Abstract
Climate change has worsened water salinization and acidification, creating challenges for animal health and productivity. This study assessed the resilience of indigenous sheep and goats that drink naturally saline water from Lake Basaka, focusing on their physiological and biochemical responses as stress indicators under pastoral system conditions. A cross-sectional study was conducted with a random systematic sample of 260 healthy local adult sheep and goats from 50 households for this study. The current finding revealed that physicochemical analysis of Lake Basaka water showed high salinity, with electrical conductivity at 3,992.53 μS/cm and sodium levels at 1,180.69 mg/L, significantly higher than those in freshwater (641.53 μS/cm and 35.28 mg/L, respectively; p < 0.05). The physiological data indicated that goats and sheep drinking saline water had higher rectal temperatures (39.3°C vs. 39.1°C; p < 0.05) and pulse rates (85.1 vs. 83.2 beats/min; p < 0.05) compared to animals drinking freshwater. Hematologic results revealed elevated hemoglobin levels (13.74 g/dL vs. 9.48 g/dL; p < 0.05) and red blood cell counts (11.51 × 106/µL vs. 9.28 × 106/µL; p < 0.05) in animals consuming saline water. Biochemical profiles showed decreased glucose (60.07 mg/dL vs. 71.33 mg/dL; p < 0.05) and cholesterol levels (54.13 mg/dL vs. 64.93 mg/dL; p < 0.05), along with increased urea (75.18 mg/dL vs. 68.96 mg/dL; p < 0.05) and creatinine (3.48 mg/dL vs. 2.68 mg/dL; p < 0.05). Although physiological and blood parameters were higher in the saline water group, most values remained within normal ranges, indicating the animals’ adaptive capacity. However, prolonged exposure to saline water may impact their long-term productivity and health. The study recommends further research on seasonal changes, interspecies resilience, and sustainable water management strategies to enhance livestock resilience and productivity in dryland regions.
Introduction
Today, global warming has become a threat to ecosystems and agriculture through rising heat extremes and shocks, hydrological fluctuations, and water scarcity (Abbass et al., 2022). These impacts are particularly severe in Africa’s arid and semi-arid regions, where pastoral systems support the livelihoods of large populations. Elevated temperatures and altered rainfall patterns reduce livestock production and productivity by impairing growth, reproduction, and lactation (Kochewad et al., 2018). However, small ruminants are well known for their relative tolerance to adverse environmental conditions under changing climate scenarios, making them vital for the pastoral production system (Aboul-Naga et al., 2021). Their adaptability to extreme environmental conditions and stress factors, remarkably in the drylands of East Africa, has been extensively studied (Adeniji et al., 2020; Tulu et al., 2022, 2023; Yirga et al., 2024).
However, despite their adaptive capacity to extreme climates (AL-Ramamneh, 2023; Hussein et al., 2020), their productivity remains constrained by compounding stressors, including heat stress, limited forage availability, and worsening water scarcity and quality (Addisie, 2022; Castro et al., 2019). The availability and quality of drinking water, particularly in terms of salinity, significantly impact the health and productivity of small ruminants, as evidenced by previous research, importance its detrimental effects on the physiological and biochemical parameters of livestock species (Castro et al., 2019; Rahardja et al., 2011; Samira et al., 2016). This issue is particularly persistent in Ethiopia’s Rift Valley, where a larger number of pastoralists depend mainly on indigenous sheep and goats for their livelihoods (Wodajo et al., 2020). In these areas, Lake Basaka is one of the rapidly expanding (Dinka, 2017a). A water body that represents a significant water source for livestock drinking, especially during dry seasons. However, the saline content of this water could present unique challenges to health and productivity (Addisie, 2022; Gebremichael et al., 2022; Regassa, 2016). This issue is critical because water quality in pastoral areas is highly variable, influenced by seasonal, geographical, and anthropogenic factors, and thus directly affects the physiological responses of livestock (Abd El-Hack et al., 2018; Abebe and Kebede, 2017; Abedin et al., 2014; Akinmoladun et al., 2019).
Despite growing interest, research on Lake Basaka has been limited in scope. Most prior studies have either examined the lake’s hydrological dynamics, including its expansion and solute accumulation (Dinka, 2016; 2016, 2017b; 2020; Dinka et al., 2014) or conducted controlled laboratory experiments to assess physiological responses and performance in confined animals consuming high-TDS water (Abera et al., 2024; Tulu et al., 2022, 2024; Yirga et al., 2018; 2024). While several studies have investigated the impacts of heat stress and forage quality in small ruminants, research on the effects of saline water on their physiological resilience is limited (Tulu et al., 2021, 2022; 2023). Consumption of saline water has been shown to influence key physiological parameters such as hydration status, blood chemistry, and electrolyte balance, but the adaptive mechanisms that allow Indigenous sheep and goats to cope with saline water are poorly understood (Cardoso et al., 2021; Mcgregor, 2004) furthermore, although selective breeding for thermotolerance has been investigated as an option to respond to environmental stresses (AL-Ramamneh, 2023; Kombat, 2023).
Despite their importance, significant knowledge gaps remain unaddressed in the study of small ruminants regarding water scarcity impacts under pastoral systems (Armson et al., 2021; Solomon et al., 2010). These include the scarcity of in situ investigations involving free-ranging adult small ruminants managed under typical pastoral conditions (Al-Khaza’leh et al., 2020). Limited data exists on species-specific physiological responses to the combined stressors of heat and salinity, and insufficient consideration is given to ecological factors such as behavioral thermoregulation and microclimatic heterogeneity that influence stress adaptation in natural environments. The present study was designed to evaluate the quality of drinking water sources and to assess the physiological and biochemical responses of indigenous sheep and goats consuming saline water from Lake Basaka in the Ethiopian Rift Valley. By investigating these responses, the study aimed to explain adaptive mechanisms that support improved water resource management and enhance livestock resilience under climate change conditions. Specifically, this research provides innovative contributions to a biologically effective assessment of thermoregulatory parameters and stress-related biomarkers, field-based empirical data on the interactive effects of heat and salinity stress on small ruminant physiology, and the identification of species-specific resilience traits with potential application in climate-adaptive breed selection programs. The findings offer valuable evidence to support improved animal welfare, productivity, and the sustainability of pastoral systems in arid and semi-arid regions.
Materials and methods
Study site
The study was carried out in the Fentalle district (Figure 1) located in the eastern Shewa zone, Oromia National Regional State, Ethiopia. The district is found in the Great Rift Valley, 193 km from Addis Ababa, and borders the Arsi Zone, Boset District, Amhara, and Afar National Regional States. The geographic coordinates of the areas are 8°45′ to 9°10′ N latitude and 39°48′to 40°00′ E longitude. The predominant vegetation consists of shrublands and grasslands, which account for approximately 51.3% and 37.3% of the total area, respectively (OPADC, 2021; Yohannes, 2011). The area is distinguished by significant water features such as the Awash River, Kesem, and Lake Basaka. Pastoralism plays an important role in the local economy, which is mainly based on livestock and small-scale mixed farming. However, the district faces challenges such as scarcity of water, disease and pests, and scarcity of pasture (Abdinoor, 2014; UNDP, 2020). Basaka Lake is a shallow saline lake, which has no natural exits and has grown significantly since the 1960s because of factors such as groundwater discharge, hot spring inflow, and excess irrigation water entering the lake through subsurface flows. It has a catchment area of approximately 500 km2 and receives about 0.28 billion m3 of rain each year. The lake’s surface area has grown from 3 km2 in the 1960s to about 48.5 km2 in 2010 (Dinka, 2012; Dinka et al., 2015; Eleni, 2009). Lake Basaka serves as a source of drinking water for cattle, particularly during the dry seasons.
