Abstract
Background: Wheat-dependent exercise-induced anaphylaxis (WDEIA) is induced not by the intake of wheat-based food alone, but by the combination of exercise stress and such intake. Provocation tests have been reported to be useful for the diagnosis of this condition, but they are not always accurate. Furthermore, provocation tests are associated with the risk of anaphylactic shock, so safer testing is required.
Subjects and methods: Thirty-three patients (mean age 35.5 ± 16.0 years) who underwent provocation tests from April 2019 to July 2023 were included in this study. We investigated associations of their provocation test results with their medical history, blood test results before the provocation tests, exercise load at the time of the test, and severity of the induced symptoms.
Results: Symptoms were induced and the diagnosis of WDEIA was made in 28 cases, while 2 cases were confirmed not to have WDEIA. Overall, 25 of the 28 positive cases had symptoms induced by an exercise load greater than 70% of the heart rate calculated by the Karvonen formula, 7 of which required Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) loading to induce symptoms. None of the patients with negative wheat-specific IgE titers and positive gluten and/or ω-5 gliadin ones required NSAIDs loading. No patients required the administration of adrenaline.
Conclusion: Wheat-specific IgE as well as gluten and ω-5 gliadin antibody titers are associated with WDEIA symptom severity. Exercise loading with a target heart rate of 70%–90% of that calculated by the Karvonen formula is safe and useful for diagnosis this condition.
Introduction
Wheat-dependent exercise-induced anaphylaxis (WDEIA) is not triggered by the intake of wheat-based foods alone, so its diagnosis is challenging but significantly contributes to the quality of life of patients [1–5]. Although provocation tests have been shown to be useful for diagnosing WDEIA, the risk of inducing anaphylaxis is high and these tests are not always accurate. Alcohol loading has been reported to increase the rate at which symptoms are induced in such tests; Brockow et al. [6] and Christensen et al. [7] reported that alcohol-loaded tests induced WDEIA symptoms with 100% probability. However, to obtain findings relevant to daily life, we consider it important to investigate how much patients should be allowed to exercise after wheat ingestion in such provocation tests. The criteria for defining tolerance of exercise among those with wheat allergy are not clear, and such provocation tests cannot be easily performed at many hospitals and clinics. Moreover, few reports have been published on provocation tests in adults. We have sought a safer and more reliable way of performing provocation tests by investigating blood test results and examining the loading intake and exercise load before performing such tests. In cases with suspected WDEIA, the purpose of performing a provocation test is to confirm the diagnosis and severity of this condition; however, we considered it important that the testing method be useful to the patient’s daily life and thought that this method should be in line with the patient’s daily routine, such as the amount of wheat to be consumed and the type of NSAIDs to be taken.
Materials and methods
Thirty-three patients (15 males and 18 females) who underwent wheat provocation tests at the Department of Allergology, Fukuoka National Hospital, from April 2019 to August 2023 were included in this study. We obtained the patients’ medical history and background, results of wheat-, gluten-, and ω-5 gliadin-specific IgE antibody titers, exercise load at the time of the provocation test, the use of NSAIDs, and the results of the provocation test, and examined the relationship among these factors.
This study was approved by the Ethics Committee of the National Hospital Organization Fukuoka National Hospital (Approval No. F5-25).
For the exercise intensity, the Karvonen formula was used to set the predicted maximum heart rate at (220—age), and the exercise intensity % was calculated using the maximum heart rate at loading as the target heart rate [8, 9].
Exercise intensity = (target heart rate − resting heart rate)/(maximum heart rate − resting heart rate)
Methods of provocation tests
A flowchart of the provocation test is shown in Figure 1.
