Abstract
Vascularized composite allotransplantation (VCA) has revolutionized restorative surgery of devastating injuries. Unfortunately, these grafts undergo significant injury during prolonged cold ischemia and subsequent reperfusion. Ex-vivo machine perfusion (EVMP) is a technique that has shown significant promise in solid organ transplant, but study of its utility in VCA has been limited. A systematic review was conducted to identify preclinical publications investigating perfusion in limb VCAs. Articles published through June 2023 were screened. 29 articles met inclusion criteria, comprising 370 VCA limbs from swine, rats, canines, and humans. EVMP was conducted under normothermic (n = 6), near-normothermic (n = 11), sub-normothermic (n = 3), or hypothermic (n = 13) conditions. While each study used a unique perfusate recipe, most were based on a premade medium. Many incorporated additives, including antibiotics and red blood cells. The duration varied from 3 to over 24 h. Multiple studies showed improved or equivalent biomarkers, histology, and outcomes for normothermic or near-normothermic EVMP (n = 4) and hypothermic EVMP (n = 8) compared to static cold storage, suggesting that EVMP may be a superior storage method to SCS. While there is no definitive evidence regarding the optimal temperature, perfusate composition, or perfusion time for VCAs, each perfusion factor should be chosen and adapted based on the individual goals of the study. This review offers a summary of the current literature to serve as an accessible reference for the design of future protocols in this field.
Introduction
Vascularized composite allotransplantation (VCA) is a pioneering reconstructive approach wherein transfer of a multi-tissue allograft is used to return form and function to a site of severe tissue injury or loss [1]. In the last 25 years, more than 150 patients have undergone successful VCA, including hand, face, uterus, abdominal wall, penis, scalp, and vascularized parathyroid gland transplantation [2, 3]. Despite the life-enhancing role of VCA, these procedures carry considerable ethical and psychosocial burdens, as well as high rates of postoperative complications [4–10]. A significant challenge facing VCA is the requirement for lifelong immunosuppression and incremental allograft monitoring. While many VCAs have seen long-term success without chronic rejection, VCA procedures initially yield a disproportionate incidence of acute rejection relative to all other transplant procedures [11–16]. Graft inflammation and staged rejection are strongly influenced by allograft ischemia, temperature changes, and mechanical trauma associated with organ recovery and preservation, even under traditional static cold storage conditions [17, 18]. Interruption of allograft perfusion, and therefore cellular respiration, causes the accumulation of toxic substances and free radicals, which trigger apoptosis and tissue necrosis [19]. Sudden reperfusion increases the production of reactive oxygen species and triggers innate and adaptive immunologic responses that may impair both short- and long-term organ function [19–22]. The low ischemic tolerance of these grafts furthermore significantly limits their accessibility and utility. In response, continued advancement in VCA necessitates novel preservation strategies that decrease reperfusion injury, enhance aerobic cellular respiration, and improve outcomes.
Ex-vivo machine perfusion (EVMP) is an innovative technique designed to prolong preservation time and improve the function of solid organ transplants, and therefore has become an area of interest in VCA [23]. In solid organ transplantation, EVMP has enabled safe transportation while prolonging preservation time and expanding the donor pool [24]. Further, this highly modifiable system has enabled non-acceptable organs to be reconditioned for successful transplantation [25, 26]. A central asset of this technique is the ability to modify fluid pressure, flow rate, and temperature, enabling normothermic and near-normothermic tissue perfusion [27]. Independent from standard cold preservation, EVMP reduces the tissue damage and subsequent functional impairments associated with prolonged cold ischemia times and reperfusion injury [28–30]. Within the past decade, use of EVMP in animal models and solid organ transplantation has made promising strides toward improved post-transplant function and expansion of organ donor pools [30–33].
Given the disproportionate burden of tissue injury and rejection in VCA, application of EVMP has the capacity to revolutionize transplant protocols and outcomes in the field. Still, application of this technology in VCA is neoteric and nuanced. The complexities of perfusing a diversity of tissues, each with unique metabolic needs, warrant careful investigation of perfusate composition and preservation methodologies. Currently, only a modest cohort of studies have been published that document protocols and outcomes of this technique in experimental VCA models.
Despite a clear need for improved methods of VCA preservation, there is a paucity of literature evaluating successful alternative transplant perfusion protocols. The purpose of this study is to conduct a systematic review of the literature on EVMP for VCA. Specific aims include identification of all current literature on EVMP in VCA, characterization of these studies in terms of perfusion protocols, perfusate composition, monitoring, and outcomes, and comparison of these protocol attributes and outcomes to assess optimal preservation of allografts. Synthesis of results will contribute to an optimized EVMP technique in VCA and guide future research in this evolving field.
Methods
Literature Search
A comprehensive literature search of manuscripts listed in PubMed, Scopus, EMBASE, Cochrane Library, and ClinicalTrials.gov databases was conducted in June 2023 in compliance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [34]. Titles, Abstracts, Keywords, and Mesh terms (PubMed only) were searched using the following terms: ((vascularized composite allotransplantation) OR (vascularized composite allotransplant) OR (vascularized composite allograft) OR (vascularized allograft) OR (vascularized allogeneic tissue) OR (vascularized composite tissue transplantation) OR (vascularized composite tissue transplant) OR (composite tissue allotransplantation) OR (composite tissue allotransplant) OR (composite tissue allograft) OR (composite tissue allografting) OR (composite tissue transplantation) OR (composite tissue transplant) OR (reconstructive transplant)) AND ((machine perfusion) OR (machine preservation) OR (ex vivo perfusion) OR (extracorporeal perfusion) OR (extracorporeal circulation)). The following filters were used in each database to fit within the inclusion criteria: “Full text” in PubMed, “Article” in Scopus, and “Article” and “Article in Press” in EMBASE. The “Trials” tab was used in Cochrane Library, and no filters were applied for ClinicalTrials.gov.
Predetermined inclusion criteria for selecting studies were [1]: preclinical articles studying normothermic, near-normothermic, sub-normothermic, and hypothermic perfusion [2]; perfusion of limbs within VCA [3]; randomized control trials, prospective and retrospective case-control and cohort studies, cross-sectional cohort studies, case reports, and technique papers. Exclusion criteria were [1]: reviews without presentation of new data [2]; abstracts, conference papers, editorials, or comments [3]; articles about solid-organ perfusion [4]; articles about non-limb perfusion; and [5] articles reporting little data on perfusion technique or outcomes.
Papers meeting exclusion criteria, duplicate publications, and articles unrelated to limb perfusion were eliminated. Remaining works were sought for retrieval as full texts, and their reference lists screened for additional relevant articles meeting inclusion criteria that were missed in the electronic search. Two independent authors (TEM and AHL) conducted the search, screening, and eligibility assessment to agree upon a comprehensive list of included articles. Controversies were resolved by discussion with a third reviewer (YG and YZ).
Variables and Outcomes of Interest
The following variables were recorded for each included study: model species, tissue undergoing perfusion, perfusion device, perfusion temperature, perfusion flow type and rate, perfusion pressure, perfusion duration, perfusate composition (where this data was available), monitoring techniques, post-perfusion findings, and post-replant outcomes.
Results
Study Design
Initial literature search yielded 776 unique articles, of which 29 met inclusion criteria (see Figure 1) [17, 35–62]. Despite the search terms specific to vascularized composite allotransplantation, the majority of these articles were focused on solid organ perfusion and were therefore excluded from the study. All included studies were randomized control trials published between 1985 and 2023 and cumulatively represent perfusion of 370 vascularized composite grafts (see Table 1). All grafts were limbs, of which 20 (5.4%) were human. The remainder were animal models, with the majority were harvested from swine (223, 60.3%), followed by rat (81, 21.9%) and canine (46, 12.4%). Among swine studies, 218 (97.8%) limbs were forelimbs. Eleven (36.7%) studies compared outcomes of perfused limbs against limbs placed in static cold storage. Twelve (40.0%) studies investigated outcomes after replantation (141 limbs). Most perfused grafts underwent cannulation of a single artery (335, 90.5%), although grafts perfused via two arteries were investigated by a single institution (35, 9.5%). Study comparison groups and outcomes are summarized in Table 1.
FIGURE 1
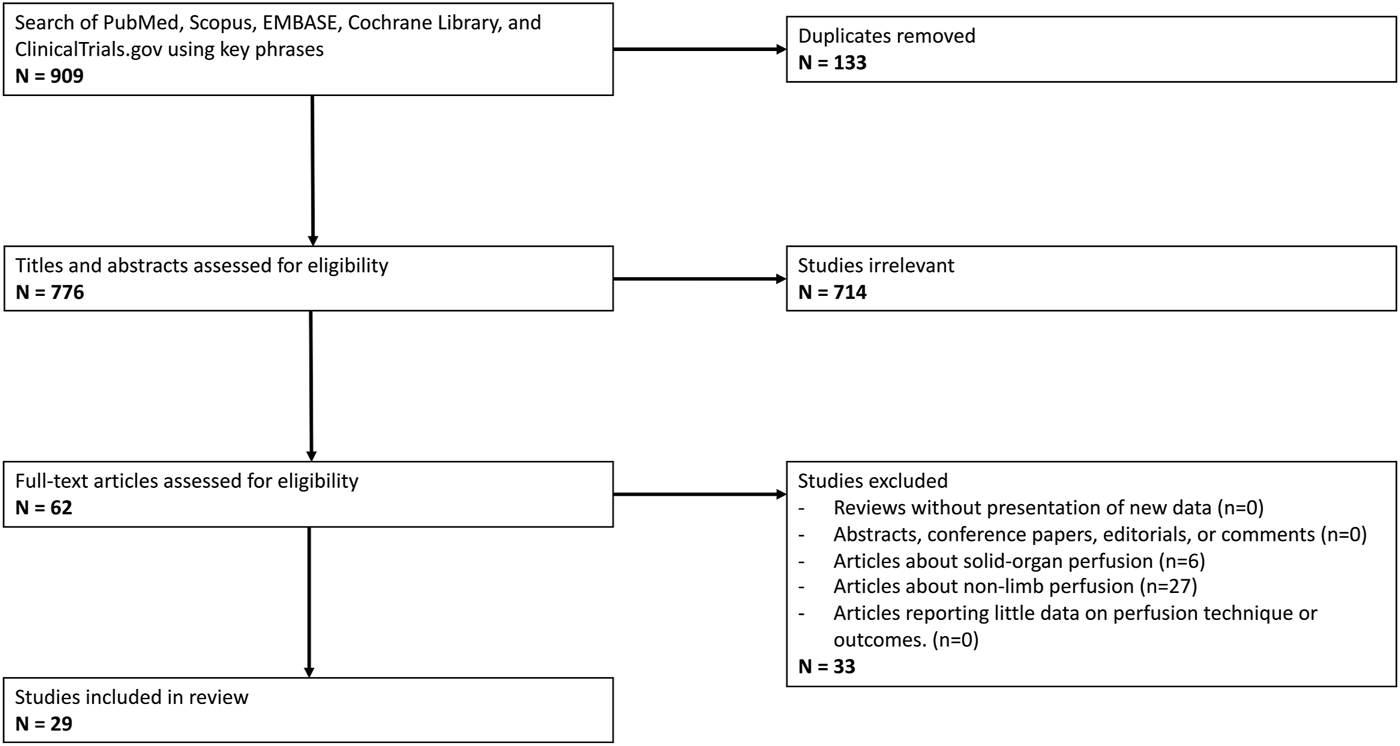
PRISMA Flow Diagram outlining inclusion and exclusion criteria, number of abstracts screened, and full texts retrieved.
