Abstract
Background:
Roflumilast, a highly selective phosphodiesterase 4 inhibitor, is used to treat with chronic obstructive pulmonary disease and psoriasis. We aim to determine potential roflumilast-associated adverse events (AEs) and the differences in AE signals among diverse populations.
Methods:
Roflumilast’s AE reports between the first quarter of 2011 and the fourth quarter of 2024 were obtained from the FDA Adverse Event Reporting System (FAERS) and Canada Vigilance Adverse Reaction Database (CVARD). The signal strength was measured by four disproportionality analysis methods, including Reporting Odds Ratio (ROR), Proportional Reporting Ratio (PRR), Bayesian Confidence Propagation Neural Network (BCPNN), and Multi-item Gamma Poisson Shrinker (MGPS).
Results:
In FAERS, population aged ≥65 years and oral medication users accounted for a predominant proportion in the reported cases. FDA-unlabeled respiratory, thoracic and mediastinal disorders was the only one signal categorized by system organ class met all four algorithms. Newly identified AEs such as dyspnea, condition aggravated, cough, and tachycardia could contribute valuable safety considerations for clinical practice. The analysis of the available time-to-onset data suggested that cases often occurred within the first 30 days post-treatment. These results were externally validated in CVARD, suggesting consistent findings. Notably, headache was more frequently reported among users of topical formulations and female patients, while suicidal ideation and weight loss were more commonly reported in male patients and oral medications, respectively.
Conclusion:
This study confirmed established adverse reactions and identified novel AEs in real-world clinical practice by dual-database pharmacovigilance analysis. Clinicians should remain vigilant for AEs that differ by gender and route to enable early intervention and improve prognosis. The findings highlight personalized safety management, while underscoring the necessity of prospective studies to validate results and further characterize roflumilast’s safety profile.
Introduction
Roflumilast is the first phosphodiesterase 4 (PDE4) inhibitor indicated for chronic obstructive pulmonary disease (COPD) in 2011, subsequently for plaque psoriasis approved by the U.S. Food and Drug Administration (FDA) and Health Canada [1, 2]. Notably, COPD has been found to be a complication of psoriasis [3]. T lymphocytes play an important role in the immunopathogenesis of both psoriasis and COPD, driving chronic inflammation [4, 5]. These two diseases impact millions of individuals worldwide and represent a huge economic burden [6, 7]. PDE4 expression is higher in patients with inflammatory conditions than in healthy people [8]. The PDE4 enzyme family (isoforms PDE4A-D) specifically catalyzes the hydrolysis of cyclic adenosine monophosphate (cAMP) [9]. Roflumilast elevates cAMP levels in inflammatory and immune cells to reduce the release of inflammatory factors, such as tumor necrosis factor α (TNFα), and interleukin (IL)-17, thereby improving hyperactive immune responses [10].
Phase III clinical trials have demonstrated good efficacy and tolerability profile of roflumilast [11, 12]. However, safety concerns could limit roflumilast’s clinical use [13]. Immune-related disease treatment is a prolonged procedure. Continuous safety monitoring is essential to fully understand roflumilast’s adverse events (AEs) during treatment [14]. Nevertheless, large-scale data analysis of roflumilast-related adverse reactions in the real world is still insufficient. An analysis based on the U.S. MarketScan database identified potential safety signals through investigating roflumilast’s concomitant medications [15]. Our study draws on FDA and Canadian pharmacovigilance databases, facilitating the capture of a broader spectrum of global AE reports, meanwhile, providing a novel perspective on signal detection that differs from prescription sequence analysis.
The FDA Adverse Event Reporting System (FAERS) and Canada Vigilance Adverse Reaction Database (CVARD) are spontaneous reporting databases that monitor post-marketing drug safety [16, 17]. To provide a clinical rationalization of drug administration, we conducted disproportionality analyses to identify potential AEs associated with roflumilast and systematically evaluated adverse reactions across different patient subgroups.
Methods
Data source and processing
The FAERS and CVARD are publicly available pharmacovigilance databases for detecting new drug safety signals. They contain structured data fields, including demographics (DEMO), drug information (DRUG), adverse events (REAC), report sources (RPSR), and indication for use (INDI). Spontaneous AE reports are gathered from patients, healthcare providers, and others [16]. Using the medicine’s generic name (roflumilast) and trade name (DALIRESP, DAXAS, ZORYVE), we downloaded AE reports relevant to roflumilast from the first quarter (Q1) of 2011 to the fourth quarter (Q4) of 2024. Roflumilast was collected as the primary suspect (PS) from the FAERS and suspect in CVARD. The data were extracted, processed, and analyzed according to Figure 1. Following FDA guidelines, we performed data deduplication grounded in CASEID, FDA_DT, and PRIMARYID [18]. Then, we standardized AE terminology using Medical Dictionary of Regulatory Activities (MedDRA 27.1), which classifies events by System Organ Class (SOC) and Preferred Term (PT) levels. Possible indications (e.g., chronic obstructive pulmonary disease, bronchitis chronic, psoriasis) for roflumilast treatment as well as reactions (e.g., off-label use, intentional product misuse, medication error) unrelated to drug therapy were excluded before analyzing AEs.
FIGURE 1

The flow diagram of screening roflumilast-related AE reports from FAERS and CVARD databases. FAERS, FDA Adverse Event Reporting System; CVARD, Canada Vigilance Adverse Reaction Database; DEMO, demographics; DRUG, drug information; REAC, adverse events; PS, primary suspect.
A novelty/unexpected signal is defined as any positive adverse drug event detected that isn’t outlined in either the FDA or Canadian drug labels. The comparative methodology is as follows: First, AEs documented in the latest FDA drug labels and Canadian product monographs were systematically collated. Subsequently, Microsoft Excel 2021 was utilized to match these AEs with the preferred terms in the FAERS and CVARD. For AEs with different expressions but similar medical connotations, those with consistent or highly similar core meanings were identified as label-documented AEs following medical evaluation.
To evaluate the independent real-world safety profile of roflumilast, we conducted sensitivity analyses that excluded AE reports involving concomitant use with long-acting β2-agonists (e.g., formoterol), dual therapy (e.g., budesonide/formoterol, mometasone/formoterol, and fluticasone/formoterol), or triple therapy (e.g., budesonide/formoterol/glycopyrrolate and fluticasone/umeclidinium/vilanterol). This method could minimize potential effects from co-administered medications and enhance the specificity of AEs attribution to roflumilast therapy.
Time-to-onset and weibull distribution analysis
We calculated the time-to-onset (TTO) of AEs as the interval between roflumilast initiation and AE occurrence. TTO data were summarized using median with interquartile range (IQR). We employed Weibull distribution modeling (parameters α and β) to characterize time-dependent risk patterns [19]. The scale parameter (α) indicates the spread of distribution, whereas the shape parameter (β) describes the curve’s form. An early failure-type pattern is indicated when β < 1 and its 95% confidence interval (CI) < 1, suggesting a decreasing risk over time. A random failure-type pattern is inferred when β is close to 1 and its 95% CI includes 1, reflecting a constant hazard. A wear-out failure-type pattern is identified when β > 1 and its 95% CI excludes 1, indicating an increasing risk over the course of treatment.
Statistical analysis
In this study, we utilized four standard disproportionality metrics: Reporting Odds Ratio (ROR), Proportional Reporting Ratio (PRR), Bayesian Confidence Propagation Neural Network (BCPNN), and Multi-item Gamma Poisson Shrinker (MGPS). The two-by-two contingency table formulas and detailed calculation methods are provided in Supplementary Tables S1, S2. Significant signals that met at least one of the four analysis methods were considered as roflumilast-associated positive AEs. Among the four methods, the ROR performs well in sensitivity and early detection, the PRR offers high specificity, the BCPNN supports multi-source data integration and cross-validation, and the MGPS excels in identifying signals for rare events [20, 21]. This study comprehensively applies the four algorithms to detect drug safety signals more comprehensively and reliably. Higher values suggested a stronger link between the drug and the occurrence of AEs. Due to the small number of reports with roflumilast in CVARD database, subgroup analysis results couldn’t be effectively output. Subgroup analyses were performed for reports in the FAERS database (Supplementary Table S3). A lower limit of the 95% CI of the adjusted ROR >1 indicated greater probability of AE relevance in the target group, while 0 < 95% CI of the adjusted ROR <1 suggested higher relevance in the control group. The p value was corrected by the false discovery rate method. Adjusted p value (p.adj) < 0.05 indicated that the difference was statistically significant. R software (version 4.4.2) was applied for all statistical assessments.
