Abstract
A cross-sectional study was conducted in Isiolo and Marsabit counties, Kenya to investigate the challenges associated with high camel calf mortality. Data were collected through focus group discussions and scheduled interviews. Milk pH levels were used to ascertain subclinical mastitis. Statistical analysis was performed through recurring themes, comparing means, and multiple linear regressions. The null hypotheses for the coefficients were rejected at the p < 0.05 significance level. Gross camel calf mortality was found to be 44.11%, with the most significant risk factors being predation, tick paralysis, and microbial diseases. Increasing the suckling period and the amount of milk consumed, along with short deworming intervals were associated with reduced morbidity and mortality from microbial diseases (p < 0.05). This is attributed to improved calf immunity. Subclinical mastitis was associated with a reduction in microbial diseases. Camel pox was positively associated with long intervals between treatments for external parasites because it is tick-transmitted. The study recommends prioritising calf rearing and implementing security measures to protect calves from predators. A longitudinal study is recommended to confirm whether the associations identified are the cause of the morbidities and mortalities observed.
Introduction
Since the early 1970s, developing countries have undergone a ‘livestock revolution’ driven by increased demand for animal products (Latino et al., 2020). Urbanisation and increased income in the majority of the middle-class population have led to a change in food preferences towards animal products. In Kenya, 80% of the landmass is arid or semi-arid and supports 60% of the country’s livestock and wildlife which coexist and often cause human-wildlife conflict (Benka, 2023). The ecosystem is very fragile due to the effects of climate change and cannot support high natural resource-demanding livestock. Therefore, pastoralists have been replacing them with camels and goats. However, the replacement stock of camels is negatively affected by a short breeding season, a low conception rate, and high camel calf mortality ranging from 35% to 50% (Muluneh et al., 2022; Thiakunu et al., 2024).
Disease morbidity and mortality are measures of illness and death, respectively, within a defined period and in a specified population (Aiello et al., 2016). Feeding colostrum to young animals gives them immunity to common diseases. Furthermore, hygiene standards and nutrition are very important in determining the possible prevalence of disease and survival rates. Climate change affects calf morbidity and mortality due to direct and indirect environmental effects (Lacetera, 2019). Direct effects include poor nutrition and water scarcity, which influence immunity and hence increase disease morbidity and mortality. Indirect effects are a result of the growth of disease-causing microorganisms. Furthermore, fluctuating extreme weather conditions facilitate vector reproduction and changes in disease distribution patterns (Chikezie et al., 2024).
Information is scarce on the critical challenges associated with high calf mortality in the ASALs (Abduljami and Kumbe, 2024). The objectives of this study therefore were to identify common camel calf diseases, life-threatening challenges, and their associated factors. Understanding morbidities and mortalities informs livestock workers and institutions providing veterinary and extension services in priority areas to address policy matters. Furthermore, this knowledge helps camel farmers take preventive measures thereby improving productivity and commercialisation.
Materials and methods
Ethics statement
Ethical review and approval for this study were obtained from the Research and Ethics Committee at Kenyatta University, Kenya. A research permit was obtained from the National Commission for Science, Technology, and Innovation (NACOSTI) in Nairobi, Kenya, licence number NACOSTI/P/22/14910. Furthermore, permission was sought from the offices of the County Directorate of Veterinary Services, Isiolo and Marsabit.
Study area
The study was carried out in Isiolo and Marsabit Counties in Kenya (Figure 1). The proportion of households keeping camels is 12% and 29% for Isiolo and Marsabit, respectively. Isiolo County is 65% very arid, 30% arid, and 5% semi-arid (Jaetzold et al., 2008). Marsabit is the largest county in Kenya covering an area of 70,961 km2 at the end of Northern Kenya. The rainfall system is bimodal (April-May and November-December) ranging between 200 mm and 1,000 mm per annum (p.a). The arid and very arid areas form the most extensive part of Marsabit below 700 m above sea level and receive rainfall below 300 mm p.a. According to the Kenya National Bureau of Statistics (KNBS), Isiolo has 148,859 camels distributed among 6771 households while Marsabit has 215,234 camels distributed among 22,093 households. The study population was therefore 28,864 households.
FIGURE 1
Map of Kenya showing the locations of Isiolo and Marsabit Counties (KNBS, 2019).
Research design
The design was cross-sectional relying on recall of events from 1 year before the study. Data were collected through two focus group discussions (FGDs) each comprising 14 people including community disease reporters (CDRs), staff from the Ministry of Agriculture and Livestock Development, and pastoralists. Household surveys were conducted through questionnaires which were uploaded to a smart web-based app known as the Kobocollect toolbox.