FIGURE 1
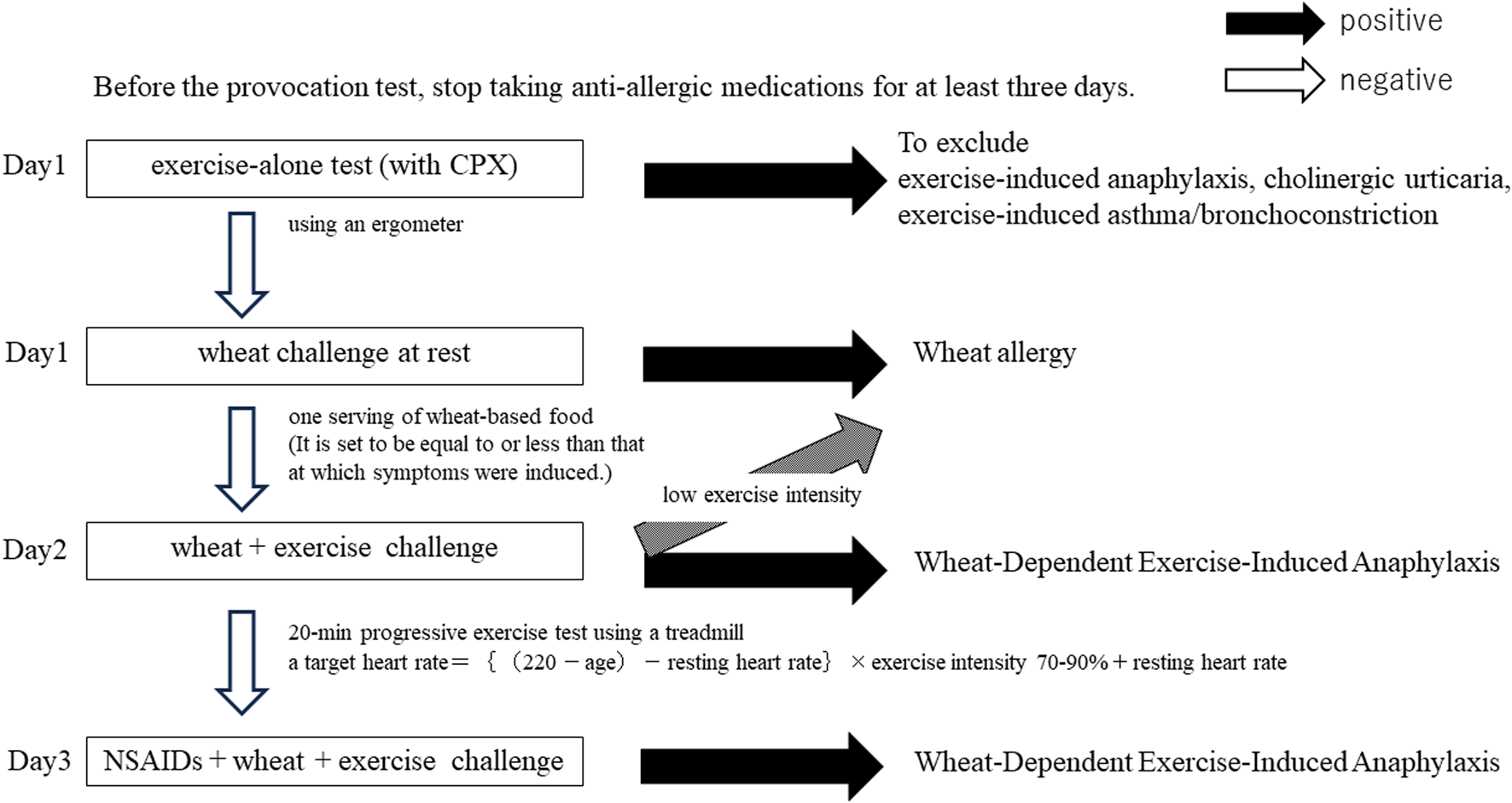
Provocation test flowchart.
Prior to the provocation test, the patients had to stop taking anti-allergic medications, including antihistamines, for at least 3 days.
Day 1: Before meals, patients were subjected to a progressive exercise load on an ergometer with an electrocardiogram monitor, blood pressure monitor, and oxygen monitor. Physical performance was checked to evaluate the safety of the exercise load and to confirm that the exercise load alone did not induce symptoms. We simultaneously carried out a cardiopulmonary exercise test (CPX) by using electrocardiogram monitoring.