Results
Descriptive characteristics
From 2011 Q1 to 2024 Q4, 19,348,490 cases were obtained from the FAERS database. After dereplication, this study ultimately contained 2,962 AE reports related to roflumilast and 8,481 roflumilast-associated AEs (Figure 1). Clinical features of AE reports related to roflumilast are described in the Table 1. Males made up 45.7% (n = 1,354) of these reports, while females accounted for 41.2% (n = 1,221). 51.6% of the age information reports was absent. 13.3% (n = 395) of all reports were from those aged 18 to 64, and 34.9% (n = 1,034) were over 65 years. Oral administration accounted for 89.5% (n = 2,650) of total reports, while topical usage was 7.7% (n = 228). Unfortunately, weight information was missing in 73% of the reports. The percentage of people weighing between 50 and 100 kg was comparatively higher, making up 21.4%. Healthcare professionals and consumers were the main reporters. COPD and psoriasis were the main indications. The top five countries with the most reports were the United States, Germany, Canada, South Korea, and Netherlands. According to reporting years, 2013 had the highest percentage of reports (26.4%), followed by 2014 (10.3%), 2023 (9.7%), 2012 (7.3%), and 2024 (7.2%).
TABLE 1
| Characteristics | Case numbers | Case proportion |
|---|---|---|
| Number of reports | 2,962 | |
| Gender | ||
| Male | 1,354 | 45.7% |
| Female | 1,221 | 41.2% |
| Missing | 387 | 13.1% |
| Age | ||
| <18 | 6 | 0.2% |
| 18–64 | 395 | 13.3% |
| ≥65 | 1,034 | 34.9% |
| Missing | 1,527 | 51.6% |
| Route | ||
| Oral | 2,650 | 89.5% |
| Topical | 228 | 7.7% |
| Other | 84 | 2.8% |
| Weight | ||
| <50 kg | 90 | 3.0% |
| >100 kg | 77 | 2.6% |
| 50–100 kg | 633 | 21.4% |
| Missing | 2,162 | 73.0% |
| Reporters | ||
| Healthcare professional | 896 | 30.2% |
| Consumer | 1,062 | 35.9% |
| Other | 686 | 23.2% |
| Missing | 318 | 10.7% |
| Indications (top 3) | ||
| COPD | 1,390 | 46.9% |
| Missing | 1,279 | 43.1% |
| Psoriasis | 86 | 2.9% |
| Reported countries (top 5) | ||
| United States | 2,275 | 76.8% |
| Germany | 450 | 15.2% |
| Canada | 32 | 1.1% |
| South Korea | 25 | 0.8% |
| Netherlands | 16 | 0.5% |
| Reporting year (top 5) | ||
| 2013 | 783 | 26.4% |
| 2014 | 305 | 10.3% |
| 2023 | 288 | 9.7% |
| 2012 | 217 | 7.3% |
| 2024 | 214 | 7.2% |
Characteristics of roflumilast-related adverse event reports in the FAERS database (2011 Q1 to 2024 Q4).
Signals detection at the SOC level
Following statistical analysis, we discovered that roflumilast-induced AEs occurrence targeted 26 organ systems. The top five SOCs by frequency were general disorders and administration site conditions (n = 1,481), gastrointestinal disorders (n = 1,257), psychiatric disorders (n = 983), respiratory, thoracic and mediastinal disorders (n = 898), and nervous system disorders (n = 736). Positive SOCs were included gastrointestinal disorders (ROR 1.85, PRR 1.72, EBGM 1.72, IC 0.78), psychiatric disorders (ROR 2.16, PRR 2.03, EBGM 2.03, IC 1.02), respiratory, thoracic and mediastinal disorders (ROR 2.34, PRR 2.20, EBGM 2.20, IC 1.14), investigations (ROR 1.14, PRR 1.13, EBGM 1.13, IC 0.18) and metabolism and nutrition disorders (ROR 1.66, PRR 1.63, EBGM 1.63, IC 0.71). Notably, only respiratory, thoracic and mediastinal disorders met all four criteria simultaneously (Table 2).
TABLE 2
| SOC | Case number | ROR (95% Cl) | PRR (χ2) | EBGM (EBGM05) | IC (IC025) |
|---|---|---|---|---|---|
| General disorders and administration site conditions | 1,481 | 0.99 (0.94, 1.05) | 0.99 (0.05) | 0.99 (0.95) | −0.01 (-0.09) |
| Gastrointestinal disorders* | 1,257 | 1.85 (1.74, 1.96)* | 1.72 (415.8) | 1.72 (1.64) | 0.78 (0.7)* |
| Psychiatric disorders* | 983 | 2.16 (2.02, 2.31)* | 2.03 (541.95)* | 2.03 (1.92) | 1.02 (0.92)* |
| Respiratory, thoracic and mediastinal disorders* | 898 | 2.34 (2.19, 2.51)* | 2.2 (617.68)* | 2.2 (2.08)* | 1.14 (1.04)* |
| Nervous system disorders | 736 | 1.02 (0.95, 1.1) | 1.02 (0.26) | 1.02 (0.96) | 0.03 (-0.09) |
| Investigations* | 600 | 1.14 (1.05, 1.24)* | 1.13 (10.17) | 1.13 (1.06) | 0.18 (0.06)* |
| Injury, poisoning and procedural complications | 460 | 0.55 (0.5, 0.6) | 0.57 (164.7) | 0.57 (0.53) | −0.81 (-0.95) |
| Musculoskeletal and connective tissue disorders | 378 | 0.83 (0.75, 0.92) | 0.84 (12.48) | 0.84 (0.77) | −0.25 (-0.41) |
| Infections and infestations | 309 | 0.67 (0.6, 0.75) | 0.68 (47.6) | 0.68 (0.62) | −0.55 (-0.71) |
| Metabolism and nutrition disorders* | 300 | 1.66 (1.48, 1.86)* | 1.63 (75.59) | 1.63 (1.48) | 0.71 (0.54)* |
| Skin and subcutaneous tissue disorders | 262 | 0.55 (0.49, 0.63) | 0.57 (90.67) | 0.57 (0.51) | −0.81 (-1) |
| Cardiac disorders | 248 | 1.1 (0.97, 1.25) | 1.1 (2.28) | 1.1 (0.99) | 0.14 (-0.05) |
| Vascular disorders | 115 | 0.62 (0.52, 0.74) | 0.62 (26.59) | 0.62 (0.54) | −0.68 (-0.95) |
| Neoplasms benign, malignant and unspecified (incl cysts and polyps) | 101 | 0.44 (0.36, 0.54) | 0.45 (70.61) | 0.45 (0.38) | −1.16 (-1.45) |
| Eye disorders | 70 | 0.4 (0.32, 0.51) | 0.41 (61.44) | 0.41 (0.33) | −1.29 (-1.64) |
| Renal and urinary disorders | 56 | 0.35 (0.27, 0.46) | 0.36 (66.28) | 0.36 (0.29) | −1.49 (-1.87) |
| Social circumstances | 34 | 0.91 (0.65, 1.28) | 0.91 (0.27) | 0.91 (0.69) | −0.13 (-0.62) |
| Immune system disorders | 32 | 0.33 (0.24, 0.47) | 0.34 (42.45) | 0.34 (0.25) | −1.57 (-2.08) |
| Blood and lymphatic system disorders | 31 | 0.21 (0.15, 0.3) | 0.21 (92.08) | 0.21 (0.16) | −2.23 (-2.75) |
| Reproductive system and breast disorders | 27 | 0.38 (0.26, 0.56) | 0.39 (26.62) | 0.39 (0.28) | −1.37 (-1.92) |
| Surgical and medical procedures | 27 | 0.23 (0.16, 0.34) | 0.23 (69.45) | 0.23 (0.17) | −2.11 (-2.65) |
| Hepatobiliary disorders | 26 | 0.33 (0.22, 0.49) | 0.33 (35.16) | 0.33 (0.24) | −1.59 (-2.14) |
| Ear and labyrinth disorders | 24 | 0.65 (0.44, 0.97) | 0.65 (4.46) | 0.65 (0.47) | −0.62 (-1.19) |
| Product issues | 19 | 0.14 (0.09, 0.22) | 0.14 (101.5) | 0.14 (0.1) | −2.83 (-3.48) |
| Endocrine disorders | 6 | 0.27 (0.12, 0.61) | 0.28 (11.49) | 0.28 (0.14) | −1.86 (-2.95) |
| Congenital, familial and genetic disorders | 1 | 0.04 (0.01, 0.27) | 0.04 (24.15) | 0.04 (0.01) | −4.7 (-6.74) |
Signal strength of adverse events of roflumilast at the SOC level.
SOC, system organ class; ROR, reporting odds ratio; PRR, proportional reporting ratio; EBGM, empirical bayesian geometric mean; EBGM05, the lower limit of the 95% CI of EBGM; IC, information component; IC025, the lower limit of the 95% CI of the IC; CI, confidence interval.
Asterisks (*) indicate positive signals.
Risk signals analyses at the PT level
The signals for the top 100 PTs ranked by frequency were presented in Supplementary Table S4. We found some side effects that were mentioned in the instructions but rarely reported, such as rash (n = 31), influenza (n = 16), nasopharyngitis (n = 14), and neoplasm malignant (n = 12).