Sample size determination
The sample population comprised all camel-keeping households in Isiolo and Marsabit Counties. The sample size was determined in two stages using the formula provided by (Pfeiffer (2010). Using Equations 1, 2, the first stage determined the sample size for an infinite population and then it was adjusted for a finite population. The level of statistical significance was first set at 0.05 and the standard normal deviation was set at 1.96.
The formula used was:Where,
n = Desired sample size for infinite population.
Z = standard normal deviation at the required confidence level.
p = proportion of the population estimated to have the attributes being measured.
q = 1-p and L = set statistical significance.
In this case, there is insufficient knowledge of the population with the attribute, so a value of 0.5 is recommended; therefore, the sample size for an infinite population was,
The second stage was to get a sample for a target population of 28,864 households, the sample size was adjusted using Equations 3, 4Where:
n′ = adjusted sample size for the finite population.
n = sample size for infinite population.
N = population size. Therefore, the sample size was
Sampling design
A two-stage sampling method was employed because the study involves a wide geographical area. In the first stage, we sampled the Isiolo and Garba Tulla sub-counties in Isiolo County along with Laisamis Sub-County in Marsabit County. In the second stage, a convenient sample was drawn from camel-keeping households who were willing to participate. Samples were drawn proportionally to the number of households keeping camels; hence, there were 137 from Isiolo County and 242 from Marsabit County. Furthermore, the samples were proportional to the number of households keeping camels in three or four locations in Isiolo and Marsabit Counties respectively. In Isiolo, these locations were Kulamawe, Gotu, and Kinna while in Marsabit they were Laisamis, Lontolio, Koya, and Merille.
Data collection
Two Focus Group Discussions (FGDs) were held in the towns of Isiolo and Laisamis with stakeholders generally highlighting how camels were managed for production and the challenges encountered. Common camel calf diseases and life-threatening challenges were highlighted by the staff from the counties’ Veterinary Departments, who interpret the symptoms from the CDRs. The data collection tool in the households consisted of questionnaires administered by CDRs in specific local languages which included Somali, Turkana, and Boran mainly from Isiolo and Rendille, and Samburu from Laisamis, Marsabit. The questionnaires were administered using the Kobo Toolbox, which was uploaded to the CDRs’ smartphones. Camel calf diseases and life-threatening incidents were determined through the recall of the respondents for 1 year before the study was carried out. The frequency of controlling internal and external parasites, the method of feeding colostrum, the suckling period and the proportion of milk suckled were measured as factors predisposing camel calves to diseases and life-threatening challenges. The level of subclinical mastitis was determined by randomly selecting one lactating camel from each herd. This method relies on an indicator paper that detects milk pH levels since they become elevated in cases of mammary gland inflammation. The indicator paper undergoes a colour change, shifting from orange to blue when there is inflammation as a result of subclinical mastitis (Plummer and Plummer, 2011). The level of subclinical mastitis was determined by the number of udder quarters affected.
Data analysis
The FGD analysis was carried out by determining recurring themes, particularly with regard to the common life-threatening challenges faced by camel calves. The CDRs describe symptoms, which are then interpreted by qualified animal health workers to make tentative diagnoses. The means and frequencies of disease occurrence and the predisposing factors, morbidities, and mortalities of common camel calf diseases were determined separately for the two counties. The mean was calculated to determine the mortality and morbidity rates for each disease in each sampled location separately. Linear regression analysis was performed with the null hypothesis for the coefficient rejected at a 0.05 level of significance. The dependent variable was tested for normality by comparing it with the expected normal distribution curve. After it was found not to show normal distribution, it was transformed with Log10 and tested again. To interpret the outcomes, the associations between disease morbidity and mortality and the risk factors were examined. This is because regression builds upon correlation (Wisniewski and Brannan, 2024).
Results
After the focus group discussions, common life-threatening challenges affecting camel calves were listed. These included diarrhoea, pneumonia, ORF, pox, eye infections, predation, tick paralysis, excess milk consumption, and calf diphtheria.
Camel calf morbidities and mortalities
The morbidity rate for diarrhoea, pneumonia, ORF, and pox was higher in Marsabit than in Isiolo locations. Predation was highest in Kinna, Gotu, and Kulamawe, all of which are in Isiolo County, and Koya in Marsabit. Tick paralysis and calf diphtheria showed higher morbidity in Lontolio and Koya than in any of the other locations in Marsabit. Gross mortality was 44.11% mainly attributed to predation, tick paralysis, and microbial diseases. Consumption of excess milk and eye infections contributed least to gross mortality (Table 1).