In the case of elderly patients or those with underlying medical conditions such as hypertension, we consulted a cardiologist beforehand and had the cardiologist present during CPX if necessary. The results of this exercise-alone test were used to exclude patients with exercise-induced anaphylaxis, cholinergic urticaria and exercise-induced asthma/bronchoconstriction.
Thereafter, patients consumed wheat-based foods (200 g of udon noodles or 2–3 bread rolls) at rest. It was a single intake. The loading dose of wheat-based foods was set to be equal to or less than that at which symptoms were induced, based on the intake of one serving of wheat-based food.
Day 2: The same wheat-based foods as on Day 1 were consumed, and 10 min after intake, a 20-min progressive exercise test was performed on a treadmill, with the Borg Scale used to check respiratory status and lower-extremity muscle fatigue. The exercise intensity was evaluated every minute while monitoring the heart rate. The first 10 min was used for warming up, and the load was gradually increased to the point that the patient became sweaty, and the load was adjusted so that aerobic exercise could be sustained for a total of 20 min.
Day 3: One hour before food intake, the patients took aspirin or NSAIDs, in line with their typical daily use, and performed the same exercise load as on Day 2. Aspirin loading was performed after ruling out the possibility of aspirin hypersensitivity beforehand. The basic oral loading dose of aspirin was set at 300 mg–330 mg (due to a change in the dosage form adopted in our hospital), and 100 mg in the case that severe symptoms were expected to be induced. For patients who frequently use NSAIDs on a daily basis, a single dose of NSAIDs was given.
Results
The history of anaphylactic shock and blood test results of the 33 patients are shown in Table 1. There were 15 males and 18 females with a mean age of 35.5 ± 16.0 years.
TABLE 1
| sex | age | T-IgE(IU/mL) | wheat (UA/mL) | gluten(UA/mL) | ω-5gliadin(UA/mL) | History of anaphylactic shock | Results of provocation tests | NSAIDs loading | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 35 | 86.9 | 0.1 | NA | NA | + | + | − |
| 2 | F | 18 | 113 | 1.1 | 1.71 | 0.73 | + | + | − |