Among positive PTs with ≥30 reported cases, half of these PTs were marked in the drug’s label, containing diarrhea (n = 423, ROR 4.92), weight decreased (n = 315, ROR 8.26), nausea (n = 296, ROR 2.74), insomnia (n = 248, ROR 6.68), headache (n = 217, ROR 2.49), decreased appetite (n = 208, ROR 6.62), dizziness (n = 166, ROR 2.39), tremor (n = 119, ROR 5.07), back pain (n = 115, ROR 3.51), anxiety (n = 110, ROR 2.72), suicidal ideation (n = 99, ROR 7.69), depression (n = 84, ROR 2.56), upper abdominal pain (n = 57, ROR 2.00), muscle spasms (n = 47, ROR 1.81) and atrial fibrillation (AF, n = 32, ROR 2.32). In addition, 14 potential adverse reactions were FDA-unlabeled, including dyspnea (n = 283, ROR 3.63), malaise (n = 109, ROR 1.74), asthenia (n = 88, ROR 1.66), condition aggravated (n = 76, ROR 1.87), cough (n = 70, ROR 1.81), feeling abnormal (n = 69, ROR 1.99), influenza-like illness (n = 55, ROR 4.64), heart rate increased (n = 46, ROR 3.31), abdominal discomfort (n = 46, ROR 1.98), chest pain (n = 39, ROR 1.47), nervousness (n = 37, ROR 4.85), palpitations (n = 35, ROR 2.13), myalgia (n = 35, ROR 1.46), and sleep disorder (n = 30, ROR 3.13) (Figure 2).
FIGURE 2
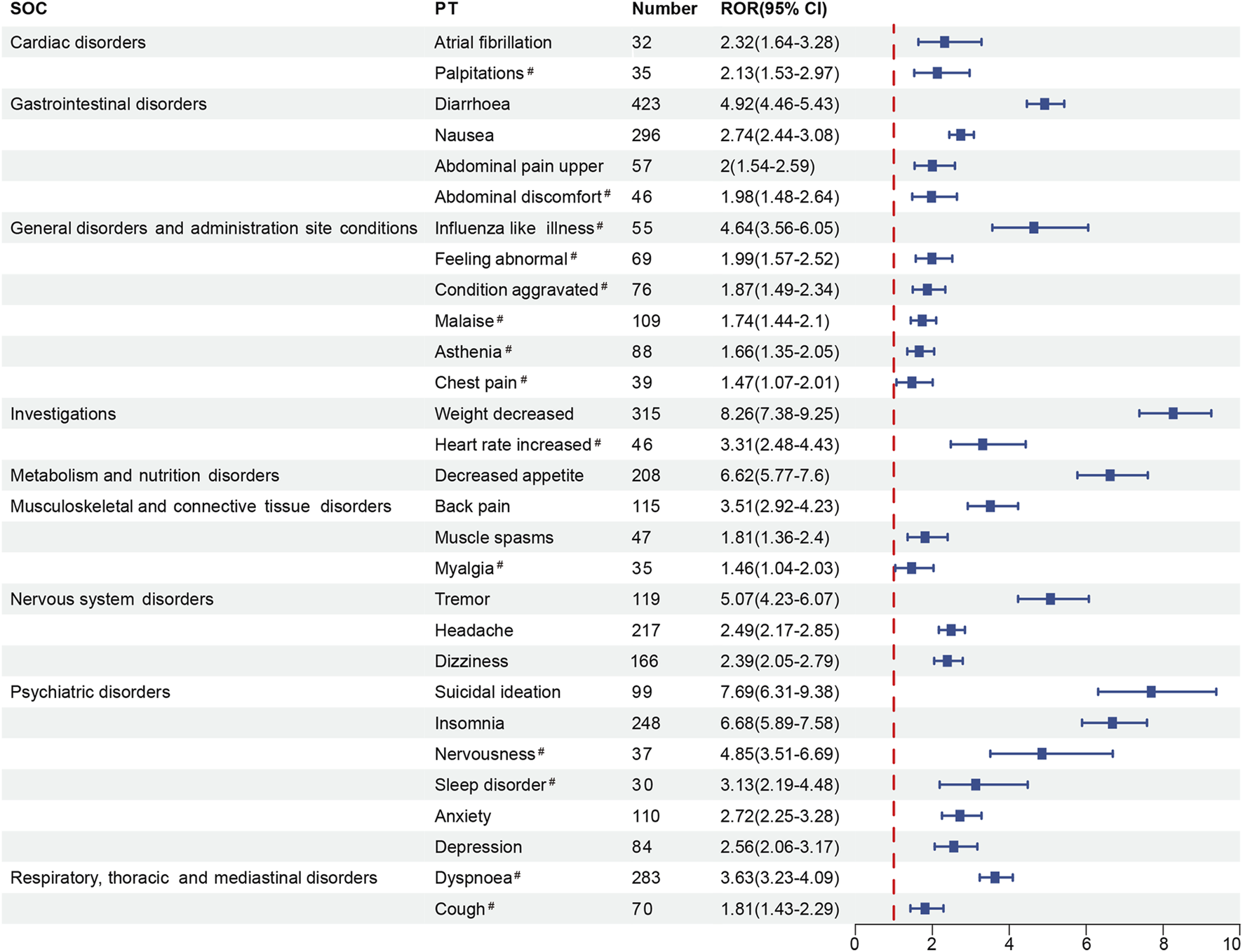
The forest plot of positive signals (n ≥ 30) related to roflumilast at the PT level. SOC, System Organ Class; PT, Preferred Term; ROR, Reporting Odds Ratio; CI, confidence interval. Hashtags (#) indicate AEs not marked in the FDA-drug’s label.
Stratified subgroup analysis
Gender-stratified analyses were performed on the 50 most frequent AEs (Supplementary Tables S5, S6). Of the top 50 signals, pruritus (n = 19), depressed mood (n = 12), and influenza (n = 11) reported only in the female population, while AF (n = 22) and suicide attempt (n = 13) required extra clinical surveillance in male patients. Our analysis suggested a potential association between males and suicidal ideation (ROR 0.57, 95% CI: 0.37–0.88), but no significant sex-based differentiation was observed. Notably, comparative analyses revealed that females exhibited significantly higher risk of headache (ROR 1.66, 95% CI: 1.23–2.23), nausea (ROR 1.77, 95% CI: 1.37–2.27), back pain (ROR 1.97, 95% CI: 1.32–2.94), and upper abdominal pain (ROR 2.42, 95% CI: 1.35–4.32) than male patients (Figures 3A,B).
FIGURE 3
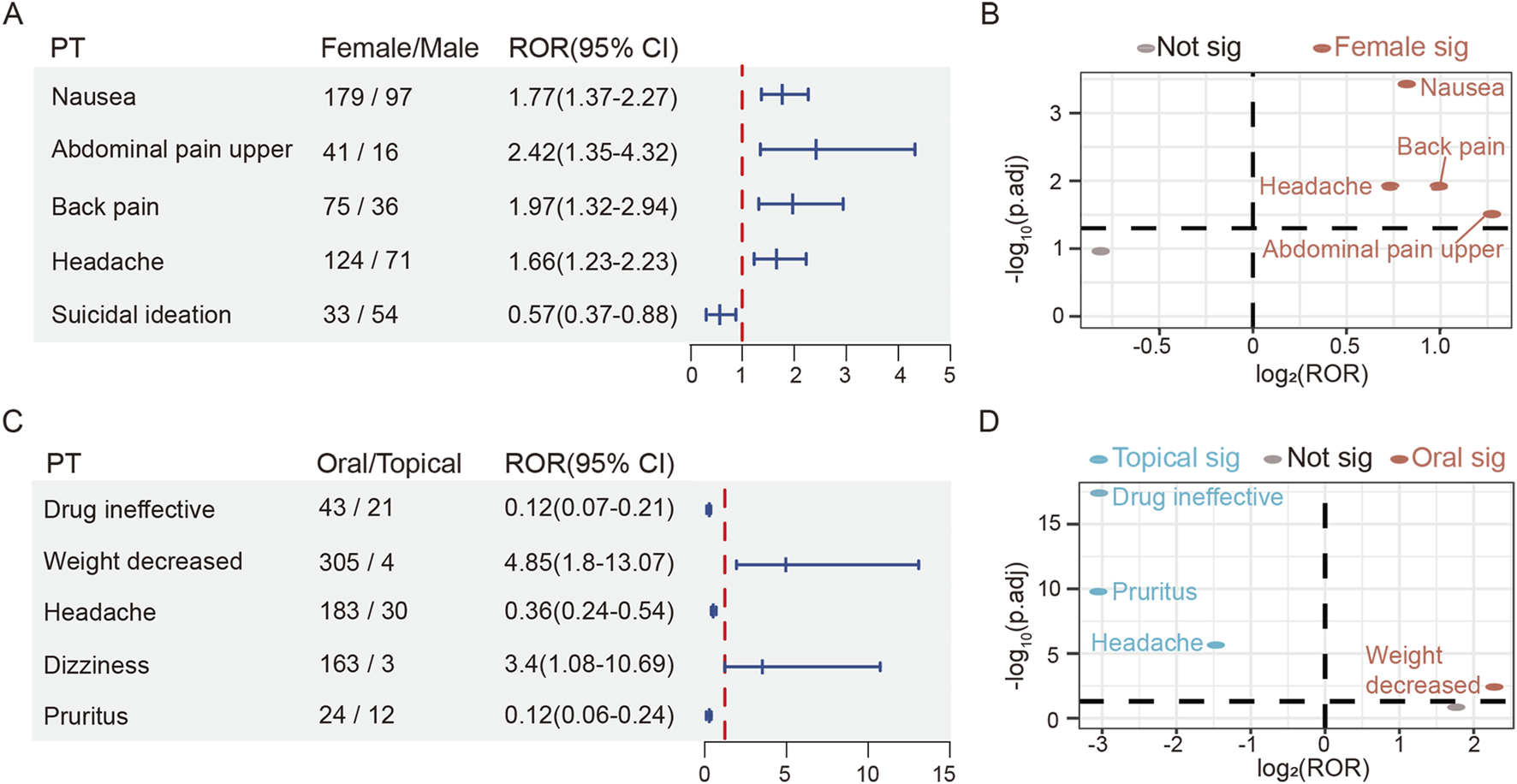
The risk differences of roflumilast in gender and route subgroup. (A) The forest plot of adjusted ROR for gender-related AEs. (B) Gender-differentiated risk signals volcano plot for roflumilast. (C) The forest plot of adjusted ROR for route-related AEs. (D) Route-differentiated risk signals volcano plot for roflumilast. SOC, System Organ Class; PT, Preferred Term; ROR, Reporting Odds Ratio; CI, confidence interval.
