney disease and kidney transplants undergo significant changes to their overall physical and psycho-social health and tend to focus on their current kidney health and are less inclined to prioritize cancer screening over imminent health problems.
Other guidelines suggest routine ultrasonographic screening (either annual or biennially) for renal cell cancers. However, evidence to support these recommendations are limited. For instance, the accuracy of ultrasonography in detecting malignant lesions in those with kidney failure is uncertain. Ultrasonography is largely operator-dependent and test performance varies with patient habitus, the kidneys and the size of the lesion (12). In the general population, test sensitivity and specificity are lower in detecting tumours <3 cm in size. In patients with kidney failure, who have scarred native kidneys with acquired cystic disease, the accuracy of detecting small renal cell cancers is ambiguous. Moreover, prior Markov modelling studies have suggested that routine surveillance for renal cancers may not be cost effective in the low to moderate risk population (13). Screening is not without harm as uncertain lesions may lead to further investigations or treatments, and therefore undue delays for transplant waitlisting. Currently, there is no clear consensus on screening for post-transplant renal cell cancers as data are limited (14). There are similar concerns regarding the risk-benefit ratio of screening high risk population for lung cancers with annual low-dose computed tomography even in the general population (15) and this modality has not been validated in the transplant population.
Viral linked cancers such as lymphoma or post-transplant lymphoproliferative disorders (PTLD) have a higher incidence in the transplant population, compared to the general population, with standardized mortality rates (SMRs) being as high as 10.7 for PTLD (3, 6, 16). A quarter of our cohort with early cancers developed PTLD within the first year of transplant with a younger median age of 48 years compared to other cancers and nearly 40% died. This is consistent with previous findings of a bimodal distribution in the incidence of PTLD development after transplantation (17). PTLD most commonly occurs within the first year of transplant affecting younger (<25 years) or older (>60 years) patients (18) and has a high mortality rate of ∼50% (19, 20). Primary Epstein-Barr virus (EBV) infection and pre-transplant EBV sero-negativity are risk factors for early onset PTLD, especially in younger transplant recipients, while late B-cell PTLD involves EBV-negative lesions (in 40–50%) (17). Once PTLD occurs, the risk of death is high (>14 fold higher than in recipients without PTLD) with median time of 6 months from diagnosis to death (21).
Older recipient age and donor age were both associated with an increased risk of cancer-related death. Over the past decades, there has been a changing demographic of transplant recipients. We are increasingly transplanting older patients with higher comorbidity burden and this in turn may have implications on screening procedures, cancer monitoring and degree of immunosuppression.
This study has several limitations. ANZDATA registry does not collect information on the uptake, adherence, type and timing of cancer screening for each transplant recipient. We lacked information on histological cancer data and treatment specific data, EBV data, relevant habits such as tobacco or alcohol use, therapeutic drug levels of immunosuppressive drugs, patients who were listed for kidney transplantation or were subsequently delisted (including those who may have developed incident cancer on the waiting list), quality of life measures and the severity of comorbid disease. There is a likelihood of selection bias due to systematic differences in the management of recipients who developed cancer.
Conclusion
In conclusion, early cancer is an infrequent complication after kidney transplantation but once it occurs, outcomes are generally poor. Clinicians should be more cognizant of the development of early cancers especially in the older population. Examination of granular data and the development of screening and management approaches to decrease post-transplant cancers without increasing the risk of allograft failure, with clear considerations of patient preferences and values may improve outcomes in this population.
Statements
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: Data was obtained from the Australian and New Zealand Dialysis and Transplant registry. Requests to access these datasets should be directed to https://www.anzdata.org.au.
Ethics statement
The studies involving human participants were reviewed and approved by the Research Ethics Committee of Western Australia, Australia. The patients/participants provided their written informed consent to participate in this study.
Author contributions
AK and WL participated in the research design, data analysis, performance of research and in writing of the paper. GW participated in research design and in writing of the paper. AT-P participated in data analysis and review of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2022.10024/full#supplementary-material
Supplementary Figure S1Proportion of recipients who developed a de novo cancer post-transplant stratified by transplant era.
Abbreviations
ANZDATA, Australian and New Zealand dialysis and transplant; ANOVA, analysis of variance; EBV, epstein-barr virus; ESKD, end stage kidney disease; HLA, human leukocyte antigen; ICD, international classification of disease; IQR, inter-quartile range; NMSC, non-melanoma skin cancer; PTLD, post-transplant lymphoproliferative disease; SD, standard deviation; SMR, standardized mortality rates.
References
1.
Wong G Chapman JR Craig JC . Death from Cancer: A Sobering Truth for Patients with Kidney Transplants. Kidney Int (2014) 85(6):1262–4. 10.1038/ki.2013.494
2.
Farrugia D Mahboob S Cheshire J Begaj I Khosla S Ray D et al Malignancy-Related Mortality Following Kidney Transplantation Is Common. Kidney Int (2014) 85(6):1395–403. 10.1038/ki.2013.458
3.
Vajdic CM McDonald SP McCredie MRE van Leeuwen MT Stewart JH Law M et al Cancer Incidence Before and after Kidney Transplantation. JAMA (2006) 296(23):2823–31. 10.1001/jama.296.23.2823
4.