| 3 | M | 38 | 274 | 0.46 | 0.03 | 0 | + | + | − |
| 4 | F | 17 | 53 | 0.28 | 0.73 | 1.82 | + | + | − |
| 5 | F | 36 | 35.7 | 0.01 | 0.01 | 0.64 | + | − pending | Asp330mg |
| 6 | F | 28 | 61.9 | 0.2 | 0.42 | 2.49 | + | + | − |
| 7 | F | 36 | 378 | 0.13 | 0.36 | 6.06 | + | + | − |
| 8 | F | 20 | 438 | 0.06 | 0.02 | 0.01 | + | − not WDEIA | Asp330mg |
| 9 | M | 38 | 318 | 0.48 | 1.31 | 14.9 | + | + | Asp100mg |
| 10 | M | 39 | 603 | 0.49 | 0.1 | 0.02 | − | − not WDEIA | Lox60mg |
| 11 | F | 18 | 1170 | 0.18 | 0.7 | 0.03 | + | + | − |
| 12 | F | 40 | 140 | 0.04 | 0 | 0 | + | + | Lox60mg |
| 13 | M | 17 | 773 | 2.79 | 0.89 | 0.07 | + | + | − |
| 14 | M | 26 | 314.8 | 0.37 | 0.88 | 0.1 | + | + | − |
| 15 | F | 68 | 2924 | 2.78 | 0.51 | 0.14 | + | + | Asp330mg |
| 16 | F | 29 | 129 | 10.4 | 0.13 | 0.02 | + | + | Asp330mg |
| 17 | M | 17 | 1510 | 2.72 | 3.46 | 11.3 | + | − | Asp330mg |
| 18 | F | 19 | 260 | 12.2 | 10.8 | 0.1 | + | + | Lox60mg |
| 19 | M | 52 | 155 | 0.04 | 0.14 | 1.66 | + | + | − |
| 20 | F | 24 | 700 | 0.31 | 1.11 | 3.8 | + | + | − |
| 21 | F | 36 | 63.8 | 0.22 | 0.01 | 0 | − | + | − |
| 22 | M | 54 | 1370 | 0.42 | 0.61 | 3.66 | + | + | − |
| 23 | F | 41 | 115 | 0.09 | 0.2 | 1.4 | + | + | − |
| 24 | M | 16 | 415 | 4.12 | 0.68 | 0.03 | + | + | − |
| 25 | F | 48 | 301 | 0.13 | 0.8 | 12 | + | − | − |
| 26 | M | 36 | 787 | 0.08 | 0.26 | 10.4 | + | + | − |
| 27 | F | 26 | 1040 | 0.27 | 0.56 | 3.28 | + | + | − |
| 28 | M | 16 | 725 | 4.05 | 0.5 | 0.03 | + | + | − |
| 29 | F | 45 | 2040 | 1.95 | 12.3 | 43.9 | + | + | − |
| 30 | M | 48 | 29 | 0.06 | 0.41 | 1.68 | + | + | − |
| 31 | M | 69 | 1160 | 0.41 | 4.41 | 15.6 | + | + | Asp300mg |
| 32 | M | 49 | 72.8 | 0.03 | 0.01 | 0.28 | + | + | Lox60mg |
| 33 | M | 73 | 70.5 | 0.06 | 0.3 | 2.59 | + | + | − |
Patient background and results of provocation tests
Thirty-one of the 33 patients had a history of anaphylactic shock, and 21 patients (60%) had a background of atopic diseases. We performed the prick test for in all but three of the 33 cases, and 28 were positive in this study. In all 33 cases, we had confirmed that the patient was positive for sensitization to wheat with blood tests or prick tests.