FIGURE 1
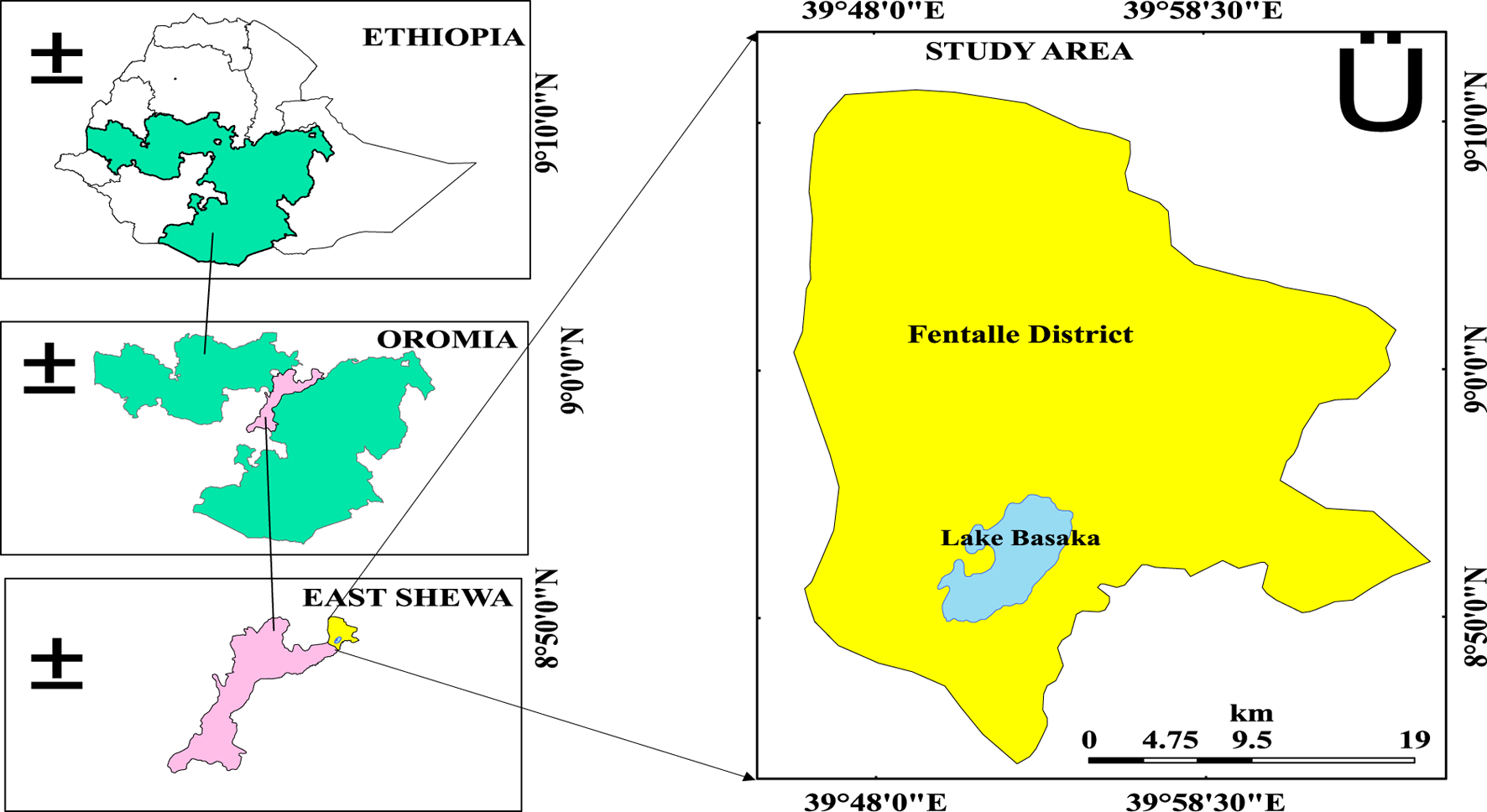
Map of the study area (Fentalle district).
Climate variables
The basic climatic characteristics of the study area (1999–2020) are summarized in Figure 2. Precipitation displayed a weakly bimodal distribution, with a mean annual accumulation of 543 mm, predominantly occurring during July and August. Air temperatures remain elevated throughout the year, with monthly highs reaching 36.1°C (April–May) and rarely descending below 20°C, except during October-December (Gashaw et al., 2023).
FIGURE 2
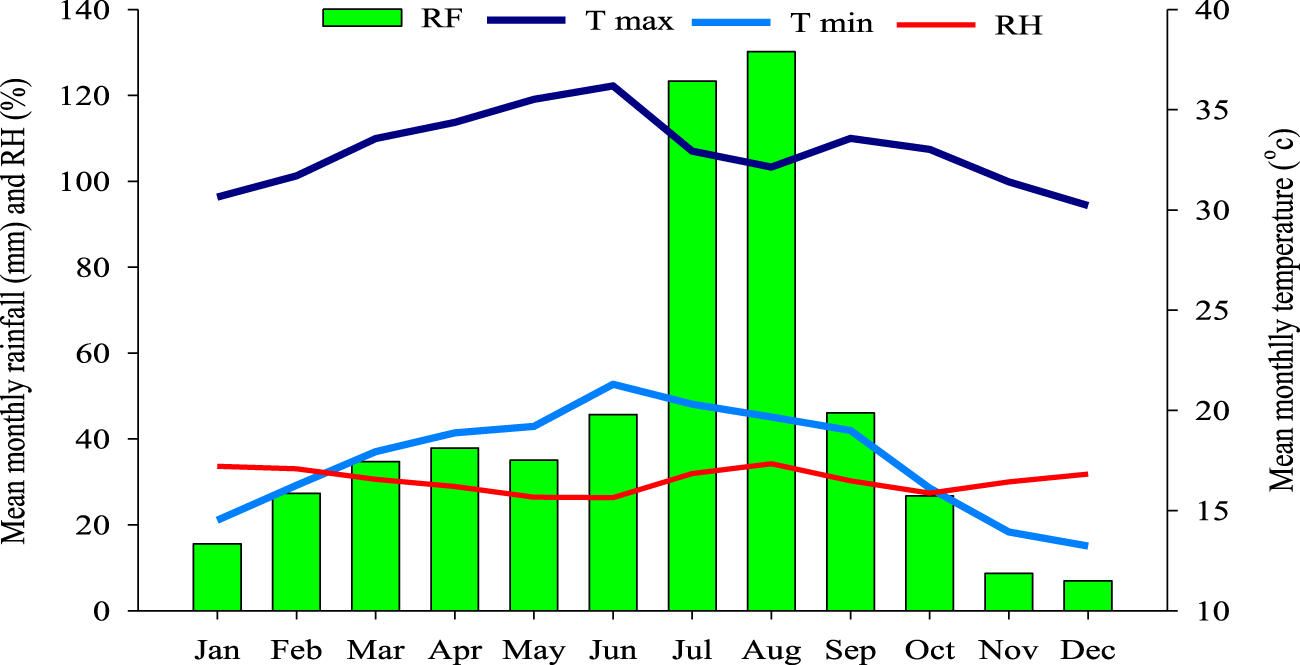
The climate of the Fentalle district during the 1990–2020 period. Source: Meteorological data from 1990 to 2019.
Relative humidity (RH) varies seasonally, climaxing during the rainy season (July–September) and declining in dry periods. During February 2019, a study month characterized by extreme aridity, maximum and minimum temperatures were 32.5°C and 21.0°C, respectively, yielding a daily mean of 26.75°C. Mean RH was 36%. These conditions elevated thermal load and evaporative water loss in livestock, exacerbating physiological stress from combined heat and water scarcity (Aboul-Naga et al., 2021; Egbe-Nwiyi T et al., 2000).
The temperature-humidity index (THI) was calculated using the modified small ruminant-specific equation (Marai et al., 2007):where AT is the air temperature in degrees Celsius (°C), and RH is the relative humidity expressed as a fraction. Substituting February’s values (AT = 26.75°C; RH = 0.36) into the equation yielded a THI of 24.3°C. This value means severe heat stress according to established livestock thresholds, possibly increasing observed physiological and biochemical responses in animals consuming saline water under pastoral management systems.
Study design, study animal, and sampling technique
A comparative cross-sectional study was conducted to investigate the physiological effects of chronic consumption of saline water from Lake Basaka on small ruminants managed under a traditional pastoral production system in Fentalle District, East Shewa Zone, Ethiopia (Figure 3). This district was purposefully selected due to its proximity to Lake Basaka, a prominent source of naturally saline water. A multistage sampling strategy was employed. Initially, 50 households were selected using systematic random sampling, based on criteria including ownership of both species, depending on either Lake Basaka or Awash River as the sole water source, and the presence of clinically healthy adult female animals. A total of 260 adult female indigenous small ruminants (130 sheep and 130 goats) were selected. Of these, 65 sheep and 65 goats consumed saline water from Lake Basaka, while the remaining 65 sheep and 65 goats consumed freshwater from the Awash River. Only non-pregnant, non-lactating females with one parity were included to control for physiological variation and better represent the demographic structure of pastoral herds, while males were excluded due to seasonal migration (Jemberu et al., 2022; Solomon et al., 2010). Animals with known health disorders, recent medical treatment, or old age were excluded. All study animals were managed under traditional extensive grazing systems, traveling 1–4 km daily in search of forage and water, and primarily grazing on natural pasture dominated by Chrysopogon plumulosus, followed by different species of Sporobolus, and in the dry seasons supplemented with very few agro-industrial by-products from the Metehara Sugar Factory. Baseline physiological parameters (body temperature, heart rate, and respiratory rate) and hematological indices were recorded to establish reference values. A one-month pre-sampling monitoring period was conducted to ensure exclusive use of the elected water sources and validate exposure classification. Informed consent was obtained from all pastoralists following FAO ethical guidelines for livestock research in indigenous communities (FAO, 2024).
FIGURE 3
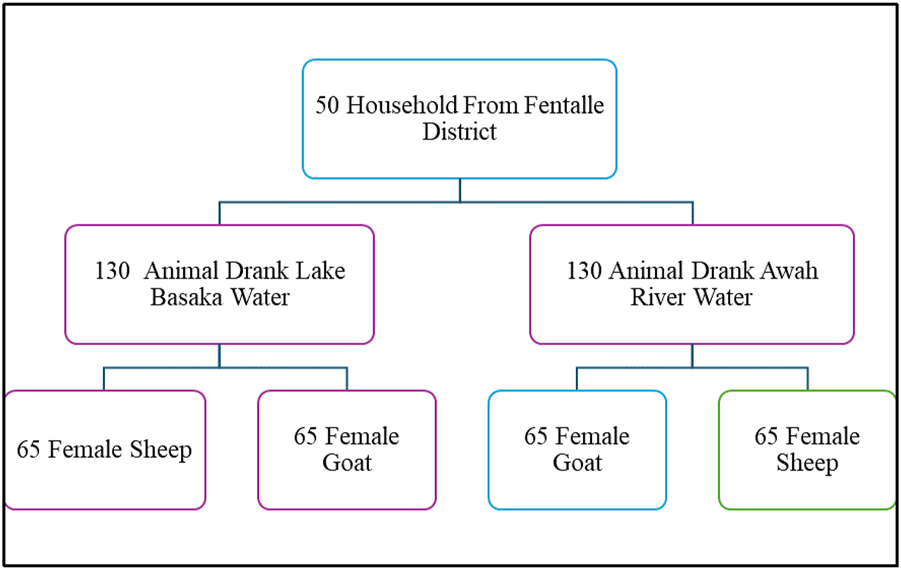
Sampling procedure of the study animal.