Among the top 50 PTs according to age subgroup analyses, patients over 64 had higher risk of decreased appetite rather than those within the group of 18–64 years (ROR 1.61, 95% CI: 1.08–2.39). The incidences of headache (ROR 0.67, 95% CI: 0.47–0.95) and suicidal ideation (ROR 0.52, 95% CI: 0.31–0.89) should be monitored in the group aged 18–64. Although these reactions suggest different associations of adverse effects by age group, there is no effective difference (Supplementary Figure S1). The <18 age group contained insufficient PTs for robust statistical analysis, it might need attention to respiratory distress, sensitive skin, and gastritis. Signal values for age-related analyses are provided in Supplementary Tables S7–S9.
The top 50 prominent AEs were also subjected to route subgroup analyses. Both oral and topical therapy with roflumilast could cause diarrhea, nausea, insomnia, headache, heart rate increased, rash, and pruritus. Additionally, it was revealed that orally treated patients required additional attention, including decreased appetite (n = 204), anxiety (n = 107), suicidal ideation (n = 97), AF (n = 32) and sleep disorder (n = 29). On the other hand, topical group requested caution for skin burning sensation (n = 15), skin exfoliation (n = 10), application site pain (n = 8), dermatitis contact (n = 7), urinary tract infection (n = 5), skin discoloration (n = 4), and urticaria (n = 4) (Supplementary Tables S10, S11). Compared to the topically treated group, the oral group showed a higher adjusted ROR signal strength for weight decreased (ROR 4.85, 95% CI: 1.8–13.07). However, topical preparation was more likely to cause headache (ROR 0.36, 95% CI: 0.24–0.54), pruritus (ROR 0.12, 95% CI: 0.06–0.24) and drug ineffective (ROR 0.12, 95% CI: 0.07–0.21) (Figures 3C,D).
TTO and weibull distribution analysis
The data on AE onset were available for only 494 reports (16.7%) in the FAERS database. The analysis of the available reports with complete TTO data suggested that cases often occurred within the first 30 days after roflumilast administration, and then the incidence of AEs decreased gradually (Figure 4A). Moreover, TTO data suggested a median onset time of 17 days and an IQR of 3–60 days (Figure 4B). Weibull distribution analysis of roflumilast-associated AEs revealed an early failure pattern, indicating decreasing AEs incidence over time (Figure 4C).
FIGURE 4
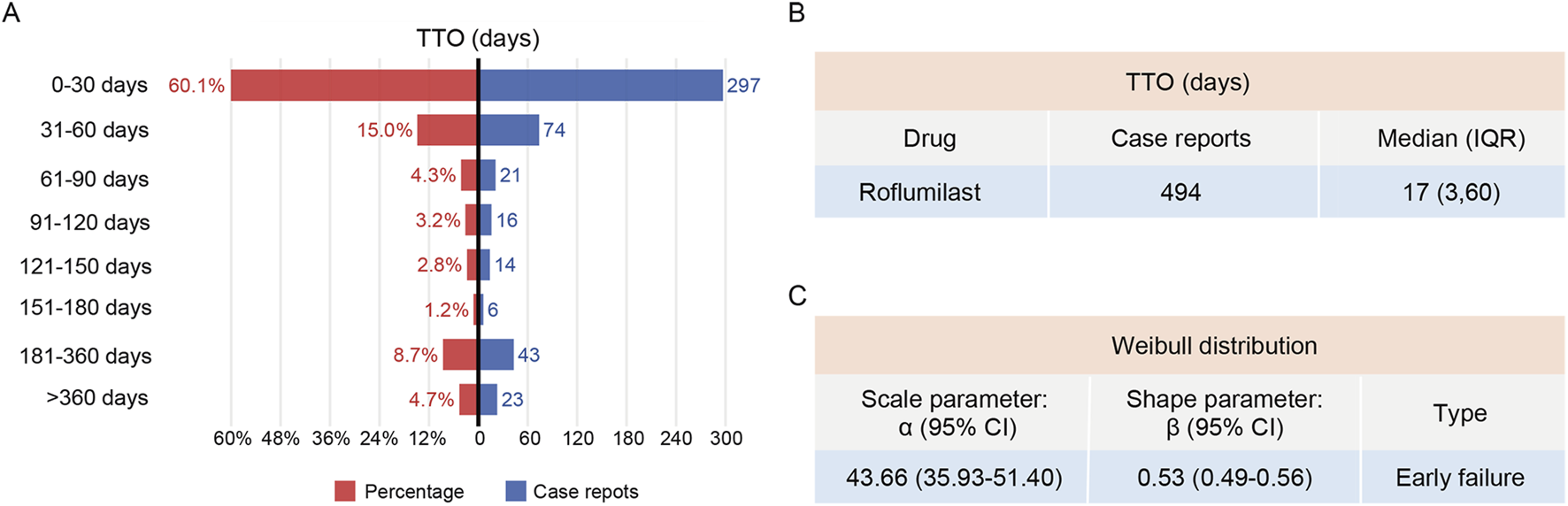
TTO and Weibull distribution analysis of roflumilast-induced adverse reactions. (A) The frequency and percentage distribution of TTO reports across various time periods. (B) TTO of roflumilast-induced adverse reactions. (C) Weibull distribution analysis of TTO reports. TTO, time-to-onset; IQR, interquartile range; CI, confidence interval.
Sensitivity analysis
Roflumilast is frequently prescribed as add-on therapy to long-acting bronchodilator, including formoterol, dual therapy (e.g., budesonide/formoterol, mometasone/formoterol, and fluticasone/formoterol), or triple therapy (e.g., budesonide/formoterol/glycopyrrolate and fluticasone/umeclidinium/vilanterol). After excluding these commonly co-administered drugs, a reanalysis of the top 100 PT signals was performed in Supplementary Table S12. The identified adverse reactions almost corresponded to previous findings, such as psychiatric symptoms, gastrointestinal diseases, respiratory tract disorders, and skin problems.
External validation in CVARD
A total of 394 reports were linked to roflumilast-related AEs in the CVARD database from 2011 to 2024 (Figure 1). Our analysis showed a comparable proportion of male (46.2%) and female (51.3%) patients treated with roflumilast, with the highest number of cases occurring in individuals aged ≥65 years (53.6%) or receiving oral formulation (99.2%) (Figure 5A). In 2013, the total number of reports (n = 155) exceeded those of any other year (Figure 5B). At the SOC level, respiratory, thoracic and mediastinal disorders (n = 377, ROR 4.83, PRR 3.94, EBGM 3.93, IC 1.97) was the only positive signal that matched the four algorithms (Figure 5C). These findings were consistent with FAERS data.