Navarro MD López-Andréu M Rodríguez-Benot A Agüera ML Del Castillo D Aljama P . Cancer Incidence and Survival in Kidney Transplant Patients. Transplant Proc (2008) 40(9):2936–40. 10.1016/j.transproceed.2008.09.025
5.
ChapmanWebster JR Webster AC Wong G . Cancer in the Transplant Recipient. Cold Spring Harb Perspect Med (2013) 3(7):677. 10.1101/cshperspect.a015677
6.
Au EH Chapman JR Craig JC Lim WH Teixeira-Pinto A Ullah S et al Overall and Site-Specific Cancer Mortality in Patients on Dialysis and After Kidney Transplant. J Am Soc Nephrol : JASN. (2019) 30:471–80. 10.1681/asn.2018090906
7.
Chadban SJ Ahn C Axelrod DA Foster BJ Kasiske BL Kher V et al Summary of the Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline on the Evaluation and Management of Candidates for Kidney Transplantation. Transplantation (2020) 104(4S1 Suppl. 1):708–14. 10.1097/TP.0000000000003137
8.
Wong G Chapman JR Craig JC . Cancer Screening in Renal Transplant Recipients: What Is the Evidence?Clin J Am Soc Nephrol (2008) 3(Suppl. 2):S87–S100. 10.2215/CJN.03320807
9.
Webster AC Supramaniam R O'Connell DL Chapman JR Craig JC . Validity of Registry Data: Agreement Between Cancer Records in an End-Stage Kidney Disease Registry (Voluntary Reporting) and a Cancer Register (Statutory Reporting). Nephrology (Carlton). (2010) 15(4):491–501. 10.1111/j.1440-1797.2010.01297.x
10.
Fritz AG . In: International Classification of Diseases for Oncology : ICD-O. Third Edition, First Revision. Geneva: World Health Organization (2013). p. 242. viii.
11.
Wong G Hayward JS McArthur E Craig JC Nash DM Dixon SN et al Patterns and Predictors of Screening for Breast and Cervical Cancer in Women with CKD. Clin J Am Soc Nephrol (2017) 12(1):95–104. 10.2215/cjn.05990616
12.
Fenton JJ Weiss NS . Screening Computed Tomography. Cancer (2004) 100(5):986–90. 10.1002/cncr.20055
13.
Wong G Howard K Webster AC Chapman JR Craig JC . Screening for Renal Cancer in Recipients of Kidney Transplants. Nephrol Dial Transplant (2011) 26(5):1729–39. 10.1093/ndt/gfq627
14.
Hickman LA Sawinski D Guzzo T Locke JE . Urologic Malignancies in Kidney Transplantation. Am J Transpl (2018) 18(1):13–22. 10.1111/ajt.14533
15.
Jonas DE Reuland DS Reddy SM Nagle M Clark SD Weber RP et al Screening for Lung Cancer with Low-Dose Computed Tomography. JAMA (2021) 325(10):971–87. 10.1001/jama.2021.0377
16.
Kasiske BL Snyder JJ Gilbertson DT Wang C . Cancer After Kidney Transplantation in the United States. Am J Transpl (2004) 4(6):905–13. 10.1111/j.1600-6143.2004.00450.x
17.
Luskin MR Heil DS Tan KS Choi S Stadtmauer EA Schuster SJ et al The Impact of EBV Status on Characteristics and Outcomes of Posttransplantation Lymphoproliferative Disorder. Am J Transpl (2015) 15(10):2665–73. 10.1111/ajt.13324
18.
Smith JM Rudser K Gillen D Kestenbaum B Seliger S Weiss N et al Risk of Lymphoma After Renal Transplantation Varies with Time: An Analysis of the United States Renal Data System. Transplantation (2006) 81(2):175–80. 10.1097/01.tp.0000188687.18972.a8
19.
Dharnidharka VR Naik AS Axelrod D Schnitzler MA Xiao H Brennan DC et al Clinical and Economic Consequences of Early Cancer After Kidney Transplantation in Contemporary Practice. Transplantation (2017) 101(4):858–66. 10.1097/tp.0000000000001385
20.
Opelz G Döhler B . Lymphomas After Solid Organ Transplantation: A Collaborative Transplant Study Report. Am J Transpl (2004) 4(2):222–30. 10.1046/j.1600-6143.2003.00325.x
21.
Francis A Johnson DW Craig J Teixeira-Pinto A Wong G . Post-Transplant Lymphoproliferative Disease May Be An Adverse Risk Factor for Patient Survival But Not Graft Loss in Kidney Transplant Recipients. Kidney Int (2018) 94(4):809–17. 10.1016/j.kint.2018.06.009
Summary
Keywords
kidney transplantation, early cancer, cancer, ANZDATA, registry, cancer outcome, cancer death
Citation
Krishnan A, Wong G, Teixeira-Pinto A and Lim WH (2022) Incidence and Outcomes of Early Cancers After Kidney Transplantation. Transpl Int 35:10024. doi: 10.3389/ti.2022.10024
Received
05 September 2021
Accepted
05 April 2022
Published
03 May 2022
Volume
35 - 2022
Updates
Copyright
© 2022 Krishnan, Wong, Teixeira-Pinto and Lim.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: A. Krishnan, anoushka.krishnan@health.wa.gov.au
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.