Sample transportation and analysis
Water sample and analysis
The water sampling plastic bottles were labelled and transported to the sampling site. The bottles were rinsed twice with the water sampled before being filled with the sample. Water samples were collected at six sites on the lake’s surface, four at the four corners of the lake, and two at the central part of the lake and river during the August season of 2019, and the mean values of the sites were presented. The same procedure was applied for sampling a water sample from the drinking water body of the Awash River. The sites were chosen based on animal water drinking sites and the guidelines for water analysis. The water sampling and analysis were performed according to the (APHA, 2005) standard test guidelines (Dinka et al., 2015) (Table 1). The previous water quality condition of the lake was referred to from previous studies (Ayenew, 2007; Dinka, 2012) for comparison purposes.
TABLE 1
| Quality parameters | Symbol | Method used |
|---|---|---|
| pH | pH | Potentiometric (1:2.5 H2O v/v) |
| Electrical Conductivity | EC | Conductometry (1:2.5 H2O v/v) |
| Calcium | Ca2+ | EDTA titrimetric |
| Magnesium | Mg2+ | EDTA titrimetric |
| Sodium | Na+ | Flame photometric |
| Potassium | K+ | Flame photometric |
| Chloride | Cl− | Titration |
| Carbonate | CO3 | Titration |
| Bicarbonate | HCO3 | Titration (with H2SO4) |
Methods adopted for water quality analysis (Dinka et al., 2015).
Physiological parameters
The animal’s rectal temperature, respiratory rate, and heart rate were collected for seven consecutive days during the experimental period, in the morning (8:00 am) before the animals were released for grazing, and in the afternoon (5:00 pm), a couple of minutes after the animals were returned to their corral. Rectal temperatures (RT) were monitored using a digital clinical thermometer with a temperature range of 32.0°C–43.9°C. The thermometer was placed into each animal’s rectum, with the bulb in contact with the mucosa, and held in the rectum until the thermometer made a beep, indicating temperature stabilisation. The respiratory rate (RR) and the heart rate (HR) were measured using a flexible stethoscope at the level of the laryngotracheal region by counting the number of movements and beats for 20 s and multiplying the results by 3 to report on a minute time scale (Mengistu et al., 2007).
Blood collection and analysis
After disinfecting the neck at the jugular vein area with iodine alcohol, two blood samples (one for hematological analysis and the other for serum chemistry) were obtained from each animal from 8:00 am to 10:00 am on two consecutive days to reduce blood cell damage and hemolysis (Fanta et al., 2024; Polizopoulou, 2010; Zaher et al., 2022). Immediately after blood collection, the animals were examined for the presence of ectoparasites, lymphadenitis, or other types of skin disorders. Blood was taken in 5 mL vacuum tubes containing 10% anticoagulant ethylene diamine tetraacetic acid (EDTA) for hematological examination. The volumetric impedance state analysis method was used to determine hematologic parameters such as hemoglobin, PCV, WBC, RBC, MCV, MCH, and MCHC (Jain, 1993). Further blood samples were taken in 7 mL vacuum tubes containing gel and clot to investigate biochemical and hormonal parameters. For 15 min, the blood was spun in a digital centrifuge at 10°C at 2,500 g. The supernatant was divided into 1.5 mL aliquots for biochemical and hormonal analyses. The analysis of the blood was performed the day after collection. The biochemical parameters glucose, total protein, albumin, triglycerides, cholesterol, urea, creatinine, aspartate aminotransferase (AST), and alanine aminotransferase (ALT) were determined using a biochemical analysis apparatus (VegaSys) with a multiple-wavelength photometer.
Data analysis
All statistical analyses were conducted using SAS software (version 9.4; SAS Institute Inc., Cary, NC, USA). Data are presented as least-squares means with their corresponding standard errors of the mean (SEM). The probability value of *p* <0.05 was considered statistically significant. Post hoc comparisons were adjusted using the Tukey-Kramer method to control for multiple testing. The experimental design included two fixed factors: water type (Basaka water consumers vs. non-consumers) and species (sheep vs. goats). Due to the longitudinal nature of physiological measurements, separate statistical models were applied for repeated and single-time-point outcomes.
Analysis of repeated physiological parameters
The repeated measures were analyzed using a linear mixed-effects model (LMM) with fixed effects for water type, species, time of day, and their two-way interactions.
The general linear mixed model for repeated measures was structured as follows:
Where: Yijklm: Observed physiological parameter for the mth animal under the ith water type, jth species, kth time of day, and lth day; μ: Overall mean; Wi: Fixed effect of water type (i = Basaka, control); Sj: Fixed effect of species (j = sheep, goats); Tk: Fixed effect of time of day (k = morning, afternoon); (W × S)ij + (W × T)ik + (S × T)jk +: Two-way interaction terms; εijklm: Residual error term.
Analysis of blood biochemical parameters
Blood parameters, including hematology and selected serum biochemistry and electrolytes, were measured once during the experimental period and analyzed using a two-way factorial analysis of variance (ANOVA).
The two-way ANOVA model was specified as:
Where: Yijk: Observed value of the biochemical parameter for the k-th animal
μ: Overall mean; Wi: Fixed effect of water type; Sj: Fixed effect of species; (W × S)ij: Interaction between water type and species; εijk: Residual error term.
All statistical procedures were implemented in SAS (version 9.4). Repeated-measures analyses were conducted using the PROC MIXED procedure, specifying the REPEATED statement for covariance structure selection. Blood parameter data were analyzed using the PROC GLM procedure, with LSMEANS and ADJUST = TUKEY options applied for post hoc pairwise comparisons.
Results and discussion
Physical parameters of drinking water sources
The physicochemical parameters of animal drinking water sources revealed significant differences between the Basaka lake water and the freshwater, highlighting potential implications for animal health and productivity (Table 2). The pH of Lake Basaka water was significantly higher (p < 0.05) than that of freshwater across all sampling periods, with mean values of 9.52 ± 0.05, compared to 7.65 ± 0.05 for freshwater. In agreement with earlier studies that reported average pH values of 8.55 (Melese and Debella, 2023), 9.30 (Tenagashaw and Tamirat, 2022), and 9.16 (Yirga et al., 2019). These values exceed the recommended range of 6.5–8.5 for livestock drinking water. Alkaline, poor-quality water with a pH greater than 9 has negative impacts on digestion, water and feed intake, and altered feed conversion efficiency (Cervantes et al., 2024; Higgins et al., 2000). Prolonged exposure can also result in metabolic alkalosis, B vitamin deficiencies, and symptoms similar to moderate acidosis (Cardoso et al., 2021; Mcgregor, 2004). The high pH observed in Basaka lake water, particularly during the dry season, highlights the need to monitor and mitigate pH imbalances to protect the wellbeing of livestock, especially young and growing animals.
TABLE 2
| Parameters | Fresh water (FW) | Lake Basaka (BL) water | Upper limits for small ruminants* |
|---|---|---|---|
| pH | 7.65 ± 0.05 | 9.52 ± 0.05 | 8.5 |
| EC | 641.53 ± 19.2 | 3,992.53 ± 37.28 | 6,000 |
| TDS | 430.33 ± 16.33 | 2,728.00 ± 25.06 | 3,000 |
| Calcium (Ca2+) | 1.20 ± 0.01 | 4.97 ± 0.05 | 1,000 |
| Magnesium (Mg2+) | 1.72 ± 0.01 | 1.59 ± 0.04 | 200 |
| Sodium (Na+) | 35.28 ± 0.35 | 1,180.69 ± 14.73 | 400 |
| Potassium (K+) | 15.38 ± 0.25 | 61.38 ± 0.04 | 20 |
| Chloride (Cl−) | 122.11 ± 1.49 | 506.44 ± 1.06 | 1,500 |
| Carbonate (CO32-) | 31.17 ± 0.69 | 258.80 ± 5.89 | — |
| Bicarbonate (HCO3−) | 62.33 ± 1.38 | 1,124.60 ± 0.46 | 1,000 |
Chemical composition of Lake Basaka and freshwater samples (mean ± SEM).
All are expressed in units of mg L−1, except electric conductivity (µScm-1); * (AGRI-FACTS, 2007; Andrew A. olkowski, 2009; Crooks, 2020; DPIRD, 2024).
The electrical conductivity (EC) of Lake Basaka water was significantly higher (p < 0.05) than that of freshwater throughout the measurement period, with mean values of 3,992.53 ± 37.28 μS/cm, compared to 641.53 ± 19.20 μS/cm for freshwater. Elevated EC reflects increased levels of dissolved ionic solutes, which serve as a substitute for salinity. Previous studies have reported EC values for Basaka lake water that range from 1,407 to 3,321 μS/cm (Umer et al., 2020) and up to 2.72 mS/cm (Melese and Debella, 2023), and 2,978 μs/cm (Tenagashaw and Tamirat, 2022). Water with an EC below 5,800 μS/cm is considered safe for most livestock species (ANZG, 2023; DPIRD, 2024; Emon, 2018; NSW, 2014). However, prolonged exposure to higher levels of EC, particularly during the dry season, can adversely affect livestock productivity. The elevated EC values observed in Lake Basaka are likely attributable to runoff, pollutants, and natural evaporation processes that concentrate dissolved salts in the lake. These findings underline the importance of addressing salinity concerns to mitigate the impact on livestock health and productivity.