FIGURE 5
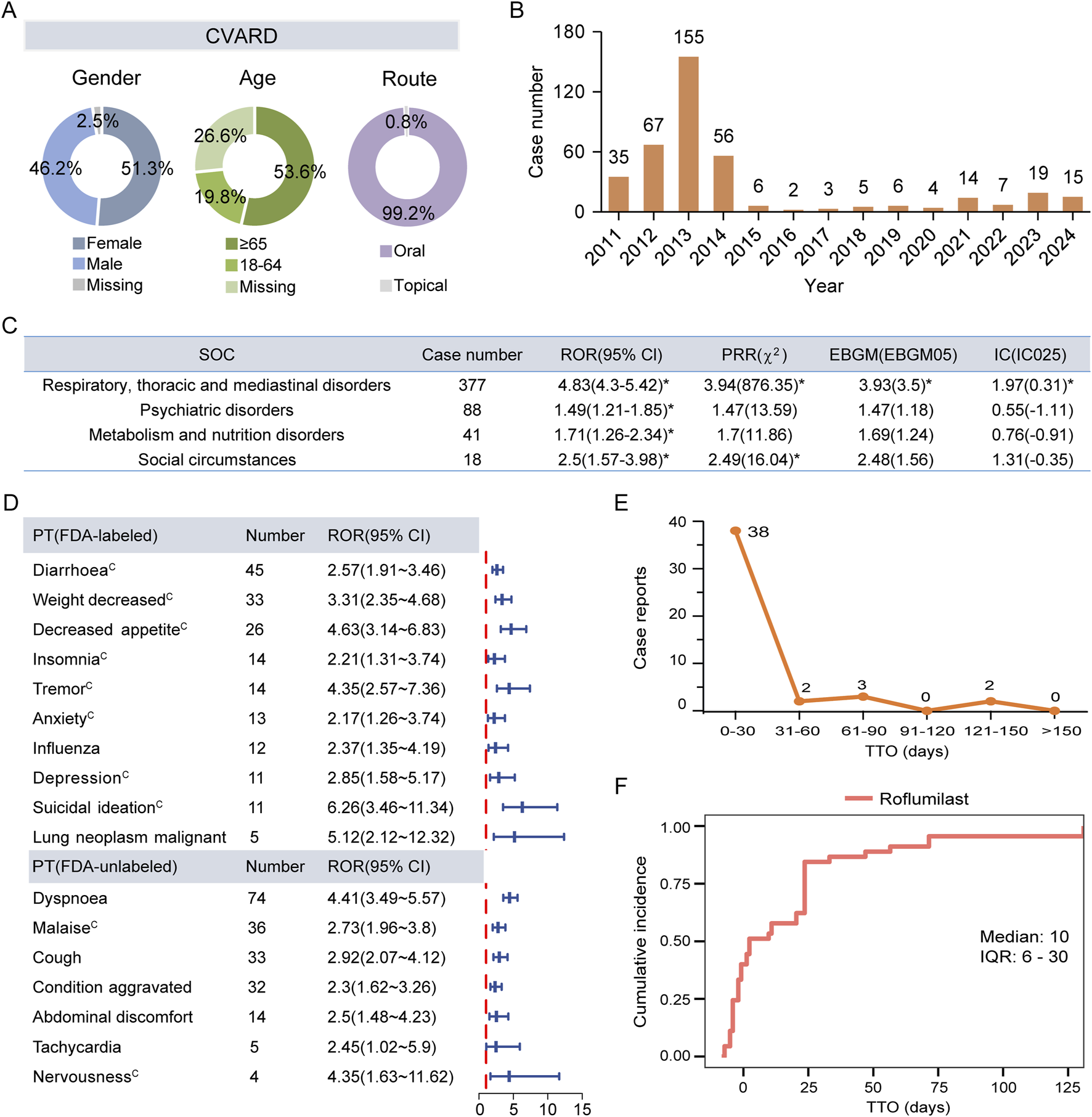
External validation using the CVARD. (A) Basic features of AE reports for roflumilast. (B) Temporal distribution of adverse drug event reports from 2011 Q1 to 2024 Q4. (C) Positive signals detection at the SOC level. (D) The forest plot of significant ROR signals at the PT level. (E) The frequency distribution of TTO reports. (F) The Kaplan-Meier curve depicts the cumulative incidence. CVARD, Canada Vigilance Adverse Reaction Database; SOC, System Organ Class; ROR, Reporting Odds Ratio; PRR, Proportional Reporting Ratio; EBGM, Empirical Bayesian Geometric Mean; EBGM05, the lower limit of the 95% CI of EBGM; IC, Information Component; IC025, the lower limit of the 95% CI of the IC; CI, confidence interval. PT, Preferred Term; TTO, time-to-onset; IQR, interquartile range. Asterisks (*) indicate positive signals. Alphabets(C) indicate AEs documented in the Canadian Product Monographs.
Further analysis of ROR-positive AEs revealed that 15 PTs overlapped between the Top 30 signals in FAERS and the Top 40 signals in CVARD. The identified AEs comprised 8 labeled events and 7 FDA-unlabeled potential safety concerns. These potential AEs included dyspnea (n = 74, ROR 4.41), malaise (n = 36, ROR 2.73), cough (n = 33, ROR 2.92), condition aggravated (n = 32, ROR 2.3), abdominal discomfort (n = 14, ROR 2.5), tachycardia (n = 5, ROR 2.45), and nervousness (n = 4, ROR 4.35). In contrast to the FDA-approved labeling, the Canadian product monograph lists malaise and nervousness as documented AEs. Influenza (n = 12, ROR 2.37) and lung neoplasm malignant (n = 5, ROR 5.12) were identified among the top 40 significant PTs in CVARD. However, these events are documented in the FDA-approved labeling but absent from the Canadian product monograph (Figure 5D; Supplementary Figure S2). Consistent with FAERS data, AE reporting frequency of CVARD peaked within the first 30 days post-administration period followed by a gradual decline (Figure 5E). The median TTO for roflumilast-mediated adverse reactions was 10 days (IQR: 6–30 days) (Figure 5F).
Discussion
In this study, we systematically analyzed the real-world reports of roflumilast from 2011 Q1 to 2024 Q4 through the FAERS and CVARD databases. Analysis of patient characteristics revealed that the AE reporting rates were higher among the older adults (≥65 years) and oral administration population. The data suggested that similar counts of roflumilast’s adverse reaction reports were observed in males and females. Roflumilast-related AEs were reported most frequently in 2013, showing the first peak. This may be attributed to the Weber effect, which describes the characteristic pattern of spontaneously reported adverse reactions peaking within the first 2 years post-approval and subsequently declining [22]. While the second peak was in 2023, following its application for psoriasis. These results highlight the extensive clinical application of roflumilast and the requirement for surveillance.
We found some adverse reactions contained in the drug instructions, including diarrhea, nausea, decreased appetite, weight loss, headache, dizziness, insomnia, anxiety, depression, suicidal ideation and lung neoplasm malignant, which validated the reliability of our results. Our analysis identified distortional reporting of roflumilast-associated AEs that were not documented in the prescribing information, such as heart rate increased, tachycardia, palpitations, and condition aggravated.
The meta-analysis showed that AF was more frequent in the roflumilast group than in the placebo group (0.4% vs. 0.2%) [23]. An accelerated and irregular heart rhythm is the hallmark of AF, which can be non-symptomatic or cause symptoms like palpitations, increased heart rate, chest pain, nausea, dizziness, dyspnea, and general fatigue [24]. Immune remodeling is an important mechanism in AF which can increase the release of cytokines (e.g., IL-6) and exosomes by activating macrophages. In turn, pacing cardiomyocytes may further promote macrophage activation [25]. PDE4, a key factor in the regulation of heart rate, is able to alter cAMP concentrations of cardiac ryanodine receptor 2 microstructural domain. Likewise, the upregulation of PDE4-dependent cAMP levels in sinus node myocytes increases heart rate [26, 27]. It has been shown that administration of the high-dose PDE4 inhibitor rolipram significantly increased heart rate [28]. In chronic kidney disease, elevated levels of fibroblast growth factor 23 inhibited PDE4B expression in cardiomyocytes, further increasing sarcoplasmic reticulum Ca2+ leakage as well as promoting ventricular arrhythmias [29]. Roflumilast exacerbated tachycardia and hypotension in septic rats while improving renal perfusion and liver damage [30]. Notably, knockdown of PDE4D increased heart rate in hypertensive mice, helping them respond to β-adrenergic receptor stimulation of the sinus node normally [26]. The mechanisms by which roflumilast induces cardiac AEs like AF and palpitations are not yet fully understood, whereas animal studies suggest several potential pathways mediated by PDE4 inhibition. COPD or psoriasis patients who are experiencing hypertension may benefit from roflumilast medication. For patients with chronic nephritis and sepsis, blood pressure and electrocardiogram should be monitored regularly after applying roflumilast.