Symptoms were induced and the diagnosis of WDEIA was confirmed in 28 cases (85%). Of the 5 cases with a negative provocation test, 2 (case 8 and case 10) were confirmed not to have WDEIA because they had freely consumed wheat in their daily lives and had no symptoms. Case 5 showed one wheal on day 2, but did not reproduce on day 3, so the diagnosis was pending. For case 17 and case 25, judging from the blood test results and the severity of the medical history, we determined that it was too risky to further accelerate the provocation test.
Eleven patients were loaded with aspirin or NSAIDs: 5 with 300–330 mg of aspirin, 1 with 100 mg of aspirin, and 4 with 60 mg of loxoprofen.
According to these results, 30 of the 33 cases (91%) were diagnosed by the provocation test. In 25 of the 28 patients with a positive provocation test, symptoms were induced at an exercise load intensity of more than 70% of the heart rate calculated by the Karvonen formula (Figure 2), and 7 of the 25 patients required an NSAIDs load for symptoms to be induced. There was a significant correlation between exercise intensity and time to symptom onset, with higher-intensity exercise being associated with symptoms taking longer to appear (p = 0.014).
FIGURE 2
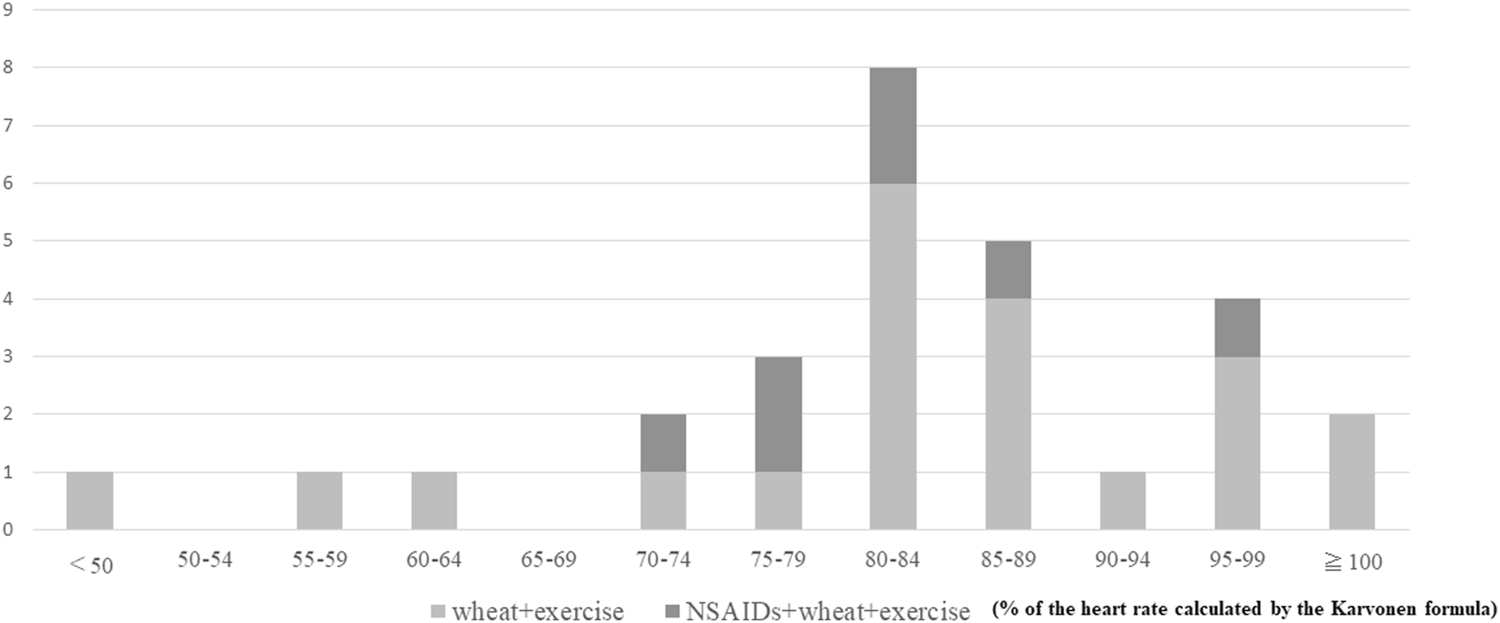
Amount of exercise load and number of positives.
It was shown that NSAIDs loading improved the induction rate, but did not change the intensity of the exercise load required. Of the 11 patients with negative wheat-specific IgE antibody titers and positive gluten- or (and) ω-5 gliadin-specific IgE antibody titers, no cases required NSAIDs loading for symptoms to be induced (Table 2). NSAIDs loading was less common in younger patients, and there was a significant correlation between age at testing and NSAIDs loading (p = 0.02) (Figure 3). None of the 28 patients who had a positive provocation test were treated with adrenaline.
TABLE 2
| specific IgE | food+exercise | NSAIDs+food+exercise | Total |
|---|---|---|---|
| w(−)g(−) | 5 | 2 | 7 |
| w(−)g(+) | 6 | 0 | 6 |
| w(+)g(−) | 1 | 1 | 2 |
| w(+)g(+) | 7 | 4 | 11 |
| w(−)ω5(−) | 2 | 2 | 4 |
| w(−)ω5(+) | 10 | 0 | 10 |
| w(+)ω5(−) | 5 | 3 | 8 |
| w(+)ω5(+) | 3 | 2 | 5 |
Association between specific IgE antibody titer and number of positive provocation tests.