TABLE 1
| Disease/condition | Diarrhoea | Pneumonia | Orf | Pox | Eye infection | Worm | Predation | Tick paralysis | Milk | Calf diphtheria | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kulamawe n = 62 | Mob | 14.32 | 26.37 | 24.54 | 11.27 | 15.34 | 16.97 | 27.31 | 26.48 | 3.47 | 14.81 | |
| Mot | 2.52 | 6.99 | 3.64 | 3.4 | 1.15 | 1.68 | 13.13 | 11.01 | 0 | 3.14 | 33.77 | |
| Gotu n = 30 | Mob | 4.55 | 13.18 | 2.61 | 5.69 | 1.49 | 8.97 | 28.55 | 7.72 | 3.3 | 0.58 | |
| Mot | 0 | 2.09 | 0 | 0 | 0 | 0 | 9.25 | 1.99 | 0.58 | 0 | 12.99 | |
| Kinna n = 45 | Mob | 17.12 | 28.92 | 32.23 | 22.72 | 13.3 | 10.19 | 38.71 | 37.6 | 3.3 | 15.42 | |
| Mot | 4.37 | 8.86 | 1.92 | 4.98 | 0.61 | 0.99 | 14.54 | 11.17 | 0 | 4.58 | 36.5 | |
| Laisamis n = 27 | Mob | 16.84 | 12.19 | 28.92 | 9.65 | 3.09 | 40.41 | 9.97 | 18.66 | 0 | 11.15 | |
| Mot | 7.8 | 5.23 | 10.42 | 5.38 | 0 | 14.1 | 5.92 | 10.42 | 2.15 | 8.74 | 43.64 | |
| Lontolio n = 28 | Mob | 33.94 | 47.83 | 38.07 | 31.44 | 17.31 | 23.72 | 12.46 | 33.86 | 19.52 | 23.74 | |
| Mot | 8.71 | 10.42 | 9.44 | 9.31 | 6.79 | 10.59 | 13.55 | 12.78 | 7.53 | 6.46 | 51.54 | |
| Koya n = 42 | Mob | 30.64 | 37.27 | 39.8 | 31.01 | 28.7 | 29.62 | 22.89 | 31.72 | 24.56 | 25.6 | |
| Mot | 9.81 | 8.04 | 3.06 | 9.47 | 4.93 | 2.76 | 5.05 | 10.56 | 10.09 | 9.11 | 44.33 | |
| Merille n = 23 | Mob | 19.68 | 14.7 | 14.7 | 17.42 | 16.51 | 18.32 | 14.7 | 18.78 | 10.38 | 15.77 | |
| Mot | 12.23 | 13.49 | 12.51 | 10.27 | 12.48 | 12.63 | 16.98 | 11.85 | 11.29 | 11.57 | 58.97 | |
| Total n = 257 | Mob | 19.15 | 26.82 | 26.8 | 18.39 | 14.53 | 20.15 | 24.23 | 26.36 | 8.83 | 15.63 | |
| Mot | 6.99 | 8.27 | 6.35 | 6.55 | 4.12 | 6.67 | 11.43 | 10.51 | 5 | 6.73 | 44.11 | |
Calf morbidities and mortalities.
Disease predisposing factors
The routine herd, calf management, and milking practices are shown in Table 2. Of the routine management practices, the majority of locations in Marsabit either did not perform external parasite control or did so at intervals of more than 2 weeks. In contrast, Isiolo County pastoralists were found to control external parasites either weekly or every 2 weeks. In Isiolo County, the majority of pastoralists were observed to deworm their camels every 6 months to 1 year while in Marsabit the majority dewormed after 1 year or did not deworm at all. Pastoralists in Isiolo were generally found to implement better calf management practices than those in Marsabit. They allowed calves to suckle immediately after birth and started selling milk within one to 2 months. In contrast, Marsabit herders delayed colostrum feeding and started selling milk within a month. However, Isiolo pastoralists were observed to restrict suckling to one teat for a shorter duration compared to those in Marsabit. Notably, lactating camels in Marsabit had a lower incidence of subclinical mastitis than those in Isiolo.