TABLE 1
| Author (Year) | Institution | Species (details) | Limb (total #) | Cannulated arteries | Intervention (# limbs) | Comparator (# limbs) | Outcomes | Conclusion |
|---|---|---|---|---|---|---|---|---|
| Amin [17] | University of Manchester, UK | Swine (Landrace, 80 kg) | Fore (5) | 2: brachial artery (dominant) and radial artery (collateral) | NT perfusion (5) | (0) | Cytokine concentration and leukocyte count at perfusion t = 0 and t = end (6 h) | At 6 h, there was a cumulative increase in pro-inflammatory cytokines and significant leukocyte diapedesis and depletion from the graft |
| Amin [35] | University of Manchester, UK | Swine (Landrace, 80 kg) | Fore (35) | 2: brachial artery (dominant) and radial artery (collateral) | Experiment 1: NT at 70 mmHg (10) SNT at 70 mmHg (5) SNT at 50 mmHg (5) HT at 30 mmHg (5) Experiment 2: 2 h SCS + Optimal condition perfusion (5) | Experiment 1: Each other Experiment 2: SCS (8 h) (5) | Experiment 1: Hemodynamic and biochemical stability, to identify optimal perfusion conditions for Experiment 2 Experiment 2: Reperfusion with matched blood from unrelated donor for 4 h without immunosuppression: hemodynamic and biochemical stability | Experiment 1: NT perfusion had best outcomes and was deemed to have “optimal conditions” Experiment 2: 2 h SCS + NT perfusion was superior to 8 h SCS. |
| Gok [36] | UMich | Rat (275 ± 25 g) | Hind (25) | 1: femoral artery or common iliac artery | NNT perfusion using: Experiment 1: Femoral artery cannulation (5) Experiment 2: Hemofilter (5) Experiment 3: 6 h NNT perfusion (5) | Experiment 1: NNT perfusion using common iliac artery cannulation (5) Experiment 2: No hemofilter (Experiment 1 limbs) Experiment 3: Contralateral limbs: No perfusion (5) | Experiment 1: Flow rate, perfusion pressure, barotrauma Experiment 2: Lactate and potassium clearance Experiment 3: Hemodynamic and biochemical stability, histology | Experiment 1: Common iliac artery cannulation offers better hemodynamics and less shear stress Experiment 2: Lactate and potassium were maintained at low levels using a hemofilter Experiment 3: Using the common iliac artery and a hemofilter, metabolic outcomes were good without barotrauma, however muscle cells were more damaged than in controls |
| Werner [37] | UMich | Human (3M:2F, 37–69y, BMI 22.5–43.9 kg/m2) | Upper (5) | 1: brachial artery | NNT perfusion (5) | (0) | Hemodynamic and biochemical stability, histology, muscle contractility | Human limb allografts appeared viable after 24 h NNT perfusion |
| Ozer [38] | UMich | Swine | Fore (8) | 1: brachial artery | NNT perfusion with autologous blood for 24 h (4) | SCS for 6 h at 4°C (4) | Hemodynamic and biochemical stability, histology; Post-perfusion transplantation to recipients (12 h monitoring) | Limb survival up to 24 h |
| Ozer [39] | UMich | Swine (40 ± 5 kg) | Fore (7) | 1: brachial artery | NNT perfusion with autologous blood for 12 h (4) | SCS for 6 h at 4°C (3) | Hemodynamic and biochemical stability, histology; Post-perfusion transplantation to recipients (7) (12 h monitoring) | Achieved transplantation of limbs after 6 h NNT perfusion with promising contractility and biochemical stability |
| Constantinescu [40] | Bern University Hospital, Switzerland | Swine (Large white, 37.5 ± 5.5 kg) | Fore (16) | 1: axillary artery | NNT 12 h (8) | Contralateral limbs: SCS at 4°C (8) | Hemodynamic and biochemical stability, histology | Perfused limbs demonstrated superior biochemical stability and muscle contractility compared to controls |
| Fahradyan [41] | Cleveland Clinic | Swine (Yorkshire, 45 kg) | Fore (20) | 1: subclavian artery | 12h group: NT perfusion for 12 h (5) >24h group: NT perfusion until vascular resistance increased: Systolic pressure >115 mmHg, compartment fullness, weight gain, O2 decrease by 20% (5) | Contralateral limbs: SCS at 4°C (10) | Muscle contractility, compartment pressure, tissue O2 saturation, indocyanine green angiography, thermography | Outcomes of prolonged NT perfusion (>24 h) are not significantly different from 12 h NT perfusion |
| Duraes [42] | Cleveland Clinic | Swine (Yorkshire, 45 kg) | Fore (36) | 1: subclavian artery | NT perfusion for 12 h (18), with evolving protocol of WIT, CIT, perfusate contents, and perfusate temperature | Contralateral limbs: SCS at 4°C for 12 h (18) | Muscle contractility, compartment pressure, tissue O2 saturation, indocyanine green angiography, thermography | Perfusion preserved limb physiology and function for up to 12 h. Limbs with best outcomes: Colloid + washed RBC perfusate at 39°C for 12 h |
| Haug [43] | BWH | Swine (Yorkshire, 40 kg) | Fore (8) | 1: axillary artery | HT perfusion for 12h, using either modified STEEN (2), balanced electrolyte Phoxilium (2), or dextran-enriched Phoxilium (PHODEX) (2) | SCS at 4°C for 12 h (2) | Hemodynamic and biochemical stability, histology, HIF1a | PHODEX is an affordable substitute for STEEN, with exception to elevated creatine kinase and lactate dehydrogenase |
| Haug [44] | BWH | Human (2M:1F, 24–51y, BMI 22.3–29.1 kg/m2) | Upper (6) | 1: brachial artery | HT perfusion for 24 h (3) | Contralateral limbs: SCS for 24 h (3) | Hemodynamic and biochemical stability, histology, HIF1a | HT perfusion extended preservation time to 24 h |
| Kueckelhaus [45] | BWH and Germany | Swine (Yorkshire, 38.4 ± 1.5 kg) | Fore (7) | 1: Unspecified | HT perfusion for 12 h using portable perfusion machine and subsequent heterotopic replantation (3) | SCS at 4°C for 4 h and subsequent heterotopic replantation (4) | Hemodynamic and biochemical stability, histology, cytokine levels | Perfused limbs were superior to SCS limbs after transplantation |
| Kueckelhaus [46] | BWH and Germany | Swine (Female Yorkshire, 50–60 kg) | Hind (10) | 1: femoral artery | HT perfusion using portable perfusion machine (5) | SCS for 12 h (5) | Hemodynamic and biochemical stability, histology | Successful perfusion via portable device, superior to SCS. |
| Krezdorn [47] | BWH and Germany | Swine (Female Yorkshire, 35–45 kg) | Fore (8) | 1: axillary artery | HT perfusion for 24 h and subsequent replant onto same animal (4) | SCS at 4°C for 4 h and subsequent replant onto same animal (4) | Hemodynamic and biochemical stability, histology, 7-day monitoring of animals | Perfused limbs were comparable to SCS limbs and may reduce muscle damage and systemic reactions on replantation |
| Krezdorn [48] | BWH | Swine (Female Yorkshire, 35–45 kg) | Fore (8) | 1: axillary artery | HT perfusion at 10°C for 2 h and subsequent replantation onto same animal (3) Or HT perfusion at 10°C for 12 h and subsequent replant onto same animal (3) | SCS at 4°C for 2 h and subsequent replant onto same animal (2) | Hemodynamic and biochemical stability, histology, PCR of target genes | Perfused limbs demonstrated downregulation of genes involved in glycolysis, angiogenesis, and DNA damage compared with SCS limbs |
| Kruit [49] | Radboud University Medical Center, Netherlands | Swine (Female Dutch Landrace, ∼69 kg) | Fore (24) | 1: brachial artery | HT perfusion for 18 h and subsequent replant onto the same animal (6) | SCS at 4°C–6°C for 4 h and subsequent replant onto the same animal (6) Sham surgery in contralateral limbs (12) | Hemodynamic and biochemical stability, histology, nerve stimulation, 12 h monitoring of animals | Muscle contraction comparable between perfused, SCS, and sham limbs, perfused limbs had greater edema than SCS limbs. There was no correlation between muscle function and histology |
| Domingo-Pech [50], | Spain | Canine (Mongrel) | Hind (21) | 1: iliac artery | Perfusion for 24 h (9) Perfusion for 24 h and subsequent replantation onto same animal (6) | Limb harvest and immediate replant (6) | Hemodynamic and biochemical stability, histology, 6 h monitoring of animals | Edema was managed with peripheral vasodilators, steroids, and cool perfusate temperature |
| Usui [51] | Japan | Canine (Mongrel, 10–15 kg) | Hind (46) | 1: femoral artery | Intermittent perfusion with fluorocarbon at room temp (9) or HT (6); Continuous perfusion with fluorocarbon at room temp (6) or HT (5); Continuous perfusion with Lactated Ringer’s at HT (5) All limbs were replanted | Limb harvest and immediate replantation (15) | 6 h monitoring of animals | Fibrocarbon inhibited anaerobic metabolism and creatine phosphokinase leak from the limb and was more pronounced under continuous and HT perfusion conditions |
| Muller [52] | Bern University Hospital, Switzerland | Swine (Large white, 39 ± 5.5 kg) | Fore (61) | 1: unspecified | 6 h SCS/12 h perfusion (7) 12 h SCS/5 h perfusion (6) No SCS/12 h perfusion/replantation (11) 6 h SCS/12 h perfusion/replantation (8) | Contralateral limbs SCS for 18 h (10) Contralateral limb biopsies at euthanasia (19) | Hemodynamic and biochemical stability, histology, inflammatory markers, 7-day monitoring of replanted animals | No significant difference in markers for ischemia/reperfusion injury |
| Adil [53] | University of Toronto | Rat (Male Lewis, 300–430 g) | Hind (4) | 1: femoral artery | Decellularization perfusion for 5 days (4) | (0) | Hemodynamic and biochemical stability, histology | Successful decellularization |
| Burlage [54] | MGH | Rat (Lewis, 250–300 g) | Hind (39) | 1: femoral artery | HT perfusion with BSA for 6 h (4) HT perfusion with BSA/PEG for 6 h (4) HT perfusion with HBOC-201 for 6 h (4) HT perfusion with HBOC-201 for 6h, then transplant (13) | SCS 6h, transplant (4) SCS 24h, transplant (5) Direct transplant after harvest (5) | Hemodynamic and biochemical stability, histology | Lower edema with HBOC-201 perfusate compared to BSA and BSA/PEG, decreased energy charge ratios in SCS compared to HBOC-201 |
| Figueroa [55] | Cleveland Clinic | Swine (Yorkshire, 45 kg) | Fore (24) | 1: subclavian artery | NNT perfusion with HBOC-201 (6) NNT perfusion with RBC perfusate (6) | SCS at 4°C (12) | Hemodynamic and biochemical stability, histology | No significant differences between HBOC-201 and RBC-perfused limbs |
| Gok [56] | UMich | Rat (Male Lewis, 250 ± 2.5 g) | Hind (25) | 1: unspecified | HT perfusion with HTK for 6h, then transplant (5) | No intervention (5) Sciatic nerve transected and directly repaired (5) Limb harvest and immediate transplant (5) HTK flush, 6h SCS, then transplant (5) | Hemodynamic and biochemical stability, histology, muscle contractility after 12 weeks | No significant differences in myocyte injury in HT perfusion group compared to controls, decreased muscle force in HT perfusion after 12 weeks compared to controls |
| Goutard [57] | MGH | Rat (Lewis, 250 ± 50 g) | Hind (32) | 1: femoral artery | HT perfusion 3 h (4) 12h SCS, HT perfusion 3 h (4) 18h SCS, HT perfusion 3 h (4) 12h SCS, HT perfusion 3h, transplant (4) | Direct transplant (4) SCS 12–48h, transplant (16) | Hemodynamic and biochemical stability, histology, 21-day monitoring of animals | No differences in survival for 0–24 h SCS, frequent delayed graft failure for 48h SCS, increased edema in 18 h SCS perfusion compared to 12h SCS, improved clinical appearance 12 h SCS perfusion transplants compared to 12 h SCS only |
| Mayer [58] | Humboldt Univerty, Berlin, Germany | Swine | Fore (60) | 1: unspecified | NNT perfusion (60) | (0) | Hemodynamic and biochemical stability | Viability of flaps for up to 27 h |
| Rezaei [59] | Cleveland Clinic | Human (Adult DBD) | Upper (20) | 1: brachial artery | NT perfusion 48 h at 38°C (10) | SCS at 4°C (10) | Hemodynamic and biochemical stability, histology | Improved histology and decreased edema in perfusion compared to SCS |
| Stone [60] | University of Manchester, UK | Swine (Landrace, 80 kg) | Fore (10) | 1: brachial artery | NT limb + kidney perfusion 5 h (5) NT limb only perfusion 5 h (5) | (0) | Hemodynamic and biochemical stability, histology, inflammatory markers, thermal imaging | Addition of a kidney rapidly stabilized lactate, bicarbonate, and pH levels, more homogenous global perfusion in kidney group compared to limb only |
| Taeger [61] | University Hospital Regensburg, Germany | Human (Adult traumatic amputations) | Lower (2) | 1: femoral artery | HT perfusion followed by reattachment to patient (2) | (0) | 3-month follow-up | Successful replantation in both patients |
| Valdivia [62] | Hannover Medical School, Germany | Rat (Lewis, 227–400 g) | Hind (30) | 1: femoral artery | HT perfusion 4 h with lentiviral vectors (15) HT perfusion 4 h (15) | (0) | Hemodynamic and biochemical stability, histology, cytokine levels, bioluminescence detection, cell phenotyping | No significant tissue damage from lentiviral vector use |
Articles included in systematic review, n = 30.