w: wheat g: gluten ω5:ω-5 gliadin (−) : class 0
FIGURE 3
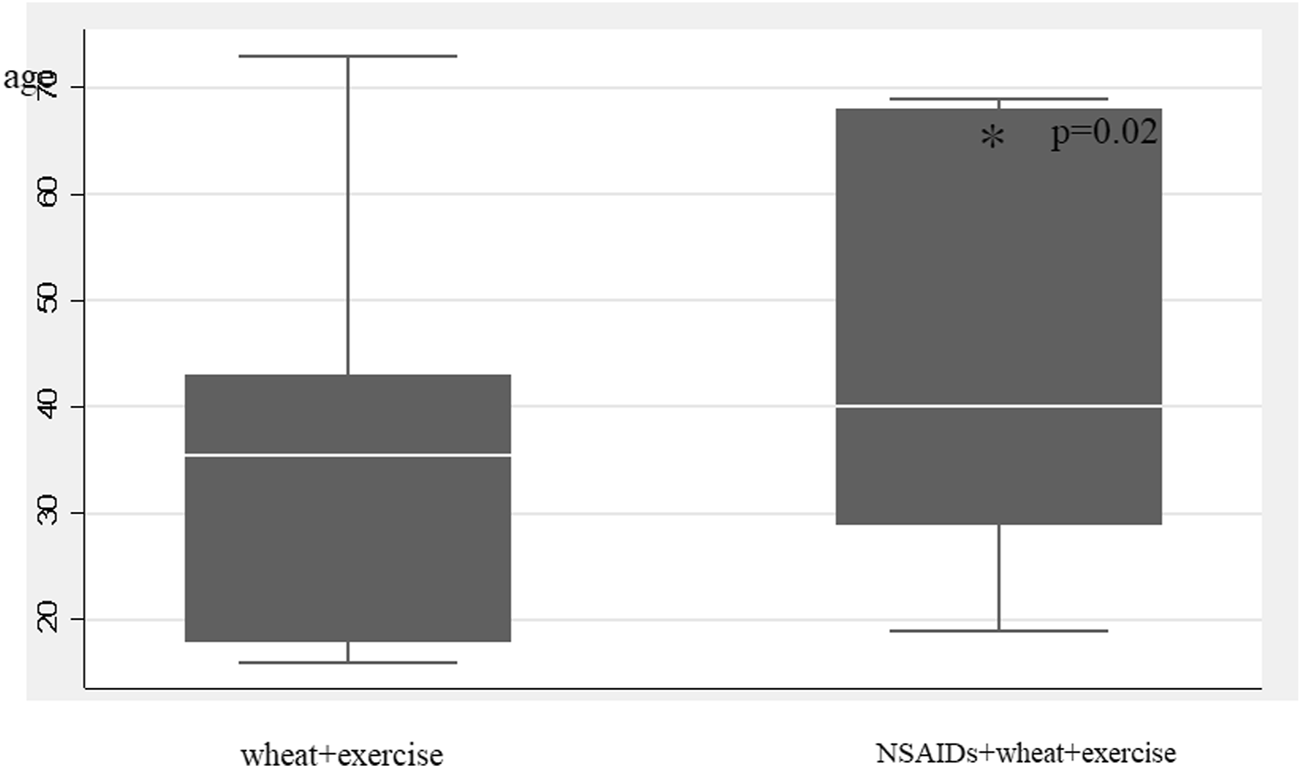
Association between age and NSAIDs load.
Discussion
WDEIA is a condition in which symptoms are not induced by the causative food alone or by exercise alone, but by a combination of the causative food intake, exercise, NSAIDs, alcohol, and excessive fatigue [1–5, 10, 11]. Wheat is the most common causative food of exercise-induced anaphylaxis, followed by shellfish and fruits. Although the peak age of onset is considered to be in adolescence and males are more frequently affected, in the present study females were more frequently affected, with the age ranging from 35 to 73 years old. Thongngarm et al. [12] similarly reported that women were predominant among those with adult-onset WDEIA. As shown in these results, adult-onset WDEIA appears to occur in a wide range of age groups, with no difference in the sex ratio of affected individuals compared with adolescent-onset WDEIA. As a wide range of age groups are affected, the intensity of the exercise load that induces symptoms varies from person to person and is also dependent on the individual’s original physical fitness and exercise habits. In many cases, patients have primary diseases, for whom safer loading tests are required. Provocation tests are useful not only in diagnosing the cause of the disease but also in evaluating its severity, which would certainly improve the quality of life of patients. However, it has been reported that provocation tests often do not induce symptoms [5, 13–15]. The risk of anaphylactic shock is also high, and few medical institutions in Japan perform provocation tests for WDEIA in adults. In our provocation test, we were able to make a definitive diagnosis in more than 90% of the cases, which is considered a high diagnostic rate. It has been reported that alcohol loading induced symptoms at a higher rate in provocation tests [6, 7]. We believe that alcohol loading lowers the symptom induction threshold for WDEIA, but alcohol intake is not difficult to avoid on a daily life. However, the intensity of exercise performed regularly varies greatly among individuals, and in daily life it is difficult to avoid exercise with an intensity level above a certain threshold. A notable finding here is that none of the cases required the administration of adrenaline.