Total dissolved solids (TDS) concentrations further support the increased salinity of Lake Basaka compared to freshwater sources. In this study, mean TDS levels were 2,728.00 ± 25.06 mg/L, significantly higher than freshwater at 430.33 ± 16.33 mg/L. Although these concentrations remain below the maximum tolerable limits established for most livestock species, typically between 3,000 mg/L (Emon, 2018; Meehan et al., 2021) and 4,000–5,000 mg/L (DPIRD, 2024; NSW, 2014), which indicates moderate salinity stress with potential impacts under unfavorable exposure. These results align with previous reports of TDS in Lake Basaka, ranging from 2,304.5 mg/L in the dry season to 840.3 mg/L in the wet season (Umer et al., 2019), demonstrating seasonal variability. Elevated TDS reduces water palatability, which can decrease water intake and impair livestock productivity. This issue is exacerbated in semi-arid regions where limited freshwater availability increases reliance on saline sources. The persistent salinity is primarily driven by the area’s hydrogeological and climatic factors, including high evaporation, low freshwater input, and solute accumulation, posing long-term risks to livestock health and agricultural sustainability.
The chemical composition of Lake Basaka water also reflects elevated levels of some key minerals, including sodium, potassium, chloride, carbonate, and bicarbonate, compared to freshwater. Sodium concentrations reached 1,180.69 mg/L, far exceeding the recommended limit of 300 mg/L for livestock drinking water (Davis, 2016; Higgins et al., 2000). Similarly, potassium and chloride levels were significantly higher than tolerable thresholds. Chloride levels exceeding 300 mg/L impart a salty taste and have the potential to reduce water intake (Davis, 2016; Higgins et al., 2000). Excess chloride intake has also been linked to changes in rumen function and reduced milk production. The combined impact of these high concentrations of minerals poses considerable challenges for livestock in the study area. Long-term consumption of mineral-rich saline water is particularly challenging for animals in dry and semi-dry areas, where the quality and quantity of feed are already a stress for livestock (Cardoso et al., 2021; Hussein et al., 2022; Mcgregor, 2004). Short-term exposure to water above the recommended limits may not immediately affect growth or reproduction; however, long-term consumption can lead to chronic health problems and reduced productivity (Cervantes et al., 2024). These findings emphasise the need for regular water quality monitoring and the implementation of mitigation strategies to ensure sustainable livestock management (Çapar et al., 2020) in areas dependent on natural saline water sources.
Physiological parameters
The physiological responses of sheep and goats to different water sources and diurnal variations are presented in Table 3. The finding revealed that, animals drinking saline Lake Basaka water showed significantly higher rectal temperature (RT: 39.3°C ± 0.04°C vs. 39.1°C ± 0.04°C) and pulse rate (PR: 85.1 ± 0.39 vs. 83.2 ± 0.39 beats/min) compared to those on freshwater (p < 0.05), while respiration rate (RR) remained unaffected. Diurnal changes were marked, with afternoon values significantly higher than morning for RT, RR, and PR (p < 0.05). The higher RT may result from increased heat production during mineral excretion via urine (Thiet et al., 2022), greater energy use for mineral absorption and excretion (Mdletshe et al., 2017; Tsukahara et al., 2016), or the impacts of increasing Temperature-Humidity Index (THI) during daytime in this study. A significant species × water interaction (p < 0.05) showed that sheep had the highest physiological responses on saline water (RT: 39.4°C ± 0.04°C; PR: 85.4 ± 0.39 beats/min), while goats revealed lower values (RT: 38.9°C ± 0.04°C; PR: 82.9 ± 0.39 beats/min). Although the values in this study were significantly different, they remained within the normal range for the species (Li et al., 2021; Reece and Prater, 2015). Sheep’s higher water intake may be due to their evaporative cooling mechanisms, as they pant more frequently than goats. Another reason sheep require more water for thermoregulation is their longer fleece, which increases endogenous heat production (AL-Ramamneh, 2023). The absence of an influence on RR in the current study, along with values within the normal range, indicates that these breeds of sheep and goats, which are ideal for tropical conditions, were tolerant to drinking saline water, and their thermoregulation was not significantly compromised (Adeniji et al., 2020). Previous studies by Yirga et al. (2024) found no significant effects of water salinity on respiratory rate (RR), rectal temperature (RT), and pulse rate (PR) in Blackhead Ogaden sheep and Somali goats. This underscores the variability in physiological responses to saline water among different animal breeds. These findings are consistent with previous studies (Abera et al., 2024; Nguyen et al., 2024; Rahardja et al., 2011; Yirga et al., 2024), all of which contributed to a growing body of evidence regarding the physiological adaptations of these animals in response to varying saline water and availability.
TABLE 3
| Variables | Water types | Time | Species | SE | P-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LB | FW | AM | PM | Goat | Sheep | WT | Time | Spp | WT*Spp | ||
| RT (oC) | 39.3a | 39.1b | 38.5b | 39.8a | 38.9b | 39.4a | 0.04 | ** | ** | ** | ** |
| RR (mov/min) | 34.6 | 35.0 | 29.3b | 40.3a | 30.4b | 39.2a | 0.43 | ns | ** | ** | ** |
| PR (beat/min) | 85.1a | 83.2b | 81.5b | 86.8a | 82.9b | 85.4a | 0.39 | ** | ** | ** | ** |
Physiological parameters of goats and sheep that drank Lake Basaka or freshwater.
ab means within the row bearing different superscript letters differs significantly (** = p < 0.05); RR, Respiration Rate; RT, Rectal temperature, PR, Pulse Rate; LB, Lake Basaka; FW, Fresh water; AM, Morning; PM, Afternoon; WT, Water types; Spp, Species; ns, nonsignificant; SEM, Standard error of the mean.
The different physiological responses can be attributed to a variety of factors, including species differences, age, adaptability to water stress, and the nutritional status of grazing animals (Mane et al., 2022; Mdletshe et al., 2017; Nguyen et al., 2024). Increased levels of rectal temperature (RT), respiratory rate (RR), and pulse rate (PR) in grazing sheep and goats can be associated with environmental factors such as high ambient temperatures and physical stress from grazing and movement. Increased physiological parameters do not necessarily indicate heat stress, as effective thermoregulation can help maintain homeostasis. For example, Gupta et al. (2013) found that goats exposed to high temperatures for 6 hours showed an increase in rectal temperature from 38.97°C to 39.35°C and in respiratory rate from 43.66 to 77.33 breaths per minute. Similarly, Sejian et al. (2017) reported significant increases in these parameters in sheep during a 14 km daily grazing routine under walking stress. Since water quality affected the physiological parameters in the present study, implementing measures to decrease water-related stress, such as improving the drinking water quality and monitoring grazing patterns, could increase livestock productivity and welfare.
Hematology profile
The hematology profiles of sheep and goats that consumed different water sources were evaluated, and the results are presented in Table 4. Sheep and goats consuming saline Lake Basaka (LB) water exhibited significantly higher (P < 0.05) hemoglobin (Hb: 13.74 vs. 9.48 g/dL) and red blood cell counts (RBC: 11.51 vs. 9.28 ×106/mL) compared to freshwater (FW). A significant species effect was observed for RBC count (P < 0.05), with sheep (11.13 × 106/μL) recorded as higher than goats (9.65 × 106/μL), suggesting that sheep may have a healthier erythropoietic response to osmotic stress. The elevated values of Hb and RBC in animals consuming Lake Basaka water could be explained by the higher levels of salt in the drinking water, which is likely to induce hemoconcentration. This phenomenon may occur due to dehydration or fluid loss associated with the osmotic effects of high sodium chloride (NaCl) content in the water, particularly under arid conditions (Egbe-Nwiyi T et al., 2000; Hussein et al., 2022). Moreover, the erythrocyte indices, mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC), also showed significant differences between water types, with higher values observed in sheep and goat consuming Lake Basaka water (MCH: 12.26 vs. 9.08 pg; MCHC: 37.39 vs. 26.72 g/dL) compared to those in freshwater (FW). Increased MCH and MCHC values are revealing of a compensatory hematological response to the osmotic imbalance and potential chronic dehydration caused by high salt intake, which leads to hemoconcentration and an increase in red blood cell density and hemoglobin content, suggesting an adaptive response to hyperosmolar and hypovolemic conditions (Antunović et al., 2022; Barsila et al., 2020). However, other hematological parameters, including packed cell volume (PCV), white blood cell count (WBC), and mean corpuscular volume (MCV), showed insignificant variations between water sources or species. A related study was reported by Abera et al. (2024), and Yirga et al. (2024) observed that Blackhead Ogaden sheep and Somali goats exposed to different total dissolved solids (TDS) levels have varied hematological parameters under controlled experimental conditions. These compare and contrasting results in with a previous study, indicating that species, breed, exposure time, and water composition significantly influence hematological parameters of animals exposed to saline water. Despite these variations, all measured haematological parameters remained within their respective normal ranges between species and water sources (Fanta et al., 2024; Mane et al., 2022; Tschuor et al., 2008). This indicates that sheep and goats can adapt to saline water without immediate negative effects. However, the long-term health impacts of saline water intake require further study to understand the mechanisms involved and guide effective livestock management. This research is essential for maintaining the health and productivity of small ruminants in areas reliant on saline water for drinking.