C, continuous flow; Fore, forelimbs; h, hours; Hind, hindlimbs; HT, hypothermic; N2, nitrogen; NR, not reported; NT, normothermic; NNT, near-normothermic, P, pulsatile flow; q#time, to indicate frequency a medication was administered; SCS, static cold storage; SNT, sub-normothermic; Upper, upper limbs.
Perfusion Technique
Perfusion was achieved under varying temperature conditions: normothermic (NT, 38°C–39°C) in 6 studies, near-normothermic (NNT, 27°C–35°C) in 11 studies, sub-normothermic (SNT, 20°C–22°C) in 3 studies, and hypothermic (HT, 4°C–12°C) in 13 studies (see Table 2). Pump-controlled perfusate flow was pulsatile (7 studies), continuous (12 studies), or intermittent (cyclically paused and resumed, 1 study), although 9 studies provided insufficient detail to determine flow pattern. Seven studies discussed a technique to initiate perfusion, requiring up to 1 h to reach target pressure, flow, and temperature parameters. Perfusion was performed for 3–6 h (9 studies), 12 h (10 studies), 18 h (1 study), 24 h (5 studies), or longer (4 studies), with the longest perfusion achieved via normothermic pulsatile perfusion for 44 h [41]. While perfusate gas composition varied widely, all studies applied oxygen to the perfusion circuit.
TABLE 2
| Author (Year) | WIT | CIT (target) | Perfusion device | Flow type | Relative perfusate temp | Actual perfusate temperature (target) (˚C) | Perfusion initiation technique | Perfusate flow rate (% of in vivo baseline measurements) | Perfusion pressure (target) (mmHg) | Vascular resistance | Gas content | Perfusion duration (h) (target) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amin [17] | 25 ± 2.7 min | 124.6 ± 6.2 min (120 min) | Centrifugal pump | NR | NT | 37.1 ± 0.1 (38) | Pressure increase 5 mmHg Q5 min | 119.8 ± 12.75 mL/min/Kg; 356 ± 131.5 mL/min | MAP: 69.5 ± 0.4; (70) | Decreased until t = 1 h, stable thereafter | 95% O2/5% CO2 | 6 |
| Amin [35] | NR | NR | Centrifugal pump | NR | NT NNT HT | NR (38) NR (28) NR (10) | Pressure increase 5 mmHg Q5 min | 102.3 ± 34.8 mL/kg/min | MAP: 65.6 ± 6.7 | NT at 70 mmHg: 0.4 ± 0.3 mmHg/min/mL, stable, uniform | 95% O2/5% CO2 | 6 |
| Gok [36] | NR | NR | Peristaltic roller pump (Masterflex L/S peristaltic pump | P | NNT | NR (30–35) | Flow at t = 0 0.1 mL, increased incrementally to 2.5 mL/min over first 20 min | Experiment 3: 0.9 ± 0.24 mL/min | Experiment 3: 33.74 ± 14.83 | Gradual decrease | 95–100% O2; adjusted to maintain pO2 225–400 mmHg/0%–5% CO2 | 6 |
| Werner [37] | 76min | NR | Roller pump (Shiley Roller Pump) | P | NNT | 32.0 ± 0.2 (30–33) | NR | 310 ± 20 mL/min (6%–10%) | Systolic: 93 ± 2 | 0.4 ± 0.3 mmHg/min/L | 40–60% O2/5–10% CO2/Remaining% N2 | 24 |
| Ozer [38] | NR | NR | Perfusion pump (Waters Medical Systems, Minneapolis, MN) | P | NNT | NR (27–32) | NR | 80 mL/h | MAP: 60–80 | Increased until t = 1 h, decreased after t = 2 h | 95% O2/5% CO2 | 24 |
| Ozer [39] | NR | NR | RM3 pulsatile perfusion pump (Waters Medical Systems, Minneapolis, MN) | P | NNT | NR (27–32) | NR | 80–120 mL/h | MAP: 60–80 | High at t0 = 3 h, later normalized | 95% O2/5% CO2 | 12 |
| Constantinescu [40] | 1 h | NR | Turbine pump (MEDOS Deltastream Blood Pump, Model DP2; Medos Medizintechnik AG, Stolberg, Germany) | C | NNT | NR (32) | NR | 100–150 mL/min (50%) | MAP: 33.73 ± 2.06 | NR | 21% O2; arterial pO2 128.81 ± 8.82 mmHg | 12 |
| Fahradyan [41] | NR | NR | Roller pump (Terumo Sarns 8000) fitted with a pulse module (Terumo Sarns) | P | NT | NR (38) | Flow and temp were gradually increased during first hour | 12 h group: 0.77 ± 0.1 L/min >24 h group: 0.43 ± 0.03 L/min | 12 h group: Systolic: 107.25 ± 31.02 Diastolic: 44.69 ± 21.10 >24 h group: Systolic: 111.14 ± 12.48 Diastolic: 64.25 ± 14.15 | 12 h group: +6.4% ± 18.4% >24 h group: +33.3% ± 23.6% | 12 h group: 100% O2/7% CO2/93% N2 >24 h group: 100% O2 1 L/min | 12 h group: 12 >24 h group: 24–44 |
| Duraes [42] | 12h 39°C colloid/wRBC: 112 ± 68 min | 12h 39°C colloid/wRBC: None | Roller pump (Terumo Sarns 8000) fitted with a pulse module (Terumo Sarns) | P | NNT NT | n = 1 (N/A) n = 7 (32) n = 8 (39) | NR | NR | NR | NR | 100% O2 + 7% CO2/93% N2 | 6–12 (12) |
| Haug [43] | 77.5 ± 5.24 min | NR | Peristaltic machine pump (Master Flex Pump L/S, Cole-Parmer, Illinois, USA) | C | HT | (10) | NR | 20 mL/min | 24.48 ± 10.72 | NR | 377.22 ± 89.58 mmHg | 12 |
| Haug [44] | Median: 90min (65–155 min) | Median: 67 min (37–148 min) | Peristaltic machine pump (Master Flex Pump L/S, Cole-Parmer, IL) | C | HT | Median: 9.43 (Range 4.8–14.3) (10) | NR | Median: 30.4 mL/min | 30 | NR | 385.4–609.7 mmHg, median 555.8 mmHg | 24 |
| Kueckelhaus [45] | NR | NR | NR | C | HT | 10 ± 1.9 (10) | NR | NR | 30 | NR | Oxygenator used | 12 |
| Kueckelhaus [46] | NR | NR | Peristaltic pump | C | HT | (10–12) | NR | NR | 30 | NR | Oxygenator used | 12 |
| Krezdorn [47] | 26.2 ± 14.4 min | NR | Pump | C | HT | (8) | NR | Fluctuating | 29.4 ± 0.6 | 8.2 ± 0.7mL/100 mL | 24 | |
| Krezdorn [48] | NR | NR | NR | NR | HT | (10) | NR | NR | NR | NR | Oxygenated | 12 |
| Kruit [49] | NR | NR | Centrofugal pump (BP-50 Bio-Pump Centrifugal Blood Pump, Medtronic) | NR | HT | (8–10) | NR | 16 ± 1.7 mL/min | <30 | NR | 95% O2/5%CO2 | 18 |
| Domingo-Pech [50] | NR | NR | Sarns low velocity pump | NR | HT | “Cold” | NR | NR | >100 | NR | Oxygenated | 24 |
| Usui [51] | NR | NR | NR | C or I | SNT HT | (∼20) (4) | NR | NR | 50 | NR | Oxygenated, >400 mmHg in Fluorocarbon group | C: 6 h I: 20min perfusion for 3 or 5 cycles |
| Muller [52] | NR | Group 1: 6.2 ± 0.03 h (6) Group 2: 12.9 ± 1.5 h (12) Group 4: 6.2 ± 0.2 h (6) | MEDOS DataStream blood pump, model DP2 (Medos Medizintechnik AG, Germany) | NR | NNT | (32) | NR | 100–150 mL/min | NR | NR | Oxygenated | Group 1: 12.1 ± 0.2 (12) Group 2: 4.9 ± 1.9 Group 3: 12.0 ± 0.3 (12) Group 4: 12.0 ± 0.1 (12) |
| Adil [53] | NR | NR | Peristaltic pump | C | NT | NR | NR | 1 mL/min | NR | NR | NR | 120 |
| Burlage [54] | 10–15 min | NR | Rotating pump (Drive Mflex L/S, Cole-Parmer, IL) | C | HT | NR | NR | HBOC-201: median 0.4 mL/min | 30–40 | Decreased within 1st hour, stable afterwards | Oxygenated | 6 |
| Figueroa [55] | HBOC-201: 35.50 ± 8.62 min RBC: 30.17 ± 8.03 min | NR | Roller pump (Terumo Sarns 8000) | C | NNT | HBOC-201: 33.23 ± 1.11 RBC: 33.12 ± 1.69 (38) | Temperature raised from 27°C to 38°C over 1 h | HBOC-201: 325 ± 25.00 mL/min RBC: 444.73 ± 50.60 mL/min | HBOC-201: 78.50 ± 10.75 RBC: 85.70 ± 19.90 (MAP 90) | HBOC-201: 214.80 ± 69.80 mmHg/min RBC: 190.90 ± 58.33 mmHg/min | Oxygenated | HBOC-201: 22.50 ± 1.71 RBC: 28.17 ± 7.34 |
| Gok [56] | 30 min avg | NR | Peristaltic roller pump (Masterflex L/S peristalitic pump, Cole-Palmer, IL) | C | HT | (10–15) | NR | NR | 20–40 | NR | Oxygenated | 6 |
| Goutard [57] | NR | NR | Roller pump (Drive Mflex L/S, Cole-Palmer, IL) | C | SNT | (21) | NR | 0.8 mL/min | 30–50 | Decreased over 3 h | Oxygenated | 3 |
| Mayer [58] | NR | NR | NR | NR | NNT | NR | NR | NR | NR | NR | Oxygenated | NR |
| Rezaei [59] | 59.6 ± 20.9 min | NR | Roller pump | C | NNT | 35.1 ± 1.7 (38) | Flow gradually increased over 1 h | 0.41 ± 0.06 L/min | MAP 90 | 187.3 ± 26.7 mmHg x min/L | Humidified 100% O2 | 41.6 ± 9.4 h (48) |
| Stone [60] | 20.6 ± 3.0 min | 195.4 ± 13.7 min (180 min) | NR | NR | NT | (38) | Pressure 55 mmHg, increased by 5 every 5min to reach target, limbs added after 1 h renal perfusion | Limb/Kidney: 496 ± 78.29 mL/min Limb Only: 232 ± 106.6 mL/min | 75 | NR | 95% O2, 5% CO2 | 5 |
| Taeger [61] | NR | NR | ECMO Pediatric Set (Quadrox, Maquet, Germany) | P | SNT | (20) | NR | NR | NR | NR | 100% O2 | Patient 1: 15 h 49 min Patient 2: 12 h 27 min |
| Valdivia [62] | NR | NR | NR | NR | HT | NR | NR | NR | NR | NR | Oxygenated | 4 |
Details of perfused limbs.