AEs that strongly associated with roflumilast were condition aggravated, chest pain, influenza-like illness, dyspnea, and cough, despite these actions being not labeled in the insert. Few studies have found the existence of the above five adverse actions. The DERMIS-2 trial observed that one case of psoriasis progression was reported in the topical roflumilast group [12]. In a randomized controlled trial utilizing oral roflumilast for the treatment of moderate-to-severe psoriasis, a non-ischemic chest pain event occurred at weeks 12–24 [31]. A pooled analysis showed that topical roflumilast caused upper respiratory tract infections [32]. CHEST guideline suggests that respiratory tract infections are a major cause of cough [33]. In a 16-week, randomized, double-blind, placebo-controlled trial, a lower number of serious AEs experienced in the roflumilast treatment group (n = 8), including COPD worsening, dyspnea, and influenza A virus. Meanwhile, cough belonged to mild-to-moderate side effect [34]. Although the exact mechanism of exacerbations during roflumilast treatment remains unknown, we hypothesize that it might be connected to the dual effects of cAMP, which reduces T cell proliferation by facilitating the release of IL-10. However, higher dosages of roflumilast caused bone marrow-derived dendritic cells to elevate PDE4B and PDE4D expressions in response to increased cAMP levels. The result was followed by Th17 cell polarization and high expression of IL-23 that stimulated the release of IL-17 and IL-6 [35]. It is crucial to monitor the changes of inflammatory cytokine levels before and after the clinical application of roflumilast, considering the prognosis and quality of life of patients. Physicians should timely detect and assess exacerbation, chest pain, and respiratory dysfunctions in roflumilast-treated patients. Depending on the situation, adjusting the therapeutic dosage, the combination of medications, or symptomatic supportive therapy may be required.
Multiple clinical trials demonstrated that the roflumilast-related predominant AEs involved the gastrointestinal and psycho-neurological systems, as well the majority of AEs were transient and mild-to-moderate in severity. Long-term treatment of roflumilast in COPD or plaque psoriasis revealed higher incidence rates of diarrhea, nausea, headache, and insomnia than placebo [1, 36]. An analysis of 15 trials involving 11,168 COPD subjects found higher incidences of psychiatric-related adverse reactions in roflumilast 500 μg group than those in the control group (OR 2.13, 95% CI: 1.79–2.54), particularly an increased possibility of sleep disorder, anxiety, and depressed mood. Notably, participants receiving roflumilast reported 3 cases of suicidal behavior and 2 suicide attempts, whereas no related behaviors were noted in placebo-treated participants. No statistically significant difference was observed in psychiatric AEs between the 250 μg roflumilast and placebo groups (OR 0.87, 95% CI: 0.56–1.33) [37]. Although nervousness and sleep disorders are not listed in the FDA-approved labeling while being included in Canadian labels, their identification in our real-world data illustrates the dynamic nature of drug safety knowledge, which in turn underscores the importance of ongoing, systematic pharmacovigilance to capture and validate safety signals across diverse information sources.
Present studies have found that the regulatory role of PDE4 in gastrointestinal systems strongly associated with common AEs, such as nausea and diarrhea. The following postulated mechanisms may contribute to these two AEs. There is evidence that nausea is closely connected to delayed stomach transit. Roflumilast inhibited gastric transit more effectively than selective PDE4B inhibitors, but PDE4 inhibitor-induced gastroparesis wasn’t influenced by gene deletion of any one PDE4 subtype, illustrating that two or more PDE4 subtypes may be involved in this condition. Mechanistically, roflumilast-induced PDE4D inhibition may contribute to diarrhea through the signals of cystic fibrosis transmembrane conductance regulator and 5-hydroxytryptamine 4 receptor [38]. Animal research revealed that a prolonged overdose of roflumilast led to diarrhea, as well as increasing serum levels of cytokine-induced neutrophil chemotactic factor 1 and leucocytes. Diclofenac, a non-selective cyclooxygenase inhibitor, could protect against the toxic effects mentioned above by roflumilast [39]. Therefore, taking diclofenac helps patients improve roflumilast-induced diarrhea and abdominal pain. Anti-diarrheal drugs like oral rehydration salts can be used to treat dehydration induced by severe diarrhea. In addition, roflumilast inhaler is being developed to avoid gastrointestinal problems [40].
PDE4 also plays a critical role in the central nervous system. When compared to wild-type (WT) mice, PDE4A KO or PDE4B KO mice proved anxiety characteristics along with higher corticosterone levels, while PDE4B KO mice also showed increased long-term depression [41–43]. Furthermore, PDE4D KO mice slept for a shorter time than their WT littermates under xylazine/ketamine-induced anesthesia [44]. The above mechanistic hypothesis could be the reason for induction of insomnia, sleep disorder or vomiting with roflumilast. Thus, physicians should proactively inquire about the patient’s psychological history or conduct a psychological examination before prescribing roflumilast. To lower the patient’s risk of adverse mental events, dose reduction or gradual dose escalation should be considered.
In our study, there were only a limited number of reports regarding AEs associated with cancer. Reports of cancer in clinical trials were consistently rare and not considered to be directly caused by roflumilast [1]. Recent research has indicated that roflumilast may inhibit the proliferation of liver and ovarian cancer cells [45, 46]. However, PDE4-dependent cAMP was also found to suppress both innate and adaptive immunity, which could potentially facilitate immunological escape. Evidence suggested that roflumilast increased the volume of B-cell lymphomas while decreasing CD3+ cell and CD4/CD8 ratios, indicating its potential immunosuppressive properties. Specifically, inhibition of PDE4 enhanced cytokines (IL-10, IL-8, and IL-6) gene transcription through activation of the cAMP/PKA/CREB signaling pathway. These cytokines subsequently bond to their respective receptors in an autocrine manner, further phosphorylating JAK/STAT signaling and elevating transcription as well as surface expression levels of the immune checkpoint PD-L1 [47]. The aforementioned speculative mechanisms for roflumilast-associated malignancies require validation with additional clinical samples. Despite the ongoing debate on roflumilast’s carcinogenic potential, close surveillance of cancer-related AEs in clinical practice is indispensable.
Roflumilast plasma concentrations were similar among children, adolescents, and adults; however, gender and age were found to influence roflumilast clearance and metabolic fraction in vivo. A pharmacokinetic study of roflumilast revealed higher total PDE4 inhibitory activity in female and older patients with COPD [48, 49]. Multivariate analysis of Korean patients revealed that older age was significantly associated with higher rates of roflumilast treatment withdrawal [50]. Thus, it was considered that drug discontinuation is more likely to occur in females and the older adults. In terms of gender, females were prone to develop AEs such as nausea, headache, and back pain. Notably, males were more inclined to AF and suicide attempt. This could be attributed to males having higher hazards of AF and suicide [51]. So as to properly manage and intervene with side effects, it is crucial to consider age and gender differences into account when evaluating drug safety. Females and the older adults should also pay special attention to the reasons for roflumilast discontinuation.
Psoriasis has been approved for topical treatment with roflumilast cream [36]. Clinical trials treating oral roflumilast for psoriasis have been shown equally excellent safety and tolerability. Similarly to the analysis of a randomized controlled trial, our study thought that headache was the most common AEs in the topical group. It was worth noting that decreased weight was more prevalent in the oral roflumilast group [2, 31]. In phase II clinical trials, topical roflumilast caused minimal weight change in patients with psoriasis [52]. Studies have shown that roflumilast may help obese patients decrease weight by inhibiting adipogenesis and promoting lipolysis through AMPKα activation [53]. A multicenter study showed that patients with low body mass index (BMI <23 kg/m2) were more likely to experience adverse reactions and then choose to discontinue treatment with roflumilast [13]. Therefore, patients with BMI <23 kg/m2 must be cautious with roflumilast or even avoid choosing an oral dosage form. But if applying the topical formulation of roflumilast, concern should be taken to deal with headache, drug ineffective and dermatologic manifestations that haven’t yet been listed on the label (e.g., burning sensation, exfoliation, and discoloration).
For TTO analysis, only 16.7% reports of roflumilast-related reports in the FAERS database were usable. Based on the available TTO data, 60.1% reports occurred within the first month after initiating therapy. Previous studies similarly found that AEs associated with oral roflumilast occur in the first 4 weeks, whereas roflumilast cream-associated AEs were usually reported in the first 2 weeks after treatment [12, 54]. Long-acting bronchodilators such as long-acting β2-agonists and long-acting muscarinic antagonists, which together with inhaled corticosteroid are the basic drugs for long-term management of COPD. Whether used as monotherapy or combination therapy (e.g., dual or triple therapy), roflumilast has demonstrated the greatest benefit in patients with moderate-to-severe COPD [11, 55]. More efficiently than either medication alone, formoterol and roflumilast combination prevented LPS-induced production of TNFα and chemokines that recruited monocytes and T cells in human bronchus model in vitro [56]. Finally, we performed sensitivity analyses to identify side effects influenced by roflumilast alone, especially gastrointestinal issues, psychological disorders, pulmonary conditions and heart problems. These AEs may challenge patients’ medication adherence and compromise the effectiveness of roflumilast.