TABLE 4
| Variables | Water types | Species | SE | SL | Normal range | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| LB | FW | Goat | Sheep | WT | Spp | WT*Spp | Goat | Sheep | ||
| Hb (g/dL) | 13.74a | 9.48b | 11.53 | 11.69 | 0.32 | ** | ns | ns | 8–12 | 9–15 |
| PCV (%) | 37.71 | 35.52 | 36.30 | 36.93 | 0.58 | ns | ns | ns | 22–38 | 27–45 |
| RBC (x106/ml) | 11.51a | 9.28b | 9.65b | 11.13a | 0.21 | ** | ** | ns | 8–18 | 9–15 |
| WBC (x103/ml) | 11.96 | 11.92 | 12.09 | 11.80 | 0.38 | ns | ns | ns | 4–13 | 4–8 |
| MCV (fL) | 32.82 | 34.01 | 33.40 | 33.43 | 0.42 | ns | ns | ** | 6–25 | 28–40 |
| MCH (pg) | 12.26a | 9.08b | 10.90 | 10.44 | 0.26 | ** | ns | ** | 5–8 | 8–12 |
| MCHC (g/dL) | 37.39a | 26.72b | 32.44 | 31.67 | 0.77 | ** | ns | ns | 30–36 | 31–34 |
Haematology of sheep and goats consuming both Lake Basaka and freshwater.
** Show a significant difference (P < 0.05) between Lake Basaka water drinkers and freshwater drinkers within species in the same row. Hb, hemoglobin; RBC, red blood cell; WBC, white blood cell; PVC, packed cell volume; MCV, mean cell volume; MCH, mean corpuscular hemoglobin and MCHC, mean corpuscular hemoglobin concentration; SEM, standard error of means; LB,Lake Basaka; FW, fresh water; SL, Significant level; ns, nonsignificant.
Biochemical profile
Glucose and cholesterol levels
Blood biochemical profile results revealed a significant reduction in blood glucose concentrations in both goats and sheep consuming Lake Basaka water (Table 5). The decline can be related to decreased feed intake and nutrient absorption, and metabolic adaptations to salty conditions (Abbas et al., 2020). This is also reported in studies on feed consumption under high saline water consumption (Yirga et al., 2024). Saline stress can affect insulin secretion and glucose metabolism. The present findings are consistent with previous research indicating that saline water intake reduces blood glucose concentrations in ruminants (Albuquerque et al., 2020; Costa et al., 2021; Hussein et al., 2022). The reduced blood cholesterol levels observed in saline drinkers could indicate an alteration in lipid metabolism, likely due to changes in feeding behaviour or nutrient availability under saline stress (Patra et al., 2024).
TABLE 5
| Parameters | Water types | Species | SE | SL | ||||
|---|---|---|---|---|---|---|---|---|
| BL | FW | Goat | Sheep | WT | Spp | WT*Spp | ||
| Glucose (mg/dL) | 60.07b | 71.33a | 68.07 | 63.33 | 2.09 | ** | ns | Ns |
| TP (g/dL) | 6.62 | 7.11 | 7.01 | 6.71 | 0.20 | Ns | ns | Ns |
| Albumin (mg/dL) | 3.95 | 3.24 | 4.17 | 3.02 | 0.60 | Ns | ns | Ns |
| Urea (mg/dL) | 75.18a | 68.96b | 74.42a | 69.72b | 1.81 | * | ns | ** |
| Cholesterol (mg/dL) | 54.13b | 64.93a | 61.20 | 57.87 | 2.56 | * | ns | ns |
| Creatinine (mg/dL) | 3.48a | 2.68b | 3.13 | 3.03 | 0.25 | * | ns | ns |
| Na+ (mmol/L) | 136.07a | 126.44b | 138.10a | 124.41b | 1.92 | ** | ** | ** |
| Cl− (mmol/L) | 101.33 | 107.20 | 106.50 | 102.03 | 3.94 | Ns | ns | ns |
| K+ (mmol/L) | 7.67 | 5.99 | 7.28 | 6.38 | 0.78 | Ns | ns | ns |
| Mg2+ (mmol/L) | 2.73 | 2.79 | 2.70 | 2.82 | 0.13 | Ns | ns | ns |
| AST (U/L) | 92.17 | 80.93 | 97.57a | 75.54b | 7.55 | Ns | * | ns |
| ALT (U/L) | 34.44b | 53.59a | 47.33 | 40.69 | 2.76 | ** | ns | ** |
Blood biochemicals of sheep and goats that drank Lake Basaka or fresh water.
*Show a significant difference (*P < 0.05) between Lake Basaka water drinkers and nondrinkers within species. TP, Total Protein; Na, sodium; Cl, Chlorine; K, Potassium; Mg, Magnesium; AST, aspartate aminotransferase; ALT, alanine aminotransferase; SL, Significant level; ns, nonsignificant.
Urea and creatinine concentrations
Saline water drinkers exhibited significantly higher concentrations of urea and creatinine (Table 5), suggesting altered renal function, probably due to an increased renal load due to higher water intake and the possible osmotic effect of salt. The increased concentrations of urea in saline drinkers could be attributed to increased protein catabolism or increased protein intake, compounded by the dehydration stress imposed by high salt consumption. Interestingly, urea metabolism differed by species, with goats exhibiting higher urea levels than sheep, a finding that may reflect species-specific differences in salt tolerance and renal filtration capacity (Ghanem et al., 2018). Such variations have previously been documented in studies examining the effects of saline water on ruminant physiology (Zoidis and Hadjigeorgiou, 2017; Nguyen et al., 2024). Elevated creatinine levels could indicate kidney stress or subclinical renal dysfunction, a phenomenon frequently associated with prolonged exposure to saline environments (Patra et al., 2024).
Serum minerals and enzyme activity
Except for sodium (Na+), no statistically significant differences (p > 0.05) were recorded in the serum concentrations of chloride (Cl−), potassium (K+), or magnesium (Mg2+) across different water types, animal species, or their interactions (Table 5). Serum sodium (Na+) levels were significantly higher in animals that consumed Lake Basaka water (136.07 vs. 126.44 mmol/L, p < 0.05). This increase in blood sodium levels is expected, as the water from Lake Basaka contains higher concentrations of sodium chloride (NaCl), which is absorbed and retained by the body. Prolonged exposure to high sodium levels can disrupt homeostasis and put pressure on the kidney system as it attempts to maintain electrolyte balance. Significant differences observed in sodium levels between species, with goats consistently showing higher values than sheep (138.10 vs. 124.41 mmol/L, p < 0.05), could indicate species-specific adaptations to saline environments, and goats possibly possess a higher tolerance to sodium than sheep. This finding aligns with the observations of Zoidis and Hadjigeorgiou (2017) and Nguyen et al. (2024), Who also noted higher serum sodium concentrations in goats exposed to saline conditions.
Liver function, assessed through serum transaminase activity, showed significant variation based on water sources in animals (Table 5). Higher alanine aminotransferase (ALT) levels were observed in sheep and goats drinking saline water (53.59 U/L) compared to controls with freshwater (34.44 U/L; p < 0.05), indicating hepatocellular stress. This response is primarily due to hypernatremia-induced oxidative damage that damages membrane integrity and allows cytoplasmic enzyme leakage (Albuquerque et al., 2020; Paiva et al., 2017; Reneu et al., 2020). It is also driven by increased metabolic demand for maintaining homeostasis, requiring enhanced transaminase-mediated amino acid catabolism (Aboul-Naga et al., 2021; Chedid et al., 2014; Ranganatha, 2022). The significant species × water interaction (p < 0.05) demonstrates different adaptive capacities, with goats showing higher ALT activity (47.33 U/L vs. sheep: 40.69 U/L), likely due to higher metabolic rates and greater hepatocellular enzyme expression (AL-Ramamneh, 2023; Kaliber et al., 2016; Silanikove, 2000). In contrast, aspartate aminotransferase (AST) remained unaffected by salinity (92.17 U/L vs. 80.93 U/L; p > 0.05), though it was higher in goats (97.57 U/L vs. sheep: 75.54 U/L; p < 0.05). This difference stems from AST’s main role in mitochondrial energy metabolism (malate-aspartate shuttle) rather than in signaling cytoplasmic damage (Jerry Kaneko et al., 2008). The specific change in goat AST might reflect homeostatic adjustments of gluconeogenic pathways under osmotic stress without histological signs of necrosis, aligning with adaptive responses noted during thermal and saline challenges (Nguyen et al., 2024; Thiet et al., 2022). In this study, there was no significant difference between water types regarding ALT, which could suggest that the liver’s ability to manage saline-induced stress does not significantly impair salt regulation capability.
Other biochemical parameters
There were no significant differences in mean for total protein, albumin, potassium, magnesium, and chloride levels between water types, indicating that these parameters are less affected by the salinity of the water. The current study was in agreement with studies conducted on sheep and goat exposed to water stress conditions in tropical regions (Zoidis and Hadjigeorgiou, 2017; Cardoso et al., 2021; Nguyen et al., 2024; Patra et al., 2024). This stability in protein and electrolyte concentrations implies that while renal and metabolic performance varies, as demonstrated by urea and creatinine levels, the overall balance of protein and minerals in the animal’s body remains normal. These findings show that the nutritional condition and electrolyte balance of the animals are preserved even when they consume saline water and suggest that local sheep and goat breeds may be able to better withstand drinking saline water.
Conclusion and future directions
This study examined the physiological and biochemical profile of indigenous sheep and goats in Ethiopia’s Mid-Rift Valley subjected to naturally saline water intake from Lake Basaka under the pastoral production system. Despite exposure to markedly elevated salinity parameters electrical conductivity (3,992.53 μS/cm), sodium concentration (1,180.69 mg/L), alkaline pH (9.52), and total dissolved solids (2,728 mg/L), which exceed established livestock safety thresholds, these small ruminants maintained basic physiological homeostasis within species-specific standardizing limits. Noteworthy responses to salinity stress included uncertain increases in rectal temperature (+0.2°C) and pulse rate (+1.9 beats/min), alongside hematological adaptations such as increased hemoglobin concentration (+4.26 g/dL) and erythrocyte counts (+2.23 × 106/µL), revealing hemoconcentration. Biochemically, saline water exposure corresponded with significant reductions in circulating glucose (−11.26 mg/dL) and cholesterol (−10.8 mg/dL), along with higher urea (+6.22 mg/dL) and creatinine (+0.8 mg/dL), indicating metabolic alarms and increased renal toxic load. A relative study revealed that goats have higher thermoregulatory capacity and homeostasis relative to sheep, indicating interspecies variation in adaptive resilience.