It is necessary to undertake adequate risk assessment before performing provocation tests. The present results showed that cases with wheat-specific IgE antibody titers that were negative and ω-5 gliadin and/or gluten-specific IgE antibody titers that were positive did not require NSAIDs loading for symptoms to occur. Therefore, it would be useful to evaluate risk based on gluten and ω-5 gliadin titers based on the presence or absence of wheat-specific IgE antibody titers. As reported previously, not only ω-5 gliadin but also gluten-specific IgE antibody titer is considered to be a risk factor of WDEIA severity [16].
In addition, grass pollen-related wheat allergy with negative ω-5 gliadin-specific IgE has been reported [17]. In this study, specific IgE for orchardgrass was tested in 25 of the 33 cases, and 9 of these were positive and patients with positive orchardgrass-specific IgE tended to have more negative ω5 gliadin-specific IgE (p = 0.073). In the future, grass pollen-related wheat allergy should also be examined.
By targeting a heart rate around 80% of the Karvonen formula and setting the intensity of exercise load accordingly, a high rate of symptom induction can be achieved. This heart rate setting is simple enough to apply at any medical institution and is a useful indicator for patients to manage their own risk. Furthermore, the greater the exercise intensity, the longer time required for symptoms to be induced, suggesting that highly intense exercise is not necessary. This may be due to the activation of adrenaline secretion when intense exercise is performed.
Although the rate of symptom induction in cases suspected of WDEIA increased with NSAIDs, the intensity of exercise required for such induction remained the same. Therefore, if it is clinically unsafe to increase the intensity of exercise due to limited physical capacity or underlying diseases such as hypertension, it would be better to consider switching to NSAIDs-loaded exercise if it is possible for NSAIDs to be taken without changing the intensity of exercise.
Based on these results, it is useful to use the pre-calculated maximum predicted heart rate as a guide when performing WDEIA provocation tests, and to control the exercise load so that the heart rate can be kept at that level.
Our results show that our provocation test method is effective, while also highlighting some potential room for improvement. We will continue to examine safer and more effective methods of performing provocation tests by performing them on more cases suspected of having WDEIA in the future.
Statements
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving humans were approved by the Ethics Committee of the National Hospital Organization Fukuoka National Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because Participants are included in this research using an opt out approach.
Author Contributions
AS, TF, KO, KS, and RK treated the patients and obtained clinical data. SH, YS, and KK developed the statistical analysis plan and conducted statistical analyses. AS and KS contributed to the interpretation of the results. AS drafted the original manuscript. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1
Romano A Di Fonso M Giuffreda F Papa G Artesani MC Viola M et al Food-dependent exercise-induced anaphylaxis: clinical and laboratory findings in 54 subjects. Int Arch Allergy Immunol (2001) 125:264–72. 10.1159/000053825
2
Morita E Kohno K Matsuo H . Food-dependent exercise-induced anaphylaxis. J Dermatol Sci (2007) 47:109–17. 10.1016/j.jdermsci.2007.03.004
3
Wolańczyk-Medrala A Barg W Radlińska A Panaszek B Medrala W . Food-dependent exercise-induced anaphylaxis-sequence of causative factors might be reversed. Ann Agric Environ Med (2010) 17:315–7.