CIT, Cold ischemia time, time in cold storage. I, intermittent; WIT, Warm ischemia time, time from limb procurement until cold flush. Numbers formatted as mean ± standard deviation (SD).
Perfusate Composition
Among the studies, 29 unique perfusate recipes were used and four studies experimented with different perfusate recipes (see Table 3). Twenty studies (69.0%) used a premade medium, including STEEN (6 studies), Perfadex (3 studies), Ringer’s solution (3 studies), Lactated Ringer’s solution (3 studies), Custodiol HTK (2 studies), Phoxilium (1 study), Dulbecco’s Modified Eagle’s Medium (1 study), University of Wisconsin solution (1 study), Fluosol-43 (1 study), PromoCell skeletal muscle cell growth medium (1 study), and HAM’s solution (1 study). (see Table 4). Seventeen studies (58.6%) incorporated antibiotics into the perfusate, including Cefazolin (4 studies), Vancomycin (4 studies), Meropenem (3 studies), Penicillin-streptomycin (3 studies), Piperacillin-Tazobactam (2 studies), and unnamed coverage for skin flora (1 study). One study added antifungal coverage with Amphotericin B [58], and another study wrapped the limb in an antiseptic-diluted sodium hypochlorite solution dressing for the duration of perfusion [38]. Fourteen studies (48.3%) included either red blood cells or whole blood in the perfusate, whereas the remaining 15 studies (51.7%) used acellular perfusate. Common yet inconsistently used additives were metabolic carbohydrates (e.g., glucose, dextrose, dextran; 20 studies), buffer (e.g., sodium bicarbonate, trometamol, potassium dihydrogen phosphate; 20 studies), steroids (e.g., methylprednisolone, hydrocortisone, dexamethasone; 19 studies), heparin (19 studies), insulin (17 studies), calcium (15 studies), and albumin (15 studies). Many protocols included either continuous (4, 13.8%) or periodic (12, 41.4%) partial plasma exchange, with a maximum of 13 exchanges [41].
TABLE 3
| Author (date) | TCV | Base Medium | pRBCs | Albumin | Heparin | Antibiotics | Glucose | Buffer | Methyl-prednisolone | Insulin | Calcium | Other Additives or Notes | Perfusate exchange (volume and freq) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amin [17] | 1.1 L | 500 mL Ringer’s | pRBCs: 500 mL Hct: 20% | BSA | 5000 iU | 500 mg meropenem | 15% 10 mL/h | 8.4% NaHCO3 10 mL/h | 500 mg | Actrapid, 10 mL/h, C | Calcium in Nutriflex, CaCl2 in Ringer’s | Nutriflex 10 mL/h, continuous infusion | X |
| Amin [35] | 1.1 L | 500 mL Ringer’s | pRBCs: 500 mL Hct: 20% | BSA | 5000 iU | 500 mg meropenem | 15% 10 mL/h | 8.4% NaHCO3 10 mL/h | 500 mg | Actrapid, 10 mL/h, C | Calcium in Nutriflex, CaCl2 in Ringer’s | Nutriflex 10 mL/h, continuous infusion | X |
| Gok [36] | >25 mL | STEEN (25 mL) | Swine RBCs to Hb 6–9 g/dL | X | 2000 U | 5 mg cefazolin | Glucose in STEEN | NaHCO3 given in 0.5-1 m Eq increments to maintain >5.0 mmol/L | 10 mg | X | 15 mg calcium gluconate | X | Continuous plasma filtration at 6 mL/h, replaced with equal volume Plasma-Lyte A, with 30 m Eq/L NaHCO3 and 1000 U/L heparin |
| Werner [37] | 250–300 mL | X | Hb 4–6 g/dL | Albumin | Sodium heparin | Yes; skin flora coverage | Dextrose given to maintain >100 mg/dL | NaHCO3 | 200 mg at t = 0 and with each PPE | Regular insulin given if glucose >300 mg/dL | CaCl2 | Tromethamine | PPE q3-5 h |
| Ozer [38] | NR | X | 1:2 pRBCs:plasma, 20–30 mmHg colloid pressure | X | 10,000 U | Limb in antiseptic-diluted sodium hypochlorite solution dressing | Dextran; 1 mL D50 given if < 4.5 mmol/L, Q2h | X | X | 2U given if glucose >14 mmol/L, Q2h | X | Leukocyte and platelet fractions removed | Continuous PPE at 80 mL/h |
| Ozer [39] | 300 mL | Dulbecco’s Modified Eagle’s Medium (200 mL) | Hct 10% | X | 10,000 U | X | Dextrose in DMEM, 1 mL D50 given if < 4.5 mmol/L, Q2h | NaHCO3 in DMEM | X | 2U given if glucose >14 mmol/L, Q2h | CaCl2 in DMEM | Leukocyte and platelet fractions removed | PPE 160 mL q2h (fresh contained 10% hemoglobin with similar parameters of plasma oncotic pressure) |
| Constantinescu [40] | NR | X | Hb 5 mg/mL | X | 10,000 U | X | 20 mL 10% glucose given if potassium >5.5 mmol/L | X | 40 mg | 15IU Actrapid given if potassium >5.5 mmol/L | X | Circuit primed with 250 mL colloid solution | X |
| Fahradyan [41] | 2.5 L | X | Washed RBCs, Hct 10%–15% | Albumin | 10,000 U | 500 mg vancomycin | Maintenance D50 | NaHCO3 (12 h group) or THAM (>24 h group) given if pH < 7.1 | 500 mg | Regular insulin 1U/h | X | X | 400 mL PPE at t = 6 h and q3h thereafter |
| Duraes [42] | >0.5 L | X | Whole blood, Washed RBCs (Hct 10%–15%) or none | Albumin | 10,000 U | 500 mg vancomycin | Glucose | THAM to correct base deficit | 500 mg | Regular insulin 1U/h | X | X | 500 mL PPE q3h |
| Haug [43] | NR | (1) Modified STEEN, (2) balanced electrolyte Phoxilium, or (3) Phoxilium enriched with dextran (PHODEX) | Acellular | HSA in STEEN | 2,500 U/L | X | Glucose in STEEN, 0.1% D50 | NaHCO3 in STEEN and Phoxilium | 125 mg | 0.0075% Insulin R | CaCl2 in STEEN and Phoxilium | X | PPE at t = 1 h and t = 6 h |
| Haug [44] | NR | STEEN (XVIVO Perfusion AB, Göteborg, Sweden) | Acellular | HSA in STEEN | 2,500 U/L | X | Glucose in STEEN, 0.1% D50 | NaHCO3 in STEEN | 125 mg | 0.0075% Insulin R | CaCl2 in STEEN | X | PPE at 1, 6, 12, and 18 h |
| Kueckelhaus [45] | 5.6 L | 5.6 L Perfadex (XVIVO Perfusion AB, Göteborg, Sweden) | Acellular | X | X | X | D40 in Perfadex | 4 mL THAM at t = 0 | 500 mg at t = 0 | 30U at t = 0 | X | X | 4 mLD50, 30 U insulin, and 500 mg methylprednisolone were replenished at 7 h |
| Kueckelhaus [46] | 5.6 L | 5.6 L Perfadex (Vitrolife, Göteborg, Sweden) | Acellular | X | X | X | D40 in Perfadex | 4 mL THAM at t = 0 | 500 mg at t = 0 | 30U at t = 0 | X | X | 4 mL D50, 30 U insulin, and 500 mg methylprednisolone were replenished at 7 h |
| Krezdorn [47] | 4 L | 4 L STEEN (XVIVO Perfusion AB, Göteborg, Sweden) | Acellular | HSA in STEEN | X | X | Glucose in STEEN, 4 mL D50 | NaHCO3 in STEEN | 500 mg | 0.3 mL | CaCl2 in STEEN | X | PPE at 1, 6, 12, and 18 h |
| Krezdorn [48] | NR | 5.6 L “Modified” Perfadex (XVIVO Perfusion AB, Göteborg, Sweden) | Acellular | X | X | X | D40 in Perfadex | KH2PO4 in Perfadex | X | X | X | X | X |
| Kruit [49] | 1 L | 1 L University of Wisconsin solution | Acellular | X | X | X | X | KH2PO4 in UW | 40 mg | X | X | X | X |
| Domingo-Pech [50] | 727 mL | 27.5% Lactated Ringer’s solution | 27.5% preserved whole blood | X | 3 mg/kg initial dose | Piperacillin | 20.6% Rheomacrodex (LMW dextran) | 10.3% NaHCO3 | Prednisolone | X | CaCl2 in LR | 13.7% Mannitol | PPE q4h with addition of sodium heparin 5%, 3 mg/kg, Prednisolone 20mg, Piperacillin Na 1g, Nitroglycerin 5 mg |
| Usui [51] | NR | (1) Fluorocarbon (Fluosol-43) diluted in Lactated Ringer’s or (2) Lactated Ringer’s alone | Acellular | X | X | X | Glucose in Fluosol-43 | NaHCO3 in Fluosol-43 | X | X | CaCl2 in Fluosol-43 and LR | X | X |
| Muller [52] | NR | Heparinized autologous blood | NR | X | Heparinized | X | X | X | X | X | X | Initially flushed with synthetic colloid hydroxyethyl starch solution | X |
| Adil [53] | 5 L | Sodium dodecyl sulfate | Acellular | X | X | X | X | X | X | X | X | X | X |
| Burlage [54] | 500 mL | PromoCell skeletal muscle cell growth medium or HBOC-201 | Acellular | 10 g BSA | 1 mL heparin | 2 mL penicillin-streptomycin | X | X | 100 μL hydrocortisone, 8 μg dexamethasone | 100 μL | X | 5 mL L-glutamine | X |
| Figueroa [55] | 2500 mL | HBOC-201 or washed RBC | Hct 10%–15% | 800 mL | 5000U/L | 500 mg vancomycin | X | X | 500 mg | 1U/L | 2300 mg calcium gluconate | X | 400 mL exchange every 3 h starting at 6 h |
| Gok [56] | NR | Custodiol HTK | Acellular | 2.5 g | 1000U | 5 mg Cefazolin | X | NaHCO3 1mEq | X | X | X | X | Hemofiltration 0.1–0.3 mL/min |
| Goutard [57] | 200 mL | Modified STEEN solution | Acellular | BSA | 2000U/L | 4 mL/L Penicillin-streptomycin | Glucose in STEEN | NaHCO3 in STEEN | 16 mg/L dexamethasone, 200 mg/L hydrocortisone | 20U/L | CaCl2 in STEEN | X | X |
| Mayer [58] | NR | HAM’s solution | Acellular | BSA | X | Penicillin-streptomycin, amphotericin B | Glucose in HAM’s solution | X | X | X | CaCl2 in HAM’s solution | L-glutamine | Hemofiltration |
| Rezaei [59] | 2500 mL | pRBC, FFP | 1200mL pRBC, 900 mL FFP | 350 mL 25% albumin | 5000U | 250 mg vancomycin, 250 mg cefazolin | X | X | 500 mg | As needed | X | X | 500 mL every 3 h starting at 6 h |
| Stone [60] | NR | Ringer’s solution | Hct 25%–30% | BSA | 4000U | 500 mg meropenem | 30 mL 15% glucose | 50 mL NaHCO3 | 13.2 mg dexamethasone | X | CaCl2 in LR | 40 mL 20% mannitol GTN infusion 10 mL/hr Nutriflex infusion 10 mL/hr | X |
| Taeger [61] | 42–50 L | Heparinized Custodiol HTK or lactated Ringer’s | Erythrocyte concentrates | X | Heparinized | 4g –0.5 g piperacillin-tazobactam every 3 h | X | X | X | X | CaCl2 in LR | X | X |
| Valdivia [62] | NR | STEEN solution and Sterofundin ISO | Acellular | HSA in STEEN | X | 500 μL/mL cefazolin | Glucose in STEEN | NaHCO3 in STEEN | X | X | CaCl2 in STEEN | 0.06% sodium hydrogen carbonate | X |
Perfusate content.