These findings emphasize the tolerance of indigenous small ruminants to osmotic and electrolyte challenges, yet they also expose potential subclinical risks that could compromise long-term health and production under critical saline exposure. Therefore, sustainable livestock management in a saline-prone environment requires an integrated, multidisciplinary approach. Strategies should encompass enhanced water resource management, incorporating rainwater harvesting, aquifer recharge, and cost-effective desalination techniques such as solar distillation, alongside systematic water quality observation. Selective keeping of salt-tolerant species like goats, supported by rotational grazing regimes to optimize freshwater access and nutritional interventions involving supplementation with energy-dense feeds and critical micronutrients (phosphorus, vitamin D), are essential to mitigate metabolic and renal dysfunction. Routine health assessments, including biochemical monitoring, are essential to detect early renal impairment and prevent permanent organ harm, thereby maintaining herd productivity and welfare.
Future research
To improve the understanding and management of saline water impacts on livestock, continuous studies will be required to determine effects on productive and reproductive performance, growth, and carcass quality across different livestock species, breeds, sexes, age groups, and physiological states, under different seasonal and environmental conditions. Genomic and molecular investigations will identify genes associated with salinity tolerance could inform targeted breeding programs to enhance adaptive resilience. Furthermore, comprehensive water quality analyses from all available drinking sources across seasons should be prioritized to establish baseline data and guide sustainable water resource management strategies.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal studies were approved by Haramaya University’s Institutional Animal Care and Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was not obtained because the study involved non-invasive procedures (physiological measurements and blood sampling) commonly practiced in routine animal health management. Additionally, the study was conducted under the oversight of the Institutional Animal Care and Ethics Committee of Haramaya University, and verbal consent was obtained from all animal owners after explaining the study objectives and procedures in the local language, consistent with accepted ethical practices in pastoral communities where formal written agreements are uncommon.
Author contributions
DT: conceptualize, research design, data collection, formal analysis, investigation, data curation, writing original draft, review, and editing, ML: conceptualize, research design, investigation, writing review, editing, supervision, and resources mobilization, YM: conceptualization, research design, writing review, editing, and supervision, FH: methodology, writing-review and editing, DK: methodology, writing review, and editing. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was conducted as part of a master’s thesis project and received financial support from the Africa Center of Excellence for Climate Smart Agriculture and Biodiversity Conservation (ACE for Climate SABC), Haramaya University (Grant number: 0000001212).
Acknowledgments
The authors would like to acknowledge the financial support provided by ACE for the Climate SABC project at Haramaya University, which enabled the successful completion of this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
References
1
AbbasZ.SammadA.HuL.FangH.XuQ.WangY. (2020). Glucose metabolism and dynamics of facilitative glucose transporters (GLUTs) under the influence of heat stress in dairy cattle. Metabolites10 (8), 312. 10.3390/metabo10080312
2
AbbassK.QasimM. Z.SongH.MurshedM.MahmoodH.YounisI. (2022). A review of the global climate change impacts, adaptation, and sustainable mitigation measures. Environ. Sci. Pollut. Res.29 (28), 42539–42559. 10.1007/s11356-022-19718-6
3
Abd El-HackM. E.SamakD. H.NoreldinA. E.ArifM.YaqoobH. S.SwelumA. A. (2018). Towards saving freshwater: Halophytes as unconventional feedstuffs in livestock feed: A review. Environ. Sci. Pollut. Res.25 (15), 14397–14406. 10.1007/s11356-018-2052-9
4
AbdinoorM. (2014). Pastoralist areas resilience improvement through market expansion (PRIME) project funded. Prime: the United States Agency for International Development. 10.1128/AEM.70.2.837-844.2004
5
AbebeE.KebedeA. (2017). Assessment of climate change impacts on the water resources of megech river catchment, abbay basin, Ethiopia. Open J. Mod. Hydrology07 (02), 141–152. 10.4236/ojmh.2017.72008
6
AbedinM. A.HabibaU.ShawR. (2014). Community perception and adaptation to safe drinking water scarcity: salinity, arsenic, and drought risks in coastal Bangladesh. Int. J. Disaster Risk Sci.5 (2), 110–124. 10.1007/s13753-014-0021-6
7
AberaF.UrgeM.YirgaH.YousufY. (2024). Effect of drinking saline water on physiological, haematological and biochemical parameters of Blackhead ogaden sheep and Somali goats. J. Animal Physiology Animal Nutr.109, 633–645. 10.1111/jpn.14080
8
Aboul-NagaA. M.ElshafieM. H.KhalifaH.OsmanM.Abdel KhalekT. M.El-BeltagiA. R.et al (2021). Tolerance capability of desert sheep and goats to exercise heat stress under hot dry conditions, and its correlation with their production performance. Small Ruminant Res.205. 10.1016/j.smallrumres.2021.106550
9
AddisieM. B. (2022). Evaluating drinking water quality using water quality parameters and esthetic attributes. Air, Soil Water Res.15. 10.1177/11786221221075005
10
AdenijiY. A.SanniM. O.AbdounK. A.SamaraE. M.Al-BadwiM. A.BahadiM. A.et al (2020). Resilience of lambs to limited water availability without compromising their production performance. Animals10 (9), 1491–13. 10.3390/ani10091491
11
AGRI-FACTS (2007). Water analysis interpretation for livestock. Water. Available online at: https://www.beefresearch.ca/content/uploads/2022/05/Water-Analysis-Interpretation-for-Livestock.pdf.
12
AkinmoladunO. F.MuchenjeV.FonF. N.MpenduloC. T. (2019). Small ruminants: farmers’ hope in a world threatened by water scarcity. Animals9 (7), 456. 10.3390/ani9070456
13
AlbuquerqueI.AraújoG.SantosF.CarvalhoG.SantosE.NobreI.et al (2020). Performance, body water balance, ingestive behavior and blood metabolites in goats fed with cactus pear (Opuntia ficus-indica L. Miller) silage subjected to an intermittentwater supply. Sustain. Switz.12 (7), 1–15. 10.3390/su12072881
14
Al-Khaza’lehJ.AbdelqaderA.AbuajamiehM.HayajnehF. M. F. (2020). Assessment of water source availability and quality for small ruminant consumption in the Northern Badia region of Jordan. Veterinary World13 (6), 1073–1082. 10.14202/vetworld.2020.1073-1082
15
AL-RamamnehD. (2023). Thermoregulation in sheep and goats: a review. Asian J. Biol.17 (1), 34–42. 10.9734/ajob/2023/v17i1315
16
Andrew A. Olkowski (2009). Livestock water quality A field guide for cattle, horses, poultry and swine.
17
AntunovićZ.ErcegO.ŠalavardićŽ. K.MiočB.ĐidaraM.NovoselecJ. (2022). Hematological and biochemical parameters in the indigenous Croatian white goat in relation to age. Veterinarski Arh.92 (6), 713–722. 10.24099/vet.arhiv.1962
18
ANZG (2023). Livestock drinking water guidelines. Australian and New Zealand guidelines for fresh and marine water quality, Australian and New Zealand governments and Australian state and territory governments. Canberra.
19
APHA (2005). Standard methods for the examination of water and wastewater. 21st ed.Washington DC: American Public Health Association/American Water Works Association/Water Environment Federation.
20
ArmsonB.EkiriA. B.AlafiatayoR.CookA. J. (2020). Small ruminant production in Tanzania, Uganda, and Ethiopia: a systematic review of constraints and potential solutions. Veterinary Sci.8 (1), 5–13. 10.3390/vetsci8010005
21
AyenewT. (2007). Water management problems in the Ethiopian rift: challenges for development. J. Afr. Earth Sci.48, 222–236. 10.1016/j.jafrearsci.2006.05.010
22
BarsilaS. R.BhattK.DevkotaB.DevkotaN. R. (2020). Haematological changes in transhumant Baruwal sheep (Ovis aries) grazing in the western Himalayan mountains in Nepal. Pastoralism10 (1), 4. 10.1186/s13570-019-0156-6
23
ÇaparG.DilcanÇ.ArslanŞ. (2020). Assessment of livestock drinking water quality: a case study in a special environmental protection area. Erciyes Univ. J. Institue Of Sci. Technol.36 (1), 61–75. Available online at: https://search.trdizin.gov.tr/en/yayin/detay/386651/assessment-of-livestock-drinking-water-quality-a-case-study-in-a-special-environmental-protection-area.
24
CardosoE. A.FurtadoD. A.RibeiroN. L.MedeirosA. N.SaraivaE. P.NascimentoJ. W. B.et al (2021). Biochemical and hormonal parameters of goats kept in a controlled environment consuming water with different levels of salinity. Arq. Bras. Med. Veterinaria Zootec.73 (4), 853–860. 10.1590/1678-4162-12186
25
CastroM. S. M.de VasconcelosA. M.FontenelleR. O.JuliãoM. S. d. S.SoaresA. T. L.da SilvaL. C.et al (2019). Water quality of small ruminant production systems in the Brazilian semiarid region. Biol. Rhythm Res.52, 934–945. 10.1080/09291016.2019.1608731
26
CervantesA.LópezG.MongeF. J.EstradaA.PlascenciaA. (2024). Importance of water quality in small ruminants’ productivity. Iran. J. Appl. Animal Sci.14 (1), 1–9.