4
Asaumi T Ebisawa M . How to manage food dependent exercise induced anaphylaxis (FDEIA). Curr Opin Allergy Clin Immunol (2018) 18(3):243–7. 10.1097/ACI.0000000000000442
5
Asaumi T Yanagida N Sato S Shukuya A Nishino M Ebisawa M . Provocation tests for the diagnosis of food-dependent exercise-induced anaphylaxis. Pediatr Allergy Immunol (2016) 27(1):44–9. 10.1111/pai.12489
6
Brockow K Kneissl D Valentini L Zelger O Grosber M Kugler C et al Using a gluten oral food challenge protocol to improve diagnosis of wheat-dependent exercise-induced anaphylaxis. J Allergy Clin Immunol (2015) 135(4):977–84. 10.1016/j.jaci.2014.08.024
7
Christensen MJ Eller E Mortz CG Brockow K Bindslev-Jensen C . Wheat-dependent cofactor-augmented anaphylaxis: a prospective study of exercise, aspirin, and alcohol efficacy as cofactors. J Allergy Clin Immunol Pract (2019) 7(1):114–21. 10.1016/j.jaip.2018.06.018
8
Yabe H Kono K Onoyama A Kiyota A Moriyama Y Okada K et al Predicting a target exercise heart rate that reflects the anaerobic threshold in nonbeta-blocked hemodialysis patients: the Karvonen and heart rate reserve formulas. Ther Apher Dial (2021) 25(6):884–9. 10.1111/1744-9987.13628
9
Camarda SR Tebexreni AS Páfaro CN Sasai FB Tambeiro VL Juliano Y et al Comparison of maximal heart rate using the prediction equations proposed by Karvonen and Tanaka. Arq Bras Cardiol (2008) 91(5):311–4. 10.1590/s0066-782x2008001700005
10
Faihs V Kugler C Schmalhofer V Scherf KA Lexhaller B Mortz CG et al Wheat-dependent exercise-induced anaphylaxis: subtypes, diagnosis, and management. J Dtsch Dermatol Ges (2023) 21(10):1131–5. 10.1111/ddg.15162
11
Scherf KA Brockow K Biedermann T Koehler P Wieser H . Wheat-dependent exercise-induced anaphylaxis. Clin Exp Allergy (2016) 46(1):10–20. 10.1111/cea.12640
12
Thongngarm T Wongsa C Pacharn P Piboonpocanun S Sompornrattanaphan M . Clinical characteristics and proposed wheat-cofactor challenge protocol with a high diagnostic yield in adult-onset IgE-mediated wheat allergy. J Asthma Allergy (2020) 13:355–68. 10.2147/JAA.S271429
13
Christensen MJ Eller E Mortz CG Brockow K Bindslev-Jensen C . Exercise lowers threshold and increases severity, but wheat-dependent, exercise-induced anaphylaxis can be elicited at rest. J Allergy Clin Immunol Pract (2018) 6(2):514–20. 10.1016/j.jaip.2017.12.023
14
Srisuwatchari W Sompornrattanaphan M Jirapongsananuruk O Visitsunthorn N Pacharn P . Exercise-food challenge test in patients with wheat-dependent exercise-induced anaphylaxis. Asian Pac J Allergy Immunol (2021) 42:43–9. 10.12932/AP-250520-0856
15
Srisuwatchari W Kanchanaphoomi K Nawiboonwong J Thongngarm T Sompornrattanaphan M . Food-dependent exercise-induced anaphylaxis: a distinct form of food allergy-an updated review of diagnostic approaches and treatments. Foods (2023) 12(20):3768. 10.3390/foods12203768
16
Sugiyama A Kishikawa R Honjo S Nishie H Iwanaga T Furue M . Anti-gluten IgE titer is associated with severity of provocation test-evoked symptoms in wheat-dependent exercise-induced anaphylaxis. Allergol Int (2019) 68(4):541–3. 10.1016/j.alit.2019.04.007
17
Ogino R Chinuki Y Yokooji T Takizawa D Matsuo H Morita E . Identification of peroxidase-1 and beta-glucosidase as cross-reactive wheat allergens in grass pollen-related wheat allergy. Allergol Int (2021) 70(2):215–22. 10.1016/j.alit.2020.09.005
Summary
Keywords
provocation test, anaphylaxis, food allergy, immunoglobulin E (IgE), specific IgE
Citation
Sugiyama A, Fukushima T, Okabe K, Shimada K, Kojima K, Shigeoka Y, Honjo S and Kishikawa R (2024) Effective and safe provocation test for wheat-dependent exercise-induced anaphylaxis (WDEIA) in adults results of testing on 33 cases. J. Cutan. Immunol. Allergy 7:12896. doi: 10.3389/jcia.2024.12896
Received
24 February 2024
Accepted
05 April 2024
Published
18 April 2024
Volume
7 - 2024
Updates
Copyright
© 2024 Sugiyama, Fukushima, Okabe, Shimada, Kojima, Shigeoka, Honjo and Kishikawa.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Akiko Sugiyama, a.sugiyama47@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.