BSA, bovine serum albumin; D50, Dextrose 50% in normal saline; HSA, human serum albumin; MW, molecular weight; NaHCO3, sodium bicarbonate; NR, not reported; pRBCs, packed red blood cells; PPE, partial perfusate exchange; q#time, to indicate frequency a medication was administered; TCV, total circulating volume; THAM, trometamol or tris-hydroxymethyl aminomethane; X, not used or tested.
TABLE 4
| Base Medium | Contents |
|---|---|
| STEEN | Albumin, D40, glucose, KCl, NaCl, CaCl2, MgCl2, NaH2PO4, NaHCO3, NaOH |
| Perfadex | D40, NaCl, KCl, MgS, Na2HPO4, KH2PO4, glucose monohydrate |
| Phoxilium | CaCl2, MgCl2, NaCl, NaHCO3, KCl, Na2HPO4 |
| Dulbecco’s Modified Eagle’s Medium | Amino acids, vitamins, CaCl2, Fe(NO3)3, MgSO4, KCl, NaHCO3, NaCl, NaH2PO4, dextrose |
| University of Wisconsin (UW) solution | Potassium lactobionate, KH2PO4, MgSO4, raffinose, adenosine, glutathione, allopurinol, hydroxyethyl starch |
| Fluosol-43 | FC-43, Pluronic F-68, NaCl, KCl, CaCl2, MgCl2, NaHCO3, glucose, hydroxyethyl starch |
| Lactated Ringer’s solution | NaCl, KCl, CaCl2, sodium lactate |
| Ringer’s solution | NaCl, KCl, CaCl2, NaHCO3, +/- other minerals |
| Custodiol HTK | NaCl, KCl, MgCl2, CaCl2, histidine, tryptophan, mannitol, potassium hydrogen 2-ketoglutarate |
| PromoCell skeletal muscle cell growth medium | Amino acids, vitamins, fetal calf serum, fetuin, EGF, bFGF, insulin, dexamethasone |
| HAM’s solution | Amino acids, vitamins, glucose, NaCl, KCl, CaCl2, MgCl2, CuSO4, FeSO4, Na3PO4, ZnSO4 |
Contents of base media used in perfusate preparation.
Graft and Perfusate Monitoring
During perfusion, grafts were often monitored via capillary refill, skin or muscle temperature, skin color, neuromuscular electrical stimulation, and compartment pressure (see Table 5). All but three studies used sequential tissue samples for histological staining, single-muscle fiber contractility testing, TUNEL apoptosis assay, and/or quantification of various markers of ischemia-reperfusion injury and hypoxia. Change in graft weight during perfusion was noted in 20 studies. Perfusate levels of potassium, lactate, myoglobin, and creatine kinase were monitored and reported in 20, 20, 9, and 6 studies, respectively.
TABLE 5
| Author (date) | Limb monitoring | Graft weight | Potassium (mmol/L) | Lactate (mmol/L) | CK (U/L) | Mb (ng/mL) |
|---|---|---|---|---|---|---|
| Amin [17] | - Capillary refill, skin temp, and color Q15-60 min - Samples: Skin, muscle, vessel, at t < 0, t = end | X | 6.3 ± 0.5 | X | X | X |
| Amin [35] | - Capillary refill, skin temp, and color Q15-60min - Samples: Skin, muscle, vessel, at t < 0, t = end | NT at 70 mmHg: −0.3% ± 1.7% | NT at 70 mmHg: 7.0 ± 1.7 | NT at 70 mmHg: 15.1 ± 4.8 | X | X |
| Gok [36] | - Samples: 100 mg gastrocnemius sample, flash frozen, and stored at −80°C - Metabolomics profiling | +3.1% ± 0.4% | Increased; 6.3 ± 1.2 | Increased; 4.3 ± 1.3 | X | X |
| Werner [37] | - Palm skin temp Qh - Median and ulnar nerve electrical stimulation Q2h - Samples: Flexor carpi radialis samples at 0, 12, and 24 h of perfusion | −0.4% (−7%-+7%) | Varied, 3.0–5.5 | Steadily increased from 5 to 15 | X | 43 K at t = 0; 92 K at t = 24 h |
| Ozer [38] | - Capillary refill - Skin temp - Functional electostimulation Qh; Single-fiber contractility testing - Samples: Muscle biopsies, 10 mm x 5 mm | +20% after perfusion; decreased to +15% after transplantation | Stable, no change after transplantation | Increased steadily during perfusion, no change after transplantation | X | X |
| Ozer [39] | - Functional electostimulation Qh; Single-fiber contractility testing - Samples: Muscle biopsies, 10 mm x 5 mm | Significant gain after perfusion; No significant gain after transplantation | Stable | Gradual increase during perfusion, normalized after transplantation | X | X |
| Constantinescu [40] | - Capillary refill Qh - Electrical stimulation of 3 proximal nerve bundles - Skin and muscle color Qh; Compartment pressure - Samples: Muscle, nerve, vessel biopsies at t = end; Immunofluorescence staining | Maximum of +1.32% | 4.27 ± 1.38 | 16.83 ± 2.46 | X | X |
| Fahradyan [41] | - Peripheral perfusion via ICG angiography, t = end - Muscle surface temp Qh - Muscle and motor nerve electrical stimulation and contractility - Flexor and extensor compartment pressure - Samples: Muscle biopsies | 12 h group: −1.28% ± 8.59% >24 h group: +7.28% ± 15.05% | 12 h group: 5.7 ± 1.7 >24 h group: 6.5 ± 1.8 | 12 h group: 9.2 ± 4.4 >24 h group: 9.6 ± 4.7 | 12h group: 53 K ± 15 K >24 h group: 64 K ± 32 K | 12 h group: 875 ± 294 >24 h group: 1134 ± 538 |
| Duraes [42] | - Peripheral perfusion, ICG angiography, t = end - Muscle temp - Muscle and motor nerve electrical stimulation and contractility - Flexor and extensor compartment pressure, Tissue O2 sat - Samples: Muscle, skin, and nerve biopsies collected at 0 and 12 h | 12 h 39°C colloid/wRBC: +0.54% ± 7.35% | 12 h 39°C colloid/wRBC: 5.4 ± 1.1 | 12 h 39°C colloid/wRBC: 9.4 ± 2.4 | 12h 39°C colloid/wRBC: 53 K ± 15 K | 12 h 39°C colloid/wRBC: 875 ± 291.4 |
| Haug [43] | - Samples: Muscle biopsies, hematoxylin/eosin stain, HIF-1α Western blot | SCS: +3% STEEN: +25% Phoxilium: +58% PHODEX: +36% | Decreased during first 1–2 h, increased to 6 h, stable to 12 h | Decreased during first 1–2h, increased to 6h, stable to 12 h | STEEN: +1.2 K Phoxilium: +1.5 K PHODEX: +5.5 K | STEEN: +1 Phoxilium: +121 PHODEX: +140 |
| Haug [44] | - Samples: Muscle biopsies, HIF-1α Western blot - Cytokine analysis with ELISA | SCS: +1.4% Perfusion: +4.3% | 9.6 (0 h) 5.77 (24 h) | 6.9 (0 h) 2.8 (24 h) | 1.4 K (0 h) 4 K (24 h) | 4.4 K (0 h) 9 K (24 h) |
| Kueckelhaus [45] | - Samples: Muscle biopsy, histology, TEM - PCR quantification of hypoxia/ischemia markers, cytokine assay | SCS: None Perfusion: +10% ± 2% | Peaked at 3 h perfusion; SCS>perfusion after transplant | Perfusion: Increased steadily to 2.43 mM | X | Peaked at 3 h perfusion; SCS>perfusion after transplant |
| Kueckelhaus [46] | - Samples: Muscle biopsy, histology | SCS: None Perfusion: +44.06% | 5.73 (0 h) 9.35 (12 h) | X | X | X |
| Krezdorn [47] | - ATP and glycogen assay - 3-Tesla MRI of muscle changes - Samples: Muscle biopsy histology | +40% | Increased during perfusion; decreased after replantation | Increased during perfusion; increased in 3 h after replantation | X | Increased during perfusion; decreased after replantation |
| Krezdorn [48] | - Samples: Muscle biopsies after replant, histology - PCR of genes involved in glycolysis, angiogenesis, and DNA damage | X | X | X | X | X |
| Kruit [49] | - Muscle core temp - Nerve stimulation, muscle contractility - Samples: Flexor and extensor muscle histology | SCS: +1.6% Perfusion: −2.7% | SCS and perfused limb potassium increased after replantation (P = 0.4), remained wnl | 0.7 (18 h), remained low throughout perfusion, similar to SCS (P = 0.4) | 15.6 K (18 h), higher in perfused group than SCS after replantation (P < 0.01) | X |
| Domingo-Pech [50], | - Samples: Muscle biopsy, H&E stain | +20–50% | X | X | X | X |
| Usui [51] | - In vivo basic metabolic panel, enzymes | Continuous perfusion with fluorocarbon: −3.4% ± 1.2% after perfusion; +26.8 ± 2.7 after replant | After replant: Immediate marked increase, stable after 30 min | After replant: Immediate marked increase, normal at 6 h only in continuous perfusion with fluorocarbon group | X | X |
| Muller [52] | - Samples: muscle biopsy, peripheral nerve biopsy, blood vessel biopsy, histology - Inflammatory markers, serum complement activity | X | X | X | X | X |
| Adil [53] | - Samples: muscle, nerve, bone, skin, vessels | X | X | X | X | X |
| Burlage [54] | - Samples: muscle biopsy | HBOC-201: +4.9 g BSA: +48.8 g BSA/PEG: +27.3 g | HBOC-201: 5.8 after 1 h BSA: 1.8 after 1 h BSA/PEG: 4.4 after 1 h Initially increased, stabilized after 3 h | X | X | X |
| Figueroa [55] | - Samples: muscle biopsy - ICG angiography - Compartment pressures | HBOC-201: +23.10% ± 3.00% RBC: +13.18% ± 22.70% | HBOC-201: 6.45 ± 1.69 RBC: 6.78 ± 1.94 | HBOC-201: 14.66 ± 4.26 RBC: 13.11 ± 6.68 | X | X |
| Gok [56] | - Samples: muscle biopsy - Muscle contractility | +3.5% | X | <2 | X | X |
| Goutard [57] | - Samples: skin, muscle | X | Decreased over 3 h | Decreased over 3 h | X | X |
| Mayer [58] | X | X | X | X | X | X |
| Rezaei [59] | - Samples: muscle biopsy Muscle and nerve functionality - Compartment pressures | +0.4% ± 12.2% | 7.6 ± 0.9 | 20 at median time point 15 h | 956 within 1 h, 49020 at endpoint | 5370 initially, 34730 at endpoint |
| Stone [60] | - Samples: muscle, skin, vessel - Thermal imaging | X | X | Limb/Kidney: 10.9 ± 3.5 after 1h, 7.5 ± 1.7 at endpoint Limb Only: 14.6 ± 2.2 after 1 h, 13.8 ± 3.7 at endpoint | X | X |
| Taeger [61] | X | X | X | X | X | X |
| Valdivia [62] | - Samples: skin, muscle, vessels - Bioluminescence detection - Cell phenotyping | X | X | Vector: 562.3 ± 38.9 μM Non-Vector: 577 ± 26.8 μM | X | Vector: 224.9 ± 10.3 ng/mL Non-Vector: 222.9 ± 44.8 ng/mL |
Limb monitoring and common outcome measurements.