TABLE 2
| Isiolo N = 137 | Marsabit N = 242 | Total N = 379 | Isiolo N = 137 | Marsabit N = 242 | Total N = 379 | ||
|---|---|---|---|---|---|---|---|
| Practices | Percentage | Practices | Percentage | ||||
| Frequency of controlling for external parasites | How soon after birth does the calf suckle | ||||||
| Weekly | 4.3 | 19.4 | 14.0 | Immediately | 77.9 | 33.2 | 49.4 |
| Two times a week | 57.9 | 15.8 | 31.0 | 0–6 h | 22.1 | 59.9 | 46.3 |
| Beyond 2 weeks | 37.9 | 51.0 | 46.3 | 7–12 h | 6.5 | 4.1 | |
| None | 13.8 | 8.8 | Beyond 12 h | 0.4 | 0.3 | ||
| Interval between deworming treatments | Proportion of milk suckled | ||||||
| Less than 3 months | 0.7 | 2.0 | 1.6 | Two-quarters | 2.1 | 30.4 | 20.2 |
| Every 3–6 months | 3.6 | 4.9 | 4.4 | One-quarter | 76.4 | 32.8 | 48.6 |
| 6 months to 1 year | 49.3 | 23.5 | 32.8 | Not quantified | 9.3 | 30.0 | 22.5 |
| Yearly | 13.6 | 14.6 | 14.2 | ||||
| Beyond 1 year | 17.1 | 32.8 | 27.1 | None | 12.1 | 6.9 | 8.8 |
| None | 15.7 | 22.3 | 19.9 | Suckling period in months | |||
| How soon is milk sold (in months) | 19–24 | 53.6 | 17.4 | 30.5 | |||
| Beyond 4 months | 20.0 | 1.6 | 8.3 | 13–18 | 40.7 | 19.4 | 27.1 |
| 3–4 months | 2.9 | 18.6 | 12.9 | 7–12 | 5.7 | 57.1 | 38.5 |
| 1–2 months | 77.1 | 38.5 | 52.5 | 0–6 | 6.1 | 3.9 | |
| Less than 1 month | 41.3 | 26.4 | Quarters affected by subclinical mastitis | ||||
| Activities before milking | None | 37.1 | 74.1 | 60.7 | |||
| Hands/Udder | 62.9 | 2.4 | 24.3 | One | 45.0 | 9.3 | 22.2 |
| Udder | 15.7 | 91.9 | 64.3 | Two | 17.1 | 3.6 | 8.5 |
| Nothing | 21.4 | 5.7 | 11.4 | Three | 2.4 | 1.6 | |
| Four | 0.7 | 10.5 | 7.0 | ||||
Routine herd and calf management practices.
Risk factors associated with camel calf morbidity
Figure 2 illustrates the risk factors linked to calf morbidity. A decrease in both the proportion of milk suckled and the duration of suckling was significantly associated with increased cases of diarrhoea (p < 0.05). Higher levels of subclinical mastitis, however, were linked to a lower incidence of diarrhoea at the same significance level. An increase in deworming intervals, starting milk sales soon after calving, and shorter suckling periods were all significantly associated with a rise in pneumonia cases in calves (p < 0.05). Similarly, increased subclinical mastitis was connected to a decrease in pneumonia morbidity (p < 0.05). Earlier milk sales from individual camels and shorter suckling periods were strongly linked to increased morbidity due to ORF while a drop in disease morbidity was correlated with higher levels of subclinical mastitis (p < 0.01).