This research is the most comprehensive in-depth, and detailed pharmacovigilance study of roflumilast-related AEs depending on two databases. Nevertheless, it is crucial to acknowledge the limitations of this study. First, the databases have inherent limitations, such as under-reporting, reporting bias, and incomplete data. Under-reporting may occur due to patients’ lack of reporting awareness or clinicians’ failure to identify events. Reporting bias can stem from the inconsistent understanding of reporters, obstructing an accurate evaluation of roflumilast’s safety profile. Additionally, incomplete report details may reduce representativeness and introduce selection bias, which necessitate cautious interpretation of findings like TTO. Second, there are confounding variables in the study. Although this study only selected primary suspected drugs for analysis and conducted sensitivity analyses to exclude concomitant medications, potential confounding variables (e.g., duration of use, comorbidities, demographic variations) may affect the accuracy of the results and hinder stratified assessments based on patient characteristics. Third, signal detection in pharmacovigilance primarily provides associative information, estimating signal strength rather than establishing a definitive causal relationship between roflumilast and AEs. Confirming causality requires further real-world validation, including large-scale prospective cohort studies, electronic health record-based case-control studies, and mechanism-based research (animal models and cell experiments) to verify causality and clarify biological mechanisms. Despite the above limitations, the cross-validation approach using the FAERS and CVARD databases still provides valuable insights and guidance for the post-marketing safety monitoring and rare signal detection of roflumilast.
Conclusion
This study conducted a comprehensive analysis of roflumilast-associated AEs reported in the FAERS and CVARD databases. Our analysis identified disproportional reporting of several labeled risks while detecting novel safety concerns. Valuable risk suggestions were provided for different populations through subgroup analysis. The findings emphasize that individualized evaluation of patient characteristics and comorbidities before prescribing roflumilast is a key strategy for risk mitigation and rational pharmacotherapy.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The FDA Adverse Event Reporting System and Canada Vigilance Adverse Reaction Database are spontaneous reporting systems. The publicly available data in both are anonymized, so obtaining informed consent for participant involvement is not applicable. This pharmacovigilance study utilized public databases of spontaneous reports. Due to the use of deidentified data, ethical approval was deemed unnecessary.
Author contributions
RX: Writing – original draft, Data curation, Methodology, Formal analysis, Visualization, and Funding acquisition. HP: Writing – original draft and Conceptualization. CS: Writing – original draft and Conceptualization. ML: Writing – review and editing and Conceptualization. PG: Writing – review and editing, Methodology, Conceptualization, and Funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This study was supported by the funding for Scientific Research Projects from Wuhan Children's Hospital (No. 2024FEBSJJ003) and National Natural Science Foundation of China (No. 82104300).
Acknowledgments
We would like to express our sincere gratitude to the FAERS database provided by FDA and Canada Vigilance Program conducted by Health Canada.
Conflict of interest
The author(s) declared that this work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declared that generative AI was not used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/jpps.2025.15678/full#supplementary-material
References
1.
RabeKF. Update on roflumilast, a phosphodiesterase 4 inhibitor for the treatment of chronic obstructive pulmonary disease. Br J Pharmacol (2011) 163:53–67. 10.1111/j.1476-5381.2011.01218.x
2.
O'TooleAGooderhamM. Topical roflumilast for plaque psoriasis. Skin Ther Lett (2023) 28:1–4.
3.
ElmetsCALeonardiCLDavisDMRGelfandJMLichtenJMehtaNNet alJoint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol (2019) 80:1073–113. 10.1016/j.jaad.2018.11.058
4.
CaramoriGRuggeriPDi StefanoAMumbySGirbinoGAdcockIMet alAutoimmunity and COPD: clinical implications. Chest (2018) 153:1424–31. 10.1016/j.chest.2017.10.033
5.
SugumaranDYongACHStanslasJ. Advances in psoriasis research: from pathogenesis to therapeutics. Life Sci (2024) 355:122991. 10.1016/j.lfs.2024.122991
6.
MehrmalSUppalPNedleyNGieseyRLDelostGR. The global, regional, and national burden of psoriasis in 195 countries and territories, 1990 to 2017: a systematic analysis from the global burden of disease study 2017. J Am Acad Dermatol (2021) 84:46–52. 10.1016/j.jaad.2020.04.139
7.
ChenSKuhnMPrettnerKYuFYangTBärnighausenTet alThe global economic burden of chronic obstructive pulmonary disease for 204 countries and territories in 2020-50: a health-augmented macroeconomic modelling study. Lancet Glob Health (2023) 11:e1183–e1193. 10.1016/s2214-109x(23)00217-6
8.
SchaferPHTruzziFPartonAWuLKosekJZhangLHet alPhosphodiesterase 4 in inflammatory diseases: effects of apremilast in psoriatic blood and in dermal myofibroblasts through the PDE4/CD271 complex. Cell Signal (2016) 28:753–63. 10.1016/j.cellsig.2016.01.007
9.
LugnierC. The complexity and multiplicity of the specific cAMP phosphodiesterase family: PDE4, open new adapted therapeutic approaches. Int J Mol Sci (2022) 23:23. 10.3390/ijms231810616
10.
LiHZuoJTangW. Phosphodiesterase-4 inhibitors for the treatment of inflammatory diseases. Front Pharmacol (2018) 9:1048. 10.3389/fphar.2018.01048
11.
ReidDJPhamNT. Roflumilast: a novel treatment for chronic obstructive pulmonary disease. Ann Pharmacother (2012) 46:521–9. 10.1345/aph.1Q646
12.
LebwohlMGKircikLHMooreAYStein GoldLDraelosZDGooderhamMJet alEffect of roflumilast cream vs vehicle cream on chronic plaque psoriasis: the DERMIS-1 and DERMIS-2 randomized clinical trials. Jama (2022) 328:1073–84. 10.1001/jama.2022.15632
13.
KimKHKangHSKimJSYoonHKKimSKRheeCK. Risk factors for the discontinuation of roflumilast in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis (2017) 12:3449–56. 10.2147/copd.S143967
14.
Beau-LejdstromRCrookSSpanuAYuTPuhanMA. Adverse drug reaction risk measures: a comparison of estimates from drug surveillance and randomised trials. Pharmaceut Med (2019) 33:331–9. 10.1007/s40290-019-00287-y
15.
AbdelazizAGaberCEGuptaPLeeTA. High throughput pharmacovigilance screening for roflumilast adverse effects in real-world settings: a sequence symmetry analysis. Basic Clin Pharmacol Toxicol (2025) 136:e70038. 10.1111/bcpt.70038
16.
ZhengYGaoXJHuangJJChenXMLiaoYLiuJMet alThe safety assessment of ustekinumab on psoriasis and psoriatic arthritis: a real-world analysis based on the FDA adverse event reporting system database. Int Immunopharmacol (2025) 151:114339. 10.1016/j.intimp.2025.114339
17.
AliTBSchleretTRReillyBMChenWYAbagyanR. Adverse effects of cholinesterase inhibitors in dementia, according to the pharmacovigilance databases of the united-states and Canada. PLoS One (2015) 10:e0144337. 10.1371/journal.pone.0144337
18.
ZouFCuiZLouSOuYZhuCShuCet alAdverse drug events associated with linezolid administration: a real-world pharmacovigilance study from 2004 to 2023 using the FAERS database. Front Pharmacol (2024) 15:1338902. 10.3389/fphar.2024.1338902
19.
HuangHHuCLiuFJiFFuYCaoM. Glucagon-like peptide-1 receptor agonists and impaired gastric emptying: a pharmacovigilance analysis of the US food and drug administration adverse event reporting system. Br J Anaesth (2025) 134:1486–96. 10.1016/j.bja.2024.10.013
20.
ChenYGuoJJSteinbuchMLinXBuncherCRPatelNC. Comparison of sensitivity and timing of early signal detection of four frequently used signal detection methods. Pharmaceut Med (2008) 22:359–65. 10.1007/BF03256733
21.
ZouFZhuCLouSCuiZWangDOuYet alA real-world pharmacovigilance study of mepolizumab in the FDA adverse event reporting system (FAERS) database. Front Pharmacol (2023) 14:1320458. 10.3389/fphar.2023.1320458
22.
HartnellNRWilsonJP. Replication of the weber effect using postmarketing adverse event reports voluntarily submitted to the United States food and drug Administration. Pharmacotherapy (2004) 24:743–9. 10.1592/phco.24.8.743.36068
23.
ObaYLoneNA. Efficacy and safety of roflumilast in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Ther Adv Respir Dis (2013) 7:13–24. 10.1177/1753465812466167
24.
NesheiwatZGoyalAJagtapM. Atrial fibrillation. StatPearls (2025).
25.
YaoYYangMLiuDZhaoQ. Immune remodeling and atrial fibrillation. Front Physiol (2022) 13:927221. 10.3389/fphys.2022.927221
26.
DoreyTWMcRaeMDBelkeDDRoseRA. PDE4D mediates impaired β-adrenergic receptor signalling in the sinoatrial node in mice with hypertensive heart disease. Cardiovasc Res (2023) 119:2697–711. 10.1093/cvr/cvad138
27.
PavlakiNFroeseALiWDe JongKAGeertzBSubramanianHet alGene therapy with phosphodiesterases 2A and 4B ameliorates heart failure and arrhythmias by improving subcellular cAMP compartmentation. Cardiovasc Res (2024) 120:1011–23. 10.1093/cvr/cvae094
28.