C, continuous monitoring; CK, creatine kinase; Hb, hemoglobin; ICG, indocyanine green; Mb, myoglobin; PPE, partial perfusate exchange; TEM, transmission electron microscopy; wnl, within normal limits.
Perfusion Outcomes
While the designs and objectives varied between studies, multiple studies showed improved biomarkers, histology, and outcomes for EVMP limbs compared to static cold storage (SCS) at 4°C. Four studies [35, 40, 52, 59] showed equivalent or improved outcomes in NT or NNT EVMP compared to SCS, of which one involved transplantation [52]. Eight studies [44–49, 56, 57] showed equivalent or improved outcomes in HT EVMP compared to SCS, including six which involved transplantation [45, 47–49, 56, 57].
Human Limb Studies
Of note, four articles [37, 44, 59, 61] utilized human limbs for machine perfusion studies. Three studies [37, 44, 59] looked at upper limbs, all of which showed hemodynamically stable perfusions up to 24 h, with improved histology as compared to SCS in one study. The fourth human limb study [61] looked at traumatic lower extremity amputations; lower limbs were perfused for 12–15 h at SNT temperatures, with successful replantation in both cases.
Discussion
EVMP is an innovative and evolving approach to solid organ preservation and reconditioning for transplantation, with great potential for clinical application to VCA. The current literature in VCA EVMP is focused mainly on upper or lower extremities, but is expanding to include a variety of perfusion protocols and subsequent structural and immunological outcomes.
Cellular Composition of Perfusate
In transplantation, perfusion media plays a crucial role in maintaining the viability and function of the graft. These media can broadly be categorized into two types: cellular and acellular. Despite both being designed to preserve the organ, their composition and mechanisms vary significantly.
Cellular media often incorporate contents like red blood cells (RBC) or hemoglobin-based oxygen carriers which facilitate the transport of oxygen to the tissue. The inclusion of cellular components aims to create an environment that is similar to in vivo conditions, which may especially benefit organs or tissues with high metabolic rates. The presence of cellular elements can also enhance oxygen transport and provide essential nutrients, thereby reducing ischemic injury. Werner and Ozer both adopt cellular media and show its efficacy in preserving the viability of human and swine limbs for up to 24 h [37, 38]. However, cellular media may pose challenges such as inflammation and increased risk of thrombosis. Amin has observed a cumulative increase in pro-inflammatory markers at 6 h in swine forelimb perfusion [17]. Additionally, cellular blood-based perfusate is limited by blood bank accessibility, blood refrigeration, and the short shelf life of blood products, limiting its utility in military and emergency settings [63, 64]. Blood-based perfusates also carry risk of infection and coagulation, as well as HLA-sensitization and transfusion-related reactions [64–66].
By contrast, acellular media lacks cellular components and therefore generally relies on the dissolving of oxygen. Several studies in porcine lung EVMP suggest that acellular perfusates are a suitable alternative to blood-based perfusate [67–69]. Therefore, acellular perfusates have gained increasing interest as a more accessible and low-maintenance approach, evidenced by nearly half of the studies in this cohort using acellular perfusate. Importantly, while simpler and easier to manage, the absence of specialized oxygen carriers like RBCs may limit the efficiency of O2 transport. Thus, acellular media often need additional oxygenation such as adding synthetic oxygen carriers or pumping with oxygen [70].
Base Medium
The base medium (see Table 4) can be roughly categorized into 3 different types: 1) cell culture, 2) electrolyte balance, 3) preservation and perfusion. They share many common functions, including basic functions like maintaining osmotic balance, cellular homeostasis, and regulation of pH. Some of the media contains nutrients like amino acids, glucose, or specialized carbohydrates, which can provide cells with additional substrates for metabolism support during preservation. Certain media like HTK has tryptophan which can protect the graft against oxidative stress during ischemic conditions [71].
Supplements and Additives
There are a variety of supplements that can be added to tailor the perfusate to specific experimental conditions. Electrolytes are a common inclusion, especially sodium chloride, which is necessary to maintain the osmotic balance. Additionally, calcium and magnesium compounds serve important roles in cellular signaling and enzymatic functions. Potassium is important in maintaining a high intracellular-to-extracellular gradient via the Na + K + ATPase pump, as most total body potassium is stored within muscle.
The base media chosen also contains different additives that can help modulate the perfusate. Cell culture media like DMEM usually contain general nutritional components for cellular division. By contrast, STEEN and Perfadex include unique components like albumin and D40, which is specialized for specific organs like lungs. Fluosol-43 is designed to promote tissue oxygenation [72]. University of Wisconsin solution (UW) contains potassium lactobionate and raffinose, where the former compound is critical for minimizing cellular edema and the latter one is crucial in providing carbohydrate sources for metabolism. Custodiol HTK include histidine and tryptophan, amino acids that can help in maintaining pH balance and protecting cells during ischemic or hypothermic conditions.
Perfusion Time
The duration of perfusion is a pivotal factor that may influence cellular viability, organ functionality, and the risk of ischemic injury. Even brief periods of ischemia can lead to significant tissue damage. Shorter perfusion times, generally around 6 h, are beneficial for minimizing logistical challenges and reducing the risk of complications. However, perfusion times ranging between 6 and 24 h can allow for better equilibration with the perfusion solution and potentially offer a broader window for assessing organ viability prior to transplant or replant. Extended perfusion durations that exceed 24 h are usually employed for experimental settings. While they allow for in-depth monitoring and potentially improved transplantation outcomes, these extended durations are logistically complex and pose an elevated risk of complications like delayed graft function. The decision regarding duration of perfusion requires thorough consideration of the aforementioned factors and should be tailored to the type of organ, logistical challenges, and overall objective of the perfusion.
Limitations and Suggestions for Future Research
This systematic review presents with several limitations. Literature search was conducted with the assumption that all relevant studies would be discoverable via six large databases and a predetermined set of search terms. Additionally, non-English studies, abstracts, posters, conference presentations, and unpublished data were excluded from this study. In consideration of the small cohort of included studies, it is possible that we excluded other research that would offer valuable insight into the development of research in VCA EVMP. Specifically, the exclusion of non-English papers may have unintentionally limited this review, and further insights might be gleaned from supplementary examination of non-English VCA EVMP articles. Additionally, this review excludes articles published after June 2023. As VCA research is rapidly evolving, multiple studies may have been published on this topic in the intervening time.
The conclusions drawn from this review are limited by the quality and design of published research in VCA EVMP. As the swine forelimb represents the dominant model in this review, outcomes of these studies may not be generalizable to humans or other models with more complex forearm and hand anatomy. Future investigations in EVMP of monkey or ape limbs and subsequent functional testing may help to bridge this gap in knowledge. Additionally, the included studies are not representative of the breadth of VCA (e.g., face, calvarium, abdominal wall, and genital transplantation). As such, these studies may not be applicable to preservation of these structures.
While this paper details the technical aspects and limitations of VCA EVMP, these are not the only barriers to clinical translation. VCA is performed by a limited number of institutions, and on a significantly smaller scale than solid organ transplants. The low numbers of yearly VCAs are cost-prohibitive for a standardized perfusion machine, and severely limit the sample size for any potential clinical trials. VCAs also carry unique ethical considerations, including vulnerability of recipients, as well as racial and socioeconomic disparities [73]. These logistical and ethical barriers further hinder the successful clinical translation of EVMP in VCA.