FIGURE 2
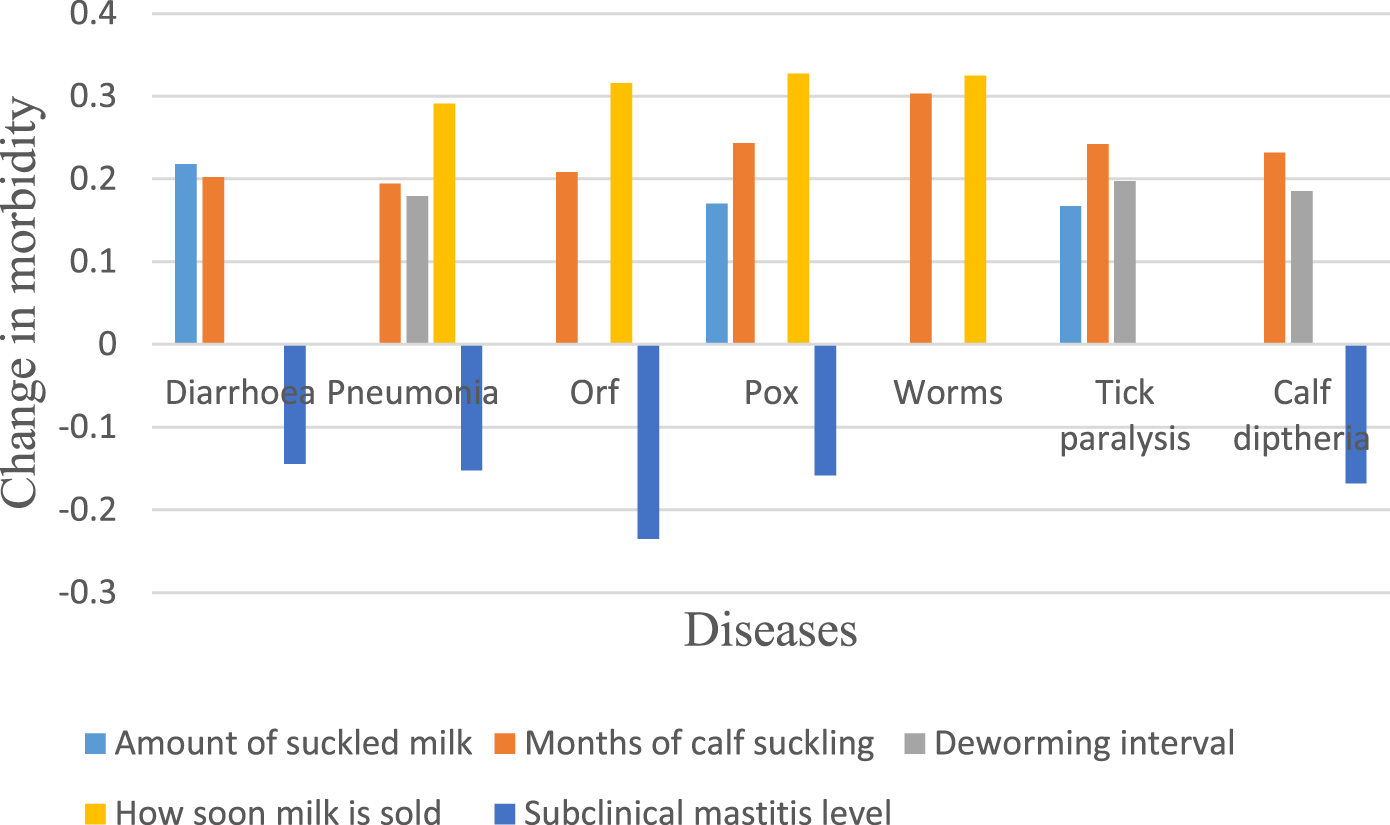
Practices associated with morbidity.
Pox morbidity rose significantly when milk was sold sooner after calving when calves suckled less milk, and for a shorter duration. Conversely, higher levels of subclinical mastitis were associated with a decrease in pox cases (p < 0.05). A shorter time before selling milk and fewer months of suckling were linked to an increase in worm-related illness with 1% significance. Incidence of tick paralysis showed a significant positive correlation with longer intervals between deworming, along with reduced milk consumption and suckling time (p < 0.05). Longer deworming intervals and shorter suckling durations were associated with an increased number of diphtheria cases (p < 0.05).
Risk factors associated with camel calf mortality
The risk factors associated with camel calf mortality are shown in Figure 3. A shorter time taken to start selling milk and a lower proportion of milk suckled were positively correlated with diarrhoeal mortality. (p < 0.05). Mortality attributed to calf pneumonia, ORF, and tick paralysis was significantly associated with the speed at which milk from a specific camel was sold (p < 0.05). Increasing the interval between treatments for external parasites and reducing the time taken to start selling milk were associated with increased mortality due to pox, while an increasing number of quarters affected by subclinical mastitis was negatively associated with it (p < 0.05).
FIGURE 3
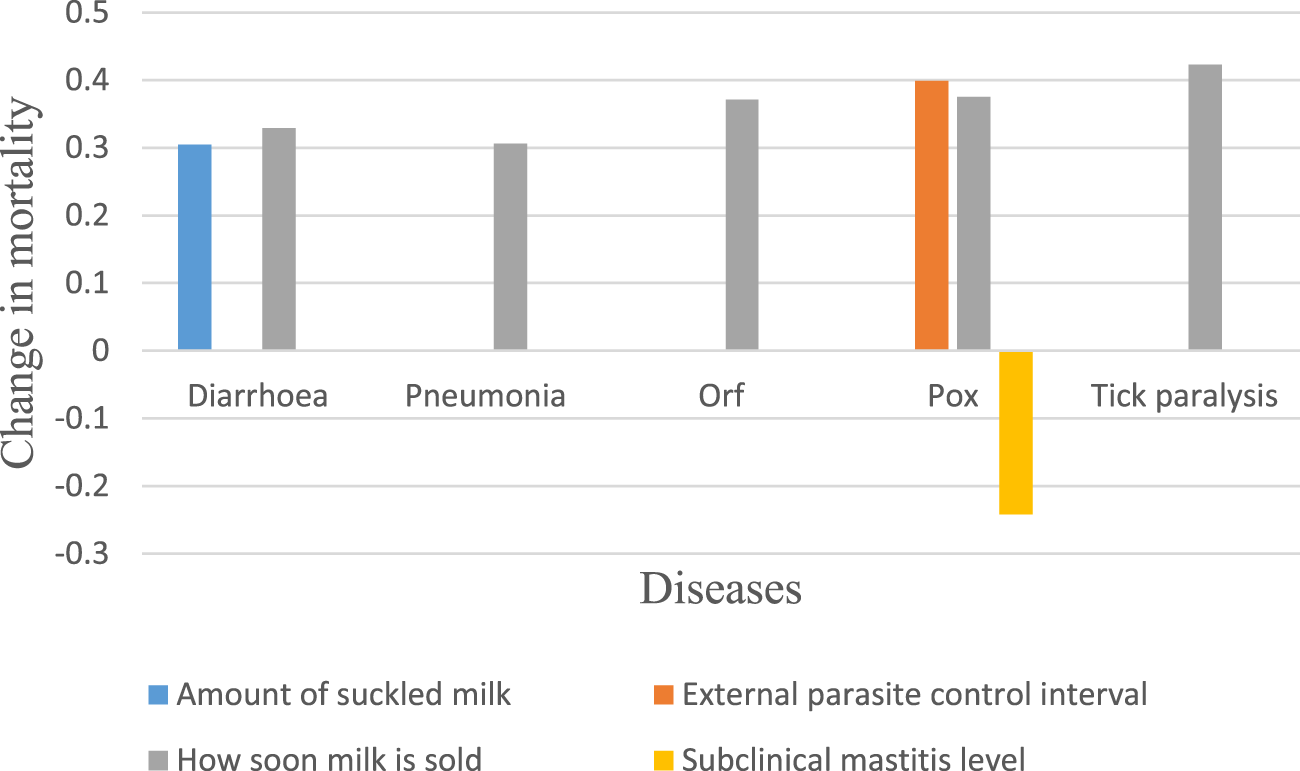
Practices associated with mortality.
Discussion
Calf-threatening challenges and calf management
Predation was the main cause of mortality in camel calves, which is consistent with other studies. Tick paralysis also contributed significantly to the calf mortality (10.51%). Other studies found that the highest contributors to mortality were septicaemia, diarrhoea, and Pneumonia (Tora et al., 2021). Calf milk intake was found to be sufficient, contradicting previous findings. Camel keepers in Isiolo took longer to commence selling milk than those in Marsabit, and this is probably because they had a higher proportion of lactating camels. The gross mortality rate for pox was lower than the 25%–100% rate established in other studies (Tadesse et al., 2018).