ChenWYLinCHLeeYSTsaoPCJengMJ. Pathophysiological effects of intravenous phosphodiesterase type 4 inhibitor in addition to surfactant lavage in meconium-injured newborn piglet lungs. Pediatr Pulmonol (2020) 55:2272–82. 10.1002/ppul.24880
29.
LindnerMMehelHDavidALeroyCBurtinMFriedlanderGet alFibroblast growth factor 23 decreases PDE4 expression in heart increasing the risk of cardiac arrhythmia; klotho opposes these effects. Basic Res Cardiol (2020) 115:51. 10.1007/s00395-020-0810-6
30.
FerreiraAGOliveiraJGNakashimaMADelfrateGSordiRAssreuyJet alCardiovascular effects of roflumilast during sepsis: risks or benefits?Eur J Pharmacol (2024) 983:177015. 10.1016/j.ejphar.2024.177015
31.
GyldenløveMMeteranHSørensenJAFageSYaoYLindhardsenJet alEfficacy and safety of oral roflumilast for moderate-to-severe psoriasis-a randomized controlled trial (PSORRO). Lancet Reg Health Eur (2023) 30:100639. 10.1016/j.lanepe.2023.100639
32.
de Moraes-SouzaRChahine ChaterRPera CalviIMesquitaYSartoRLapendaIet alEfficacy and safety of topical roflumilast for the treatment of psoriasis: a systematic review and meta-analysis of randomized controlled trials. Clin Drug Investig (2024) 44:655–65. 10.1007/s40261-024-01368-w
33.
IrwinRSFrenchCLChangABAltmanKW, CHEST Expert Cough Panel*. Classification of cough as a symptom in adults and management algorithms: CHEST guideline and expert panel report. Chest (2018) 153:196–209. 10.1016/j.chest.2017.10.016
34.
RabeKFWatzHBaraldoSPedersenFBiondiniDBagulNet alAnti-inflammatory effects of roflumilast in chronic obstructive pulmonary disease (ROBERT): a 16-week, randomised, placebo-controlled trial. Lancet Respir Med (2018) 6:827–36. 10.1016/s2213-2600(18)30331-x
35.
BrosMMontermannECholaszczyńskaAReske-KunzAB. The phosphodiesterase 4 inhibitor roflumilast augments the Th17-promoting capability of dendritic cells by enhancing IL-23 production, and impairs their T cell stimulatory activity due to elevated IL-10. Int Immunopharmacol (2016) 35:174–84. 10.1016/j.intimp.2016.03.025
36.
GuptaAKRaviSPVincentKAbramovitsW. ZORYVE(TM) (roflumilast) cream: a topical Phosphodiesterase-4 inhibitor for the treatment of psoriasis. Skinmed (2023) 21:357–9.
37.
JanjuaSFortescueRPooleP. Phosphodiesterase-4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev (2020) 5:Cd002309. 10.1002/14651858.CD002309.pub6
38.
PaesDSchepersMRombautBvan den HoveDVanmierloTPrickaertsJ. The molecular biology of phosphodiesterase 4 enzymes as pharmacological targets: an interplay of isoforms, conformational states, and inhibitors. Pharmacol Rev (2021) 73:1016–49. 10.1124/pharmrev.120.000273
39.
PeterDGöggelRColbatzkyFNickolausP. Inhibition of cyclooxygenase-2 prevents adverse effects induced by phosphodiesterase type 4 inhibitors in rats. Br J Pharmacol (2011) 162:415–27. 10.1111/j.1476-5381.2010.01035.x
40.
SuzukiÉYAmaroMIde AlmeidaGSCabralLMHealyAMde SousaVP. Development of a new formulation of roflumilast for pulmonary drug delivery to treat inflammatory lung conditions. Int J Pharm (2018) 550:89–99. 10.1016/j.ijpharm.2018.08.035
41.
HansenRT3rdContiMZhangHT. Mice deficient in phosphodiesterase-4A display anxiogenic-like behavior. Psychopharmacology (Berl) (2014) 231:2941–54. 10.1007/s00213-014-3480-y
42.
ZhangHTHuangYMasoodAStolinskiLRLiYZhangLet alAnxiogenic-like behavioral phenotype of mice deficient in phosphodiesterase 4B (PDE4B). Neuropsychopharmacology (2008) 33:1611–23. 10.1038/sj.npp.1301537
43.
RuttenKWallaceTLWorksMPrickaertsJBloklandANovakTJet alEnhanced long-term depression and impaired reversal learning in phosphodiesterase 4B-knockout (PDE4B-/-) mice. Neuropharmacology (2011) 61:138–47. 10.1016/j.neuropharm.2011.03.020
44.
RobichaudAStamatiouPBJinSLLachanceNMacDonaldDLalibertéFet alDeletion of phosphodiesterase 4D in mice shortens alpha(2)-adrenoceptor-mediated anesthesia, a behavioral correlate of emesis. J Clin Invest (2002) 110:1045–52. 10.1172/jci15506
45.
GongSChenYMengFZhangYLiCZhangGet alRoflumilast enhances cisplatin-sensitivity and reverses cisplatin-resistance of ovarian cancer cells via cAMP/PKA/CREB-FtMt signalling axis. Cell Prolif (2018) 51:e12474. 10.1111/cpr.12474
46.
RenHChenYAoZChengQYangXTaoHet alPDE4D binds and interacts with YAP to cooperatively promote HCC progression. Cancer Lett (2022) 541:215749. 10.1016/j.canlet.2022.215749
47.
SasiBEthirajPMyersJLinAPJiangSQiuZet alRegulation of PD-L1 expression is a novel facet of cyclic-AMP-mediated immunosuppression. Leukemia (2021) 35:1990–2001. 10.1038/s41375-020-01105-0
48.
NevilleKASzeflerSJAbdel-RahmanSMLahuGZechKHerzogRet alSingle-dose pharmacokinetics of roflumilast in children and adolescents. J Clin Pharmacol (2008) 48:978–85. 10.1177/0091270008319466
49.
LahuGHünnemeyerADilettiEElmlingerMRuthPZechKet alPopulation pharmacokinetic modelling of roflumilast and roflumilast N-oxide by total phosphodiesterase-4 inhibitory activity and development of a population pharmacodynamic-adverse event model. Clin Pharmacokinet (2010) 49:589–606. 10.2165/11536600-000000000-00000
50.
ParkTSKangJLeeJSOhYMLeeSDLeeSW. Adherence to roflumilast under dose-escalation strategy in Korean patients with COPD. Int J Chron Obstruct Pulmon Dis (2019) 14:871–9. 10.2147/copd.S191033
51.
AndradeJGDeyellMWLeeAYKMacleL. Sex differences in atrial fibrillation. Can J Cardiol (2018) 34:429–36. 10.1016/j.cjca.2017.11.022
52.
SteinGLAdamDNAlbrechtLAlonso-LlamazaresJFerrisLKGooderhamMJet alLong-term safety and effectiveness of roflumilast cream 0.3% in adults with chronic plaque psoriasis: a 52-week, phase 2, open-label trial. J Am Acad Dermatol (2024) 91:273–80. 10.1016/j.jaad.2024.03.030
53.
XuWZhangJXiaoJ. Roflumilast suppresses adipogenic differentiation via AMPK mediated pathway. Front Endocrinol (Lausanne) (2021) 12:662451. 10.3389/fendo.2021.662451
54.
CalverleyPMSanchez-TorilFMcIvorATeichmannPBredenbroekerDFabbriLM. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med (2007) 176:154–61. 10.1164/rccm.200610-1563OC
55.
VogelmeierCFCrinerGJMartinezFJAnzuetoABarnesPJBourbeauJet alGlobal strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Eur Respir J (2017) 49:49. 10.1183/13993003.00214-2017
56.
SalvatorHBuenestadoABrolloMNalineEVictoniTLongchampEet alClinical relevance of the anti-inflammatory effects of roflumilast on human bronchus: potentiation by a long-acting beta-2-agonist. Front Pharmacol (2020) 11:598702. 10.3389/fphar.2020.598702
Summary
Keywords
roflumilast, adverse events, disproportionality analysis, FAERS, CVARD
Citation
Xu R, Peng H, Shu C, Liu M and Gao P (2026) Real-world safety profile of roflumilast: a pharmacovigilance analysis using FDA adverse event reporting system and Canada vigilance database. J. Pharm. Pharm. Sci. 28:15678. doi: 10.3389/jpps.2025.15678
Received
30 September 2025
Revised
14 November 2025
Accepted
23 December 2025
Published
12 January 2026
Volume
28 - 2025
Edited by
Kenneth McCall, Binghamton University, United States
Updates
Copyright
© 2026 Xu, Peng, Shu, Liu and Gao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Gao, gaoping@hust.edu.cn; Maochang Liu, liumaochang007@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.