Conclusion
VCA EVMP is a versatile platform through which grafts may be preserved and optimized prior to replantation or replantation. There is significant evidence to suggest that EVMP may be superior to SCS as a preservation method. While methods greatly varied throughout the literature reviewed, the major factors of each perfusion protocol remained the same: temperature, perfusate composition, and perfusion time. As in solid organ transplant perfusion, there is currently no consensus on the optimal temperature for VCA perfusion. Studies reviewed in this paper showed promising results for both HMP and NMP/NNT, and no recent evidence has definitively suggested the benefit of one temperature over the other. Rather than attempting to condense VCA EVMP down to a singular optimal perfusion protocol, perfusion factors should be chosen and adapted based on the individual needs and goals of each future study. For instance, the choice of a blood-based perfusate might be more suitable for NMP given the higher metabolic rate, or for a shorter perfusion duration given the limitations of obtaining and storing blood. An acellular perfusate might be more suitable for HMP given the lower metabolic rate, or for a longer perfusion duration to facilitate perfusate exchange. Overall, preclinical studies offer promising results regarding the feasibility of VCA preservation via machine perfusion, but additional experimental studies are needed to overcome technical barriers to clinical translation.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
TM manuscript writing, literature review, data analysis. ED manuscript writing, literature review, data analysis. AL manuscript writing, literature review, data analysis. YG literature review, manuscript review. YZ literature review, manuscript review. CL literature review, manuscript writing, manuscript review. AG literature review, manuscript writing, manuscript review. IL literature review, manuscript review. BH literature review, manuscript writing, manuscript review. RK literature review, manuscript writing, manuscript review. BO study conceptualization, literature review, manuscript review. GB study conceptualization, literature review, manuscript review. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors would like to acknowledge the support of the Department of Defense (DoD) and the Reconstructive Transplantation Research Program (RTRP) under award W81XWH-20-RTRP-IIRA (RT200031P1), W81XWH-20-RTRP-IIRA (RT200042P1) and W81XWH-19-1-0744.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
References
1.
BuenoEMDiaz-SisoJRSiskGCChandawarkarAKiwanukaHLamparelloBet alVascularized Composite Allotransplantation and Tissue Engineering. J Craniofac Surg (2013) 24(1):256–63. 10.1097/SCS.0b013e318275f173
2.
LewisHCCendalesLC. Vascularized Composite Allotransplantation in the United States: A Retrospective Analysis of the Organ Procurement and Transplantation Network Data after 5 Years of the Final Rule. Am J Transpl (2021) 21(1):291–6. 10.1111/ajt.16086
3.
MessnerFSarduCPetruzzoP. Grasping Time - Longevity of Vascularized Composite Allografts. Curr Opin Organ Transpl (2024) 29(6):376–81. 10.1097/MOT.0000000000001177
4.
StarzlRBrandacherGLeeWPACarbonellJZhangWSchniderJet alReview of the Early Diagnoses and Assessment of Rejection in Vascularized Composite Allotransplantation. Clin Dev Immunol (2013) 2013:402980. 10.1155/2013/402980
5.
KueckelhausMFischerSSeydaMBuenoEMAycartMAAlhefziMet alVascularized Composite Allotransplantation: Current Standards and Novel Approaches to Prevent Acute Rejection and Chronic Allograft Deterioration. Transpl Int (2016) 29(6):655–62. 10.1111/tri.12652
6.
UluerMCBrazioPSWoodallJDNamAJBartlettSTBarthRN. Vascularized Composite Allotransplantation: Medical Complications. Curr Transpl Rep (2016) 3(4):395–403. 10.1007/s40472-016-0113-x
7.
HomsyPHuelsboemerLBarretJPBlondeelPBorsukDEBulaDet alAn Update on the Survival of the First 50 Face Transplants Worldwide. JAMA Surg (2024) 159(12):1339–45. 10.1001/jamasurg.2024.3748
8.
LopezCDGirardAOLakeIVOhBCBrandacherGCooneyDSet alLessons Learned from the First 15 Years of Penile Transplantation and Updates to the Baltimore Criteria. Nat Rev Urol (2023) 20(5):294–307. 10.1038/s41585-022-00699-7
9.
BenedictJMagillG. Upper Extremity and Craniofacial Vascularized Composite Allotransplantation: Ethics and Immunosuppression. Emerg Top Life Sci (2019) 3(6):681–6. 10.1042/ETLS20190060
10.
CaplanALParentBKahnJDeanWKimberlyLLLeeWPAet alEmerging Ethical Challenges Raised by the Evolution of Vascularized Composite Allotransplantation. Transplantation (2019) 103(6):1240–6. 10.1097/TP.0000000000002478
11.
HuelsboemerLKauke-NavarroMBoroumandSParikhNHosseiniHYuCTet alTen-Year Follow-Up after Face Transplantation-A Single-Center Retrospective Cohort Study. Am J Transpl (2025) 25(3):611–22. 10.1016/j.ajt.2024.10.007
12.
MessnerFGrahammerJHautzTBrandacherGSchneebergerS. Ischemia/reperfusion Injury in Vascularized Tissue Allotransplantation: Tissue Damage and Clinical Relevance. Curr Opin Organ Transpl (2016) 21(5):503–9. 10.1097/mot.0000000000000343
13.
KadonoKGruszynskiMAzariKKupiec-WeglinskiJW. Vascularized Composite Allotransplantation versus Solid Organ Transplantation: Innate-Adaptive Immune Interphase. Curr Opin Organ Transpl (2019) 24(6):714–20. 10.1097/mot.0000000000000705
14.
PetruzzoPDubernardJM. The International Registry on Hand and Composite Tissue Allotransplantation. Clin Transpl (2011) 247–53. 10.1097/TP.0b013e3181ff1472
15.
GoutardMLellouchAGDussolBLantieriLA. Facial Trauma 8 Years after a Face Transplantation. Plast Reconstr Surg Glob Open (2021) 9(5):e3575. 10.1097/GOX.0000000000003575
16.
KanitakisJPetruzzoPBadetLGazarianAThaunatOTestelinSet alChronic Rejection in Human Vascularized Composite Allotransplantation (Hand and Face Recipients): An Update. Transplantation (2016) 100(10):2053–61. 10.1097/TP.0000000000001248
17.
AminKRStoneJPKerrJGeraghtyAJosephLMontero-FernandezAet alRandomized Preclinical Study of Machine Perfusion in Vascularized Composite Allografts. Br J Surg (2021) 108(5):574–82. 10.1002/bjs.11921
18.
YehHMartinsPMarkmannJ. In: Textbook of Organ Transplantation. Wiley (2014). p. 1267–79. 10.1002/9781118873434.ch104
19.
HosgoodSANicholsonHFLNicholsonML. Oxygenated Kidney Preservation Techniques. Transplantation (2012) 93(5):455–9. 10.1097/TP.0b013e3182412b34
20.
VajdováKGrafRClavienPA. ATP-Supplies in the Cold-Preserved Liver: A Long-Neglected Factor of Organ Viability. Hepatology (2002) 36(6):1543–52. 10.1053/jhep.2002.37189
21.
LandinLCavadasPCGarcia-CosmesPThioneAVera-SempereF. Perioperative Ischemic Injury and Fibrotic Degeneration of Muscle in a Forearm Allograft: Functional Follow-Up at 32 Months Post Transplantation. Ann Plast Surg (2011) 66(2):202–9. 10.1097/SAP.0b013e318206a365
22.
PanizoAPardoFJLozanoMDde AlavaESolaIIdoateMA. Ischemic Injury in Posttransplant Endomyocardial Biopsies: Immunohistochemical Study of Fibronectin. Transpl Proc (1999) 31(6):2550–1. 10.1016/s0041-1345(99)00495-9
23.
MareckiHBozorgzadehAPorteRJLeuveninkHGUygunKMartinsPN. Liver Ex Situ Machine Perfusion Preservation: A Review of the Methodology and Results of Large Animal Studies and Clinical Trials. Liver Transpl (2017) 23(5):679–95. 10.1002/lt.24751
24.
ElgharablyHShafiiAEMasonDP. Expanding the Donor Pool: Donation after Cardiac Death. Thorac Surg Clin (2015) 25(1):35–46. 10.1016/j.thorsurg.2014.09.011
25.
UrbanMBishawiMCastleberryAWMarkinNWChaconMMUmJYet alNovel Use of Mobile Ex-Vivo Lung Perfusion in Donation after Circulatory Death Lung Transplantation. Prog Transpl (2022) 32(2):190–1. 10.1177/15269248221087437
26.
SteenSIngemanssonRErikssonLPierreLAlgotssonLWierupPet alFirst Human Transplantation of a Nonacceptable Donor Lung after Reconditioning Ex Vivo. Ann Thorac Surg (2007) 83(6):2191–4. 10.1016/j.athoracsur.2007.01.033
27.
QuaderMTorradoJFManginoMJToldoS. Temperature and Flow Rate Limit the Optimal Ex-Vivo Perfusion of the Heart - an Experimental Study. J Cardiothorac Surg (2020) 15(1):180. 10.1186/s13019-020-01223-x
28.
FurukawaHTodoSImventarzaOCasavillaAWuYMScotti-FoglieniCet alEffect of Cold Ischemia Time on the Early Outcome of Human Hepatic Allografts Preserved with UW Solution. Transplantation (1991) 51(5):1000–4. 10.1097/00007890-199105000-00013
29.
AdamRBismuthHDiamondTDucotBMorinoMAstarciogluIet alEffect of Extended Cold Ischaemia with UW Solution on Graft Function after Liver Transplantation. Lancet (1992) 340(8832):1373–6. 10.1016/0140-6736(92)92559-x
30.
BralMGala-LopezBBigamDLFreedDHShapiroAMJ. Ex situ Liver Perfusion: Organ Preservation into the Future. Transpl Rev (Orlando) (2018) 32(3):132–41. 10.1016/j.trre.2018.03.002
31.
CarrelALindberghCA. The Culture of Whole Organs. Science (1935) 81(2112):621–3. 10.1126/science.81.2112.621
32.
LaingRWMergentalHMirzaDF. Normothermic Ex-Situ Liver Preservation: The New Gold Standard. Curr Opin Organ Transplant (2017) 22(3):274–80. 10.1097/MOT.0000000000000414
33.
KatariaAMagoonSMakkarBGundrooA. Machine Perfusion in Kidney Transplantation. Curr Opin Organ Transpl (2019) 24(4):378–84. 10.1097/MOT.0000000000000675
34.
MoherDLiberatiATetzlaffJAltmanDGPRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J Clin Epidemiol (2009) 62(10):1006–12. 10.1016/j.jclinepi.2009.06.005
35.
AminKRStoneJPKerrJCWongJKFildesJE. Normothermic Ex Vivo Perfusion of the Limb Allograft Depletes Donor Leukocytes Prior to Transplantation. J Plast Reconstr Aesthet Surg (2021) 74(11):2969–76. 10.1016/j.bjps.2021.03.071
36.
GokEAlghanemFMoonRGuyERojas-PenaABartlettRHet alDevelopment of an Ex-Situ Limb Perfusion System for a Rodent Model. ASAIO J (2019) 65(2):167–72. 10.1097/MAT.0000000000000786
37.
WernerNLAlghanemFRakestrawSLSarverDCNicelyBPietroskiREet alEx Situ Perfusion of Human Limb Allografts for 24 Hours. Transplantation (2017) 101(3):e68–e74. 10.1097/TP.0000000000001500
38.