The morbidity of subclinical mastitis was significantly higher in Isiolo and lower in Marsabit County, which is inconsistent with the findings of other studies (Seligsohn et al., 2020). The high incidence in Isiolo is associated with the prevalence of high-yielding Somali-type dairy camels. Animals that produce large quantities of milk are more susceptible to host factors that increase their risk of mastitis compared to those with moderate yields (Cheng and Han, 2020). Similarly, Somali camels were more prone to mastitis than the lower-yielding Rendille camels. Additionally, a challenging hygienic environment, including limited access to water, contributes to udder infections (Azevedo et al., 2016). Basic milking hygiene practices, such as washing hands and cleaning the udder before milking, are poorly practised by camel herders (Seligsohn et al., 2020).
Correlations of disease morbidity and mortality with herd management practices
The factors that contributed to diarrhoea and pneumonia morbidity were as expected because they all improve calf immunity. Pox was positively influenced by increasing the interval of external parasite control. This can be explained by the fact that the pox virus is transmitted by mosquitoes, the population of which increases during the rainy season. The virus that causes pox has been isolated in the Amblyomma tick species identified on a severely affected camel with clinical signs of pox (Achalu, 2019). Pox is one of the diseases designated as notifiable according to the Disease Control Act of Kenya and therefore, it requires that systems must be put in place to control it. However, there are several constraints including poor mobility and inadequate staffing (Irungu, 2021).
The interval between treatments for external parasites had a positive influence on worm-related morbidity due to the use of drugs such as Ivermectin, a broad-spectrum drug used to control both ecto- and endoparasites. However, reducing the frequency of controlling external parasites increases mortality due to tick paralysis, which is caused by toxins that are produced by ticks. Tick infestation was found to be high, especially in hot climatic conditions in the ASALs (Moshaverinia and Moghaddas, 2015). Calves are easy targets for predators because of their weakness and inability to defend themselves.
The prevalence of subclinical mastitis was found to be associated with fewer microbial infections, probably because the early colonisation of the gastrointestinal tract by pathogens is essential for proper immune system development (Zheng et al., 2020). The timing of colostrum feeding differed from that reported in other studies (Tadesse et al., 2014).