27
ChedidM.JaberL. S.Giger-ReverdinS.Duvaux-PonterC.HamadehS. K. (2014). Review: water stress in sheep raised under arid conditions. Can. J. Animal Sci.94 (2), 243–257. 10.4141/CJAS2013-188
28
CostaR. G.FreireR. M. B.de AraújoG. G. L.QueirogaR.PaivaG. N.RibeiroN. L.et al (2021). Effect of increased salt water intake on the production and composition of dairy goat milk. Animals11 (9), 2642. 10.3390/ani11092642
29
CrooksA. (2020). Understanding limits for livestock water. Weld. Lab. Available online at: https://weldlabs.com/livestock-water-guide.pdf.
30
DavisR. (2016). Feedlot design and construction 5. Water Qual. Available online at: https://www.mla.com.au/globalassets/mla-corporate/research-and-development/program-areas/feeding-finishing-and-nutrition/feedlot-design-manual/05-water-quality-2016_04_01.pdf.
31
DinkaM. (2012). Analysing the extent (size and shape) of Lake Basaka expansion (Main Ethiopian Rift Valley) using remote sensing and GIS. Lakes and Reservoirs Sci. Policy Manag. Sustain. Use17 (2), 131–141. 10.1111/j.1440-1770.2012.00500.x
32
DinkaM. O. (2016). Quality composition and irrigation suitability of various surface water and groundwater sources at Matahara Plain. Water Resour.43 (4), 677–689. 10.1134/S0097807816040114
33
DinkaM. O. (2017a). Delineating the drainage structure and sources of groundwater flux for Lake Basaka, central rift valley region of Ethiopia. WaterSwitzerl.9 (12), 797. 10.3390/w9120797
34
DinkaM. O. (2017b). Lake Basaka expansion: challenges for the sustainability of the matahara irrigation scheme, Awash River basin (Ethiopia). Irrigation Drainage66 (3), 305–315. 10.1002/ird.2114
35
DinkaM. O. (2020). Estimation of groundwater contribution to Lake Basaka in different hydrologic years using conceptual netgroundwater flux model, Journal of Hydrology: regional Studies. J. Hydrology Regional Stud.30 (February), 100696. 10.1016/j.ejrh.2020.100696
36
DinkaM. O.LoiskandlW.NdambukiJ. M. (2014). Hydrologic modelling for Lake Basaka: development and application of a conceptual water budget model. Environ. Monit. Assess.186 (9), 5363–5379. 10.1007/s10661-014-3785-7
37
DinkaM. O.LoiskandlW.NdambukiJ. M. (2015). Hydrochemical characterization of various surface water and groundwater resources available in Matahara areas, Fantalle Woreda of Oromiya region. J. Hydrology Regional Stud.3, 444–456. 10.1016/j.ejrh.2015.02.007
38
DPIRD (2024). “Water quality for livestock,” in Department of primary industries and regional development, 69. Perth, WA. Factsheet DPIRD-17, Veterinary Medicine, Small Animal Clinician : VM, SAC. Available online at: https://library.dpird.wa.gov.au/cgi/viewcontent.cgi?article=1012&context=ap_factsheets.
39
Egbe-NwiyiT.NwaosuS. C.SalamiH. A. (2000). Haematological values of appararently healthy sheep and goats as influenced by age and sex in arid zone of Nigeria. Afr. J. Biomed. Res.3, 109–115. Available online at: https://www.bioline.org.br/pdf?md00031.
40
EleniA. B. (2009). Growing Lake with growing problems: integrated hydrogeological investigation on Lake beseka, Ethiopia. (Dissertation) Rheinische Friedrich-Wilhelms-Universität Bonn. Available online at: https://nbn-resolving.org/urn:nbn:de:hbz:5N-16453.
41
EmonM. V. (2018). Water quality for livestock”, extension beef cattle specialist. Montana State University. Available online at: https://www.montana.edu/extension/climate/documents/WaterQualityforLivestock_remediated.pdf.
42
FantaY.KecheroY.YemaneN. (2024). Hematological parameters of sheep and goats fed diets containing various amounts of water hyacinth (Eichhornia crassipes). Front. Veterinary Sci.11, 1286563. 10.3389/fvets.2024.1286563
43
FAO (2024). FAO code of ethical conduct. 2nd ed. Rome.
44
GashawT.WubayeG. B.WorqlulA. W.DileY. T.MohammedJ. A.BirhanD. A.et al (2023). Local and regional climate trends and variabilities in Ethiopia: implications for climate change adaptations. Environ. Challenges, 13. 10.1016/j.envc.2023.100794
45
GebremichaelE.SeyoumW. M.IshimweB.SataerG. (2022). Lake surface area expansion: insights into the role of volcano-tectonic processes, Lake Beseka, East Africa. J. Hydrology Regional Stud., 41. 10.1016/j.ejrh.2022.101093
46
GhanemM.ZeineldinM.EissaA.El EbissyE.MohammedR.AbdelraofY. (2018). The effects of saline water consumption on the ultrasonographic and histopathological appearance of the kidney and liver in Barki sheep. J. Veterinary Med. Sci.80 (5), 741–748. 10.1292/jvms.17-0596
47
GuptaM.KumarS.DangiS.JangirB. (2013). Physiological, biochemical and molecular responses to thermal stress in goats. Int. J. Livest. Res.3 (2), 27. 10.5455/ijlr.20130502081121
48
HigginsS. F.AgouridisC. T.EngineeringA.GumbertA. A.ProgramsA. (2000). ID-170: drinking water quality guidelines for cattle, 1–4. No. 1956.
49
HusseinA. H.PatraA. K.PuchalaR.WilsonB. K.GoetschA. L. (2022). Effects of restricted availability of drinking water on blood characteristics and constituents in dorper, katahdin, and st. Croix sheep from different regions of the USA. Animals12 (22), 3167. 10.3390/ani12223167
50
HusseinA. H.PuchalaR.GipsonT. A.TadesseD.WilsonB. K.GoetschA. L. (2020). Effects of water restriction on feed intake, digestion, and energy utilization by mature female St. Croix sheep, Veterinary and Animal Science. Veterinary Animal Sci.10 (June), 100132. 10.1016/j.vas.2020.100132
51
JainN. C. (1993). Essentials of veterinary hematology. Lea and Febiger: Wiley-Blackwell.
52
JemberuW. T.LiY.AsfawW.MayberryD.SchrobbackP.RushtonJ.et al (2022). Population, biomass, and economic value of small ruminants in Ethiopia. Front. Veterinary Sci.9, 972887. 10.3389/fvets.2022.972887
53
Jerry KanekoJ.HarveyJ. J.BrussM. L. (2008). “Clinical biochemistry of domestic animals,” in Clinical biochemistry of domestic animals. Elsevier Inc. 10.1016/B978-0-12-370491-7.X0001-3
54
KaliberM.KolumanN.SilanikoveN. (2016). Physiological and behavioral basis for the successful adaptation of goats to severe water restriction under hot environmental conditions. Animal10 (1), 82–88. 10.1017/S1751731115001652
55
KochewadS. A.RaghunandanT.Sarjan RaoK.KumariN. N.RamanaD. b. V.KumarD. A.et al (2018). Productive performance, body condition score and carcass characteristics of deccani lambs reared under different farming systems. Indian J. Animal Res.52 (3), 444–448. 10.18805/ijar.B-3478
56
KombatR. (2023). Adaptation capacity of indigenous sheep to Saline Lake drinking water in dry area of Ethiopia under climate change, Vol. 7 No. 28, pp. 358–368.
57
LiM.WangY. S.Elwell‐CuddyT.BaynesR. E.TellL. A.DavisJ. L.et al (2021). Physiological parameter values for physiologically based pharmacokinetic models in food-producing animals. Part III: sheep and goat. J. Veterinary Pharmacol. Ther.44 (4), 456–477. 10.1111/jvp.12938
58
ManeD. U.MK. K.AS. C.NagalakshmiD.SakaramD.VenkataramanaK.et al (2022). Physiological, haematological and serum biochemical parameters of Mahabubnagar local goats under different systems of rearing, Vol. 11 No. 10, pp. 1263–1271.
59
MaraiI. F. M.El-DarawanyA. A.FadielA.Abdel-HafezM. A. M. (2007). Physiological traits as affected by heat stress in sheep-A review. Small Ruminant Res., 71, 1–12. 10.1016/j.smallrumres.2006.10.003
60
McgregorB. A. (2004). Water quality and provision for goats A report for the rural industries research and development corporation.
61
MdletsheZ. M.ChimonyoM.MarufuM. C.NsahlaiI. V. (2017). Effects of saline water consumption on physiological responses in Nguni goats. Small Ruminant Res.153, 209–211. 10.1016/j.smallrumres.2017.06.019
62
MeehanM. A.StokkaG.MostromM. (2021). Livestock water quality (AS1764). NDSU Ext. Serv.1764 (February). Available online at: https://www.ndsu.edu/agriculture/sites/default/files/2022-08/as1764.pdf.