OzerKRojas-PenaAMendiasCLBrynerBSToomasianCBartlettRH. The Effect of Ex Situ Perfusion in a Swine Limb Vascularized Composite Tissue Allograft on Survival up to 24 Hours. J Hand Surg Am (2016) 41(1):3–12. 10.1016/j.jhsa.2015.11.003
39.
OzerKRojas-PenaAMendiasCLBrynerBToomasianCBartlettRH. Ex Situ Limb Perfusion System to Extend Vascularized Composite Tissue Allograft Survival in Swine. Transplantation (2015) 99(10):2095–101. 10.1097/tp.0000000000000756
40.
ConstantinescuMAKnallEXuXKiermeirDMJenniHGygaxEet alPreservation of Amputated Extremities by Extracorporeal Blood Perfusion; a Feasibility Study in a Porcine Model. J Surg Res (2011) 171(1):291–9. 10.1016/j.jss.2010.01.040
41.
FahradyanVSaidSAOrdenanaCDalla PozzaEFrautschiRDuraesEFRet alExtended Ex Vivo Normothermic Perfusion for Preservation of Vascularized Composite Allografts. Artif Organs (2020) 44(8):846–55. 10.1111/aor.13678
42.
DuraesEFRMadajkaMFrautschiRSolimanBCakmakogluCBarnettAet alDeveloping a Protocol for Normothermic Ex-Situ Limb Perfusion. Microsurgery (2018) 38(2):185–94. 10.1002/micr.30252
43.
HaugVKollarBEndoYKadakiaNVeeramaniAKaukeMet alComparison of Acellular Solutions for Ex-Situ Perfusion of Amputated Limbs. Mil Med (2020) 185(11-12):e2004–e2012. 10.1093/milmed/usaa160
44.
HaugVKollarBTasigiorgosSEndoYKaukeMSafiAFet alHypothermic Ex Situ Perfusion of Human Limbs with Acellular Solution for 24 Hours. Transplantation (2020) 104(9):e260–e270. 10.1097/tp.0000000000003221
45.
KueckelhausMFischerSSiskGKiwanukaHBuenoEMDermietzelAet alA Mobile Extracorporeal Extremity Salvage System for Replantation and Transplantation. Ann Plast Surg (2016) 76(3):355–60. 10.1097/SAP.0000000000000681
46.
KueckelhausMDermietzelAAlhefziMAycartMAFischerSKrezdornNet alAcellular Hypothermic Extracorporeal Perfusion Extends Allowable Ischemia Time in a Porcine Whole Limb Replantation Model. Plast Reconstr Surg (2017) 139(4):922e–932e. 10.1097/PRS.0000000000003208
47.
KrezdornNMacleodFTasigiorgosSTurk M DMWoLKiwanuka B AHet alTwenty-Four-Hour Ex Vivo Perfusion with Acellular Solution Enables Successful Replantation of Porcine Forelimbs. Plast Reconstr Surg (2019) 144(4):608e–618e. 10.1097/prs.0000000000006084
48.
KrezdornNSakthivelDTurkMAycartMATasigiorgosSBuenoEMet alReduced Hypoxia-Related Genes in Porcine Limbs in Ex Vivo Hypothermic Perfusion versus Cold Storage. J Surg Res (2018) 232:137–45. 10.1016/j.jss.2018.05.067
49.
KruitASBrouwersKvan MiddenDZegersHKoersEvan AlfenNet alSuccessful 18-h Acellular Extracorporeal Perfusion and Replantation of Porcine Limbs - Histology versus Nerve Stimulation. Transpl Int (2021) 34(2):365–75. 10.1111/tri.13802
50.
Domingo-PechJGarrigaJMToranNRusinolMGirventFRosinesDet alPreservation of the Amputated Canine Hind Limb by Extracorporeal Perfusion. Int Orthop (1991) 15(4):289–91. 10.1007/BF00186863
51.
UsuiMSakataHIshiiS. Effect of Fluorocarbon Perfusion upon the Preservation of Amputated Limbs. An Experimental Study. J Bone Joint Surg Br (1985) 67(3):473–7. 10.1302/0301-620X.67B3.3997959
52.
MüllerSConstantinescuMAKiermeirDMGajanayakeTBongoniAKVollbachFHet alIschemia/reperfusion Injury of Porcine Limbs after Extracorporeal Perfusion. J Surg Res (2013) 181(1):170–82. 10.1016/j.jss.2012.05.088
53.
AdilAKaroubiGHaykalS. Procurement and Decellularization of Rat Hindlimbs Using an Ex Vivo Perfusion-Based Bioreactor for Vascularized Composite Allotransplantation. J Vis Exp (2022) 184. 10.3791/64069
54.
BurlageLCLellouchAGTaveauCBTratnig-FranklPPendexterCARandolphMAet alOptimization of Ex Vivo Machine Perfusion and Transplantation of Vascularized Composite Allografts. J Surg Res (2022) 270:151–61. 10.1016/j.jss.2021.09.005
55.
FigueroaBASaidSAOrdenanaCRezaeiMOrfahliLMDubéGPet alEx vivo Normothermic Preservation of Amputated Limbs with a Hemoglobin-Based Oxygen Carrier Perfusate. J Trauma Acute Care Surg (2022) 92(2):388–97. 10.1097/TA.0000000000003395
56.
GokEKubiakCAGuyEPonderMHoenerhoffMJRojas-PenaAet alLong-Term Effects of Hypothermic Ex Situ Perfusion on Skeletal Muscle Metabolism, Structure, and Force Generation after Transplantation. Transplantation (2019) 103(10):2105–12. 10.1097/TP.0000000000002800
57.
GoutardMde VriesRJTawaPPendexterCARosalesIATessierSNet alExceeding the Limits of Static Cold Storage in Limb Transplantation Using Subnormothermic Machine Perfusion. J Reconstr Microsurg (2023) 39(5):350–60. 10.1055/a-1886-5697
58.
MayerBA. An Extracorporeal Warm Perfusion Device for Basic Research: Possibility of Avoiding Some Animal Experiments. Artif Organs (1999) 23(12):1126–8. 10.1046/j.1525-1594.1999.06173-2.x
59.
RezaeiMOrdenanaCFigueroaBASaidSAFahradyanVDalla PozzaEet alEx Vivo Normothermic Perfusion of Human Upper Limbs. Transplantation (2022) 106(8):1638–46. 10.1097/TP.0000000000004045
60.
StoneJPAminKRGeraghtyAKerrJShawMDabareDet alRenal Hemofiltration Prevents Metabolic Acidosis and Reduces Inflammation during Normothermic Machine Perfusion of the Vascularized Composite Allograft: A Preclinical Study. Artif Organs (2022) 46(2):259–72. 10.1111/aor.14089
61.
TaegerCDLambyPDoldererJPhilippAKehrerAHorchREet alExtracorporeal Perfusion for Salvage of Major Amputates. Ann Surg (2019) 270(1):e5–e6. 10.1097/sla.0000000000003226
62.
ValdiviaERotherTYuzefovychYHackFWenzelNBlasczykRet alGenetic Modification of Limbs Using Ex Vivo Machine Perfusion. Hum Gene Ther (2022) 33(7-8):460–71. 10.1089/hum.2021.199
63.
KnowlesS. Blood Transfusion: Challenges and Limitations. Transfus Alternatives Transfus Med (2007) 9(s2):2–9. 10.1111/j.1778-428X.2007.00062.x
64.
NgMSYDavidMMiddelburgRANgASYSuenJYTungJPet alTransfusion of Packed Red Blood Cells at the End of Shelf Life Is Associated with Increased Risk of Mortality - a Pooled Patient Data Analysis of 16 Observational Trials. Haematologica (2018) 103(9):1542–8. 10.3324/haematol.2018.191932
65.
FongIW. Blood Transfusion-Associated Infections in the Twenty-First Century: New Challenges. Curr Trends Concerns Infect Dis (2020) 191–215. 10.1007/978-3-030-36966-8_8
66.
WeinstockCSchnaidtM. Human Leucocyte Antigen Sensitisation and its Impact on Transfusion Practice. Transfus Med Hemotherapy (2019) 46(5):356–69. 10.1159/000502158
67.
BeckerSSteinmeyerJAvsarMHöfflerKSalmanJHaverichAet alEvaluating Acellular versus Cellular Perfusate Composition during Prolonged Ex Vivo Lung Perfusion after Initial Cold Ischaemia for 24 Hours. Transpl Int (2016) 29(1):88–97. 10.1111/tri.12649
68.
SteinmeyerJBeckerSAvsarMSalmanJHöfflerKHaverichAet alCellular and Acellular Ex Vivo Lung Perfusion Preserve Functional Lung Ultrastructure in a Large Animal Model: A Stereological Study. Respir Res (2018) 19(1):238. 10.1186/s12931-018-0942-5
69.
RomanMGjorgjimajkoskaONeilDNairSColahSParmarJet alComparison between Cellular and Acellular Perfusates for Ex Vivo Lung Perfusion in a Porcine Model. J Heart Lung Transpl (2015) 34(7):978–87. 10.1016/j.healun.2015.03.023
70.
MartinsPNBerendsenTAYehHBruinsmaBGIzamisMLOp den DriesSet alOxygenated UW Solution Decreases ATP Decay and Improves Survival after Transplantation of DCD Liver Grafts. Transplantation (2019) 103(2):363–70. 10.1097/TP.0000000000002530
71.
MohrABrockmannJGBeckerF. HTK-N: Modified Histidine-Tryptophan-Ketoglutarate Solution-A Promising New Tool in Solid Organ Preservation. Int J Mol Sci (2020) 21(18):6468. 10.3390/ijms21186468
72.
GouldSARosenALSehgalLRSehgalHLLangdaleLAKrauseLMet alFluosol-DA as a Red-Cell Substitute in Acute Anemia. N Engl J Med (1986) 314(26):1653–6. 10.1056/NEJM198606263142601
73.
KumnigMJowsey-GregoireSGGordonEJWerner-FelmayerG. Psychosocial and Bioethical Challenges and Developments for the Future of Vascularized Composite Allotransplantation: A Scoping Review and Viewpoint of Recent Developments and Clinical Experiences in the Field of Vascularized Composite Allotransplantation. Front Psychol (2022) 13:1045144. 10.3389/fpsyg.2022.1045144
Summary
Keywords
vascularized composite allotransplantation, vascularized composite allograft, composite tissue transplantation, machine perfusion, machine preservation
Citation
Muss TE, Drivas EM, Loftin AH, Guo Y, Zhang Y, Lopez CD, Girard AO, Lake IV, Hassan B, Kalsi R, Oh BC and Brandacher G (2025) Ex-Vivo Perfusion of Limb Vascularized Composite Allotransplants: A Systematic Review of Published Protocols. Transpl. Int. 38:14132. doi: 10.3389/ti.2025.14132
Received
27 November 2024
Accepted
28 April 2025
Published
19 May 2025
Volume
38 - 2025
Updates
Copyright
© 2025 Muss, Drivas, Loftin, Guo, Zhang, Lopez, Girard, Lake, Hassan, Kalsi, Oh and Brandacher.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gerald Brandacher, brandacher@jhmi.edu
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.