The amount of milk consumed through suckling influenced morbidity from pox, diarrhoea, and pneumonia, along with the mortality rate from diarrhoea. Furthermore, deworming intervals were linked to the incidence of diarrhoea, pneumonia, and calf diphtheria. This could be attributed to the fact that helminths feed on blood, negatively affecting the animal’s nutritional status and, consequently, its immune response (Montout et al., 2021).
Conclusion and recommendations
To improve the health of camel calves and reduce the incidence of disease, it is important to encourage longer suckling periods and delay the sale of milk. The major threats to calf survival include predators, tick paralysis, and microbial infections. Calves that do not receive enough milk often due to premature marketing of the milk, face a higher risk of illness and death. In Isiolo County, the rapid growth in camel production has not been matched by investment in animal healthcare and extension services. This has resulted in an increase in subclinical mastitis. Although this condition is unexpectedly associated with immunity, it can progress into clinical mastitis, ultimately lowering milk yield and quality. Pathogens present in milk further compromise its safety and reduce its shelf life.
Regular deworming, control of external parasites, timely vaccinations, and prompt treatment for sick animals should be emphasised to improve calf survival. Predation remains a pressing issue, so measures such as predator-proof fencing and solar lighting should be explored. Furthermore, Kenya’s pending Agriculture and Livestock Extension Bill proposes establishing a board to identify extension service priorities. Incorporating camel calf care and disease prevention into these priorities is critical. Implementing Kenya’s 2020 wildlife policy and its proposed compensation programme for wildlife-induced losses requires the allocation of resources to become operational. Overall, a combination of improved husbandry, enhanced veterinary services, and policy support is essential for sustainable camel production in the ASALs. Furthermore, studies are recommended to prove the association between diseases and risk factors.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Research ethical review and approval for this study was obtained from Kenyatta University, Kenya, Research and Ethics Committee. Research permit was obtained from the National Commission for Science, Technology and Innovation (NACOSTI), in Nairobi, Kenya, license number NACOSTI/P/22/14910. Further, permission was sought from the office of the County Directorate of Veterinary Services, Isiolo and Marsabit.
Author contributions
FT: conceptualization, data collection, and administration. BN: supervision. PN: supervision. JA: funding acquisition, data collection, data analysis, and referencing. JK: Proofreading the final document.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article. The authors acknowledge receiving financial support for the research from Kenya’s National Research Fund (NRF), reference number NRF/2/MMC/176 under the project titled: Development of Value-Added Camel Milk Products to Enhance Food Security in Arid and Semi-Arid Areas of Northern Kenya.
Acknowledgments
The authors express their gratitude to the National Research Fund (NRF) of Kenya for funding this study under the project titled: Development of Value-Added Camel Milk Products to Enhance Food Security in Arid and Semi-Arid Areas of Northern Kenya.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
References
1
Abduljami G. Kumbe A. (2024). A retrospective study on the mortality rate of camel calves, leading causes, and associated risk factors in borana zone, Oromia regional state, Ethiopia. J. Appl. Veterinary Sci. Technol.5 (1), 12–19. 10.20473/javest.V5.I1.2024.12-19
2
Achalu D. (2019). Review on camel pox: epidemiology, public health and diagnosis. (2019). ARC J. Animal Veterinary Sci.5 (4), 1–12. 10.20431/2455-2518.0504003
3
Aiello S. E. Moses M. A. Allen D. G. (2016). The merck veterinary manual. 11th ed. (KENILWORTH, NJ, USA: Merck & Co., Inc.).
4
Azevedo C. Pacheco D. Soares L. Romão R. Moitoso M. Maldo- nado J. et al (2016). Prevalence of contagious and environmental mastitis-causing bacteria in bulk tank milk and its relationships with milking practices of dairy cattle herds in Sao Miguel island (azores). Trop. Animal Health Prod.48, 451–459. 10.1007/s11250-015-0973-6
5
Benka V. A. (2023). Expanding the scope of challenges to human-wildlife coexistence, and the implications for conservation: a case study of Laikipia, Kenya. Hum. Dimensions Wildl.28 (6), 585–601. 10.1080/10871209.2022.2136421
6
Cheng W. N. Han S. G. (2020). Bovine mastitis: risk factors, therapeutic strategies, and alternative treatments — a review. Asian-Australasian J. Animal Sci.33 (11), 1699–1713. 10.5713/ajas.20.0156
7
Chikezie F. M. Opara K. N. Ubulom P. M. E. (2024). Impacts of changing climate on arthropod vectors and diseases transmission. Niger. J. Entomology40 (1), 179–192. 10.36108/NJE/4202/04.0161
8
Irungu P. (2021). Constraints and opportunities in livestock service delivery in northern Kenya rangelands. Feinstein International Center at Tufts University, USAID Nawiri project.