63
MeleseH.DebellaH. J. (2023). Comparative study on seasonal variations in physico-chemical characteristics of four soda lakes of Ethiopia (Arenguade, Beseka, Chitu and Shala). Heliyon, 9. 10.1016/j.heliyon.2023.e16308
64
MengistuU.DahlbornK.OlssonK. (2007). Effect of intermittent watering on growth, thermoregulation and behaviour of Ethiopian Somali goat kids. Small Ruminant Res.72 (2–3), 214–220. 10.1016/j.smallrumres.2006.10.012
65
NguyenT.Nguyen TrongN.ChaiyabutrN.ThammacharoenS. (2024). Diluted seawater decreased weight gain and altered blood biochemical parameters in Bach Thao goats. J. Appl. Animal Res., 52. 10.1080/09712119.2024.2371123
66
NSW (2014). “Water requirements for sheep and cattle Environmental factors”, Department of Primary Industries.
67
Oromiya Pastoral Area Development Commission (OPADC) (2021). Livestock production and productivity improvement towards pastoral livelihood enhancement in the pastoral areas of Oromia region; unpublished report; oromiya pastoral area development commission: Addis Ababa, Ethiopia, 1–40.
68
PaivaG. N.De AraújoG. G. L.HenriquesL. T.MedeirosA. N.FilhoE. M. B.CostaR. G.et al (2017). Water with different salinity levels for lactating goats. Semina:Ciencias Agrar. Univ. Estadual Londrina38 (4), 2065–2074. 10.5433/1679-0359.2017v38n4p2065
69
PatraA. K.dos Santos RibeiroL. P.YirgaH.SonibareA. O.AskarA. R.HusseinA. H.et al (2024). Effects of the concentration and nature of total dissolved solids in drinking water on feed intake, nutrient digestion, energy balance, methane emission, ruminal fermentation, and blood constituents in different breeds of young goats and hair sheep. Anim. Nutr.16, 84–95. 10.1016/j.aninu.2023.10.002
70
PolizopoulouZ. S. (2010). Haematological tests in sheep health management. Small Ruminant Res., 92, 88–91. 10.1016/j.smallrumres.2010.04.015
71
RahardjaD. P.TolengA. L.LestariV. S. (2011). Thermoregulation and water balance in fat-tailed sheep and Kacang goat under sunlight exposure and water restriction in a hot and dry area. Animal5 (10), 1587–1593. 10.1017/S1751731111000577
72
RanganathaS. K. (2022). Effect of drinking water salinity on productive performance and blood biochemical parameters in Surti kids under tropical conditions Effect of drinking water salinity on productive performance and blood biochemical parameters in Surti kids under tropical. 10.56093/ijans.v92i7.116126
73
ReeceW. O.PraterM. R. (2015). Dukes’ physiology of domestic animals (13th ed.). 44. Wiley-Blackwell. 10.1088/1751-8113/44/8/085201
74
RegassaA. E. (2016). Determinants of agro pastoralists’ participation in irrigation scheme: The case of fentalle agro pastoral district, oromia regional state, Ethiopia. Int. J. Agric. Res. Innovation Technol.5 (2), 44–50. 10.3329/ijarit.v5i2.26269
75
Reneu Rosas de AlbuquerqueÍ.Garcia Leal de AraujoG.Vinhas VoltoliniT.Helder de Andrade MouraJ.Germano CostaR.Costa GoisG.et al (2020). Saline water intake effects performance, digestibility, nitrogen and water balance of feedlot lambs. Animal Prod. Sci.60, 1591–1597. 10.1071/an19224
76
SamiraA. M.MohammedA. R.AnaamE. O.SheebaA.WaleedM. A. G. (2016). Biochemical and hematological profile of different breeds of goat maintained under intensive production system. Afr. J. Biotechnol.15 (24), 1253–1257. 10.5897/ajb2016.15362
77
SejianV.KumarD.GaughanJ. B.NaqviS. M. K. (2017). Effect of multiple environmental stressors on the adaptive capability of Malpura rams based on physiological responses in a semi-arid tropical environment. J. Veterinary Behav.17, 6–13. 10.1016/j.jveb.2016.10.009
78
SilanikoveN. (2000). The physiological basis of adaptation in goats to harsh environments. Small Ruminant Res.35 (3), 181–193. 10.1016/S0921-4488(99)00096-6
79
SolomonG.AzageT.BerhanuG.HoekstraD. (2010). Sheep and goat production and marketing systems in Ethiopia: characteristics and strategies for improvement, Working Paper 23.
80
TenagashawD. Y.TamiratD. M. (2022). Spatial and temporal variations of the physicochemical parameters of the water quality of Lake Basaka, oromia region, Ethiopia. Water Conservation Sci. Eng.7 (2), 143–155. 10.1007/s41101-022-00134-3
81
ThietN.HonN. V.NguN. T.ThammacharoenS. (2022). “Effects of high salinity in drinking water on behaviors, growth, and renal electrolyte excretion in crossbred Boer goats under tropical conditions”, Veterinary World. Veterinary World15 (4), 834–840. 10.14202/vetworld.2022.834-840
82
TschuorA. C.RiondB.BraunU.LutzH. (2008). Hämatologische und klinisch-chemische Referenzwerte für adulte Ziegen und Schafe. Schweiz. Arch. für Tierheilkd.150 (6), 287–295. 10.1024/0036-7281.150.6.287
83
TsukaharaY.PuchalaR.SahluT.GoetschA. L. (2016). Effects of level of brackish water on feed intake, digestion, heat energy, and blood constituents of growing Boer and Spanish goat wethers. J. Animal Sci.94 (9), 3864–3874. 10.2527/jas.2016-0553
84
TuluD.GadissaS.HundessaF. (2023). Impact of water stress on adaptation and performance of sheep and goat in dryland regions under climate change scenarios: A systematic review. J. Animal Behav. Biometeorology11 (2), 1–13. 10.31893/JABB.23012
85
TuluD.HundessaF.GadissaS.TemesgenT. (2024). Review on the influence of water quality on livestock production in the era of climate change: Perspectives from dryland regions. Cogent Food and Agric.10 (1), 2306726. 10.1080/23311932.2024.2306726
86
TuluD.UrgeM.MummedY. Y. (2022). Physiological, hematological, and biochemical responses in Hararghe-highland lamb subjected to water salinity levels of Lake Basaka in a semiarid area of Ethiopia. Heliyon8. 10.1016/j.heliyon.2022.e12616
87
TuluD.UrgeM.YusufY. (2021). Assessment of pastorals’ perceptions of Lake Basaka’s water quality concerning its impact on sheep and goat production in Mid Rift Valley of Ethiopia. J. Biol. Agric. Healthc.11 (17), 4–13. 10.7176/jbah/11-17-02
88
UmerA.AssefaB.FitoJ. (2020). Spatial and seasonal variation of lake water quality: beseka in the Rift Valley of Oromia region, Ethiopia. Int. J. Energy Water Resour., 4, 47–54. 10.1007/s42108-019-00050-8
89
UNDP. (2020). Oromia pastoralist association (OPA) equator initiative case studies
90
WodajoH. D.GemedaB. A.KinatiW.MulemA. A.van EerdewijkA.WielandB. (2020). Contribution of small ruminants to food security for Ethiopian smallholder farmers. Small Ruminant Res.184. 10.1016/j.smallrumres.2020.106064
91
YirgaH.PuchalaR.TsukaharaY.TesfaiK.SahluT.MengistuU. L.et al (2018). “Effects of level of brackish water and salinity on feed intake, digestion, heat energy, ruminal fluid characteristics, and blood constituent levels in growing Boer goat wethers and mature Boer goat and Katahdin sheep wethers”, Small Ruminant Research. Small Ruminant Res.164 (May), 70–81. 10.1016/j.smallrumres.2018.05.004
92
YirgaH.UrgeM.GoetschA. L.ToleraA. (2019). Quality of water from Rift Valley lakes of Ethiopia for livestock drinking. East Afr. J. Veterinary Animal Sci.3 (1), 9–16. 10.20372/eajvas.v3i1.402
93
YirgaH.UrgeM.GoetschA. L.ToleraA.PuchalaR.PatraA. K. (2024). Effects of salinity levels of drinking water on water intake and loss, feed utilization, body weight, thermoregulatory traits, and blood constituents in growing and mature Blackhead ogaden sheep and Somali goats. Animals14 (11), 1565. 10.3390/ani14111565
94
YohannesG. (2011). Large scale irrigation management and critical environmental concern: the case of fentale irrigation project in central Oromia. MSc. Thesis. Addis Ababa, Ethiopia: Addis Ababa University.
95
ZaherH. A.MesalamA.Al BloushiA. I.TolbaA.SwelumA. A.Abu-AlrubI. (2022). Hematological and biochemical indices, growth performance, and puberty of goats fed with Mombasa and blue panic as salt-tolerant alternatives to alfalfa under arid conditions. Front. Veterinary Sci.9, 961583. 10.3389/fvets.2022.961583
96
ZoidisE.HadjigeorgiouI. (2018). Effects of drinking saline water on food and water intake, blood and urine electrolytes and biochemical and haematological parameters in goats: a preliminary study. Animal Prod. Sci.58 (10), 1822–1828. 10.1071/AN16539
Summary
Keywords
goat, Lake Basaka, resilience, salinity, sheep
Citation
Tulu D, Letta MU, Mummed YY, Hundessa F and Kitila DB (2025) Thermoregulatory and biochemical responses of indigenous sheep and goats to naturally saline water under the pastoral system, a study at the Mid-Rift Valley of Ethiopia. Pastoralism 15:14829. doi: 10.3389/past.2025.14829
Received
28 April 2025
Accepted
09 July 2025
Published
25 July 2025
Volume
15 - 2025
Edited by
Ioannis Hadjigeorgiou, Agricultural University of Athens, Greece
Updates
Copyright
© 2025 Tulu, Letta, Mummed, Hundessa and Kitila.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Diriba Tulu, dirotulu@gmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.