9
Jaetzold R. Schmidt H. Holmetz B. Shisanya C. (2008). Farm management handbook of Kenya. Nairobi: Ministry of Agriculture in cooperation with GTZ.
10
KNBS (2019). National population and housing census. Nairobi: Government Printers.
11
Lacetera N. (2019). Impact of climate change on animal health and welfare. Anim. Front.9 (1), 26–31. 10.1093/af/vfy030
12
Latino L. R. Pica-Ciamarra U. Wisser D. (2020). Africa: the livestock revolution urbanizes. Glob. Food Secur.26 (100399), 100399–18. 10.1016/j.gfs.2020.100399
13
Montout L. Poullet N. Bambou J.-C. (2021). A systematic review of the interaction between nutrition and immunity in livestock: effect of dietary supplementation with synthetic amino acids. Animals11 (10), 1–13. 10.3390/ani11102813
14
Moshaverinia A. Moghaddas E. (2015). Prevalence of tick infestation in dromedary camels (Camelus dromedarius) brought for slaughter in mashhad abattoir, Iran. J. Parasit. Dis.39 (3), 452–455. 10.1007/s12639-013-0367-5
15
Muluneh B. Shiferaw D. Teshome D. Al-Khaza'leh J. Megersa B. (2022). Constraints and incidence of camel calf morbidity and mortality in borana rangeland, southern Ethiopia. J. Arid Environ.206, 104841–104849. 10.1016/j.jaridenv.2022.104841
16
Pfeiffer D. U. (2010). Veterinary epidemiology: an introduction. Oxford, UK: Wiley Blackwell.
17
Plummer P. J. Plummer C. (2011). Diseases of the mammary gland in sheep and goat medicine. 2nd ed., 442–465.
18
Seligsohn D. Nyman A.-K. Younan M. Sake W. Persson Y. Bornstein S. et al (2020). Subclinical mastitis in pastoralist dairy camel herds in Isiolo, Kenya: prevalence, risk factors, and antimicrobial susceptibility. J. Dairy Sci.103 (5), 4717–4731. 10.3168/jds.2019-17701
19
Tadesse T. Mulatu E. Bekuma A. (2018). Review on camel pox: an economically overwhelming disease of pastorals. Int. J. Adv. Res. Biol. Sci.5 (9), 65–73. 10.22192/ijarbs.2018.05.09.006
20
Tadesse Y. Urge M. Abegaz S. Kurtu M. Y. Kebede K. Dessie T. (2014). Husbandry, breeding practices, and production constraints of camel in the pastoral communities of Afar and Somali, Ethiopia. J. Agric. Environ. Int. Dev.108 (2), 167–189. 10.12895/jaeid.20142.238
21
Thiakunu F. Njehia B. K. Nguhiu P. N. Arimi J. M. (2024). Effects of concentrate supplementation on lactating dromedary camels during mating season in Isiolo, Kenya. Afr. J. Sci. Technol. Soc. Sci.2 (2), 70–82. 10.58506/ajstss.v2i2.147
22
Tora E. Abayneh E. Seyoum W. Shurbe M. (2021). Longitudinal study of calf morbidity and mortality on smallholder farms in southern Ethiopia. Plos One16 (9), e0257139–18 e0257139. 10.1371/journal.pone.0257139
23
Wisniewski S. J. Brannan G. D. (2024). “Correlation (coefficient, partial, and spearman rank) and regression analysis,” in StatPearls (Treasure Island (FL): StatPearls Publishing). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK606101/.
24
Zheng D. Liwinski T. Elinav E. (2020). Interaction between microbiota and immunity in health and disease. Cell Res.30 (6), 492–506. 10.1038/s41422-020-0332-7
Summary
Keywords
mortality, morbidity, immunity, predation, association
Citation
Thiakunu FK, Njehia B, Nguhiu P, Arimi J and Kirimi J (2025) Camel calf diseases, life-threatening challenges, and associated risk factors in Isiolo and Marsabit counties, Kenya. Pastoralism 15:14696. doi: 10.3389/past.2025.14696
Received
28 March 2025
Accepted
11 July 2025
Published
06 August 2025
Volume
15 - 2025
Edited by
Derradji Harek, Algerian National Institute for Agronomic Research INRAA, Algeria
Updates
Copyright
© 2025 Thiakunu, Njehia, Nguhiu, Arimi and Kirimi.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Florence Karimi Thiakunu, karimithiakunu@gmail.com
† Present address: Florence Karimi Thiakunu, Department of Animal Science, Meru University of Science and Technology, Meru, Kenya
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.